Meizoso-Huesca and Launikonis describe multiple effects of BTP2, an inhibitor of the Ca2+ channel Orai1, on Ca2+ handling in skeletal muscle, which include impairment of Ca2+ release from the sarcoplasmic reticulum and indirect effects on the activity of the ryanodine receptors.
Abstract
BTP2 is an inhibitor of the Ca2+ channel Orai1, which mediates store-operated Ca2+ entry (SOCE). Despite having been extensively used in skeletal muscle, the effects of this inhibitor on Ca2+ handling in muscle cells have not been described. To address this question, we used intra- and extracellular application of BTP2 in mechanically skinned fibers and developed a localized modulator application approach, which provided in-preparation reference and test fiber sections to enhance detection of the effect of Ca2+ handling modulators. In addition to blocking Orai1-dependent SOCE, we found a BTP2-dependent inhibition of resting extracellular Ca2+ flux. Increasing concentrations of BTP2 caused a shift from inducing accumulation of Ca2+ in the t-system due to Orai1 blocking to reducing the resting [Ca2+] in the sealed t-system. This effect was not observed in the absence of functional ryanodine receptors (RYRs), suggesting that higher concentrations of BTP2 impair RYR function. Additionally, we found that BTP2 impaired action potential–induced Ca2+ release from the sarcoplasmic reticulum during repetitive stimulation without compromising the fiber Ca2+ content. BTP2 was found to have an effect on RYR-mediated Ca2+ release, suggesting that RYR is the point of BTP2-induced inhibition during cycles of EC coupling. The effects of BTP2 on the RYR Ca2+ leak and release were abolished by pre-exposure to saponin, indicating that the effects of BTP2 on the RYR are not direct and require a functional t-system. Our results demonstrate the presence of a SOCE channels–mediated basal Ca2+ influx in healthy muscle fibers and indicate that BTP2 has multiple effects on Ca2+ handling, including indirect effects on the activity of the RYR.
Introduction
Store-operated Ca2+ entry (SOCE) is a retrograde Ca2+ regulatory mechanism activated by a depletion of calcium in the ER/SR that causes an influx of Ca2+ into the cell. Two components, an SR Ca2+ sensor (STIM1) and a Ca2+ channel in the plasma membrane (Orai1; Soboloff et al., 2006; Feske et al., 2006) conduct SOCE. In skeletal muscle, SOCE is distinguishable by its rapid rate of activation, likely due to its specialized internal Ca2+ store, the SR, and to its specialized cell membrane, the sarcolemma (Kurebayashi and Ogawa 2001; Launikonis and Ríos, 2007; Edwards et al., 2010; Duke et al., 2010). The sarcolemma is largely internalized as a network of regularly spaced tubules, called the tubular system (t-system). The t-system wraps around each myofibril, forming junctions with the terminal SR at every sarcomere (Peachey, 1965). The primary role of the t-system is to support action potential propagation to all parts of the muscle fiber. Excitation of the t-system causes rapid release of SR Ca2+ through RYRs, communicated through protein–protein interactions with the t-system voltage sensors across these junctional membranes, in a process known as excitation–contraction (EC) coupling (Melzer et al., 1995; Stephenson, 2006).
The activity of the RYR is central to both EC coupling and SOCE, intimately linking these processes in muscle. The static platform of junctional membranes established for EC coupling allows the STIM1 isoform STIM1L to be permanently placed at the SR terminal cisternae (Darbellay et al., 2011), where RYRs are located. This provides STIM1 direct access to the transverse tubules, the site of store-dependent Ca2+ entry (Launikonis et al., 2003). The close arrangement of these Ca2+-regulatory proteins at the junctional membranes allows RYR activity to be pivotal for SOCE. In response to local RYR activity, the temporal presentations of SOCE are a small, chronic influx activated by increased levels of RYR Ca2+ leak, as observed in human muscle fibers with RYR variants and in CASQ1-null mouse fibers (Cully et al., 2018; Michelucci et al., 2020), and a phasic, greater amplitude influx activated briefly with a submillisecond delay following voltage-controlled SR Ca2+ release (Koenig et al., 2018, 2019). Both chronic and phasic SOCE—cSOCE and pSOCE, respectively—magnitude is sensitive to Ca2+ depletion within the SR (Koenig et al., 2018).
SOCE is a small Ca2+ flux, three to four orders of magnitude smaller than the release flux from the SR during EC coupling (Launikonis et al., 2010; Koenig et al., 2018). The SOCE flux has not been imaged during the release of Ca2+ from the SR in intact muscle fiber preparations because of problems separating SOCE from the contribution of cytoplasmic Ca2+ released from the SR during EC coupling. In the absence of direct measurements of SOCE in intact fiber preparations during Ca2+ release, examination of SOCE in muscle has relied on the use of pharmacologic agents, such as 2-APB, SKF-26365, and 3,5-bis(trifluoromethyl) pyrazole derivative (BTP2; also called YM-58483), that inhibit SOCE. Since its discovery and characterization as an Orai1 inhibitor, BTP2 (Trevillyan et al., 2001; Zitt et al., 2004; Ishikawa et al., 2003) has been broadly used to study cellular and physiologic aspects of SOCE; however, the effects of BTP2 on Ca2+ handing in skeletal muscle have not been defined.
Exploring the effects of BTP2 on skeletal muscle Ca2+ handling in detail would significantly aid our understanding of the physiologic roles of SOCE in skeletal muscle. To do this, we used the mechanically skinned fiber preparation that allows for simultaneous measurements of SOCE and SR Ca2+ release in the presence of a functional SR Ca2+ pump (Launikonis et al., 2003; Launikonis and Ríos, 2007). Mechanical skinning of skeletal muscle fibers is the removal of the outer plasma membrane with fine forceps, which causes the former interface between the outer plasma membrane and the t-tubular mouths to immediately seal over upon their separation (Launikonis and Stephenson, 2004; Lamb and Stephenson, 2018). The pre-exposure of the fiber to an impermeant Ca2+-sensitive dye before skinning traps the dye inside the t-system (Stephenson, 2006), while the cytoplasm is opened to experimental manipulation. Addition of a Ca2+-sensitive dye to the cytoplasm allows for the measurement of SR Ca2+ release, and the t-system–trapped dye monitors net changes in t-system [Ca2+] ([Ca2+]t-sys) from which SOCE or other t-system Ca2+ fluxes can be isolated (Launikonis and Ríos, 2007; Cully et al., 2016, 2018). Here, we applied BTP2 from the cytoplasm and localized its application to a subsection of the t-system lumen—the extracellular space—of skinned fibers to provide both in-preparation test and reference areas for assessing the effect of the compound on the t-system and SR Ca2+ handling. With these approaches, we observed that BTP2 blocked both a t-system Ca2+ channel and a SR Ca2+ leak. Additionally, we found that BTP2 impaired RYR Ca2+ release function during repetitive electrical stimulation or under lowered Mg2+ without compromising the calcium loading of the SR. The effect of BTP2 on RYR appears to be indirect and dependent on a functional t-system membrane, presumably implicating regulated Ca2+ fluxes and/or protein interactions across the junctional membranes.
Materials and methods
Muscle preparation
All experimental methods using rodents were approved by the Animal Ethics Committee at The University of Queensland. Male Wistar rats were sacrificed by asphyxiation via CO2 exposure, and the extensor digitorum longus muscles were rapidly excised from the animals. Muscles were then placed in a Petri dish under paraffin oil above a layer of Sylgard.
All chemicals were obtained from Sigma-Aldrich. BTP2, tetracaine, and N-benzyl-p-toluene sulphonamide (BTS) were prepared in stocks dissolved in DMSO. Equivalent levels of DMSO used in solutions containing BTP2 were added as vehicle to solutions used in control experiments.
Orai1 resting Ca2+ conductance experiment
Rhod-5N salt was trapped in the sealed t-system as originally described by Stephenson and Lamb (1993). Briefly, single fibers from extensor digitorum longus muscles were isolated by using fine forceps and exposed to an Na+-based physiologic solution (external solution) containing the following (in mM): 2.5 rhod-5N, 2.5 CaCl2, 132 NaCl, 1 MgCl2, 3.3 KCl, and 20 HEPES, and pH was adjusted to 7.4 with NaOH. The dye was allowed 2 min to diffuse into the t-system from the surrounding bubble of solution containing fluorescent dye. After this equilibration period, the fibers were mechanically skinned and transferred to an experimental chamber and bathed in a cytoplasmic solution containing the following (in mM): 1 Mg2+, 50 EGTAtotal, 90 HEPES, 126 K+, 36 Na+, 8 ATP, 10 creatine phosphate, and 0.05 BTS, with pH adjusted (with KOH) to 7.1. Free [Ca2+] was set to 200 nM in this solution to promote a loaded SR.
In these experiments and others, we used 1 mM tetracaine to block SR Ca2+ leak. Tetracaine in DMSO was diluted in K+-based cytoplasmic solutions to reach a final concentration of 1 mM. 1 mM tetracaine has been shown to block the leak of Ca2+ in cardiomyocytes and skeletal muscle fibers from rodent and human skeletal muscle without inducing off-target effects on t-system Ca2+ handling properties (Shannon et al., 2002; Cully et al., 2018; Rebbeck et al., 2020).
