Abstract
Background:
Although emerging evidence has suggested the relationship of chronic obstructive pulmonary disease (COPD) with atrial fibrillation (AF), little is known about whether acute exacerbation of COPD (AECOPD) increases the risk of repeated AF-related healthcare utilization.
Methods and Results:
This is a self-controlled case series study using the population-based ED and inpatient databases of five U.S. states from 2007 through 2012. Among patients with existing AF, we identified patients with an AECOPD hospitalization and at least one ED visit or hospitalization for AF during the observation period. We constructed conditional Poisson regression models to compare the rate of AF-related ED visits or hospitalizations during sequential 90-day periods after the AECOPD hospitalization, with pre-AECOPD days 1–90 as the reference. Of 944 patients eligible for the study, the median age was 77 years and 41% were male. Compared to the reference period, the rate of AF-related ED visits or hospitalizations significantly increased in the post-AECOPD days 1–90 (7.3 vs 14.1 per 100 person-months; rate ratio [RR] 1.93; 95%CI 1.63–2.29; P<0.001). Then, the rate decreased to the reference-level in the post-AECOPD days 91–180 (7.5 per 100 person-months; RR 1.03; 95%CI 0.85–1.25; P=0.77) and remained at the reference-level during post-AECOPD days 181–270 (RR 0.84; 95%CI 0.68–1.03; P=0.09) and days 271–360 (RR 0.90; 95%CI 0.73–1.10; P=0.29). These temporal associations persisted with stratification by age, sex, and season.
Conclusions:
Among patients with existing AF, AECOPD was associated with a higher risk of AF-related ED visit or hospitalization in the first 90-day post-AECOPD period.
Keywords: Atrial fibrillation, acute exacerbation of COPD, epidemiology, healthcare utilization
INTRODUCTION
Atrial fibrillation (AF) is a major public health problem that affects 6 million Americans1 and accounts for 540,000 emergency department (ED) visits and 400,000 hospitalizations annually.1 Among patients with AF, one-third of patients are hospitalized each year.2 Additionally, with aging in the population, the number of AF-related hospitalization has increased by 23% over the last decade.3 Because of its clinical and public health importance,3 understanding risk factors for AF-related morbidities is instrumental to developing preventive strategies.
In parallel to AF, chronic obstructive pulmonary disease (COPD) is another major public health problem, with 600,000 hospitalizations for acute exacerbation of COPD (AECOPD) in the U.S. each year.1 Recent studies have shown the linkage between COPD and AF. For example, cohort studies have reported that COPD is a risk factor for new-onset AF.4–7 In addition, another cohort study revealed that, in patients with paroxysmal AF, concurrent COPD is a risk factor for AF progression, from paroxysmal AF to persistent AF.8 These emerging evidence suggest the relationship of COPD (as a chronic morbidity) with both the development and progression of AF. Despite the clinical and physiological importance of AECOPD, little is known about whether, in patients with existing AF, AECOPD is associated with a higher risk of AF-related morbidities.
To address the knowledge gap, we investigated the temporal association between AECOPD and AF-related ED visits or hospitalizations. Specifically, we hypothesized, among adults with AF, that an AECOPD hospitalization increases the rate of AF-related ED visits or hospitalizations within one-year period after the hospitalization, compared to that in the pre-AECOPD hospitalization period.
