Abstract
Background
Prior research demonstrated statistically significant racial disparities related to lung cancer treatment and outcomes. We examined differences in initial imaging and survival between blacks, Hispanics, and non-Hispanic whites.
Methods
The linked Surveillance, Epidemiology, and End Results-Medicare database between 2007 and 2015 was used to compare initial imaging modality for patients with lung cancer. Participants included 28 881 non-Hispanic whites, 3123 black, and 1907 Hispanics, patients age 66 years and older who were enrolled in Medicare fee-for-service and diagnosed with lung cancer. The primary outcome was comparison of positron emission tomography (PET) imaging with computerized tomography (CT) imaging use between groups. A secondary outcome was 12-month cancer-specific survival. Information on stage, treatment, and treatment facility was included in the analysis. Chi-square test and logistic regression were used to evaluate factors associated with imaging use. Kaplan-Meier method and Cox proportional hazards regression were used to calculate adjusted hazard ratios and survival. All statistical tests were two-sided.
Results
After adjusting for demographic, community, and facility characteristics, blacks were less likely to undergo PET or CT imaging at diagnosis compared with non-Hispanic whites odds ratio (OR) = 0.54 (95% confidence interval [CI] = 0.50 to 0.59; P < .001). Hispanics were also less likely to receive PET with CT imaging (OR = 0.72, 95% CI = 0.65 to 0.81; P < .001). PET with CT was associated with improved survival (HR = 0.61, 95% CI = 0.57 to 0.65; P < .001).
Conclusions
Blacks and Hispanics are less likely to undergo guideline-recommended PET with CT imaging at diagnosis of lung cancer, which may partially explain differences in survival. Awareness of this issue will allow for future interventions aimed at reducing this disparity.
In 2018, there were an estimated 234 030 new cases of lung cancer, with 158 770 estimated deaths (1). Despite improvement in survival of patients with lung cancer, it remains the leading cause of cancer death (1). Although the lung cancer survival rate for all patients remains low (1), blacks have worse outcomes with a 5-year all-stages survival rate of 15% compared with 18% for non-Hispanic whites (1). Both blacks and Hispanics are often advanced stage at diagnosis relative to non-Hispanic whites (1,2). Numerous factors that contribute to lung cancer disparities, including early detection, smoking, biology, environmental, and societal factors, have been described (3). Recent medical innovations provide new therapeutic options and are improving outcomes, but the optimization of such treatments depends on the patient’s stage. Appropriate imaging is imperative for improving the accuracy of staging and optimizing the choice of therapeutic interventions (4).
Current NCCN guidelines recommend a CT of the Chest and upper abdomen with contrast for initial evaluation of non-small cell lung cancer (NSCLC) (5). A 18F-fludeoxyglucose positron emission tomography (PET) with computerized tomography (CT) imaging is then recommended for stage I to stage IV NSCLC patients. In addition, the European Society for Medical Oncology, the Pan-Asian adapted Clinical Practice Guidelines, and the American College of Radiology Appropriateness Criteria all support a role for PET imaging in cases of newly diagnosed NSCLC (4,6–8). The national and international level of support for PET imaging is due to numerous prior studies demonstrating both prognostic and management improvement in patients who receive a PET exam. One recent study showed PET serves as an independent prognostic factor for disease recurrence for early-stage IA NSCLC (9). A prospective multicenter randomized trial found combining PET with conventional workup reduced futile thoracotomies by 51% (10). A second prospective multicenter trial found PET imaging resulted in a change of management in 72% of cases (11). Although the benefits PET offers in staging and patient management have been reported (10,11), the inequity of its use for patients with newly diagnosed NSCLC has not been evaluated in a national Medicare population. Given the impact of PET on patient management and outcomes, this study examines differences in imaging use of blacks, Hispanics, and white patients with newly diagnosed lung cancer and the impact on patient survival.
Methods
Data
We conducted a retrospective cohort observational study using patients from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. SEER, a program of the National Cancer Institute (NCI), encompasses person-level information on cancer survival and incidence from 18 population-based tumor registries that cover approximately 28% of the United States (1). Medicare claims and census information were linked to the SEER tumor registry data, which have additional information on patient demographic and tumor characteristics. By linking SEER data to Medicare claims, dates of service, payments, procedures, and diagnosis codes are also captured.
