Abstract
Background
Brain metastases are associated with considerable morbidity and mortality. Integration of hospice at the end of life offers patients symptom relief and improves quality of life, particularly for elderly patients who are less able to tolerate brain-directed therapy. Population-level investigations of hospice utilization among elderly patients with brain metastases are limited.
Methods
Using the Surveillance, Epidemiology and End Results–Medicare database for primary cancer sites that commonly metastasize to the brain, we identified 50 148 patients (aged 66 years and older) diagnosed with brain metastases between 2005 and 2016. We calculated the incidence, timing, and predictors of hospice enrollment using descriptive techniques and multivariable logistic regression. All statistical tests were 2-sided.
Results
The incidence of hospice enrollment was 71.4% (95% confidence interval [CI] = 71.0 to 71.9; P < .001), a rate that increased over the study period (P < .001). The odds of enrollment for black (odds ratio [OR] = 0.76, 95% CI = 0.71 to 0.82; P < .001), Hispanic (OR = 0.80, 95% CI = 0.72 to 0.87; P < .001), and Asian patients (OR = 0.52, 95% CI = 0.48 to 0.57; P < .001) were substantially lower than white patients; men were less likely to be enrolled in hospice than women (OR = 0.78, 95% CI = 0.74 to 0.81; P < .001). Among patients enrolled in hospice, 32.6% (95% CI = 32.1 to 33.1; P < .001) were enrolled less than 7 days prior to death, a rate that was stable over the study period.
Conclusions
Hospice is used for a majority of elderly patients with brain metastases although a considerable percentage of patients die without hospice services. Many patients enroll in hospice late and, concerningly, statistically significant sociodemographic disparities exist in hospice utilization. Further investigations to facilitate targeted interventions addressing such disparities are warranted.
Hospice provides important services for patients with advanced solid malignancies and is associated with reduced symptom burden and improved quality of life among patients with terminal disease (1–5). Hospice also minimizes the utilization of surgical, radiotherapeutic, and systemic therapy-based approaches near the end of life, with associated reduction in health-care expenditure (4–6). For these reasons, guidelines from the American Society for Clinical Oncology have identified hospice utilization as a key measure of quality in end-of-life care in oncology (7,8).
Brain metastases occur commonly among patients with advanced solid malignancies, with an estimated incidence in the United States of 150 000–300 000 per annum (9,10). The prognosis for patients with brain metastases is poor, with median survival typically ranging from 4 to 10 months depending on the primary site, among other factors (11). Brain metastases are also associated with considerable morbidity, utilization of health-care resources, and health-care expenditure (9,12). Patients with brain metastases often experience neurologic symptoms that impair their functional status (9), particularly elderly patients who are less able to tolerate aggressive brain-directed interventions (13,14). Consequently, appropriate utilization of hospice in the management of elderly patients with brain metastases at the end of life may be especially beneficial to both patients and the health-care system.
Prior studies have investigated end-of-life care among patients with malignant brain tumors or other oncologic conditions (4,15–26). However, population-level investigations of hospice utilization focusing on patients with brain metastases are limited. We sought to use population-level data from the Surveillance, Epidemiology and End Results (SEER)–Medicare database to investigate the rate and timing of hospice enrollment at the end of life among elderly patients with brain metastases and to identify sociodemographic, clinical, and hospital-based predictors of hospice utilization. Identification of gaps in hospice enrollment may permit targeted interventions to increase hospice utilization when appropriate.
Materials and Methods
Data Source
Sponsored by the National Cancer Institute, the SEER program collects cancer data from registries that capture approximately 34.6% of the US population (27). Medicare is a federal health insurance program for the elderly, some people with disabilities, and people with end-stage renal disease (28). In 2018, Medicare covered 59.9 million US citizens including 51.2 million people aged 65 years and older (29). The SEER-Medicare database links SEER datasets with Medicare claims for covered health-care services from the time a patient is eligible for Medicare until death. We used SEER-Medicare datasets over the period of 2005–2015 (SEER cases) and 2004–2016 (Medicare claims) for primary cancer sites with considerable potential to metastasize to the brain (lung cancer, melanoma, breast cancer, renal cancer, and ovarian cancer). Despite the lower incidence of brain metastases among patients with colorectal and esophageal cancer, we also included these cancers in our analysis because the incidence of brain metastases among these primaries has been shown to increase with improvement of systemic therapy (30–33). The study was determined to be exempt by our local institutional review board.
