Abstract
Background
The National Cancer Institute Moonshot research initiative calls for improvements in the analysis and reporting of treatment toxicity to advise key stakeholders on treatment tolerability and inform regulatory and clinical decision making. This study illustrates alternative approaches to toxicity evaluation using the National Surgical Adjuvant Breast and Bowel Project R-04 clinical trial as an example.
Methods
National Surgical Adjuvant Breast and Bowel Project R-04 was a neoadjuvant chemoradiation trial in stage II–III rectal cancer patients. A 2 x 2 factorial design was used to evaluate whether the addition of oxaliplatin (Oxa) to 5-fluorouracil (5FU) or capecitabine (Cape) with radiation therapy improved local-regional tumor control. The toxicity index (TI), which accounts for the frequency and severity of toxicities, was compared across treatments using multivariable probabilistic index models, where Pr A < B indicates the probability that higher values of TI were observed for A when compared with B. Baseline age, sex, performance status, body mass index, surgery type, and stage were evaluated as independent risk factors.
Results
A total of 4560 toxicities from 1558 patients were analyzed. Results from adjusted probabilistic index models indicate that oxaliplatin-containing regimens had statistically significant (P < .001) probability (Pr) for higher TI compared with regimens without oxaliplatin (Pr 5FU < 5FU + Oxa = 0.619, 95% confidence interval [CI] = 0.560 to 0.674; Pr 5FU < Cape + Oxa = 0.627, 95% CI = 0.568 to 0.682; Pr Cape < 5FU + Oxa = 0.587, 95% 0.527 to 0.644; and Pr Cape < Cape + Oxa = 0.596, 95% 0.536 to 0.653). When compared with other existing toxicity analysis methods, TI provided greater power to detect differences between treatments.
Conclusions
This article uses standard data collected in a cancer clinical trial to introduce descriptive and analytic methods that account for the additional burden of multiple toxicities. These methods may provide a more accurate description of a patient’s treatment experience that could lead to individualized dosing for better toxicity control. Future research will evaluate the generalizability of these findings in trials with similar drugs.
For more than 60 years, cancer clinical trials have used an observer-rated toxicity grading system, Common Terminology Criteria for Adverse Events (CTCAE), which assesses the severity of various organ system toxicities associated with treatment (1). CTCAE data collection in a trial provides detailed longitudinal information on the severity and types of toxicity. However, standard methods for summarizing CTCAE toxicities do not capture the complete toxicity experience over the course of treatment. For instance, maximum grade analysis involves the aggregation of toxicities by highest grade experienced over time and does not account for the cumulative burden that multiple toxicities may introduce or the persistence and chronicity of some lower-grade toxicities (2–6).
Toxicity reports are further limited by ignoring baseline risk factors that may contribute to treatment burden. Although information on demographic and clinical characteristics is collected for most clinical trials, it is often presented separately and seldom evaluated within the context of toxicity. Understanding which factors predict greater toxicity is critical to determining optimal treatment approaches and identifying those at higher risk for toxicity. For instance, host factors such as baseline performance status, older age, and sex, or disease-specific factors such as clinical stage or surgery type received, are known predictors of survival and treatment outcomes and should be considered when evaluating toxicity (7–10). In recognition of deficiencies in toxicity reporting, the National Cancer Institute launched a Cancer Moonshot funding opportunity to accelerate research on improved approaches to evaluating the tolerability of cancer treatments.
This article examines new strategies for understanding treatment toxicity applied to existing data from a large randomized clinical trial, the National Surgical Adjuvant Breast and Bowel Project (NSABP R-04) (11,12). Using new statistical approaches and graphical displays to summarize the toxicity data, we demonstrate how one can optimize the use of available information and provide a more complete and accurate account of which patients are at greatest risk for toxicity at the completion of a trial.
Methods
Methods for Analyzing Toxicity
We applied three methods for analyzing toxicity: the toxicity index (TI) (13), the maximum grade, and average toxicity. The TI was developed as a summary measure to better discriminate patients based on their overall toxicity experiences, accounting for all observed toxicity grades rather than just the most severe one (13). A patient's TI score is defined as a function of the ordered toxicity grades, where the toxicity grades are represented in descending order by the sequence. The TI is computed according to the following algorithm (14):
The TI has the following properties: any score 3 corresponds to the usual definition of dose limiting toxicity, and the maximum toxicity grade is the integer part of the final score. For example, a TI score of 3.0 indicates a single grade 3 toxicity, whereas a score of 3.5 indicates at least one grade 3 toxicity plus additional toxicity; all toxicity grades are represented in the score, although lower grades contribute less to the final score; the score is a number between 0 and 5.83 (see Supplementary Material for explanation of upper limit, available online); multiple toxicities of the same grade yield a TI score slightly less than that generated by a single toxicity of the next higher grade; and when several patients are compared with relation to their toxicity profile, the TI preserves their ranking.
The second approach, maximum grade analysis, yields an incidence rate that is summarized by the most severe grade observed across all events, independent of time of occurrence (2–4). For example, a patient experiencing multiple high-grade toxicities across organ systems is noted as having experienced only a single high-grade toxicity overall. We also compute the average toxicity, which is the summary statistic used in the Toxicity over Time approach (13,15), which requires analysis across multiple treatment cycles.