METHODS
Study Design and Setting
We performed a self-controlled case series study using the Healthcare Cost and Utilization Project (HCUP) State Emergency Department Databases (SEDD) and State Inpatient Databases (SID). Briefly, we created a cohort of patients with AF, and then compared each subject’s rate of AF-related ED visits or hospitalizations during a 450-day period (i.e., 90-day period before and 360-day period after AECOPD). Based on the data use agreement, the datasets and study materials will not be made publically available. The self-controlled case series design allows each subject to serve as his/her own control. Therefore, it minimizes confounding by time-invariant factors and removes inter-person variations. The data were extracted from the HCUP SEDD and SID of geographically-diverse five U.S. states (California, Florida, Nebraska, New York, and Utah), from January 2007 through December 2012. The HCUP is the largest collection of longitudinal hospital care data in the US, with all-payer, encounter-level information.9 The SEDD encompass all ED visits, including treat-and-release encounters and transfers; the SID capture all hospitalizations, regardless of source or disposition, from acute care, non-federal, general, and other specialty hospitals within the participating states. Taken together, the SEDD and SID capture all ED visits regardless of disposition and all hospitalizations regardless of admission source. Additional details of the HCUP SEDD and SID can be found elsewhere.9 The five states were selected for their geographical diversity, data quality, and chiefly because that their data contain unique subject identifiers that enable us to follow specific subject longitudinally. The institutional review board of Massachusetts General Hospital approved this study.
Study Population
First, we identified all adults who had at least one ED visit or hospitalization for AF (defined by the International Classification of Disease-Ninth Revision-Clinical Modification [ICD-9-CM] code of 427.31)3 in any diagnostic field in 2007. From these adults, to create the analytic cohort, we further identified patients (aged ≥40 years) with an exposure – at least one unplanned hospitalization for AECOPD (ICD-9-CM codes, 491.21, 491.22, 491.8, 491.9, 492.8, 493.20, 493.21, 493.22, and 496) or those with a primary diagnosis of respiratory failure (ICD-9-CM codes, 518.81, 518.82, 518.84, and 799.1) and a secondary diagnosis of COPD10 – as well as an outcome – at least one ED visit or hospitalization for AF – during 2008 to 2011. To minimize the potential misclassification of COPD, we excluded patients aged <40 years because they are less likely to have COPD.10, 11 We excluded patients who live outside of the study states, those who died during the index hospitalization, and those who left the hospital against medical advice. Selection of study samples is shown in Figure 1.
Figure 1.
Selection of study samples
Visualized presentation of the selection flow of the study samples.
Measurements
The databases recorded patient characteristics, including demographics (age, sex, and race/ethnicity), primary insurance type (payer), quartiles for estimated household income, patient residence, ICD-9-CM diagnostic and procedure codes, and patient comorbidities (29 Elixhauser comorbidity measures). We determined the baseline characteristics at the time of hospitalization for AECOPD.
Primary Exposure
The primary exposure was hospitalization for AECOPD as defined above.10–12 For the analysis, we used the data of the first AECOPD hospitalization for each individual. To isolate the effects of a single AECOPD hospitalization on the outcomes, we excluded patients who had an additional AECOPD hospitalization during the follow-up period.
Outcome Measure
The outcome measure of interest was the composite of ED visit or hospitalization with a primary diagnosis of AF3, 13–15 during the 90-day period preceding the AECOPD hospitalization and the 360-day period following the hospitalization.
Statistical Analysis
We used a self-controlled case series method to perform within-person comparisons among those who experienced both the exposure (i.e., hospitalization for AECOPD) and outcome (ED visit or hospitalization for AF) during a 450-day period. No separate control group was necessary as this method allows each patient to function as his or her own control.16 We computed the rate of AF-related ED visits or hospitalizations for 450 consecutive days (90 days before and 360 days after the AECOPD hospitalization; Supplemental Figure 1). We described the rate of AF-related ED visits or hospitalizations – by computing the number of outcome events / 100 person-months – at each 90-day interval over the 450-day period (90 days before and 360 days after the AECOPD hospitalization). To compare each patient’s rate of the outcomes with pre-AECOPD days 1 to 90 as the reference period, we fit conditional Poisson regression models calculating the rate ratios (RRs) and the 95% confidence intervals (95%CIs) for post-AECOPD days 1 to 90, days 91 to 180, days 181 to 270, and days 271 to 360. For the graphic presentation, we also calculated the RRs in 30-day intervals with pre-AECOPD days 61–90 as the reference period.