Patient Population and Study Design
We identified 82 637 patients with brain metastases based on the presence of at least three International Classification of Diseases, Clinical Modification, ninth or tenth edition, claim-based billing codes for a diagnosis inclusive of brain metastasis (ICD-9-CM: 198.3; ICD-10-CM: C79.31, C79.32). This approach has been successfully validated for the identification of brain metastases using health insurance claims data with high sensitivity (97%) and specificity (99%) relative to manual chart review (34). We excluded patients alive as of the follow-up date of December 31, 2016, as well as those who were 65 years or younger at date of death, had a date of death in Medicare and SEER that differed by more than 3 months, were diagnosed at autopsy or via death certificate, lacked continuous enrollment in Medicare parts A and B for 12 consecutive months prior to death, or were enrolled in a health maintenance organization during any of the 12 months preceding death. We removed 468 patients (0.9%) from our model because of nonconvergence on logistic regression related to high levels of hospice enrollment in patients for whom information regarding receipt of care at a medical school–affiliated hospital or at a government hospital within 1 year of death was missing, leaving 50 148 patients in the final cohort.
Statistical Analysis
We tabulated patient sociodemographic and clinical information including age at death, sex, race, marital status, urban vs rural residence, primary cancer site, and Charlson Comorbidity Index (35). We calculated the Charlson Comorbidity Index using the Deyo adaptation for ICD-9-CM (36) and the Quan adaptation for ICD-10-CM (37). Race and ethnicity were classified as white, black, Hispanic, and Asian as determined by SEER. Patients of other races or ethnicities were combined because of small numbers. Zip code–based educational status (percentage of residents aged 25 years and older with a high school education) and median household income were obtained by linking patient zip codes to information from the 2000 US Census and the 2008–2012 America Community Survey. Hospital-related variables were used to identify whether patients received care at a medical school–affiliated hospital vs not, an urban hospital vs not, or a government hospital vs not, within 1 year of their date of death. The primary outcome was hospice enrollment (yes vs no).
Baseline characteristics in patients receiving hospice vs no hospice at the end of life were assessed using the t test for continuous covariates or chi-squaretest for categorical covariates. The incidence of hospice enrollment was calculated as the quotient of the number of hospice enrollees over the number of patient deaths, and a 95% confidence interval (CI) was calculated around the incidence estimate. Aforementioned sociodemographic-, clinical-, and hospital-related covariates were used in univariable and multivariable logistic regression to identify unadjusted and adjusted predictors of hospice enrollment. As a subset analysis, among patients enrolled in hospice before death, we determined the percentage of cases for which hospice was initiated less than 7 days prior to death, a metric of quality end-of-life care (8). The same covariates referenced above were used to identify predictors of enrollment on hospice less than 7 days vs at least 7 days prior to death in univariable and multivariable logistic regression models. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC). All tests were 2-sided, and a P value less than .05 was considered statistically significant.
Results
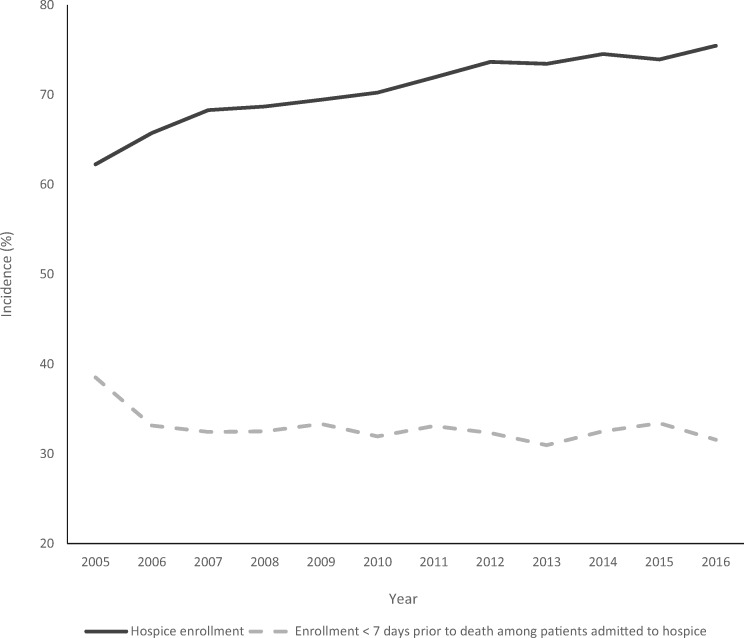
Baseline patient characteristics are shown in Table 1. Among the 50 148 patients with brain metastases included in our final cohort, 35 801 were enrolled in hospice prior to death, whereas 14 347 were not enrolled in hospice prior to death. The incidence of hospice enrollment for patients with brain metastases between 2005 and 2016 was 71.4% (95% CI = 71.0 to 71.9; P < .001). Rates of enrollment by cancer type ranged from 70% (renal cancer) to 80% (ovarian cancer). The median length of stay among all hospice enrollees was 13 days with an interquartile range of 5–33 days. The incidence of hospice utilization for patients with brain metastases gradually increased from 62.3% in 2005 to 75.5% in 2016 (Figure 1; P < .001). Among patients enrolled in hospice, 32.6% (95% CI = 32.1 to 33.1; P < .001) were enrolled less than 7 days prior to death, a rate that remained relatively stable for the duration of the study period (range = 31.0–33.4% during 2006–2016; Figure 1).
