Abstract
Marginal keratitis, also known as catarrhal infiltrates, is a common, self-limiting condition characterized by inflammation at the peripheral aspect of the cornea. This non-infectious process is most typically a reaction to bacteria such as Staphylococcus, and results from a cell-mediated immune response to the bacterial antigens. This hypersensitivity reaction leads to the formation of stromal infiltrates that run parallel to the limbus. These infiltrates may extend around the limbal edge and can lead to the formation of marginal ulcers. Often the patient will have associated blepharoconjunctivitis. Both marginal keratitis and blepharoconjunctivitis are treated with topical steroids, with or without antibiotics, and good lid hygiene. We report a case of a patient who previously underwent small incision lenticule extraction (SMILE) who presented with marginal keratitis and secondary diffuse lamellar keratitis (DLK) in the right eye following recent initiation of continuous positive airway pressure (CPAP) therapy. There was no antecedent ocular trauma. With the initiation of steroid therapy, the patient returned to baseline visual acuity within one week. Though recurrence may be common in cases of marginal keratitis, our patient has not had any recurrence of symptoms or disease. DLK has previously been reported in the literature; however, there has been no reported case of marginal keratitis with secondary DLK after initiation of CPAP therapy to date.
Keywords: marginal keratitis, diffuse lamellar keratitis, small incision lenticule extraction, continuous positive airway pressure, CPAP
Introduction
Marginal keratitis is a self-limiting, common inflammatory condition that most often occurs secondary to staphylococcal blepharoconjunctivitis (ie, an overgrowth of Staphylococcus in the margins of the eyelid and conjunctiva), but may also be associated with other conditions including meibomitis, and rosacea.1 Staphylococcus-associated marginal keratitis occurs in immunocompetent individuals as a result of an abnormal immune response of the ocular surface rather than direct infection of the cornea. This enhanced immune response leads to an inflammatory spread towards the corneal limbus.2 The Fc portion of IgG and IgM antibodies then fuse with protein A, ribitol teichoic acid, peptidoglycan, and other bacterial antigens within the cell wall of Staphylococcus aureus. These antibody-antigen immune complexes deposit in the peripheral corneal stroma, resulting in a type III delayed hypersensitivity reaction.2,3 Additionally, Staphylococcal superantigens, (ie, anergy inducing molecules produced by a pathologic organism or bacteria) have been found to be associated with this disease.4 The peripheral cornea’s unique response to these antigens is a result of it having a more robust and complement activating immunologic response compared to the central cornea.5 This may also explain why the infiltrates may extend circumferentially, but rarely extend more centrally.4 Deposition of immune complexes classically presents on slit lamp examination as multiple discrete infiltrates, occasionally referred to as “catarrhal” or “peripheral sterile” infiltrates. These infiltrates typically run parallel to, but remain separated from, the corneal limbus, often with a band of unaffected cornea between the infiltrate and limbus. Staphylococcus-associated marginal keratitis is traditionally seen in areas where the eyelid rubs on the limbal ridge. This includes the 2 o’clock and 10 o’clock regions, corresponding to the upper lid, and the 4 o’clock and 8 o’clock regions, corresponding to the lower lid. This is also consistent with the understanding that this disease is often found in conjunction with Staphylococcus blepharitis and/or conjunctivitis. Treatment of marginal keratitis with topical corticosteroids and antibiotics generally results in rapid resolution of symptoms with good visual outcomes.4 The aim of this study is to describe a case of marginal keratitis with secondary DLK that occurred shortly after initiation of CPAP therapy for obstructive sleep apnea (OSA). This association has not previously been reported in the literature.
