Abstract
BACKGROUND
Delta-24-RGD, an oncolytic adenovirus, shows promise against glioblastoma. To enhance virus delivery, we recently demonstrated that human bone marrow-derived mesenchymal stem cells loaded with Delta-24-RGD (hMSC-D24) can eradicate glioblastomas in mouse models. There are no studies examining the safety of endovascular selective intra-arterial (ESIA) infusions of MSC-D24 in large animals simulating human clinical situations.
OBJECTIVE
To perform canine preclinical studies testing the feasibility and safety of delivering increasing doses of hMSCs-D24 via ESIA infusions.
METHODS
ESIA infusions of hMSC-D24 were performed in the cerebral circulation of 10 normal canines in the target vessels (internal carotid artery [ICA]/P1) via transfemoral approach using commercially available microcatheters. Increasing concentrations of hMSC-D24 or particles (as a positive control) were injected into 1 hemisphere; saline (negative control) was infused contralaterally. Toxicity (particularly embolic stroke) was assessed on postinfusion angiography, diffusion-weighted magnetic resonance imaging, clinical exam, and necropsy.
RESULTS
ESIA injections were performed in the ICA (n = 7) or P1 (n = 3). In 2 animals injected with particles (positive control), strokes were detected by all assays. Of 6 canines injected with hMSC-D24 through the anterior circulation, escalating dose from 2 × 106 cells/20 mL to 1 × 108 cells/10 mL resulted in no strokes. Two animals had ischemic and hemorrhagic strokes after posterior cerebral artery catheterization. A survival experiment of 2 subjects resulted in no complications detected for 24-h before euthanization.
CONCLUSION
This novel study simulating ESIA infusion demonstrates that MSCs-D24 can be infused safely at least up to doses of 1 × 108 cells/10 mL (107 cells/ml) in the canine anterior circulation using commercially available microcatheters. These findings support a clinical trial of ESIA infusion of hMSCs-D24.
Keywords: Endovascular, Cerebrovascular, Glioma, Glioblastoma, Microcatheter, Superselective, Intra-arterial
ABBREVIATIONS
- ADC
apparent diffusion coefficient
- ESIA
endovascular selective intra-arterial
- GBM
Glioblastoma
- GFP
green fluorescent protein
- IA
intra-arterial
- ICA
internal carotid artery
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MTD
maximal tested dose
- PCA
posterior cerebral artery
- SAH
subarachnoid hemorrhage
Glioblastoma (GBM) is recalcitrant to conventional therapies.1 We have developed a novel tumor-selective oncolytic adenovirus, Delta-24-RGD (Delta-24) to combat GBM.2 In a recent clinical trial, we showed that Delta-24 is capable of replicating in human tumors after direct intratumoral injection, resulting in cell death through oncolysis, while also triggering an anti-glioma CD8 T cell immune-mediated response, all of which is capable of resulting in long term tumor eradication.3 This trial has positioned Delta-24 as a promising novel viro-immunotherapy.4
Unfortunately, this trial also showed that direct intratumoral injection of Delta-24 is not an ideal method for delivery. Intratumoral injection results in limited initial distribution as the virus was localized primarily around the injection catheter. Alternative delivery methods that distribute large amounts of virus widely through the tumor would be of benefit and increase the potential for more robust oncolytic effects. Intravascular delivery meets these requirements, but naked adenovirus is prohibited by immune-mediated viral inactivation and by peripheral organ toxicity, particularly viral-induced liver failure.
We recently showed that human bone marrow-derived mesenchymal stem cells (BM-hMSC) are capable of homing to gliomas.5-7 BM-hMSC loaded with Delta-24 (hMSCs-D24) maintain their ability to home to gliomas after intra-arterial (IA) injection, and are capable of delivering and releasing Delta-24 into human glioma xenografts in immune-deficient mice.5 In several independent experiments, hMSCs-D24 treatment resulted in a significant improvement in median survival and cures in 30% to 40% of mice.5 These data provide a rationale for using hMSCs to deliver Delta-24 to patients with GBMs in an effort to enhance viral delivery.
The clinical implementation of IA delivery of hMSCs-D24 in patients with GBM has received little attention up to now. IA delivery of “empty” hMSCs has been tested in clinical trials for other neurological disorders,8,9 but the safety10 and feasibility of delivering virally loaded BM-hMSC have not been assessed. To begin to address this, we recently reported in Vitro studies demonstrating that hMSCs-D24 are compatible with several clinical-grade microcatheters, and co-infusion with medications commonly used in endovascular procedures (verapamil, heparin), either alone or in combination does not alter hMSC-D24 viability.11
We now build upon these in Vitro studies by assessing the in Vivo feasibility of endovascular selective intra-arterial (ESIA) delivery12 of hMSCs-D24 in a nontumor-bearing canine model. We establish the technical feasibility, safety, and maximum tolerated dose in a large animal model on a scale similar to humans.
METHODS
Preparation, Labeling, and Transduction of MSCs
hMSCs were prepared and transfected with DNX-2401 (Delta-24-RGD) as previously described,11 with green fluorescent protein (GFP) labeling of the vector.5 Full description is provided in Supplemental Digital Content 1. The stem cells were approximately 18 to 20 microns in size.