Data Source
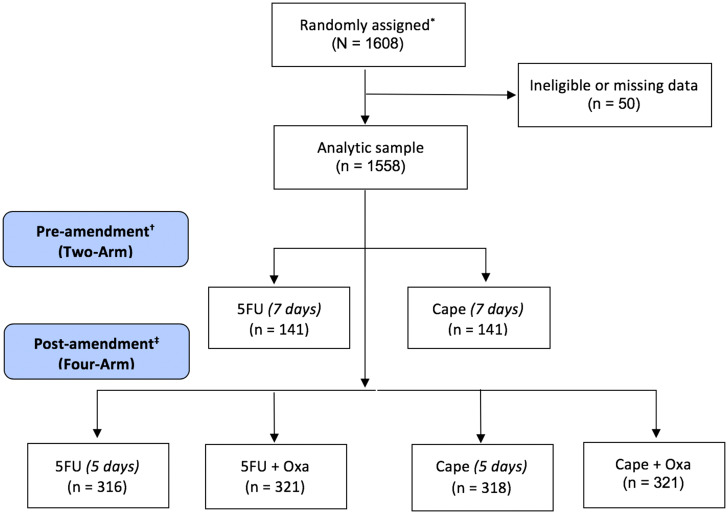
Data from the NSABP R-04 rectal cancer clinical trial were used as a case example for this research (12). NSABP R-04 was a phase III trial conducted between July 2004 and August 2013 (NCT00058474). Eligible patients were diagnosed with surgically resectable stage II or III rectal adenocarcinoma. The trial was approved by the local institutional review boards, and all patients provided written consent, as detailed in the main trial report; however, these secondary analyses were deemed exempt by our institutional review boards (12). When the trial first opened, patients were randomized to two treatment groups: infusional 5-fluorouracil (5FU) with pelvic radiation therapy (RT) compared with oral capecitabine (Cape) with pelvic RT. In 2005, the protocol was amended to add an oxaliplatin (Oxa) option to 5FU and Cape, resulting in a 2 x 2 factorial design with four treatment groups: 5FU + RT; 5FU + Oxa + RT; Cape + RT; and Cape + Oxa + RT. The doses for 5FU and Cape for the four-arm amended trial were reduced from 7 to 5 days a week postamendment to allow for the addition of Oxa (12) (Figure 1). Surgery was performed within 6 to 8 weeks after RT completion. The primary outcome was local-regional tumor control, defined as time to local or regional recurrence or surgery if an R0 resection was not achieved. Oxa did not improve the primary outcome, and there was no statistically significant difference between the Cape and 5FU-alone arms (12). As a result, Cape with RT has now become the standard of care in subsequent trials.
Figure 1.
CONSORT diagram. *Main study description available in Allegra (12). All treatment arms included radiation therapy. †Preamendment: In 2004, patients were randomly assigned to either RT + 5FU or RT + Cape for 7 days a week beginning the day of RT start and ending on the last dose of RT. ‡Postamendment: In 2005, the protocol was amended to add Oxa and resulted in a 2 x 2 factorial design. Doses were reduced from 7 days to 5 days. 5FU = 5-fluorouracil; Cape = capecitabine; Oxa = oxaliplatin; RT = radiation therapy.
Baseline assessments included demographics, medical history, height, weight, vitals, physical exam, quality of life, imaging, and bloodwork. Laboratory tests (eg, complete blood count with differential, platelets, bilirubin, alkaline phosphate, aspartate aminotransferase) were evaluated weekly during treatment and 2 weeks prior to surgery. Toxicity assessment was conducted using CTCAE version 4.0 graded from 0 (least severe) to 5 (most severe) and grouped by 26 system organ classes. Adverse events (AEs) were collected at a single time point after chemoradiation treatment within 2 weeks of surgery. More than 50 AEs of special interest were selected a priori based on clinical expertise concerning the study regimens and evaluated systematically during treatment (Supplementary Table 1, available online). Quality-of-life questionnaire data were collected prior to treatment, at the end of chemoradiation prior to surgery, and then 12 months after surgery and have been reported in part elsewhere (11) and are not included here. Patient follow-up for survival and disease progression occurred every 12 months from surgery for the first 2 years. The trial included 1608 participants, with complete toxicity data available for 1558 patients (our analysis sample). Additional information about the trial design and study population is reported elsewhere (11,12).
Statistical Analysis
Graphical summaries of the toxicity data included box plots, histograms, and combinations of graphical and tabular results. All graphical summaries were produced in the R statistical package.
Probabilistic index models (PIMs), a rank-based method that generalizes the Kruskal-Wallis test, were fit to compute the probability (Pr) of higher toxicity between groups (16–20). For example, considering a score S for groups A and B, a probability Pr(SA < SB) equal to 0.5 indicates that both groups have similar score S distributions; a probability statistically significantly greater than 0.5 (Pr > 0.5) gives evidence that group B has higher score S than group A; a probability statistically significantly less than 0.5 (Pr < 0.5) gives evidence that group B has a lower score S than group A. The probability that a score S for one group is greater than or equal to a score S for another group was estimated with a Wald-type 95% confidence interval (CI). P values were calculated using the Wald statistic, and P values for multiple comparisons were corrected using Holm adjustment (21). In addition, we defined body system-specific TI as the TI calculated considering only toxicities in a given specific body system. Separate PIMs were then fit for each body system that had at least 10 nonzero TI values. All PIMs incorporated covariables of interest, including sex, age, Karnofsky Performance Status (KPS), clinical stage, and intended surgery (sphincter or nonsphincter preserving) at entry. Tests for interactions between sex and treatment were assessed, where an interaction effect was present if the interaction term in the PIMs was statistically significant (P < .05). If the interaction term was not statistically significant, the term was removed from the model. To compare the performance of different analytic approaches, the power to detect treatment differences was estimated for sample sizes of 50, 75, 100, …, 300 patients for each method (TI, maximum grade, average toxicity) based on 2000 resamples. Calculations were performed using the R-package “pim” (22), and all hypotheses were two-tailed and tested at the 5% statistical significance level.