Cohort Selection
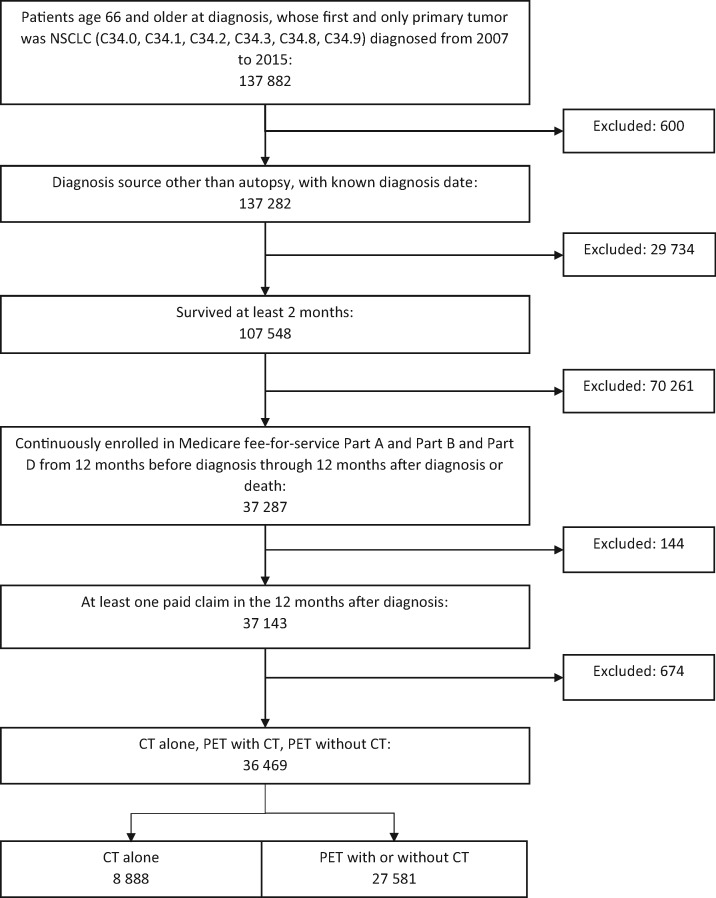
We selected patients aged 66 years or older at diagnosis whose first and only primary tumor was lung cancer (International Classification of Diseases [ICD]-O-3 site codes: C34.0, C34.1, C34.2, C34.3, C34.8, and C34.9) diagnosed between 2007 and 2015. We limited the study to patients with the following non-small cell histology types: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and other non-small cell carcinoma (n = 137 882). Patients who were diagnosed by autopsy, had unknown diagnosis dates, or survived less than 2 months were excluded, leaving 107 548 patients. We further limited the sample to patients continuously enrolled in fee-for-service Medicare Parts A and B for 12 months before through 12 months after the month of diagnosis (or until death if it occurred within 12 months) to ensure complete claims history. Additionally, we required that patients were enrolled in Medicare Part D prescription drug plans from the month of diagnosis through the following 12 months to capture oral chemotherapy treatment. We further limited the sample to patients who underwent PET with CT imaging during the diagnostic period (n = 36 469) (Figure 1). We used ICD, 9th and 10th revision, Clinical Modification codes, Current Procedural Terminology codes, and Healthcare Common Procedure Coding System codes to identify the diagnoses and procedures. The study was approved by the University of Colorado Multiple Institutional Review Board.
Figure 1.
Sample derivation. Newly diagnosed non-small cell lung cancer (NSCLC) in patients 66 years and older who survived at least 2 months and continuously participated in Medicare fee-for-service for 12 months before and following diagnosis. CT = computerized tomography imaging; PET = positron emission tomography imaging.
Outcomes
The primary outcome was imaging modality at diagnosis, and the secondary outcome was cancer-specific survival (CSS) differences between imaging modality. We identified imaging modality using Healthcare Common Procedure Coding System and Current Procedural Terminology procedure codes reported during the diagnostic period, defined as 3 months before diagnosis through the earlier of two potential dates: 2 months after the month of diagnosis or 30 days after the initiation of therapy. Patients were categorized as having 1) any PET imaging with or without CT imaging, or 2) CT imaging alone. CSS and overall survival (OS) were estimated 12 months after diagnosis. CSS time was determined using SEER dates of death, which include cause of death and are reported through December 2015. OS time was determined using Medicare dates of death reported through December 2016. Patients surviving longer than 12 months were censored at 12 months.
Statistical Analysis
Chi-square tests were performed to assess the univariate association of categorical variables and imaging modalities at diagnosis. Logistic regression was used to evaluate the adjusted impact of demographic, socioeconomic, and facility characteristics on choice of diagnostic imaging modality. A generalized estimating equation model was used to obtain standard errors that accounted for clustering by the facility that provided initial treatment.
Survival curves were generated using the Kaplan-Meier method, and Cox proportional hazards regression was used to estimate adjusted hazard ratios (HRs) for 12-month CSS and OS. In multivariable survival analysis, we used a robust sandwich covariance matrix estimate to account for clustering by facility (12).
We examined a series of Cox proportional hazards models. In the base model, we adjusted for year of diagnosis, patient sex (male or female), age at diagnosis (66–69 years, 70–74 years, and 75 years or older), type of NSCLC (squamous or other), race or ethnicity (white, black, Hispanic, other), marital status (married or partnered, or single), SEER registry site, census tract Rural Urban Commuting Area Codes (urban commuting area or not an urban commuting area), census tract poverty level (less than study sample median or greater than or equal to study sample median level), census tract-level percentage high school education or less (less than study sample median level or greater than or equal to study sample median level), additional imaging (Magnetic Resonance Imaging [MRI] brain yes or no), and whether first cancer-directed therapy was provided by an NCI-designated center, teaching hospital, or community hospital (Supplementary Table 1, available online). We used Medicare claims from the year before diagnosis to estimate the Charlson Comorbidity Index according to the NCI’s adaptation of the algorithm described by Klabunde et al. (13)
In subsequent Cox proportional hazards models, we added the following variables in a stepwise fashion: imaging modality, derived American Joint Committee on Cancer Stage Group 6th edition, and initial treatment. Initial treatment was defined as treatment initiated within 6 months of diagnosis, categorized as no treatment, chemotherapy, radiation therapy, surgery, as well as combinations of these treatments. We used Schoenfeld residuals to validate the proportional hazard assumption in the Cox model. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC), were two-sided and were evaluated at a statistical significance level of P less than .05.
