Abstract
Aims
The aim of this study was to define the natural history of patients with mitral annular calcification (MAC)-related mitral valve dysfunction and to assess the prognostic importance of mean transmitral pressure gradient (MG) and impact of concomitant mitral regurgitation (MR).
Methods and results
The institutional echocardiography database was examined from 2001 to 2019 for all patients with MAC and MG ≥3 mmHg. A total of 5754 patients were stratified by MG in low (3–5 mmHg, n = 3927), mid (5–10 mmHg, n = 1476), and high (≥10 mmHg, n = 351) gradient. The mean age was 78 ± 11 years, and 67% were female. MR was none/trace in 32%, mild in 42%, moderate in 23%, and severe in 3%. Primary outcome was all-cause mortality, and outcome models were adjusted for age, sex, and MAC-related risk factors (hypertension, diabetes, coronary artery disease, chronic kidney disease). Survival at 1, 5, and 10 years was 77%, 42%, and 18% in the low-gradient group; 73%, 38%, and 17% in the mid-gradient group; and 67%, 25%, and 11% in the high-gradient group, respectively (log-rank P < 0.001 between groups). MG was independently associated with mortality (adjusted HR 1.064 per 1 mmHg increase, 95% CI 1.049–1.080). MR severity was associated with mortality at low gradients (P < 0.001) but not at higher gradients (P = 0.166 and 0.372 in the mid- and high-gradient groups, respectively).
Conclusion
In MAC-related mitral valve dysfunction, mean transmitral gradient is associated with increased mortality after adjustment for age, sex, and MAC-related risk factors. Concomitant MR is associated with excess mortality in low-gradient ranges (3–5 mmHg) but gradually loses prognostic importance at higher gradients, indicating prognostic utility of transmitral gradient in MAC regardless of MR severity.
Keywords: Mitral annular calcification, Mitral stenosis, Mitral valve gradient, Mitral regurgitation, Echocardiography
Graphical Abstract
See page 4329 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa828)
Introduction
Mitral annular calcification (MAC) is a common degenerative mitral valve (MV) disease characterized by calcification at the level of the mitral annulus that can be associated with significant MV dysfunction including both stenosis and regurgitation.1 , 2 MAC is associated with female sex, advanced age, chronic kidney disease (CKD), and multiple cardiovascular risk factors, and prevalence ranges from 8% to 15% in the general population and reaches as high as 40% among the elderly.3 , 4 Significant mitral stenosis (MS) is reported in ∼8% of patients with MAC,5 frequently combined with mitral regurgitation (MR).6 The presence of MAC by itself is associated with poor outcome,3 which limits our understanding of the prognostic impact of MAC-related MV dysfunction.
Despite the relatively high prevalence of MAC, especially in an aging population, the assessment of patients with MAC remains problematic. Standard echocardiographic metrics for assessing stenosis severity lack validation in patients with calcific disease and are largely extrapolated from the rheumatic MS literature.7 It remains unclear if the transmitral gradient—affected by not only the degree of stenosis but also concomitant MR (i.e. increased flow)—has prognostic value in patients with MAC-related MV dysfunction. These limitations impair our ability to define disease severity and thresholds for intervention, a gap that is taking on increasing importance as the array of therapeutic options to treat patients with MAC-related MV dysfunction expands.8 At the same time, the high 1-year mortality observed in contemporary valve-in-MAC registries highlights the critical need to define outcomes in this patient population.9
The aim of this study was therefore to define the natural history in patients with MAC-related MV dysfunction and to assess the prognostic importance of mean transmitral pressure gradient. We hypothesized that the transmitral gradient would be an independent predictor of outcome in MAC-related MV dysfunction and that this would hold true irrespective of whether gradients were driven by stenosis, regurgitation, or a combination of both as is frequently seen in MAC.
Methods
Study population
All patients with MAC and a documented transmitral gradient on echocardiography between 2001 and 2019 were included from a large institutional echocardiography database (Massachusetts General Hospital, Boston, MA, USA). For patients with multiple echocardiograms, the earliest examination was included. After exclusions for rheumatic and congenital MS, mitral prostheses or repair, and missing clinical follow-up, the starting sample consisted of 6620 patients with MAC and documented transmitral gradient (Figure 1).
Figure 1.
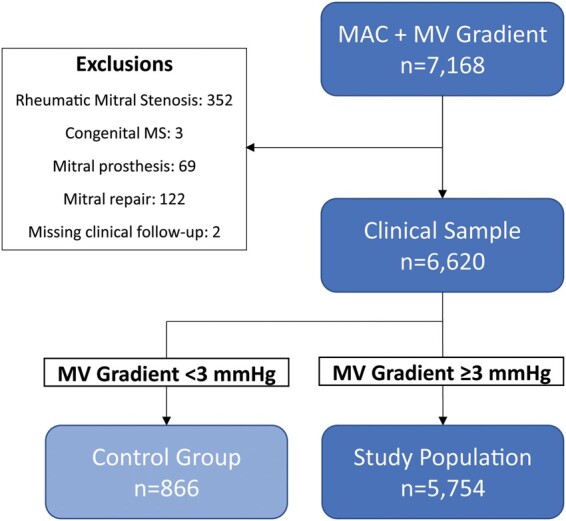
Study population. Derivation of the study population and control group after exclusions is shown. MAC, mitral annular calcification; MV, mitral valve.