Table 1.
Demographic-, clinical-, and hospital-related characteristics of hospice recipients vs hospice nonrecipients
| Characteristic | Hospice recipients (n = 35 801) | Hospice nonrecipients (n = 14 347) | P ‖ |
|---|---|---|---|
| Age quintiles, No. (%), y | <.001 | ||
| 66–69 | 7713 (70.2) | 3274 (29.8) | |
| 70–72 | 6121 (70.8) | 2520 (29.2) | |
| 73–76 | 7484 (71.2) | 3023 (28.8) | |
| 77–81 | 7266 (70.5) | 3037 (29.5) | |
| 82–104 | 7217 (74.3) | 2493 (25.7) | |
| Sex, No. (%) | |||
| Female | 19 172 (73.8) | 6807 (26.2) | <.001 |
| Male | 16 629 (68.8) | 7540 (31.2) | |
| Race/Ethnicity, No. (%) | |||
| White | 30 277 (72.9) | 11 283 (27.1) | <.001 |
| Black | 2691 (66.5) | 1358 (33.5) | |
| Hispanic | 1455 (66.8) | 724 (33.2) | |
| Asian | 1247 (57.4) | 924 (42.6) | |
| Other or unknown | 131 (69.3) | 58 (30.7) | |
| Marital status, No. (%) | |||
| Married | 19 010 (71.7) | 7785 (28.3) | .02 |
| Unmarried | 15 095 (71.0) | 5946 (29.0) | |
| Unknown | 1696 (73.4) | 616 (26.6) | |
| Residence, No. (%) | |||
| Urban | 29 723 (70.3) | 11 672 (29.7) | <.001 |
| Rural | 3940 (71.8) | 1661 (28.2) | |
| Unknown | 2138 (67.8) | 1014 (32.2) | |
| Median household income by zip code (USD), mean (SD)* | 57 386 (26 523) | 56 632 (27 026) | .004 |
| High school graduates by zip code (%), mean (SD)* | 85.2 (10.6) | 84.1 (11.5) | <.001 |
| Primary cancer site, No. (%)† | |||
| Lung cancer | 21 671 (70.5) | 9069 (29.5) | <.001 |
| Breast cancer | 4269 (73.9) | 1509 (26.1) | |
| Melanoma | 2492 (75.5) | 808 (24.5) | |
| Renal cancer | 1274 (69.8) | 551 (30.2) | |
| Colorectal cancer | 1920 (71.7) | 757 (28.3) | |
| Ovarian cancer | 350 (79.9) | 88 (20.1) | |
| Esophageal cancer | 440 (74.3) | 152 (25.7) | |
| Other | 3385 (70.5) | 1413 (29.5) | |
| Charlson Comorbidity Index, No. (%) | |||
| 0–2 | 24 286 (71.9) | 9490 (28.1) | <.001 |
| ≥3 | 3463 (68.9) | 1564 (31.1) | |
| Unknown | 8052 (71.0) | 3293 (29.0) | |
| Received care at a medical school–affiliated hospital, No. (%)‡ | |||
| Yes | 12 166 (71.1) | 4756 (28.9) | .07 |
| No | 23 635 (71.9) | 9591 (28.1) | |
| Unknown | N/A§ | N/A§ | |
| Received care at an urban hospital, No. (%)‡ | |||
| Yes | 27 917 (70.8) | 10 992 (29.2) | <.001 |
| No | 4862 (71.8) | 2007 (28.2) | |
| Unknown | 3022 (69.1) | 1348 (30.9) | |
| Received care at a government hospital, No. (%)‡ | |||
| Yes | 11 156 (71.2) | 4373 (28.8) | .14 |
| No | 24 645 (71.8) | 9974 (28.2) | |
| Unknown | N/A | N/A§ |
Median household income and high school graduates were obtained by linking patient zip codes to information from the 2000 US Census and the 2008–2012 America Community Survey. USD = US dollars.
First cancer type was used for patients with multiple primary tumors.
Within 1 year of date of death.
468 observations (0.9%) were removed because of nonconvergence.
The 2-sided t test was used for continuous variables, and the chi-squaretest was used for categorical variables.
Figure 1.
Hospice incidence and timing of hospice enrollment by year of death among patients with brain metastases.