Results
Descriptives
The 6-year study period identified 36 469 total patients who met eligibility criteria, of which 28 881 (79.2%) were non-Hispanic white, 3123 (8.6%) were black, and 1907 (5.2%) were Hispanic (Table 1). Approximately 78% of non-Hispanic whites received a PET at diagnosis compared with 63% of blacks and 70% of Hispanics. Numerous other factors were associated with decreased PET use, including older age, unmarried, higher number of comorbidities, and nonteaching facility.
Table 1.
Descriptive statistics, SEER-Medicare, 2007–2015
| Characteristic | Overall | CT alone | PET with or without CT | Patients with PET | P * |
|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | % | ||
| Total | 36 469 | 8888 | 27 581 | 75.6 | — |
| Year of diagnosis | |||||
| 2007 | 3768 (10.3) | 1174 (13.2) | 2594 (9.4) | 68.8 | <.001 |
| 2008 | 3764 (10.3) | 1097 (12.3) | 2667 (9.7) | 70.9 | |
| 2009 | 3769 (10.3) | 1015 (11.4) | 2754 (10) | 73.1 | |
| 2010 | 3772 (10.3) | 942 (10.6) | 2830 (10.3) | 75 | |
| 2011 | 3814 (10.5) | 919 (10.3) | 2895 (10.5) | 75.9 | |
| 2012 | 4159 (11.4) | 1010 (11.4) | 3149 (11.4) | 75.7 | |
| 2013 | 4625 (12.7) | 976 (11) | 3649 (13.2) | 78.9 | |
| 2014 | 4748 (13) | 951 (10.7) | 3797 (13.8) | 80 | |
| 2015 | 4050 (11.1) | 804 (9.1) | 3246 (11.8) | 80.1 | |
| Age, y | |||||
| 66–69 | 7942 (21.8) | 1710 (19.2) | 6232 (22.6) | 78.5 | <.001 |
| 70–74 | 10 342 (28.4) | 2282 (25.7) | 8060 (29.2) | 77.9 | |
| 75 and older | 18 185 (49.9) | 4896 (55.1) | 13 289 (48.2) | 73.1 | |
| Sex | |||||
| Female | 19 972 (54.8) | 4910 (55.2) | 15 062 (54.6) | 75.4 | .30 |
| Male | 16 497 (45.2) | 3978 (44.8) | 12 519 (45.4) | 75.9 | |
| Race or ethnicity | |||||
| Non-Hispanic white | 28 881 (79.2) | 6387 (71.9) | 22 494 (81.6) | 77.9 | <.001 |
| Non-Hispanic black | 3123 (8.6) | 1145 (12.9) | 1978 (7.2) | 63.3 | |
| Hispanic | 1907 (5.2) | 581 (6.5) | 1326 (4.8) | 69.5 | |
| Other | 2558 (7) | 775 (8.7) | 1783 (6.5) | 69.7 | |
| Marital status | |||||
| Not married | 19 470 (53.4) | 5446 (61.3) | 14 024 (50.9) | 72 | <.001 |
| Married or partnered | 16 999 (46.6) | 3442 (38.7) | 13 557 (49.2) | 89.8 | |
| Geographic region | |||||
| East | 7639 (21) | 1803 (20.3) | 5836 (21.2) | 76.4 | <.001 |
| Midwest | 4509 (12.4) | 1059 (11.9) | 3450 (12.5) | 76.5 | |
| South | 10 538 (28.9) | 2461 (27.7) | 8077 (29.3) | 76.6 | |
| West | 13 783 (37.8) | 3565 (40.1) | 10 218 (37.1) | 74.1 | |
| Patient residence† | |||||
| Urban | 31 427 (86.3) | 7702 (86.8) | 23 725 (86.1) | 75.5 | .11 |
| Rural | 5002 (13.7) | 1173 (13.2) | 3829 (13.9) | 76.5 | |
| Census tract poverty‡ | |||||
| Less than median level | 18 255 (50.2) | 4030 (45.5) | 14 225 (51.7) | 77.9 | <.001 |
| Greater than or equal to median level | 18 126 (49.8) | 4819 (54.5) | 13 307 (48.3) | 73.4 | |
| Census tract education | |||||
| Less than median level | 18 227 (50.1) | 4088 (46.2) | 14 139 (51.4) | 77.6 | <.001 |
| Greater than or equal to median level | 18 154 (49.9) | 4761 (53.8) | 13 393 (48.6) | 73.8 | |
| Charlson Comorbidity Index | |||||
| 0 | 17 158 (47.1) | 3848 (43.3) | 13 310 (48.3) | 77.6 | <.001 |
| 1 | 8548 (23.4) | 2030 (22.8) | 6518 (23.6) | 76.3 | |
