Abstract
Background
Adjuvant trastuzumab for early-stage (I-III) HER2-positive breast cancer (BC) has led to statistically significant improvement in cancer outcomes but carries a risk of cardiotoxicity. Trastuzumab is discontinued early in many patients for asymptomatic changes in left ventricular ejection fraction. We evaluated the impact of early discontinuation of trastuzumab on cancer outcomes.
Methods
We conducted a retrospective population-based cohort study of early BC patients treated with adjuvant trastuzumab in Ontario, Canada, 2007-2016. Four groups were analyzed: group A was full treatment, 17-18 cycles trastuzumab; group B was cardiac event (CE) within treatment period; group C was ≤16 cycles, no CEs, stopped within 30 days from last cardiac imaging; and group D was ≤16 cycles, no CEs, stopped more than 30 days from cardiac imaging. Primary outcome was disease-free survival (DFS); secondary outcomes were: overall survival, cancer-specific mortality, and cardiovascular mortality. Sensitivity analyses were performed 14 months after cycle 1 trastuzumab to control for early relapse.
Results
A total of 5547 patients met the inclusion criteria: group A = 3921, group B = 309, group C = 362, and group D = 955. The 5-year DFS was 94.1% in group A, 80.1% in group B, 81.4% in group C, and 82.4% in group D. Using a Cox model, the hazard ratio for 5-year DFS was 3.15 (95% confidence interval [CI] = 2.13 to 4.65) for group B, 1.94 (95% CI = 1.30 to 2.89) for group C, and 1.92 (95% CI = 1.46 to 2.53) for group D. Overall, 26 patients (0.5%) died of cardiac causes.
Conclusions
BC patients in Ontario who did not complete adjuvant trastuzumab had a statistically significantly higher risk of BC relapse and death and low incidence of cardiac death. These findings support 1 year of adjuvant trastuzumab in early-stage BC.
Since the early 2000s, 1 year of adjuvant trastuzumab has been standard of care for patients with HER2-positive early breast cancer (BC) (1,2). Clinically significant rates of heart failure (HF) were observed when trastuzumab was administered concurrently with anthracyclines in advanced BC, leading to adoption of intensive cardiac monitoring in the adjuvant setting. International guidelines recommend cardiac monitoring every 3-4 months for early-stage (I-III) BC patients receiving adjuvant trastuzumab (3–6). Despite almost 15 years of clinical experience with trastuzumab, there is little high-quality evidence to support the clinical utility of routine cardiac monitoring in preventing long-term cardiac dysfunction or optimizing cancer care in this patient population (7,8).
The risk of clinically significant HF with adjuvant trastuzumab is low. A meta-analysis of adjuvant trastuzumab trials demonstrated the risk of symptomatic HF was 2.5% (range = 0%-4%); 11.2% of patients experienced an asymptomatic drop in left ventricular ejection fraction (LVEF) (1). A recent population-based study found that the cumulative incidence of HF in BC patients who received chemotherapy and trastuzumab was 3.08% compared with 0.96% in an age-matched sample of women without breast cancer (9). Trastuzumab-associated cardiotoxicity, particularly when administered without anthracyclines, is often considered reversible. Long-term follow-up of the pivotal adjuvant trastuzumab trials has failed to demonstrate an increased risk of cardiotoxicity (10–15) after trastuzumab completion. Outside of clinical trials, the evidence on long-term risk is mixed, with some evidence showing that the HF risk did not extend beyond 1.5 years after initiation of therapy (16), whereas elsewhere the risk was shown to persist up to 5 years after completing trastuzumab (17).
Concerns about cardiotoxicity can lead to disruptions in the curative treatment plan for HER2+ BC patients. In our own experience with patients in a dedicated cardio-oncology clinic, 33% of patients experienced delays in trastuzumab therapy because of asymptomatic declines in LVEF detected during routine cardiac monitoring (18) and 15% had trastuzumab terminated for cardiotoxicity.
In Ontario, Canada, the majority (75%) of early BC patients undergo cardiac screening every 3-4 months during treatment with adjuvant trastuzumab (19). We hypothesized that there is a population of patients who have trastuzumab discontinued early because of asymptomatic changes in cardiac function, potentially compromising cancer outcomes. We conducted a retrospective population-based cohort study in BC patients who received adjuvant trastuzumab in Ontario, Canada, to assess the impact of stopping trastuzumab early on disease-free survival (DFS) and overall survival (OS) as well as cancer and cardiac specific mortality.
Methods
Study Design
This was a retrospective population-based cohort study of early-stage (I-III) BC patients, aged 18 years or older, who received their first cycle of adjuvant trastuzumab in Ontario, Canada, between January 1, 2007, and March 31, 2016. The main exposure was trastuzumab-based therapy, calculated as the number of (every 3 weeks) cycles each patient received. The secondary exposure was development of a cardiac event (CE) between first treatment and 90 days after completion of trastuzumab. A clinically significant CE was defined as a new diagnosis of HF, pulmonary edema, or cardiomyopathy. We categorized patients into 4 groups based on the number of completed cycles of trastuzumab and incidence of CEs.
The comparator arm—group A—completed a full course of adjuvant trastuzumab (17-18 cycles) without any CEs. Group B had a CE between first dose and 90 days after completion of trastuzumab. Group C had no CEs but received 16 or fewer cycles of trastuzumab and stopped within 30 days of their last cardiac imaging. Group D had no CEs, they received 16 or fewer cycles of trastuzumab, and treatment was discontinued more than 30 days since last cardiac imaging. We initially planned the study with only arms A, B, and C in an effort to isolate patients with asymptomatic changes in LVEF (group C). However, the protocol was revised after finding a large population that did not fit our prespecified cohort definitions.