Dose Escalation
A stepwise dose escalation was used with each subsequent subject, starting at 2 × 106 cells/20 mL. Doses were chosen based on prior mouse studies and human studies.3,5
Animal Procedure
Full descriptions of the procedure are available by protocol (Supplemental Digital Content 1), and for each individual procedure (Supplemental Digital Content 2). Briefly, adult mongrel canines were anesthetized with complete general anesthesia. All procedures were approved by the institutional animal care and use committee (IACUC). In the animal angiography suite, via right femoral arterial access, the target vessel (distal internal carotid artery [ICA] for anterior circulation, P1 posterior cerebral artery (PCA) for posterior circulation) was selected with the microcatheter (Echelon 14, Medtronic, inner diameter 0.017″). Animals were systemically heparinized. Baseline angiography was performed in each hemisphere. Infusion of the experimental agent was performed in one hemisphere over 25 to 30 min. The opposite ICA or PCA was then accessed and a normal saline infusion of equal duration and volume was performed as an internal control. The experimental hemisphere was infused first followed by the control injections of normal saline to maximize the infusion to magnetic resonance imaging (MRI) time. Postinfusion angiography was performed to assess for angiographic stroke. The anesthetized animals then underwent MRI, at least 1-h postinfusion to allow complications to be detected while accounting for the logistics of maintaining anesthesia. Animals were then euthanized. Gross anatomical pathology and histological analyses were performed by independent veterinary pathologists.
Positive Controls
In 2 animals, a “positive control” was performed, with the intention to induce a stroke by endovascular means that would be detectable on MRI and pathology. In these controls, LC Bead Lumi™ 70-micron particles (BTG, London, United Kingdom) were used to embolize 1 hemisphere to induce an ischemic stroke. Otherwise, the procedure was performed as above.
Survival Experiments
Two animals were infused at the maximum dose as a survival experiment. Immediately after the procedure, the animals were recovered from anesthesia and extubated. They were monitored for 24 h with neurological13 and systemic assessments. After 24 h postprocedure, an MRI was performed. The animals were then euthanized and assessed as above.
Postprocedure Assessments
Imaging protocol
The MRI studies were performed on a 3-Tesla MRI system (Trio Tim, Siemens Healthcare, Erlangen, Germany). T1, T2, T2 FLAIR, and diffusion weighted imaging with apparent diffusion coefficient (ADC) maps were generated. Please refer to Supplemental Digital Content 1 for further MRI acquisition detail.
Magnetic resonance image processing
The magnetic resonance (MR) images were transferred to Horos (The Horos Project, Horosproject.org). Two regions of interest were drawn on the ADC maps in the centrum semiovale and cerebellum in each dog. In addition, a region of interest was drawn on the ADC map in focal areas of abnormally low signal intensity. The ADC mean values and minimum values were recorded. A board-certified diagnostic radiologist (MC) read all the MRIs and was blinded to the clinical protocol and pathologic findings.
Tissue Analyses
Organs (eyes, brain, lungs, heart, liver, spleen, and kidneys) were harvested and representative sections were taken. Gross inspection was performed to assess for large vessel injury or organ injury. Histological analysis was performed for any signs of end-organ damage. A board-certified veterinary pathologist performed all necropsy and histological analyses. GFP was used to localize the GFP-labeled BM-hMSCs in the tissues and brain. Histological sections (N = 10/organ) were analyzed by fluorescent microscopy to identify hMSCs-D24 in brain and peripheral organs.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism (GraphPad Software, San Diego, California). Standard deviation is given for means where appropriate.
RESULTS
Subjects and Procedural Outcome
Ten adult canines were included in this study. Infusion was achieved in all 10 (Supplemental Digital Content 1). The ICAs were catheterized in 7 canines and the PCAs were accessed in three. The procedural results and animal subject details are summarized in Table 1. Microcatheter access and selective angiography of the anterior and posterior circulation are shown in Figure 1.
TABLE 1.
Angiography and Infusion Summary
| Subject | Age | Weight | Infusion concentration/volume | Cell infusion duration; Location | Control injection duration; Location | Angiographic result | Procedure outcomes |
|---|---|---|---|---|---|---|---|
| Canine 1 | 10 mo 13 d | 35 kg (77 lbs) | 2 × 106 cells/20 mL | 32 min; L ICA | 17 min; R ICA | No angiographic occlusions | No complications |
| Canine 2 | 8 mo 6 d | 27.5 kg (60.5 lbs) | 1 × 107 cells/20 mL | 40 min 50 s; L ICA | 15 min 28 s; R ICA | No angiographic occlusions | No complications |
| Canine 3 | 9 mo 22 d | 28.3 kg (61.8 lbs) | 2 × 107 cells/20 mL | 22 min 21 s; L ICA | 20 min 34 s; R ICA | No angiographic occlusions | No complications |
| Canine 4 | 10 mo 26 d | 28.8 kg (63.5 lbs) | 1 × 108 cells/20 mL | 21 min; R ICA | 17 min; L ICA | No angiographic occlusions | No complications |
| Canine 5 | 8 mo 22 d | 33.1 kg (72.9 lbs) | 1 × 108 cells/20 mL | 21 min 34 s; L PCA | 18 min; R PCA | No angiographic occlusions | Technical difficulty in PCA access |
| Canine 6 | 9 mo 14 d | 32.7 kg (72.1 lbs) | 1 × 108 cells/10 mL | 11 min 26 s; L PCA | 10 min 12 s; R PCA | No angiographic occlusions | Basal cistern/perivascular SAH, no stroke or other complications |
| Canine 7 | 8 mo 15 d | 27.6 kg (60.8 lbs) | Lumi Particles; Positive control #1 | R PCA | L PCA no injection | Slowed flow in R PCA without occlusion | Basal cistern/perivascular SAH, no stroke or other complications |
| Canine 8 | 9 mo 27 d | 33.6 kg (74.1 lbs) | Lumi Particles; Positive control #2 | R ICA | No injection | Complete occlusion of R ICA, stagnant LICA flow | Numerous particles found in meningeal vessels and brain on both hemispheres. |
| Canine 9 | 8 mo 20 d | 26.0 kg (57.3 lbs) | 1 × 108 cells/10 mL Survival #1 |
R ICA | L ICA | No angiographic occlusions | No complications |
| Canine 10 | 9 mo 2 d | 29.3 kg (64.6 lbs) | 1 × 108 cells/10 mL Survival #2 |
L ICA | R ICA | No angiographic occlusions | No complications |
Angiographic result is summarized in comparison to baseline angiography prior to infusion. Technical notes or complications are also described. Full procedural reports are available in Supplemental data. For all ICA injections, a Synchro2 microwire (0.014”, Stryker Neurovascular) over an Echelon 14 microcatheter was used. PCA injections were more technically challenging. When inaccessible with the Synchro2/Echelon 14 system, a Marathon microcatheter (Medtronic) over a Mirage microwire (.008”, Medtronic) was used.