Multivariable logistic regression (Table 2) identified that enrollment in hospice was more likely among older patients (odds ratio [OR] for patients aged 82–104 years vs 66–69 years = 1.19, 95% CI = 1.12 to 1.27; P < .001), urban residents (OR = 1.10, 95% CI = 1.02 to 1.18; P = .02), patients living in areas with higher educational status (OR per 1% increase in high school graduates by zip code = 1.01, 95% CI = 1.01 to 1.01; P < .001), patients who received care at a government hospital within 1 year of death (OR =1.07, 95% CI = 1.03 to 1.12; P = .002), and patients with primary ovarian cancer (OR = 1.44, 95% CI = 1.13 to 1.82; P = .003), esophageal cancer (OR = 1.26, 95% CI = 1.04 to 1.51; P = .02), or melanoma (OR = 1.24, 95% CI = 1.14 to 1.35; P < .001) relative to the reference of lung cancer. The odds of enrollment in hospice were lower for Asian patients (OR = 0.52, 95% CI = 0.48 to 0.57; P < .001), black patients (OR = 0.76, 95% CI = 0.71 to 0.82; P < .001), and Hispanic patients (OR = 0.80, 95% CI = 0.72 to 0.87; P < .001) compared with white patients, as well as male patients (OR = 0.78, 95% CI = 0.74 to 0.81; P < .001), patients with greater comorbidity (OR for Charlson Comorbidity Index ≥3 vs 0–2 = 0.90, 95% CI = 0.84 to 0.96; P = .001), and patients living in areas with higher household income (OR per US $10 000 increase in median household income by zip code = 0.98, 95% CI = 0.97 to 0.99; P < .001).
Table 2.
Demographic-, clinical-, and hospital-related predictors of hospice utilization and late hospice enrollment (length of stay <7 days vs ≥7 days before death) among patients with brain metastases
| Variable | Multivariable OR for hospice enrollment (95% CI) | P ¶ | Multivariable OR for late hospice enrollment* (95% CI) | P ¶ |
|---|---|---|---|---|
| Age quintiles, y | ||||
| 66–69 | 1.00 (Referent) | 1.00 (Referent) | ||
| 70–72 | 1.02 (0.96 to 1.09) | .49 | 1.03 (0.95 to 1.10) | .50 |
| 73–76 | 1.04 (0.98 to 1.10) | .24 | 0.93 (0.87 to 1.00) | .04 |
| 77–81 | 1.00 (0.94 to 1.06) | .94 | 0.97 (0.91 to 1.04) | .44 |
| 82–104 | 1.19 (1.12 to 1.27) | <.001 | 0.90 (0.83 to 0.96) | .003 |
| Sex | ||||
| Female | 1.00 (Referent) | 1.00 (Referent) | ||
| Male | 0.78 (0.74 to 0.81) | <.001 | 1.36 (1.29 to 1.43) | <.001 |
| Race/Ethnicity | ||||
| White | 1.00 (Referent) | 1.00 (Referent) | ||
| Black | 0.76 (0.71 to 0.82) | <.001 | 0.97 (0.89 to 1.06) | .54 |
| Hispanic | 0.80 (0.72 to 0.87) | <.001 | 0.85 (0.76 to 0.96) | .007 |
| Asian | 0.52 (0.48 to 0.57) | <.001 | 0.82 (0.72 to 0.93) | .002 |
| Other or unknown | 0.87 (0.63 to 1.18) | .36 | 1.19 (0.83 to 1.70) | .35 |
| Marital status | ||||
| Single | 1.00 (Referent) | 1.00 (Referent) | ||
| Married | 1.03 (0.99 to 1.07) | .19 | 1.06 (1.01 to 1.11) | .03 |
| Unknown | 1.09 (0.99 to 1.21) | .08 | 1.14 (1.02 to 1.26) | .02 |
| Residence | ||||
| Rural | 1.00 (Referent) | 1.00 (Referent) | ||
| Urban | 1.10 (1.02 to 1.18) | .02 | 1.11 (1.02 to 1.21) | .02 |
| Unknown | 0.86 (0.78 to 1.06) | .19 | 0.92 (0.81 to 1.03) | .14 |
| Zip-code level household income, per US $10 000 increase† | 0.98 (0.97 to 0.99) | <.001 | 1.01 (1.00 to 1.02) | .22 |
| Zip-code level high school graduates, per percent increase† | 1.01 (1.01 to 1.01) | <.001 | 1.00 (1.00 to 1.00) | .67 |
| Primary cancer site‡ | ||||
| Lung cancer | 1.00 (Referent)§ | 1.00 (Referent)§ | ||
| Breast cancer | 1.04 (0.97 to 1.12) | .25 | 1.05 (0.98 to 1.14) | .19 |
| Melanoma | 1.24 (1.14 to 1.35) | <.001 | 1.05 (0.96 to 1.15) | .26 |
| Renal cancer | 0.99 (0.89 to 1.10) | .83 | 0.89 (0.79 to 1.01) | .06 |
| Colorectal cancer | 1.07 (0.98 to 1.17) | .13 | 0.84 (0.76 to 0.93) | .001 |
| Ovarian cancer | 1.44 (1.13 to 1.82) | .003 | 0.90 (0.71 to 1.14) | .39 |
| Esophageal cancer | 1.26 (1.04 to 1.51) | .02 | 1.00 (0.82 to 1.22) | 1.00 |
| Other | 1.04 (0.97 to 1.12) | .28 | 1.04 (0.96 to 1.14) | .33 |
| Charlson Comorbidity Index | ||||
| 0–2 | 1.00 (Referent) | 1.00 (Referent) | ||
| ≥3 | 0.90 (0.84 to 0.96) | .001 | 1.23 (1.14 to 1.32) | <.001 |
