Abstract

Van der Waals heterostructures have attracted increasing interest, owing to the combined benefits of their constituents. These hybrid nanostructures can be realized via epitaxial growth, which offers a promising approach for the controlled synthesis of the desired crystal phase and the interface between van der Waals layers. Here, the epitaxial growth of a continuous molybdenum disulfide (MoS2) film on large-area graphene, which was directly grown on a sapphire substrate, is reported. Interestingly, the grain size of MoS2 grown on graphene increases, whereas that of MoS2 grown on SiO2 decreases with an increasing amount of hydrogen in the chemical vapor deposition reactor. In addition, to achieve the same quality, MoS2 grown on graphene requires a much lower growth temperature (400 °C) than that grown on SiO2 (580 °C). The MoS2/graphene heterostructure that was epitaxially grown on a transparent platform was investigated to explore its photosensing properties and was found to exhibit inverse photoresponse with highly uniform photoresponsivity in the photodetector pixels fabricated across a full wafer. The MoS2/graphene heterostructure exhibited ultrahigh photoresponsivity (4.3 × 104 A W–1) upon exposure to visible light of a wide range of wavelengths, confirming the growth of a high-quality MoS2/graphene heterostructure with a clean interface.
Keywords: graphene, MoS2, large area growth, heterostructure, photodetector
Introduction
Transition metal dichalcogenides (TMDs), which are two dimentional (2D) layered materials, have been extensively studied as one of promissing candidates for the realization of various emerging applications such as optoelectronics,1,2 flexible electronics,3 and quantum technologies.4,5 The true potential of TMDs is believed to lie in the possibility of mixing different materials to form their heterostructure, which offers excellent features that can be applied to next-generation electronic applications. Researchers have been exploring interesting physical phenomena and engineering applications for new ultrathin devices6−8 by creating 2D heterostructures by conventionally exfoliating and transferring materials from bulk crystals. However, the rapid evolution of new device technologies requires the development of synthetic techniques to realize large-area thin films based on heterostructures. Several efforts have been made toward this end, including the growth of TMD heterostructures using chemical vapor deposition (CVD),9 atomic layer deposition,10 molecular beam epitaxy,11 and metal–organic chemical vapor deposition (MOCVD).12 In recent years, the strategy of using graphene as a platform for TMD growth has been widely applied to obtain either lateral or vertical heterostructures covering a large area.13,14 In general, a large area of graphene is first grown on the metal foil used as the catalyst, followed by transferring onto a targeted substrate for TMDC growth. However, the surface of graphene becomes contaminated owing to residual polymers or chemicals involved in the transfer process, thus losing its pristine properties and resulting in drastic degradation in the properties of the heterostructure. To secure the characteristics of the heterostructure, an ultraclean interface between layers is a necessary and critical challenge.
The existence of grain boundaries (GBs) is another concern that strongly affects the mobility and transport characteristics of TMDCs.15,16 The density of GBs, which is further suppressed by the seamless stitching that occurs under the oriented lattice domains, can be reduced by enlarging the domain size of the TMDC grains and precisely controlling the lattice alignment.17,18 Therefore, the in situ growth of layered 2D TMDC heterostructures and an understanding of the growth mechanism would enable the large-area synthesis of higher quality materials on the desired substrate. To date, MOCVD has been the most promising method for large-area thin film growth according to precisely controlled precursors and growth parameters. Compared to conventional CVD which requires high growth temperature,19,20 MOCVD shows the superiority of growth at a low temperature by taking advantage of metal–organic precursors. In addition, the synthesis of high-quality, electronic-grade materials using MOCVD systems has improved,21,22 but the direct synthesis of large-area heterostructures inside a CVD system has rarely been reported.
In this paper, we report the synthesis of a wafer-scale MoS2/graphene heterostructure using a MOCVD system. Wafer-scale high-quality graphene synthesized on sapphire using a metal-free method is used for epitaxial growth. MoS2 is then grown on the as-grown graphene/sapphire substrate, thus achieving a clean MoS2/graphene interface as well as preserving the epitaxial growth behavior with highly ordered orientation. In addition, we conducted a comprehensive investigation to provide an understanding of the MoS2 growth behavior on graphene. Particularly, we found that the MoS2 grain size is increased by increasing the amount of H2, as opposed to the behavior of MoS2 grown on a conventional SiO2 substrate. Apart from this, the growth temperature and precursor species also affect the grain size of MoS2. The photosensing capability of MoS2/graphene is then characterized by fabricating 900 photodetector (PD) devices directly on the sapphire substrate without any transfer. These gating-free devices show an ultrahigh photoresponsivity of ∼4.3 × 104 A W–1 for blue illumination and ∼6.8 × 103 A W–1 for red illumination, demonstrating the high-quality of the MoS2/graphene heterostructure. We expect the high-quality MoS2/graphene heterostructure grown in situ to be utilized for realizing large-scale transparent optoelectronic devices.
Results and Discussion
Figure 1a schematically illustrates the epitaxial growth of MoS2 on a graphene/sapphire substrate. In this study, high-quality
graphene was grown on a 4 in. sapphire substrate using a cold-wall
LPCVD system as reported previously.23 Briefly,
before it was used for graphene growth, the sapphire wafer was annealed
in a hydrogen atmosphere, resulting in the loss of surface oxygen
and therefore rendering the surface sapphire Al-rich. This reconstruction
of the sapphire surface enables the Al sites to catalyze the dissociation
of the methane molecules, which are used as the carbon source for
graphene synthesis. Therefore, no external metal catalyst is required
for graphene growth. The low-energy electron diffraction (LEED) pattern
(blue square box) recorded after graphene synthesis at a beam energy
of 64 eV presents the  structure, confirming the reconstruction of the sapphire surface
by hydrogen etching as well as showing that graphene (black arrows)
preferentially aligns along the R30 direction of
the sapphire substrate (yellow arrows). The characterization of as-grown
graphene indicates that continuous monolayer graphene with high quality
was successfully grown on the sapphire substrate (Figure S1).
structure, confirming the reconstruction of the sapphire surface
by hydrogen etching as well as showing that graphene (black arrows)
preferentially aligns along the R30 direction of
the sapphire substrate (yellow arrows). The characterization of as-grown
graphene indicates that continuous monolayer graphene with high quality
was successfully grown on the sapphire substrate (Figure S1).
Figure 1.
Large-area heterostructure of MoS2/graphene. (a) Schematic representation of the growth of graphene and MoS2/graphene and respective photographic images (the blue box contains the LEED pattern of graphene grown on sapphire). (b) Raman spectrum of MoS2/graphene. (c) Optical absorption and PL of MoS2/graphene. (d) Raman spectra of graphene before and after MoS2 growth. (e,f) XPS analysis of graphene before and after MoS2 growth. (g) XPS analysis of MoS2.
The use of a metal-free synthesis process serves to eliminate contamination on the surface of the synthesized graphene layer caused by the postgrowth transfer step (i.e., residue polymer or chemicals), thus providing a chemically pristine starting surface that can be directly used for MoS2 growth. The MoS2 film is uniformly grown over the entire graphene wafer by using the MOCVD system, except at the edge site that was in contact with the substrate holder. Photographic images of the wafer scale of graphene before (grayish color) and after MoS2 growth (yellowish color) are shown, respectively.