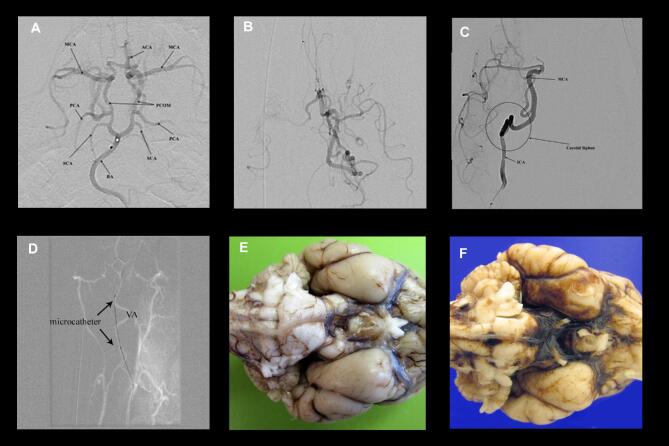
FIGURE 1.
Angiography in canine subjects. A, Diagnostic cerebral angiogram (DSA) of the circle of Willis in the canine. The microcatheter is positioned near the basilar apex (BA). Both the anterior circulation (anterior cerebral artery (ACA), middle cerebral artery (MCA)) and posterior circulation (posterior communicating artery (PCOM), posterior cerebral artery (PCA), and superior cerebellar arteries (SCAs)) are visible. B, DSA of the posterior circulation with the microcatheter positioned in the P1 PCA. Via the PCOM, the anterior circulation is partially opacified along with the PCA. This demonstrates that the canine has a posterior circulation dominance compared the anterior circulation. C, DSA of the anterior circulation with the microcatheter positioned in the proximal ICA at the bottom of the panel. The carotid siphon is very tortuous, precluding further selective access. D, microcatheter access via the vertebral artery, roadmap view. The microcatheter can be seen outside of the roadmap of the artery. This demonstrates that the vessel is highly mobile and being easily straightened by a flexible, soft microcatheter. It is likely this manipulation that led to vessel injury in posterior circulation subjects. E, Gross pathology looking at the ventral brainstem and cerebrum in subject 1, with no procedural complications. F, Same view in subject 6 demonstrating diffuse basal cistern SAH.
Infusion Doses and Angiographic Outcomes After Infusions
In 8 animals, hMSCs-D24 was infused in one hemisphere and saline (negative control) was infused into the opposite hemisphere (Table 1). Specifically, in each animal hMSC-D24 was infused over 20 to 30 min using a handheld technique. The cell dose was increased with each subsequent animal starting at 2 × 106 cells in 20 mL up to 1 × 108 cells in 20 mL (Table 1). This was then concentrated to 1 × 108 cells in 10 mL. No strokes were found on baseline angiography. Post-infusion angiography showed no evidence of embolic strokes after infusion of hMSC-D24 at all dose levels or after infusion of saline into the ICA (N = 5) or PCA (N = 3).
MRI Outcomes
Postprocedure MRI was performed in all subjects to assess for intracerebral complications, particularly ischemic stroke (Table 2, Supplemental Digital Content 3). Quantitative analysis was based on previous canine MRI studies.14,15 The normal mean ADC values have been determined to be above 700 μm2/s (Table 3).
TABLE 2.
MRI Results
| Subject | Cell concentration | Cell infusion location/duration | Saline infusion location/duration | MR findings | MRI notes |
|---|---|---|---|---|---|
| Canine 1 | 2 × 106 cells/20 mL | 32 min 0 s L ICA | 17 min 0 s; R ICA | normal | |
| Canine 2 | 1 × 107 cells/20 mL | 40 min 50 s; L ICA | 15 min 28 s; R ICA | left thalamic/left brainstem infarct | Infarct uncorrelated with cell infusion; technical issue, related to access |
| Canine 3 | 2 × 107 cells/20 mL | 22 min 21 s; L ICA | 20 min 34 s; R ICA | normal | |
| Canine 4 | 1 × 108 cells/20 mL | 21 min 0 s; R ICA | 17 min 0 s; L ICA | normal | |
| Canine 5 | 1 × 108 cells/20 mL | 21 min 34 s; L PCA | 18 min 0 s; R PCA | left cerebellar infarct, IVH | Left PCA cell infusion, likely access-related stroke |
| Canine 6 | 1 × 108 cells/10 mL | 11 min 26 s; L PCA | 10 min 12 s; R PCA | diffuse stroke, IVH | Left PCA cell infusion, likely access-related stroke |
| Canine 7 | Positive stroke control | R PCA particles Positive control #1 |
no injection | diffuse stroke, IVH, SAH | Right PCA PVA particle embolization, but likely access-related stroke |
| Canine 8 | Positive stroke control | R ICA particles Positive control #2 |
no injection | diffuse stroke | Right ICA Lumi particle embolization; penetrated bilaterally |
| Canine 9 | 1 × 108 cells/10 mL | R ICA | L ICA | normal | Survival |
| Canine 10 | 1 × 108 cells/10 mL | L ICA | R ICA | normal | Survival |
The stroke in subject 2 was in the contralateral posterior circulation territory relative to the cell infusion; thus it was determined (independently by the reading radiologist) that it was likely related to access rather than cell infusion. The remaining MRI-detected complications occurred in posterior circulation access cases. Quantitative analysis of MRI results is shown in Table 3. MRI was found to be sensitive to hemorrhagic and ischemic complications in the 2 positive control subjects (7 and 8), which were accessed via the right PCA and ICA, respectively. The PCA infusion subject had embolic particles in the vessel infused and had diffuse ischemic stroke, but also intraventricular hemorrhage (IVH) and SAH, which would be attributable to microcatheterization of the posterior circulation rather than the particles themselves. Subject 8 had a more characteristic global ischemic caused from right ICA infusion of embolic particles. This confirmed the sensitivity of MRI to detect hemorrhagic as well as ischemic complications.