Data Sources
Patients were identified using health administrative databases in Ontario, Canada, that contain patient-level information on cancer diagnosis and cancer drug administration as well as inpatient and outpatient data, cancer registry data, and demographics. Deidentified databases were accessed through the Institute for Clinical Evaluative Sciences (ICES), and all data sources were linked through a unique encrypted identifier and analyzed at ICES. ICES is an independent, nonprofit research institute funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health-care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures approved by the Information and Privacy Commissioner of Ontario. Details on the databases used in this study are presented in the Supplementary Methods (available online). Ethics approval was obtained from the Ottawa Health Sciences Research Ethics Board, Ottawa, Canada.
Patients
All patients in Ontario, Canada, with early-stage (I-III) HER2-positive BC, aged 18 years or older, who initiated adjuvant trastuzumab-based therapy between January 1, 2007, and March 31, 2016 were included. Patients were excluded if they had a diagnosis of HF or cardiomyopathy within 12 months before trastuzumab treatment or were treated with neoadjuvant trastuzumab. Only 1 patient was lost to follow-up.
Endpoints
The primary endpoint in this study was DFS, defined as time between initial BC diagnosis and new diagnosis of BC, disease recurrence (defined as starting new systemic therapy ≥6 months after completion of trastuzumab), or death from any cause (20). Secondary endpoints were OS, BC-specific mortality, cardiovascular morality, and noncancer mortality.
Statistical Methods
Descriptive statistics were performed to report baseline characteristics of patients in each group. Categorical variables were compared using χ2 test or Fisher exact test; continuous variables were compared using an analysis of variance. Statistical significance was tested at an alpha of .05. All tests were 2-sided.
For survival outcomes, Kaplan-Meier analysis was performed. Cumulative incidence functions were performed for disease-specific mortality (BC and cardiovascular). The 5-year survival estimates were calculated with 95% confidence intervals (CI). Survival outcomes were reported as an overall result for each group and also stratified by stage to compare results for stages I, II, and III.
Cox-proportional hazards (PH) model was used to adjust for all the covariates (age at treatment, disease stage, estrogen receptor or progesterone receptor status, Charlson comorbidity index, anthracycline treatment history [yes or no], cardiologist visit history, teaching vs community hospital, and neighborhood income quintile) to estimate the hazard risk of DFS and OS in arms B, C, and D compared with arm A. The PH model assumptions were tested by calculating Martingale and Schoenfeld residuals. Subdistribution hazard models were performed to estimate hazard risk of disease-specific mortality to adjust for all covariates in arms B, C, and D compared with arm A. Results are presented as hazard ratios (HRs) with 95% confidence intervals. The analyses were performed with SAS Software (version 9.4.3.0).
Sensitivity analysis was performed on the results by repeating survival analyses 14 months after initiation of trastuzumab. In Ontario, Canada, where the study population was based, treatment costs are covered by Cancer Care Ontario, which mandates that providers have a maximum of 14 months to provide 18 cycles of trastuzumab. This was done as an internal control for early relapse.
Results
Patient Characteristics
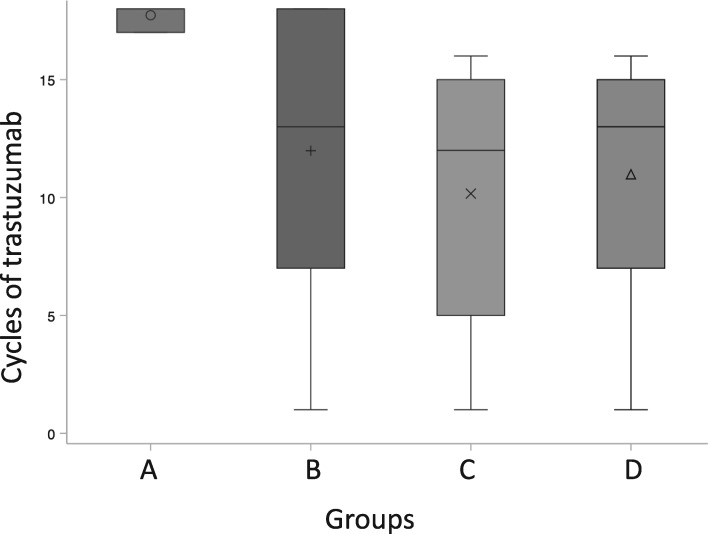
There were 7588 patients, aged 18 years or older, with early-stage HER2-positive BC started on adjuvant trastuzumab in Ontario, Canada, between January 1, 2007, and March 31, 2016. A total of 34 patients were excluded for history of HF and 2007 were excluded because they were treated with neoadjuvant trastuzumab, leaving 5547 for analysis. There were 3921 patients who met the criteria for group A (70.7%), 309 for group B (5.6%), 362 for group C (6.5%), and 955 for group D (17.2%). Baseline characteristics are listed in Table 1. The median age at diagnosis for the entire group was 56 (±11) years. Median cycles of trastuzumab in each arm were 18, 13, 12, and 13, respectively (see Figure 1). All 4 arms had a median of 4 cardiac imaging tests performed. The median follow-up was 6.4 years for the entire cohort, 6.8 years for group A, 5.7 years for group B, and 5.2 years each for groups C and D.
Table 1.