Figure 1b shows the representative E2g1 and A1g modes, which clearly indicate the existence of MoS2 on the Raman spectrum of MoS2/graphene (red line) and which are not observed on the Raman spectrum of bare graphene (black line). The continuity of MoS2 grown on graphene was confirmed using scanning electron microscopy (SEM) and atomic force microscopy (AFM) (Figure S2). The photoluminescence (PL) and UV–vis absorbance spectra presented in Figure 1c are evident of the good optical quality of MoS2 with a direct band gap of ∼1.86 eV. The A and B excitons created by the splitting of the topmost valence bands are clearly observed at 1.87 and 2.01 eV, respectively. The C peak—associated with van Hove singularities is observed at 2.8 eV on the sapphire substrate. The PL and UV–vis absorbance spectra both indicate that the MoS2 deposited on graphene is largely a monolayer with high crystalline quality; the coverage of the bilayer region is only about 2% (Figure S2). The additional Raman and PL spectra of MoS2 demonstrate the uniformity of MoS2 throughout a 4 in. wafer (Figure S3). The Raman spectra shown in Figure 1d have no observable D peak, which implies that the quality of graphene was preserved after MoS2 growth. Alternatively, after MoS2 growth, both the G and 2D peaks of graphene undergo blue-shift, indicating that a significant amount of strain on graphene was induced by the thermal effect during MoS2 growth or the existence of MoS2 or a combination of both. The role of strain in MoS2 growth is explained in the next section. Our MOCVD systems utilize molybdenum hexacarbonyl (MHC) and dimethyl sulfide (DMS). Therefore, it should be noted that the thermal decomposition of the sulfur precursor (i.e., DMS) at a high temperature can cause the grown MoS2 to be contaminated with carbon as reported previously.24−27 Optimization of the growth parameters enabled us to obtain a MoS2/graphene film from which amorphous carbon is absent as confirmed by the absence of the D peak from the Raman spectrum of the MoS2/graphene sample. In addition, the cross-sectional transmission electron microscopy image clearly indicates the clean van der Waals interface between MoS2 and graphene layers (Figure S4).
High-resolution X-ray photoelectron spectroscopy (HR-XPS) was conducted for the chemical characterization of graphene before and after MoS2 growth (Figure 1e). The presence of MoS2 on graphene is clearly shown by the result of the XPS survey with the representative peaks distinguishable from the spectrum of bare graphene. On both of the graphene and MoS2/graphene samples, the C 1s core level analysis shows an intense and sharp peak resulting from C–C sp2 bonding (∼284.2 eV), together with the absence of C–C sp3 bonding at 284.9 eV, clearly confirming the existence of high-quality graphene on the sapphire (Figure 1f). It also indicates that the quality of graphene was preserved after MoS2 growth. Here, it is worth noting that the C 1s spectrum of graphene in the MoS2/graphene sample shows the C–C peak at 284.2 eV, whereas the C–C peak of the bare graphene sample is located at 283.8 eV. This peak shift is likely caused by the interaction of graphene with the Al-rich sapphire substrate. The Al 2s peak is shifted from 118.1 to 119.1 eV, the Al 2p peak is shifted from 73.1 to 74.1 eV, and the O 1s peak is shifted from 529.8 to 530.7 eV after MoS2 deposition, indicating breakage of the graphene–Al interaction after the growth of the heterostructure (Figure S5). The Mo 3d core level spectrum of MoS2 presents the Mo4+ 3d3/2 and 3d5/2 peaks at 232.6 eV and 229.4 eV, respectively, together with the S 2s peak at 226.7 eV (Figure 1g). The absence of the Mo6+ 3d3/2 and 3d5/2 peaks indicates that the MoS2 film was not oxidized. In addition, the peaks at 163.5 and 162.3 eV on the S 2p core level spectrum correspond to S 2p1/2 and 2p3/2 of MoS2, respectively. The atomic ratio of Mo/S is 1:2.065, indicating the good stoichiometry of the synthesized MoS2.
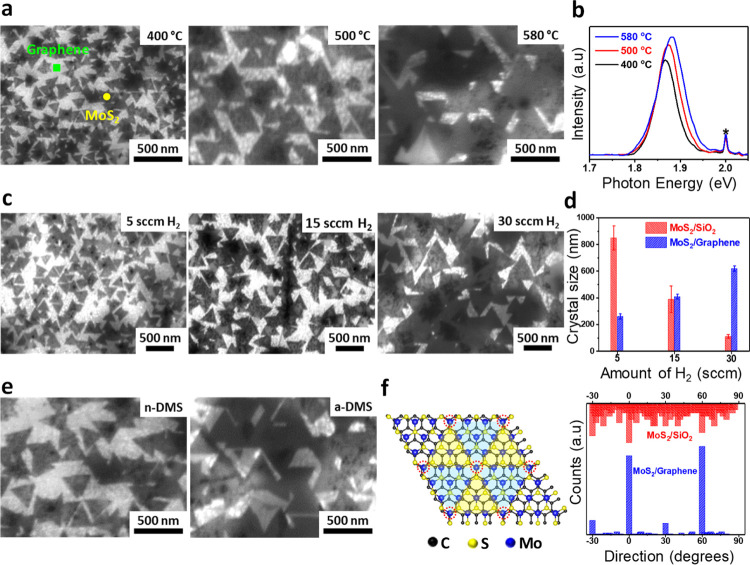
Aiming to develop a comprehensive understanding of the MoS2 growth mechanism, we reduced the growing time of the heterostructure to observe the grain size and orientation. First, we carried out a morphological study of MoS2 evolution by varying the growth temperature in the range of 400–580 °C while maintaining the amount of H2 at a constant level of 5 sccm. At a low temperature of 400 °C, MoS2 grown on graphene exhibits a sharp triangular morphology (Figure 2a). The black dots visible at the edge sites of the MoS2 grains are supposedly Mo-rich MoS2 particulates28 which are absent inside the grain region. The grain size of MoS2 grown on graphene increases with an increasing growth temperature, which can be explained by the Ostwald ripening model.29 In contrast, MoS2 grown on SiO2 evolves from a fractal structure at 400 °C, to an anisotropic shape in the middle range of 500 °C, and to a compact triangular shape at a high temperature of 580 °C, as observed on the AFM images of the morphology (Figure S6a–c). The mechanism of the transition in the MoS2 morphology on SiO2 can be described as a competition between the formation of bonds between adatoms at the edges and the diffusion of adatoms along the domain edge.30 As the edge diffusion is thermally activated, it is suppressed at low temperatures. Therefore, the diffusion of adatoms along the domain edge is dominant, resulting in the islands being ramified owing to the rapid attachment of adatoms. On the other hand, by increasing the growth temperature, the mean free time and the mean free path of both Mo and S adatoms are decreased according to the theoretical calculation.31 Hence, this slow growth rate provides sufficient edge diffusion for reshaping the domain, and a compact shape can be obtained. The islands reshape into a thermodynamically stable configuration, which implies that the triangular shape is grown near the adsorption–desorption equilibrium condition. In contrast, the low diffusion energy barrier of the graphene substrate32,33 allows the Mo and S adatoms to easily identify the energetically favorable site to restructure the MoS2 domain34 even at a low temperature of 400 °C. Because the graphene substrate maintains the equilibrium to preserve the triangular morphology, the growth of MoS2 at a high temperature focuses on slowing down the growth rate and therefore enlarging the grain size. PL spectroscopy is utilized to optically probe the defect states of MoS2 without destroying the sample. As expected, the PL spectra of MoS2 on graphene were observed to be well resolved with the intensity increasing as the growth temperature increases (Figure 2b). The full width at half-maximum (fwhm) of the PL at 400, 500, and 580 °C is 69.9, 60.6, and 55.8 meV, respectively. The reduction in the fwhm indicates that the MoS2 quality is enhanced as the growth temperature increases. The low growth rate at higher growth temperature can improve the film quality. The reduction in thermal-induced strain because of the lower growth temperature might result in PL peaks undergoing red-shift from 1.882 to 1.866 eV. The increasing grain size could also contribute to this peak shift. The PL of MoS2 grown on SiO2 was measured under the same conditions, and the results are shown in Figure S6d. As predicted, MoS2 grown at 400 °C has poor crystallinity, and therefore, no PL peak was observed, whereas MoS2 grown at 500 °C exhibits wider PL peak located at 1.879 eV with a fwhm of 75.5 meV, together with an observable B exciton, as shown in the normalized intensity graph, indicating higher defect density of MoS2.35 On the other hand, MoS2 grown at 580 °C exhibits a high intensity, sharp peak at 1.860 eV with a fwhm of 72.6 meV, confirming the high quality of the MoS2 film. At the reduced growth temperature, the shift in the PL peak of MoS2 grown on SiO2 seems to be related to the quality of the sample rather than the strain effect. The PL investigation again corroborates the advantage of graphene as an ideal substrate for MoS2 growth.