Case Report
A 37-year-old Caucasian male, with a history of uneventful bilateral small incision lenticule extraction (SMILE) six months prior, presented to our clinic following five days of unilateral redness in the right eye. There was photophobia of 3 days duration and pain. Slight blurriness in the right eye was also noted. The patient denied any ocular trauma, recent illness or cold sores. The patient was on artificial tears. Of note, he had begun nighttime continuous positive airway pressure (CPAP) therapy two weeks prior for newly diagnosed OSA. Since initiation of CPAP therapy, he felt that his right eye was drier upon awakening.
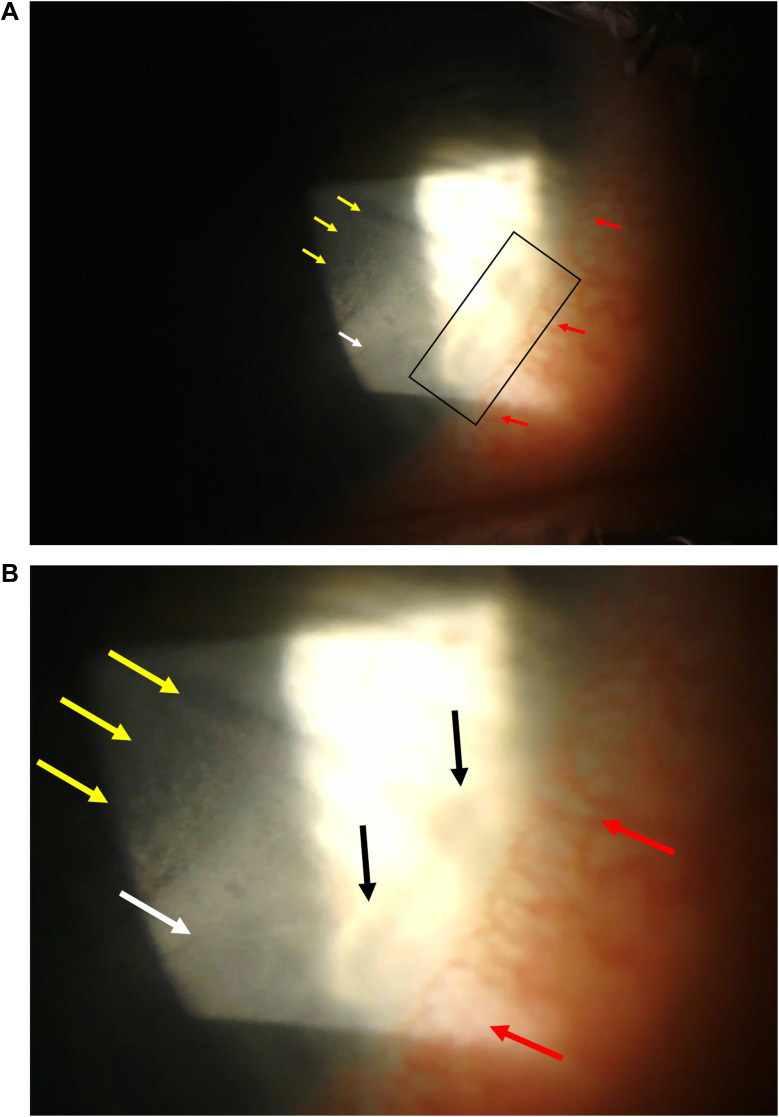
On examination, uncorrected visual acuity had declined to 20/25 in the right eye. Slit lamp examination of the right eye revealed an injected conjunctiva inferonasally and corresponding sectoral ciliary flush with limbitis. In the 4 o’clock region of the right cornea, small peripheral infiltrates were noted with no epithelial defect or corneal staining. DLK grade I was noted inside the margin of the SMILE cap, corresponding to the area of ciliary flush and limbitis (Figure 1). Examination of the left eye revealed mild superficial punctate keratopathy. Inspissated Meibomian glands and posterior marginal telangiectasias were seen in both eyes. Although desiccation from CPAP has been reported to cause keratitis with an epithelial defect or sterile ulcers,6 this was not observed in our patient. Visual acuity was 20/20 in each eye.