Results
Patient Characteristics
The analytic sample consisted of 1558 eligible patients. There were 141 patients analyzed in each treatment group from the two-arm trial (preamendment): (group 1) 5FU + RT and (group 2) Cape + RT. In the four-arm 2 x 2 factorial trial, 316 patients were randomized to 5FU + RT (group 3), 321 to 5FU + Oxa + RT (group 4), 318 to Cape + RT (group 5), and 321 to Cape + Oxa + RT (group 6) (Figure 1). Demographic characteristics including age, sex, clinical stage, and surgical treatment intent were well balanced across groups as previously reported (12).
Treatment Toxicity
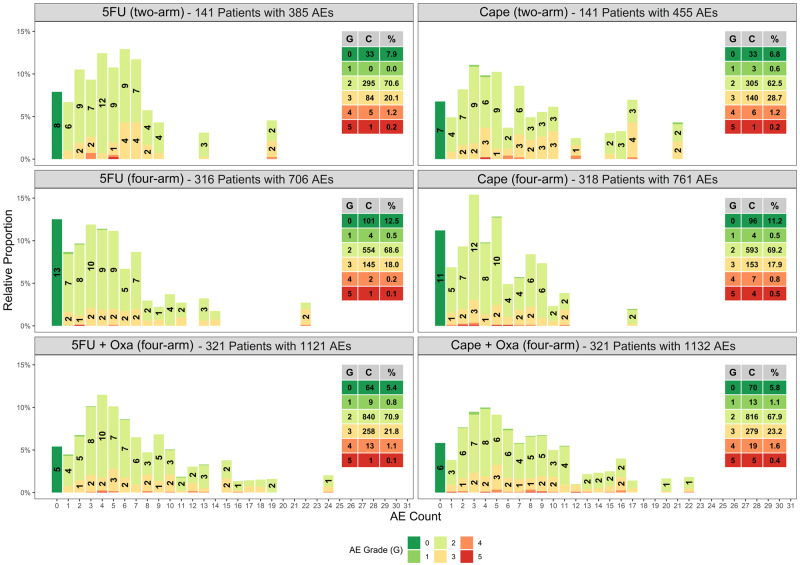
In this study, our only toxicity assessment time point was at the end of chemoradiation therapy and before surgery. Among 1558 eligible patients from all treatment groups (two-arm and four-arm), there were a total of 4560 toxicities of which 3720 toxicities occurred in the subgroup of 1276 patients in the four-arm trial (postamendment). Figure 2 shows the relative proportion of toxicities for each toxicity severity (y-axis) by the number of toxicities that occurred per patient (x-axis). From this figure, it can be observed that the number ranged from 0 to 24 toxicities per patient, with the most frequent and severe toxicities occurring in patients treated with Oxa combined with 5FU or Cape (Figure 2).
Figure 2.
Relative percentages of toxicities per patient by treatment arm. The x-axis represents the number of adverse events observed per patient. The y-axis represents the percentage of the total associated with each number of adverse events within each treatment arm. Each column is further broken down and color coded by grade from no adverse events (grade 0) represented as green to most severe (grade 5) represented as red. The labeling within each column represents the relative percentage of a given grade among patients with the specified number of adverse events (grades with less than 1% are omitted). The table inset presents the grade (G), count (C), and percent (%) of each grade observed for a given treatment arm. 5FU = 5-fluorouracil; AE = adverse event; Cape = capecitabine; Oxa = oxaliplatin.
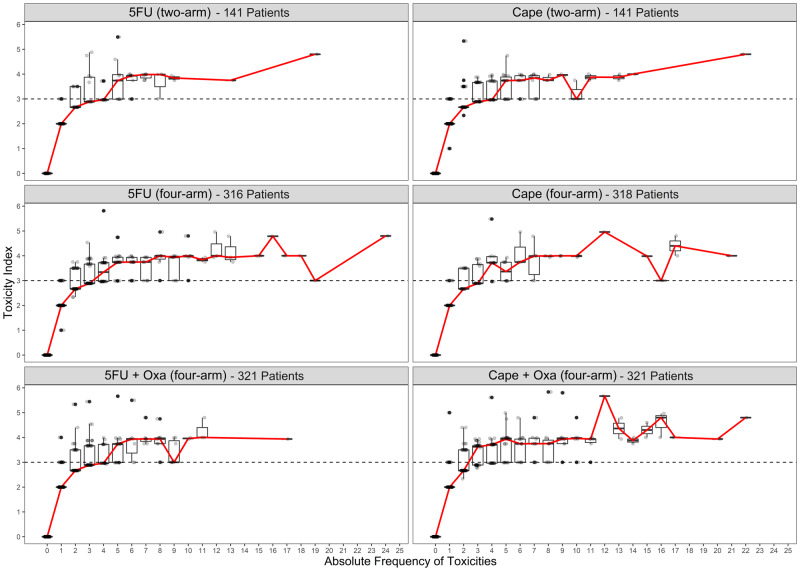
TI was calculated to provide a quantitative measure of the cumulative burden of treatment toxicity. A summary of the mean, median, and interquartile ranges for TI is provided in Table 1. TI was lowest in the 5FU four-arm trial (median = 2.33) and highest in the Cape + Oxa and 5FU + Oxa arms (median = 2.98) (Table 1). The mode of the distribution of toxicities per patient was 0 for 5FU, 3 for Cape, and 4 for both 5FU + Oxa and Cape + Oxa (Supplementary Figure 1, available online). Patients with one or two toxicities tended to have a median TI less than 3, whereas patients with more than toxicities displayed a median TI no less than 3, which is typically classified as dose-limiting toxicity. Figure 3 shows that TI increased with the increasing number of toxicities per patient in each treatment group, thus demonstrating that the severity of toxicities also increases with the number of toxicities occurring per patient.
Table 1.