TABLE 3.
Quantitative MRI Analysis
| Supratentorial | Infratentorial | ||||
|---|---|---|---|---|---|
| Subject | ADC mean | ADC min | ADC mean | ADC min | Lesions (Location, ADC mean/min) |
| Canine 1 | 768.9 | 628 | 816 | 718 | |
| Canine 2 | 804 | 630 | 894 | 684 | L Thalamus (596/564) |
| L Brainstem (555/339) | |||||
| Canine 3 | 764 | 710 | 821 | 789 | |
| Canine 4 | 848 | 685 | 746 | 614 | |
| Canine 5 | 868 | 694 | 878 | 709 | L Cerebellum (485/276) |
| L Medial Temporal (593/364) | |||||
| R Parietal (853/186) | |||||
| L Parietal (710/403) | |||||
| Canine 6 | 519 | 472 | 452 | 371 | Global ischemia |
| Canine 7 | 492 | 360 | 503 | 342 | Global ischemia |
| Canine 8 | 469 | 397 | 533 | 375 | Global ischemia |
| Canine 9 | 756 | 682 | 702 | 591 | |
| Canine 10 | 823 | 676 | 827 | 691 | |
ADC map was used to assess for ischemia as described in the Methods section. Based on previous canine MRI studies the normal mean ADC values have been determined to be above 700 μm2/s.
Embolic Particle-Infusion (Positive Control)
To test the sensitivity of our assays for their ability to detect stroke, Lumi radio-opaque embolic particles were infused (subject 7 in the PCA and subject 8 in the ICA) and demonstrated angiographic occlusion, radiographic stroke, and pathologic occlusion (Figure 2).
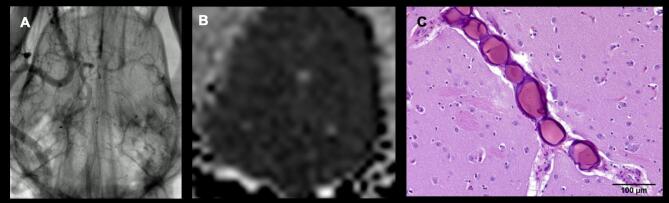
FIGURE 2.
Multimodality assessment of toxicity. Toxicity of the cell infusion (stroke) was assessed by angiography, MRI, and necropsy. These are shown in the positive control subjects 7 and 8. A, Angiography demonstrating contrast stagnation and subtracted vascular cast of the brain caused by radio-opaque embolic particle injection. This demonstrates the expected appearance and detectability of large vessel stroke by angiography. The result was most dramatic in subject 8, shown here, with complete occlusion of the right, demonstrating our ability to identify angiographic occlusion in this animal model. B, MRI Brain, FLAIR sequence demonstrating diffuse sulcal effacement and gray-white matter blurring. The paired ADC sequence shows diffusely low ADC signal. C, In all sections of the cerebrum and midbrain examined there were numerous intravascular, spherical, laminated foreign bodies approximately 40-100 microns in diameter; these were seen especially along the middle cerebral artery and in small arterioles. While the vessels were occluded, histological development of stroke was not yet observed.
MSC-D24-Infusion
No strokes were observed in the anterior circulation infusions with hMSCs-D24 (n = 6). MRI was normal at doses up to 1 × 108 cells/10 mL (Figure 3), including in both survival experiments (Table 2, Figure 4). However, in 2 canines with infusions through the posterior circulation, ischemia in and out of the territory of MSC infusion was noted.
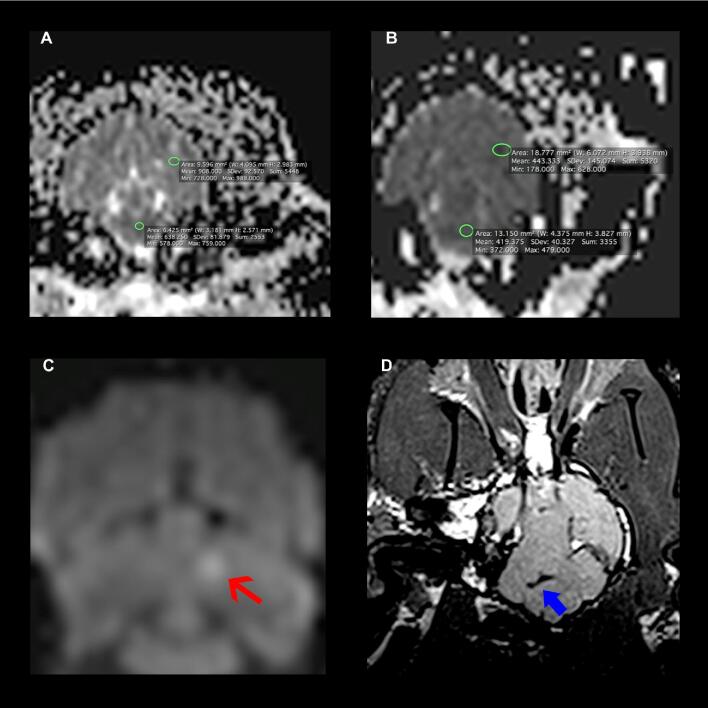
FIGURE 3.
