Abstract
Background
BRCA1 methylation has been associated with homologous recombination deficiency, a biomarker of platinum sensitivity. Studies evaluating BRCA1-methylated tubal and ovarian cancer (OC) do not consistently support improved survival following platinum chemotherapy. We examine the characteristics of BRCA1-methylated OC in a meta-analysis of individual participant data.
Methods
Data of 2636 participants across 15 studies were analyzed. BRCA1-methylated tumors were defined according to their original study. Associations between BRCA1 methylation and clinicopathological characteristics were evaluated. The effects of methylation on overall survival (OS) and progression-free survival (PFS) were examined using mixed-effects models. All statistical tests were 2-sided.
Results
430 (16.3%) tumors were BRCA1-methylated. BRCA1 methylation was associated with younger age and advanced-stage, high-grade serous OC. There were no survival differences between BRCA1-methylated and non–BRCA1-methylated OC (median PFS = 20.0 vs 18.5 months, hazard ratio [HR] = 1.01, 95% CI = 0.87 to 1.16; P = .98; median OS = 46.6 vs 48.0 months, HR = 1.02, 95% CI = 0.87 to 1.18; P = .96). Where BRCA1/2 mutations were evaluated (n = 1248), BRCA1 methylation displayed no survival advantage over BRCA1/2-intact (BRCA1/2 wild-type non–BRCA1-methylated) OC. Studies used different methods to define BRCA1 methylation. Where BRCA1 methylation was determined using methylation-specific polymerase chain reaction and gel electrophoresis (n = 834), it was associated with improved survival (PFS: HR = 0.80, 95% CI = 0.66 to 0.97; P = .02; OS: HR = 0.80, 95% CI = 0.63 to 1.00; P = .05) on mixed-effects modeling.
Conclusion
BRCA1-methylated OC displays similar clinicopathological features to BRCA1-mutated OC but is not associated with survival. Heterogeneity within BRCA1 methylation assays influences associations. Refining these assays may better identify cases with silenced BRCA1 function and improved patient outcomes.
Epithelial tubal, primary peritoneal, and ovarian cancer, hereafter referred to as ovarian cancer (OC), are aggressive diseases with poor patient outcomes. High-grade serous cancer (HGSC) is the most common and lethal form of OC (1). Targeting homologous recombination deficiency (HRD), a molecular hallmark in approximately 50% of HGSC, could improve outcomes for a substantial number of women with OC. BRCA1/2 germline and somatic mutations are observed in 15%–20% of OC and account for approximately one-third of HRD tumors (2,3). These mutations are predictors of platinum and poly-ADP-ribose polymerase inhibitors (PARPi) response and are prognostic for improved outcomes in OC (4,5). Identifying other mechanisms producing HRD could expand the number of women with OC benefiting from PARPi. Another possible although less well characterized mechanism of HRD is BRCA1 promoter methylation, occurring in approximately 10%–15% of HGSC (3,6–8), although reported rates vary between 5% and 89.9% (9,10). By virtue of epigenetic silencing of BRCA1, BRCA1-methylated OC is postulated to compare to BRCA1-mutated OC in terms of HRD, platinum chemotherapy and PARPi sensitivity, clinical characteristics, and survival outcomes.
Cell line models of BRCA1-methylated OC display specific sensitivity to platinum chemotherapy and PARPi (11). Analysis of BRCA1-methylated OC specimens, albeit in small cohorts, consistently display low BRCA1 protein and mRNA expression (7,12–14). In the clinical setting, few retrospective studies have addressed the implication of BRCA1 methylation on clinical characteristics and patient outcomes after platinum chemotherapy in OC, with inconsistent results. Recent large studies utilizing genome-wide methylation arrays (GWMA) correlated to BRCA1 mRNA expression to detect BRCA1 methylation demonstrate no prognostic impact on survival (3,15). One study, however, shows similar hazard ratios (HR) for overall survival (OS) for both BRCA1-methylated (HR = 0.74, 95% confidence interval [CI] = 0.49 to 1.14) and BRCA1-mutated OC (HR = 0.75, 95% CI = 0.46 to 1.22) (15) as compared with BRCA1/2-intact disease, although neither were statistically significant. In contrast, smaller studies (n = 27 to 332) utilizing methylation sensitive or methylation-specific polymerase chain reaction (PCR) (MSP) as a diagnostic assay have conflicting findings with regards to associations with platinum sensitivity and survival. Whereas some report statistically significant improvements in survival (16,17), as compared with non–BRCA1-methylated OC, others observe trends toward a worse outcome (18). A comprehensive study of the clinical implications of BRCA1-methylated OC is required.
Methods
Search Strategy and Study Selection
The conduct of this meta-analysis followed the Meta-Analysis of Observational Studies in Epidemiology guidelines. Original investigations were sought in PubMed and Medline through April 1, 2018, with no restrictions on publication date or language. The search strategy followed the syntax (BRCA*[Title/Abstract] OR BRCA1*[Title/Abstract]) AND methylat*[Title/Abstract] AND ovar*[Title/Abstract] and was performed independently by 2 investigators (RK and BS), who independently reviewed abstracts for eligibility. Additionally, abstracts from the 2009–2018 American Society of Clinical Oncology, European Society for Medical Oncology, and Society of Gynecologic Oncology annual meetings were searched. Reference lists for eligible articles were reviewed for further potential studies. The inclusion of published and unpublished studies regardless of publication language or date attempts to minimize publication bias.
Eligible studies needed to assess BRCA1 methylation in fallopian tube, primary peritoneal, or ovarian cancer specimens; report on participant and disease characteristics; report on progression-free survival (PFS) and/or OS; and provide suitable methodology on their BRCA1 methylation assay. Clinical trials involving PARPi were not eligible. There were no restrictions on the BRCA1 methylation assay used. As BRCA2 methylation seldom occurs in ovarian cancer, we did not investigate its role in OC. Studies reporting solely on BRCA2 methylation in OC were therefore excluded.
Data Acquisition
The following anonymized individual participant data (IPD) was requested from the authors of eligible studies, using a prespecified template and coding: BRCA1 methylation assay details, participant and disease characteristics [age, histology, International Federation of Gynecology and Obstetrics (FIGO) stage (19), grade, HRD score, BRCA1 methylation, BRCA1/2 mutation], treatment details (receipt of adjuvant and neoadjuvant platinum chemotherapy, degree of surgical cytoreduction, platinum sensitivity), and survival outcomes (platinum-free interval [PFI], PFS, and OS). Tumor HRD score is the unweighted sum of loss of heterozygosity (LOH), telomeric allelic imbalance, and large-scale state transitions (20). A score of no less than 42 defines a tumor as HR deficient (21).
Data Integrity and Risk of Bias Assessment
IPD was checked for errors, missing data, and consistency with study publications. Study authors were contacted to resolve discrepancies or obtain missing data. Studies with partially missing data were analyzed on the basis of available data.
Bias assessment for the studies internal validity was performed using the ROBINS-I tool (22) recommended by the Cochrane collaboration for nonrandomized studies.
Statistical Analysis
This meta-analysis aims to clinically characterize BRCA1-methylated OC. Its primary and secondary objectives were to determine the clinicopathological characteristics associated with BRCA1 methylation and ascertain the prognostic impact of BRCA1 methylation on PFS and OS, respectively, in OC. A tumor was considered BRCA1 methylated if it was defined as such within its original study. OS was the time from diagnosis until death; participants were censored at the last known survival date. PFS was the time from diagnosis until CA125 and/or RECIST disease progression or death, whichever occurred first; participants were censored at the last known progression-free date.
Descriptive statistics were calculated for the combined IPD to summarize participants and disease characteristics for BRCA1-methylated OC vs non–BRCA1-methylated OC. The characteristics of interest were age, histotype, stage, grade, residual disease after surgical cytoreduction, and platinum sensitivity. Comparisons between BRCA1-methylated and non–BRCA1-methylated OC were made using the generalized Cochran-Mantel-Haenzel test for repeated tests of independence with continuity correction to facilitate combining the multiple cohorts. Where available, HRD scores of BRCA1-methylated OC were compared with BRCA1/2-intact, BRCA1-mutated, and BRCA2-mutated OC using unpaired t tests. The Kaplan-Meier method (log-rank test) was used to generate survival plots via the R package survival (23). Forest plots were generated using the R package survcomp (24). Univariate and multivariate analysis of PFS and OS were performed using Cox proportional hazards regression models, which estimated hazard ratios and 95% confidence intervals for each individual dataset. A mixed effects Cox model was then used to perform univariate and multivariate analysis of the combined dataset, comparing BRCA1-methylated OC with non–BRCA1 methylated OC. The assumptions of proportional hazards were tested using the Schoenfeld residuals ensuring that they were independent of time. These models were generated using the R packages survival and coxme (25), respectively. Multivariate models were adjusted for the following clinical variables: age, grade, stage, and residual disease after surgical cytoreduction. For cohorts with available germline and/or somatic BRCA1/2 mutation data, the above analyses were repeated comparing BRCA1-methylated with BRCA1/2-intact (BRCA1/2–wild-type non–BRCA1-methylated) OC to eliminate the potential survival bias attributed by BRCA1/2-mutated OC in the non–BRCA1-methylated population. P values were adjusted for multiple testing [Benjamini-Hochberg method (26)]. Heterogeneity was quantified using the I2 statistic (27), which provides a numerical value ranging between 0% and 100%. This value and its 95% confidence interval were interpreted according to ranges described in the Cochrane Handbook for Systematic Review of Interventions, with values between 0% and 40%, 30% and 60%, 50% and 90%, and 75% and 100% suggesting low, moderate, considerable, and substantial heterogeneity, respectively (28). For all analyses, P values less than .05 (2-tailed) were considered statistically significant. All calculations were performed in the R statistical environment (https://www.r-project.org/).