Baseline characteristics of study population
| Variable and value | Group Aa | Group Bb | Group Cc | Group Dd | Total | P e |
|---|---|---|---|---|---|---|
| Patients, No. (%)f | 3,921 (70.7) | 309 (5.6) | 362 (6.5) | 955 (17.2) | 5547 | |
| Follow-up time, median (IQR), y | 6.83 (4.80-8.82) | 5.67 (3.35-8.33) | 5.15 (3.98-8.00) | 5.18 (3.93-7.59) | 6.41 (4.40-8.66) | <.001 |
| Age, y | ||||||
| Median (IQR) | 55.00 (48.00-64.00) | 58.00 (50.00-67.00) | 57.00 (49.00-66.00) | 56.00 (48.00-66.00) | 56.00 (48.00-64.00) | <.001 |
| <40, No. (%) | 342 (8.7) | 10 (3.2) | 18 (5.0) | 80 (8.4) | 450 (8.1) | <.001 |
| 40-49, No.(%) | 955 (24.4) | 68 (22.0) | 91 (25.1) | 243 (25.4) | 1357 (24.5) | |
| 50-59, No. (%) | 1268 (32.3) | 98 (31.7) | 105 (29.0) | 267 (28.0) | 1738 (31.3) | |
| 60-69, No. (%) | 977 (24.9) | 87 (28.2) | 94 (26.0) | 218 (22.8) | 1376 (24.8) | |
| 70-79, No. (%) | 347 (8.8) | 40 (12.9) | 49 (13.5) | 125 (13.1) | 561 (10.1) | |
| ≥80, No. (%) | 32 (0.8) | —g | — | 22 (2.3) | 65 (1.2) | |
| Charlson comorbidity index, No. (%) | ||||||
| 0 | 1793 (45.7) | 127 (41.1) | 167 (46.1) | 460 (48.2) | 2547 (45.9) | .30 |
| 1-2 | 609 (15.5) | 55 (17.8) | 50 (13.8) | 154 (16.1) | 868 (15.6) | |
| ≥3 | 1519 (38.7) | 127 (41.1) | 145 (40.1) | 341 (35.7) | 2132 (38.4) | |
| Nearest census-based neighborhood income quintile | ||||||
| Lowest income | 610 (15.6) | 68 (22.0) | 62 (17.1) | 166 (17.4) | 906 (16.3) | <.001 |
| Q2 | 739 (18.8) | 51 (16.5) | 66 (18.2) | 166 (17.4) | 1022 (18.4) | |
| Q3 | 826 (21.1) | 51 (16.5) | 86 (23.8) | 194 (20.3) | 1157 (20.9) | |
| Q4 | 873 (22.3) | 50 (16.2) | 72 (19.9) | 211 (22.1) | 1206 (21.7) | |
| Highest income | 858 (21.9) | 78 (25.2) | 63 (17.4) | 193 (20.2) | 1192 (21.5) | |
| Missing | 15 (0.4) | 11 (3.6) | 13 (3.6) | 25 (2.6) | 64 (1.2) | |
| Hospital setting, No. (%) | ||||||
| Community | 2,99 (56.1) | 175 (56.6 | 222 (61.3) | 582 (60.9 | 3178 (57.3) | .02 |
| Teaching | 1722 (43.9) | 134 (43.4) | 140 (38.7) | 373 (39.1) | 2369 (42.7) | |
| Estrogen receptor status, No. (%) | ||||||
| Negative | 801 (20.4) | 69 (22.3) | 77 (21.3) | 211 (22.1) | 1158 (20.9) | .58 |
| Positive | 1423 (36.3) | 116 (37.5) | 138 (38.1) | 361 (37.8) | 2038 (36.7) | |
| Missing | 1697 (43.3) | 124 (40.1) | 147 (40.6) | 383 (40.1) | 2351 (42.4) | |
| Progesterone receptor status, No. (%) | ||||||
| Negative | 1120 (28.6) | 93 (30.1) | 112 (30.9) | 299 (31.3) | 1624 (29.3) | .51 |
| Positive | 1104 (28.2) | 92 (29.8) | 103 (28.5) | 273 (28.6) | 1572 (28.3) | |
| Missing | 1697 (43.3) | 124 (40.1) | 147 (40.6) | 383 (40.1) | 2,351 (42.4) | |
| Breast cancer stage, No. (%) | ||||||
| Stage I | 1054 (26.9) | 67 (21.7) | 75 (20.7) | 206 (21.6) | 1402 (25.3) | <.001 |
| Stage II | 1645 (42.0) | 133 (43.0) | 135 (37.3) | 372 (39.0) | 2285 (41.2) | |
| Stage III | 679 (17.3) | 62 (20.1 | 77 (21.3) | 183 (19.2) | 1001 (18.0) | |
| Missing | 543 (13.8) | 47 (15.2) | 75 (20.7) | 194 (20.3) | 859 (15.5) | |
| No. of trastuzumab cycles | ||||||
| Mean ± SD | 17.73 ± 0.44 | 11.98 ± 5.71 | 10.17 ± 5.40 | 10.98 ± 4.96 | 15.76 ± 4.19 | <.001 |
| Median (IQR) | 18.00 (17.00-18.00) | 13.00 (7.00-18.00) | 12.00 (5.00-15.00) | 13.00 (7.00-15.00) | 18.00 (16.00-18.00) | <.001 |
| Prior anthracycline treatment, No. (%) | ||||||
| No | 1846 (47.1 | 159 (51.5) | 191 (52.8) | 544 (57.0) | 2740 (49.4) | <.001 |
| Yes | 2075 (52.9) | 150 (48.5) | 171 (47.2) | 411 (43.0) | 2807 (50.6) | |
| Cardiac test within 30 d of last trastuzumab cycle, No. (%) | ||||||
| Missing or no imaging | 284 (7.2) | 26 (8.4) | 0 (0.0) | 216 (22.6) | 526 (9.5) | <.001 |
| No | 2463 (62.8) | 180 (58.3) | 0 (0.0) | 739 (77.4) | 3382 (61.0) | |
| Yes | 1174 (29.9) | 103 (33.3) | 362 (100.0) | 0 (0.0) | 1639 (29.5) | |
| Cardiologist visit during study period, No. (%) | ||||||
| No | 3540 (90.3) | 79 (25.6) | 290 (80.1) | 850 (89.0) | 4759 (85.8) | <.001 |
| Yes | 381 (9.7) | 230 (74.4) | 72 (19.9) | 105 (11.0) | 788 (14.2) | |
| No. of cardiac imaging tests | ||||||
| Median (IQR) | 4.00 (4.00-5.00) | 4.00 (2.00-6.00) | 4.00 (2.00-5.00) | 2.00 (1.00-4.00) | 4.00 (3.00-5.00) | <.001 |
| Cause of death, No. (%) | ||||||
| All-cause mortality | 234 (6.0) | 50 (16.2) | 68 (18.8) | 171 (17.9) | 523 (9.4) | <.001 |
| Cancer mortality | 110 (2.8) | 21 (6.8) | 34 (9.4) | 102 (10.7) | 267 (4.8) | |
| Cardiac mortality | 11 (0.3) | — | — | 7 (0.7) | 26 (0.5) | |
Group A received a full course of trastuzumab (17-18 cycles) without any cardiac events between day 1, cycle 1, and 90 days after the final cycle. CI = confidence interval; IQR = interquartile range.