Measures of central tendency of the toxicity index by treatment*
| Treatment | No. patients | No. toxicities | Mean (SD) | Median (IQR) |
|---|---|---|---|---|
| 5FU (two-arm) | 141 | 385 | 3.56 (2.81) | 3 (1–5) |
| 5FU (four-arm) | 316 | 706 | 3.28 (2.85) | 2 (1–4) |
| 5FU + Oxa (four-arm) | 321 | 1121 | 4.36 (3.65) | 3 (2–6) |
| Cape (two-arm) | 141 | 455 | 4.21 (3.83) | 3 (2–5) |
| Cape (four-arm) | 318 | 761 | 3.43 (2.59) | 3 (1–5) |
| Cape + Oxa (four-arm) | 321 | 1132 | 4.51 (3.73) | 3 (2–6) |
5FU = 5-fluorouracil; Cape = capecitabine; IQR = interquartile range; Oxa = oxaliplatin.
Figure 3.
Relationship between toxicity index and number of toxicities per patient by treatment arm. 5FU = 5-fluorouracil; Cape = capecitabine; Oxa = oxaliplatin.
Probabilistic Index Models
In univariable analysis (Supplementary Table 2, available online), in older women who underwent planned nonsphincter-saving surgery and had poor KPS (50–60) and body mass index less than 18.5 were statistically significantly associated with increased probability (Pr > 0.5) for higher TI. Treatment with Cape + Oxa and 5FU+ Oxa also had increased probability of higher TI than either 5FU or Cape alone (four-arm) (Supplementary Table 2, available online). Additionally, the higher dose of 5FU (two-arm) was associated with greater toxicity as compared with the four-arm regimen. There were no statistical differences observed between 5FU and Cape (four-arm) or 5FU + Oxa and Cape + Oxa.
Multivariable PIMs for the two-arm and four-arm trials are shown in Tables 2 and 3, respectively. The adjusted probability that the 5FU two-arm had a higher TI than four-arm (Pr = 0.57, 95% CI = 0.51 to 0.63; P = .02), and the probability the Cape two-arm had a higher TI than the four-arm (Pr = 0.56, 95% CI = 0.50 to 0.62; P = .05) were greater than 0.5, showing that two-arm single treatments were more toxic than four-arm single treatments, but only the comparison between the 5FU two-arm and four-arm trials was statistically significant (Table 2).
Table 2.
Multivariable probabilistic index for toxicity index comparing four-arm and two-arm trials
| Variable | Comparison* | 5FU |
Cape |
||
|---|---|---|---|---|---|
| A < B | Probability (95% CI) | P† | Probability (95% CI) | P † | |
| Treatment | Four-arm < Two-arm‡ | 0.570 (0.513 to 0.625) | .02 | 0.558 (0.499 to 0.615) | .054 |
| Sex | Male < Female | 0.571 (0.513 to 0.628) | .02 | 0.609 (0.551 to 0.664) | <.001 |
| Age, y | Every 5 years | 0.511 (0.499 to 0.522) | .07 | 0.508 (0.496 to 0.520) | .18 |
| Karnofsky PS | 90–100 < 70–80 | 0.605 (0.532 to 0.673) | .005 | 0.579 (0.503 to 0.653) | .043 |
| 90–100 < 50–60 | N/A | N/A | 0.933 (0.916 to 0.947) | <.001 | |
| Clinical stage N | Negative < Positive | 0.492 (0.438 to 0.547) | .78 | 0.522 (0.468 to 0.576) | .41 |
| Sphincter-saving surgery | Yes < No | 0.503 (0.445 to 0.562) | .92 | 0.508 (0.448 to 0.568) | .79 |
| Clinical stage T | T1/T2/T3 < T4 | 0.537 (0.383 to 0.684) | .64 | 0.505 (0.384 to 0.626) | .93 |
| BMI (kg/m2) | LT 18.5 < 18.5–25 | 0.467 (0.241 to 0.708) | .80 | 0.369 (0.226 to 0.541) | .13 |
| LT 18.5 < 2–30 | 0.445 (0.224 to 0.690) | .67 | 0.362 (0.221 to 0.532) | .11 | |
| LT 18.5 < GE 30 | 0.417 (0.206 to 0.664) | .52 | 0.309 (0.183 to 0.471) | .02 | |
Probabilistic Model Interpretation: Comparison A < B denotes the probability that toxicity index for B is higher than toxicity index for A. Probability of 0.5 indicates no difference between comparisons (A = B). If probability is greater than 0.5, then probability of toxicity index for B is greater than A is high, indicating that B has higher toxicity. If the probability is less than 0.5, then probability of toxicity index for B is greater than A is small, indicating that A has higher toxicity. Multivariable models were adjusted for sex, four-arm treatments, age, body mass index (BMI), clinical T stage, clinical N stage, sphincter-saving surgery, and Karnofsky Performance Status (PS). 5FU = 5-fluorouracil; Cape = capecitabine; CI = confidence interval; GE = greater or equal to; LT = less than.
All P values are two-sided and were calculated using the Wald statistic. P values for multiple comparisons were corrected using Holm adjustment.
The trial was amended in 2005 to add oxaliplatin to each of the arms. The doses for 5FU and Cape for the four-arm clinical trial were reduced from 7 days (two-arm trial) to 5 days (four-arm trials) a week at the same daily dose.
Table 3.