Results
Study Selection
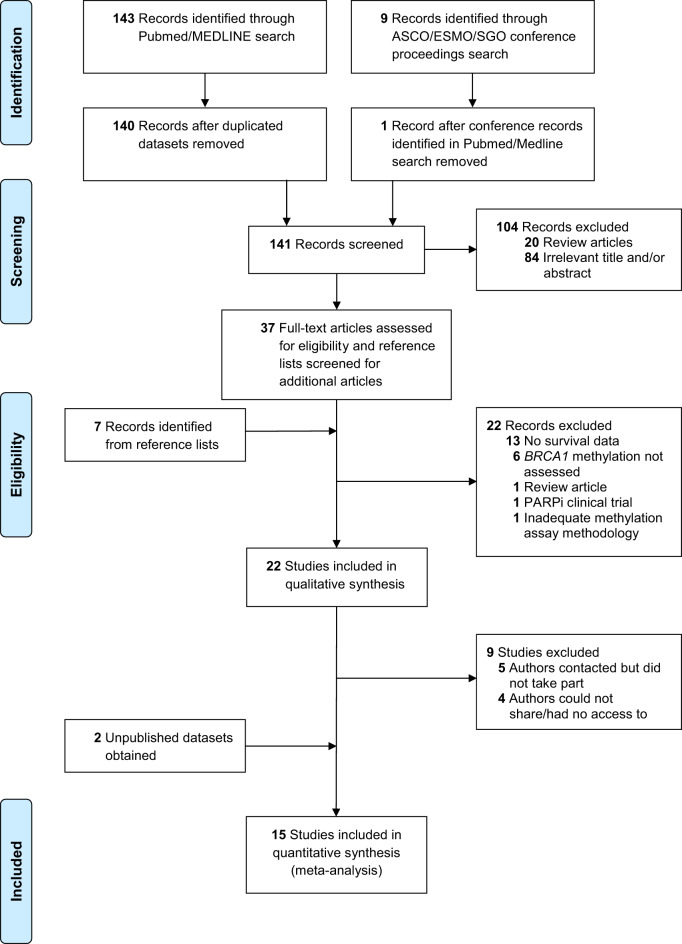
The literature search retrieved 159 records (Figure 1). Three were duplicated datasets, 8 were conference abstracts identified in the PubMed search, 90 were irrelevant, 21 were reviews, 13 had no survival data, 1 had unsatisfactory methodology details, 1 concerned a PARPi clinical trial, and 22 were eligible. Following contact with authors of eligible studies, 5 did not participate, and 4 could not access or share the data (Supplementary Table 1, available online) (14,17,18,29–34). In addition, we accessed 2 unpublished cohorts. One author provided data on additional patients not included in the original publication. Overall, 15 observational studies (430 cases; 2206 controls) were included (3,13,15,16,35–43) (Supplementary Tables 2–4, available online).
Figure 1.
Flow chart of study. ASCO = American Society of Clinical Oncology; ESMO = European Society for Medical Oncology; PARPi = poly-ADP-ribose polymerase inhibitors; SGO = Society of Gynecologic Oncology.
Quality Assessment
Individual study assessments were made on the basis of raw IPD, study manuscript, and any necessary clarifications with study authors (Supplementary Table 5, available online). The interval validity of studies was deemed overall good, with a likely low risk of bias on the meta-analysis results. Of the 15 studies, 13 had an overall moderate risk of bias, mainly owing to the potential for confounding baseline factors that were nevertheless adjusted for appropriately. In addition, 4 studies had a moderate risk in patient selection with regards to determining the clinicopathological characteristics of BRCA1-methylated OC, because only HGSC were included. A moderate risk was also found for 5 studies where intended BRCA1 methylation assessment failed because of inadequate tumor tissue or DNA. Two studies had an overall serious risk of bias, owing to missing data. One did not collect tumor grade and OS data for its entire cohort (n = 35) (39). The other provided IPD for 61.0% (n = 147 of 241) of study participants due to time constraints in data collection and provision (36). Neither of these 2 studies collected data pertaining to BRCA1/2 mutation status or HRD score. The exclusion of these 2 studies from the entire meta-analysis cohort did not alter the results of the meta-analysis for any of the endpoints measured (data not shown). It was therefore deemed reasonable to include these 2 studies in this meta-analysis of individual patient data.
Participant Characteristics
Data was obtained on 2645 participants; 9 participants with dual aberrations (BRCA1 methylation and/or BRCA1/2 mutations) were excluded (Supplementary Table 2, available online), leaving 2636 participants within the analysis. Among participants with known BRCA1/2 mutation status (n = 1257), BRCA1 methylation and BRCA1 mutation were mutually exclusive (odds ratio [OR] = 0.18; P = .003), as were BRCA1 methylation and BRCA2 mutation (OR = 0.33; P = .04). In the entire cohort, the median age was 59 years old. Fallopian tube and primary peritoneal cancers comprised 0.4% (n = 7 of 2022) and 1.3% (n = 26 of 2022) of the entire cohort, respectively; 85.4% (n = 2247 of 2630) participants presented with advanced stage disease (FIGO stage III and IV); 95.6% (n = 2396 of 2506) participants received (neo)adjuvant platinum-based therapy; and 79.7% (n = 2065 of 2592) were HGSC. Stage III/IV HGSC comprised 73.9% participants (n = 1904 of 2576). Surgical cytoreduction less than 1 cm residual disease was achieved in 71.9% (n = 1757 of 2444) participants (Supplementary Table 6, available online).
Association of BRCA1 Methylation With Participants, Disease, and Molecular Characteristics
The BRCA1 methylation rate varied in studies from 6.2% to 73.7% (Supplementary Table 2, available online), with a pooled rate of 16.3% (n = 430 of 2636). BRCA1-methylated OC was statistically significantly associated with younger age (P = .005) and high grade disease (P = .03) (Table 1). No other statistically significant clinicopathological correlations were observed.
Table 1.
Association between BRCA1 methylation status and clinicopathological factorsa
| Parameter | BRCA1-methylated (n = 430) | Non–BRCA1 methylated (n = 2206) | Adjusted Pb |
|---|---|---|---|
| Age, years No. (%) | .005 | ||
| <59 y | 245 (57.0) | 1090 (49.6) | |
| ≥59 y | 185 (43.0) | 1109 (50.4) | |
| Missing | 0 | 7 | |
| Grade, No. (%) | .03 | ||
| Low | 25 (6.0) | 198 (9.1) | |
| High | 389 (94.0) | 1979 (90.9) | |
| Missing | 16 | 29 | |
| FIGOc stage, No. (%) | .47 | ||
| I/II | 48 (11.2) | 339 (15.4) | |
| III/IV | 381 (88.8) | 1862 (84.6) | |
| Missing | 1 | 5 | |
| Histology, No. (%) | .97 | ||
| Serous | 354 (82.3) | 1799 (81.6) | |
| Nonserous | 76 (17.7) | 407 (18.4) | |
| Missing | 0 | 0 | |
| Residual disease post-cytoreduction, No. (%) | .47 | ||
| Macro <1 cm | 306 (73.6) | 1451 (71.5) | |
| Macro ≥1 cm | 110 (26.4) | 577 (28.5) | |
| Missing | 14 | 178 | |
| Platinum sensitivity, No. (%) | .34 | ||
| <6 months | 66 (19.4) | 463 (28.2) | |
| ≥6 months | 275 (80.6) | 1177 (71.8) | |
| No platinum | 17 | 106 | |
| Missing | 72 | 460 |
Percentages reflect percentage of total nonmissing data.