Figure 1.
(A) Peripheral sterile infiltrates and diffuse lamellar keratitis in the inferonasal area of the right cornea. Yellow arrows: Area of diffuse lamellar keratitis with “sands of Sahara” appearance. White arrow: Edge of the small incision lenticular extraction (SMILE) cap. Red arrows: Limbal inflammation. Black rectangle: Peripheral sterile (catarrhal) infiltrate of marginal keratitis. Slit lamp light has overexposed the area. (B) Enlarged view of panel A. Yellow arrows showing diffuse lamellar keratitis. White arrow showing the edge of the SMILE cap. Red arrows showing limbal inflammation. Black arrows showing the catarrhal infiltrates.
Differential diagnosis considerations were given to various other possibilities including herpetic keratitis, peripheral stromal keratitis, and peripheral ulcerative keratitis including Mooren’s ulcer. In the setting of the appropriate patient past medical history or other applicable findings differential diagnoses could also have included ocular rosacea and keratitis associated with systemic vascular diseases or rheumatoid arthritis.
Given the presence of limbitis and the absence of corneal staining or defect overlying the infiltrate, a diagnosis of marginal keratitis was made. The patient was started on topical prednisolone acetate 1% (Pred Forte®) eye drops every two hours for two days, then every 4 hours until follow-up. At follow-up, 4 days after diagnosis and steroid therapy, the patient felt his subjective visual acuity had returned to baseline and denied symptoms of ocular pain or photophobia. Visual acuity had returned to 20/20 in the right eye. Slit lamp examination revealed the complete resolution of ciliary flush and peripheral infiltrates without the presence of DLK in the corresponding area. The patient was tapered off prednisolone 1% eye drops over the following week. He has since fully recovered and remains asymptomatic.
Discussion
Our patient is a 37-year-old man with a history of SMILE, 6 months prior, and initiation of CPAP therapy, two weeks prior, who presented with pain, photophobia, and decreased visual acuity. Examination showed limbitis, DLK, and marginal keratitis. Prednisolone acetate 1% was started, and the patient’s slit lamp exam findings and visual changes resolved.
Though the development of catarrhal infiltrates as a rare complication of refractive procedures has been well documented, this generally arises in the 48 hours immediately following the procedure in patients with a history of blepharitis.7 Furthermore, DLK has been reported in patients following SMILE;8 however, our case is unique in that DLK arose only secondary to marginal keratitis six months after an uneventful procedure.
As the name suggests, CPAP therapy provides constant positive pressure throughout the phases of both inhalation and exhalation. Therapy is delivered by a machine which attaches to a mask which fits tightly over the patient’s mouth and nose. This helps increase alveoli recruitment and thus oxygenation. CPAP treatment is commonly employed in OSA as it helps keep the airway open.9
Complications of CPAP therapy commonly revolve around discomfort from the mask or the treatment itself and include congestion, runny nose, nosebleeds, as well as dry mouth, and dry nose.9 Ocular pathology has also been reported including dry eyes and cases of limbal keratitis, corneal ulcers, and bacterial keratitis.6
CPAP therapy has been taught to be associated with increased upper respiratory infections, though this teaching has been brought into question.10 This increased risk of infection may be associated with keratitis due to air flow from a poorly fitting mask to the eyes, or through air passage through the nasolacrimal duct into the eye.6 These theories explain the introduction of microorganisms to the ocular surface or periocular area secondary to CPAP therapy. Furthermore, CPAP therapy has been associated with ocular irritation and tear evaporation. These findings tend to differentially impact the right eye and are speculated to be caused by leakage of air from a poorly fitted mask or propulsion of bacteria from the mask into the eyes.6,11 We hypothesize two possible mechanisms by which new-onset blepharitis may have developed in our patient secondary to CPAP therapy. First, existing Staphylococcus aureus meibomian gland disease may have formed a biofilm in the margin of the eyelid which was exacerbated by dryness from an ill-fitting CPAP mask; this biofilm would have promoted the production of virulence factors that cause Meibomian gland dysfunction, a potential exacerbating factor to his morning dry eye.6 Second, decreased tear production likely led to keratoconjunctivitis sicca, or “dry eye,” which is associated with a pro-inflammatory cascade of cytokines (IL-1, IL-6, IL-8) in the cornea, conjunctiva, and Meibomian and lacrimal glands. Dry eye may also cause dysfunction of the peripheral nervous system, which is responsible for regulating recruitment of leukocytes.12,13 The underlying inflammation and growth of bacteria within the Meibomian glands would have triggered an antibody response, thereby causing immune complex formation as mentioned above.