Selected postprocedure brain MRIs. A, Subject 1, normal MRI ADC map. Circled are selected regions showing supratentorial and infratentorial minimum ADC values of 908 and 578 respectively. B, Subject 6, MRI ADC map demonstrating global ischemia. Circled are selected regions showing supratentorial and infratentorial minimum ADC values of 443 and 372 respectively. C, Subject 5, MRI diffusion weighted imaging (DWI) sequence demonstrating focal signal abnormality that had a corresponding low ADC signal, consistent with acute infarct in the left cerebellum (red arrow). D, Subject 6, MRI FLAIR sequence demonstrating low signal consistent with layering of blood (SAH) in the basal cisterns (blue thick arrow).
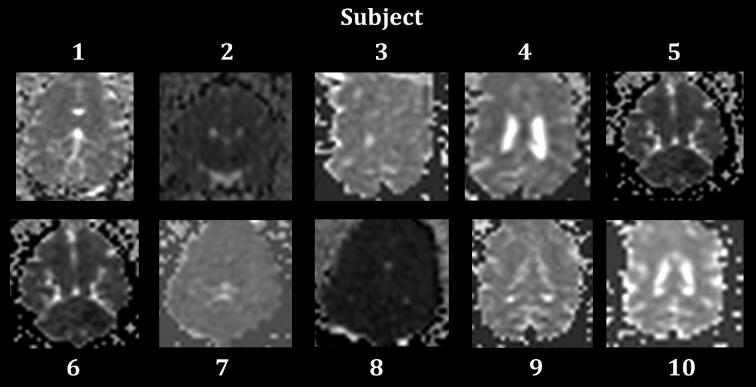
FIGURE 4.
Selected ADC slices from all 10 subjects. MRI results are summarized for all 10 in Table 2. Subject 2 had a left thalamic stroke thought to be a result of the control saline infusion through the left ICA, given the large posterior communicating arteries in the canine (Figure 1). Subject 5 had a left cerebellar stroke. Subject 6 had a diffuse stroke. Subjects 7 and 8, positive stroke controls in the right PCA and right ICA respectively, also had diffuse strokes.
Saline-Infusion
Normal saline infusion was performed in each of the 8 MSC-D24-infused subjects in the contralateral hemisphere as a control for the baseline risk of ischemic stroke from the access and infusion. ADC analysis confirmed ischemic stroke in 1 of 8 hemispheres infused (12.5%) with saline (Figure 4). In this subject, the MSC-infused hemisphere did not demonstrate any complications.
Pathology–Gross, Histology, and Fluorescence
Gross pathology and histological analyses of representative sections were performed on the brain, eyes, liver, kidneys, lungs, and liver of all cell infusion subjects (Supplemental Digital Content 4).
Brain
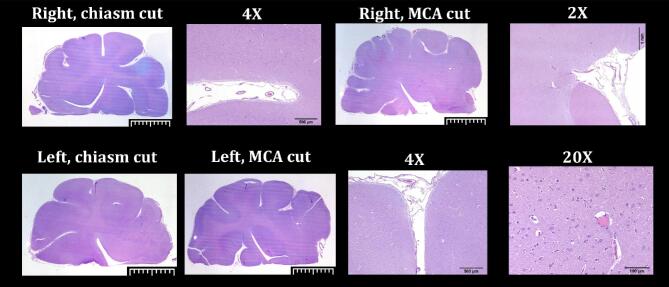
Cerebral complications were noted by pathology in subjects 6 and 7, which were both accessed via the posterior circulation. Subarachnoid hemorrhage (SAH) around the basilar artery and caudal brain stem was seen, likely as a result of traumatic microcatheterization (Figure 1). In the remaining 6 animals that received hMSC-D24 into the anterior circulation, there was no gross or histological evidence of strokes on necropsy (Table 4, Figure 5). GFP-labeled hMSC-D24 cells were not identified in histological analysis in any cerebral sections (in either hemisphere).
TABLE 4.