| 2 or more | 10 763 (29.5) | 3010 (33.9) | 7753 (28.1) | 72 | |
| Facility of first treatment | |||||
| NCI center | 3092 (8.5) | 489 (5.5) | 2603 (9.5) | 84.2 | <.001 |
| Teaching hospital | 17 250 (47.4) | 4068 (46) | 13 182 (47.9) | 76.4 | |
| Other facility | 16 043 (44.1) | 4278 (48.4) | 11 765 (42.7) | 73.3 | |
| AJCC Stage Group 6th ed. | |||||
| Stage I | 9974 (27.4) | 1657 (18.7) | 8317 (30.2) | 83.4 | <.001 |
| Stage II | 1862 (5.1) | 230 (2.6) | 1632 (5.9) | 87.6 | |
| Stage III | 8971 (24.7) | 1926 (21.8) | 7045 (25.6) | 78.5 | |
| Stage IV | 13 237 (36.4) | 4226 (47.7) | 9011 (32.7) | 68.1 | |
| Unknown stage | 2351 (6.5) | 817 (9.2) | 1534 (5.6) | 65.2 | |
| Type of treatment | |||||
| No treatment | 6011 (16.5) | 3097 (34.8) | 2914 (10.6) | 48.5 | <.001 |
| Radiation | 6391 (17.5) | 1704 (19.2) | 4687 (17) | 73.3 | |
| Surgery | 6545 (18) | 1082 (12.2) | 5463 (19.8) | 83.5 | |
| Chemotherapy | 4595 (12.6) | 1141 (12.8) | 3454 (12.5) | 75.2 | |
| Surgery and radiation | 473 (1.3) | 59 (0.7) | 414 (1.5) | 87.5 | |
| Chemotherapy and radiation | 9611 (26.4) | 1541 (17.3) | 8070 (29.3) | 84 | |
| Chemotherapy and surgery | 1738 (4.8) | 185 (2.1) | 1553 (5.6) | 89.4 | |
| Chemotherapy, surgery, and radiation | 1105 (3.0) | 79 (0.9) | 1026 (3.7) | 92.9 | |
| MRI brain imaging | |||||
| No MRI | 21 503 (59) | 6130 (69) | 15 373 (55.7) | 71.5 | <.001 |
| MRI | 14 966 (41) | 2758 (31) | 12 208 (44.3) | 81.6 | |
| Histology | |||||
| Other NSCLC | 25 155 (69) | 6514 (73.3) | 18 641 (67.6) | 74.1 | <.001 |
| Squamous cell | 11 314 (31) | 2374 (26.7) | 8940 (32.4) | 79 | |
P value comparing PET with or without CT vs CT alone using a two-sided chi-square test. AJCC = American Joint Committee on Cancer; CT = computerized tomography imaging; MRI = Magnetic Resonance Imaging; NCI = National Cancer Institute; NSCLC = non-small cell lung cancer; PET = positron emission tomography imaging; SEER = Surveillance, Epidemiology, and End Results.
Urban or rural classification is based on US Department of Agriculture’s Rural Urban Commuting Area Codes.
Area-level poverty defined at the census tract, and for those with few observations area level is at the zip code.
Imaging
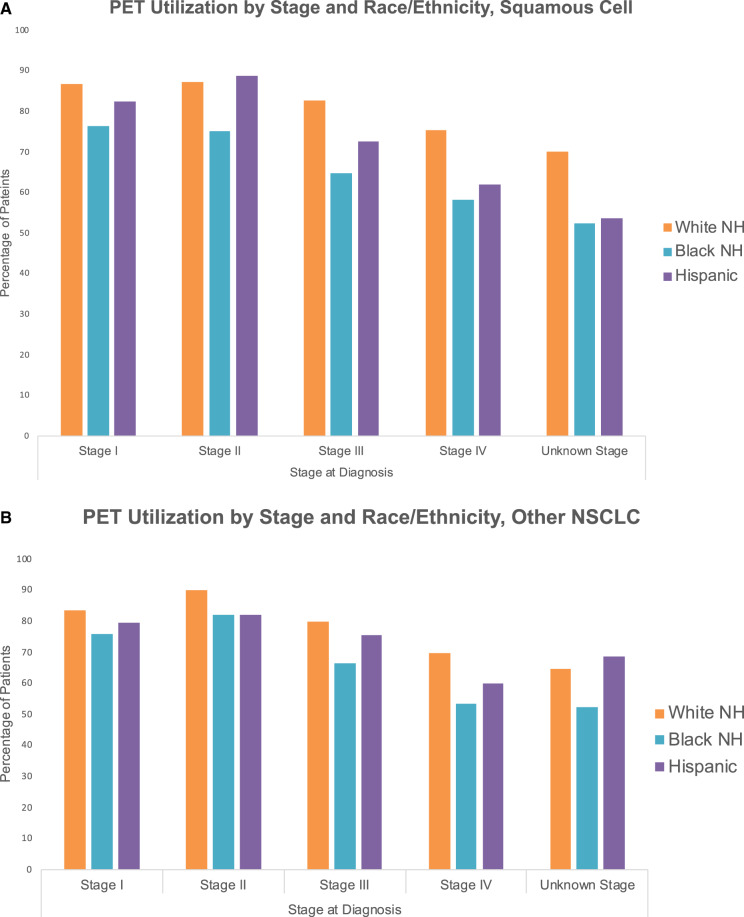
Based on odds ratios adjusted for demographic, socioeconomic, and facility characteristics, black patients with squamous cell NSCLC were about one-half as likely to receive a PET (OR = 0.51, 95% CI = 0.44 to 0.60; P < .001) and Hispanic patients were about two-thirds as likely to receive a PET (OR = 0.67, 95% CI = 0.54 to 0.83; P < .001) compared with non-Hispanic whites. In patients with nonsquamous cell NSCLC, the likelihood of receiving a PET was also lower for black (OR = 0.57, 95% CI = 0.51 to 0.64; P < .001) and Hispanic patients (OR = 0.76, 95% CI = 0.67 to 0.88; P < .001). Differences in PET use were evident throughout all stages for both squamous cell carcinoma patients (Figure 2A) and all other histologies (Figure 2B). The PET imaging OR of all histologies for black patients was 0.54 (95% CI = 0.50 to 0.59; P < .001) and 0.72 (95% CI = 0.65 to 0.81; P < .001) for Hispanic patients.