Multivariable probabilistic index for toxicity index comparing four-arm and two-arm trials*
| Variable | Comparison | Probability (95% CI) | P † |
|---|---|---|---|
| A < B | |||
| Treatment | 5FU < 5FU + Oxa | 0.619 (0.560 to 0.674) | <.001 |
| 5FU < Cape | 0.533 (0.472 to 0.593) | .30 | |
| 5FU < Cape + Oxa | 0.627 (0.568 to 0.682) | <.001 | |
| Cape < 5FU + Oxa | 0.587 (0.527 to 0.644) | <.001 | |
| Cape < Cape + Oxa | 0.596 (0.536 to 0.653) | <.001 | |
| 5FU + Oxa < Cape + Oxa | 0.509 (0.449 to 0.569) | .70 | |
| Sex | Male < Female | 0.623 (0.589 to 0.655) | <.001 |
| Age, y | Every 5 years | 0.507 (0.500 to 0.515) | .04 |
| Karnofsky PS | 90–100 < 70–80 | 0.575 (0.529 to 0.619) | .001 |
| Clinical stage N | Negative < Positive | 0.480 (0.447 to 0.513) | .24 |
| Sphincter-saving surgery | Yes < No | 0.540 (0.504 to 0.577) | .03 |
| Clinical stage T | T1–3 < T4 | 0.551 (0.468 to 0.632) | .23 |
| BMI (kg/m2) | LT 18.5 < 18.5–25 | 0.441 (0.311 to 0.580) | .49 |
| LT 18.5 < 25–30 | 0.403 (0.278 to 0.542) | .17 | |
| LT 18.5 < GE 30 | 0.360 (0.243 to 0.495) | .04 |
Probabilistic Model Interpretation: Comparison A < B denotes the probability that toxicity index for B is higher than toxicity index for A. Probability of 0.5 indicates no difference between comparisons (A = B). If probability is greater than 0.5, then probability of toxicity index for B is greater than A is high, indicating that B has higher toxicity. If the probability is less than 0.5, then probability of toxicity index for B is greater than A is small, indicating that A has higher toxicity. Multivariable models were adjusted for sex, four-arm treatments, age, body mass index (BMI), clinical T stage, clinical N stage, sphincter-saving surgery, and Karnofsky Performance Status (PS). The trial was amended in 2005 to add oxaliplatin (Oxa) to each of the arms. The doses for 5-fluorouracil (5FU) and capecitabine (Cape) for the four-arm clinical trial were reduced from 7 days (two-arm trial) to 5 days (four-arm trials) a week at the same daily dose. CI = confidence interval; GE = greater or equal to; LT = less than.
All P values are two-sided and were calculated using the Wald statistic. P values for multiple comparisons were corrected using Holm adjustment.
Oxa-containing regimens also had statistically significant probability (Pr > 0.5) for higher TI compared with regimens without Oxa in the four-arm trials (Pr 5FU < 5FU + Oxa = 0.619, 95% CI = 0.56 to 0.674; Pr 5FU < Cape + Oxa = 0.627, 95% CI = 0.568 to 0.682; Pr Cape < 5FU + Oxa = 0.587, 95% CI = 0.527 to 0.644; and Pr Cape < Cape + Oxa = 0.596, 95% CI = 0.536 to 0.653) (Table 3). Baseline characteristics independently associated with increased probability of higher toxicity included women, poor KPS, low body mass index (<18.5), and planned nonsphincter-preserving surgery (Table 3). No statistically significant interaction between sex and treatment was observed (P = .97). We did observe that women had statistically significant higher toxicity than men using body system–specific TI for the following body systems: blood, gastrointestinal, general, investigations, metabolism, and reproductive (Table 4).
Table 4.
Multivariable probabilistic index models for system organ class–specific toxicity index comparing sex*
| System organ class | No. observations with nonzero SOC-specific toxicity index† | Probability‡ (95% CI§) | P ¶ |
|---|---|---|---|
| Blood | 136 | 0.553 (0.522 to 0.584) | <.001 |
| Cardiac | 14 | Not examined due to a small number of nonzero values | |
| Ear | 2 | Not examined due to a small number of nonzero values | |
| Endocrine | 1 | Not examined due to a small number of nonzero values | |
| Eye | 8 | Not examined due to a small number of nonzero values | |
| Gastrointestinal | 672 | 0.616 (0.566 to 0.662) | <.001 |
| General | 362 | 0.563 (0.521 to 0.604) | <.001 |
| Hepatobiliary | 3 | Not examined due to a small number of nonzero values | |
| Immune | 26 | 0.501 (0.489 to 0.514) | 1.00 |
| Infections | 91 | 0.518 (0.494 to 0.543) | .26 |
| Injury | 198 | 0.511 (0.479 to 0.544) | 1.00 |
| Investigations | 299 | 0.572 (0.532 to 0.612) | <.001 |
| Metabolism | 251 | 0.554 (0.517 to 0.591) | <.001 |
| Musculoskeletal | 71 | 0.507 (0.486 to 0.528) | 1.00 |
| Nervous | 116 | 0.506 (0.480 to 0.533) | 1.00 |
| Psychiatric | 62 | 0.500 (0.480 to 0.519) | 1.00 |
| Renal | 130 | 0.500 (0.472 to 0.527) | 1.00 |
| Reproductive | 22 | 0.522 (0.506 to 0.538) | .001 |
| Respiratory | 29 | 0.501 (0.488 to 0.515) | 1.00 |
| Skin | 114 | 0.512 (0.486 to 0.538) | 1.00 |
| Vascular | 70 | 0.519 (0.497 to 0.540) | .10 |
Multivariable models were adjusted for sex, four-arm treatments, age, body mass index, clinical T stage, clinical N stage, sphincter-saving surgery, and Karnofsky Performance Status. CI = confidence interval; SOC = system organ class.
From a total of 1276 observations.
Probability that SOC-specific toxicity index for women is higher than SOC-specific toxicity index for men.
Adjusted for multiple tests using the Bonferroni procedure.
All P values are two-sided and were calculated using the Wald statistic. P values for multiple comparisons were corrected using Holm adjustment.
Comparison With Existing Toxicity Methods
Results from adjusted PIMs for each analysis method (TI, maximum grade, average toxicity) are graphically represented in Figure 4. The corresponding numerical estimates and 95% confidence intervals are available in Supplementary Table 3 (available online). Overall, point estimates for the probability of higher score were of greater or equal value when TI was used as compared with maximum grade for all comparisons, except Cape (two-arm) was less than Cape (four-arm).