MAC-related MV dysfunction was defined as MAC with associated increase in mitral inflow velocity and/or turbulent flow by colour Doppler mapping at a normal Nyquist limit (50–70 cm/s) and colour gain. In our experience, this corresponds to a mean transmitral gradient ≥3 mmHg, i.e. the upper limit of normal (mean + 1.95 × SD) of a random sample of 200 patients with MAC and normal mitral inflow (Supplementary material online). As such, the final study population consisted of 5754 unique patients with significant MAC-related MV dysfunction, i.e. transmitral gradient ≥3 mmHg (Figure 1).
A control group of patients with MAC but without haemodynamically significant MV dysfunction consisted of all patients with MAC and gradient <3 mmHg (n = 866).
Echocardiographic and clinical data
The presence of MAC and associated quantitative echocardiographic data were extracted from the official clinical interpretation by an attending cardiologist with level III certification in echocardiography. MAC was defined as ‘areas of echodense thickening with associated acoustic shadowing’ at the level of the mitral annulus and potentially extending onto the anterior and posterior leaflets. Based on the MAC location and the extent of calcium, MAC was described as (i) confined to either anterior or posterior mitral annulus, (ii) present on both the anterior and posterior annulus, or (iii) ‘extensive’ MAC that encroaches upon the leaflets resulting in a restriction of leaflet motion and/or narrowing of the mitral orifice. Transmitral gradients were measured from continuous wave spectral Doppler tracings in the apical four-chamber view and averaged over ≥3 cardiac cycles in sinus rhythm and ≥5 cardiac cycles in atrial fibrillation. Heart rate during the echocardiogram was available from the institutional database in 1041 (18%) consecutive patients, all since 2016. Median heart rate at the time of the echocardiogram was additionally extracted from 2986 patients with electronically available image data, resulting in a total of 4027 patients (70% of the study population) with heart rate data. Valvular regurgitation was assessed integrating both quantitative and qualitative parameters in accordance with society guidelines.10 , 11 Aortic stenosis was classified as none/mild, moderate, or severe. Severe aortic stenosis was defined inclusively as either mean transaortic gradient ≥40 mmHg, aortic valve area ≤1.0 cm2, or dimensionless index [ratio of peak aortic velocity to left ventricular (LV) outflow tract velocity] ≤0.25, whereas moderate aortic stenosis was defined by mean transaortic gradient 20–39 mmHg, aortic valve area >1 cm2, and dimensionless index >0.25.
Demographics, baseline comorbidities, and laboratory data at the time of echocardiography were determined using the electronic health record (EHR). Mortality data were obtained from the EHR, which integrated clinical records and the social security death index to identify dates of death. A total of 24 patients were reported deceased but no date of death could be identified and were censored at the date of last visit. All aspects of this study comply with the Declaration of Helsinki and were approved by the Massachusetts General Hospital/Partners Institutional Review Board. The need for informed consent was waived.
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median (interquartile range) and compared using independent samples’ t-test and one-way ANOVA or Wilcoxon sign rank and Kruskal–Wallis tests as appropriate. Categorical variables are expressed as raw numbers with percentages and compared using Chi-squared test.
The primary outcome was all-cause mortality. To allow the assessment of natural disease prognosis, patients who underwent MV intervention (surgical or percutaneous, n = 210) were censored at the time of intervention. Survival was analyzed using Cox proportional hazards regression using three models with increasing depth of adjustment: (1) age and sex; (2) Model 1 + hypertension, diabetes, coronary artery disease (CAD), and CKD (defined as Stage III CKD or above), and (3) Model 2 + left ventricular ejection fraction (LVEF), severity of aortic stenosis, estimated right ventricular systolic pressure, and atrial fibrillation. Survival stratified by transmitral gradient groups (mean gradient 3–5, 5–10, and ≥10 mmHg) was analyzed by Cox regression survival analysis after adjustment for age, sex, and common comorbidities (Model 2). Knotted spline curves of the adjusted hazard ratio for all-cause mortality relative to the control group were calculated for MV gradient as a continuous variable. Model 2 was selected as the preferred model for additional outcome analyses due to the completeness of the data and the established prognostic importance of these co-morbidities associated with MAC. Kaplan–Meier survival analysis and adjusted Cox regression survival analysis stratified by the degree of MR (none/trace, mild, moderate, and severe) was additionally performed in each gradient subgroup (3–5, 5–10, and ≥10 mmHg). Finally, the impact of the extent of calcium, rheological factors (haematocrit), and heart rate on the gradient’s prognostic importance was analyzed in adjusted Cox regression models (Model 2). Two-sided p-values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Version 25 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Table 1 summarizes the characteristics of the total study population and of the three predefined subgroups of stenosis severity (transmitral gradient 3–5, 5–10, and ≥10 mmHg, respectively). Overall age in the study population was 78 ± 11 years, and 67% were female. With increasing haemodynamic severity as stratified by the transmitral gradient, female sex, CKD, the extent of annular calcium, and concomitant valve diseases were more common, although a younger age and less associated ischaemic heart disease were observed.
Table 1.