Figure 2.
Positron emission tomography imaging (PET) use by stage and race. A) Use of PET with or without computerized tomography imaging (CT) by stage and race or ethnicity for squamous cell carcinoma. B) Use of PET with or without CT by stage and race or ethnicity for all other histologies of non-small cell lung cancer (NSCLC). Stage based on American Joint Committee on Cancer Staging system. NH = non-Hispanic.
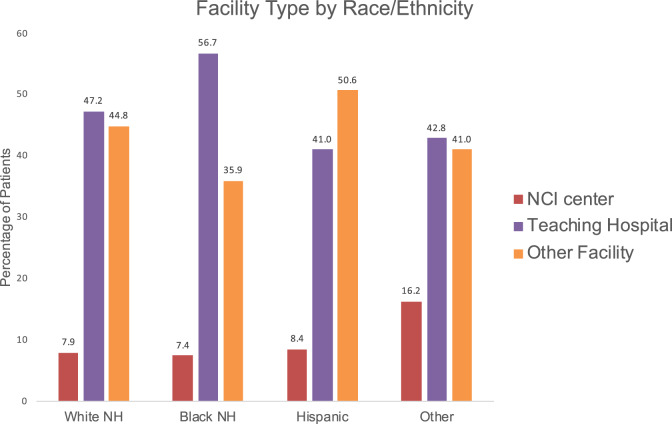
Treatment facility type also predicted PET use. Both NCI centers and teaching hospitals were more likely to use PET (OR = 3.00, 95% CI = 2.17 to 4.16, P < .001; and OR = 1.22, 95% CI = 1.07 to 1.4, P = .004, respectively). Less than 10% of patients received care at an NCI center (7.9% of non-Hispanic whites, 7.4% of blacks, and 8.4% of Hispanics). Teaching hospitals were the most common site of treatment for non-Hispanic whites and blacks, with 47.2% of non-Hispanic whites, 56.7% of blacks, and 41% of Hispanics receiving their treatment from a teaching hospital. The remainder received their care at nonteaching, non-NCI facilities (Figure 3). Additional factors associated with a lower likelihood of PET use included higher census tract poverty level, lower census tract education level, more comorbidity, and whether additional MRI imaging of the brain was also obtained (Table 1).
Figure 3.
Utilization of positron emission tomography imaging (PET) with or without computerized tomography imaging (CT) by race or ethnicity and treatment facility. NH = non-Hispanic; NCI = National Cancer Institute.
Survival
Table 2 reports hazard ratios for CSS. After controlling for demographics, socioeconomic factors, stage, initial imaging, and treatment, black patients are less likely to die (HR = 0.84, 95% CI = 0.75 to 0.94; P = .002). Hispanic patients had a hazard ratio similar to that of white patients and it was not statistically significant. In patients with squamous cell carcinoma histology, PET was associated with a lower probability of death (HR = 0.61, 95% CI = 0.57 to 0.65; P < .001). Likewise, in patients with other histologies, the CSS for patients who had PET was about twice that of patients without a PET (HR = 0.62, 95% CI = 0.60 to 0.65; P < .001) after the first year. Combining all histologies, the probability of survival at 12 months was more than 20% higher in patients imaged with PET than those imaged with CT alone (Figure 4A).
Table 2.