Group B had a cardiac event within the study period.
Group C had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment within 30 days of the last cardiac imaging test.
Group D had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment more than 30 days after the last cardiac imaging test.
P values were calculated using χ2 test or Fisher exact test for categorical variables; continuous variables were compared using an analysis of variance.
Number of patients per group are row percentages; all other percentages listed are column percentages.
In instances where n is ≤5, values are suppressed per ICES policy.
Figure 1.
Box plot displaying distribution of cycles of trastuzumab in groups A-D. Group A received a full course of trastuzumab (17-18 cycles) without any (CEs) between day 1, cycle 1, and 90 days after the final cycle. Group B had a CE within the study period. Group C had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment within 30 days of the last cardiac imaging test. Group D had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment more than 30 days after the last cardiac imaging test. Symbolson each boxplot indicate the mean value, horizantal line the median and the boxes represent the interquartile range of values for each group. Whiskers above and below the boxes represent the minimum and maximum values for each group.
DFS and OS
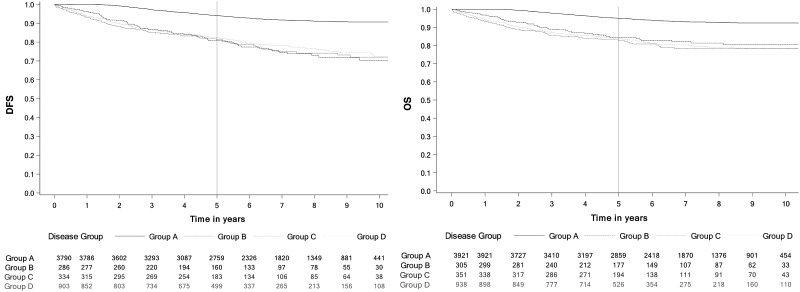
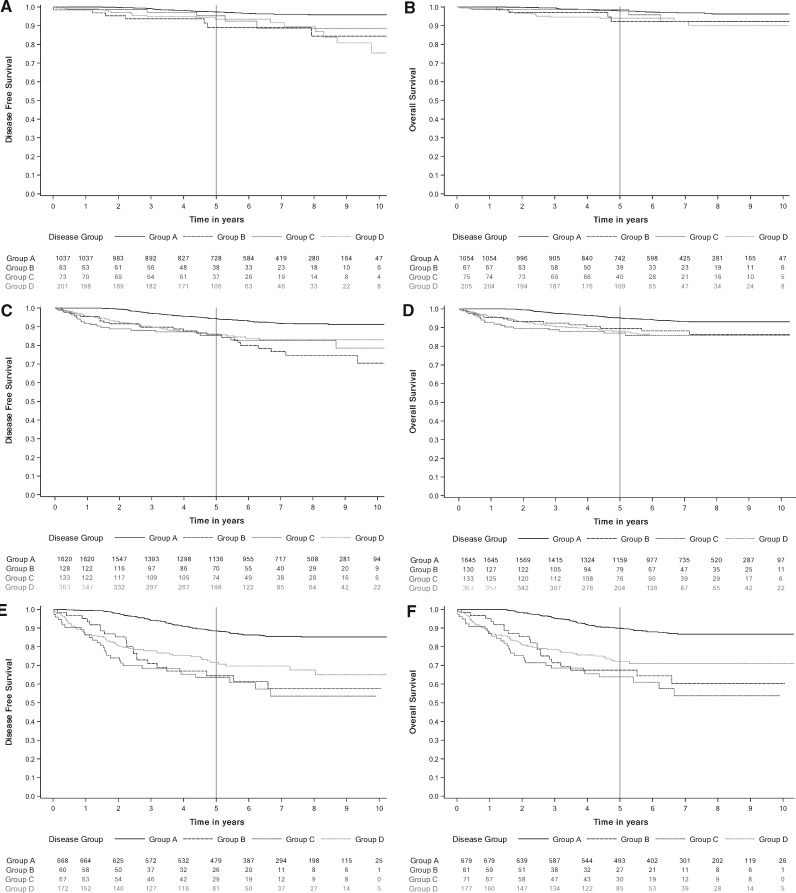
For the primary endpoint, all groups had statistically significantly lower estimated 5-year DFS compared with group A. The 5-year DFS was estimated to be 94.1% (95% CI = 93.3% to 94.9%) in group A compared with 80.1% (95% CI = 74.8% to 84.6%) in group B, 81.4% (95% CI = 76.7% to 85.2%) in group C, and 82.4% (95% CI = 79.7% to 84.8%) in group D. Figure 2 shows Kaplan-Meier survival estimates for DFS and OS. Five-year survival estimates are listed in Table 2 along with estimates from the sensitivity analysis for survival 14 months after initiation of trastuzumab. OS was statistically significantly lower in all groups (B, C, D) compared with arm A. The 5-year OS was estimated to be 95.0% (95% CI = 94.3% to 95.7%) in group A vs 84.4% (95% CI = 79.6% to 88.2%) in group B, 82.8% (95% CI = 78.4% to 86.4%) in group C, and 83.7% (95% CI = 81.2% to 86.0%) in group D. A similar difference in DFS and OS estimates was seen during the sensitivity analysis done with time zero starting 14 months after initiation of trastuzumab (see Table 2 for DFS and OS estimates). Kaplan-Meier curves illustrating DFS and OS are presented in Figure 2. Kaplan-Meier curves for DFS and OS stratified by stage are presented in Figure 3.