Figure 4.
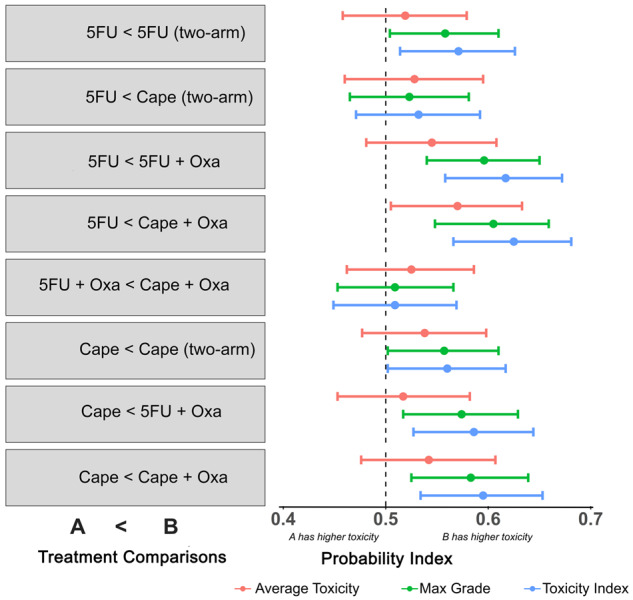
Multivariable probabilistic index model results by treatment comparison and analytic method. Each bar represents the probability index and 95% confidence intervals. 5FU = 5-fluorouracil; Cape = capecitabine; Oxa = oxaliplatin.
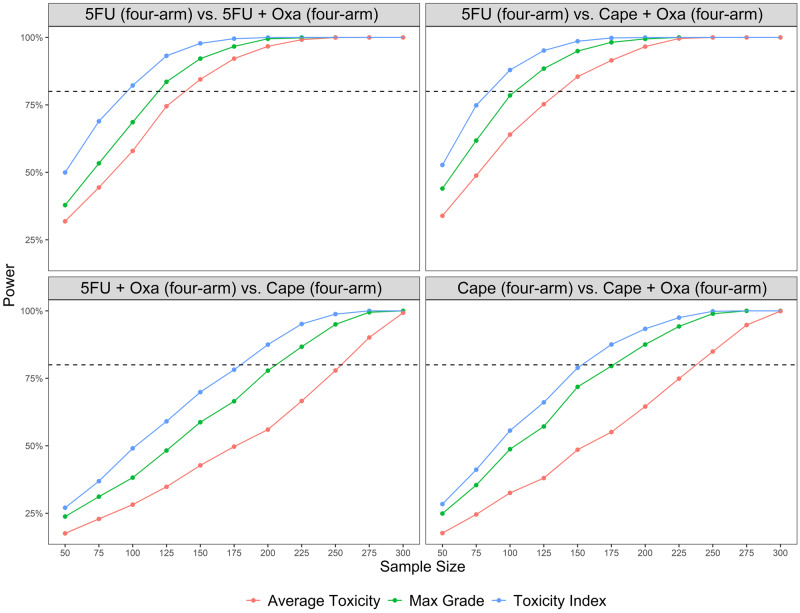
Although point estimates and measures of precision were comparable for TI and maximum grade, TI had greater power to detect differences between treatments (Figure 5). Thus, the use of TI results in a smaller number of patients needed to detect differences in treatments. For example, a sample size of 95 would be required to detect a difference between 5FU and 5FU + Oxa using TI. The same comparison would require a sample of 117 patients for the maximum grade method or 137 for the average toxicity method, resulting in a 19% and 31% difference in required sample sizes, respectively (Figure 5; Supplementary Table 3, available online).
Figure 5.
Power to detect treatment differences for toxicity index, maximum grade, and mean toxicity methods. 5FU = 5-fluorouracil; Capeape = capecitabine; Oxa = oxaliplatin
Discussion
Current approaches for analyzing and reporting clinical trial toxicity data are limited and do not capture the complete picture of a patient’s treatment experience. Most analyses have defaulted to the maximum grade approach, which collapses toxicities across all grades and organ systems and ignores the extensive toxicity data and baseline risk factors that are available. In this article, we demonstrate the feasibility of a more comprehensive approach to the presentation and analysis of toxicity data using the NSABP R-04 clinical trial as a case example.
Findings from this analysis revealed important differences in toxicity across treatment arms. By supplementing visual displays with the computation of TI scores, we were able to demonstrate the positive relationship between the frequency and severity of toxicities.
The TI also allowed for treatment comparisons, where the probability of a treatment having higher toxicity can be adjusted for baseline factors using PIMs. Applying this method to NSABP R-04 toxicity data resulted in statistically significant differences in toxicity between treatment arms that combined 5FU or Cape with Oxa and RT. The TI was also sensitive to differences in doses of 5FU where toxicity in the two-arm trial, at a higher dose (7 days), was statistically greater than the lower dose (5 days) in the four-arm trials. Although the primary NSABP R-04 trial publication described differences in the percentage of grade 3–4 toxicities in the two-arm and four-arm trials, it did not reach statistical significance (12). Further, there was no information about the frequency or occurrence of less-severe toxicities, using the standard maximum grade approach to present safety results.