Extracellular administration of rhod-5N ± BTP2
To expose single fibers immersed in paraffin oil to BTP2, we added 10 µM BTP2 to the Na+-based physiologic solution (external solution; same formulation as above).
Local exposure of t-system lumen to BTP2
To expose discrete segments of single fibers immersed in paraffin oil to BTP2, an approximate 200-µm subsection of ∼1-mm-long single, intact section of fibers were exposed to a Na+-based physiologic solution (same formulation as above) but also containing 10 µM BTP2, using a 2-µl microcapillary tube. The localized application of BTP2 so that the same fiber had a BTP2-exposed section and a nonexposed section was possible through leveraging (1) the restriction on diffusion set by the paraffin oil surrounding the intact fiber and physiologic solution applied to it (Lamb et al., 1995) and (2) the restriction on longitudinal diffusion of small molecules within the t-system (Edwards and Launikonis, 2008). After the 2-min equilibration period, the fiber was mechanically skinned along its length, encompassing the extracellular solution–exposed region and unexposed region. The preparation was transferred to a custom-built chamber and placed under 50–70 µl K+-based cytoplasmic solution, described in Orai1 resting Ca2+ conductance experiment, for imaging on the confocal microscope.
SR Ca2+ release experiments
In experiments in which Ca2+ was released from the SR by stimulation with low [Mg2+]cyto or activated by field stimulation, fibers were mechanically skinned and transferred to the experimental chamber and bathed in a cytoplasmic solution containing the following (in mM): 1 Mg2+, 1 EGTAtotal, 90 HEPES, 126 K+, 36 Na+, 8 ATP, 10 creatine phosphate, 0.01 rhod-2, and 0.05 BTS, with pH adjusted (with KOH) to 7.1. [Ca2+] in solution was set to 100 nM to load the SR with Ca2+. Unless otherwise indicated, [Mg2+] was lowered to 0.01 mM to stimulate the thorough release of Ca2+ from the SR.
Disruption of the t-system membrane
Saponin was prepared from a stock solution of 2 mg ml−1 dissolved in a K+-based cytoplasmic solution. To selectively disrupt the t-system membrane, 50 µg ml−1 saponin was applied to the skinned fiber preparation in the cytoplasmic solution containing 67 nM [Ca2+]cyto for not longer than 2 min. Previously, we have shown that 50 µg ml−1 saponin causes a disruption of the t-system membrane within seconds to cause chronic depolarization fast enough to elicit voltage-controlled Ca2+ release observed as a force response (Launikonis and Stephenson, 2001).
SR calcium detection
To load the SR with the Ca2+-sensitive fluorescent dye fluo-5N, we adopted the method of Kabbara and Allen (2001), with minor modifications. Individual mechanically skinned fibers were mounted in an experimental chamber and bathed in 67 nM [Ca2+]cyto cytoplasmic solution (same formulation as above) with 10 µM fluo-5N acetoxymethyl (AM) ester. 10 µM carbonylcyanide p-trifluoromethoxyphenylhydrazone and 0.05% Pluronic F-127 detergent were added to decouple mitochondria and to help disperse the AM ester, respectively. Fibers were incubated for 1 h at 37°C. Thereafter, the solution was exchanged to the same internal solution but without fluo-5N AM. Fibers were then incubated for an additional 1 h at 37°C to allow for complete hydrolysis of the acetyl moiety.
Confocal imaging
Mounted skinned fibers were imaged using an Olympus FV1000 confocal microscope equipped with an Olympus 0.9NA 40× Plan-Apochromat objective. Rhod-5N trapped in the sealed t-system or cytoplasmic rhod-2 were excited with a 543-nm HeNe laser and the emission was filtered using the Olympus spectral detector. For tracking Ca2+ transients in the t-system or during direct activation of Ca2+ release with low Mg2+ in single skinned fibers, images were continuously recorded in xyt mode with an aspect ratio of 256 × 512, with the long aspect of the image parallel with that of the preparation. The temporal resolution of imaging in this mode, where the fluorescence signal from within the borders of the fiber, was 0.8 s. For imaging action potential–induced Ca2+ release, xt scanning was performed at 2 ms line−1 with the scanning line parallel to the long axis of the fiber. Scanning was always initiated before the field pulses. Field pulses were delivered at a rate of 0.5 Hz and strength of 30–50 V cm−2 (Posterino et al., 2000) using a Grass stimulator box.
Image analysis for Ca2+ measurements
t-system rhod-5N fluorescence (t) (F (t)) was collected during continuous xyt imaging during multiple internal solution changes. At the end of the experiment, each fiber was exposed to ionophore and 5 mM Ca2+, followed by 0 Ca2+ to obtain the fluorescence maximum (Fmax) and minimum (Fmin), respectively. These values were used in conjunction with the previously determined Kd of rhod-5N in the t-system of 0.872 mM (Cully et al., 2016) to determine [Ca2+]t-sys, with the relationship:
In some experiments, the Fmax solution induced vacuoles and could not be used as a suitable calibration point for the determination of [Ca2+]t-sys. Under such conditions, Fmax was determined by rearranging the above equation to
with F and [Ca2+], the respective free Ca2+ concentration in the cytoplasm (200 nM) and the t-system (calibrated in an independent set of experiments), respectively.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 8. ANOVA followed by the post hoc Tukey test for repeated measures was used to compare different groups (e.g., effect of increasing concentration of BTP2 on [Ca2+]t-sys and effect of extracellular exposure of BTP2 on SR Ca2+ release). Unpaired two-tailed Student’s t test was performed to compare means between two groups (e.g., effect of cytosolic exposure of BTP2 on BTP2–pre-exposed fibers; see Fig. 2). Paired two-tailed Student’s t test was performed when the experiment involved in-preparation reference and test areas (e.g., saponin disrupts BTP2 effect on SR Ca2+ release). Multiple t tests using the Holm-Sidak method were used to determine the significance between the fluo-5N SR resting signal in the absence and presence of 10 µM BTP2 at different [Ca2+]cyto as well as to determine the effect of 10 µM BTP2 on electrically evoked Ca2+ transients. For all cases, differences were considered statistically significant at P < 0.05.
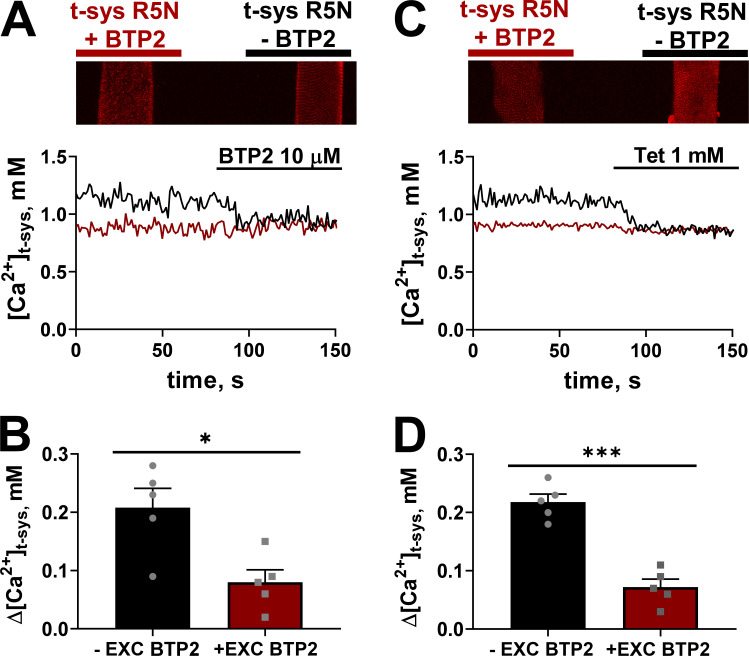
Figure 2.
Cytosolic administration of BTP2 does not induce further effects on [Ca2+]t-sys on fibers previously exposed to BTP2 extracellularly. (A) Top: Representative image of two skinned fibers with t-system–trapped rhod-5N, with (left) and without (right) 10 µM BTP2 trapped within the t-system placed parallel to each other in the same experimental chamber. Bottom: Spatially averaged [Ca2+]t-sys profiles of the two fibers simultaneously exposed to cytosolic BTP2 (10 µM). Black and red font (top) corresponds to the fiber with the same color trace (bottom). (B) Mean ± SEM of Δ[Ca2+]t-sys of fibers with and without extracellular (EXC) 10 µM BTP2 exposed to 10 µM cytosolic BTP2 (n = 5). Two-tailed unpaired t test shows a significant difference between −EXC BTP2 versus +EXC BTP2 (*, P < 0.05). (C) Top: Representative image of two skinned fibers with t-system–trapped rhod-5N, with (left) and without (right) 10 µM BTP2 trapped within the t-system placed parallel to each other in the same experimental chamber. Bottom: Spatially averaged [Ca2+]t-sys profiles of the two fibers simultaneously exposed to cytosolic tetracaine (Tet; 1 mM). Black and red font (top) corresponds to the fiber with the same color trace (bottom). (D) Mean ± SEM of Δ[Ca2+]t-sys is the difference between [Ca2+]t-sys in the presence and absence of tetracaine in fibers with and without EXC applied 10 µM BTP2 (n = 5). Data are shown as mean ± SEM, with individual data points also shown. Two-tailed unpaired t test showed a significant difference between no EXC BTP2 versus EC BTP2 (***, P < 0.0001).