Multivariable analysis of CSS† for squamous cell carcinoma and all other types of NSCLC‡, SEER-Medicare, 2007–2015
| Characteristics | Squamous cell carcinoma NSCLC (N = 11 257) | All other NSCLC (N = 25 001) |
|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio 95% CI | |
| Age | ||
| 66–69 | 1.00 (Referent) | 1.00 (Referent) |
| 70–74 | 1.00 (0.92 to 1.08) | 1.07 (1.01 to 1.14) |
| 75 and older | 1.06 (0.98 to 1.15) | 1.13* (1.07 to 1.19) |
| Sex | ||
| Male | 1.00 (Referent) | 1.00 (Referent) |
| Female | 0.85* (0.79 to 0.91) | 0.76* (0.73 to 0.80) |
| Race or ethnicity | ||
| White non-Hispanic | 1.00 (Referent) | 1.00 (Referent) |
| Black non-Hispanic | 0.84* (0.75 to 0.94) | 0.89* (0.83 to 0.96) |
| Hispanic | 0.95 (0.82 to 1.11) | 0.91* (0.83 to 0.99) |
| Other | 0.99 (0.86 to 1.14) | 0.74* (0.68 to 0.81) |
| Marital status | ||
| Married or partnered | 1.00 (Referent) | 1.00 (Referent) |
| Not married | 1.06 (0.99 to 1.13) | 1.08* (1.03 to 1.13) |
| Patient residence§ | ||
| Urban community | 1.00 (Referent) | 1.00 (Referent) |
| Nonurban community | 1.10* (1.00 to 1.21) | 1.01 (0.94 to 1.08) |
| Census tract poverty‖ | ||
| Less than median | 1.00 (Referent) | 1.00 (Referent) |
| Greater than or equal to median | 1.00 (0.94 to 1.08) | 1.02 (0.97 to 1.07) |
| Census tract education | ||
| Less than median | 1.00 (Referent) | 1.00 (Referent) |
| Greater than or equal to median | 1.00 (0.93 to 1.07) | 1.05 (1.00 to 1.10) |
| Charlson Comorbidity | ||
| 0 | 1.00 (Referent) | 1.00 (Referent) |
| 1 | 0.96 (0.88 to 1.05) | 1.09* (1.03 to 1.15) |
| 2 or more | 0.99 (0.91 to 1.07) | 1.13* (1.07 to 1.19) |
| Type of facility | ||
| Nonteaching | 1.00 (Referent) | 1.00 (Referent) |
| NCI center | 0.88 (0.74 to 1.04) | 0.76* (0.69 to 0.83) |
| Teaching hospital | 1.00 (0.93 to 1.07) | 0.98 (0.94 to 1.03) |
| Initial imaging | ||
| CT alone | 1.00 (Referent) | 1.00 (Referent) |
| PET | 0.61* (0.57 to 0.65) | 0.62* (0.60 to 0.65) |
| MRI brain imaging | ||
| No MRI | 1.00 (Referent) | 1.00 (Referent) |
| MRI | 1.24* (1.17 to 1.32) | 1.19* (1.14 to 1.24) |
| AJCC Stage Group 6th ed. | ||
| Stage I + | 1.00 (Referent) | 1.00 (Referent) |
| Stage II | 1.91* (1.65 to 2.22) | 2.58* (2.18 to 3.05) |
| Stage III | 2.79* (2.49 to 3.13) | 3.98* (3.57 to 4.45) |
| Stage IV | 4.79* (4.25 to 5.39) | 6.48* (5.81 to 7.22) |
| Treatment | ||
| No treatment | 1.00 (Referent) | 1.00 (Referent) |
| Chemotherapy | 0.69* (0.61 to 0.77) | 0.64* (0.60 to 0.69) |
| Chemotherapy, surgery, and radiation | 0.33* (0.27 to 0.41) | 0.34* (0.29 to 0.40) |
| Chemotherapy and radiation | 0.57* (0.52 to 0.63) | 0.60* (0.57 to 0.65) |
| Chemotherapy and surgery | 0.16* (0.12 to 0.22) | 0.23* (0.19 to 0.27) |
| Radiation | 0.84* (0.76 to 0.93) | 1.05 (0.99 to 1.13) |
| Surgery | 0.29* (0.24 to 0.35) | 0.28* (0.24 to 0.32) |
| Surgery and radiation | 0.62* (0.46 to 0.84) | 0.63* (0.50 to 0.79) |
P < .05. AJCC = American Joint Committee on Cancer; CI = confidence interval; CSS = cancer-specific survival; CT = computerized tomography imaging; MRI = Magnetic Resonance Imaging; NCI = National Cancer Institute; NSCLC = non-small cell lung cancer; PET = positron emission tomography imaging; SEER = Surveillance, Epidemiology, and End Results.
CSS was estimated using Cox proportional hazards model.
Year of diagnosis and registry site were included in the multivariable analysis.
Urban or rural classification is based on US Department of Agriculture’s Rural Urban Commuting Area Codes.
Area-level poverty defined at the census tract, and for those with few observations area level is at the zip code.
Figure 4.
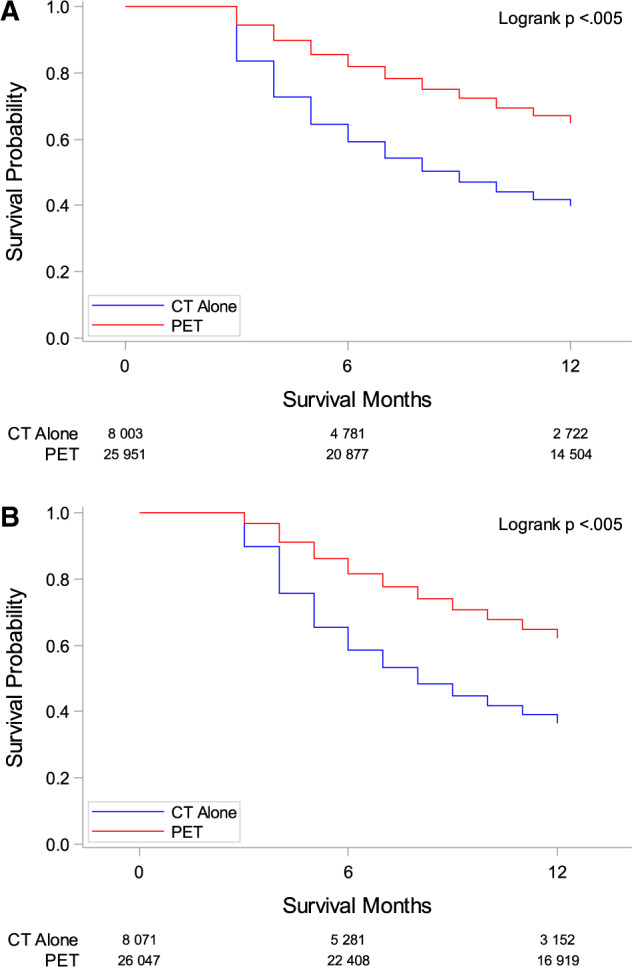
Survival curves comparing computerized tomography imaging (CT) and positron emission tomography imaging (PET) with or without CT. A) Cancer-specific Kaplan-Meier survival curves comparing CT alone vs PET. P value was generated using the two-sided log-rank test method. B) Overall Kaplan-Meier survival curves comparing CT alone vs PET. P value was generated using the two-sided log-rank test method.