Existing trial reports also failed to describe the additional risk that baseline factors may contribute to our understanding of the overall toxicity burden and tolerability of treatment regimens on subgroups of patients within the setting of a randomized trial. Using adjusted PIMs, we compared TI across treatments and patient characteristics. We found that older women with worse KPS and clinician intent for nonsphincter-preserving surgery were statistically associated with higher probability of subsequent toxicity. Although the prognostic values of some of these host factors for survival (eg, age, KPS) are established in the literature, we know of few reports that describe the impact of baseline host factors on treatment toxicity (23–26). When reported, these analyses usually occur in secondary analyses long after the primary trial result and thus may not be promptly reported to the clinicians adopting a treatment regimen. Reporting on baseline characteristics that are risk factors for greater toxicity can better prepare clinicians who apply trial results to the treatment of patients in their clinical practice.
Our analysis also uncovered interesting differences in toxicity that are independently associated with sex. Overall, women had statistically significantly higher toxicity across treatments and body systems than men. It is unclear whether these differences are a result of differences in clinician reports by sex or if women are at greater risk for toxicity. Earlier studies, more than 2 decades ago, reported sex-related differences in 5FU toxicity related to hematological toxicity and mucositis, but the sample size and quality of these studies were limited (23,27–29). Thus, this analysis greatly expands on these past observations, showing multisystem toxicities. We plan to evaluate our sex-related findings in another adjuvant colon cancer trial comparing 5FU with or without Oxa. We have also begun to explore whether there are sex differences in the patient-reported outcome (PRO) data that were collected in the R04 trial (11). The National Institutes of Health (NIH) now requires all research applications to discuss sex as a biological variable, and there are increasing reports of sex differences in the newer immunotherapy treatment trials, where there are known differences between men and women with regard to the immune system as well as other factors (30). In retrospect, we may have missed an opportunity to identify an important variable that is closely related to treatment toxicity and tolerability for some regimens, and future evaluations of treatment toxicity should consider sex-specific evaluations of toxicity.
Several strengths are associated with the use of TI for toxicity analysis. We show that it contains more information than other toxicity analysis methods by accounting for both the multiplicity and severity of toxicities, without losing the natural interpretability of the maximum grade approach. This added information provides greater power to examine comparisons across treatment types when compared with the maximum grade and average toxicity approaches, resulting in the least number of patients required to detect differences between treatments, and consequently saving trial resources and time.
As a limitation, the use of TI requires rank-based methods because it does not follow any well-known probability distribution such as the normal distribution. These methods are less powerful than parametric approaches, and rank-based regressions such as PIM are less disseminated. Although one could argue that the decreased power is mitigated because of the large sample sizes used in phase III clinical trials, the lack of a distribution assumption makes our conclusions more robust. Furthermore, the TI can be applied to other ordinal scales such as the PRO-CTCAE, which is increasingly being introduced into clinical trial data collection and analysis (31,32). The use of patient-reported toxicities also addresses deficiencies of clinician-rated CTCAE toxicities that lack standardization, are not systematically rated, and are difficult to assess because of their subjective nature (eg, pain, fatigue, anxiety), leading to underreporting of the frequency and severity of symptoms. Detailed analysis of PRO data from the quality-of-life questionnaire from the NSABP R-04 trial will be presented in an independent report. Future applications of TI may also incorporate weights for different toxicities as determined a priori by investigators or patients and included in the analysis of toxicities in clinical trials.
A limitation of this study was the availability of only a single AE assessment time point in the NSABP R-04 clinical trial. We plan to explore the use of TI for longitudinal evaluations and compare it with other methods that require repeated measures such as Toxicity over Time (15) and TAME (33) to assess whether the benefits of the TI approach hold. Although longitudinal analyses were previously challenging using rank-based approaches, recently developed methods are now available (34,35).
In conclusion, this research used standard data collected in a cancer clinical trial to introduce descriptive and analytic methods that account for the additional burden of multiple toxicities. Our findings demonstrate initial feasibility of TI and its added value in the analysis of toxicity data to improve our understanding of the comparative tolerability across different treatments. These methods may provide a more accurate account of treatment tolerability that could lead to individualized dosing for better toxicity control. Future research will validate the clinical findings observed in the R-04 trials with additional trials that used similar drugs.
Funding
This work was supported in part by the National Cancer Institute of the NIH (1U01CA232859-01) (GG, MAD, ZSR, ML, SK, RDH, SP, MT, GY, PAG, AR); National Cancer Institute (R01 CA188480-01A1) (MT, AR); and NIH National Center for Advancing Translational Science UCLA CTSI (UL1 TR001881-01) (MAD, MT, AR). Additional funding included support from the NIH for the original trial (U10-CA180868, U10-CA180822, UG1-CA189867, U10-CA180888, U10-CA180820, and U10-CA180821).
Notes
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The authors report no conflicts of interest (GG, MAD, ZR, ML, SK, RDH, SP, MT, GY, PAG, AR).
The authors acknowledge members of the scientific advisory committee (Lari Wenzel, PhD; Claire Snyder, PhD; Michael Brundage, MD; N. Lynn Henry, MD, PhD; and Elisa Long, PhD) and NRG Oncology statisticians (Hanna Bandos, PhD, and Reena Cecchini, PhD).
Author contributions: Gillian Gresham: formal analysis, visualization, writing (original draft, review, and editing). Márcio A. Diniz: data curation, formal analysis, methodology, visualization, writing (review and editing). Zahra S. Razaee: formal analysis, writing (review and editing). Michael Luu: formal analysis, visualization, writing (review and editing). Sungjin Kim: formal analysis, methodology, visualization, writing (review and editing). Ron D. Hays: conceptualization, formal analysis, investigation, methodology, supervision, writing (review and editing). Steven Piantadosi: conceptualization, funding acquisition, methodology, writing (review and editing). Mourad Tighiouart: conceptualization, formal analysis, methodology, writing (review and editing). Greg Yothers: data curation, methodology, writing (review and editing). Patricia A. Ganz: conceptualization, funding acquisition, methodology, supervision, writing (original draft, review, and editing). André Rogatko: conceptualization, formal analysis, funding acquisition, methodology, supervision, visualization, writing (original draft, review, and editing).