Results
Experimental evidence from myotubes studies suggest that there is a resting Ca2+ influx across the plasma membrane that is sensitive to low [BTP2] (Li et al., 2010; Eltit et al., 2013). By leveraging the weak Ca2+ buffering of the sealed t-system that provides high sensitivity for detecting small changes in [Ca2+]t-sys, we assessed whether BTP2 could inhibit a resting Ca2+ conductance in adult skeletal muscle fibers. This was possible by monitoring the t-system [Ca2+] transient ([Ca2+]t-sys (t)) during the application of known BTP2 doses to the cytoplasmic solution of the skinned fiber, where a change in the steady state of [Ca2+]t-sys must be due to a change in the balance between the t-system Ca2+ leak rate and t-system Ca2+ uptake rate. Therefore, a BTP2-sensitive resting Ca2+ flux in the skinned fiber could be observed as an increase in [Ca2+]t-sys when Orai1 is inhibited.
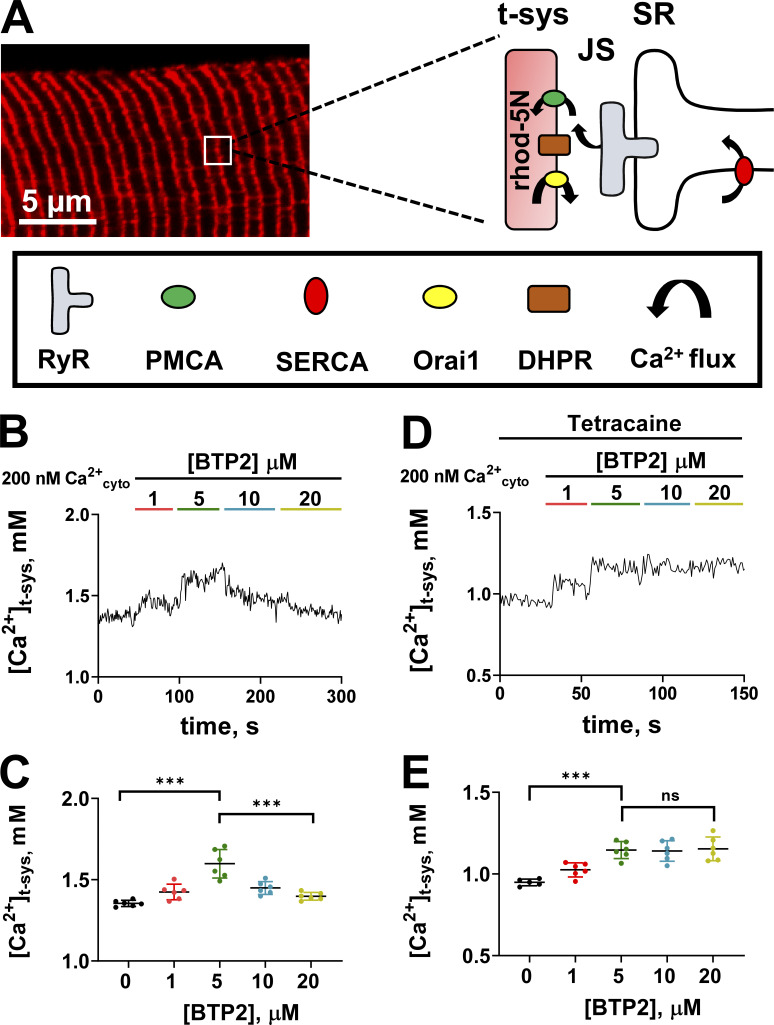
Fig. 1 A shows an example of a confocal image of rhod-5N fluorescence from the t-system of a skinned fiber. The characteristic two transverse tubules per sarcomere are clearly visible in the image. The sealed t-system with the trapped Ca2+-sensitive dye is able to detect net Ca2+ movements across the t-system membrane. Key components that influence this are shown in the expanded diagram from the image. The sealed t-system with rhod-5N has pumps and leak pathways that control the net [Ca2+]t-sys, and the major proteins are plasma membrane Ca2+ ATPase (PMCA) and Orai1, respectively. The dihydropyridine receptor (DHPR) sits on the t-system membrane opposite RYR, but is not expected to contribute to net changes in [Ca2+]t-sys at rest. Transient receptor potential (TRP) channels also exist on the t-system membrane and could be regulated by changes in [Ca2+]SR (He et al., 2005; Lopez et al., 2020b)—for simplicity, we will refer to any inhibition observed in store-operated calcium channels as Orai1 inhibition. The SR is adjacent to the t-system, with RYR almost spanning the junctional space (JS) between the SR and t-system. The RYR Ca2+ leak increases the JS [Ca2+] ([Ca2+]JS), thereby increasing the activity of the PMCA, which is observed as an increase in [Ca2+]t-sys (Cully et al., 2016, 2018).
Figure 1.
Effect of BTP2 on resting [Ca2+]t-sys. (A) t-system–trapped rhod-5N in a mechanically skinned fiber and a schematic representation of the key players of calcium handling in the junctional membranes. (B) Representative t-system calcium transient from a fiber exposed to increasing concentrations of BTP2. (C) Mean ± SD of [Ca2+]t-sys of fibers exposed to increasing concentrations of BTP2 (n = 6). One-way ANOVA followed by post hoc Tukey’s test revealed significant differences between 0 versus 5 µM BTP2 (P < 0.0001) and 5 versus 20 μM BTP2 (P < 0.0001). (D) Representative t-system calcium transient from a fiber exposed to increasing concentrations of BTP2 in the constant presence of RYR inhibitor 1 mM tetracaine. (E) Mean ± SD of [Ca2+]t-sys of fibers exposed to increasing concentrations of BTP2 in the presence of RYR inhibitor tetracaine (1 mM; n = 6). In the presence of tetracaine, one-way ANOVA followed by post hoc Tukey’s test revealed significant differences between 0 versus 5 µM BTP2 (***, P < 0.0001). Data are shown as mean ± SEM, with individual data points also shown. No differences between 5 versus 20 µM BTP2 were found (P > 0.999).
The necessary resolution for detecting tiny leaks of Ca2+ across the t-system membrane is obtained by optimizing the signal-to-noise ratio, which requires averaging the rhod-5N fluorescence signal from the many transverse tubules within the optical plane of the 100–150-µm length of the fiber in the field of view. This approach adequately captures rhod-5N transients while continuously imaging at one frame every 0.87 s, without causing bleaching. This absence of bleaching is necessary for an accurate calibration of the rhod-5N signal and [Ca2+]t-sys (Cully et al., 2016).
Fig. 1 B shows [Ca2+]t-sys (t) continuously monitored during the addition of known [BTP2] to a resting cytoplasmic solution containing 200 nM Ca2+. The sequential addition of 1 and then 5 µM BTP2 each induced increases in [Ca2+]t-sys, indicating a progressive decrease in Ca2+ leak from the t-system lumen; however, administration of 10 and 20 µM BTP2 induced the opposite effect, lowering the [Ca2+]t-sys. The biphasic effect observed suggests that higher [BTP2] initiates a secondary effect that causes the decline in [Ca2+]t-sys. A summary of these experiments is shown in Fig. 1 C. The reduction of [Ca2+]t-sys observed could be due to an increase in Ca2+ flux from the t-system lumen into the bathing solution or to a compromised ability of the t-system to uptake calcium. Previously, we have shown that the inhibition of RYR Ca2+ leak causes a decrease in [Ca2+]t-sys (Cully et al., 2018).
To test whether higher [BTP2] affected RYR Ca2+ leak and contributed to the decrease in [Ca2+]t-sys observed, we performed experiments similar to those in Fig. 1 B, with the variation that the RYR Ca2+ leak was inhibited with 1 mM tetracaine (Fig. 1 D; Shannon et al., 2002; Cully et al., 2018). In this experiment, 1 and 5 µM BTP2 exerted a comparable increase in [Ca2+]t-sys steady state, as observed in Fig. 1 B. In contrast, when the fibers were exposed to 10 and 20 µM BTP2, [Ca2+]t-sys remained steady, suggesting that the [Ca2+]t-sys decrease observed in Fig. 1 B when RYRs were functional was due to a negative modulation of RYR by BTP2. In the presence of tetracaine, the increase in [Ca2+]t-sys is saturated from 5 µM BTP2, suggesting this is sufficient to block the basal extracellular Ca2+ flux. A summary of these experiments is shown in Fig. 1 E.
Additionally, attempts to wash out BTP2 in these experiments did not result in a reversal of the effect imposed by the agent (data not shown).
In the experiments described above, we administered BTP2 to the cytoplasm of the skinned fibers; however, when using intact fibers, BTP2 is applied from the extracellular side of the plasma membrane. Therefore, we next asked whether the site of BTP2 application would affect the results observed. To test if the site of BTP2 administration affected the RYR modulation observed, we designed an approach where BTP2 could be applied extracellularly while RYR basal activity could still be assessed. To do this, intact fibers in paraffin oil were exposed to a physiologic solution from a microcap pipette containing either solution 1 (rhod-5N with 10 µM BTP2) or solution 2 (rhod-5N without BTP2). Fibers exposed to solutions 1 and 2 were mechanically skinned, trapping the solutions inside the t-system. Under these conditions, a potential inhibition of the RYR-mediated SR Ca2+ leak would result in reduced [Ca2+]t-sys compared to fibers with functional RYRs. To reduce noise that might interfere with comparisons between the reference and test fibers, we positioned two skinned fibers that represented the two conditions (rhod-5N ± BTP2) in the same experimental chamber, allowing them to be imaged simultaneously under identical ionic conditions (Fig. 2, A and C, top).