Figure 2.
Kaplan-Meier curves disease-free survival (DFS) and overall survival (OS) to estimate survival for arms A-D. A) DFS. B) OS. Group A received a full course of trastuzumab (17-18 cycles) without any cardiac events (CEs) between day 1, cycle 1, and 90 days after the final cycle. Group B had a CE within the study period. Group C had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment within 30 days of the last cardiac imaging test. Group D had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment more than 30 days after the last cardiac imaging test.
Table 2.
Five-year survival estimates and cumulative incidence estimates from time of initiation of trastuzumab and sensitivity analysis 14 months after initiation of trastuzumab
| Study outcome | Primary analysis, % (95% CI) | Sensitivity analysis, % (95% CI) |
|---|---|---|
| 5-year survival estimates | ||
| DFS | ||
| Group Aa | 94.1 (93.3 to 94.9) | 92.6 (91.6 to 93.5) |
| Group Bb | 80.2 (74.8 to 84.6) | 80.6 (74.7 to 85.3) |
| Group Cc | 81.4 (76.7 to 85.2) | 84.9 (79.7 to 88.8) |
| Group Dd | 82.4 (79.7 to 84.8) | 84.7 (81.7 to 87.2) |
| OS | ||
| Group A | 95.0 (94.3 to 95.7) | 93.7 (92.9 to 94.5) |
| Group B | 84.4 (79.6 to 88.2) | 86.2 (81.2 to 89.9) |
| Group C | 82.8 (78.4 to 86.4) | 86.7 (82.0 to 90.3) |
| Group D | 83.7 (81.2 to 86.0) | 86.3 (83.5 to 88.7) |
| 5-year cumulative incidence estimates | ||
| Cancer-specific death | ||
| Group A | 2.7 (2.2 to 3.3) | 3.1 (2.5 to 3.7) |
| Group B | 6.5 (4.0 to 9.7) | 6.0 (3.5 to 9.3) |
| Group C | 9.2 (6.5 to 12.5) | 6.5 (4.0 to 9.8) |
| Group D | 10.2 (8.3 to 12.2) | 7.7 (5.8 to 9.8) |
| Cardiovascular-specific death | ||
| Group A | 0.3 (0.1 to 0.5) | 0.3 (0.1 to 0.5) |
| Group B | 2.0 (0.7 to 4.4) | 2.1 (0.8 to 4.6) |
| Group C | 0.6 (0.1 to 1.9) | 0.3 (0.0 to 1.7) |
| Group D | 0.4 (0.2 to 1.1) | 0.7 (0.2 to 1.8) |
Group A received a full course of trastuzumab (17-18 cycles) without any cardiac events between day 1, cycle 1, and 90 days after the final cycle. CI = confidence interval; DFS = disease-free survival; OS = overall survival.
Group B had a cardiac event within the study period.
Group C had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment within 30 days of the last cardiac imaging test.
Group D had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment more than 30 days after the last cardiac imaging test.
Figure 3.
Kaplan-Meier curves for disease-free survival (DFS) and overall survival (OS) stratified by stage. A) Stage I DFS. B) Stage I OS. C) Stage II DFS. D) Stage II OS. E) Stage III DFS. F) Stage III OS. Group A received a full course of trastuzumab (17-18 cycles) without any cardiac events (CEs) between day 1, cycle 1, and 90 days after the final cycle. Group B had a CE within the study period. Group C had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment within 30 days of the last cardiac imaging test. Group D had 16 or fewer cycles of trastuzumab, had no CEs, and stopped treatment more than 30 days after the last cardiac imaging test.
The PH model was used to calculate hazard ratios for groups B, C, and D compared with group A accounting for baseline patient covariates (see Table 3 for primary and sensitivity analysis). When the PH assumptions were tested, the calculated Martingale residuals deviated from what was observed for groups C and D and the Schoenfeld residuals were not independent of time in groups C and D. To account for the nonproportionality, instead of 1 hazard ratio estimate, we have reported 4 time-point hazard ratio estimates at 6 months, 1 year, 5 years, and 10 years, which are each statistically valid, although change with time as can be seen in Table 3. Groups B-D had a statistically significantly increased risk of DFS or OS events at time points of 6 months, 1 year, and 5 years (except group C for OS), but by 10 years the effect was lost. The hazard ratio for 5-year DFS was 3.15 (95% CI = 2.13 to 4.65, P < .001) in group B, 1.94 (95% CI = 1.30 to 2.89, P = .001) in group C, and 1.92 (95% CI = 1.46 to 2.53, P < .001) in group D. The hazard ratio for 5-year OS was 2.12 for group B (95% CI = 1.25 to 3.58, P = .005), 1.24 (95% CI = 0.72 to 2.13, P = .44) for group C, and 1.56 (95% CI = 1.11 to 2.18, P = .01) for group D. In the adjusted survival analysis, older age (>70 years), higher disease stage, Charlson comorbidity score greater than 3, and not completing adjuvant chemotherapy had statistically significantly increased hazard ratios for DFS. A full list of hazard ratios for all variables can be found in the Supplementary Tables 1-4 (available online).
Table 3.