Baseline clinical and echocardiographic characteristics of the study population
| Variable | MAC-related MV dysfunction |
Control group (n = 866) | |||||
|---|---|---|---|---|---|---|---|
| Study population (n = 5754) | P-value* | 3–5 mmHg (n = 3927) | >5 mmHg to <10mmHg (n = 1476) | ≥10 mmHg (n = 351) | P-value † | ||
| Age (years) | 77.5 ± 11 | <0.001 | 78.4 ± 10.5 | 76 ± 12 | 74 ± 13 | <0.001 | 79 ± 10 |
| Male | 1916 (33.3) | <0.001 | 1373 (35.0) | 439 (30) | 104 (30) | <0.001 | 409 (47) |
| BSA (m²) | 1.8 ± 0.3 | 0.82 | 1.8 ± 0.3 | 1.8 ± 0.3 | 1.8 ± 0.3 | 0.22 | 1.8 ± 0.3 |
| Medical history | |||||||
| Hypertension | 4579 (79.6) | 0.03 | 3210 (81.7) | 1127 (76) | 242 (69) | <0.001 | 717 (82.9) |
| Hyperlipidemia | 3715 (64.6) | <0.001 | 2622 (66.8) | 907 (61) | 186 (53) | <0.001 | 631 (72.9) |
| Diabetes | 2440 (42.4) | <0.001 | 1667 (42.5) | 638 (43) | 135 (39) | 0.27 | 296 (34.2) |
| CKD Stage ≥3 | 988 (17.2) | 0.03 | 638 (16.3) | 282 (19) | 68 (19) | 0.02 | 123 (14.2) |
| CAD | 3243 (56.4) | 0.003 | 2282 (58.1) | 778 (53) | 183 (52) | <0.001 | 535 (61.8) |
| Myocardial infarction | 871 (15.1) | 0.19 | 639 (16.3) | 197 (13) | 35 (10) | 0.001 | 146 (16.8) |
| Atrial fibrillation | 2392 (41.6) | <0.001 | 1655 (42.1) | 590 (40) | 147 (42) | 0.35 | 421 (48.6) |
| Stroke | 491 (8.5) | 0.36 | 339 (8.6) | 127 (9) | 25 (7) | 0.62 | 82 (9.5) |
| Chest radiation | 163 (2.8) | 0.92 | 114 (2.9) | 42 (2.8) | 7 (2.0) | 0.62 | 24 (2.8) |
| Laboratory data | |||||||
| Hct (%) | 33.7 ± 5.5 | <0.001 | 34.0 ± 5.5 | 33.3 ± 6 | 33.2 ± 6 | <0.001 | 35.0 ± 5.7 |
| Echocardiographic data | |||||||
| LV ejection fraction (%) | 64.5 ± 13 | <0.001 | 63.9 ± 13 | 66 ± 13 | 67 ± 12 | <0.001 | 61.7 ± 13 |
| LV end diastolic diameter (mm) | 42.8 ± 7 | <0.001 | 43.0 ± 7 | 42 ± 7 | 41 ± 7 | <0.001 | 44.7 ± 7 |
| LV end systolic diameter (mm) | 28.1 ± 7 | <0.001 | 28.4 ± 7 | 28 ± 7 | 27 ± 7 | <0.001 | 30.1 ± 8 |
| Septal thickness (mm) | 12.6 ± 2.5 | <0.001 | 12.5 ± 2.4 | 12.6 ± 2.6 | 12.9 ± 2.7 | 0.004 | 11.9 ± 2.6 |
| LA anteroposterior dimension (mm) | 41 ± 7 | 0.89 | 41 ± 7 | 42 ± 6 | 43 ± 6 | 0.003 | 41 ± 7 |
| Heart rate (bpm) | 75 ± 15 | 72 ± 14 | 80 ± 15 | 83 ± 16 | <0.001 | 65 ± 11 | |
| Transmitral mean gradient (mmHg) | 5.2 ± 2.5 | <0.001 | 3.9 ± 0.8 | 6.9 ± 1.0 | 12.4 ± 2.7 | <0.001 | 1.8 ± 0.5 |
| Transmitral peak gradient (mmHg) | 12.7 ± 5.2 | <0.001 | 10.4 ± 2.8 | 15.8 ± 3.8 | 25.1 ± 6.0 | <0.001 | 5.7 ± 2.2 |
| RVSP (mmHg) | 48.5 ± 15 | <0.001 | 46.4 ± 14 | 51 ± 14 | 59 ± 17 | <0.001 | 40.9 ± 14 |
| Extent/location of annular calcium | |||||||
|
1249 (22) | <0.001 | 1011 (26) | 202 (14) | 36 (10) | <0.001 | 415 (49) |
|
905 (16) | 679 (18) | 192 (13) | 34 (10) | 188 (22) | ||
|
3524 (62) | 2186 (56) | 1062 (73) | 276 (80) | 236 (28) | ||
| Mitral regurgitation | |||||||
| None/trace | 1794 (32) | 0.13 | 1282 (34) | 422 (30) | 90 (26) | <0.001 | 280 (32) |
| Mild | 2326 (42) | 1655 (43) | 536 (38) | 135 (39) | 362 (42) | ||
| Moderate | 1270 (23) | 796 (21) | 381 (27) | 93 (27) | 180 (21) | ||
| Severe | 191 (3) | 88 (2) | 78 (5.5) | 25 (7.3) | 17 (2.0) | ||
| Aortic stenosis | |||||||
| None/mild | 3729 (65) | 0.023 | 2577 (66) | 933 (63) | 219 (62) | 0.261 | 600 (69) |
| Moderate | 538 (9) | 353 (9) | 144 (10) | 41 (12) | 63 (7) | ||
| Severe | 1487 (26) | 997 (25) | 399 (27) | 91 (26) | 203 (23) | ||
| Tricuspid regurgitation, ≥ moderate | 1284 (24) | 0.04 | 815 (22) | 371 (26) | 98 (29) | <0.001 | 170 (20) |
Values are reported as mean ± standard deviation or n (%). BSA, body surface area; CKD, chronic kidney disease; CAD, coronary artery disease; LA, left atrium; LV, left ventricle; RVSP, right ventricular systolic pressure.