Fig. 2 A shows the [Ca2+]t-sys (t) of two adjacent fibers (left [red trace] with BTP2 and right [black trace] without BTP2 trapped inside the t-system) as they are imaged in the presence of a control solution with 200 nM [Ca2+]cyto for 80 s. After this period, the fibers were exposed to 10 µM cytoplasmic BTP2. Application of 10 µM cytoplasmic BTP2 reduced the [Ca2+]t-sys in the fibers that were not pre-exposed to BTP2 from the lumen of the t-system. In contrast, the fiber with 10 µM BTP2 trapped within the t-system showed no effect under cytosolic administration of BTP2. The change in [Ca2+]t-sys in the two preparations following exposure to cytoplasmic BTP2 is summarized in Fig. 2 B. The lack of effect of cytosolic exposure to BTP2 on fibers that were previously exposed extracellularly to the same compound suggests that cytosolic administration does not exert further effects following preloading of the t-system lumen with BTP2.
Assuming that BTP2 affects the RYR Ca2+ leak, then the preloading of the t-system lumen with BTP2 should show minimal sensitivity to any subsequent application of tetracaine. Fig. 2 C shows the experimental arrangement of two skinned fibers in the same experimental chamber, one fiber preloaded with solution 1 (rhod-5N + BTP2) and the other with solution 2 (rhod-5N). The [Ca2+]t-sys (t) in Fig. 2 C shows sensitivity of the fiber to tetracaine that was not preloaded with BTP2, whereas tetracaine had no effect on the [Ca2+]t-sys (t) in the fiber preloaded with BTP2. The change in steady-state [Ca2+]t-sys in the presence of tetracaine following preloading of solution 1 or 2 is summarized in Fig. 2 D. Results of Fig. 2 collectively show that BTP2 impairs the RYR Ca2+ leak regardless of the nature of application (extracellular or cytosolic).
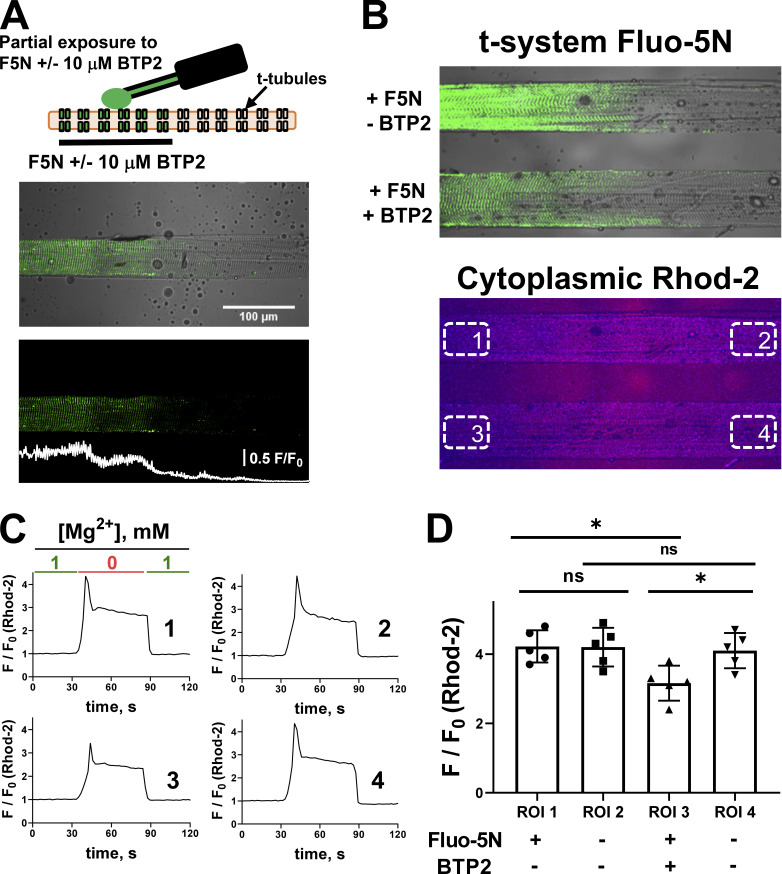
After observing a negative modulation of the basal state of RYR by BTP2, we asked whether BTP2 could impair the activation of RYR Ca2+ release. To control for variations in Ca2+ release between preparations, we designed a new approach in which the extracellular BTP2 application was restricted to a subsection of the t-system, providing a reference region for Ca2+ measurements using rhod-2, which was simultaneously present throughout the cytoplasm. To do this, a physiologic solution containing fluo-5N or fluo-5N + 10 µM BTP2 was applied to only a ∼200-μm-length section of an intact fiber in paraffin oil with a 2-µl microcapillary tube. The fiber was then skinned to trap the physiologic solution in the t-system (Fig. 3 A). The partial exposure of the fiber to BTP2 is visualized by the local presence of fluo-5N fluorescence, helping to establish the test region of the fiber. The rest of the fiber was therefore unexposed to the physiologic solution, providing both a reference and test region in the same preparation for Ca2+ release measurements, with rhod-2 present in the cytoplasm.
Figure 3.
Extracellular exposure of BTP2 impairs RYR Ca2+ release. (A) Top: Schematic illustration of partial exposure of a single fiber immersed in paraffin oil to fluo-5N (F5N) ± 10 µM BTP2. Middle and bottom: Representative images of a skinned fiber with F5N + 10 µM BTP2 trapped on a segment of the t-system as a transmitted light and confocal image overlay (middle) and confocal image only (bottom). (B) Top: Transmitted light and confocal image overlay of two skinned fibers with t-system F5N. Within the top image, one fiber has F5N and 10 µM BTP2 partially trapped (bottom) or F5N only trapped (top) inside the t-system. Bottom: A simultaneously acquired image shows the rhod-2 fluorescence signal from the cytoplasmic solution of the two fibers. ROIs used for analysis in C and D are indicated in this panel, representing the four conditions (ROI1: F5N, No BTP2; ROI2: No F5N, No BTP2; ROI3: F5N, BTP2; ROI4: No F5N, No BTP2). (C) Representative confocal images and intensity plots of four different ROIs analyzed during time in response to [Mg2+]cyto removal. (D) Mean ± SD of Ca2+ transient amplitudes induced by [Mg2+] removal (n = 5). One-way ANOVA followed by post hoc Tukey’s test revealed significant differences between ROI1 and ROI3 (*, P < 0.05) and ROI3 and ROI4 (*, P < 0.05). ns, not significant.
In this experiment, two fibers are placed in parallel, one partially exposed to fluo-5N and the second partially exposed to fluo-5N + 10 µM BTP2. This helps to reduce experimental variables and noise that may otherwise complicate analysis. In this experiment, we can consider there are four conditions spaced across the two fibers in the one experimental chamber (Fig. 3 B). The chamber has been positioned so that the long axis of the fibers are parallel with the scanning line. This reduces the time required to capture a single xy image while maximizing the visualization of fiber area per image area. This also allows regions marked 1 and 2 and regions 3 and 4 to be imaged simultaneously by the scanning laser. Fig. 3 C shows an example of the Ca2+ release experiment, where there is an exchange of a standard resting solution (1 mM Mg2+) to a low Mg2+, Ca2+-releasing solution (0.01 mM Mg2+ solution) during continuous imaging in xy mode. Ca2+ release is monitored under the four conditions by spatially averaging the fluorescence transient from the cytoplasm (Fig. 3 C, traces from within each of the four boxed areas indicated in Fig. 3 B). In this example, the fiber segment with fluo-5N and BTP2 showed a lower Ca2+ release amplitude than the other three regions of interest (ROIs) that represent the three reference conditions (ROI1 F/F0 = 4.37; ROI2 F/F0 = 4.42; ROI3 F/F0 = 3.16; ROI4 F/F0 = 4.35). The results from a total of five similar experiments using different preparations is summarized in Fig. 3 D. This observation suggests that BTP2 applied extracellularly impairs SR Ca2+ release through RYRs.