Two-sided Cochran-Mantel-Haenzel test with P value adjusted for study.cInternational Federation of Gynecology and Obstetrics
Germline and/or somatic BRCA1/2 mutation status was available for 1248 participants from 7 of 15 included studies. Of these, 10.6% (n = 132) were BRCA1-mutated, 6.5% (n = 81) were BRCA2-mutated, and 10.3% (n = 128) were BRCA1 methylated. Within this cohort with known germline and/or somatic BRCA1/2 mutation status, BRCA1 methylation was also associated with younger age (P = .007) and high grade disease (P = .005) when compared with patients with BRCA1/2-intact OC. Furthermore, BRCA1-methylated OC was associated with advanced stage (P = .01) and serous histology (P = .009) compared with BRCA1/2-intact OC. BRCA1 mutation was associated with younger age (P < .001), high grade (P = .006), serous histology (P = .005), advanced stage (P = .02), and platinum sensitivity (P = .008) when compared with BRCA1/2-intact disease. The clinicopathological profile of BRCA1-methylated OC did not differ statistically significantly from that of BRCA1-mutated OC (Supplementary Table 7, available online).
Tumor HRD score was available for 447 participants (MD Anderson Cancer Center 2010 and The Cancer Genome Atlas [TCGA] 2011 cohorts). HRD scores were statistically significantly higher in BRCA1-methylated OC (median = 68, interquartile range [IQR] = 62–74) compared with BRCA1/2-intact disease (median = 26, IQR = 18–38.8), BRCA1-mutated (median = 63, IQR = 56–70), and BRCA2-mutated (median = 56, IQR = 44.5–65.5) disease (Figure 2).
Figure 2.
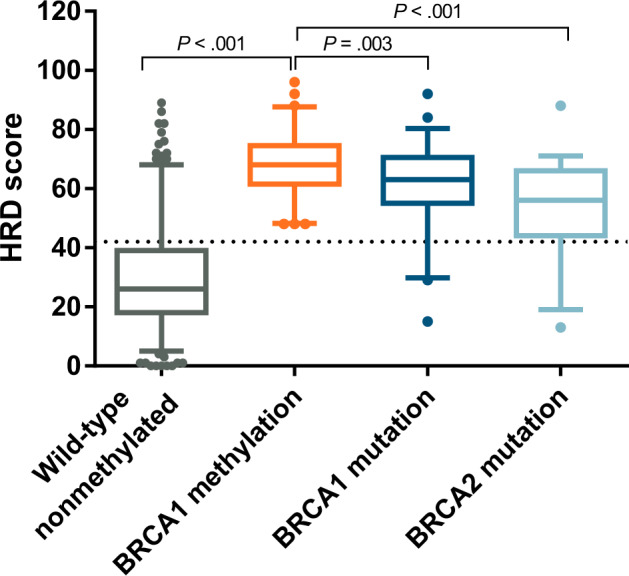
HRD score as assessed on 447 tumor samples obtained from TCGA 2011 and MDACC 2010 cohorts, according to underlying BRCA1/2 aberration. Box plots depict the median and 95% confidence intervals of the HRD scores according to the underlying BRCA1/2 aberration in TCGA 2011 and MDACC 2010 cohorts. The dotted line represents the threshold value of 42 above which samples are considered to be homologous recombination deficient as per the HRD score assay. P values denote the level of statistical significance between sets of groups (unpaired t tests). Numbers in each subgroup are as follows: wild-type, n = 286; BRCA1 methylation, n = 65; BRCA1 mutation, n = 57; BRCA2 mutation, n = 39. HRD = homologous recombination deficiency; MDACC = MD Anderson Cancer Center; TCGA = The Cancer Genome Atlas
Association of BRCA1 Methylation With Survival
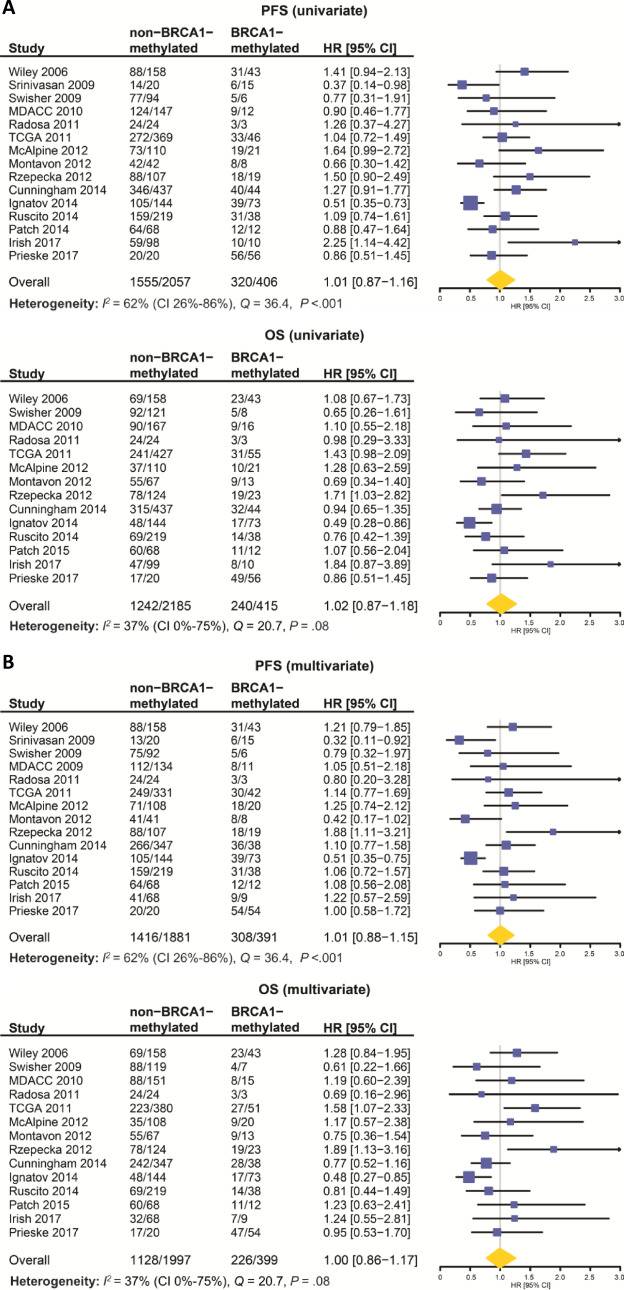
The median follow-up time was 2.8 years (range = 0–18.3, IQR = 1.5–4.8). There was a statistically significant constituent study effect on the Cox regression model first used to associate BRCA1 methylation with PFS and OS (P < .001 for both PFS and OS). Statistically significant moderate to substantial heterogeneity was observed between studies for PFS (I2 = 62.0%, 95% CI = 26.0% to 86.0%, Cochran’s Q = 36.4, df = 14; P < .001). Although not statistically significant, we note low heterogeneity between studies for the assessment of OS (I2 = 37.0%, 95% CI = 0.0% to 75.0%, Q = 20.7, df = 13; P = .08), although the 95% confidence interval is wide. Clinically, heterogeneity is expected given observed differences between cohorts with regard to patient and disease clinical characteristics and study characteristics. A mixed-effect model was therefore employed to adjust for study heterogeneity. To justify our acceptance of the null hypothesis of this meta-analysis, we performed a power calculation utilizing observed information (study heterogeneity) and assumptions (predicted effect size). Using this information, we calculated that we have an 84.0% power to detect a modest effect size (Cohen d of 0.2) across the 15 studies with an average BRCA1 methylation rate of 29 samples per study and an average non-BRCA1 methylation rate of 147 samples per study with an alpha value of 0.05 and a moderate level of heterogeneity.
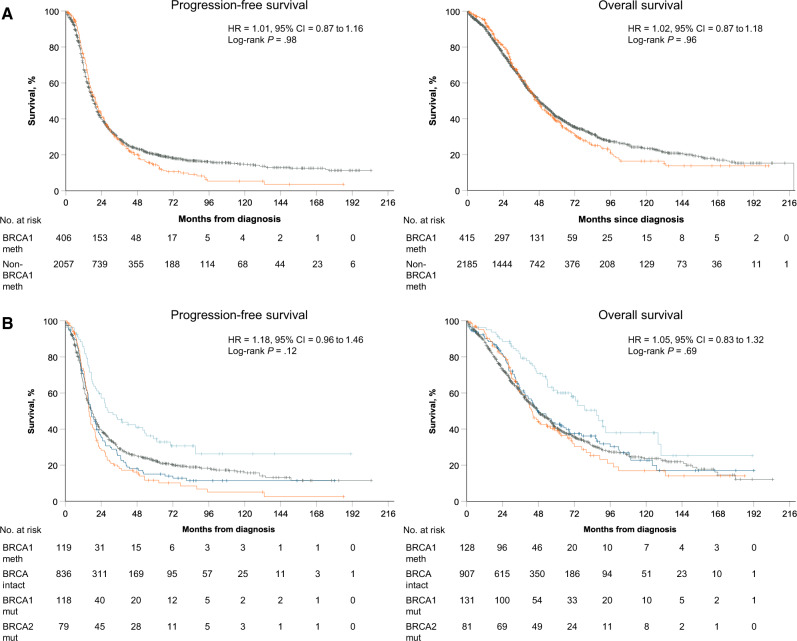
In the combined population, there was no statistically significant difference in PFS and OS between BRCA1-methylated and non–BRCA1-methylated OC (median PFS = 20.0 vs 18.5 months, HR = 1.01, 95% CI = 0.87 to 1.16; P = .98; median OS = 46.6 vs 48.0 months, HR = 1.02, 95% CI = 0.87 to 1.18; P = .96, respectively). This lack of association persisted in a multivariate model adjusted for age, stage, grade, and cytoreduction (Figures 3–4A).