P-value reported relative to control group.
P-value indicating differences across three gradient groups.
Clinical outcome
During a median follow-up of 1.3 years (interquartile range 0.3–3.5 years) with maximum follow-up until 17.4 years, 2935 (51%) patients died. Mean transmitral gradient as a continuous variable was independently associated with increased risk of mortality in univariate analyses and in multivariable Cox regression analyses with increasing depth of adjustment (Table 2). The adjusted hazard ratio (Model 2) for each 1 mmHg increase in the transmitral gradient was 1.064 (95% CI 1.049–1.080).
Table 2.
Impact of the mitral valve gradient on mortality in univariable and multivariable Cox model analyses
| Analysis | n | HR of death (per mmHg increase in transmitral gradient) | P-value |
|---|---|---|---|
| Univariate analysis | 5754 | 1.048 (95% CI 1.033–1.063) | <0.001 |
| Model 1: adjusted for age and sex | 5754 | 1.067 (95% CI 1.052–1.083) | <0.001 |
| Model 2: Model 1 + hypertension, diabetes, CAD, and CKD | 5754 | 1.064 (95% CI 1.049–1.080) | <0.001 |
| Model 3: Model 2 + LV ejection fraction, severity of AS, RVSP, and atrial fibrillation | 4448 | 1.041 (95% CI 1.024–1.059) | <0.001 |
AS, aortic stenosis; CAD, coronary artery disease; CKD, chronic kidney disease; LV, left ventricle; RVSP, right ventricular systolic pressure.
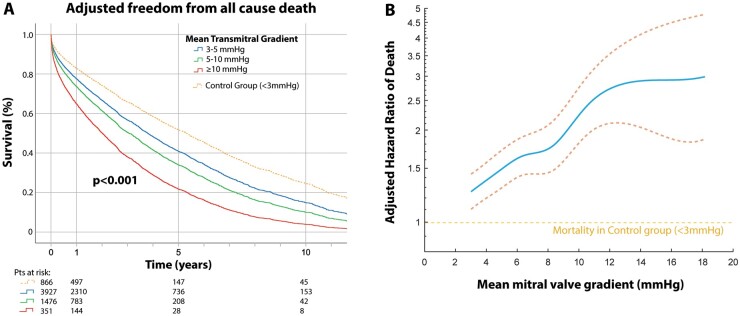
Figure 2 shows the Cox regression freedom from all-cause death stratified by the transmitral gradient (3–5, 5–10, and ≥10 mmHg), after adjustments for age, sex, hypertension, diabetes, CAD, and CKD (Model 2). The median survival time and the observed 1-year, 5-year, and 10-year survival in patients with MAC-related MV dysfunction decreased incrementally with increasing gradient (Table 3).
Figure 2.
Impact of transmitral gradient on all-cause mortality. (A) Adjusted Cox regression survival curves are shown among patients with mitral annular calcification-related mitral valve dysfunction stratified by transmitral gradient (3–5, 5–10, and ≥10 mmHg) and compared to a control group of mitral annular calcification with low/normal gradient. (B) In spline survival analysis, the adjusted hazard ratio of death relative to controls steadily increases for increasing transmitral gradient. Survival was adjusted for age, sex, and common mitral annular calcification-associated comorbidities (diabetes, hypertension, coronary artery disease, and chronic kidney disease).
Table 3.
Observed 1-, 5-, and 10-year survival in patients with mitral annular calcification-related mitral valve dysfunction according to mean transmitral gradient
| Survival | n | Median survival (95% CI) | 1 year (%) | 5 years (%) | 10 years (%) |
|---|---|---|---|---|---|
| Control group (MAC, gradient <3 mmHg) | 866 | 5.5 (4.5–6.4) | 82 | 52 | 25 |
| 3 ≤ transmitral gradient ≤5 mmHg | 3926 | 3.8 (3.6–4.1) | 77 | 42 | 18 |
| 5 < transmitral gradient <10 mmHg | 1476 | 3.1 (2.7–3.4) | 73 | 38 | 17 |
| Transmitral gradient ≥10 mmHg | 351 | 2.1 (1.6–2.5) | 67 | 25 | 11 |
MAC, mitral annular calcification.