Effects of BTP2 on SR Ca2+ loading
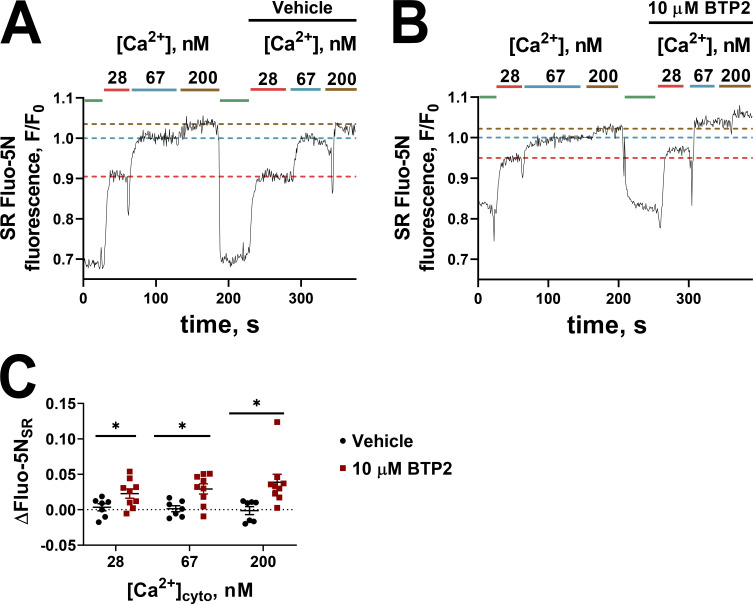
Next, we wished to determine whether BTP2 affected the ability of the SR to load Ca2+. To do this, we loaded the SR of skinned fibers with fluo-5N and tracked the SR Ca2+-dependent fluorescence transient during Ca2+ release with caffeine and reloading the SR with Ca2+ at known [Ca2+]cyto. Fig. 4 A shows the SR fluo-5N fluorescence transients in the absence of BTP2, where the SR is initially depleted in the presence of caffeine, and the [Ca2+]cyto is stepped up from 28 to 67 to 200 nM under resting ionic conditions. This protocol was repeated on the same fiber to test the reproducibility of SR Ca2+ loading in the preparation, indicated by the broken horizontal lines on Fig. 4 A to compare the steady-state SR fluo-5N fluorescence at the repeated exposure to the same [Ca2+]cyto. In another preparation, the same protocol was performed including 10 µM BTP2 in the second series of exposures to 28, 67, and 200 nM [Ca2+]cyto (Fig. 4 B). In this example, the SR fluo-5N fluorescence transient showed that the SR could hold more Ca2+ in the presence of BTP2 than in its absence. The increase in SR fluo-5N fluorescence in the presence of BTP2 from these and similar experiments is summarized in Fig. 4 C. Our results are consistent with a blocking action of BTP2 on RYR Ca2+ leak and the absence of inhibitory effects of BTP2 on the SR Ca2+ pump.
Figure 4.
BTP2 does not compromise SR Ca2+ loading. (A and B) Normalized spatially averaged SR fluo-5N fluorescence during exposure to a thorough depletion of SR calcium with 30 mM caffeine (shown in green). The SR was then loaded in increasing concentrations of free cytoplasmic Ca2+ (28, 67, and 200 nM). This procedure was repeated on the same fiber, depleting the SR of Ca2+ with 30 mM caffeine and re-exposing the fiber to the increasing [Ca2+], this time adding vehicle (A) or 10 µM BTP2 (B). The fluo-5N steady states of the two rounds were compared. F0 was established as the fluo-5N fluorescence intensity at 67 nM [Ca2+]cyto. The horizontal broken lines on A and B provide a guide to the effect of vehicle or BTP2 on the steady-state SR fluo-5N fluorescence signal in the same [Ca2+]cyto. (C) Summary of ΔFluo-5N values ± SEM obtained from seven to nine experiments. Multiple t tests by using the Holm-Sidak method revealed significant differences between vehicle and 10 µM BTP2 across different [Ca2+]cyto. *, P < 0.05.
Effects of BTP2 on the SR from the t-system
The similar efficacy observed with 10 µM BTP2 on RYR function when applied intra- or extracellularly suggested that this inhibition was unlikely to come from a direct effect on RYR. If extracellular BTP2 affected the RYR by diffusing through the t-system membrane and interacting with this channel, we would expect this effect to at least be partially reduced due to dilution of [BTP2] in the bathing solution and competition with Orai1, compared with the case where the cytosol is loaded with a known final concentration of the compound. Since the known target of BTP2 (Orai1) is found in the t-system membrane, we decided to test whether altering the t-system functionality affected the ability of BTP2 to inhibit RYR function.
The integrity and functionality of the t-system can be selectively disrupted by exposure to saponin (Endo and Iino, 1980; Launikonis and Stephenson, 2001). The presence of saponin causes saponin–cholesterol subunits to become mobile to form pores in the t-system. Cholesterol is enriched in plasma membranes, making this treatment specific to the t-system (Bangham et al., 1962). Thus, we could determine whether the effect of BTP2 on the SR was dependent on the functional status and/or lipid environment of the t-system by pre-exposing the skinned fiber to saponin.
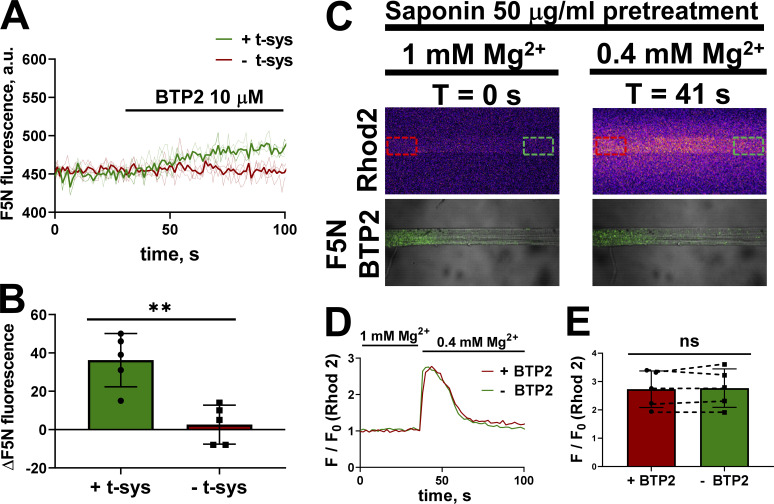
We tested whether saponin pretreatment altered the effect of BTP2 on SR Ca2+ leak and release in two independent experiments. First, the SRs of two mechanically skinned fibers were loaded with fluo-5N, but only one of the fibers was pretreated with saponin. These fibers were placed in the same experimental chamber for imaging on the confocal microscope, as shown in Fig. 2 A. The SR fluo-5N transient from the two fibers was monitored continuously during the exchange of a standard cytoplasmic solution for a similar one containing 10 µM BTP2 (Fig. 5 A). The saponin-pretreated fiber—indicated as − t-sys—did not respond to the introduction of BTP2, whereas the fiber with the intact t-tubules—indicated as + t-sys—showed an increase in SR Ca2+-dependent fluo-5N signal after exposure to 10 µM BTP2, which is consistent with a negative effect on RYR (Fig. 5, A and B).
Figure 5.
The negative modulation of BTP2 on SR Ca2+ release requires t-system integrity. (A) Effect of 10 µM BTP2 on spatially averaged SR fluo-5N (F5N) signal (thin lines represent individual traces, and thick line represent the average of the individuals) on fibers with (green) and without (red) a functional t-system. (B) Mean ± SD of ΔF5N signal after BTP2 treatment. Two-tailed unpaired t test showed significant difference between intact t-system versus disrupted t-system (**, P < 0.005). (C) Selected, simultaneous confocal images of a fiber partially exposed extracellularly to F5N + 10 µM BTP2 and bathed in a solution with rhod-2, in bathing solutions with 1 mM Mg2+ (t = 0 s) and 0.4 mM Mg2+ (t = 41 s). The red and green dashed squares indicate the ROI exposed to and free of BTP2, respectively. (D) Spatially averaged rhod-2 cytoplasmic fluorescence in an ROI with BTP2 (red) and an ROI without BTP2 (green) over time. (E) Mean ± SD of Ca2+ transient amplitudes induced by [Mg2+] decrease under the two conditions (n = 5). Dotted lines between the red and green bars indicate data points obtained on the same fiber during the same Ca2+ release event. Two-tailed paired t test revealed no significant differences between ROI1 (+BTP2) versus ROI2 (−BTP2). a.u., arbitrary units; ns, not significant.
To determine whether the effect of BTP2 on SR Ca2+ release required a functional t-system, a similar approach to that described in Fig. 3 was used. A portion of the fiber t-system was loaded with 10 µM BTP2 (identified by the presence of simultaneously loaded Fluo-5N; Fig. 3). After the skinning process, the fiber was exposed to 50 µg ml saponin for 2 min and then transferred to a cytoplasmic solution with rhod-2. Enough fluo-5N remained inside the t-system to identify the differently treated sections of the preparations. To stimulate a direct release of SR Ca2+, [Mg2+]cyto was lowered from 1 mM to 0.4 mM while the cytoplasmic Ca2+-sensitive rhod-2 transients were continuously monitored in xyt mode (selected images in Fig. 5 C). Following saponin pretreatment, 10 µM BTP2 no longer had an effect on the cytoplasmic Ca2+ transient in low Mg2+ (Fig. 5, D and E). The lack of effect of BTP2 on RYR activation following saponin pretreatment suggests that, even though BTP2 impairs RYR function, it is unlikely to be due to a direct interaction with this channel.
BTP2 and EC coupling
The major role of the RYR is to release Ca2+ during excitation for the purpose of force generation. Given the results presented so far, it was important to determine whether BTP2 affected RYR activity during EC coupling. The t-system of skinned fibers is able to repolarize in the presence of a K+-based cytoplasmic solution, allowing field pulses to initiate all the steps in EC coupling (Posterino et al., 2000; Choi et al., 2017; Lamb and Stephenson, 2018). Using mechanically skinned fibers in this way, it was possible to directly explore the effect of BTP2 on voltage-controlled Ca2+ release; in these experiments, contractile proteins were blocked from generating force by the presence of myosin II ATPase inhibitor BTS in the cytoplasmic solution. Importantly, this could be done without altering the fiber calcium content, as the cytoplasmic solution bathing the skinned fiber is effectively an infinite pool of buffered [Ca2+]cyto, not influenced by any small amount of Ca2+ that would enter from the sealed t-system during pSOCE (Koenig et al., 2018, 2019).