The pattern of sterile infiltrates often reflects the circumstances surrounding their development: infiltrates localize to the flap margin following LASIK,14 whereas diffuse infiltrates are seen in individuals with poor contact lens hygiene.15 The restriction of catarrhal infiltrates to the inferonasal region of the right eye in our patient is significant as it is highly suggestive of air leakage from a poorly fitted CPAP mask as the underlying etiology. Our patient additionally presented with DLK underneath the corneal cap; this pattern of development is consistent with previously reported cases of DLK in the setting of marginal keratitis following refractive surgeries other than SMILE.16
Topical steroids remain the mainstay of therapy for sterile marginal keratitis;17 however, especially in patients with atypical presentations, physicians should inquire about inciting events to make recommendations and modify potential risk factors. Coexisting blepharitis may be treated with lid hygiene since steroid and antibiotic use may be less effective.18 We advised our patient to avoid using his CPAP machine for several days to allow for inflammation to resolve and to meet with a respiratory therapist to find a better fitting CPAP mask.
The diagnosis in this case is strengthened by the classic presentation, slit lamp examination findings and excellent response to topical corticosteroids consistent with marginal keratitis. The connection with newly diagnosed OSA and treatment with nocturnal CPAP provides a strong temporal association and probable cause. However, there are some limitations to our report. As classic marginal keratitis is a clinical diagnosis, the diagnosis was not confirmed with a smear or culture, which is expected to be negative. Furthermore, culture and gram-stain were not done because any de-epithelialization resulting from diagnostic scraping can cause or exacerbate DLK after SMILE8 or other excimer laser ablative procedures.19 Future studies should report additional associations of marginal keratitis with CPAP therapy and help establish a causal link between the two. Additionally, CPAP and other immune or bacteria- mediated ocular pathologies should be explored for potential correlations.
Conclusion
We present a unique case of localized sterile marginal keratitis with secondary DLK that arose several months following SMILE, most likely due to initiation of CPAP therapy for newly diagnosed OSA. The link between non-traumatic exogenous stimuli and the development of non-infectious keratitis warrants further investigation.
Acknowledgment
We would like to acknowledge Dr Yasmyne Castillo-Ronquillo for critical revision and editing.
Funding Statement
This study was funded by an unrestricted grant from Research to Prevent Blindness (RPB), 360 Lexington Avenue, 22nd Floor New York, NY 10017. No support was received for the publication of this article.
Ethics Approval and Informed Consent
This case report was approved by the Hoopes Vision Ethics Board to publish the case details and the patient signed an written informed consent to have the case details and any accompanying images published. The patient signed an informed consent for the release of clinical information and images for education and research purposes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
None of the authors have any conflict of interest related to this work.