Hazard ratios for DFS and OS calculated for both standard analysis and sensitivity analysis at 14 months after cycle 1 trastuzumab using Cox model
| Group Ba |
Group Cb |
Group Dc |
|||||
|---|---|---|---|---|---|---|---|
| Study outcome | HRd (95% CI) | P e | HRd (95% CI) | P e | HRd (95% CI) | P e | |
| Standard analysis | |||||||
| Time since 1st cycle trastuzumab | |||||||
| DFS | 6 mo | 5.3 (3.32 to 8.58) | <.001 | 6.13 (4.04 to 9.30) | <.001 | 5.30 (3.83 to 7.32) | <.001 |
| 1 y | 5.03 (3.27 to 7.73) | <.001 | 5.39 (3.73 to 7.79) | <.001 | 4.73 (3.55 to 6.31) | <.001 | |
| 5 y | 3.15 (2.13 to 4.65) | <.001 | 1.94 (1.30 to 2.89) | .001 | 1.92 (1.46 to 2.52) | <.001 | |
| 10 y | 1.75 (0.69 to 4.40) | .24 | 0.54 (0.20 to 1.48) | .23 | 0.62 (0.31 to 1.24) | .18 | |
| OS | 6 mo | 8.11 (4.71 to 13.98) | <.001 | 11.76 (7.45 to 18.56) | <.001 | 9.32 (6.44 to 13.49) | <.001 |
| 1 y | 6.99 (4.31 to 11.33) | <.001 | 9.16 (6.18 to 13.57) | <.001 | 7.64 (5.54 to 10.54) | <.001 | |
| 5 y | 2.12 (1.25 to 3.58) | .01 | 1.24 (0.72 to 2.13) | .44 | 1.56 (1.11 to 2.18) | .01 | |
| 10 y | 0.48 (0.13 to 1.75) | .26 | 0.10 (0.03 to 0.40) | .001 | 0.21 (0.09 to 0.52) | <.001 | |
| Sensitivity analysis | |||||||
| Time since 1st cycle trastuzumab + 14 mo | |||||||
| DFS | 6 mo | 2.96 (1.81 to 4.82) | <.001 | 2.11 (1.31 to 3.38) | .002 | 2.03 (1.45 to 2.82) | <.001 |
| 1 y | 2.94 (1.90 to 4.55) | <.001 | 2.09 (1.38 to 3.17) | <.001 | 2.01 (1.50 to 2.69) | <.001 | |
| 5 y | 2.78 (1.68 to 4.62) | <.001 | 1.94 (1.18 to 3.20) | .01 | 1.89 (1.34 to 2.66) | .001 | |
| 10 y | 2.60 (0.78 to 8.63) | .12 | 1.78 (0.51 to 6.21) | .37 | 1.74 (0.74 to 4.12) | .20 | |
| OS | 6 mo | 3.61 (2.09 to 6.21) | <.001 | 3.31 (2.02 to 5.43) | <.001 | 2.92 (2.05 to 4.16) | <.001 |
| 1 y | 3.31 (2.05 to 5.35) | <.001 | 2.96 (1.94 to 4.51) | <.001 | 2.74 (2.02 to 3.71) | <.001 | |
| 5 y | 1.68 (0.82 to 3.47) | .16 | 1.21 (0.59 to 2.48) | .61 | 1.61 (1.04 to 2.51) | .03 | |
| 10 y | 0.72 (0.13 to 4.04) | .71 | 0.39 (0.07 to 2.31) | .30 | 0.83 (0.28 to 2.53) | .75 | |
Group B had a cardiac event within the study period. CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; OS = overall survival.
Group C had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment within 30 days of the last cardiac imaging test.
Group D had 16 or fewer cycles of trastuzumab, had no cardiac events, and stopped treatment more than 30 days after the last cardiac imaging test.
Hazard ratios are adjusted for age at treatment, Charlson comorbidity index, hospital type (teaching or community), breast cancer stage, estrogen receptor status, progesterone receptor status, neighborhood income quintile, rurality, anthracycline treatment history, and cardiologist visit history.
P values were calculated using 2-sided Wald χ2 test.
In total, 9.4% (523 of 5547) of patients died during the study follow-up period. Group A had 234 (6.0%) deaths compared with 50 (16.2%) deaths in group B, 68 (18.8%) deaths in group C, and 171 (17.9%) deaths in group D. The majority of the 523 deaths were related to cancer (267 of 523), with 5.0% of deaths due to cardiac causes (26 of 523).
BC-Specific Mortality
A statistically significant difference between groups B, C, and D compared with group A was found in the incidence of BC-specific mortality. Estimating 5-year BC-specific mortality was done using a cumulative incidence function: group A had 2.7% (95% CI = 2.2% to 3.3%) compared with 6.5% in group B (95% CI = 4.0% to 9.7%), 9.2% in group C (95% CI = 6.5% to 12.5%), and 10.2% (95% CI = 8.3% to 12.2%) in group D. Using a subdistribution model for risk analysis to control for covariates, the hazard ratio for group B was 3.30 (95% CI = 1.89 to 5.75, P < .001), group C was 3.47 (95% CI = 2.31 to 5.20, P < .001), and group D was 4.11 (95% CI = 3.12 to 5.41, P < .001).
Cardiovascular Mortality
Although we planned an analysis to examine cardiovascular mortality, there was a very small number (n = 26, 0.5%) of cardiovascular deaths in the entire population. Using the subdistribution model, there was an increased risk of cardiovascular mortality in group B compared with group A (HR = 3.64, 95% CI = 1.04 to 12.70, P = .04), which just met statistical significance although the absolute difference was very low.
Discussion
We examined real-world outcomes of patients treated with adjuvant trastuzumab therapy in Ontario, Canada. We were able to identify 3 groups of patients who did not complete a standard course of adjuvant trastuzumab (17-18 cycles) treatment. In the absence of patient-level data on cardiac monitoring (ie, LVEF results), we attempted to infer causality as to why patients stopped trastuzumab prematurely. In group B, patients developed a clinically significant CE, so we assumed trastuzumab was stopped for that reason. In group C, there was no evidence of a CE, but there was a temporal association between timing of cardiac imaging and stopping trastuzumab, suggesting discontinuation could be related to an asymptomatic decline in LVEF. Group D included patients who stopped trastuzumab early with no apparent CE and no temporal association with cardiac imaging. This may have been due to patient preference, comorbidities, or oncologist recommendation, but without patient-level data we were unable to ascertain the cause so treated them separately for analysis. All BC patients (groups B, C, D) who received fewer than 17-18 cycles of adjuvant trastuzumab had statistically significantly inferior BC outcomes.