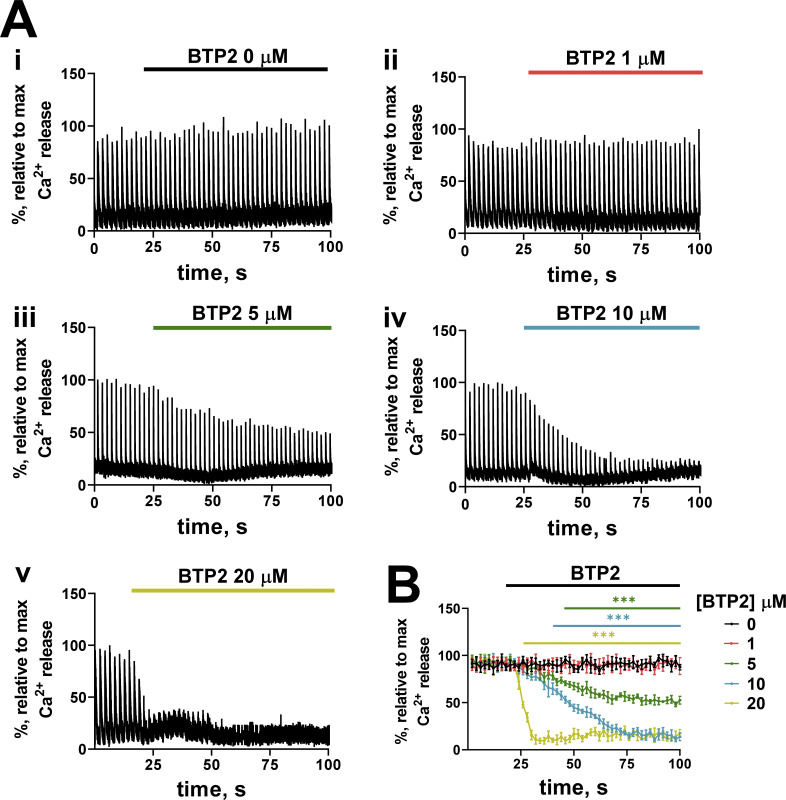
We imaged cytoplasmic rhod-2 with confocal line-scanning during field pulse shocks to the fiber at 0.5 Hz to assess the effect of BTP2 on EC coupling. Fig. 6 A shows examples of the spatially averaged rhod-2 fluorescence transients. Approximately 20 s of field stimulation of the fiber in a control solution showed roughly equal peak amplitudes of the electrically evoked Ca2+ transients, after which BTP2 was directly added to the bathing solution at 0 (control), 1, 5, 10, or 20 µM. A dose-dependent decline in the amplitude of the electrically evoked Ca2+ transients was observed after the addition of BTP2. Fig. 6 B shows the summary of the experiments under each experimental condition.
Figure 6.
Effect of BTP2 on electrically evoked SR Ca2+ release. (A) Original recordings of mechanically skinned fibers exposed to increasing concentrations of BTP2 (i–v) obtained with confocal line scanning parallel to the fiber long axis. Ca2+ transients were normalized to the maximum peak value of rhod-2 fluorescence during electrical stimulation. (B) Mean of transient amplitude over time (n = 4–7). Data are presented as mean ± SD. Horizontal lines (color matched to [BTP2]) indicate where multiple t tests performed using the Holm-Sidak method revealed a significant decrease compared with 0 µM BTP2 treatment (***, P < 0.005).
Discussion
The experiments presented in this work provide novel evidence regarding the effect of BTP2 on Ca2+-handling in skeletal muscle fibers. We show that low µM concentrations of BTP2 block a resting Ca2+ influx across the t-system membrane, likely corresponding to Orai1 inhibition; however, at concentrations above 5 µM, RYR Ca2+ leak and release was impaired by BTP2. The inhibitory effect of BTP2 on the RYR was observed following either extracellular or intracellular application of the agent and was abolished by saponin treatment. The sensitivity of a t-system Ca2+ channel to low [BTP2] has implications for understanding extracellular Ca2+ influx at rest, which is important in our fundamental understanding of the maintenance of fiber calcium content (Ríos, 2010) and conditions, such as muscular dystrophy (Turner et al., 1991) and malignant hyperthermia (Eltit et al., 2013; Lopez et al., 2020a). Additionally, the promiscuity of BTP2 effects at commonly used doses highlights a need for re-evaluation of some conclusions regarding SOCE function in skeletal muscle that have been based on the premise of a selective action of BTP2 on Orai1.
The mechanically skinned fiber represents an effective cellular preparation for testing of Ca2+-handling modulators as a part of drug and modulator discovery pipelines. The progression of modulators from identification in a high-throughput, noncellular, or molecular protein modulator assay to a cell-based assay is essential for confirmation of specificity for a particular target and analysis of off-target effects (Rebbeck et al., 2020). In skinned fibers, basal Ca2+-handling activity, including RYR Ca2+ leak to Ca2+ release during EC coupling, can be assessed (Choi et al., 2017; Rebbeck et al., 2020). In addition to the controlled delivery of modulators to the cytoplasmic environment (Lamb and Stephenson, 2018), here we provide a way to isolate modulator application within a single fiber by local application to a subsection of the t-system lumen. The t-system lumen can be manipulated by the delivery of physiologic solution containing compounds of interest while the fibers are intact and in paraffin oil. Paraffin oil maintains the physiologic solution to the area of the fiber where it was applied, preventing diffusion along the outside of the sarcolemma. Furthermore, as the t-system is diffusionally restricted longitudinally, there is negligible penetration of the modulator throughout the t-system network of the fiber. The applied modulator is maintained in the t-system region of application while the fibers are intact and in paraffin oil or following skinning (Fig. 3; Edwards and Launikonis, 2008). Testing the effects of modulators on low-amplitude t-system membrane Ca2+ fluxes particularly benefits from this approach, where the capacity to have in-preparation reference and test regions improves the resolution of the comparison, as the variability of comparing reference and test conditions between fibers is eliminated (Fig. 3). We further strengthened the approach by monitoring two fibers in the same chamber, side-by-side, that therefore encounter exactly the same cytoplasmic ionic conditions. The examples we provide in the current study (Figs. 3 and 5) can be changed to other drugs or modulators against desired reference conditions in future studies.
BTP2, t-system Ca2+ leak, and RYR Ca2+ leak
In mechanically skinned fibers, the t-system is a very weakly Ca2+-buffered compartment with [Ca2+]t-sys in the low millimolar (physiologic) range. The high [Ca2+] and poor Ca2+ buffering makes the t-system membrane leaky to Ca2+. A constant action of the t-system PMCA is required to balance the steady-state [Ca2+]t-sys with the leak of Ca2+ across the membrane (Cully et al., 2016). In the skinned fiber, the low volume–to–surface area ratio of the sealed t-system and weak Ca2+ buffering of this compartment provide the sensitivity needed to detect small Ca2+ fluxes across its membrane. This makes the skinned fiber an appropriate preparation for detecting the effects of drugs or modulators on t-system Ca2+ leak. With this approach, we found that in the presence of [BTP2] ≤5 µM, steady-state [Ca2+]t-sys increased, indicating that BTP2 blocked resting extracellular Ca2+ influx across the t-system membrane in adult muscle fibers. As [BTP2] was increased to 10 µM, steady-state [Ca2+]t-sys was reduced compared with that at 1 µM BTP2. The depressive effect of 10 µM BTP2 was abolished in the presence of tetracaine, a blocker of RYR Ca2+ leak (Shannon et al., 2002).
Our results are consistent with the fact that steady-state [Ca2+]t-sys is modulated by the RYR Ca2+ leak, as this influences the JS [Ca2+] ([Ca2+]JS), and that there is a constant Ca2+ leak across the t-system membrane. Importantly, we show here that BTP2 can separate these variables. In resting myotubes, Eltit et al. (2013) found a similar inhibition of the influx of Ca2+ in myotubes in the presence of 5 µM BTP2 and in dominant-negative Orai1E190Q myotubes. The well-established effect of BTP2 on Orai1 (Wei-LaPierre et al., 2013), in combination with our results (Figs. 1 and 2) and those of Eltit et al. (2013), suggest that BTP2 inhibits a basal extracellular Ca2+ influx through Orai1. It is possible that this BTP2-sensitive Ca2+ influx is low-magnitude SOCE, regulated by RYR Ca2+ leak to modulate fiber calcium content (Ríos, 2010; Cully et al., 2018); however, we must also bear in mind that BTP2 has been shown to have an effect on TRP channels, which may conduct part of the Ca2+ flux across the t-system membrane (He et al., 2005). Looking forward, BTP2 can be an important tool used to separate t-system Ca2+ flux and RYR Ca2+ leak to assess basal Ca2+ handling in conditions such as muscular dystrophy or malignant hyperthermia, where excess Ca2+ entry through the t-system has been proposed to be pathophysiologic through both Orai1 and TRP channels (Turner et al., 1991; Eltit et al., 2013; Cully et al., 2018; Lopez et al., 2020a).
It is possible that BTP2 also affects other RYR isoforms. In the sinoatrial node, Liu et al. (2015) showed a negative effect of BTP2 on spontaneous and caffeine-induced SR Ca2+ release. BTP2 may indirectly affect RYR2 in a similar fashion to RYR1 as we have shown here.