Figure 2.
Grain size evolution of MoS2 grown on graphene. (a) SEM images of MoS2 with increasing growth temperature and (b) respective PL spectra. (c) SEM images of MoS2 with the increasing H2 content. (d) Crystal orientation of MoS2 grown on graphene and SiO2. (e) SEM images of MoS2 with normal DMS (n-DMS) and anhydrous DMS (a-DMS) used as source of sulfur. (f) Atomic arrangement and crystal orientation of MoS2 grown on SiO2 (red) and on graphene (blue).
To further investigate the mechanism underlying the growth of MoS2 on graphene, we studied the dependence of the growth parameters on the amount of hydrogen at a growth temperature of 400 °C. We found the lateral size of MoS2 grains to vary depending on the amount of hydrogen gas. In particular, the grain size of MoS2 grown on graphene increases when the flow of H2 increases from 5 to 30 sccm (Figure 2c). Surprisingly, by synthesizing MoS2 at 400 °C and 30 sccm H2, it was possible to achieve a MoS2 lateral size comparable to that obtained at 580 °C with 5 sccm H2. In contrast, the growth of MoS2 on the SiO2 substrate at 580 °C shows the reverse phenomenon; that is, the MoS2 grains become smaller in size as the amount of H2 increases (Figure S7). The conflicting result can be explained by the strain effects experienced by graphene during the growth. The lattice constants of graphene and 2H-MoS2 are 2.47 and 3.16 Å, respectively;36 therefore, the lattice mismatch between graphene and MoS2 is approximately 22%. Thus, a certain amount of strain is introduced into the graphene during MoS2 deposition. Evidence thereof is found on the Raman spectrum of the MoS2/graphene film transferred onto 300 nm-thick SiO2/Si, which is red-shifted compared to the as-grown film (Figure S8) and which indicates that the strain was released after transfer. In addition, the peaks of the transferred graphene are still blue-shifted compared to those of pristine graphene, providing evidence for the MoS2 contribution to the graphene strain. In contrast, rigid insulating substrates such as SiO2 do not create strain. It should be noted that the use of 5 sccm H2 results in the grain size of MoS2 grown on graphene being much smaller than that grown on the SiO2 substrate. The interaction between MoS2 and graphene induces strain in the MoS2 because of lattice mismatch, which is one of the factors responsible for reducing the size of the MoS2 grains. In addition, the difference in the thermal expansion coefficient between MoS2/graphene and the substrate contributes additional strain known as thermal-induced strain during the cooling step. Based on this result, we hypothesize that the reverse phenomenon of MoS2 grown on graphene and the SiO2 substrate can be explained as follows: an increase in the flow of H2 causes the intercalation of H2 between graphene and the sapphire substrate and/or between MoS2 and graphene; therefore, the strain is reduced and the MoS2 grain size increases. Reportedly, in the case of graphene grown on a SiC(0001) substrate, H2 intercalation can induce decoupling by terminating the dangling bonds, thus releasing the graphene from the substrate.37 In our case, graphene is deposited on the sapphire by using metal-free CVD. Because of the reconstruction of the sapphire surface, the graphene is supposed to strongly interact with Al sites on the surface. During the H2 intercalation process, the Al-rich surface might be terminated with hydrogen, thus reducing the graphene–Al interaction. As mentioned above, the C–C sp2 bonding energy is 284.2 eV after MoS2 growth (Figure 1f), which, together with the shifting of the Al 2s and 2p peaks and O 1s peak (Figure S5) suggests the reduction of interaction between graphene and the substrate. In addition, as depicted in Figure S9, the magnified Raman spectra of the G and 2D peaks show that these peaks undergo red-shift as the amount of H2 increases, thereby implying reduced strain in the graphene. Thus, the intercalation of H2 offers an explanation for the released strain, which verifies our hypothesis. Moreover, the possibility exists of H2-passivated Stone–Wales defects and bi-vacancies of graphene,38 which can reduce the nucleation density of MoS2, which is an important factor explaining the increase in the grain size.
We also consider another assumption: by increasing the amount of hydrogen, the formation rate of MoS2 on graphene is higher than the desorption rate, which is reversed for the growth of MoS2 on SiO2. In addition to the pyrolysis process, H2 also accelerates the decomposition of DMS by breaking the C–S bonds in DMS,39 which further increases the concentration of sulfur. A high sulfur-to-molybdenum (S/Mo) ratio is reported to be preferred to grow a MoS2 film with a large domain size on sapphire,40,41 whereas the grain size of MoS2 grown on SiO2 is decreased by increasing the S/Mo ratio.21 The lower surface energy of SiO2 compared to the sapphire substrate results in easier desorption of precursors from the SiO2 surface than from the sapphire surface. The grains are enlarged by energetically adsorbing Mo and S adatoms at the MoS2 edge sites. However, owing to the high adsorption energy and high diffusion energy barrier of the SiO2 substrate as well as the increase in the amount of S adatoms, the Mo adatoms cannot reach the preferred sites. Therefore, the Mo adatoms are located randomly on the SiO2 surface, resulting in an increase in the nucleation density and reduction in the grain size. In contrast, graphene has a lower diffusion energy barrier than the SiO2 surface; thus, the diffusion of Mo and S adatoms on the graphene surface is easier than on the SiO2 surface.33,34 Therefore, MoS2 grown on graphene is likely to occur similarly to MoS2 grown on Al2O3. Noteworthy is that the van der Waals growth of MoS2 on graphene is more preferable than growth on an amorphous substrate such as the SiO2 surface.42 To further examine the effect of the sulfur concentration on the grain size of MoS2, we reduced the amount of DMS and kept the other parameters constant. As presented in Figure S10, the grain size of MoS2 is increased by increasing the amount of DMS, and similar results are obtained by keeping the amount of DMS constant and increasing the amount of H2. Therefore, the assumption that the formation rate dominates the etching rate is validated. Note that the S/Mo ratio cannot be increased too much when using an organic compound such as DMS.41 The H2-dependent grain size of MoS2 grown on graphene at 400 °C and on SiO2/Si at 580 °C is summarized in Figure 2d.