Figure 3.
Univariate and multivariate analyses of BRCA1 methylation on progression-free survival (PFS) and overall survival (OS) by study and for the combined dataset. Forest plots of the association of BRCA1 methylation with PFS and OS on univariate and multivariate analyses. A) Univariate analyses of PFS and OS; (B) Multivariate analyses of PFS and OS, adjusted for binary clinical variables as follows: Age: 0 for <60 (median age) and 1 for ≥60; Grade: 0 for low grade, 1 for high grade; Stage: 0 for stage I/II, 1 for stage III/IV; Residual disease: 0 for <1 cm, 1 for ≥1 cm. Numbers in non–BRCA1-methylated and BRCA1-methylated columns represent number of events/total numbers. Squares determine study-specific estimates, and horizontal lines indicate 95% confidence interval; diamond depicts summary estimate with its associated 95% confidence interval (CI). Hazard ratios (HR) and 95% confidence intervals were determined using a Cox proportional hazards mixed effects model. All statistical tests were two-sided.
Figure 4.
Survival in the entire pooled dataset and a pooled subset with known BRCA1/2 mutation status. Estimates of progression-free survival (PFS) and overall survival (OS) from Kaplan-Meier curves, with tests of differences by 2-sided log-rank test. A) PFS and OS in the entire pooled cohort (n = 2636). Kaplan-Meier curves for BRCA1 methylation (orange) and no BRCA1 methylation (grey) curves are depicted. B) PFS and OS in a pooled subset of the entire cohort with known BRCA1/2 mutation status (n = 1248). Kaplan-Meier curves for BRCA1 methylation (orange), BRCA1 mutation (dark blue), BRCA2 mutation (light blue), and BRCA1/2 intact (non–BRCA1-methylated BRCA1/2 wild-type) (grey) curves are depicted. In all graphs, univariate hazard ratios (HR), 95% confidence interval (CI), and P value for BRCA1 methylation vs BRCA1/2 intact, adjusted for study, are given. BRCA1 meth = BRCA1 methylation; non–BRCA1-meth = no BRCA1 methylation.
Within the subgroup with known BRCA1/2 mutation status, BRCA1-methylated OC was associated with a worse PFS than BRCA1/2-intact OC on univariate analysis (median PFS = 15.7 vs 18.0 months, HR = 1.26, 95% CI = 1.02 to 1.56; P = .03), although this statistical significance was lost on multivariate analysis. There was no OS difference on univariate or multivariate analyses between the BRCA1-methylated and the BRCA1/2-intact groups (median OS = 43.5 vs 47.5 months, univariate HR = 1.05, 95% CI = 0.83 to 1.32; P = .70). BRCA1-mutated OC was only associated with a statistically significant improvement in PFS and OS as compared with BRCA1/2-intact OC on multivariate analysis (PFS: median = 17.3 months, univariate HR = 0.94, 95% CI = 0.75 to 1.17; P = .57; multivariate HR = 0.78, 95% CI = 0.62 to 0.99; P = .04; OS: median = 47.4 months, univariate HR = 0.81, 95% CI = 0.64 to 1.03; P = .09; multivariate HR = 0.76, 95% CI = 0.58 to 0.98; P = .03). BRCA2 mutation conferred a clear PFS and OS benefit compared with BRCA1/2-intact OC on univariate and multivariate analyses (median PFS = 28.6 months, univariate HR = 0.58, 95% CI = 0.44 to 0.78; P < .001; median OS = 87.0 months, univariate HR = 0.55, 95% CI = 0.40 to 0.77; P < .001) (Figure 4B and Table 2).
Table 2.
Univariate and multivariate analysis for PFS and OS according to BRCA1/2 aberrations
| Variables | Univariate analysis |
Multivariate analysisa |
||||||
|---|---|---|---|---|---|---|---|---|
| PFS |
OS |
PFS |
OS |
|||||
| HR (95% CI) | P b | HR (95% CI) | P b | HR (95% CI) | P b | HR (95% CI) | P b | |
| Entire cohort (n = 2636) | ||||||||
| Age | — | — | — | — | 1.10 (1.00 to 1.25) | .05 | 1.22 (1.10 to 1.37) | <.001 |
| Grade | — | — | — | — | 2.38 (1.84 to 3.07) | <.001 | 1.80 (1.35 to 2.41) | <.001 |
| Stage | — | — | — | — | 2.89 (2.36 to 3.47) | <.001 | 2.76 (2.19 to 3.48) | <.001 |
| Residual disease | — | — | — | — | 1.59 (1.41 to 1.80) | <.001 | 1.79 (1.57 to 2.03) | <.001 |
| BRCA1 methylation | 1.01 (0.87 to 1.16) | .98 | 1.02 (0.87 to 1.18) | .96 | 1.01 (0.88 to 1.15) | .92 | 1.00 (0.86 to 1.17) | .97 |
| Cohort with known BRCA1/2 mutations (n = 1248) | ||||||||
| Age | — | — | — | — | 1.08 (0.93 to 1.25) | .33 | 1.18 (1.00 to 1.37) | .04 |
| Grade | — | — | — | — | 2.30 (1.48 to 3.57) | <.001 | 1.97 (1.19 to 3.27) | .01 |
| Stage | — | — | — | — | 4.07 (3.00 to 5.52) | <.001 | 3.10 (2.18 to 4.42) | <.001 |
| Residual disease | — | — | — | — | 1.37 (1.15 to 1.62) | <.001 | 1.54 (1.29 to 1.83) | <.001 |
| BRCA1 methylation | 1.26 (1.02 to 1.56) | .03 | 1.11 (0.88 to 1.41) | .35 | 1.06 (0.85 to 1.34) | .59 | 0.97 (0.75 to 1.24) | .78 |
| BRCA1 mutation | 0.99 (0.80 to 1.23) | .91 | 0.87 (0.69 to 1.10) | .24 | 0.78 (0.62 to 0.99) | .04 | 0.76 (0.58 to 0.98) | .03 |
| BRCA2 mutation | 0.58 (0.43 to 0.77) | <.001 | 0.57 (0.41 to 0.80) | <.001 | 0.51 (0.37 to 0.69) | <.001 | 0.51 (0.35 to 0.73) | <.001 |
All clinical variables within the multivariate model are binary, as follows: Age: 0 for <60 (median age) and 1 for ≥60; Grade: 0 for low grade, 1 for high grade; 0 for stage I/II, 1 for stage III/IV; Residual disease: 0 for <1 cm, 1 for ≥1 cm. CI = confidence interval; HR = hazard ratio; OS= overall survival; PFS = progression-free survival.
Two-tailed mixed-effects Cox proportional hazards regression model with P value adjusted for study.