We performed a series of sensitivity analyses to examine the robustness of our inference. First, we repeated the analysis with stratification by age category (age 40–64 and ≥65 years) sex, and presence of AF in any diagnostic fields at the index hospitalization. Second, to account for the seasonal variation of AF,17 we repeated the analysis with stratification by season of the index hospitalization for AECOPD: spring (March-May), summer (June-August), fall (September-November), and winter (December-February).18 Third, to address the potential misclassification of AF, we repeated the analysis including the patients with a primary diagnosis of heart failure (ICD-9-CM diagnosis code of 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.0)19, 20 and secondary diagnosis of AF. Fourth, to account for the potential effects of separate mild-to-moderate AECOPD episodes (in addition to the AECOPD hospitalization) on the outcomes during the reference period, we repeated the analysis limiting to the patients who did not have an AECOPD-related ED visit (treat-and-release) during the reference period. Fifth, we performed the analysis including the patients with multiple hospitalizations for AECOPD during the follow-up period. Sixth, to account for the loss-to-follow-up, we repeated the analysis limiting to those who had any healthcare utilization after the post-AECOPD day of 360 (i.e., those who are known to be alive for at least 360 days after the AECOPD hospitalization). Lastly, we fit conditional logistic regressions modeling the outcome as a binomial variable (instead of a count variable) in 90-day intervals. All analyses used STATA 14.0 (STATA Corp, College Station, TX). All P values were two-tailed, with P<0.05 considered statistically significant.
RESULTS
Patient characteristics
We identified all adults with existing AF who were hospitalized for AECOPD (the exposure) during 2008–2011 in the five U.S. states (n=25,227). Of these, a total of 1,547 patients had an ED visit or hospitalization for AF (the outcome) during a 450-day period. From these samples, we excluded 603 patients who had an additional AECOPD hospitalization, leaving 944 patients eligible for the analysis (Figure 1). Table 1 summarizes the patient characteristics. The median age was 77 years (IQR 67–83 years), 59% were female, and 79% were non-Hispanic white; 51% had congestive heart failure, 35% had diabetes, and 71% had hypertension.
Table 1.
Characteristics of patients with atrial fibrillation hospitalized for acute exacerbation of chronic obstructive pulmonary disease
| Characteristics | n=944 |
|---|---|
| Age (year), median (IQR) | 77 (67–83) |
| Female sex | 560 (59.3) |
| Race/ethnicity | |
| Non-Hispanic white | 714 (78.5) |
| Non-Hispanic black | 84 (9.2) |
| Hispanic | 77 (8.5) |
| Others | 35 (3.8) |