Importance of the extent of annular calcium
Annular calcium was isolated to the posterior or anterior annulus in 1249 (22%) patients, was present on both the anterior and posterior annulus in 905 (16%), and was ‘extensive’ with encroachment onto the leaflets and/or narrowing of the valve orifice in 3524 (61%). The proportion of ‘extensive’ MAC was higher in the high-gradient group (Table 1). In multivariable Cox regression for all-cause death (Model 2, excluding transmitral gradient), increasing calcium extent was associated with increased risk of death (P = 0.004; HR 1.168, 95% CI 1.062–1.284 for ‘extensive’ MAC vs. isolated posterior/anterior calcium, but no difference between ‘extensive’ vs. both anterior and posterior calcium: HR 0.988, 95% CI 0.896–1.091). When mean transmitral gradient was added back to this multivariable Cox regression model, the gradient remained strongly associated with mortality (adjusted HR 1.061, 95% CI 1.045–1.077, P < 0.001), but the prognostic importance of the calcium burden decreased, with an adjusted hazard ratio for death at borderline significance (P = 0.048). Moreover, in the subgroup with ‘extensive MAC’ (61% of the population), mean transmitral gradient was strongly associated with mortality, similar to the overall results of this study (detailed analysis in Supplementary material online, Tables S3 and S4 and Supplementary material online, Figure S3).
Prognostic importance of concomitant mitral regurgitation
MR was none/trace in 1794 (32%) patients, mild in 2326 (42%), moderate in 1270 (23%), and severe in 191 (3%), with higher proportions of moderate or severe MR in the higher-gradient groups (Table 1). In 173 (3%) patients, no data on MR severity were available, and these patients were excluded from the MR analysis. A comparison of baseline demographics, clinical history, and echocardiographic findings relative to MR severity is presented in Supplementary material online, Table S1. There was evidence of leaflet pathology (prolapse or flail) in 110 (2%) patients, with MR being none/trace in 3, mild in 17, moderate in 42, and severe in 48. Patients with prolapse/flail represented a minority of patients with severe MR (48/191, 25%) and of patients with high gradients ≥10 mmHg (5/351, 1.3%), and MAC was ‘extensive’ in over 1/3 of the 110 patients with prolapse/flail.
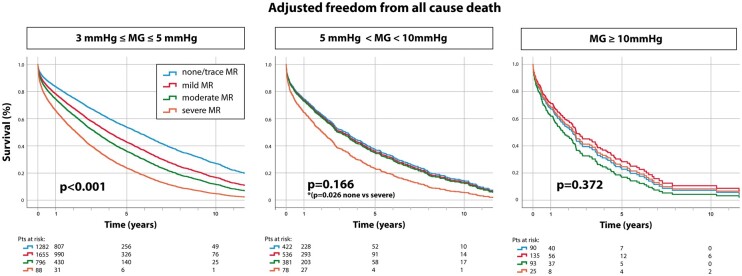
The impact of MR severity on all-cause mortality in the study population is presented in Figure 3. For patients with mean gradient 3–5 mmHg, an incremental increase in mortality was observed between none/trace, mild, moderate, and severe MR (P < 0.001, adjusted for Model 2). In patients with mean gradient 5–10 mmHg, the difference in survival between none/trace, mild, and moderate MR severities was no longer observed, resulting in an overall P = 0.166 for prognostic importance of MR severity in this gradient subgroup. However, survival remained significantly worse for severe MR compared to none/trace MR (P = 0.026). Finally, in the high-gradient (≥10 mmHg) group, outcome was similar across all MR severities (P = 0.372, Figure 3).
Figure 3.
Impact of mitral regurgitation severity on all-cause mortality in patients with mitral annular calcification-related mitral valve dysfunction according to transmitral gradient. Among patients with mitral annular calcification-related mitral valve dysfunction, adjusted Cox regression survival curves are shown stratified by mitral regurgitation severity within each transmitral gradient group (3–5, 5–10, and ≥10 mmHg). In the low-gradient group (3–5 mmHg), increasing mitral regurgitation severity was associated with worse outcome, while among patients with gradient 5–10 mmHg, only severe mitral regurgitation was associated with increased mortality. In the high (≥10 mmHg) gradient group, by contrast, mitral regurgitation severity was not associated with outcome.
Heart rate
In 4027 patients with available heart rate data during the echocardiogram, the average heart rate was 75 ± 15 bpm, and 95% of heart rates were ≤100 bpm. Heart rate and mean gradient were weakly correlated (r = 0.28, P = 0.001), and heart rate was higher in the high-gradient groups (Table 1). After adjusting for heart rate in the multivariable Cox regression model (Model 2), the transmitral gradient remained strongly and independently prognostic for mortality (HR 1.048, 95% CI 1.028–1.069, P < 0.001). Supplementary material online, Table S5 shows the impact on the prognostic value of the mean transmitral gradient when adjusting for heart rate in different Cox regression models.
Rheological factors
Haematocrit levels were available in 4987 (87%) of patients (Table 1), with a median time difference between the laboratory values and the echocardiogram of 0 days (interquartile range (IQR) 0–1 days). Lower haematocrit levels were associated with worse outcome (hazard ratio (HR) 0.950 per haematocrit point, 95% confidence interval (CI) 0.943–0.957, P < 0.001). In the multivariable model (Model 2) incorporating both the transmitral gradient and haematocrit, the transmitral gradient remained strongly associated with outcome independent of haematocrit (adjusted HR 1.043, 95% CI 1.026–1.059, P < 0.001).