Necropsy and Histology Results
| Subject | Cell concentration | Cell infusion location | Saline infusion location | Gross pathology | Histology and GFP |
|---|---|---|---|---|---|
| Canine 1 | 2 × 106 cells/20 mL | L ICA | R ICA | No significant lesions observed | No significant lesions observed. |
| Canine 2 | 1 × 107cells/20 mL | L ICA | R ICA | No significant lesions observed | Brain edema, meningeal, moderate, multifocal, acute. No difference observed between hemispheres. |
| No significant lesions observed in peripheral organs. | |||||
| Canine 3 | 2 × 107cells/20 mL | L ICA | R ICA | No significant lesions observed | No significant lesions observed. |
| Canine 4 | 1 × 108cells/20 mL | R ICA | L ICA | No significant lesions observed | No significant lesions observed. |
| Canine 5 | 1 × 108 cells/20 mL | L PCA | R PCA | No significant lesions observed | No evidence of stroke or vascular obstruction was observed in the brain. |
| No significant lesions observed in peripheral organs. | |||||
| Canine 6 | 1 × 108 cells/10 mL | L PCA | R PCA | Perivascular hemorrhage around distal BA | Hemorrhage, leptomeningeal, focally extensive and moderate, multifocal, acute. No evidence of stroke or vascular obstruction was observed in the brain. |
| No significant lesions observed in peripheral organs. | |||||
| Canine 7 | Positive stroke control | R PCA particles | no infusion | Cisterna magna SAH; peri-medullary hemorrhage | Hemorrhage, leptomeningeal, regional (caudal brain stem), moderate, multifocal coalescing, acute. |
| Intravascular foreign bodies, bilateral small arterioles – moderate number, acute. No evidence of stroke other than particle obstruction. | |||||
| Canine 8 | Positive stroke control | R ICA particles | no infusion | No significant lesions observed | Intracerebral intravascular beads, marked, diffuse, acute, also in the eye (choroid and optic nerve), and meninges. No morphologic evidence of ischemia was observed in the brain or eye. |
| Canine 9 | 1 × 108 cells/10 mL | R ICA | L ICA | No significant lesions observed | No morphologic indications of ischemia in the brain or other tissues are observed |
| Canine 10 | 1 × 108 cells/10 mL | L ICA | R ICA | No significant lesions observed | No morphologic indications of ischemia in the brain or other tissues are observed. Clump of large mononuclear round cells seen in a large meningeal artery. |
Incidental findings (eg, hepatocellular lipidosis) that were independently determined to be unrelated are available in supplemental data reports. BA = basilar artery.
FIGURE 5.
Cerebral pathology. H&E slides of the canine cerebrum following Delta-24-MSC infusion. No histological evidence of stroke was noticed in subjects with anterior circulation infusions. Assessment of GFP-labeled cells was also performed (not shown).
In the positive control animals (subjects 7 and 8), H&E histology demonstrated the expected intravascular sequestration and occlusion by the embolic particles. No histological changes associated with ischemia were seen, which would not be expected in the short time window following stroke (1-2 h). This confirms MRI/ADC analysis to be the more sensitive assay.
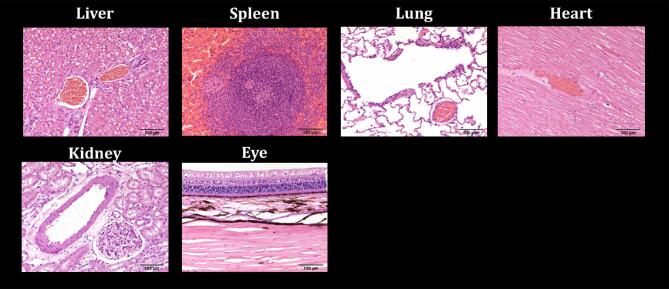
Peripheral Organs
Peripheral organs were removed in all animals; gross inspection revealed no abnormalities. Analyses by standard light microscopy in the eight animals infused with hMSC-D24 did not detect any pathological findings (Figure 6). Importantly, all sections were analyzed by fluorescent microscopy to detect GFP-labeled MSC-D24, and none were found.
FIGURE 6.
Peripheral pathology. Representative sections were taken from each organ and stained with H&E. Delta-24-MSCs were not identified in any of the peripheral organs supplied. Further, histologic and gross damage was not seen in these organs. Specifically, there was including no evidence of inflammation, ischemia, vessel occlusion or hemorrhages in any of the eight animals infused with hMSC-D24. Embolic particles were identified in the eye in subject 8 (positive control). This confirms the angiographic observation of the ophthalmic artery supply from the ICA and represents a potential complication detectable by pathology.
Survival Experiments
Two dogs were recovered after the procedure with reversal of anesthesia and were assessed by clinical exams for 24 h. There were no immediate or delayed postprocedural clinical signs in both animals. Both subjects had normal postprocedural vital signs, neurological exam (assessed by the canine stroke scale), and general systemic assessment.
DISCUSSION
We present here a translational study of ESIA infusion of hMSC-D24 in a nontumor-bearing canines. All procedural aspects of the study were designed to recapitulate the clinical setting of a human Phase 1 dose-escalation trial. We show for the first time that GMP-grade hMSC-D24 can be safely infused in the anterior circulation up to doses of 107 cells/ml with commercially available microcatheters. These data support translation of this strategy in a human clinical trial.
Maximal Tested Dose
The initial dose started at 2 × 106 cells/20 mL and was escalated in each subsequent animal to 1 × 108 cells/20 mL (5 × 106 cells/ml). Once this dose was tolerated in two subjects, the cell solution was concentrated to 1 × 108 cells/10 mL (1 × 107/ml). Qualitatively, the character of the cell solution changed from clear/non-viscous at the low dose to viscous and cloudy, necessitating continuous agitation at 1 × 108 cells/10 mL. Despite this difference in composition, no dose-related ischemic complications were identified at any level based on post-infusion angiography, MRI or necropsy. All complications were due to endovascular access (discussed below). We did not reach an maximal tested dose (MTD), suggesting that higher doses may be possible, though due to the tendency for cells to precipitate out, further escalation was not performed. Based on mouse studies, this is expected to in the high therapeutic range.