The effect of different sulfide precursors (i.e., DES, H2S, and DMDS) on the MoS2 growth was studied previously.41,43 Herein, we also investigated two types of DMS precursors: DMS (denoted n-DMS) and anhydrous DMS (denoted a-DMS). As shown in Figure 2e, MoS2 grown by using a-DMS presents a large grain size and epitaxial growth, whereas MoS2 grown by n-DMS presents relatively smaller grains and a less ordered orientation. The previous study21 reported that the presence of H2O during MOCVD synthesis had the effect of reducing the grain size. When the SiO2/Si substrate is used, a clear difference is not obvious because of the wide range of grain sizes (Figure S11a). However, compared to a-DMS, the MoS2 grown on SiO2 using n-DMS contains a small amount of amorphous carbon, as shown in Figure S11b. These results suggest that, to obtain a larger grain size and avoid the formation of amorphous carbon, the use of a-DMS is a more appropriate choice for MoS2 synthesis.
Another interesting feature of the epitaxial growth of MoS2 on graphene is the grain direction. Figure 2f presents the orientation histogram of MoS2 with the corresponding images shown in Figure S12. Compared to the random distribution on the SiO2/Si substrate, the primary orientations of 0° and 60° of MoS2 grown on graphene statistically constitute ∼85% of the coverage. Graphene has hexagonal symmetry, whereas MoS2 has a layered structure with trigonal symmetry. A proposed structural model of the MoS2/graphene heterostructure suggests that every fourth Mo atom sits nearly perfectly above every fifth C atom along the zigzag direction to minimize the energy between the two layers (Figure 2f).44 As mentioned above, the heterostructure of MoS2/graphene has a lattice mismatch of about 22% between graphene and MoS2. It has been proved that the van der Waals epitaxial growth of a thin film is still valid even for a lattice mismatch as high as 50%.45 We also observed MoS2 to grow epitaxially on graphene as a result of weak van der Waals forces and the absence of dangling bonds between layers. However, a minor orientation of −30 and 30° with ∼10% coverage and 5% of an undesirable area is covered with randomly oriented grains. Transition metal adatoms are considered to bind strongly to the dangling bonds at Stone–Wales defects and the bi-vacancies of graphene, thus controlling the orientation of the TMD grains.46−48 Therefore, Mo adatoms might be strongly attached to the graphene defect sites, eliminating the weak interaction between layers and causing the rotation of MoS2 grains. In addition, the deviation in the preferred growth direction of MoS2 is probably due to small amounts of other transition metals that are present in the precursors in the form of impurities. A similar misorientation was observed previously.49−52 A high percentage of misorientation is observed by using 5 sccm of H2, whereas a highly ordered orientation is obtained by increasing the H2 to 30 sccm, which implies that the dangling bonds on the graphene surface are reduced by increasing the amount of H2.
The quality of graphene is an important factor that can affect the MoS2 growth process. As explained above, graphene with less defect sites and a low wrinkle density is an ideal substrate to achieve a large grain size as well as epitaxial growth. Herein, we conducted an experiment by growing MoS2 with two types of graphene: graphene grown on the pristine sapphire substrate (denoted p-Gr) and graphene grown on the H2-etched sapphire substrate (denoted H2-Gr). As we reported previously,23 compared to p-Gr, the quality of graphene is drastically enhanced in H2-Gr. As shown in Figure 3a,b using the Raman intensity mapping of ID/IG, H2-Gr presents a high quality and uniform film, whereas p-Gr has a nonuniform surface and contains more defects. Figure 3c presents the Raman spectrum of H2-Gr with the D peak suppressed. In contrast, the D peak of p-Gr is observable, indicating that p-Gr contains a highly disordered sp2-bond. The absence of a significant number of defects together with lower wrinkle density made H2-Gr an ideal substrate for MoS2 growth and resulted in MoS2 with a large grain size, as shown in Figure 3d, whereas MoS2 grown on p-Gr exhibited smaller grains, as shown in Figure 3e. As we explained above, the surface defects of graphene are active sites for MoS2 growth and thus affect the nucleation density of MoS2. To assess the defect-controlled nucleation density of MoS2 growth, O2 plasma treatment was utilized to intentionally induce surface defects on the graphene substrate. After exposure to plasma for 2 s, the Raman spectrum of H2-Gr showed the appearance of the D peak, whereas the intensity of the D peak of p-Gr increased noticeably (Figure 3c) implying an increase in the number of defects. Because the phonon vibrations are suppressed after plasma treatment, the intensity of the 2D peak on the Raman spectrum of graphene decreased, which is consistent with a previous report.53 It is worth noting the fact that I2D/IG is less than 1 in this case and is not related to the formation of an additional graphene layer, but the quality of the graphene was degraded after plasma treatment. As predicted, the MoS2 domain density was significantly increased, resulting in the small grain size. Figure 3f shows an image of MoS2 grown on H2-Gr exposed to O2 plasma. The MoS2 has a triangular shape while maintaining epitaxial growth. Compared to H2-Gr, p-Gr already contains many defect sites, and thus, it resembles H2-Gr that was exposed to O2 plasma for a longer time. As shown in Figure 3g, very small size MoS2 nanoflakes are formed because p-Gr contains more defect sites than H2-Gr. The nucleation density can further increase by increasing the exposure time of graphene to O2 plasma. However, the graphene surface can be strongly damaged or could even be removed. It is worth to note that the defect of graphene does not make a big influence on the quality of MoS2, as indicated from PL spectra (Figure S13).
Figure 3.
Dependence of the MoS2 grain size on the quality and defect density of graphene. (a,b) Raman mapping of ID/IG of high-quality graphene grown on H2-treated sapphire (H2-Gr) and on pristine sapphire (p-Gr), respectively. (c) Raman spectra of H2-Gr and p-Gr without and with exposure to O2-plasma. (d,e) MoS2 grown on H2-Gr and p-Gr, respectively. (f,g) MoS2 grown on H2-Gr and p-Gr treated with O2-plasma, respectively.
