Abstract
Background
On February 21, 2020, 2 coronavirus disease 2019 (COVID-19) cases in pilgrim travelers from Korea to Israel were identified. We investigated the source of infection, clinical features of COVID-19, and transmission potential of presymptomatic and asymptomatic cases.
Methods
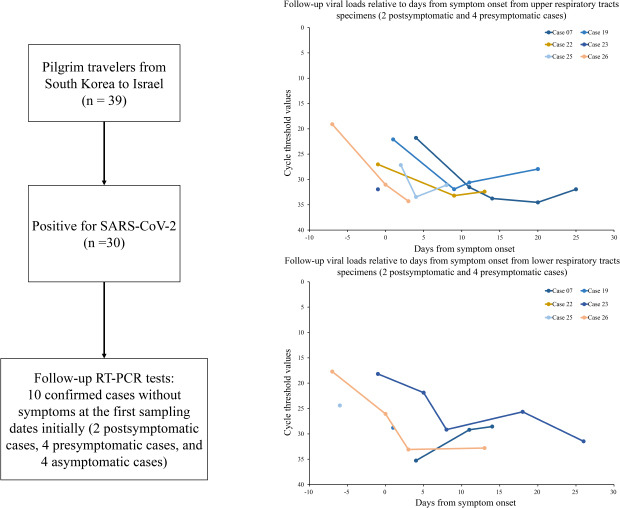
All 39 pilgrim travelers were aggressively tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Their clinical features and possible source of infection were investigated by interview and review of their medical records. Ten confirmed cases without symptoms at the first sampling dates were selected for follow-up reverse transcription polymerase chain reaction tests.
Results
Of 39 pilgrim travelers, 30 (77%) were positive for SARS-CoV-2. Among the 30 positive COVID-19 cases, 4 (13%) were asymptomatic. Available follow-up cycle threshold values from 10 cases gradually increased over time and were lower during the presymptomatic period than during the postsymptomatic period. Out of 328 contacts related to the COVID-19 cases in the pilgrim travelers, 22 additional cases (7%) were confirmed with SARS-CoV-2 infections. Three tertiary cases were identified to be transmitted by presymptomatic secondary cases.
Conclusion
To prevent transmission of COVID-19, we need to focus on presymptomatic and asymptomatic cases, and massive testing for SARS-CoV-2 is required. More research about the possibility of presymptomatic transmission over 2 days before symptom onset is required.
Keywords: COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2, Outbreaks, Transmission
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, as pneumonia of unknown cause in late December 2019.1 As of October 9, 2020, the pandemic disease has resulted in more than 36 million cases and more than a million deaths.2 With a higher fatality rate among older ages, the clinical spectrum of COVID-19 is wide, varying from asymptomatic infection to death.3,4 COVID-19 is a novel disease, and information on the transmission potential of asymptomatic and presymptomatic cases are important to develop control strategies.5
Some studies reported that people could be infected with SARS-CoV-2 in the presymptomatic period.6,7 The current definition of contacts by the WHO are persons who have been exposed to a COVID-19 case within 2 days prior to symptom onset.8 A study in 3 patients confirmed with COVID-19 reported higher viral loads during the presymptomatic period.9 However, the first sampling dates were within 2 days prior to symptom onset. Studies regarding the viral loads and the possibility of presymptomatic transmission 2 days or more before symptom onset are needed.
In Korea, the first COVID-19 case was reported on January 19, 2020, with additional sporadic cases. However, after the first confirmed COVID-19 case related to the Shincheonji Church on February 18, the number of cases increased explosively.10 In Israel, the first COVID-19 case reported on February 21, 2020, was a person who had traveled from Japan. Seven cases were then reported up to February 29, and limited transmission of household contact was identified in Israel.11,12
On February 21, 2020, 2 COVID-19 cases of 39 persons who traveled to Israel from February 8 to February 16 for a pilgrimage were reported in Korea. In this study, we aimed to investigate the source of their infection and the clinical features of COVID-19. In addition, follow-up real-time reverse transcription polymerase chain reaction (RT-PCR) tests to detect SARS-CoV-2 were conducted to evaluate the transmission potential of presymptomatic and asymptomatic cases.