Systemic Toxicity
A second aim of the experiment was to identify any systemic toxicity associated with ESIA infusion. The distribution of the agent in this experiment does not perfectly recapitulate the human scenario, as the nontumor bearing canine model does not have any tumor to attract the hMSCs-D24, and thus it would be expected for the cells to circulate through the cerebrovasculature and enter the heart to be systemically distributed. However, no systemic toxicities were identified in any of the eight canines who received infusions with hMSC-D24, including 2 survival subjects with clinical examinations. In previous experiments with intravenous infusions, MSCs have been found primarily in the lungs and secondarily in the liver and other organs.16 Intraarterial infusions have been noted to avoid pulmonary entrapment compared with intravenous infusion.17 Our findings are consistent with these reports. Specifically, fluorescent microscopy assessments of histological sections from systemic organs for distribution of ESIA-injected GFP-labeled MSCs did not identify GFP-labeled cells in any peripheral organs. In addition, GFP-labeled cells were not identified in the brains of any subjects. These results suggest that without a tumor to attract them,7,18 hMSCs-D24 pass through the normal cerebrovasculature and are not sequestered into peripheral organs, including the lungs at the concentrations tested in these studies. How the cells are eliminated from the body and will require further studies using continuous, whole body, real-time tracking methods.
Technical Feasibility
One of the goals of the experiment was to determine technical feasibility of the procedure, including hMSC-D24 production, procedural logistics, endovascular access, and infusion. Cells were successfully produced by a GMP laboratory, transported in a sterile container, and temporarily stored on ice into the procedure room. They were thawed during the access phase and then drawn up at room temperature once the target vessel was catheterized. The solution was mixed frequently during the infusion phase to avoid precipitation of the cells.
Selective catheterizations of the distal ICA were feasible and safe. Although catheterization of the P2 was feasible, the access resulted in stroke and SAH. Compared with humans, the small caliber and laxity of the canine basilar artery made catheterization technically difficult. We suspect that the SAH observed was from avulsion of small perforators due to the arterial hypermobility during catheterization.
Unintentional hemorrhagic and ischemic stroke was observed in 2 animal subjects with PCA catheterization while none of the 6 animals with anterior circulation infusions had strokes in the infusion distribution. The complication rate of posterior circulation access in these canines is higher than would be expected or tolerated in humans. All neurointerventionalists were very experienced; all found the access to be quite challenging due to the reasons above. Therefore, the dog basilar artery is not representative of the human clinical setting.
Limitations
This study is meant to be a feasibility/safety study to help define the MTD for ESIA infusion. The study has not directly assessed the efficacy of this therapy in a large animal model. Another limitation is the small sample size (limited due to cost and ethical concerns). The sensitivity of MRI in the canine may be limited by the small size of the canine cerebrum. Nevertheless, the data provide valuable information about the feasibility and safety of infusing hMSC-D24 into the cerebrovasculature of an animal whose circulation approaches that of humans in terms of size and pattern. Future study will be required in a tumor-bearing large animal model.
CONCLUSION
This first-of-its-kind study, simulating ESIA infusion of hMSCs-D24, demonstrates that hMSCs-D24 can be infused safely at least up to doses of 1 × 108 cells/10 mL. ESIA infusion is feasible in a large animal model with potential for translation to clinical practice. High-dose infusion does not result in dose-related toxicity. Complications, when encountered, were due to technical challenges inherent to the posterior circulation canine model. These findings support a clinical trial of ESIA infusion of hMSCs-D24.
Funding
This study was supported by NREF and AANS/CNS Cerebrovascular Section Dempsey Award (VMS). Previous portions of this work have been presented at the 2016 CV section meeting. Additional support is from the National Cancer Institute (1R01CA214749, 1R01CA247970 and 2P50CA127001), The University of Texas MD Anderson Moon Shots ProgramTM, The Broach Foundation for Brain Cancer Research, The Elias Family Fund, The Priscila and Jason Hiley Fund, The Baumann Family/Curefest Fund, The Jim and Pam Harris Fund, The Gene Pennebaker Brain Cancer Fund, The Schneider Memorial Fund, The Sweet Family Cancer Research Fund, The Gold Family Memorial Fund, The Sorenson Foundation (all to F.F.L.).
Disclosures
Dr Lang reports being a patent holder with DNAtrix, who manufactures Delta-24-RGD. Dr Kan reports being a consultant for Stryker Neurovascular, Medtronic (manufacturer of microcatheters used here), and Cerenovus. Dr Kan is a stockholder in Vena Medical, Inc. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
We appreciate assistance with production of the GMP human BM-MSCs by Dr Shpall's team at The MD Anderson Department of Stem Cell Transplantation. We thank Dr Collins’ team from the Center for Comparative Medicine (CCM) at Baylor College of Medicine for the excellent care of our animal subjects. We thank Dr Roger Price, DVM, PhD, our veterinary pathologist, for performing pathological analyses.
Notes
This material was previously presented as 1. Srinivasan VM, Gumin J, Camstra KM, Lang FF, Kan P. (2019, December 8). Delta-24 Mesenchymal Stem Cells for treating Gliomas in a Canine Model. Oral presentation at: Neurological Society of India Meeting; Mumbai, India, and 2. Srinivasan VM, Shpall E, Lang FF, Kan P. (2019, September 20-24). Endovascular Superselective Intra-arterial infusion of Mesenchymal Stem Cells loaded with Delta-24 in a Canine Model. Abstract included at: American Academy of Neurological Surgery meeting, Rome, Italy.
Contributor Information
Visish M Srinivasan, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas.
Joy Gumin, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas.
Kevin M Camstra, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Dalis E Collins, Center for Comparative Medicine, Baylor College of Medicine, Houston, Texas.
Melissa M Chen, Department of Diagnostic Radiology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Elizabeth J Shpall, Department of Stem Cell Transplantation, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Brittany C Parker Kerrigan, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas.