Discussion
This study investigated the natural history in patients with MAC and associated MV dysfunction in a large institutional cohort with long-term follow-up. Key findings are that (i) the transmitral gradient is associated with increased mortality in MAC with associated MV dysfunction, after adjustment for age, sex, and common MAC-related risk factors (Take home figure), and (ii) concomitant MR is an important driver of mortality in MAC with transmitral gradient ≤5 mmHg, whereas at higher gradients outcome becomes similarly and severely impaired irrespective of the degree of MR.
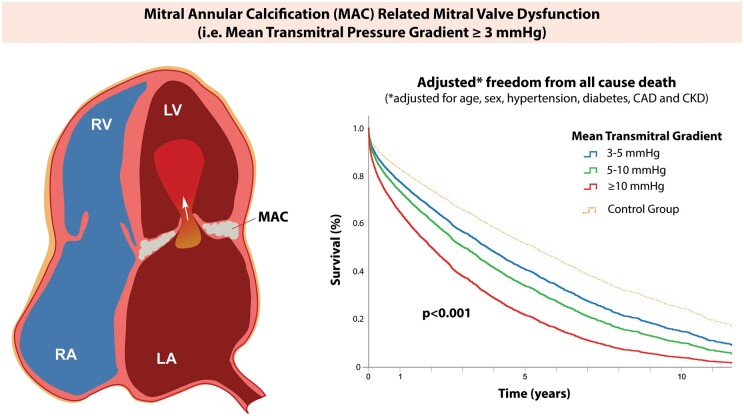
Take home figure.
In mitral annular calcification-related mitral valve dysfunction, the mean transmitral gradient is associated with increased mortality after adjustment for age, sex, and mitral annular calcification-associated risk factors such as hypertension, diabetes, coronary artery disease, and chronic kidney disease. CAD, coronary artery disease; CKD, chronic kidney disease; MAC, mitral annular calcification; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Mitral annular calcification-related mitral valve dysfunction and clinical outcomes
Patients with MAC are known to have a poor prognosis, a finding most commonly attributed to the presence of comorbidities that predispose to MAC rather than to the risk conveyed by MAC and associated valvular dysfunction itself.1 , 3 Nevertheless, recent data suggest that the adverse impact of MAC relates to associated valve dysfunction rather than to MAC itself.12 Although the present retrospective study cannot demonstrate causality, the present data suggest that MAC can partially lead to valve dysfunction since in our study (i) other valve abnormalities causing MV stenosis (rheumatic/congenital/postoperative) were excluded; (ii) the majority of patients had an ‘extensive’ MAC burden causing valve dysfunction; and (iii) there was a low prevalence (<2%) of leaflet pathology (prolapse/flail), of which over 1/3 were associated with ‘extensive’ MAC burden.
Anatomic assessments of MAC severity have been limited and heterogeneous in the literature,13 although a link between MAC thickness and incident cardiovascular events has been described.14 The prognostic importance of MV dysfunction related to MAC, by contrast, has received little attention. Pasca et al. 15 reported outcome in 1004 patients with calcific MS, defined as severe MAC without commissural fusion and a mean transmitral gradient ≥2 mmHg. In that cohort, 1-, 5-, and 10-year survival rates were 78%, 47%, and 25%, respectively, relatively in line with the outcomes in the low-gradient (3–5 mmHg) group in the present study. A recent analysis of 200 patients with calcific MV dysfunction (mean gradient 8.1 ± 3.8 mmHg) reported a 72% 1-year survival rate, again in line with outcome in our study.16 Our study in 5754 patients with MAC-related MV dysfunction provides additional data, reporting survival rates and median survival for different cut-points of the transmitral gradient (Table 3) and, importantly, demonstrating excess mortality with increasing gradients relative to a control group after adjustment for age, sex, and comorbidities (Figure 2B). In a population already at high mortality risk, the true impact of the MAC-related valvular dysfunction (and thus the maximal improvement in outcome that can be expected from MV interventions) is appreciated in the relative outcome difference between the control group and the higher-gradient groups, adjusted for age and co-morbidities (Figure 2).
This prognostic information becomes particularly relevant in light of emerging ‘valve-in-MAC’ procedures that are increasingly performed in patients with MAC and valve dysfunction. In the Transcatheter Mitral Valve Replacement (TMVR) in MAC Global Registry, 116 MAC patients with mean transmitral gradient 11.5 ± 4.2 mmHg underwent TMVR, with 30-day and 1-year all-cause mortality as high as 25% and 53.7%, respectively.9 While post-procedural LV outflow tract obstruction and paravalvular MR are implicated in these poor outcomes, questions about the natural history and outcome in this patient population arise. Our findings on prognosis and mortality, therefore, add important guidance to the interpretation of these mortality data and might aid in risk stratification and patient selection for future valvular interventions in MAC.
Impact of mitral regurgitation on transmitral gradient and outcome in mitral annular calcification-related mitral valve dysfunction
A particularly novel finding in this study is that concomitant MR—which is commonly seen in the ‘mixed’ valve lesions associated with MAC—is incrementally associated with mortality at lower transmitral gradients (3–5 mmHg), concordant with outcome data in primary MR,17 but gradually loses prognostic significance in the higher-gradient ranges (Figure 3). The latter finding suggests that in MAC-related MV dysfunction with a high transmitral gradient, outcome is similar whether this gradient is driven by more severe valve obstruction in the absence of MR or by increased MR-related flow across a mildly or moderately stenotic valve orifice. A corollary implication is that in low-gradient MAC, increasing MR severity confers additive risk and thus should be accounted for in the disease assessment.