BTP2 effects on the SR from the t-system membrane
In this study, we found that the t-system Ca2+ leak and SR Ca2+ leak or release was affected by BTP2 regardless of intra- or extracellular delivery (Figs. 2, 3, 4, 5, and 6). It was important to determine this, as the efficiency of BTP2 to diffuse through the plasma membrane is unclear. We also observed that saponin pretreatment abolished the effects of BTP2 on the SR (Fig. 5). Pre-exposure to saponin disrupts the functional status of the t-system membrane specifically because saponin binds cholesterol, which is only in high abundance in the t-system membrane (Bangham et al., 1962; Yeagle, 1985). The brief pre-exposure to saponin did not affect SR Ca2+-handling properties (Fig. 5). These results are consistent with BTP2 having its effects from the t-system membrane. This is possible through alterations in any of the multiple protein–protein interactions across the t-system–SR junction that are dependent on the organization of the t-system lipid. The high cholesterol concentration of the t-system, as in all plasma membranes, conveys the physical properties of the membrane that, in turn, affect the properties of its embedded proteins. Cholesterol provides rigidity and determines the thickness of the lipid bilayer (Yeagle, 1985). For example, the removal of cholesterol affects the t-system DHPR and K+ channel properties in muscle (Launikonis and Stephenson, 2001; Pouvreau et al., 2004). The need for a functional t-system to observe the effect of BTP2 on RYRs highlights that the effect corresponds to an indirect modulation of the channel, as well as the complexity of the protein–protein interactions and their interplay at the JS (Barone et al., 2015; Quick et al., 2017; Nakada et al., 2018).
BTP2 and EC coupling
We provide a dose–response of the effect of BTP2 on the Ca2+ transients of EC coupling in skeletal muscle (Fig. 6). The result is independent of alterations induced by BTP2 on Ca2+ flow through Orai1 during pSOCE because the low stimulation rate (0.5 Hz) would only induce the entry of a very small amount of Ca2+ from the t-system in the presence of a functional Orai1 (Koenig et al., 2018), and the cytoplasmic solution of the skinned fiber is an effectively infinite pool of cytoplasmic EGTA (1 mM) that buffers [Ca2+]cyto without restricting the capacity of the SR Ca2+ pump to resequester released Ca2+ (Stephenson, 2006; Choi et al., 2017; Lamb and Stephenson, 2018). For these reasons, the decline of the transients in the presence of BTP2 in our experiments cannot be attributed to any effect on Orai1 or SOCE. The demonstration of an indirect effect of BTP2 on RYR Ca2+ release induced by lowering [Mg2+]cyto (Fig. 3) suggests that the impaired EC coupling step in the presence of BTP2 is the opening of RYR.
To check that there was an absence of effect of pSOCE on fiber calcium content in these experiments, we can calculate the sum of calcium that was potentially blocked from entering the open cytoplasm during electrical stimulation via pSOCE in Fig. 6. To reach the point at which the electrical stimulation responses were reduced to 50% of the control in the presence of BTP2, 25, 8, and 2 pulses were required in the presence of 5, 10, or 20 µM BTP2, respectively. From Fig. 4 in Koenig et al. (2018), an upper threshold of 0.25 mM [Ca2+]t-sys was calculated to enter the cytoplasm during pSOCE (2.5 µM calcium per fiber volume). Therefore, the sum (upper limit) of calcium potentially prevented from entering from the sealed t-system in the presence of BTP2 during 0.5-Hz stimulation was 62.5, 20, and 5 µM calcium per fiber volume, respectively. A depletion of SR calcium by 62.5 µM from the endogenous level of 1 mM—assuming all calcium entering from the t-system ends up in the SR or causes it to retain its normal level of calcium—would not decrease the SR calcium content enough to affect the total amount of calcium released during action potential stimulation (Posterino and Lamb, 2003). We conclude that BTP2 inhibits electrically evoked Ca2+ transients independently of alterations in Ca2+ flow through Orai1.
A prime candidate for the indirect effect of BTP2 from the t-system on RYR is the DHPR, as DHPR is in direct contact with RYR. DHPR imposes inhibitory regulation on RYR1 activity at rest (Zhou et al., 2006; Figueroa et al., 2012; Eltit et al., 2011), and DHPR has been shown to be affected by other SOCE inhibitors, 2-APB and SKF-96365 (Olivera and Pizarro, 2010). A nonspecific effect of 10 µM BTP2 on DHPR would explain such indirect effects on RYR reported here.
Here, we have further developed the capacity of the mechanically skinned fiber to be used as a preparation suited to the assessment of modulators of Ca2+ handling in muscle. We focused on BTP2 because of its known action on Orai1 (Wei-LaPierre et al., 2013) and the high interest in understanding the physiologic roles of SOCE in muscle function. Our systematic assessment of the actions of BTP2 showed effects on EC coupling, which directly affects the regulation of SOCE. Of course, the use of BTP2 to address questions about SOCE and t-system Ca2+ fluxes remains possible when care is taken to avoid undesired effects of the modulator on the function of RYR, a key protein of SOCE and EC coupling. In our experiments, the actions of BTP2 on [Ca2+]t-sys transients provided further evidence for the important role of RYR Ca2+ leak in setting the t-system Ca2+ gradient in the resting muscle (Cully et al., 2018) and suggests how the lipid environment of the t-system can affect protein function in the SR. Finally, BTP2 is likely to be an important tool in examining basal Ca2+ influx across the t-system membrane. The BTP2-sensitive Ca2+ influx is likely an essential component of Ca2+ homeostasis in healthy muscle but contributes to pathophysiology in other conditions (Turner et al., 1991; Eltit et al., 2013; Ríos et al., 2015; Lopez et al., 2020a).
Acknowledgments
Eduardo Ríos served as editor.
We thank D. George Stephenson (La Trobe University, Melbourne, Victoria, Australia); Xaver Koenig (Medical University of Vienna, Vienna, Austria); and Cedric Lamboley, Luke Pearce, Daniel Singh, and Crystal Seng (The University of Queensland, Brisbane, Queensland, Australia) for helpful comments on the manuscript.
This work was supported by Australian Research Council Discovery Projects grants DP180100937 and DP200100435 (to B.S. Launikonis).
The authors declare no competing financial interests.
Author contributions: A. Meizoso-Huesca designed and performed experiments, analyzed and interpreted data, and cowrote the paper. B.S. Launikonis designed experiments, interpreted data, and cowrote the paper.
References
- Bangham A.D., Horne R.W., Glauert A.M., Dingle J.T., and Lucy J.A.. 1962. Action of saponin on biological cell membranes. Nature. 196:952–955. 10.1038/196952a0 [DOI] [PubMed] [Google Scholar]
- Barone V., Randazzo D., Del Re V., Sorrentino V., and Rossi D.. 2015. Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 36:501–515. 10.1007/s10974-015-9421-5 [DOI] [PubMed] [Google Scholar]
- Choi R.H., Koenig X., and Launikonis B.S.. 2017. Dantrolene requires Mg2+ to arrest malignant hyperthermia. Proc. Natl. Acad. Sci. USA. 114:4811–4815. 10.1073/pnas.1619835114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully T.R., Edwards J.N., Murphy R.M., and Launikonis B.S.. 2016. A quantitative description of tubular system Ca(2+) handling in fast- and slow-twitch muscle fibres. J. Physiol. 594:2795–2810. 10.1113/JP271658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully T.R., Choi R.H., Bjorksten A.R., Stephenson D.G., Murphy R.M., and Launikonis B.S.. 2018. Junctional membrane Ca2+ dynamics in human muscle fibers are altered by malignant hyperthermia causative RyR mutation. Proc. Natl. Acad. Sci. USA. 115:8215–8220. 10.1073/pnas.1800490115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay B., Arnaudeau S., Bader C.R., Konig S., and Bernheim L.. 2011. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J. Cell Biol. 194:335–346. 10.1083/jcb.201012157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke A.M., Hopkins P.M., Calaghan S.C., Halsall J.P., and Steele D.S.. 2010. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem. 285:25645–25653. 10.1074/jbc.M110.104976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.N., and Launikonis B.S.. 2008. The accessibility and interconnectivity of the tubular system network in toad skeletal muscle. J. Physiol. 586:5077–5089. 10.1113/jphysiol.2008.155127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J.N., Murphy R.M., Cully T.R., von Wegner F., Friedrich O., and Launikonis B.S.. 2010. Ultra-rapid activation and deactivation of store-operated Ca2+ entry in skeletal muscle. Cell Calcium. 47:458–467. 10.1016/j.ceca.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Eltit J.M., Li H., Ward C.W., Molinski T., Pessah I.N., Allen P.D., and Lopez J.R.. 2011. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc. Natl. Acad. Sci. USA. 108:7046–7051. 10.1073/pnas.1018380108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltit J.M., Ding X., Pessah I.N., Allen P.D., and Lopez J.R.. 2013. Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. FASEB J. 27:991–1000. 10.1096/fj.12-218354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., and Iino M.. 1980. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J. Muscle Res. Cell Motil. 1:89–100. 10.1007/BF00711927 [DOI] [PubMed] [Google Scholar]
- Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B., Hogan P.G., Lewis R.S., Daly M., and Rao A.. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 441:179–185. 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- Figueroa L., Shkryl V.M., Zhou J., Manno C., Momotake A., Brum G., Blatter L.A., Ellis-Davies G.C.R., and Ríos E.. 2012. Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle. J. Physiol. 590:1389–1411. 10.1113/jphysiol.2011.225854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.P., Hewavitharana T., Soboloff J., Spassova M.A., and Gill D.L.. 2005. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 280:10997–11006. 10.1074/jbc.M411797200 [DOI] [PubMed] [Google Scholar]
- Ishikawa J., Ohga K., Yoshino T., Takezawa R., Ichikawa A., Kubota H., and Yamada T.. 2003. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J. Immunol. 170:4441–4449. 10.4049/jimmunol.170.9.4441 [DOI] [PubMed] [Google Scholar]
- Kabbara A.A., and Allen D.G.. 2001. The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J. Physiol. 534:87–97. 10.1111/j.1469-7793.2001.00087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig X., Choi R.H., and Launikonis B.S.. 2018. Store-operated Ca2+ entry is activated by every action potential in skeletal muscle. Commun. Biol. 1:31 10.1038/s42003-018-0033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig X., Choi R.H., Schicker K., Singh D.P., Hilber K., and Launikonis B.S.. 2019. Mechanistic insights into store-operated Ca2+ entry during excitation-contraction coupling in skeletal muscle. Biochim. Biophys. Acta Mol. Cell Res. 1866:1239–1248. 10.1016/j.bbamcr.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Kurebayashi N., and Ogawa Y.. 2001. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 533:185–199. 10.1111/j.1469-7793.2001.0185b.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G.D., and Stephenson D.G.. 2018. Measurement of force and calcium release using mechanically skinned fibers from mammalian skeletal muscle. J Appl Physiol (1985). 125:1105–1127. 10.1152/japplphysiol.00445.2018 [DOI] [PubMed] [Google Scholar]
- Lamb G.D., Junankar P.R., and Stephenson D.G.. 1995. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J. Physiol. 489:349–362. 10.1113/jphysiol.1995.sp021056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., and Ríos E.. 2007. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J. Physiol. 583:81–97. 10.1113/jphysiol.2007.135046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., and Stephenson D.G.. 2001. Effects of membrane cholesterol manipulation on excitation-contraction coupling in skeletal muscle of the toad. J. Physiol. 534:71–85. 10.1111/j.1469-7793.2001.00071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., and Stephenson D.G.. 2004. Osmotic properties of the sealed tubular system of toad and rat skeletal muscle. J. Gen. Physiol. 123:231–247. 10.1085/jgp.200308946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., Barnes M., and Stephenson D.G.. 2003. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA. 100:2941–2944. 10.1073/pnas.0536227100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., Murphy R.M., and Edwards J.N.. 2010. Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflugers Arch. 460:813–823. 10.1007/s00424-010-0856-7 [DOI] [PubMed] [Google Scholar]
- Li H., Ding X., Lopez J.R., Takeshima H., Ma J., Allen P.D., and Eltit J.M.. 2010. Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J. Biol. Chem. 285:39171–39179. 10.1074/jbc.M110.149690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xin L., Benson V.L., Allen D.G., and Ju Y.K.. 2015. Store-operated calcium entry and the localization of STIM1 and Orai1 proteins in isolated mouse sinoatrial node cells. Front. Physiol. 6:69 10.3389/fphys.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.R., Uryash A., Faury G., Estève E., and Adams J.A.. 2020. a. Contribution of TRPC Channels to Intracellular Ca2 + Dyshomeostasis in Smooth Muscle From mdx Mice. Front. Physiol. 11:126 10.3389/fphys.2020.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J.R., Kaura V., Hopkins P., Liu X., Uryach A., Adams J, and Allen P.D.. 2020b. Transient Receptor Potential Cation Channels and Calcium Dyshomeostasis in a Mouse Model Relevant to Malignant Hyperthermia. Anesthesiology. 133(2):364–376. 10.1097/ALN.0000000000003387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., and Lüttgau H.C.. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. 10.1016/0304-4157(94)00014-5 [DOI] [PubMed] [Google Scholar]
- Michelucci A., Boncompagni S., Pietrangelo L., Takano T., Protasi F., and Dirksen R.T.. 2020. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 152 e202012617 10.1085/jgp.202012617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada T., Kashihara T., Komatsu M., Kojima K., Takeshita T., and Yamada M.. 2018. Physical interaction of junctophilin and the CaV1.1 C terminus is crucial for skeletal muscle contraction. Proc. Natl. Acad. Sci. USA. 115:4507–4512. 10.1073/pnas.1716649115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera J.F., and Pizarro G.. 2010. Two inhibitors of store operated Ca2+ entry suppress excitation contraction coupling in frog skeletal muscle. J. Muscle Res. Cell Motil. 31:127–139. 10.1007/s10974-010-9216-7 [DOI] [PubMed] [Google Scholar]
- Peachey L.D. 1965. The sarcoplasmic reticulum and transverse tubules of the frog’s sartorius. J. Cell Biol. 25:209–231. 10.1083/jcb.25.3.209 [DOI] [PubMed] [Google Scholar]
- Posterino G.S., Lamb G.D., and Stephenson D.G.. 2000. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J. Physiol. 527:131–137. 10.1111/j.1469-7793.2000.t01-2-00131.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino G.S., and Lamb G.D.. 2003. Effect of sarcoplasmic reticulum Ca2+ content on action potential-induced Ca2+ release in rat skeletal muscle fibres. J. Physiol. 551:219–237. 10.1113/jphysiol.2003.040022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau S., Berthier C., Blaineau S., Amsellem J., Coronado R., and Strube C.. 2004. Membrane cholesterol modulates dihydropyridine receptor function in mice fetal skeletal muscle cells. J. Physiol. 555:365–381. 10.1113/jphysiol.2003.055285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick A.P., Wang Q., Philippen L.E., Barreto-Torres G., Chiang D.Y., Beavers D., Wang G., Khalid M., Reynolds J.O., Campbell H.M., et al. 2017. SPEG (Striated Muscle Preferentially Expressed Protein Kinase) Is Essential for Cardiac Function by Regulating Junctional Membrane Complex Activity. Circ. Res. 120:110–119. 10.1161/CIRCRESAHA.116.309977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck R.T., Singh D.P., Janicek K.A., Bers D.M., Thomas D.D., Launikonis B.S., and Cornea R.L.. 2020. RyR1-targeted drug discovery pipeline integrating FRET-based high-throughput screening and human myofiber dynamic Ca2+ assays. Sci. Rep. 10:1791 10.1038/s41598-020-58461-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E. 2010. The cell boundary theorem: a simple law of the control of cytosolic calcium concentration. J. Physiol. Sci. 60:81–84. 10.1007/s12576-009-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Figueroa L., Manno C., Kraeva N., and Riazi S.. 2015. The couplonopathies: A comparative approach to a class of diseases of skeletal and cardiac muscle. J. Gen. Physiol. 145:459–474. 10.1085/jgp.201411321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon T.R., Ginsburg K.S., and Bers D.M.. 2002. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ. Res. 91:594–600. 10.1161/01.RES.0000036914.12686.28 [DOI] [PubMed] [Google Scholar]
- Soboloff J., Spassova M.A., Tang X.D., Hewavitharana T., Xu W., and Gill D.L.. 2006. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 281:20661–20665. 10.1074/jbc.C600126200 [DOI] [PubMed] [Google Scholar]
- Stephenson D.G., and Lamb G.D.. 1993. Visualisation of the transverse tubular system in isolated intact and mechanically skinned muscle fibres of the cane toad by confocal laser scanning microscopy. J. Physiol. 459:15P. [Google Scholar]
- Stephenson D.G. 2006. Tubular system excitability: an essential component of excitation-contraction coupling in fast-twitch fibres of vertebrate skeletal muscle. J. Muscle Res. Cell Motil. 27:259–274. 10.1007/s10974-006-9073-6 [DOI] [PubMed] [Google Scholar]
- Trevillyan J.M., Chiou X.G., Chen Y.W., Ballaron S.J., Sheets M.P., Smith M.L., Wiedeman P.E., Warrior U., Wilkins J., Gubbins E.J., et al. 2001. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J. Biol. Chem. 276:48118–48126. 10.1074/jbc.M107919200 [DOI] [PubMed] [Google Scholar]
- Turner P.R., Fong P.Y., Denetclaw W.F., and Steinhardt R.A.. 1991. Increased calcium influx in dystrophic muscle. J. Cell Biol. 115:1701–1712. 10.1083/jcb.115.6.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei-LaPierre L., Carrell E.M., Boncompagni S., Protasi F., and Dirksen R.T.. 2013. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 4:2805 10.1038/ncomms3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P.L. 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 822:267–287. 10.1016/0304-4157(85)90011-5 [DOI] [PubMed] [Google Scholar]
- Zhou J., Yi J., Royer L., Launikonis B.S., González A., García J., and Ríos E.. 2006. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am. J. Physiol. Cell Physiol. 290:C539–C553. 10.1152/ajpcell.00592.2004 [DOI] [PubMed] [Google Scholar]
- Zitt C., Strauss B., Schwarz E.C., Spaeth N., Rast G., Hatzelmann A., and Hoth M.. 2004. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 279:12427–12437. 10.1074/jbc.M309297200 [DOI] [PubMed] [Google Scholar]