To demonstrate the utility of the synthesized MoS2/graphene heterostructure in potential applications, we fabricated two-terminal PD devices to evaluate the photosensing capability of the heterostructure under ambient conditions. Figure 4a shows a photographic image of a batch of 900 devices fabricated directly on the synthesized MoS2/graphene heterostructure on a 2 in. sapphire wafer without any transfer. The devices were fabricated with a channel length and width of 14 and 400 μm, respectively (Figure S14a). The transparency of the devices is advantageous that it allows light to enter both from the top and from the reverse side of the sapphire substrate. By applying bias, the current is strong (∼10–2 A) in a dark environment because graphene does not have a band gap (Figure 4b). The obtained linearity in the source–drain I–V curve confirms the Ohmic contact between the metal electrodes and 2D heterostructure film. Interestingly, under a white light illumination of 0.1 mW, the current was significantly reduced, which is in contrast to the normal PDs fabricated with semiconducting materials. To understand this phenomenon more precisely, we performed a similar photocurrent measurement with a bare graphene film. This experiment confirmed that a negative photocurrent is generated only by the MoS2/graphene heterostructure (Figure S14b), in which MoS2 is a typical n-type semiconductor, whereas graphene exhibits p-type characteristics. When the MoS2/graphene PD is illuminated with light, the photogenerated electrons are injected from the MoS2 layer into the graphene. Thus, the net hole concentration in graphene under light exposure is reduced by the injected electrons, resulting in an increase in the Fermi level of graphene and thus decreasing its conductivity.54,55Figure 4c presents the wavelength-dependent characteristic with the source-drain current as a function of time at a fixed incident optical power of 30 μW for each wavelength. The current decreased under light illumination and recovered when the illumination was discontinued. Furthermore, the photocurrent is highest under blue light exposure and gradually decreases when light-emitting diodes (LEDs) of higher wavelengths are used. The observed wavelength-dependent photocurrent reveals that the MoS2/graphene PD exhibits good light absorption and ability to generate electron–hole pairs across a wide wavelength range. The small grain size of the grown MoS2 domains may cause a certain amount of photogenerated electrons to be trapped in the MoS2 layer, which results in a relatively slow response speed. In addition, the PD shows good cycling stability and reversibility of the photocurrent without any decay after 100 cycles (Figure S15). In addition, the photoresponsivity for each light source was calculated using the following equation
| 1 |
where R is the photoresponsivity, IP is the photocurrent, W and L are the width and length of the device, and Ps is the power density of the light source. The calculated photoresponsivity was then plotted with respect to the wavelength in Figure 4d. As the wavelength decreases, the responsivity increases up to ∼43,426 A W–1, which is much higher than that reported for MoS2-based PDs. The decrease in the photocurrent is more pronounced with the increasing power of incident light, as depicted in Figure S16. Figure 4e shows that the photocurrent decreases as the positive source-drain voltage (Vsd) increases, which suggests that holes are trapped in the MoS2 layer and play the role of a positive local potential, which contributes to producing the negative photocurrent in the MoS2/graphene heterostructure. Figure 4f presents the histogram of the on/off current ratio (Ion/Ioff) and reveals that most of the devices exhibit (Ion/Ioff) as ∼2.5–1 with the photoresponsivity of these batch-fabricated MoS2/graphene PDs being highly uniform. This suggests that high quality MoS2 is uniformly grown on the wafer scale.
Figure 4.
Wafer scale, transparent PD based on the MoS2/graphene heterostructure. (a) PD fabricated on the MoS2/graphene wafer (900 devices). (b) Photoresponse of MoS2/graphene under white light with an incident power of 0.63 mW. (c) Wavelength dependence under an fixed incident power of 30 μW. (d) Calculated photoresponsivity depending on the wavelength. (e) Voltage dependence under white light stimulation. (f) Histogram of the ION/IOFF ratio.
Conclusions
In summary, we successfully demonstrated 4 in. wafer-scale synthesis of MoS2/graphene heterostructures via van der Waals epitaxy. Even though the lattice mismatch between graphene and MoS2 was shown to be 22%, the heterostructure is commensurate with a highly ordered orientation of MoS2 grains (85% is in the same preferred direction). In addition, the MoS2 grown on graphene has high crystallinity even at a low growth temperature of 400 °C, much lower than the temperature required to grow MoS2 on SiO2 (580 °C) of the same quality. Moreover, we conducted a comprehensive investigation of the growth of MoS2 on graphene by varying the growth parameters of the MOCVD system, providing new strategies to increase the MoS2 grain size. Finally, a batch of PD using the MoS2/graphene heterojunction with a clean interface was fabricated. The MoS2/graphene heterojunction exhibited outstanding photosensing capability with ultrahigh photoresponsivity (4.3 × 104 A W–1) and good optical transmittance. We believe our study significantly contributes toward the realization of high-quality heterostructures with uniform large-area growth, as well as toward next-generation optoelectronic devices.
Experimental Section
Growth Process
The procedure to grow wafer-scale graphene on sapphire is described elsewhere.23 Briefly, the c-axis sapphire (0001) substrate was cleaned with acetone, isopropanol, and deionized water before growth. The cleaned sapphire was then placed in the cold-wall reactor at 1180 °C for 5 min in a H2 environment. The graphene for both H2-Gr and p-Gr was grown as follows: first, the substrate was annealed at 1200 °C in a chamber with a pressure of 25 mbar under 1000 sccm flow of Ar for 10 min. Thereafter, the growth was continued under 100 sccm flow of H2 and 5 sccm CH4, finally cooling the reactor under Ar.
A horizontal, hot-wall MOCVD system was utilized for MoS2 growth. The 300 nm-thick SiO2/Si substrate was cleaned with acetone, isopropanol, and deionized water and dried by N2 gas before use. The graphene/sapphire substrate was used directly for MoS2 growth. Molybdenum disulfide was synthesized using MHC (Alfa Aesar, 98%) and DMS (Sigma Aldrich, ≥99%). The precursors were stored outside the furnace using glass bubblers in order to precisely control the inserted amount. Silica gel particles with diameters of ∼2 mm were added to the MHC bubbler for dehumidification. It is important to avoid the formation of amorphous carbon during growth because of the H2O effect. Growth was accomplished by loading the substrate into the chamber and keeping it vertically at 90° to the tube axis. Before the growth, the furnace was evacuated to a vacuum of less than 10–4 Torr for 1 h. The furnace was then heated to 400–580 °C for 30 min at 10 Torr, followed by the insertion of 1.0 sccm MHC and 0.6 sccm DMS. The growth was continued for 15 h as a short-time experiment and for 20 h to obtain a continuous MoS2 film, followed by cooling of the MOCVD system up to room temperature in an Ar environment. A mixture of 300 sccm Ar and 30 sccm H2 (for MoS2 grown on graphene) or 5 sccm H2 (for MoS2 grown on SiO2) was maintained throughout the growth processing.
Raman and PL Measurement
Raman and PL spectra were recorded using a 532 nm laser (Coherent, EN60825-1) with an Andor Tech spectrometer equipped with a liquid-nitrogen-cooled CCD. A spot size of ∼1 μm with a low laser power of ∼0.2 mW was utilized. All the measurements were performed at room temperature under ambient conditions using a confocal microscope system.
Optical Absorption Measurement
Optical absorption measurements were carried out using a UV–vis spectrometer (Jasco V650) over a wide wavelength range (200–900 nm).
Morphologies of Crystal Measurement
The crystal size and distribution were observed by a scanning electron microscope (Tescan MIRA3 FE-SEM system). In addition, the morphology of MoS2 and graphene samples was also analyzed using AFM imaging (Park System, NX-10) in the noncontact mode.
HR-XPS Measurement
The chemical identification of samples was implemented by XPS (K-Alpha, Thermo Scientific). The XPS analysis was performed using a conventional monochromated Al X-ray source (Al Kα line: 1486.6 eV) with a power of 12 kV and 3 mA, a pass energy of 40 eV, and step size of 0.1 eV.