| Unknown | 0.95 (0.90 to 1.00) | .07 | 0.95 (0.89 to 1.01) | .08 |
| Received care at a medical school–affiliated hospital‖ | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | ||
| Yes | 1.02 (0.98 to 1.07) | .28 | 0.94 (0.89 to 0.98) | .009 |
| Received care at an urban hospital‖ | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | ||
| Yes | 0.99 (0.92 to 1.06) | .73 | 1.07 (0.99 to 1.16) | .11 |
| Unknown | 0.91 (0.84 to 1.06) | .23 | 0.96 (0.87 to 1.07) | .47 |
| Received care at a government hospital‖ | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | ||
| Yes | 1.07 (1.03 to 1.12) | .002 | 0.93 (0.89 to 0.98) | .007 |
Among patients enrolled in hospice. CI = confidence interval; OR = odds ratio.
Median household income and high school graduates were obtained by linking patient zip codes to information from the 2000 US Census and the 2008–2012 America Community Survey.
First cancer type was used for patients with multiple primary tumors.
Lung cancer was used as the reference group because of the highest prevalence in the study cohort.
Within 1 year of date of death.
Multivariable regression analysis was used. P values are 2-sided.
Among patients enrolled in hospice, the odds of enrollment less than 7 days vs at least 7 days prior to death were higher among men (OR = 1.36, 95% CI = 1.29 to 1.43; P < .001), urban residents (OR = 1.11, 95% CI = 1.02 to 1.21; P = .02), patients with higher Charlson Comorbidity Index (OR = 1.23, 95% CI = 1.14 to 1.32; P < .001), and married patients (OR = 1.06, 95% CI = 1.01 to 1.11; P = .03) (Table 2). The odds of enrollment in hospice less than 7 days vs at least 7 days prior to death were lower for older patients (OR for patients aged 82–104 years vs 66–69 years = 0.90, 95% CI = 0.83 to 0.96; P = .003), Asian patients (OR = 0.82, 95% CI = 0.72 to 0.93; P = .002), and Hispanic patients (OR = 0.85, 95% CI = 0.76 to 0.96; P = .007) relative to white patients, and patients who received care at a medical school–affiliated hospital (OR = 0.94, 95% CI = 0.89 to 0.98; P = .009) or a government hospital (OR = 0.93, 95% CI = 0.89 to 0.98; P = .007) within 1 year of death.
An interaction model did not reveal statistically significant differences in hospice enrollment over time by sex (Pinteraction = .36), race (Pinteraction > .05 for all races relative to white race), marital status (Pinteraction = .18), or residence (Pinteraction = .42).
Discussion
Our population-level study using the SEER-Medicare database identified an incidence of hospice utilization of 71.4% among elderly patients with brain metastases between 2005 and 2016, with a sustained increase in incidence during the study period. Despite the increasing incidence, a considerable percentage (28.6%) of elderly patients with brain metastases do not enroll in hospice, suggesting continued underutilization in a high-need group. Elderly patients are especially vulnerable to the consequences of brain metastases and brain-directed therapy, and such treatment may have limited efficacy (38). Therefore, utilization of hospice in such patients has considerable appeal.
Whereas prior investigations have evaluated hospice utilization in patients with primary brain tumors or other oncologic conditions on a population-based level, the novelty of our study is that we focused specifically on a population-based cohort of elderly patients with brain metastases. An important prior study on this topic was performed by Dover et al. (18), who evaluated 383 older patients with primary malignant brain tumors and 940 older patients with brain metastases managed within a specific health network and found that nonwhite patients and male patients were more likely not to receive hospice care. No predictive factors for late hospice enrollment were identified. Our study expands on the important work from Dover et al. by providing a larger (n = 50 148) and more generalizable (spanning all SEER registries) study population while also expanding on the robustness of the multivariable models used to predict hospice enrollment.