References
- 1.Bernardes TF, Bonfioli AA. Blepharitis. Semin Ophthalmol. 2010;25(3):79–83. doi: 10.3109/08820538.2010.488562 [DOI] [PubMed] [Google Scholar]
- 2.Ficker L, Seal D, Wright P. Staphylococcal infection and the limbus: study of the cell-mediated immune response. Eye. 1989;3(2):190–193. doi: 10.1038/eye.1989.27 [DOI] [PubMed] [Google Scholar]
- 3.Ficker L, Ramakrishnan M, Seal D, Wright P. Role of cell-mediated immunity to staphylococci in blepharitis. Am J Ophthalmol. 1991. [DOI] [PubMed] [Google Scholar]
- 4.Jayamanne DGR, Dayan M, Jenkins D, Porter R. The role of staphylococcal superantigens in the pathogenesis of marginal keratitis. Eye. 1997;11(5):618–621. doi: 10.1038/eye.1997.165 [DOI] [PubMed] [Google Scholar]
- 5.Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmology. 1988;95(4):463–472. doi: 10.1016/S0161-6420(88)33164-7 [DOI] [PubMed] [Google Scholar]
- 6.Harrison W, Pence N, Kovacich S. Anterior segment complications secondary to continuous positive airway pressure machine treatment in patients with obstructive sleep apnea. Optometry. 2007;78(7):352–355. doi: 10.1016/j.optm.2006.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Moshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and noninfectious keratitis after laser in situ keratomileusis. Occurrence, management, and visual outcomes. J Cataract Refract Surg. 2007;33(3):474–483. doi: 10.1016/j.jcrs.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, He L, Yao P, et al. Diffuse lamellar keratitis after small-incision lenticule extraction. J Cataract Refract Surg. 2015;41(2):400–407. doi: 10.1016/j.jcrs.2014.05.041 [DOI] [PubMed] [Google Scholar]
- 9.Pinto VL, Sharma S. Continuous positive airway pressure. StatPearls; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482178/#:~:text=Continuous positive airway pressure (CPAP, people who are breathing spontaneously. Accessed November30, 2020. [PubMed] [Google Scholar]
- 10.Mercieca L, Pullicino R, Camilleri K, et al. Continuous positive airway pressure: is it a route for infection in those with obstructive sleep apnoea? Sleep Sci. 2017. doi: 10.1016/j.slsci.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayirci E, Yagci A, Palamar M, Basoglu OK, Veral A. The effect of continuous positive airway pressure treatment for obstructive sleep apnea syndrome on the ocular surface. Cornea. 2012;31(6):604–608. doi: 10.1097/ICO.0b013e31824a2040 [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T. Inflammatory response in dry eye. Investig Ophthalmol Vis Sci. 2018;59(14Special Issue):DES192–9. doi: 10.1167/iovs.17-23651 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Investig Ophthalmol Vis Sci. 2018;59(14):DES94. doi: 10.1167/iovs.17-23345 [DOI] [PubMed] [Google Scholar]
- 14.Lifshitz T, Levy J, Mahler O, Levinger S. Peripheral sterile corneal infiltrates after refractive surgery. J Cataract Refract Surg. 2005;31(7):1392–1395. doi: 10.1016/j.jcrs.2004.12.057 [DOI] [PubMed] [Google Scholar]
- 15.Dart JKG. Disease and risks associated with contact lenses. Br J Ophthalmol. 1993;77(1):49–53. doi: 10.1136/bjo.77.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrosio R, Hallal R, Ramos I, Faria-Correia F. Marginal sterile corneal infiltrates after lasik and corneal procedures In: Alio JL, Azar DT, editors. Management of Complications of Refractive Surgery. 2nd ed. Springer; 2018:83–90. [Google Scholar]
- 17.Ambrósio R, Periman LM, Netto MV, Wilson SE. Bilateral marginal sterile infiltrates and diffuse lamellar keratitis after laser in situ keratomileusis. J Refract Surg. 2003;19(2):154–158. [DOI] [PubMed] [Google Scholar]
- 18.Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012. doi: 10.1002/14651858.CD005556.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah MN, Misra M, Wihelmus KR, Koch DD. Diffuse lamellar keratitis associated with epithelial defects after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26(9):1312–1318. doi: 10.1016/S0886-3350(00)00570-8 [DOI] [PubMed] [Google Scholar]