Device Measurement
A batch of 900 devices was directly fabricated on the synthesized MoS2/graphene heterostructure on a 2 in. sapphire wafer without any transfer processing. Source–drain electrodes (Cr/Au: 3/30 nm) were formed using standard photolithography and a lift-off process. To evaluate the PD devices, light beams of various wavelengths in the range of 475 nm (blue LED), 532 nm (green LED), 590 nm (yellow LED), and 685 nm (red LED) were projected normally onto the plane of the PD, and the incident optical power was measured using the power meter (Nova P/N7Z01500, Ophir) equipped with large area Si CCD as the light sensor. The current–voltage characteristics were recorded using a source meter unit (Keithley 4200 SCS parameter analyzer, Keithley Instruments Inc.).
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (NRF-2015R1A3A2066337) and the European Union’s Horizon 2020 research and innovation program under grant agreement 881603-GrapheneCore3. Ken Teo of AIXTRON Ltd. is gratefully acknowledged for support in the wafer-scale growth of graphene on sapphire.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.0c12894.
XPS of Al2O3; AFM topography images, PL spectra and SEM images of MoS2 grown on SiO2; Raman spectra investigation of MoS2 and graphene; SEM images of MoS2 with varying amounts and types of DMS; and photoresponse of bare graphene and MoS2/graphene (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Choi M.; Park Y. J.; Sharma B. K.; Bae S.-R.; Kim S. Y.; Ahn J.-H. Flexible Active-Matrix Organic Light-Emitting Diode Display Enabled by MoS2 Thin-Film Transistor. Sci. Adv. 2018, 4, eaas8721 10.1126/sciadv.aas8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T.; Malic E. Exciton Physics and Device Application of Two-Dimensional Transition Metal Dichalcogenide Semiconductors. npj 2D Mater. Appl. 2018, 2, 29. 10.1038/s41699-018-0074-2. [DOI] [Google Scholar]
- Park Y. J.; Sharma B. K.; Shinde S. M.; Kim M.-S.; Jang B.; Kim J.-H.; Ahn J.-H. All MoS2-Based Large Area, Skin-Attachable Active-Matrix Tactile Sensor. ACS Nano 2019, 13, 3023–3030. 10.1021/acsnano.8b07995. [DOI] [PubMed] [Google Scholar]
- Xu R.; Jang H.; Lee M.-H.; Amanov D.; Cho Y.; Kim H.; Park S.; Shin H.-j.; Ham D. Vertical MoS2 Double-Layer Memristor with Electrochemical Metallization as an Atomic-Scale Synapse with Switching Thresholds Approaching 100 Mv. Nano Lett. 2019, 19, 2411–2417. 10.1021/acs.nanolett.8b05140. [DOI] [PubMed] [Google Scholar]
- Ye M.; Seo H.; Galli G. Spin Coherence in Two-Dimensional Materials. npj Comput. Mater. 2019, 5, 44. 10.1038/s41524-019-0182-3. [DOI] [Google Scholar]
- Dankert A.; Dash S. P. Electrical Gate Control of Spin Current in Van Der Waals Heterostructures at Room Temperature. Nat. Commun. 2017, 8, 16093. 10.1038/ncomms16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. K.; Xu J.; Zhu T.; Wu G.; McCormick E. J.; Zhan W.; Neupane M. R.; Kawakami R. K. Opto-Valleytronic Spin Injection in Monolayer MoS2/Few-Layer Graphene Hybrid Spin Valves. Nano Lett. 2017, 17, 3877–3883. 10.1021/acs.nanolett.7b01393. [DOI] [PubMed] [Google Scholar]
- Lee W.; Liu Y.; Lee Y.; Sharma B. K.; Shinde S. M.; Kim S. D.; Nan K.; Yan Z.; Han M.; Huang Y.; Zhang Y.; Ahn J.-H.; Rogers J. A. Two-Dimensional Materials in Functional Three-Dimensional Architectures with Applications in Photodetection and Imaging. Nat. Commun. 2018, 9, 1417. 10.1038/s41467-018-03870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-C.; Chang C.-Y. S.; Ghosh R. K.; Li J.; Zhu H.; Addou R.; Diaconescu B.; Ohta T.; Peng X.; Lu N.; Kim M. J.; Robinson J. T.; Wallace R. M.; Mayer T. S.; Datta S.; Li L.-J.; Robinson J. A. Atomically Thin Heterostructures Based on Single-Layer Tungsten Diselenide and Graphene. Nano Lett. 2014, 14, 6936–6941. 10.1021/nl503144a. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Choi D.; Woo W. J.; Lee J. B.; Ryu G. H.; Lim J. H.; Lee S.; Lee Z.; Im S.; Ahn J.-H.; Kim W.-H.; Park J.; Kim H. Synthesis of Two-Dimensional MoS2/Graphene Heterostructure by Atomic Layer Deposition Using MoF6 Precursor. Appl. Surf. Sci. 2019, 494, 591–599. 10.1016/j.apsusc.2019.07.168. [DOI] [Google Scholar]
- Vishwanath S.; Liu X.; Rouvimov S.; Mende P. C.; Azcatl A.; McDonnell S.; Wallace R. M.; Feenstra R. M.; Furdyna J. K.; Jena D.; Grace Xing H. Comprehensive Structural and Optical Characterization of Mbe Grown MoSe2 on Graphite, CaF2 and Graphene. 2D Mater. 2015, 2, 024007. 10.1088/2053-1583/2/2/024007. [DOI] [Google Scholar]
- Zhang X.; Zhang F.; Wang Y.; Schulman D. S.; Zhang T.; Bansal A.; Alem N.; Das S.; Crespi V. H.; Terrones M.; Redwing J. M. Defect-Controlled Nucleation and Orientation of WSe2 on hBN: A Route to Single-Crystal Epitaxial Monolayers. ACS Nano 2019, 13, 3341–3352. 10.1021/acsnano.8b09230. [DOI] [PubMed] [Google Scholar]
- Stoica T.; Stoica M.; Duchamp M.; Tiedemann A.; Mantl S.; Grützmacher D.; Buca D.; Kardynał B. E. Vapor Transport Growth of MoS2 Nucleated on SiO2 Patterns and Graphene Flakes. Nano Res. 2016, 9, 3504–3514. 10.1007/s12274-016-1227-2. [DOI] [Google Scholar]
- Behranginia A.; Yasaei P.; Majee A. K.; Sangwan V. K.; Long F.; Foss C. J.; Foroozan T.; Fuladi S.; Hantehzadeh M. R.; Shahbazian-Yassar R.; Hersam M. C.; Aksamija Z.; Salehi-Khojin A. Direct Growth of High Mobility and Low-Noise Lateral MoS2–Graphene Heterostructure Electronics. Small 2017, 13, 1604301. 10.1002/smll.201604301. [DOI] [PubMed] [Google Scholar]