| Primary health insurance | |
| Medicare | 739 (78.3) |
| Medicaid | 67 (7.1) |
| Private | 106 (11.2) |
| Others | 32 (3.4) |
| Quartiles for median household income | |
| 1 (lowest) | 269 (29.1) |
| 2 | 263 (28.5) |
| 3 | 222 (24.0) |
| 4 (highest) | 170 (18.4) |
| Patient residence | |
| Metropolitan | 845 (89.5) |
| Non-metropolitan | 99 (10.5) |
| Selected comorbidities* | |
| Congestive heart failure | 479 (50.7) |
| Depression | 105 (11.1) |
| Diabetes | 332 (35.2) |
| Hypertension | 666 (70.6) |
| Obesity | 140 (14.8) |
| Peripheral vascular disorders | 73 (7.7) |
| Renal failure | 173 (18.3) |
| Hospital state | |
| California | 294 (31.1) |
| Florida | 363 (38.5) |
| Nebraska | 21 (2.2) |
| New York | 260 (27.5) |
| Utah | 6 (0.6) |
Data are shown as n (%) unless otherwise specified
Abbreviation: IQR, inter-quartile range
Comorbidities were selected from 29 Elixhauser comorbidity measures
Associations between AECOPD hospitalization and rate of AF-related acute care use
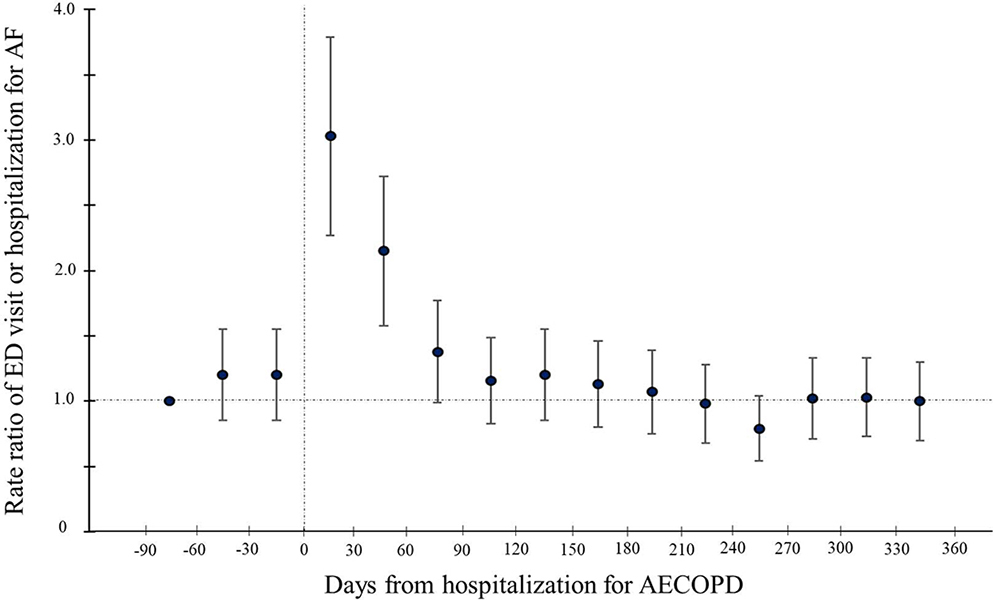
Figure 2 depicts the rate of ED visits or hospitalizations for AF during the pre- and post-AECOPD periods by 30-day intervals. In the reference period (i.e., pre-AECOPD days 1–90), the rate of AF-related ED visits or hospitalizations was 7.3 per 100 person-months (Table 2). In the first 90-day period immediately after the AECOPD hospitalization (i.e., post-AECOPD days 1–90), the rate increased significantly (14.1 per 100 person-months) with a corresponding RR of 1.93 (95%CI 1.63–2.29; P<0.001). Then, in the subsequent 90-day period (i.e., post-AECOPD days 91–180), the rate decreased to the reference-level (7.5 per 100 person-months) with a corresponding RR of 1.03 (95%CI 0.85–1.25; P=0.77). Similarly, the rate remained at the reference-level in the post-AECOPD periods of 181–270 days (6.1 per 100 person-months; RR 0.84 [95% CI 0.68–1.03]; P=0.09) and 271–360 days (6.6 per 100 person-months; RR 0.90 [95%CI 0.73–1.10]; P=0.29).
Figure 2.
Rate ratios of atrial fibrillation-related emergency department visits or hospitalizations before and after hospitalization for acute exacerbation of chronic obstructive pulmonary disease in 30-day intervals
Shown is the rate ratio (RR) of the composite outcome (ED visit or hospitalization for atrial fibrillation [AF]) and the 95% CI during 450-day period in 30-day intervals. The reference period was the pre-hospitalization for acute exacerbation of chronic obstructive pulmonary disease (AECOPD) days 61–90, The rate was highest during the 30-day period immediately after the AECOPD hospitalization (RR 3.03; 95% CI 2.27–4.05; P<0.001) followed by the subsequent 30-day period (RR 2.15; 95% CI 1.58–2.91; P<0.001). In the subsequent periods, the RR did not differ significantly from that in the reference period.
Table 2.