Clinical implications
Clinical decision-making in patients with calcific MV disease is typically based on a thorough assessment of the severity of valve dysfunction. In general, and extrapolating from rheumatic MS data, an MV area of ≤1.5 cm2 is considered to be severe MS.18 , 19 This typically corresponds to a mean gradient of 5–10 mmHg at a normal heart rate.19 However, the ‘classic’ echocardiographic metrics for stenosis assessment (the pressure half-time method, continuity equation, or MV planimetry) lack validation in (frequently mixed) MAC-related valve dysfunction and present important technical challenges.7 , 8 , 20 In this setting, measurement of the transmitral gradient might be a more reliable tool to integrate the haemodynamic impact of valve dysfunction and assess disease severity and prognosis in MAC with (mixed) valve dysfunction, as supported by the data in this study. However, the major limitation of the transmitral gradient remains its inherent dependence on flow, which in turn depends on factors including the LVEF, anaemia, comorbid valvular disease, and heart rate. We demonstrate that the prognostic importance of the transmitral gradient remains robust after adjustment for each of these factors. However, heart rate in our study was ≤100 bpm in 95% of patients, highlighting that interpretation of the gradient for the assessment of valve dysfunction and prognostication should likely be limited to normal heart rates, in line with guideline recommendations.21
Finally, the relative contribution of LV filling abnormalities to the transmitral gradient remains uncertain. As described by Reddy et al.,22 it is difficult to separate the relative contributions of intrinsic valve obstruction from diastolic dysfunction when interpreting increased transmitral gradients in MAC. While both factors have prognostic implications, the beneficial impact of MV interventions in MAC and elevated gradients might be blunted if LV filling abnormalities are a dominant contributor to the gradient.
Study limitations
This was an observational retrospective cohort study from a single quaternary care centre in an elderly cohort with very high mortality rate. Despite a total follow-up duration of up to 17 years, the median follow-up was short reflecting the high mortality rate. Nevertheless, outcome predictions at 1, 5, and 10 years remain supported by a high number of patients at risk. In the Cox analysis stratified by MR severity (Figure 3), the number of patients at risk in the high-gradient group (≥10 mmHg) was low, which could lead to underestimation of true differences in outcome between MR groups. Heart rate was not available for the entire study cohort. However, in the 70% of the population with heart rate data, the transmitral gradient was strongly associated with mortality after adjustment for heart rate. In addition, this study could not correct for measures of diastolic dysfunction, leaving unknown the relative contribution of LV filling abnormalities to the observed transmitral gradient.22 Finally, we lack data on the prevalence of malignancy in this elderly population and are thus unable to evaluate its role in the observed mortality.
Conclusions
In MAC-related MV dysfunction, the mean transmitral gradient is associated with increased mortality after adjustment for age, sex, and MAC-associated risk factors such as hypertension, diabetes, CAD and CKD. Concomitant MR is associated with excess mortality risk in low-gradient ranges (≤5 mmHg) but not at higher gradient, indicating prognostic utility of the transmitral gradient in MAC-related MV dysfunction regardless of MR severity. Future studies are needed to determine whether relief of the valve lesion can normalize the transmitral gradient and result in improved outcome.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Ellison Foundation, Boston, MA, and National Institutes of Health (NIH) grant (R01 HL141917) to R.A.L. and J.H.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Philippe B Bertrand, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Timothy W Churchill, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Evin Yucel, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Mayooran Namasivayam, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Samuel Bernard, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Yasufumi Nagata, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Wei He, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Carl T Andrews, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Michael H Picard, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Arthur E Weyman, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Robert A Levine, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
Judy Hung, Division of Cardiology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St Boston, MA 02114, USA.