- Najmaei S.; Amani M.; Chin M. L.; Liu Z.; Birdwell A. G.; O’Regan T. P.; Ajayan P. M.; Dubey M.; Lou J. Electrical Transport Properties of Polycrystalline Monolayer Molybdenum Disulfide. ACS Nano 2014, 8, 7930–7937. 10.1021/nn501701a. [DOI] [PubMed] [Google Scholar]
- Ly T. H.; Perello D. J.; Zhao J.; Deng Q.; Kim H.; Han G. H.; Chae S. H.; Jeong H. Y.; Lee Y. H. Misorientation-Angle-Dependent Electrical Transport across Molybdenum Disulfide Grain Boundaries. Nat. Commun. 2016, 7, 10426. 10.1038/ncomms10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Yang Z.; Du L.; Zhang J.; Shi J.; Chen W.; Chen P.; Liao M.; Zhao J.; Meng J.; Wang G.; Zhu J.; Yang R.; Shi D.; Gu L.; Zhang G. Precisely Aligned Monolayer MoS2 Epitaxially Grown on h-BN Basal Plane. Small 2017, 13, 1603005. 10.1002/smll.201603005. [DOI] [PubMed] [Google Scholar]
- Ji H. G.; Lin Y.-C.; Nagashio K.; Maruyama M.; Solís-Fernández P.; Sukma Aji A.; Panchal V.; Okada S.; Suenaga K.; Ago H. Hydrogen-Assisted Epitaxial Growth of Monolayer Tungsten Disulfide and Seamless Grain Stitching. Chem. Mater. 2018, 30, 403–411. 10.1021/acs.chemmater.7b04149. [DOI] [Google Scholar]
- Yu H.; Liao M.; Zhao W.; Liu G.; Zhou X. J.; Wei Z.; Xu X.; Liu K.; Hu Z.; Deng K.; Zhou S.; Shi J.-A.; Gu L.; Shen C.; Zhang T.; Du L.; Xie L.; Zhu J.; Chen W.; Yang R.; Shi D.; Zhang G. Wafer-Scale Growth and Transfer of Highly-Oriented Monolayer MoS2 Continuous Films. ACS Nano 2017, 11, 12001–12007. 10.1021/acsnano.7b03819. [DOI] [PubMed] [Google Scholar]
- Wang L.; Chen L.; Wong S. L.; Huang X.; Liao W.; Zhu C.; Lim Y. F.; Li D.; Liu X.; Chi D.; Ang K. W. Electronic Devices and Circuits Based on Wafer-Scale Polycrystalline Monolayer MoS2 by Chemical Vapor Deposition. Adv. Electron. Mater. 2019, 5, 1900393. 10.1002/aelm.201900393. [DOI] [Google Scholar]
- Kang K.; Xie S.; Huang L.; Han Y.; Huang P. Y.; Mak K. F.; Kim C.-J.; Muller D.; Park J. High-Mobility Three-Atom-Thick Semiconducting Films with Wafer-Scale Homogeneity. Nature 2015, 520, 656–660. 10.1038/nature14417. [DOI] [PubMed] [Google Scholar]
- Lin Y.-C.; Jariwala B.; Bersch B. M.; Xu K.; Nie Y.; Wang B.; Eichfeld S. M.; Zhang X.; Choudhury T. H.; Pan Y.; Addou R.; Smyth C. M.; Li J.; Zhang K.; Haque M. A.; Fölsch S.; Feenstra R. M.; Wallace R. M.; Cho K.; Fullerton-Shirey S. K.; Redwing J. M.; Robinson J. A. Realizing Large-Scale, Electronic-Grade Two-Dimensional Semiconductors. ACS Nano 2018, 12, 965–975. 10.1021/acsnano.7b07059. [DOI] [PubMed] [Google Scholar]
- Mishra N.; Forti S.; Fabbri F.; Martini L.; McAleese C.; Conran B. R.; Whelan P. R.; Shivayogimath A.; Jessen B. S.; Buss L.; Falta J.; Aliaj I.; Roddaro S.; Flege J. I.; Bøggild P.; Teo K. B. K.; Coletti C. Wafer-Scale Synthesis of Graphene on Sapphire: Toward Fab-Compatible Graphene. Small 2019, 15, 1904906 10.1002/smll.201904906. [DOI] [PubMed] [Google Scholar]
- Ferguson I. F.; Ainscough J. B.; Morse D.; Miller A. W. Decomposition of Molybdenum Hexacarbonyl. Nature 1964, 202, 1327–1328. 10.1038/2021327b0. [DOI] [Google Scholar]
- Shum L. G. S.; Benson S. W. The Pyrolysis of Dimethyl Sulfide, Kinetics and Mechanism. Int. J. Chem. Kinet. 1985, 17, 749–761. 10.1002/kin.550170705. [DOI] [Google Scholar]
- Hofmann W. K. Thin Films of Molybdenum and Tungsten Disulphides by Metal Organic Chemical Vapour Deposition. J. Mater. Sci. 1988, 23, 3981–3986. 10.1007/bf01106824. [DOI] [Google Scholar]
- Mousavipour S. H.; Emad L.; Fakhraee S. Theoretical Study on the Unimolecular Dissociation of CH3SCH3 and CH3SCH2. J. Phys. Chem. A 2002, 106, 2489–2496. 10.1021/jp010990q. [DOI] [Google Scholar]
- Eichfeld S. M.; Hossain L.; Lin Y.-C.; Piasecki A. F.; Kupp B.; Birdwell A. G.; Burke R. A.; Lu N.; Peng X.; Li J.; Azcatl A.; McDonnell S.; Wallace R. M.; Kim M. J.; Mayer T. S.; Redwing J. M.; Robinson J. A. Highly Scalable, Atomically Thin WSe2 Grown Via Metal–Organic Chemical Vapor Deposition. ACS Nano 2015, 9, 2080–2087. 10.1021/nn5073286. [DOI] [PubMed] [Google Scholar]
- Zinke-Allmang M.; Feldman L. C.; Grabow M. H. Clustering on Surfaces. Surf. Sci. Rep. 1992, 16, 377–463. 10.1016/0167-5729(92)90006-w. [DOI] [Google Scholar]
- Bales G. S.; Chrzan D. C. Transition from Compact to Fractal Islands During Submonolayer Epitaxial Growth. Phys. Rev. Lett. 1995, 74, 4879–4882. 10.1103/physrevlett.74.4879. [DOI] [PubMed] [Google Scholar]
- Nie Y.; Liang C.; Cha P.-R.; Colombo L.; Wallace R. M.; Cho K. A Kinetic Monte Carlo Simulation Method of Van Der Waals Epitaxy for Atomistic Nucleation-Growth Processes of Transition Metal Dichalcogenides. Sci. Rep. 2017, 7, 2977. 10.1038/s41598-017-02919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y.; Liang C.; Zhang K.; Zhao R.; Eichfeld S. M.; Cha P.-R.; Colombo L.; Robinson J. A.; Wallace R. M.; Cho K. First Principles Kinetic Monte Carlo Study on the Growth Patterns of WSe2 Monolayer. 2D Mater. 2016, 3, 025029. 10.1088/2053-1583/3/2/025029. [DOI] [Google Scholar]
- Shang S.-L.; Lindwall G.; Wang Y.; Redwing J. M.; Anderson T.; Liu Z.-K. Lateral Versus Vertical Growth of Two-Dimensional Layered Transition-Metal Dichalcogenides: Thermodynamic Insight into MoS2. Nano Lett. 2016, 16, 5742–5750. 10.1021/acs.nanolett.6b02443. [DOI] [PubMed] [Google Scholar]