In assessments of OS, 54.8% of all patients survived the first 12 months after diagnosis. In patients with squamous cell carcinoma histology and who had PET imaging with or without CT vs CT alone, those who had a PET had a lower probability of death (HR = 0.63, 95% CI = 0.59 to 0.67; P < .001) (Figure 4A). Findings were similar for patients with other histologies who also had a PET compared with similar patients who did not receive PET imaging (Figure 4B).
Discussion
One-fourth of patients with newly diagnosed NSCLC do not receive PET imaging. Within this sample, blacks and Hispanics had a statistically significantly lower rate of PET imaging compared with non-Hispanic whites for every stage. This discrepancy was greatest for stage IV squamous cell carcinoma, where 75% of non-Hispanic whites received a PET compared with only 58% of blacks and 62% of Hispanics. The potential impact of our findings on changing practice patterns and improving outcomes is considerable.
Ensuring equity in the US health-care system is a top priority for the National Academy of Medicine but remains a practical challenge to implement (14). We identified PET imaging as a potential contributing factor to racial differences in NSCLC survival. Improving the rate PET imaging is a targetable intervention with considerable supporting evidence of its benefit (4,10,15–17). For example, PET imaging improves staging, as shown by a prior study where PET increased the stage of disease in approximately 10% of patients (11). Blacks and Hispanics are diagnosed at later stages compared with non-Hispanic whites, which, given our results of PET use differences, suggests prior reports may underestimate racial and ethnic differences in stage at diagnosis (1,11,18). Our findings suggest that equitable use of PET imaging may more accurately stage the disease, leading to more appropriate guideline-concordant care, and ultimately reduce the NSCLC survival gap currently reported between ethnic groups.
Prior studies identified an association between race and PET usage in NSCLC (19). A Veterans Health Administration multi-site, prospective, observational study (CanCORS) from 2003 to 2005 showed 13% less PET usage in non-whites and Hispanics. Although this study drew from only four geographic regions and Veteran Health Administration patients are more likely to be from racial and ethnic minority groups, it does demonstrate that the issue is found outside of the Medicare population (20). In addition, these data were published nearly a decade ago, yet the disparate trends in PET usage continue and are independent of differences in income, education, insurance coverage, or health-care setting. These data collectively suggest that factors inherent to medical practice may explain why PET was used less frequently in insured blacks and Hispanics.
Our data showed that patients receiving treatment at NCI-designated centers were more likely to undergo PET imaging at diagnosis at a rate almost 10% higher than teaching or community hospitals. This supports previous research demonstrating better adherence to practice guidelines for NCI centers vs other hospitals (21). The commitment to treatment guidelines may also contribute to the survival benefits previously reported for patients receiving care at a NCI-designated center (22,23). Although our study supports these prior reports, it is important to note the three groups had a similar rate of use at NCI centers. The differences in PET use and survival cannot be explained by differences in treatment location.
Our findings are relevant to clinical practice, both in support of current guidelines and in confronting the problem of considerable racial and ethnic disparity. Health-care providers who stage patients with newly diagnosed NSCLC should be aware not only of the impact of PET imaging and its association with improved CSS and OS but also of the racial and ethnic gap in PET usage. Although the cause for the disparity in imaging use for blacks and Hispanics is unknown, one factor that may contribute to this difference is unconscious or institutional bias. A prior systematic review found 35 studies demonstrating implicit bias in health-care professionals, with 42 studies showing a statistically significant positive correlation between the level of implicit bias and lower quality of care (24). The simple step of being aware of potential unconscious bias can influence clinicians’ delivery of care to patients (25).
Recently, PET overuse has been a subject of controversy. A 2016 study using lung and esophageal SEER-Medicare datasets reported no change in CSS when PET is used to evaluate tumor recurrence (26). These data triggered a debate in the literature questioning the overusage of PET imaging in these disease subsites. Our data specific to NSCLC and the collective published body of evidence, however, show that PET usage for diagnosis and treatment management is associated with improved CSS (10,11,15–17). It is important to note that the Centers for Medicaid and Medicare Services continues to support PET usage for staging and treatment management (27). Additionally, multiple international guidelines, including the NCCN, European Society for Medical Oncology, Pan-Asian-adapted Clinical Practice Guidelines, and the American College of Radiology Appropriateness Criteria, support the usage of PET at diagnosis of NSCLC (4,6–8). This level of support is due to numerous prior studies demonstrating PET imaging often alters patient management or serves as a prognostic marker for future outcomes (10,11,15–17). Our results show PET with or without CT imaging is associated with statistically significant improvement in survival, for all stages, compared with patients imaged with CT alone, supporting prior studies.