Exploratory Analysis of Methylation Methodology on Survival
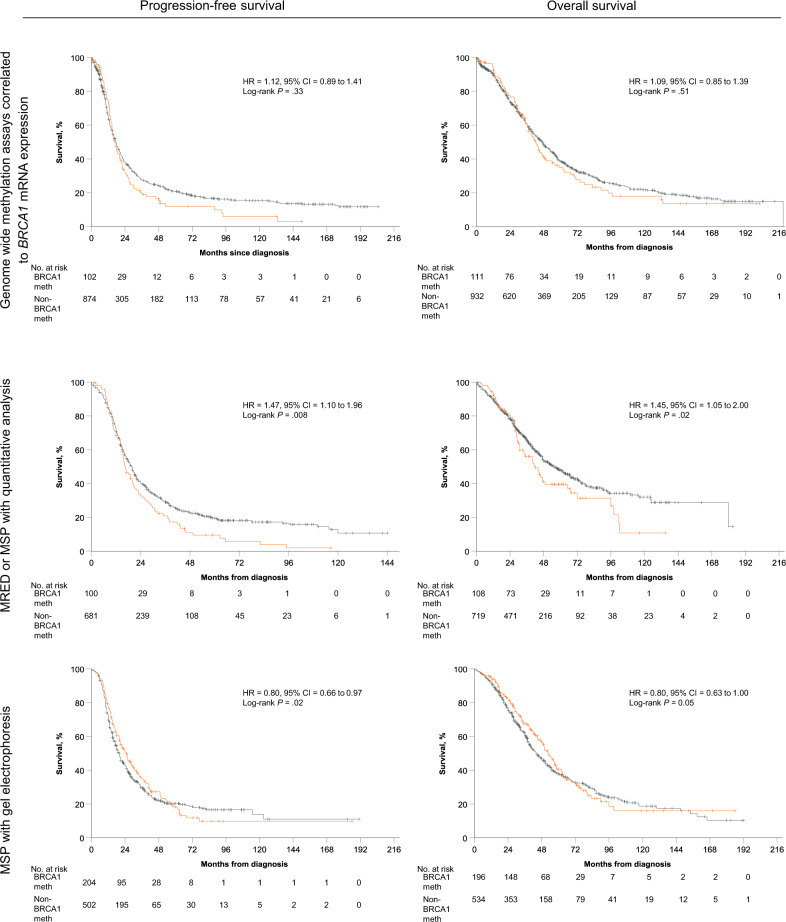
We explored PFS and OS comparing BRCA1-methylated OC with non–BRCA1-methylated OC within 3 subgroups of the meta-analysis’ entire cohort divided according to methylation assay type: those studies utilizing methylation-specific PCR (MSP) with gel electrophoresis (GE), or MSP-GE, (7 studies, n = 765), those utilizing quantitative analysis of MSP or methylation sensitive restriction endonuclease digestion (MRED; 5 studies, n = 828), and those utilizing GWMA (3 studies, n = 1043). Methylation assays are further detailed in Supplementary Table 2 and Supplementary Figure 1 (available online). In the combined cohorts utilizing MSP-GE, BRCA1-methylated OC was associated with an improved PFS and OS (univariate HR = 0.80, 95% CI = 0.66 to 0.97; P = .02; univariate HR = 0.80, 95% CI = 0.63 to 1.00; P = .05, respectively) as compared with non–BRCA1-methylated OC, although statistical significance was lost for OS on multivariate analysis (P = .08). Among cohorts utilizing MSP/MRED with quantitative analysis, BRCA1-methylated OC was associated with a worse PFS (HR = 1.47, 95% CI = 1.10 to 1.96; P = .008) and OS (HR = 1.45, 95% CI =1.05 to 2.00; P = .02), when compared with non–BRCA1-methylated OC. This persisted on multivariate analysis. There were no survival differences observed between BRCA1-methylated OC and non–BRCA1-methylated OC in the subgroup employing GWMA (Figure 5 and Table 3).
Figure 5.
Survival analyses in subgroups combined according to methylation assay. Estimates of progression-free survival (PFS) and overall survival (OS) from Kaplan-Meier curves, in subgroups combined according to methylation assay, with tests of differences by 2-sided log-rank test. A) PFS and OS in cohorts where BRCA1 methylation was determined using genome-wide methylation assays correlated to BRCA1 mRNA expression represents. B) PFS and OS in cohorts where BRCA1 methylation was determined using MSP or MRED analyzed using quantitative methods. C) PFS and OS in cohorts where BRCA1 methylation was determined using MSP and gel electrophoresis. In all graphs, univariate hazard ratios (HR) and 95% confidence interval (CI) and P value for BRCA1 methylation (orange) as compared to no BRCA1-methylation (grey), adjusted for study, are given. BRCA1 meth = BRCA1 methylation; non–BRCA1-meth = no BRCA1 methylation; MRED = methylation-sensitive restriction endonuclease digestion, MSP = methylation specific PCR.
Table 3.
Association of BRCA1 methylation with PFS and OS according to methylation assay subgroup (methylated relative to nonmethylated)
| Methylation assay subgroup | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P a | n (events) | HR (95% CI) | P a | n (events) | |
| Univariable | ||||||
| Genome-wide methylation array correlated to BRCA1 mRNA expression | 1.12 (0.89 to 1.41) | 0.33 | 976 (767) | 1.09 (0.85 to 1.39) | 0.51 | 1043 (690) |
| MRED or MSP with quantitative analysis | 1.47 (1.10 to 1.96) | 0.008 | 781 (590) | 1.45 (1.05 to 2.00) | 0.02 | 826 (382) |
| MSP with gel electrophoresis | 0.80 (0. 66 to 0.97) | 0.02 | 706 (518) | 0.80 (0.63 to 1.00) | 0.05 | 730 (411) |
| Multivariableb | ||||||
| Genome-wide methylation array correlated to BRCA1 mRNA expression | 1.11 (0.87 to 1.42) | 0.40 | 838 (657) | 1.04 (0.80 to 1.35) | 0.78 | 896 (591) |
| MRED or MSP with quantitative analysis | 1.36 (1.00 to 1.84) | 0.04 | 733 (556) | 1.40 (1.01 to 1.94) | 0.04 | 774 (354) |
| MSP with gel electrophoresis | 0.82 (0.68 to 1.00) | 0.05 | 655 (482) | 0.81 (0.64 to 1.03) | 0.08 | 724 (407) |
Two-tailed mixed-effects Cox proportional hazards regression model with P value adjusted for cohort. CI = confidence interval; HR = hazard ratio; MRED = methylation-sensitive restriction endonuclease digest; MSP = methylation-specific PCR; OS = overall survival PFS = progression-free survival.
Adjusted for binary clinical variables as follows: Age: 0 for <60 (median age) and 1 for ≥60; Grade: 0 for low grade, 1 for high grade; Stage: 0 for stage I/II, 1 for stage III/IV; Residual disease: 0 for <1 cm, 1 for ≥1 cm.
Discussion
Dysfunctional BRCA1 and BRCA2 proteins as a result of BRCA1/2 mutations render OC particularly susceptible to therapies targeting the homologous recombination DNA repair pathway. Although the association of BRCA1/2-mutated OC with improved survival resulting from sensitivity to (neo)adjuvant platinum-based chemotherapy is well established, the clinical and therapeutic implications of other BRCA1 dysfunction mechanisms are less understood. In a recent meta-analysis, loss of BRCA1 expression by immunohistochemistry was also associated with a statistically significant improved survival (44), although the mechanisms behind absent BRCA1 protein expression were not specified. Gene silencing through promoter methylation is one such mechanism, although other potential indirect or posttranslational mechanisms leading to BRCA1 inactivation or reduced expression require investigation. Whereas BRCA1 methylation is a recognized event in OC, promoter methylation of BRCA2 appears to be a rare occurrence in OC, if at all. Among 6 studies investigating BRCA2 methylation in OC (15,45–49), only 2 BRCA2-methylated cases were identified among 612 cases tested. This meta-analysis was therefore limited to studies investigating BRCA1 methylation. Reports on BRCA1-methylated OC have been conflicting in terms of clinicopathological associations, with smaller cohorts observing associations with FIGO stage I/II disease (31) or a lack of association with any histotype (18). Other cohorts limited their assessment of BRCA1 methylation to homogeneous HGSC cohorts, thereby precluding the detection of histopathological associations. Our study population comprised heterogeneous OC subtypes, although dominated by advanced stage HGSC. We show that features of BRCA1-methylated OC mirror that of BRCA1-mutated disease in terms of advanced stage, high grade, serous disease, and a younger age at diagnosis.