Rate ratio of emergency department visit or hospitalization for atrial fibrillation in patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease (n=944)
| Days from the index hospitalization | Number of outcome events | Rate (per 100 person-months) | Rate ratio* (95% CI) | P value |
|---|---|---|---|---|
| Pre-AECOPD period | ||||
| −1 to −90 days | 207 | 7.3 | 1 (reference) | - |
| Post-AECOPD period | ||||
| 1 to 90 days | 400 | 14.1 | 1.93 (1.63–2.29) | <0.001 |
| 91 to 180 days | 213 | 7.5 | 1.03 (0.85–1.25) | 0.77 |
| 181 to 270 days | 173 | 6.1 | 0.84 (0.68–1.03) | 0.09 |
| 271 to 360 days | 186 | 6.6 | 0.90 (0.73–1.10) | 0.29 |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval
Rate ratios were calculated by using conditional Poisson regression models
Sensitivity analyses
The observed temporal patterns persisted with stratification by age (Supplemental Table 1), sex (Supplemental Table 2), presence of AF at the index hospitalization (Supplemental Table 3), and season of the AECOPD hospitalization (Supplemental Table 4). Likewise, in the sensitivity analysis including the patients with a primary diagnosis of heart failure and secondary diagnosis of AF, the observed temporal patterns persisted (Supplemental Table 5). Likewise, in the analysis limiting to the patients who did not have an ED visit for AECOPD during the reference period, the result did not change materially (Supplemental Table 6). Similarly, in the analysis including the patients with multiple hospitalizations for AECOPD during the follow-up period, the results were consistent with the main findings (Supplemental Table 7). Furthermore, in the subgroup analysis limiting to the patients who were known to be alive at least 360 days after the AECOPD hospitalization, the results were also consistent with the main findings (Supplemental Table 8). Lastly, the significant association also persisted with modeling the outcome as a binomial response – e.g., the odds ratio of the outcome event was 2.55 (95%CI, 2.07–3.14; P<0.001; Supplemental Table 9) in the post-AECOPD days 1 to 90.
DISCUSSION
In this self-controlled case series study using a population-based dataset of adults with existing AF, we found that, after hospitalization for AECOPD (the exposure), there was an approximately 2-fold increase in the rate of ED visits or hospitalizations for AF (the outcome) in the first 90-day post-AECOPD period. The elevated rate decreased to the baseline thereafter. These observed temporal relationships of AECOPD with AF-related ED visits or hospitalizations persisted across the different patient subgroups and statistical assumptions. To the best of our knowledge, this is the first study that has investigated the temporal association between AECOPD and AF-related acute morbidities.
To date, the literature has shown the relations of COPD as a chronic morbidity (not AECOPD) with AF. For example, having COPD is a risk factor for incident AF.4–7 In a cohort study of the US general population, individuals with a lowest quartile of forced expiratory volume in one second (FEV1) had a 1.5-fold increased risk of incident AF compared to those with a highest quartile of FEV1. In a study of 173 patients hospitalized for AECOPD and hypercapnia, those with concurrent AF had a lower FEV1.0%, higher PaCO2, and higher pulmonary artery systolic pressure, compared to those without AF.7 In addition, studies have reported that comorbid COPD is associated with clinical AF progression,8 failure of AF treatment,21–23 and overall mortality.24, 25 Indeed, a cohort study of 1,219 patients with paroxysmal AF demonstrated that concurrent COPD was a risk factor for progression to persistent AF during the 1-year follow-up period.8 Another cohort study also showed that COPD was associated with a higher recurrence of AF after their first catheter ablation.22 Furthermore, an analysis of patients with AF in the ARISTOTLE study showed that patients with COPD had a 1.6-fold higher risk of all-cause mortality compared to those without COPD.24 These evidence collectively suggest the associations of chronic morbid COPD and AF morbidity. Our findings corroborate these earlier studies, and extend them by demonstrating the temporal association between acute exacerbations of COPD – important events in the disease course of COPD – and an increased risk of AF-related ED visits and hospitalizations.