Our study revealed that among elderly patients with brain metastases who enroll in hospice, a considerable percentage (32.6%) do so in the last few days of life, a period that is focused on improving quality of death rather than allowing patients and their families to fully benefit from the comprehensive hospice services that palliate symptoms during terminal illness (39–41). We found that the incidence of late hospice enrollment has not decreased over time from 2005 to 2016. The underlying reasons for the lack of reduction in late hospice enrollment over time are unclear and may include the inability to adequately predict prognosis, reluctance in accepting hospice services by patients and families, or a delay in referral to hospice care by health-care providers to prolong cancer-directed therapy despite terminal illness (42,43). Prior studies have demonstrated that cancer care at the end of life has become more aggressive in the United States (44). The rate of intensive care unit admission within 30 days of death has increased from 24% in 2000 to 29% in 2015, and late hospice enrollment has become more frequent (45,46). Patients who enroll in hospice at a very late stage may not experience the palliative benefits that it offers, and shorter hospice stays are associated with lower quality of life for patients and worse psychological outcomes for bereaved caregivers (47,48). Some studies have also demonstrated that no appreciable differences exist in quality of life among patients who receive less than 7 days of hospice services and those who were not enrolled in hospice at all, in contrast to those who receive at least 7 days of hospice services (48).
We identified sociodemographic disparities in hospice utilization at the end of life. Specifically, black, Hispanic, and Asian elderly patients with brain metastases were less likely to enroll in hospice prior to death compared with white patients, and such disparities have not decreased between 2005 and 2016. Health-care disparities in nonwhite patients with cancer are well documented (49–54). Five-year relative survival for all cancers at all stages is lower among black patients compared with white patients (51,52). Among patients with breast cancer, black patients and patients of lower socioeconomic status were more likely to present with brain metastases at diagnosis and displayed poorer survival (53). Hispanic patients are less likely to be diagnosed with cancer at a localized stage compared with non-Hispanic patients (54), emphasizing the existence of disparities in accessing timely high-quality cancer care. Our data suggest that such disparities extend to end-of-life care. The mechanisms for these disparities are unclear but may include structural inequality in access to hospice care, cultural and religious preferences, lack of knowledge about the benefits of palliative care and hospice services, and stigma against hospice use or the belief that palliative services hasten death (55). As the population ages substantially in the United States, it is also projected to increase its racial and ethnic diversity (56). Consequently, there is a need to eliminate racial and ethnic disparities in hospice utilization to ensure high-quality end-of-life care for all patients.
The effects of socioeconomic status on the odds of hospice enrollment were too small to be deemed clinically significant in our study. It is important to note that we cannot obtain individual-level socioeconomic status and can only comment on zip code–level estimates of this parameter. However, prior studies have shown that patients with lower socioeconomic status have worse cancer outcomes and higher death rates compared with patients with higher socioeconomic status regardless of demographic characteristics such as race or sex (52). This is likely due to structural barriers in accessing care across all stages from uptake of screening and preventive services to timely provision of curative or palliative services.
We also found that male patients and patients with higher comorbidity were less likely to utilize hospice and more likely to enroll late (within 7 days of death), whereas patients in the oldest age quintile (84–102 years) were more likely to utilize hospice and less likely to enroll late compared with the reference quintile (66–69 years). Younger age and men have been previously associated with lower hospice utilization in other cancer populations (57–60). The reasons are unclear, but prior studies suggest that such disparities may be attributed to more limited end-of-life care discussions among these patient populations (60). Patients with higher comorbidity may less likely enroll in hospice because of the increased complexity of their health issues, increased rates of hospitalization and health-care transitions, or inadequately met needs in hospice (61). Further studies are needed to elucidate the mechanisms by which sex, age, and comorbidity affect hospice utilization.
A limitation of our study is that we are unable to account for patient or family preferences regarding end-of-life care, whether hospice or palliative care referral was discussed but ultimately refused, and whether patients themselves were satisfied with the quality of care that they received. Our study population is limited to primary cancer sites that most commonly metastasize to the brain as opposed to all cancers, and the SEER-Medicare database only includes patients from specific geographic locations (SEER registries), which affects the generalizability of our study. Additionally, claims data cannot be reliably used for identification of metastatic involvement of many organs such as lungs and bones. Accordingly, the National Cancer Institute advises caution regarding identification of metastases after initial cancer diagnosis to metastatic sites, particularly those for which reimbursement is unaffected by diagnostic codes (62). However, because brain metastases are largely managed with local therapy (resection and/or radiation), billing codes are useful indicators for intracranial involvement, and the use of health insurance claims data for identification of brain metastases has been validated with high sensitivity (97%) and specificity (99%) relative to manual chart review (34). As such, SEER-Medicare datasets have been successfully used to identify patients with brain metastases in various studies (63,64). Of note, claims data can also be used to identify the date of diagnosis of brain metastases within 30 days of the true date of diagnosis with 92% sensitivity relative to chart review (65). In our study, 96.2% of patients enrolled in hospice had at least one brain metastases-related claim prior to hospice initiation. However, given the small margin of error in determining the date of diagnosis of brain metastases using claims data and that patients do not typically receive brain imaging while in hospice, we included the remaining 3.8% of patients in our analysis because we cannot definitively determine that they were diagnosed with brain metastases after hospice initiation. Lastly, our statistical results are susceptible to multiple testing concerns given the number of models and predictors evaluated and the lack of a predictor of interest. However, even when using a conservative approach such as the Bonferroni method for adjusting the P value, which may reduce the threshold for statistical significance from .05 to approximately .001 (assuming approximately 50 predictors spanning two models), the odds ratios for racial and ethnic disparities in hospice use remained relevant.