The expectation is therefore that BRCA1-methylated disease will be sensitive to platinum and PARPi by virtue of HRD. We provide evidence of HRD in BRCA1-methylated OC, as defined by the HRD score, albeit in a limited subgroup of participants derived from 2 studies. Cell line and patient-derived xenograft models of BRCA1-methylated OC demonstrate clear sensitivity to platinum and/or PARPi therapy (11,50,51). Recent data from the ARIEL2 phase II clinical trial demonstrate an encouraging 63% (n = 12 of 19) RECIST response rate among BRCA1-methylated recurrent HGSC to the PARPi rucaparib as compared with response rates of 79% (n = 23 of 29) and 13.5% (n = 7 of 52) observed in BRCA1-mutated and BRCA1-intact with low genome-wide LOH recurrent HGSC, respectively (52). However, large clinical cohorts (eg, TCGA) show no difference in PFI by BRCA1 methylation status. Within this meta-analysis, PFI data was unavailable for 56.9% (n = 1539 of 2636) of participants, thereby precluding a representative assessment. Nevertheless, we found no association between BRCA1-methylated OC and platinum sensitivity. Moreover, we observed no PFS difference between BRCA1-methylated and non–BRCA1-methylated OC in the entire cohort. When evaluated against the more appropriate comparator population that is BRCA1/2-intact OC, albeit within a smaller cohort of 1248 patients, BRCA1-methylated OC once again did not display improved survival. In contrast, both BRCA1 and BRCA2 mutations were prognostic of improved PFS and OS on multivariate analysis as compared to BRCA1/2-intact OC, despite the limited cohort size of 1248 patients. Reasons for the discrepancy in survival between BRCA1-mutated OC and BRCA1-methylated OC, relative to BRCA1/2-intact OC, are unclear and warrant further investigation. Compared to BRCA2 mutations, the survival benefit conferred by BRCA1 mutations is of a lesser magnitude, echoing recent reports evaluating survival in BRCA1/2-mutated OC. Some studies find no survival difference between BRCA1-mutated and BRCA1/2 wild-type cancers (15,49). A pooled cohort (>6500 participants) demonstrated the expected positive prognostic effect of BRCA1 mutation on OS, which was to a markedly lesser degree than observed with BRCA2 mutation (BRCA1 mutation HR = 0.83, 95% CI = 0.74 to 0.93; P < .001; BRCA2 mutation HR = 0.55, 95% CI = 0.47 to 0.65; P = .002) (53). It is likely the survival benefit is diluted by heterogeneity within BRCA1-mutated disease, whereby some cases assigned a BRCA1-mutated status actually behave in HR proficient manner. Similarly, the difference in survival patterns between BRCA1 mutation and BRCA1 methylation, despite sharing similar clinicopathological features, could be explained in part by heterogeneity within BRCA1-methylated OC, as discussed further below. Moreover, methylation as a rule is a more dynamic mechanism relative to mutation and subject to change depending on specific characteristics of the tumor microenvironment. The survival benefit observed in BRCA1/2-mutated OC results from their marked sensitivity to platinum-based chemotherapy, used as standard of care in the (neo)adjuvant treatment of OC. Potentially, chemotherapy induces changes affecting the methylation levels of the BRCA1 promoter, causing earlier and perhaps more frequent resistance to platinum-based chemotherapy than is observed in BRCA1-mutated OC. This may manifest as methylation loss in relapsed BRCA1-methylated OC, observed in 16.7% –80.0% of small, mostly retrospective, cohorts of 6 to 13 paired primary or recurrent BRCA1-methylated HGSC (43,52,54,55).
The pooled BRCA1 methylation rate was 16.3%. Although this reflects most reports of BRCA1 methylation frequency, there is marked variability in the reported occurrence of BRCA1 methylation in OC (5%–89.9%) (9,39). Among studies included in this meta-analysis, BRCA1 methylation frequency ranged from 6.2% to 73.6%, with an interquartile range of 10.1%–18.9%. To some extent, this could be explained by cohort sampling bias, in terms of size, populations, and histological subtypes included, with slightly higher rates often observed when cohorts are restricted to HGSC (Supplementary Table 2, available online). In breast cancer, BRCA1 methylation is more frequent among Asian as compared with Caucasians (56); however, ethnicity data was not available in this meta-analysis. Moreover, tissue sampling size and content introduce bias owing to variations in sampling sites (ovary vs metastatic), neoplastic cell content, and intratumor heterogeneity. Four studies report particularly high BRCA1 methylation rates: 21.4% (35), 33.6% (16), 42% (39), and 73.7% (43). This latter cohort, which confirmed all its MSP-GE determined BRCA1-methylated OC cases with Sanger sequencing, consisted solely of relapsed cases, which may account to some extent for this variation. We also observed that these 4 studies determined BRCA1 methylation using MSP-GE, whereas studies utilizing quantitative methodologies or high throughput microarrays reported rates varying between 8.2% and 16.0%. In a meta-analysis evaluating BRCA1 promoter methylation as a risk for the development of breast cancer, studies utilizing MSP were statistically significantly more likely to report higher frequencies of BRCA1 methylation (56). Nonspecific primer binding or incomplete bisulfite conversion has linked MSP with false-positive results and may account for this observation to some extent (57).
The marked variation in the methodology used to determine BRCA1 promoter methylation is also likely to contribute to differences in reported BRCA1 methylation rates (Supplementary Figure 1, available online). This epigenetic phenomenon is characterized by the methylation of CpG dinucleotides within an approximate 2.7 kB 5´CpG island containing 96 CpG dinucleotides and involving the bidirectional BRCA1 promoter and its adjacent alternative first exons (exon 1a/1b) (58,59). An essential regulatory area 202 bp downstream and 20 bp upstream of the BRCA1 transcription start site at exon 1a (according to GenBank U37574) contains sequence-specific transcription factor binding sites that prevent transcription when methylated (60). However, the individual contribution of CpG dinucleotides within this area (or elsewhere in the 2.7 kb CpG island) to the regulation of BRCA1 transcription has yet to be comprehensively evaluated in OC. Commonly used assays include MRED, MSP, methylation-sensitive multiplex ligation probe amplification, bisulfite sequencing, and more recently, GWMA. In contrast to the other methodologies, the latter correlates BRCA1 mRNA expression to the methylation status of CpG dinucleotides (9–46 assessed), to enable the selection of relevant CpG probes (3,15). Despite this common rationale, 2 studies (15,42) using the same GWMA assay (Illumina Infinium HumanMethylation 450k Beadchip) selected 8 and 21 CpG probes to determine BRCA1 methylation status within their samples. Were both assays applied to the same samples, different results may ensue depending on the level of methylation in tumor samples at these CpG sites. PCR-based assays use different primers, which assess often overlapping although different regions of the BRCA1 promoter, resulting in different CpG dinucleotides being interrogated between assays (Supplementary Table 2 and Supplementary Figure 1, available online). A study using 2 sets of primers, each targeting a different region within the BRCA1 promoter, reported different methylation levels within the same OC sample: 17.5% (region 1) and 3.3% (region 2) (61). Using an arbitrary cutoff of 10% to define methylation, and had only one set of primers been used, this sample may or may not be defined as BRCA1 methylated. Moreover, methylation of individual CpG dinucleotides, which may or may not be involved in regulation of BRCA1 transcription, cannot be distinguished within a PCR-based assay. The importance of CpG site selection to determine BRCA1 methylation status was underscored in a study demonstrating a correlation between high methylation levels at the BRCA1 promoter and the triple-negative breast cancer subtype, as determined by pyrosequencing. However, the level of methylation was variable across the 11 CpG sites evaluated, with 4 CpG sites displaying low or lacking methylation (62). Differences also exist with regards to assay interpretation, which includes GE (used in 8 of the included studies) or quantitative analyses. The latter use varying methodologies to determine nonmethylated reads and quantify the percentage of methylated reads within a sample. Quantitative analyses arbitrarily define low thresholds (4%–10%) to define methylation, without critically evaluating the threshold required for BRCA1 inactivation. Arguably, a low threshold is selected to account for potential dilution of methylated fragments in specimens with low neoplastic cell content or tumor heterogeneity, although it may result in labeling non–BRCA1-methylated samples with high neoplastic cell content and/or homogeneous tumor as BRCA1 methylated. Finally, while technically valid, these assays have not been compared with one another in the same OC dataset. A study compared methylation status of 4 genes using MSP-GE, quantitative methylation-sensitive multiplex ligation probe amplification, and quantitative multiplex MSP in 40 breast cancers and found high discrepancies between MSP-GE and the results of the quantitative assays (63). Methylation assay heterogeneity was evident between studies included in this meta-analysis. In an exploratory analysis, we identified a survival benefit within a subset of BRCA1-methylated OC identified with MSP-GE as compared with non–BRCA1-methylated OC. This subset was particularly homogeneous as 5 of the 7 cohorts included (13,16,35,41–43) evaluated the same 7 CpG sites, whereas 4 CpG sites were common to 6 cohorts (CpG details for 1 cohort were unavailable). Our findings may give credence to these particular CpG sites in terms of their essential role on BRCA1 transcription. However, only 2 of these 7 cohorts individually reported an improved survival for BRCA1-methylated OC compared with non–BRCA1-methylated OC, as did 1 of 5 eligible studies using identical PCR primers excluded from this analysis (Supplementary Table 1, available online) (14,17,29,30). Although this observation could be related to the small sample size of individual studies, our findings are exploratory and should be interpreted with caution.