The mechanisms that underlie our observation are likely multifactorial. A potential explanation includes AECOPD-related elevated systemic inflammatory status.26–29 Prior research have indicated that inflammation plays an important role in presence,30 incidence,31 and prognosis32 of AF. Alternatively, hypoxemia may be another pathological mechanism for worse AF control. Recent studies have shown that hypoxia-inducible factor-1 alpha promotes atrial structural remodeling leads to AF.33, 34 Another possible mechanism is ventricular dysfunction35 with increased atrial afterload36 in patients with AECOPD. Lastly, medications for AECOPD management (e.g., β-agonists), through modifying the atrioventricular nodal conduction,37, 38 potentially affect AF control; these medications may be held in this study population with COPD. These data should facilitate further investigations into the development of preventive strategies on AF-related morbidities in patients with COPD.
Potential limitations
Our study has several potential limitations. First, because we used administrative datasets, there may be misclassifications such as misdiagnosis of COPD and AF. However, the HCUP data are rigorously tested15, 39 and these have been widely used to investigate their morbidities.10, 15, 40 In addition, the literature has also shown that the ICD-9-CM diagnostic codes for COPD have a specificity of 99% and positive predictive value of 90%.41 Similarly, patients with latent AF might have been misclassified as patients without AF. Yet, studies showed that the codes for AF have a positive predictive value of 90%.42, 43 Second, because the datasets did not contain detailed clinical information (e.g., medications), the current analyses were unable to address the longitudinal changes in the risk factors for AF during the study periods (e.g., changes in AF medications, severity of concurrent cardiac diseases) and the specific type of AF – i.e., persistent, permanent and paroxysmal AF. However, the self-controlled case series method enabled us to address time-invariant confounders (e.g., genetics) – regardless of measured or unmeasured. Third, patients might have been lost-to-follow-up after the hospitalization for AECOPD. Yet, the sensitivity analysis limiting to those who are known to be alive at least 360 days after the hospitalization showed the consistent results. Fourth, as our study focused on AF patients hospitalized for AECOPD, generalizing our inferences beyond this population (e.g., patients without existing AF, those with mild-to-moderate AECOPD) requires caution. However, the study population accounts for a large societal burden,1, 3 and hence is the one for which the development of preventive strategies are urgently needed. Finally, the studied data are not a random sample of all individuals with COPD and AF in the U.S. However, the five study states are geographically diverse and represent approximately 27% of the US population.
CONCLUSIONS
In this self-controlled case series study using population-based data of adults with AF, we found that, after the hospitalization for AECOPD, there was an approximately 2-fold increase in the rate of ED visits or hospitalizations for AF during the first 90-day post-AECOPD period. The rate then declined to the baseline levels. The observed temporal relationship of AECOPD with AF-related acute morbidities persisted across the different subgroups and statistical assumptions. For clinicians, our findings underscore the importance of preventing AF-related events in AF patients who experience AECOPD. For researchers, the data should advance further investigations into the mechanisms underlying the AECOPD-AF linkage to develop targeted therapeutic interventions in this population with large healthcare utilization.
Supplementary Material
ACKNOWLEDGEMENTS:
AH was supported by a grant from the Fulbright Scholarship.
FUNDING SOURCES: This study was supported by the grant R01 HS023305 from the Agency for Healthcare Research and Quality (Rockville, MD, USA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
DISCLOSURES: Dr. Camargo has performed COPD-related consultation for AstraZeneca, GSK, and Mereo. The other authors have no relevant financial relationships to disclose.