In conclusion, our results demonstrate that a considerable percentage of patients with brain metastases do not enroll in hospice prior to death, and despite the rise in hospice utilization over time, approximately one-third of patients are enrolled too late to fully benefit from hospice services. Our study also identified statistically significant racial and ethnic disparities in hospice utilization with lower rates of hospice enrollment among Asian, black, and Hispanic patients. Further studies are needed to understand factors that contribute to such disparities and to guide interventions that aim to improve access to quality end-of-life care on a patient- and system-based level.
Funding
No funding was required for this study.
Notes
Disclosures: Daniel N. Cagney is a recipient of research support from NH Theraguix. Ayal A Aizer is a recipient of research support from Varian Medical Systems. The authors report no other conflicts of interest.
References
- 1. Huo J, Du XL, Lairson DR, et al. Utilization of surgery, chemotherapy, radiation therapy, and hospice at the end of life for patients diagnosed with metastatic melanoma. Am J Clin Oncol. 2015;38(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Institute of Medicine. Committee on approaching death: addressing key end-of-life issues In: Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2015:1–639. [PubMed] [Google Scholar]
- 3. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Obermeyer Z, Makar M, Abujaber S, et al. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 6. May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness: a meta-analysis. JAMA Intern Med. 2018;178(6):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campion FX, Larson LR, Kadlubek PJ, et al. Advancing performance measurement in oncology: quality oncology practice initiative participation and quality outcomes. J Oncol Pract. 2011;7(suppl 3):31s–35s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Society of Clinical Oncology. Quality Oncology Practice Initiative 2019 Reporting Tracks. https://practice.asco.org/sites/default/files/drupalfiles/QOPI-2019-Round-1-Reporting-Tracks-Public-Posting.pdf. Accessed May 1, 2019.
- 9. Aizer AA, Lee EQ. Brain metastases. Neurol Clin. 2018;36(3):557–577. [DOI] [PubMed] [Google Scholar]
- 10. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 11. Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pelletier EM, Shim B, Goodman S, et al. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat. 2008;108(2):297–305. [DOI] [PubMed] [Google Scholar]
- 13. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 14. Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 15. Bergman J, Saigal CS, Lorenz KA, et al. ; for the Urologic Diseases in America Project. Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med. 2011;171(3):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diamond EL, Russell D, Kryza-Lacombe M, et al. Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol. 2016;18(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dover LL, Dulaney CR, Fiveash JB, et al. Hospital-based end-of-life care and costs for older patients with malignant brain tumors. JAMA Oncol. 2017;3(11):1581–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dover LL, Dulaney CR, Williams CP, et al. Hospice care, cancer-directed therapy, and Medicare expenditures among older patients dying with malignant brain tumors. Neuro Oncol. 2018;20(7):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fairfield KM, Murray KM, Wierman HR, et al. Disparities in hospice care among older women dying with ovarian cancer. Gynecol Oncol. 2012;125(1):14–18. [DOI] [PubMed] [Google Scholar]
- 20. Keating NL, Herrinton LJ, Zaslavsky AM, et al. Variations in hospice use among cancer patients. J Natl Cancer Inst. 2006;98(15):1053–1059. [DOI] [PubMed] [Google Scholar]
- 21. Keating NL, Landrum MB, Guadagnoli E, et al. Care in the months before death and hospice enrollment among older women with advanced breast cancer. J Gen Intern Med. 2008;23(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuchinad KE, Strowd R, Evans A, et al. End of life care for glioblastoma patients at a large academic cancer center. J Neurooncol. 2017;134(1):75–81. [DOI] [PubMed] [Google Scholar]
- 23. Lackan NA, Ostir GV, Freeman JL, et al. Decreasing variation in the use of hospice among older adults with breast, colorectal, lung, and prostate cancer. Med Care. 2004;42(2):116–122. [DOI] [PubMed] [Google Scholar]
- 24. McCarthy EP, Burns RB, Ngo-Metzger Q, et al. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238–2245. [DOI] [PubMed] [Google Scholar]
- 25. Saito AM, Landrum MB, Neville BA, et al. Hospice care and survival among elderly patients with lung cancer. J Palliat Med. 2011;14(8):929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stavas M, Arneson K, Friedman J, et al. From whole brain to hospice: patterns of care in radiation oncology. J Palliat Med. 2014;17(6):662–666. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Incidence Data, 1975-2016 https://seer.cancer.gov/data. Accessed April 26, 2019.