Factors other than diagnostic methylation assays may contribute to heterogeneity within BRCA1-methylated OC, as is observed in BRCA1-mutated disease. Indeed, studies suggest resistance to platinum and/or PARPi therapy with BRCA1 mutations occurring within the BRCA1 RING domain (64). Moreover, mono-allelic BRCA1 mutations do not display a hazard ratio–deficient phenotype when BRCA1-locus LOH is absent, as evaluated in a cohort of 52 BRCA1-mutated OC (65). In this study, 7% of BRCA1-mutated OC had absent BRCA1-locus LOH and a worse survival compared with BRCA1-mutated OC with BRCA1-locus LOH (P = .02). Similarly, the discrepancy between preclinical findings and our analysis results with regards to platinum sensitivity and survival may lie in heterogeneity within BRCA1-methylated OC. This has been illustrated in BRCA1-methylated HGSC patient-derived xenograft models, whereby one model was cisplatin sensitive and the other cisplatin resistant, using the same MSP assay (51). Potentially, mono-allelic BRCA1 methylation with absent BRCA1-locus LOH would result in transcription of an intact BRCA1 on the nonmethylated allele, resulting in a functioning BRCA1 protein. By pooling studies evaluating LOH in BRCA1-methylated OC, we found that 19.6% (n = 18 of 92) of BRCA1-methylated OC have absent LOH, as determined by analyzing microsatellites near BRCA1 (Supplementary Table 8, available online). Current methylation assays do not routinely examine BRCA1 locus LOH, nor do they differentiate between mono- or bi-allelic methylation. In quantitative assays, greater than 50% methylation at the BRCA1 promoter may assume bi-allelic methylation. A recent study estimated percentage BRCA1 promoter methylation (adjusted for BRCA1 locus LOH, neoplastic cellularity, and BRCA1 copy number) to differentiate homozygous (>50% methylation) and heterozygous (<50% methylation) BRCA1 methylation in 21 BRCA1-methylated tumors from the ARIEL2 phase II clinical trial. Encouragingly, homozygous BRCA1-methylated OC (n = 6) was associated with a longer PFS than BRCA1/2-intact OC (n = 143), although this was not statistically significant (median PFS 14.5 months, 95% CI = 4.8 to 18.3 months, vs 5.5 months, 95% CI = 5.0 to 6.2 months; P = .06) (66). The development of allele-specific methylation methodologies that include LOH assessment, confirmed by absent or low BRCA1 mRNA expression, and ideally using specimens with 100% neoplastic cell content should minimize heterogeneity and permit a more accurate determination of BRCA1 epigenetically silenced tumors, or “true” BRCA1-methylated OC.
This is the most extensive meta-analysis to date evaluating the clinical characteristics of BRCA1-methylation in OC. The inclusion of published and unpublished studies without publication language restrictions, along with the use of IPD from studies assessed to be of overall good quality, further strengthens our results.
Limitations include incomplete inclusion of all eligible studies identified through our search strategy and statistically significant heterogeneity between cohorts in the assessment of PFS and OS, which was mitigated by the use of a mixed-effects model. Moreover, heterogeneity within the methylation assays utilized in the included studies was evident. Finally, the availability of BRCA1/2 mutation status, which enables a more thorough assessment of the prognostic effect of BRCA1 methylation on survival by using BRCA1/2-intact OC as a comparator, was limited to 1248 of 2636 patients.
In the largest meta-analysis on this topic, we show that BRCA1-methylated OC has a clinicopathological profile similar to that of BRCA1-mutated OC, presenting at a younger age as advanced stage HGSC. However, BRCA1 methylation does not predict for platinum sensitivity nor is it prognostic of survival. Although early and/or frequent platinum resistance mechanisms may account for this observation, there is marked heterogeneity between methylation assays used to detect BRCA1-methylated OC, in terms of the exact CpG sites assessed and the interpretation of the observed result. Moreover, these assays are not allele-specific and do not account for BRCA1-locus LOH. Potentially, a comprehensive assay that examines CpG dinucleotides critical to BRCA1 transcription in OC specimens in an allele-specific manner, combined with assessment of BRCA1-locus LOH, may permit a better selection of “true” BRCA1-methylated OC. Defined as such, BRCA1-methylated OC may represent a smaller subset yet permit a more accurate selection of patients with OC that would derive clear benefit from PARPi and other novel therapies targeting HR-deficient OC.
Funding
This work was supported in part by the North East Cancer Research and Education Trust (no applicable grant number) and the St Luke’s Institute for Cancer Research (no applicable grant number).
Notes
Role of the Funder: The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: RK has received conference travel reimbursement from Astra Zeneca, outside the submitted work. EB has received travel and advisory board fees from Roche Pharma, Tesaro, Incyte; travel and congress fees from Astra Zeneca; advisory board fees from Clovis, Seattle Genetics, Amgen; and institution funding from Roche Pharma, all outside the submitted work. JS has received honoraria, consulting/advisory fees, travel fees, and institutional research funding from Astra Zeneca, Clovis, PharmaMar, Pfizer, TESARO, MSD, as well as institutional research funding from Merck, Bayer; consulting/advisory fees from Lilly, Roche; honoraria from Eisai, Olympus, Johnson and Johnson, TEVA; travel fees from Roche. GM has had a consultant role or participated on scientific advisory boards of Astra Zeneca, Chrysalis, ImmunoMET, Ionis, Mills Institute for Personalized Care (MIPCC), PDX Pharma, Signalchem Lifesciences, Symphogen, Tarveda. GM has stock or options in Catena Pharmaceuticals, ImmunoMet, SignalChem, Spindletop Ventures, Tarveda, and has received research funding from Astra Zeneca, Ionis, Karus Therapeutics, Nanostring, Pfizer, Takeda/Millenium Pharmaceuticals. KT is an employee of and may have stocks in Myriad Genetics. DB has received research funding and clinical trial support from Genentech/Roche, Astra Zeneca, and Beigene. LW has received research support, honoraria or travel reimbursement from TESARO, medac oncol, GSK, MSD, Jenapharm, Roche, Medupdate GmbH. The other authors have declared no conflicts of interest.
Acknowledgments: The results shown in this meta-analysis are in part based on data generated by TCGA Research Network (https://www.cancer.gov/tcga) and the Australian Ovarian Cancer Study group (AOCS; http://www.aocstudy.org).
As outlined on the TCGA website (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga/using-tcga/citing-tcga), authors are permitted to use data from TCGA once the TCGA Research Network is acknowledged, as above, in their work.
The AOCS group was supported by the US Army Medical Research and Material Command under DAMD17-01-1-0729, the Cancer Council Victoria, Queensland Cancer Fund, the Cancer Council New South Wales, the Cancer Council South Australia, the Cancer Council Tasmania, and the Cancer Foundation of Western Australia (Multi-State Applications 191, 211, and 182) and the National Health and Medical Research Council of Australia (NHMRC; ID400413 and ID400281). The Australian Ovarian Cancer Study gratefully acknowledges additional support from S. Boldeman, the Agar family, Ovarian Cancer Action (UK), Ovarian Cancer Australia, and the Peter MacCallum Foundation. The AOCS also acknowledges the cooperation of the participating institutions in Australia and acknowledges the contribution of the study nurses, research assistants, and all clinical and scientific collaborators to the study. The complete AOCS Study Group can be found at www.aocstudy.org. We would like to thank all of the women who participated in these research programs.
Author Contributions: RK and SM had full access to all of the data in the study and take responsibility for the accuracy of the data analysis. RK, BS, and BH: Study concept and design. RK, JC, EG, IRus, EB, JS, AI, HY, DK, GM, KL, MC, KT, JK, IRze, AP, JMc, ES, SB, CO'R, SO'T, JO'L, DB, DT, KP, SJ,LW, PC, NH, and Irun: Acquisition of data. RK, BS, and BH: Analysis and interpretation of data. RK, SM, and BC: Statistical analysis. RK, BS, SM, and BH: Drafting of the manuscript. All authors: Critical revision of the manuscript for important intellectual content. BS and BH: Study supervision.