The optimal duration of trastuzumab therapy is an ongoing area of investigation with a number of studies exploring shorter durations of therapy. Several phase III trials have examined shorter durations of trastuzumab (9 weeks to 6 months vs 1 year), but only 1 was able to demonstrate noninferiority (21–25). A meta-analysis of these trials demonstrated that shorter durations of trastuzumab therapy are associated with inferior DFS and OS (26). Our findings are consistent with a previous real-world study that reported inferior OS in patients who did not complete 1 year of adjuvant trastuzumab (27). We observed that the impact of discontinuing trastuzumab early was greatest in higher stage (II/III) patients; future research on deescalation of anti-HER2 therapy should focus on stage I patients or, in the setting of neoadjuvant therapy, patients who achieve a pathologic complete response (pCR).
An initial criticism of our study was that survival differences could be explained by early relapse. To address this, we performed a sensitivity analysis 14 months after initiation of trastuzumab. In Ontario, Canada, the government funding agency Cancer Care Ontario requires all adjuvant trastuzumab be given within a 14-month window regardless of reasons for treatment delays. The sensitivity analysis did not change the main findings of the study, adding strength to our findings. The risk of cancer death exceeded cardiac death in all groups, even in group B, where patients experienced a CE. We used a Cox model for analysis of our survival outcomes. The assumptions of the model were violated, meaning that the hazard ratio changed over time. This observation is consistent with recent results from NCCTGN9837/NRG.NSABP B-31 examining the incidence of late recurrence. The authors found that the risk of recurrence in HER2-positive BC after adjuvant trastuzumab is greatest in the first 5 years and levels out in years 6-10 (28). To account for nonproportionality, we have reported hazard ratio estimates at 4 different time points, and those hazard ratio estimates are valid for those time points.
This study is not without its weaknesses. It is a retrospective cohort study derived from population-based administrative databases. This is a rich source of information; however, it does not provide patient-level data on some variables, including cardiac imaging results, cardiovascular risk factors, or clinical rationale for deviations from standard adjuvant treatment plans. Access to cardiac test results and/or clinical notes would have allowed a more granular analysis of the relationship between discontinuing trastuzumab for asymptomatic changes in LVEF and cancer outcomes. Knowledge of cardiovascular risk factors would also have added to the study results by allowing us to add further patient characteristics to our statistical model and strengthen our conclusion. Our results are limited to those treated with adjuvant trastuzumab alone; adjuvant pertuzumab was not available or funded during the study period, so we cannot comment on the optimal duration of therapy for patients treated with multiple anti-HER2 agents. We excluded patients treated with neoadjuvant therapy in an effort to keep the population as homogenous as possible; potential postsurgical complications could disrupt trastuzumab therapy in ways we could not account for. We acknowledge that neoadjuvant therapy for stage II-III HER2+ BC is now considered standard of care, based on the Katherine study that demonstrated in patients who did not achieve a pCR, switching adjuvant anti-HER2 therapy to trastuzumab emtansine led to improved OS (29). For those patients who do achieve a pCR, the optimal duration of trastuzumab remains unknown.
Clinical utility has been described as the ability of a test’s results to make a treatment decision that positively changes the outcome for a patient (30,31). Frequent cardiac monitoring in BC patients receiving trastuzumab was intended to enhance cardiac safety; however, in the real-world setting this practice may have a negative impact on patient care if it results in early discontinuation of treatment for asymptomatic changes in left ventricular function. Despite nearly 2 decades of experience treating patients with trastuzumab, there is still no direct evidence to support the clinical utility of cardiac imaging in all early-stage BC patients receiving HER2-based targeted therapy. Current guidelines (3–6) continue to support routine cardiac monitoring (every 3 to 4 months) regardless of risk and in the absence of demonstrable improvement in cardiac safety for the majority of patients. Changes in LVEF should lead to discussion between oncologists and cardiologists to discuss the risks vs benefits of disrupting anti-HER2 therapy. Recent studies have demonstrated that trastuzumab can be administered safely even in the face of mild cardiac dysfunction with concurrent cardiology management (32–34).
Rather than continue routine cardiac imaging for all patients, resources could be redirected toward optimizing cardiovascular risk factors and risk-stratifying patients before initiation of cancer treatment. In patients at high risk of cardiotoxicity, including those older than 60 years with baseline abnormal LVEF, personal history, or more than 1 risk factor for cardiovascular disease (smoking, diabetes, obesity, dyslipidemia, etc) (5,13,35,36), we suggest continuing the current approach of regular LVEF assessments every 3-4 months during anti-HER2 therapy as per current guidelines (3–6). In contrast, low- or average-risk patients could be monitored clinically to avoid unnecessary testing and mitigate the risk of treatment interruptions; this would also reduce health-care use costs. Although there has been some reluctance on the part of clinicians to consider less frequent cardiac monitoring in this patient population, in a 2015 study of Medicare patients in the United States, only 36% had cardiac monitoring as per current guidelines (37). Prospective evaluation of a risk-stratified approach to cardiac monitoring should be done to fully understand the impact this would have on cardiac safety and cancer outcomes.
In this large retrospective population-based cohort study, less than 1 year of adjuvant trastuzumab in patients with HER2+ early BC resulted in inferior clinical outcomes regardless of reason for treatment discontinuation. We hypothesized, based on our clinical experience of patients referred to a cardio-oncology clinic, that some patients had interruption or permanent discontinuation of trastuzumab therapy based on asymptomatic drops in LVEF. The overall CE rate in this study was low, suggesting that intensive cardiac monitoring of all patients treated with HER2-targeted therapy does not improve cardiac safety and may be detrimental if life-saving HER2-targeted therapy is prematurely discontinued. As the evidence evolves, we anticipate that a risk-based approach to cardiac monitoring will emerge; the results presented here will add to the evidence supporting 1 year of trastuzumab for optimal cancer outcomes.
Funding
Research funds were provided through an unrestricted educational grant 714302099. The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors of this paper make the following disclosures: Rushton: None; Lima: None; Johnson: None; Tuna: None; Ivars: None; Pritchard: None; Hawken: None; Dent: Novartis advisory board, research funding; Eli Lilly honoraria.