Jeremiah N Johnson, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Stephen R Chen, Department of Interventional Radiology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Juan Fueyo, Department of Neuro-Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Cande Gomez-Manzano, Department of Neuro-Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Frederick F Lang, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas.
Peter Kan, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas; Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center, Houston, Texas.
Supplemental Digital Content 1. Methods. Details of animal procedure, MSC production, and MRI protocol.
Supplemental Digital Content 2. Procedure Reports. Detailed procedure report of each of the 10 surgical/endovascular procedures.
Supplemental Digital Content 3. Final MRI Reports. Detailed MRI reports read by board-certified neuroradiologist.
Supplemental Digital Content 4. Canine Path Reports. Gross and histologic pathology reports for the 10 animal subjects, including brain and peripheral organ assessment.
COMMENTS
The use of BM-hMSC loaded with Delta-24 oncolytic adenovirus has been previously shown to be efficacious in murine studies, able to infiltrate gliomas following IA delivery, eradicate GBM, and prolong survival. The authors present a novel, proof-of-concept safety and feasibility study assessing the use of endovascular selective intra-arterial (ESIA) delivery of mesenchymal stem cells loaded with Delta-24 in a canine model. While IA delivery of hMSCs has been shown in both canines and human clinical trials previously, this study is the first of its kind to assess the safety and feasibility of ESIA delivery of hMSCs loaded with an oncolytic virus that specifically targets GBM. Given the significant morbidity and poor improvement in median survival in GBM to date, this presents a promising emerging adjunctive treatment modality for this disease process.
Despite low numbers in the present study, the authors effectively demonstrates the safety and feasibility of ESIA in a large animal model. Recent advancements in IA delivery of chemotherapy and endovascular delivery of neuromodulation leads and strent-electrodes have identified new minimally invasive mechanisms for the treatment of a variety of neuropathologies. ESIA delivery of hMSCs provides an exciting new treatment modality, able to deliver high concentrations of therapeutics directly to the target tissue, thereby limiting systemic side effects and improving local efficacy. This initial translational study shows promise in anticipation of later clinical applications of this treatment paradigm.
David Dornbos III
Adam S. Arthur
Memphis, Tennessee
The authors present a study investigating the safety and feasibility of delivering human bone-marrow derived mesenchymal stem cells loaded with the oncolytic adenovirus, Delta-24-RGD intraarterially by selective endovascular catheterization. Delta-24’s oncolytic potential via intratumoral injection has been previously shown, and the authors are seeking a delivery method that better covers the extent of the tumor while limiting systemic adverse effects. They were able to demonstrate the technical feasibility of intraarterial delivery of hMSC-D24 without systemic effect evidenced by the lack of hMSC in other tissues or histological signs of organ toxicity. In combination with prior referenced studies demonstrating the ability of BM-hMSC loaded with Delta-24 to deliver the virus into human glioma xenografts along with a therapeutic benefit, this study provides sufficient evidence to support a clinical trial.
While they did experience complications with posterior circulation catheterization, they attribute this to the canine anatomy, and it is reasobable to expect that the technical aspects of the endovascular procedure can be performed safely in humans. Given the lack of effective treatments for glioblastoma, short expected survival and lack of a better model to confirm these views, proceeding with future study is warranted as this represents a potentially exciting avenue for glioblastoma treatment.
Rami O. Almefty
Philadelphia, Pennsylvania
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2. Fueyo J, Alemany R, Gomez-Manzano C et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652-660. [DOI] [PubMed] [Google Scholar]
- 3. Lang FF, Conrad C, Gomez-Manzano C et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong CW, Zeng Q.. Awaiting a new era of cancer immunotherapy. Cancer Res. 2012;72(15):3715-3719. [DOI] [PubMed] [Google Scholar]
- 5. Yong RL, Shinojima N, Fueyo J et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69(23):8932-8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamizo A, Marini F, Amano T et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307-3318. [DOI] [PubMed] [Google Scholar]
- 7. Shinojima N, Hossain A, Takezaki T et al. TGF-beta mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73(7):2333-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee PH, Lee JE, Kim HS et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72(1):32-40. [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22(12):2291-2298. [DOI] [PubMed] [Google Scholar]
- 10. Cui LL, Kerkela E, Bakreen A et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srinivasan VM, Gumin J, Camstra KM et al. Microcatheter delivery of neurotherapeutics: compatibility with mesenchymal stem cells. published online: September 6, 2019. J Neurosurg. (doi:10.3171/2019.6.JNS19327). [DOI] [PubMed] [Google Scholar]
- 12. Srinivasan VM, Lang FF, Chen SR et al. Advances in endovascular neuro-oncology: endovascular selective intra-arterial (ESIA) infusion of targeted biologic therapy for brain tumors. J NeuroIntervent Surg. 2020;12(2):197-203. [DOI] [PubMed] [Google Scholar]
- 13. Christoforidis GA, Rink C, Kontzialis MS et al. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol. 2011;46(1):34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartmann A, Sager S, Failing K, Sparenberg M, Schmidt MJ. Diffusion-weighted imaging of the brains of dogs with idiopathic epilepsy. BMC Vet Res. 2017;13(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anaya Garcia MS, Hernandez Anaya JS, Marrufo Melendez O, Velazquez Ramirez JL, Palacios Aguiar R. In vivo study of cerebral white matter in the dog using diffusion tensor tractography. Vet Radiol Ultrasound. 2015;56(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12-20. [DOI] [PubMed] [Google Scholar]
- 17. Makela T, Takalo R, Arvola O et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392-402. [DOI] [PubMed] [Google Scholar]
- 18. Hata N, Shinojima N, Gumin J et al. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery. 2010;66(1):144-157; discussion 156-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.