References
- 1. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol 2015;66:1934–1941. [DOI] [PubMed] [Google Scholar]
- 2. Pressman GS, Agarwal A, Braitman LE, Muddassir SM. Mitral annular calcium causing mitral stenosis. Am J Cardiol 2010;105:389–391. [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, Benjamin EJ. Mitral annular calcification predicts cardiovascular morbidity and mortality. Circulation 2003;107:1492–1496. [DOI] [PubMed] [Google Scholar]
- 4. Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS, Eng J, Budoff MJ. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2010;213:558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Labovitz AJ, Nelson JG, Windhorst DM, Kennedy HL, Williams GA. Frequency of mitral valve dysfunction from mitral annular calcium as detected by Doppler echocardiography. Am J Cardiol 1985;55:133–137. [DOI] [PubMed] [Google Scholar]
- 6. Movahed MR, Saito Y, Ahmadi-Kashani M, Ebrahimi R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound 2007;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oktay AA, Gilliland YE, Lavie CJ, Ramee SJ, Parrino PE, Bates M, Shah S, Cash ME, Dinshaw H, Qamruddin S. Echocardiographic assessment of degenerative mitral stenosis: a diagnostic challenge of an emerging cardiac disease. Curr Probl Cardiol 2017;42:71–100. [DOI] [PubMed] [Google Scholar]
- 8. Bertrand PB, Mihos CG, Yucel E. Mitral annular calcification and calcific mitral stenosis: therapeutic challenges and considerations. Curr Treat Options Cardiovasc Med 2019;21:19. [DOI] [PubMed] [Google Scholar]
- 9. Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, Pershad A, Fang HK, O’Hair D, Jones N, Mahadevan VS, Dumonteil N, Rodés-Cabau J, Piazza N, Ferrari E, Ciaburri D, Nejjari M, DeLago A, Sorajja P, Zahr F, Rajagopal V, Whisenant B, Shah PB, Sinning J-M, Witkowski A, Eltchaninoff H, Dvir D, Martin B, Attizzani GF, Gaia D, Nunes NSV, Fassa A-A, Kerendi F, Pavlides G, Iyer V, Kaddissi G, Witzke C, Wudel J, Mishkel G, Raybuck B, Wang C, Waksman R, Palacios I, Cribier A, Webb J, Bapat V, Reisman M, Makkar R, Leon M, Rihal C, Vahanian A, O’Neill W, Feldman T. 1-Year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71:1841–1853. [DOI] [PubMed] [Google Scholar]
- 10. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL; On behalf of the Scientific Document Committee of the European Association of Cardiovascular Imaging: Thor Edvardsen, Oliver Bruder, Bernard Cosyns, Erwan Donal, Raluca Dulgheru, Maurizio Galderisi, Patrizio Lancellotti, Denisa Muraru, Koen Nieman, Rosa S; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611–644. [DOI] [PubMed] [Google Scholar]
- 11. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 12. Okuno T, Asami M, Khan F, Praz F, Heg D, Lanz J, Kassar M, Khalique OK, Grani C, Brugger N, Raber L, Stortecky S, Valgimigli M, Windecker S, Pilgrim T. Does isolated mitral annular calcification in the absence of mitral valve disease affect clinical outcomes after transcatheter aortic valve replacement? Eur Heart J Cardiovasc Imaging 2020;21:522–532. [DOI] [PubMed] [Google Scholar]
- 13. Movva R, Murthy K, Romero-Corral A, Seetha Rammohan HR, Fumo P, Pressman GS. Calcification of the mitral valve and annulus: systematic evaluation of effects on valve anatomy and function. J Am Soc Echocardiogr 2013;26:1135–1142. [DOI] [PubMed] [Google Scholar]
- 14. Kohsaka S, Jin Z, Rundek T, Boden-Albala B, Homma S, Sacco RL, Di Tullio MR. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc Imaging 2008;1:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasca I, Dang P, Tyagi G, Pai RG. Survival in patients with degenerative mitral stenosis: results from a large retrospective cohort study. J Am Soc Echocardiogr 2016;29:461–469. [DOI] [PubMed] [Google Scholar]
- 16. Kato N, Padang R, Scott CG, Guerrero M, Pislaru SV, Pellikka PA. The natural history of severe calcific mitral stenosis. J Am Coll Cardiol 2020;75:3048–3057. [DOI] [PubMed] [Google Scholar]
- 17. Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875–883. [DOI] [PubMed] [Google Scholar]
- 18. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Roffi M, Alfieri O, Agewall S, Ahlsson A, Barbato E, Bueno H, Collet J-P, Coman IM, Czerny M, Delgado V, Fitzsimons D, Folliguet T, Gaemperli O, Habib G, Harringer W, Haude M, Hindricks G, Katus HA, Knuuti J, Kolh P, Leclercq C, McDonagh TA, Piepoli MF, Pierard LA, Ponikowski P, Rosano GMC, Ruschitzka F, Shlyakhto E, Simpson IA, Sousa-Uva M, Stepinska J, Tarantini G, Tchétché D, Aboyans V, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Kzhdryan HK, Mascherbauer J, Samadov F, Shumavets V, Camp GV, Lončar D, Lovric D, Georgiou GM, Linhartova K, Ihlemann N, Abdelhamid M, Pern T, Turpeinen A, Srbinovska-Kostovska E, Cohen A, Bakhutashvili Z, Ince H, Vavuranakis M, Temesvári A, Gudnason T, Mylotte D, Kuperstein R, Indolfi C, Pya Y, Bajraktari G, Kerimkulova A, Rudzitis A, Mizariene V, Lebrun F, Demarco DC, Oukerraj L, Bouma BJ, Steigen TK, Komar M, De Moura Branco LM, Popescu BA, Uspenskiy V, Foscoli M, Jovovic L, Simkova I, Bunc M, de Prada JAV, Stagmo M, Kaufmann BA, Mahdhaoui A, Bozkurt E, Nesukay E, Brecker SJD; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 19. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 20. Karp K, Teien D, Bjerle P, Eriksson P. Reassessment of valve area determinations in mitral stenosis by the pressure half-time method: impact of left ventricular stiffness and peak diastolic pressure difference. J Am Coll Cardiol 1989;13:594–599. [DOI] [PubMed] [Google Scholar]
- 21. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M; American Society of Echocardiography, European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23, quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 22. Reddy YNV, Murgo JP, Nishimura RA. Complexity of defining severe “stenosis” from mitral annular calcification. Circulation 2019;140:523–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.