Supplementary Material
References
- 1. Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–808. [DOI] [PubMed] [Google Scholar]
- 2. Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13(24):7421–7431. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. [DOI] [PubMed] [Google Scholar]
- 6. Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baldwin RL, Nemeth E, Tran H, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60(19):5329–5333. [PubMed] [Google Scholar]
- 8. Geisler JP, Hatterman-Zogg MA, Rathe JA, Buller RE. Frequency of BRCA1 dysfunction in ovarian cancer. J Natl Cancer Inst. 2002;94(1):61–67. [DOI] [PubMed] [Google Scholar]
- 9. Catteau A, Harris WH, Xu CF, Solomon E. Methylation of the BRCA1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene. 1999;18(11):1957–1965. [DOI] [PubMed] [Google Scholar]
- 10. Pradjatmo H, Dasuki D, Anwar M, Mubarika S, Harijadi H. Methylation status and immunohistochemistry of BRCA1 in epithelial ovarian cancer. Asian Pac J Cancer Prev. 2014;15(21):9479–9485. [DOI] [PubMed] [Google Scholar]
- 11. Stordal B, Timms K, Farrelly A, et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol Oncol. 2013;7(3):567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swisher EM, Gonzalez RM, Taniguchi T, et al. Methylation and protein expression of DNA repair genes: association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol Cancer. 2009;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang C, Horiuchi A, Imai T, et al. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202(2):215–223. [DOI] [PubMed] [Google Scholar]
- 15. Cunningham JM, Cicek MS, Larson NB, et al. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep . 2015;4(1):4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ignatov T, Eggemann H, Costa SD, Roessner A, Kalinski T, Ignatov A. BRCA1 promoter methylation is a marker of better response to platinum-taxane-based therapy in sporadic epithelial ovarian cancer. J Cancer Res Clin Oncol. 2014;140(9):1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bai X, Fu Y, Xue H, et al. Promoter hypermethylation in sporadic epithelial ovarian carcinoma: association with low expression of BRCA1, improved survival and co-expression of DNA methyltransferases. Oncol Lett. 2014;7(4):1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101(3):403–410. [DOI] [PubMed] [Google Scholar]
- 19. Prat J, Oncology FCoG. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26(2):87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timms KM, Abkevich V, Hughes E, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16(6):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Telli ML, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22(15):3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355(8080):i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 24. Schröder MS, Culhane AC, Quackenbush J, Haibe-Kains B. survcomp: an R/Bioconductor package for performance assessment and comparison of survival models. Bioinformatics. 2011;27(22):3206–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Therneau TM, Grambsch PM, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12(1):156–175. [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57(1):289–300. [Google Scholar]
- 27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ . 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deeks JJ HJ, Altman DG, eds. . Chapter 10: analysing data and undertaking meta-analyses In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Hoboken, NJ: Cochrane; 2019. [Google Scholar]
- 29. Buller RE, Shahin MS, Geisler JP, Zogg M, De Young BR, Davis CS. Failure of BRCA1 dysfunction to alter ovarian cancer survival. Clin Cancer Res. 2002;8(5):1196–1202. [PubMed] [Google Scholar]
- 30. Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;6(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stefansson OA, Villanueva A, Vidal A, Marti L, Esteller M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics. 2012;7(11):1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernards SS, Pennington KP, Harrell MI, et al. Clinical characteristics and outcomes of patients with BRCA1 or RAD51C methylated versus mutated ovarian carcinoma. Gynecol Oncol. 2018;148(2):281–285. [DOI] [PubMed] [Google Scholar]
- 33. Yates MS, Timms K, Daniels MS, et al. Evaluation of BRCA1/2 and homologous recombination defects in ovarian cancer and impact on clinical outcomes. J Clin Oncol. 2017;35(15_suppl):5511. [Google Scholar]
- 34. Wang ZC, Birkbak NJ, Culhane AC, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18(20):5806–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiley A, Katsaros D, Chen H, et al. Aberrant promoter methylation of multiple genes in malignant ovarian tumors and in ovarian tumors with low malignant potential. Cancer. 2006;107(2):299–308. [DOI] [PubMed] [Google Scholar]
- 36. Rzepecka IK, Szafron L, Stys A, et al. High frequency of allelic loss at the BRCA1 locus in ovarian cancers: clinicopathologic and molecular associations. Cancer Genet. 2012;205(3):94–100. [DOI] [PubMed] [Google Scholar]
- 37. Montavon C, Gloss BS, Warton K, et al. Prognostic and diagnostic significance of DNA methylation patterns in high grade serous ovarian cancer. Gynecol Oncol. 2012;124(3):582–588. [DOI] [PubMed] [Google Scholar]
- 38. Ruscito I, Dimitrova D, Vasconcelos I, et al. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients—a study of the tumour bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD). Eur J Cancer. 2014;50(12):2090–2098. [DOI] [PubMed] [Google Scholar]
- 39. Chaudhry P, Srinivasan R, Patel FD. Utility of gene promoter methylation in prediction of response to platinum-based chemotherapy in epithelial ovarian cancer (EOC). Cancer Investig. 2009;27(8):877–884. [DOI] [PubMed] [Google Scholar]
- 40. McAlpine JN, Porter H, Kobel M, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25(5):740–750. [DOI] [PubMed] [Google Scholar]
- 41. Radosa MP, Hafner N, Camara O, et al. Loss of BRCA1 protein expression as indicator of the BRCAness phenotype is associated with favorable overall survival after complete resection of sporadic ovarian cancer. Int J Gynecol Cancer. 2011;21(8):1399–1406. [DOI] [PubMed] [Google Scholar]
- 42. Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. [DOI] [PubMed] [Google Scholar]
- 43. Prieske K, Prieske S, Joosse SA, et al. Loss of BRCA1 promotor hypermethylation in recurrent high-grade ovarian cancer. Oncotarget. 2017;8(47):83063–83074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teixeira LA, Candido dos Reis FJ. BRCA1 expression by immunohistochemistry and prognosis in ovarian cancer: a systematic review and meta-analysis. Target Oncol. 2020;15(1):37–46. [DOI] [PubMed] [Google Scholar]
- 45. Gras E, Cortes J, Diez O, et al. Loss of heterozygosity on chromosome 13q12-q14, BRCA-2 mutations and lack of BRCA-2 promoter hypermethylation in sporadic epithelial ovarian tumors. Cancer. 2001;92(4):787–795. [DOI] [PubMed] [Google Scholar]
- 46. Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, DeYoung B, Buller RE. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002;94(18):1396–1406. [DOI] [PubMed] [Google Scholar]
- 47. Bol GM, Suijkerbuijk KP, Bart J, Vooijs M, van der Wall E, van Diest PJ. Methylation profiles of hereditary and sporadic ovarian cancer. Histopathology. 2010;57(3):363–370. [DOI] [PubMed] [Google Scholar]
- 48. Goodheart MJ, Rose SL, Hattermann-Zogg M, Smith BJ, De Young BR, Buller RE. BRCA2 alteration is important in clear cell carcinoma of the ovary. Clin Genet. 2009;76(2):161–167. [DOI] [PubMed] [Google Scholar]
- 49. Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veeck J, Ropero S, Setien F, et al. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J Clin Oncol. 2010;28(29):e563–e564; author reply e565–e566. [DOI] [PubMed] [Google Scholar]
- 51. Topp MD, Hartley L, Cook M, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol Oncol. 2014;8(3):656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. [DOI] [PubMed] [Google Scholar]
- 53. Candido-dos-Reis FJ, Song H, Goode EL, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21(3):652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel JN, Braicu I, Timms KM, et al. Characterisation of homologous recombination deficiency in paired primary and recurrent high-grade serous ovarian cancer. Br J Cancer . 2018;119(9):1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bisogna M, Dao F, Olvera N, Bacares R, Zhang L, Levine DA. Abstract A05: BRCA1 promoter hypermethylation loss in recurrent high-grade serous carcinoma. Clin Cancer Res. 2016;22(suppl 2):A05–A05. [Google Scholar]
- 56. Zhang L, Long X. Association of BRCA1 promoter methylation with sporadic breast cancers: evidence from 40 studies. Sci Rep. 2016;5(1):17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lan VT, Ha NT, Uyen NQ, et al. Standardization of the methylation-specific PCR method for analyzing BRCA1 and ER methylation. Mol Med Rep. 2014;9(5):1844–1850. [DOI] [PubMed] [Google Scholar]
- 58. Brown MA, Xu CF, Nicolai H, et al. The 5’ end of the BRCA1 gene lies within a duplicated region of human chromosome 17q21. Oncogene. 1996;12(12):2507–2513. [PubMed] [Google Scholar]
- 59. Xu CF, Chambers JA, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272(34):20994–20997. [DOI] [PubMed] [Google Scholar]
- 60. Thakur S, Croce CM. Positive regulation of the BRCA1 promoter. J Biol Chem. 1999;274(13):8837–8843. [DOI] [PubMed] [Google Scholar]
- 61. Franzese E, Centonze S, Diana A, et al. Genomic profile and BRCA-1 promoter methylation status in BRCA mutated ovarian cancer: new insights in predictive biomarkers of olaparib response. Front Oncol. 2019;9:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Daniels SL, Burghel GJ, Chambers P, et al. Levels of DNA methylation vary at CpG Sites across the BRCA1 promoter, and differ according to triple negative and “BRCA-Like” status, in both blood and tumour DNA. PLoS One. 2016;11(7):e0160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Suijkerbuijk KP, Pan X, van der Wall E, van Diest PJ, Vooijs M. Comparison of different promoter methylation assays in breast cancer. Anal Cell Pathol . 2010;33(3-4):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dimitrova D, Ruscito I, Olek S, et al. Germline mutations of BRCA1 gene exon 11 are not associated with platinum response neither with survival advantage in patients with primary ovarian cancer: understanding the clinical importance of one of the biggest human exons. A study of the Tumor Bank Ovarian Cancer (TOC) Consortium. Tumor Biol. 2016;37(9):12329–12337. [DOI] [PubMed] [Google Scholar]
- 65. Maxwell KN, Wubbenhorst B, Wenz BM, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kondrashova O, Topp M, Nesic K, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9(1):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.