Role of the author: MR and SD were involved in the study design, interpretation of results, manuscript writing and decision to submit. CJ, JI and KP were involved in the interpretation of results, manuscript writing and decision to submit. IL, MT and SH were involved in the data collection and analysis, manuscript writing, and decision to submit.
Disclaimer: The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI.
Acknowledgments: This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Supplementary Material
References
- 1. Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database of Systematic Reviews. 2012;4:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;6:CD006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virani SA, Dent S, Brezden-Masley C, et al. Canadian cardiovascular society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol. 2016;32(7):831–841. [DOI] [PubMed] [Google Scholar]
- 4. Curigliano G, Cardinale D, Suter T, et al. ; on behalf of the ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012;23(suppl 7):155–166. [DOI] [PubMed] [Google Scholar]
- 5. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines)® Breast cancer version 2. 2017. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 26, 2017.
- 7. Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for traztuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34(10):1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu AF, Yin A, Liu JE, Steingart RM. Cost-effectiveness of cardiotoxicity monitoring. American College of Cardiology Expert Analysis website. 2017. http://www.acc.org/latest-in-cardiology/articles/2017/08/14/07/23/cost-effectiveness-of-cardiotoxicity-monitoring. Accessed September 23, 2017.
- 9. Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. Breast cancer therapy-related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34(19):2239–2246. [DOI] [PubMed] [Google Scholar]
- 10. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2 positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slamon D, Eiermann W, Robert N, et al. BCIRG 006 Phase 3 trial comparing AC-T with AC-TH and TCH in the adjuvant treatment of HER2 amplified early breast cancer patients: 10-year follow-up analysis. Oral abstract S5-04 presented at San Antonio Breast Cancer Symposium; December 8-12, 2015; San Antonio, TX.
- 12. Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30(31);3792–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganz PA, Romond EH, Cecchini RS, et al. Long-term follow-up of cardiac function and quality of life for patients in NSABP protocol B-31/NRG Oncology: a randomized trial comparing the safety and efficacy of doxorubicin and cyclophosphamide (AC) followed by paclitaxel with AC followed by pacitaxel ad trastuzumab in patients with node-positive breast cancer with tumors overexpressing human epidermal growth factor receptor 2. J Clin Oncol. 2017;35(35):3942–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Advani PP, Ballman KV, Travis J, et al. Long-term cardiac safety analysis of NCCTG N9831 (Allicance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34(6):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2019;37(22):1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldhar HA, Yan AT, Ko DT, et al. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst. 2016;108(1):djv301. [DOI] [PubMed] [Google Scholar]
- 17. Bowles EJA, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104(17):1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dent S, Hopkins S, Graham N, et al. The experience of a multidisciplinary clinic in the management of early-stage breast cancer patients receiving trastuzumab therapy: an observational study. Cardiol Res Pract. 2012;2012(135819):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin-Yee NJ, Yan AT, Kumachev A, et al. Association of hospital and physician case volumes with cardiac monitoring during adjuvant trastuzumab treatment for breast cancer: a retrospective cohort study. CMAJ Open. 2016;4(1):E66–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP System. J Clin Oncol. 2007;25(15):2127–2132. [DOI] [PubMed] [Google Scholar]
- 21. Joensu H, Fraser J, Wildiers H, et al. A randomized phase III study of adjuvant trastuzumab for a duration of 9 weeks versus 1 year, combined with adjuvant taxane-anthracycline chemotherapy, for early HER2 positive breast cancer (the SOLD study) AACR. Cancer Res. 2018;78(4 suppl): Abstract no GS3-04. [Google Scholar]
- 22. Pivot X, Romieu G, Debled M, et al. 6 months vs. 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. [DOI] [PubMed] [Google Scholar]
- 23. Conte P, Frassoldati A, Bisagni G, et al. Nine weeks versus 1-year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study. Ann Oncol. 2018;29(12):2328–2333. [DOI] [PubMed] [Google Scholar]
- 24. Mavroudis D, Saloustros E, Malamos N, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333–1340. [DOI] [PubMed] [Google Scholar]
- 25. Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393(10191):2599–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gong IY, Verma S, Yan AT, et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population study. Breast Cancer Res Treat. 2016;157(3):535–544. [DOI] [PubMed] [Google Scholar]
- 27. Goldvaser H, Korzets Y, Shepshelovich D, et al. Deescalating adjuvant trastuzumab in HER2-positive early-stage breast cancer: a systemic review and meta-analysis. JNCI Cancer Spectr. 2019;3(2):pkz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chumsri S, Li Z, Serie DJ, et al. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: combined analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J Clin Oncol. 2019;37(35):3425–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 30. Barron C, Alhussein M, Kaur U, et al. An evaluation of the safety of continuing trastuzumab despite overt left ventricular dysfunction. Curr Oncol. 2019;26(4):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynce F, Barac A, Tan MT, et al. SAFE‐HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2‐positive breast cancer and reduced left ventricular function. The Oncol. 2017;22(5):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leong DP, Cosman T, Alhussein M, et al. Safety of continuing trastuzumab despite mild cardiotoxicity. JACC CardioOncol. 2019;1(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teutsch SM, Bradley LA, Palomaki GE, et al. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: methods of the EGAPP working group. Genet Med. 2009;11(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bossuyt PM, Reitsma JB, Linnet K, Moons KG. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58(12):1636–1643. [DOI] [PubMed] [Google Scholar]
- 35. Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab for breast cancer. J Am Heart Assoc. 2014;3(1):e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Florido R, Sith KL, Cuomo KK, Russel SD. Cardiotoxicity from human epidermal growth factor receptor-2 (HER2) targeted therapies. J Am Heart Assoc. 2017;6(9):e006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chavez-MacGregor M, Niu J, Chang N, et al. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol. 2015;33(19):2176–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.