Our study has limitations. Our analyses were based on SEER registry data in a Medicare fee-for-service population with required coverage 12 months before and following diagnosis, decreasing our sample and limiting generalizability to all Medicare patients. Application to younger patients or patients with other forms of insurance (or uninsured) requires further study. By limiting the sample to patients who underwent PET with CT imaging during the diagnostic period, we are biasing toward the null. Improved disease detection by PET could lead to stage migration and spurious improvement in survival rates. SEER registries are reported to have greater economic disadvantage and greater racial and ethnic diversity, which may limit the results’ generalizability to the national population (28). Although our multivariable analysis controlled for numerous independent variables such as age, stage, sex, and facility, there may be unobservable characteristics that also affect the disparate usage of PET (19).
Blacks and Hispanic patients with newly diagnosed NSCLC are imaged with PET at a lower rate than their non-Hispanic white counterparts. These findings may contribute to poorer outcomes for racial and ethnic minorities but also suggest a path forward to combat racial inequality in health care. Further studies to identify underlying causes of this racial and ethnic disparity in PET imaging are warranted.
Funding
This project was supported by Population Health Shared Resource, University of Colorado Cancer Center, P30CA046934.
Notes
Drs Morgan and Bradley have no disclosures. Dr Sana D. Karam is funded by the National Institute of Dental and Craniofacial Research (R01 DE028529-01, R01 DE028282-01) and receives clinical trial funding from AstraZeneca and Cancer League of Colorado for work unrelated to this research.
PET/CT Utilization of Non-Small Cell Lung Cancer at Diagnosis: Does it Impact Survival? Radiology Society of North American, December 2019, Chicago, IL.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan BM. Lung cancer health disparities. Carcinogenesis. 2018;39(6):741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines insights: non–small cell lung cancer. J Natl Compr Canc Netw. 2018;16(7):807–821. [DOI] [PubMed] [Google Scholar]
- 5. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. [DOI] [PubMed] [Google Scholar]
- 6. Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210. [DOI] [PubMed] [Google Scholar]
- 7. de Groot PM, Chung JH, Ackman JB, et al. ACR Appropriateness Criteria((R)) noninvasive clinical staging of primary lung cancer. J Am Coll Radiol. 2019;16(5):S184–S195. [DOI] [PubMed] [Google Scholar]
- 8. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supp 4):iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 9. Park HL, Yoo IR, Boo SH, et al. Does FDG PET/CT have a role in determining adjuvant chemotherapy in surgical margin-negative stage IA non-small cell lung cancer patients? J Cancer Res Clin Oncol. 2019;145(4):1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–1392. [DOI] [PubMed] [Google Scholar]
- 11. Kubota K, Matsuno S, Morioka N, et al. Impact of FDG-PET findings on decisions regarding patient management strategies: a multicenter trial in patients with lung cancer and other types of cancer. Ann Nucl Med. 2015;29(5):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee EW, Wei LJ, Amato DA, et al. Cox-type regression analysis for large numbers of small groups of correlated failure time observations In: Klein JP, Goel PK, eds. Survival Analysis: State of the Art. Dordrecht: Springer Netherlands; 1992:237–247. [Google Scholar]
- 13. Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 14. Hostetter MK. In Focus: Reducing Racial Disparities in Health Care by Confronting Racism. The Commonwealth Fund; 2018. https://www.commonwealthfund.org/publications/newsletter-article/2018/sep/focus-reducing-racial-disparities-health-care-confronting. [Google Scholar]
- 15. Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126(6):1943–1951. [DOI] [PubMed] [Google Scholar]
- 16. Hattori A, Matsunaga T, Takamochi K, et al. Clinical significance of positron emission tomography in subcentimeter non-small cell lung cancer. Ann Thorac Surg. 2017;103(5):1614–1620. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Dong M, Sun X, et al. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One. 2016;11(1):e0146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017(1);67:7–30. [DOI] [PubMed] [Google Scholar]
- 19. Gould MK, Schultz EM, Wagner TH, et al. Disparities in lung cancer staging with positron emission tomography in the Cancer Care Outcomes Research and Surveillance (CanCORS) study. J Thorac Oncol. 2011;6(5):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanchate AD, Frakt AB, Kressin NR, et al. External determinants of Veterans' utilization of VA Health Care. Health Serv Res. 2018;53(6):4224–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paulson EC, Mitra N, Sonnad S, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248(4):675–686. [DOI] [PubMed] [Google Scholar]
- 22. Onega T, Duell EJ, Shi X, et al. Influence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patients. Med Care Res Rev. 2009;66(5):542–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfson JA, Sun C-L, Wyatt LP, et al. Impact of care at comprehensive cancer centers on outcome: results from a population-based study. Cancer. 2015;121(21):3885–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeidan AJ, Khatri UG, Aysola J, et al. Implicit bias education and emergency medicine training: step one? Awareness. AEM Educ Train. 2019;3(1):81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Healy MA, Yin H, Reddy RM, et al. Use of positron emission tomography to detect recurrence and associations with survival in patients with lung and esophageal cancers. J Natl Cancer Inst. 2016;108(7):djv429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United HealthCare Services I. Coverage summary - positron emission tomography (PET)/combined PET-CT (Computed Tomography). https://www.uhcprovider.com/content/dam/provider/docs/public/policies/medadv-coverage-sum/positron-emission-tomography-pet-combined-pet-ct-computed-tomography.pdf. Accessed December 15, 2019.
- 28. Kuo T-M, Mobley LR. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control. 2016;27(9):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.