- 28.US Centers for Medicare & Medicaid Services. What’s Medicare? https://www.medicare.gov/what-medicare-covers/your-medicare-coverage-choices/whats-medicare. Accessed December 6, 2019.
- 29.The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. 2019 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds; 2019.
- 30. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 31. Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer. 2005;5(2):108–113. [DOI] [PubMed] [Google Scholar]
- 32. Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. W J Gastroenterol. 2011;17(19):2407–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen TD, Palshof JA, Larsen FO, et al. Risk factors for brain metastases in patients with metastatic colorectal cancer. Acta Oncol. 2017;56(5):639–645. [DOI] [PubMed] [Google Scholar]
- 34. Eichler AF, Lamont EB. Utility of administrative claims data for the study of brain metastases: a validation study. J Neurooncol. 2009;95(3):427–431. [DOI] [PubMed] [Google Scholar]
- 35. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 36. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 37. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 38. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Michael N, Beale G, O’Callaghan C, et al. Timing of palliative care referral and aggressive cancer care toward the end-of-life in pancreatic cancer: a retrospective, single-center observational study. BMC Palliat Care. 2019;18(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christakis NA, Iwashyna TJ. The health impact of health care on families: a matched cohort study of hospice use by decedents and mortality outcomes in surviving, widowed spouses. Soc Sci Med. 2003;57(3):465–475. [DOI] [PubMed] [Google Scholar]
- 41. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88–93. [DOI] [PubMed] [Google Scholar]
- 42. Johnson CB, Slaninka SC. Barriers to accessing hospice services before a late terminal stage. Death Stud. 1999;23(3):225–238. [DOI] [PubMed] [Google Scholar]
- 43. Schockett ER, Teno JM, Miller SC, et al. Late referral to hospice and bereaved family member perception of quality of end-of-life care. J Pain Symptom Manage. 2005;30(5):400–407. [DOI] [PubMed] [Google Scholar]
- 44. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315–321. [DOI] [PubMed] [Google Scholar]
- 46. Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kris AE, Cherlin EJ, Prigerson H, et al. Length of hospice enrollment and subsequent depression in family caregivers: 13-month follow-up study. Am J Geriatr Psychiatry. 2006;14(3):264–269. [DOI] [PubMed] [Google Scholar]
- 48. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Institute of Medicine. Committee on understanding and eliminating racial and ethnic disparities in health care In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003:1–781. [PubMed] [Google Scholar]
- 50. Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120(10):1532–1539. [DOI] [PubMed] [Google Scholar]
- 51.American Cancer Society. Cancer Facts & Figures 2014. American Cancer Society; 2014. [Google Scholar]
- 52. DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. [DOI] [PubMed] [Google Scholar]
- 53. Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2018-2020. American Cancer Society; 2018. [Google Scholar]
- 55. Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med. 2013;16(11):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grayson VK, Velkoff VA. The Next Four Decades, the Older Population in the United States: 2010 to 2050. Washington, DC: U.S. Census Bureau; 2010. [Google Scholar]
- 57. Forst D, Adams E, Nipp R, et al. Hospice utilization in patients with malignant gliomas. Neuro Oncol. 2018;20(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tramontano AC, Nipp R, Kong CY, et al. Hospice use and end-of-life care among older patients with esophageal cancer. Health Sci Rep. 2018;1(9):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. O’Connor NR, Hu R, Harris PS, et al. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma RK, Prigerson HG, Penedo FJ, et al. Male-female patient differences in the association between end-of-life discussions and receipt of intensive care near death. Cancer. 2015;121(16):2814–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legler A, Bradley EH, Carlson MD. The effect of comorbidity burden on health care utilization for patients with cancer using hospice. J Palliat Med. 2011;14(6):751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Cancer Institute. SEER-Medicare Linked Database. https://healthcaredelivery.cancer.gov/seermedicare/considerations/measures.html#13. Accessed August 2, 2019.
- 63. Cahill KS, Chi JH, Day AL, et al. Trends in survival after surgery for breast cancer metastatic to the brain and spinal column in Medicare patients: a population-based analysis. Neurosurgery. 2011;68(3):705–713; discussion 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Halasz LM, Weeks JC, Neville BA, et al. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lamba N, Kearney RB, Mehanna E, et al. Utility of claims data for identification of date of diagnosis of brain metastases. Neuro Oncol. 2020;22(4):575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]