REFERENCES
- 1.Agency for Healthcare Research and Quality. HCUP net. http://hcupnet.ahrq.gov/. Accessed May 1, 2018
- 2.Steinberg BA, Kim S, Fonarow GC, Thomas L, Ansell J, Kowey PR, Mahaffey KW, Gersh BJ, Hylek E, Naccarelli G, Go AS, Reiffel J, Chang P, Peterson ED and Piccini JP. Drivers of hospitalization for patients with atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2014;167:735–42.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF and Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–9. [DOI] [PubMed] [Google Scholar]
- 4.Buch P, Friberg J, Scharling H, Lange P and Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. [DOI] [PubMed] [Google Scholar]
- 5.Shibata Y, Watanabe T, Osaka D, Abe S, Inoue S, Tokairin Y, Igarashi A, Yamauchi K, Kimura T, Kishi H, Aida Y, Nunomiya K, Nemoto T, Sato M, Konta T, Kawata S, Kato T, Kayama T and Kubota I. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: the Takahata study. Int J Med Sci 2011;8:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Agarwal SK, Alonso A, Blecker S, Chamberlain AM, London SJ, Loehr LR, McNeill AM, Poole C, Soliman EZ and Heiss G. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129:971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terzano C, Romani S, Conti V, Paone G, Oriolo F and Vitarelli A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur Rev Med Pharmacol Sci 2014;18:2908–17. [PubMed] [Google Scholar]
- 8.de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA and Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. https://www.hcup-us.ahrq.gov/ Accessed May 1, 2018
- 10.Goto T, Faridi MK, Gibo K, Camargo CA Jr. and Hasegawa K Sex and racial/ethnic differences in the reason for 30-day readmission after COPD hospitalization. Respir Med 2017;131:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa K, Tsugawa Y, Tsai CL, Brown DF and Camargo CA Jr. Frequent utilization of the emergency department for acute exacerbation of chronic obstructive pulmonary disease. Respir Res 2014;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Chronic obstructive pulmonary disease (COPD): hospital 30-day, all-cause, risk-standardized readmission rate following acute exacerbation of COPD hospitalization. https://www.qualitymeasures.ahrq.gov/summaries/summary/49195/chronic-obstructive-pulmonary-disease-copd-hospital-30day-allcause-riskstandardized-readmission-rate-following-acute-exacerbation-of-copd-hospitalization. Accessed May 1, 2018
- 13.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV and Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW and Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 15.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH and Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen I, Douglas I and Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 17.Censi F, Calcagnini G, Mattei E, Calo L, Curnis A, D’Onofrio A, Vaccari D, Zanotto G, Morichelli L, Rovai N, Gargaro A and Ricci RP. Seasonal trends in atrial fibrillation episodes and physical activity collected daily with a remote monitoring system for cardiac implantable electronic devices. Int J Cardiol 2017;234:48–52. [DOI] [PubMed] [Google Scholar]
- 18.Rumana N, Kita Y, Turin TC, Murakami Y, Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y and Ueshima H. Seasonal pattern of incidence and case fatality of acute myocardial infarction in a Japanese population (from the Takashima AMI Registry, 1988 to 2003). Am J Cardiol 2008;102:1307–11. [DOI] [PubMed] [Google Scholar]
- 19.Shimada YJ, Tsugawa Y, Brown DF and Hasegawa K. Bariatric Surgery and emergency department visits and hospitalizations for heart failure exacerbation: population-based, self-controlled series. J Am Coll Cardiol 2016;67:895–903. [DOI] [PubMed] [Google Scholar]
- 20.Hirayama A, Goto T, Shimada YJ, Faridi MK, Camargo CA Jr. and Hasegawa K Association of obesity with severity of heart failure exacerbation: a population-based study. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh SY, Choi JI, Lee JY, Kwak JJ, Park JS, Kim JB, Lim HE and Kim YH. Catheter ablation of atrial fibrillation in patients with chronic lung disease. Circ Arrhythm Electrophysiol 2011;4:815–22. [DOI] [PubMed] [Google Scholar]
- 22.Gu J, Liu X, Tan H, Zhou L, Jiang W, Wang Y, Liu Y and Gu J. Impact of chronic obstructive pulmonary disease on procedural outcomes and quality of life in patients with atrial fibrillation undergoing catheter ablation. J Cardiovasc Electrophysiol 2013;24:148–54. [DOI] [PubMed] [Google Scholar]