- Bayer B. C.; Kaindl R.; Reza Ahmadpour Monazam M.; Susi T.; Kotakoski J.; Gupta T.; Eder D.; Waldhauser W.; Meyer J. C. Atomic-Scale in Situ Observations of Crystallization and Restructuring Processes in Two-Dimensional MoS2 Films. ACS Nano 2018, 12, 8758–8769. 10.1021/acsnano.8b04945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary K. M.; Hanbicki A. T.; Sivaram S. V.; Jonker B. T. A- and B-Exciton Photoluminescence Intensity Ratio as a Measure of Sample Quality for Transition Metal Dichalcogenide Monolayers. APL Mater. 2018, 6, 111106. 10.1063/1.5053699. [DOI] [Google Scholar]
- Chen W.; Yang Y.; Zhang Z.; Kaxiras E. Properties of in-Plane Graphene/MoS2 Heterojunctions. 2D Mater 2017, 4, 045001. 10.1088/2053-1583/aa8313. [DOI] [Google Scholar]
- Riedl C.; Coletti C.; Iwasaki T.; Zakharov A. A.; Starke U. Quasi-Free-Standing Epitaxial Graphene on SiC Obtained by Hydrogen Intercalation. Phys. Rev. Lett. 2009, 103, 246804. 10.1103/physrevlett.103.246804. [DOI] [PubMed] [Google Scholar]
- Boukhvalov D. W.; Katsnelson M. I. Chemical Functionalization of Graphene with Defects. Nano Lett. 2008, 8, 4373–4379. 10.1021/nl802234n. [DOI] [PubMed] [Google Scholar]
- Connor R.; Adkins H. Hydrogenolysis of Oxygenated Organic Compounds. J. Am. Chem. Soc. 1932, 54, 4678–4690. 10.1021/ja01351a026. [DOI] [Google Scholar]
- Zhang X.; Al Balushi Z. Y.; Zhang F.; Choudhury T. H.; Eichfeld S. M.; Alem N.; Jackson T. N.; Robinson J. A.; Redwing J. M. Influence of Carbon in Metalorganic Chemical Vapor Deposition of Few-Layer WSe2 Thin Films. J. Electron. Mater. 2016, 45, 6273–6279. 10.1007/s11664-016-5033-0. [DOI] [Google Scholar]
- Choudhury T. H.; Simchi H.; Boichot R.; Chubarov M.; Mohney S. E.; Redwing J. M. Chalcogen Precursor Effect on Cold-Wall Gas-Source Chemical Vapor Deposition Growth of WS2. Cryst. Growth Des. 2018, 18, 4357–4364. 10.1021/acs.cgd.8b00306. [DOI] [Google Scholar]
- Suenaga K.; Ji H. G.; Lin Y.-C.; Vincent T.; Maruyama M.; Aji A. S.; Shiratsuchi Y.; Ding D.; Kawahara K.; Okada S.; Panchal V.; Kazakova O.; Hibino H.; Suenaga K.; Ago H. Surface-Mediated Aligned Growth of Monolayer MoS2 and In-plane Heterostructures with Graphene on Sapphire. ACS Nano 2018, 12, 10032–10044. 10.1021/acsnano.8b04612. [DOI] [PubMed] [Google Scholar]
- Tuxen A.; Gøbel H.; Hinnemann B.; Li Z.; Knudsen K. G.; Topsøe H.; Lauritsen J. V.; Besenbacher F. An Atomic-Scale Investigation of Carbon in MoS2 Hydrotreating Catalysts Sulfided by Organosulfur Compounds. J. Catal. 2011, 281, 345–351. 10.1016/j.jcat.2011.05.018. [DOI] [Google Scholar]
- Büch H.; Rossi A.; Forti S.; Convertino D.; Tozzini V.; Coletti C. Superlubricity of Epitaxial Monolayer WS2 on Graphene. Nano Res. 2018, 11, 5946–5956. 10.1007/s12274-018-2108-7. [DOI] [Google Scholar]
- Koma A. Van Der Waals Epitaxy for Highly Lattice-Mismatched Systems. J. Cryst. Growth 1999, 201–202, 236–241. 10.1016/s0022-0248(98)01329-3. [DOI] [Google Scholar]
- Krasheninnikov A. V.; Lehtinen P. O.; Foster A. S.; Pyykkö P.; Nieminen R. M. Embedding Transition-Metal Atoms in Graphene: Structure, Bonding, and Magnetism. Phys. Rev. Lett. 2009, 102, 126807. 10.1103/physrevlett.102.126807. [DOI] [PubMed] [Google Scholar]
- Cretu O.; Krasheninnikov A. V.; Rodríguez-Manzo J. A.; Sun L.; Nieminen R. M.; Banhart F. Migration and Localization of Metal Atoms on Strained Graphene. Phys. Rev. Lett. 2010, 105, 196102. 10.1103/physrevlett.105.196102. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Wang Y. X.; Erb C.; Wang K.; Moradifar P.; Crespi V. H.; Alem N.. Full Orientation Control of Epitaxial MoS2 on hBN Assisted by Substrate Defects. Phys. Rev. B 2019, 99, 155430. 10.1103/physrevb.99.155430 [DOI] [Google Scholar]
- Shi Y.; Zhou W.; Lu A.-Y.; Fang W.; Lee Y.-H.; Hsu A. L.; Kim S. M.; Kim K. K.; Yang H. Y.; Li L.-J.; Idrobo J.-C.; Kong J. Van Der Waals Epitaxy of MoS2 Layers Using Graphene as Growth Templates. Nano Lett. 2012, 12, 2784–2791. 10.1021/nl204562j. [DOI] [PubMed] [Google Scholar]
- Shi J.; Liu M.; Wen J.; Ren X.; Zhou X.; Ji Q.; Ma D.; Zhang Y.; Jin C.; Chen H.; Deng S.; Xu N.; Liu Z.; Zhang Y. All Chemical Vapor Deposition Synthesis and Intrinsic Bandgap Observation of MoS2/Graphene Heterostructures. Adv. Mater. 2015, 27, 7086–7092. 10.1002/adma.201503342. [DOI] [PubMed] [Google Scholar]
- Yan A.; Velasco J.; Kahn S.; Watanabe K.; Taniguchi T.; Wang F.; Crommie M. F.; Zettl A. Direct Growth of Single- and Few-Layer MoS2 on h-BN with Preferred Relative Rotation Angles. Nano Lett. 2015, 15, 6324–6331. 10.1021/acs.nanolett.5b01311. [DOI] [PubMed] [Google Scholar]
- Liu X.; Balla I.; Bergeron H.; Campbell G. P.; Bedzyk M. J.; Hersam M. C. Rotationally Commensurate Growth of MoS2 on Epitaxial Graphene. ACS Nano 2016, 10, 1067–1075. 10.1021/acsnano.5b06398. [DOI] [PubMed] [Google Scholar]
- Eckmann A.; Felten A.; Mishchenko A.; Britnell L.; Krupke R.; Novoselov K. S.; Casiraghi C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. 10.1021/nl300901a. [DOI] [PubMed] [Google Scholar]
- Roy K.; Padmanabhan M.; Goswami S.; Sai T. P.; Ramalingam G.; Raghavan S.; Ghosh A. Graphene–MoS2 Hybrid Structures for Multifunctional Photoresponsive Memory Devices. Nat. Nanotechnol. 2013, 8, 826–830. 10.1038/nnano.2013.206. [DOI] [PubMed] [Google Scholar]
- De Fazio D.; Goykhman I.; Yoon D.; Bruna M.; Eiden A.; Milana S.; Sassi U.; Barbone M.; Dumcenco D.; Marinov K.; Kis A.; Ferrari A. C. High Responsivity, Large-Area Graphene/MoS2 Flexible Photodetectors. ACS Nano 2016, 10, 8252–8262. 10.1021/acsnano.6b05109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.