Supplementary Material
References
- 1.National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. [last accessed March 3, 2020]; NIH publication # 09-7473. Published May 29, 2009; Revised Version 4.03 June 14, 2010. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 2. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. [DOI] [PubMed] [Google Scholar]
- 3. Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–98. [DOI] [PubMed] [Google Scholar]
- 4. Baselga J, Trigo JM, Bourhis J, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5568–5577. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg RM, Sargent DJ, Morton RF, Mahoney MR, Krook JE, O’Connell MJ. Early detection of toxicity and adjustment of ongoing clinical trials: the history and performance of the North Central Cancer Treatment Group’s real-time toxicity monitoring program. J Clin Oncol. 2002;20(23):4591–4596. [DOI] [PubMed] [Google Scholar]
- 6. Mahoney MR, Sargent DJ, O’Connell MJ, Goldberg RM, Schaefer P, Buckner JC. Dealing with a deluge of data: an assessment of adverse event data on North Central Cancer Treatment Group trials. J Clin Oncol. 2005;23(36):9275–9281. [DOI] [PubMed] [Google Scholar]
- 7. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laghousi D, Jafari E, Nikbakht H, Nasiri B, Shamshirgaran M, Aminisani N. Gender differences in health-related quality of life among patients with colorectal cancer. J Gastrointest Oncol. 2019;10(3):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burkeen J, Pan T, Dalia Y, et al. Gender differences in health-related quality of life (hrQOL) in patients undergoing intracranial RT. Int J Radiat Oncol Biol Phys. 2018;102(3):e730. [Google Scholar]
- 10. Unger JM, Vaidya R, Albain KS, et al. Sex differences in adverse event reporting in SWOG chemotherapy, biologic/immunotherapy, and targeted agent cancer clinical trials. J Clin Oncol. 2019;37(suppl 15):11588. [Google Scholar]
- 11. Russell MM, Ganz PA, Lopa S, et al. Comparative effectiveness of sphincter-sparing surgery versus abdominoperineal resection in rectal cancer: patient-reported outcomes in National Surgical Adjuvant Breast and Bowel Project randomized trial R-04. Ann Surg. 2015;261(1):144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogatko A, Babb JS, Wang H, Slifker MJ, Hudes GR. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin Cancer Res. 2004;10(14):4645–4651. [DOI] [PubMed] [Google Scholar]
- 14. Knuth D. The Art of Computer Programming, Vol.3, Sorting and Searching. Reading, MA: Addison-Wesley; 1973. [Google Scholar]
- 15. Thanarajasingam G, Atherton PJ, Novotny PJ, Loprinzi CL, Sloan JA, Grothey A. Longitudinal adverse event assessment in oncology clinical trials: The Toxicity over Time (ToxT) analysis of Alliance trials NCCTG N9741 and 979254. Lancet Oncol. 2016;17(5):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Neve J, Thas O. A regression framework for rank tests based on the probabilistic index model. J Am Stat Assoc. 2015;110(511):1276–1283. [Google Scholar]
- 17. De Neve J, Thas O. A Mann–Whitney type effect measure of interaction for factorial designs. Commun Stat Theory Methods. 2017;46(22):11243–11260. [Google Scholar]
- 18. De Neve J, Thas O, Ottoy J-P. Goodness-of-fit methods for probabilistic index models. Commun Stat Theory Methods. 2013;42(7):1193–1207. [Google Scholar]
- 19. Fay MP, Malinovsky Y. Confidence intervals of the Mann-Whitney parameter that are compatible with the Wilcoxon-Mann-Whitney test. Stat Med. 2018;37(27):3991–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thas O, Neve JD, Clement L, Ottoy JP. Probabilistic index models. J R Stat Soc Series B Stat Methodol. 2012;74(4):623–671. [Google Scholar]
- 21. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 22. Meys J, De Neve J, Sabbe N, Amorim G. Pim: Fit Probabilistic Index Models R Package Version 2.0. 0.2. 2016.
- 23. Ilich AI, Danilak M, Kim CA, et al. Effects of gender on capecitabine toxicity in colorectal cancer. J Oncol Pharm Pract. 2016;22(3):454–460. [DOI] [PubMed] [Google Scholar]
- 24. Greenlee H, Hershman DL, Shi Z, et al. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: the pathways study. J Natl Cancer Inst. 2017;109(2):djw206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34(25):3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bandos H, Melnikow J, Rivera DR, et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 2018;110(2):djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sloan JA, Loprinzi CL, Novotny PJ, Okuno S, Nair S, Barton DL. Sex differences in fluorouracil-induced stomatitis. J Clin Oncol. 2000;18(2):412–420. [DOI] [PubMed] [Google Scholar]
- 28. Chansky K, Benedetti J, Macdonald JS. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer. 2005;103(6):1165–1171. [DOI] [PubMed] [Google Scholar]
- 29. Zalcberg J, Kerr D, Seymour L, Palmer M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur. J. Cancer. 1998;34(12):1871–1875. [DOI] [PubMed] [Google Scholar]
- 30. Colli LM, Morton LM, Chanock SJ. Sex-related effect on immunotherapy response: implications and opportunities. J Natl Cancer Inst. 2019;111(8):749–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. [DOI] [PubMed] [Google Scholar]
- 32. Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3(8):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007;8(7):613–624. [DOI] [PubMed] [Google Scholar]
- 34. Chen T, Kowalski J, Chen R, et al. Rank-preserving regression: a more robust rank regression model against outliers. Stat Med. 2016;35(19):3333–3346. [DOI] [PubMed] [Google Scholar]
- 35. Jung SH, Ying Z. Rank‐based regression with repeated measurements data. Biometrika. 2003;90(3):732–740. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.