- 23.Pisters R, Nieuwlaat R, Prins MH, Le Heuzey JY, Maggioni AP, Camm AJ and Crijns HJ. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–74. [DOI] [PubMed] [Google Scholar]
- 24.Durheim MT, Cyr DD, Lopes RD, Thomas LE, Tsuang WM, Gersh BJ, Held C, Wallentin L, Granger CB, Palmer SM and Al-Khatib SM. Chronic obstructive pulmonary disease in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Int J Cardiol 2016;202:589–94. [DOI] [PubMed] [Google Scholar]
- 25.Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, Breithardt G, Singer DE, Hankey GJ, Hacke W, Becker RC, Berkowitz SD, Nessel CC, Mahaffey KW, Fox KA and Califf RM. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, Gaggar A, Rennard SI, Jackson PL and Blalock JE. An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014;190:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells JM, Jackson PL, Viera L, Bhatt SP, Gautney J, Handley G, King RW, Xu X, Gaggar A, Bailey WC, Dransfield MT and Blalock JE. A randomized, placebo-controlled trial of roflumilast. Effect on proline-glycine-proline and neutrophilic inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA and Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:1239–45. [DOI] [PubMed] [Google Scholar]
- 29.Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:26–33. [DOI] [PubMed] [Google Scholar]
- 30.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG and Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J 2008;155:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marott SC, Nordestgaard BG, Zacho J, Friberg J, Jensen GB, Tybjaerg-Hansen A and Benn M. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J Am Coll Cardiol 2010;56:789–95. [DOI] [PubMed] [Google Scholar]
- 32.Ederhy S, Di Angelantonio E, Dufaitre G, Meuleman C, Masliah J, Boyer-Chatenet L, Boccara F and Cohen A. C-reactive protein and transesophageal echocardiographic markers of thromboembolism in patients with atrial fibrillation. Int J Cardiol 2012;159:40–6. [DOI] [PubMed] [Google Scholar]
- 33.Ogi H, Nakano Y, Niida S, Dote K, Hirai Y, Suenari K, Tonouchi Y, Oda N, Makita Y, Ueda S, Kajihara K, Imai K, Sueda T, Chayama K and Kihara Y. Is structural remodeling of fibrillated atria the consequence of tissue hypoxia? Circ J 2010;74:1815–21. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Sharma D, Du F and Liu Y. The role of Toll-like receptor 2 and hypoxia-induced transcription factor-1alpha in the atrial structural remodeling of non-valvular atrial fibrillation. Int J Cardiol 2013;168:2940–1. [DOI] [PubMed] [Google Scholar]
- 35.Abroug F, Ouanes-Besbes L, Nciri N, Sellami N, Addad F, Hamda KB, Amor AB, Najjar MF and Knani J. Association of left-heart dysfunction with severe exacerbation of chronic obstructive pulmonary disease: diagnostic performance of cardiac biomarkers. Am J Respir Crit Care Med. 2006;174:990–6. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg MA and Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–62. [DOI] [PubMed] [Google Scholar]
- 37.Salpeter SR, Ormiston TM and Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–21. [DOI] [PubMed] [Google Scholar]
- 38.Kallergis EM, Manios EG, Kanoupakis EM, Schiza SE, Mavrakis HE, Klapsinos NK and Vardas PE. Acute electrophysiologic effects of inhaled salbutamol in humans. Chest. 2005;127:2057–63. [DOI] [PubMed] [Google Scholar]
- 39.Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S and Savani GT. Inhospital complications associated with catheter ablation of atrial fibrillation in the united states between 2000–2010: Analysis of 93,801 procedures. Circulation. 2013;128(19):2104–12. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa K, Tsugawa Y, Tsai C-L, Brown DF and Camargo CA. Frequent utilization of the emergency department for acute exacerbation of chronic obstructive pulmonary disease. Respir Res 2014;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R and Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaker GC, McGowan DJ, Boechler M, Fortune G and Gage B. Underutilization of antithrombotic therapy in elderly rural patients with atrial fibrillation. Am Heart J. 1999;137:307–12. [DOI] [PubMed] [Google Scholar]
- 43.Brass LM, Krumholz HM, Scinto JM and Radford M. Warfarin use among patients with atrial fibrillation. Stroke. 1997;28:2382–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.