METHODS
Study population
The study population was 39 pilgrim travelers comprising 1 priest, 1 travel guide, and 37 tourists. Immediately after 2 confirmed COVID-19 cases in the group, the remaining 37 pilgrim travelers were aggressively tested for SARS-CoV-2 from February 21 to February 22 regardless of symptoms. A COVID-19 case was defined as anyone who tested positive through real-time RT-PCR. All pilgrim travelers except 1 travel guide resided in Gyeongsangbuk-do. Thus, the Gyeongsangbuk-do Provincial Government initiated an outbreak investigation with the cooperation of the Korea Centers for Disease Control and Prevention (KCDC).
Control of COVID-19 outbreak
The Gyeongsangbuk-do rapid response team was organized to investigate and control the outbreak. Confirmed COVID-19 cases were admitted to 4 designated hospitals for COVID-19 patients in Gyeongsangbuk-do and the National Medical Center in Seoul. Pilgrim travelers who tested negative were home quarantined for 14 days. According to the COVID-19 response guidelines in Korea, contact tracing of COVID-19 cases was conducted from 1 day before symptom onset or 1 day before the case was sampled.13 The criteria were changed to 2 days after the revised guideline on April 2.14
Data collection
The pilgrim travelers were initially interviewed using a standardized infectious disease report form in Korea for COVID-19 that evaluated demographic characteristics, exposure history, and clinical symptoms. In addition, the pilgrim travelers were interviewed through cellular phones to identify the presence of symptoms, onset of symptoms, and possible source of infection during the pilgrimage trip. The clinical course of COVID-19 cases was obtained by reviewing medical records. Onset of symptoms was determined using information from case interview and supplemented by medical records. The passengers and aircrews list was secured through the KCDC to evaluate the possibility of exposure during the flight from Korea to Israel on February 8. Considering that the early outbreak in Korea was related to the Shincheonji Church,10 the COVID-19 patients and their families were checked if they were on the list of Shincheonji, obtained by the KCDC. In addition, the history of medical facilities and visiting pharmacies, GPS tracking of cellular phones, and card transactions were utilized to confirm if they had visited the Shincheonji Church in Daegu.
Laboratory testing
The Gyeongsangbuk-do Government Public Institute of Health and Education (GGPIHE) performed real-time RT-PCR on all samples except 1 sample from a travel guide in Seoul, which was performed by the Seoul Research Institute of Public Health and Environment (SRIPHE). These 2 organizations were certified to perform SARS-CoV-2 testing by the KCDC on January 24, 2020.10 Real-time RT-PCR was used to amplify the viral E gene as a screening test and the RdRp region of the orf1b gene as a confirmatory test in accordance with the laboratory guidelines in Korea.15 Cycle threshold (Ct) values below 40 indicated a positive result for SARS-CoV-2. According to the response guidelines in Korea, upper and lower respiratory tract specimens were obtained from nasopharyngeal plus oropharyngeal swabs and the sputum, respectively.13 The first specimens were collected by trained medical personnel in each public health center and immediately transported to the GGPIHE and the SRIPHE. The revised guideline on March 2 recommended to obtain only lower respiratory tract specimens from cases with sputum.16
The first real-time RT-PCR test for SARS-CoV-2 was performed for all 39 pilgrim travelers; those with a negative result were retested 7 days later. All were initially negative for SARS-CoV-2. Ten confirmed cases without symptoms at the first sampling dates initially were selected for follow-up RT-PCR (Case 07, 19, 22, 23, and 25–30). Two cases were revealed to later have symptoms at the first sampling dates (Cases 07 and 19). Follow-up RT-PCR tests were conducted 7 days after the first sampling dates; additional follow-up RT-PCR tests were conducted twice or more per week until hospital discharge. Follow-up specimens were obtained by medical staff in the 4 designated hospitals for COVID-19 patients in Gyeongsangbuk-do and transported to the GGPIHE within 24 hours of collection. In this study, we compared the Ct values that were analyzed by the GGPIHE.
Statistical analysis
Continuous variables are presented as the median (interquartile range [IQR]) and compared by the Mann–Whitney U test. Categorial variables are presented as the number (%) and compared with the chi-square test or Fishers' exact test. A P value less than 0.05 was considered as statistically significant. Statistical analyses were performed with SPSS (version 22.0; SPSS Inc, Chicago, IL, USA).
Ethics statement
Data were collected as part of the public health response and not considered research subject to approval of the Institutional Review Board; informed consent was not required.
RESULTS
Route of pilgrimage and outbreak situation
The pilgrim travelers boarded an airplane from Korea to Israel on February 8, 2020 (KE957). None of the 219 passengers and 15 aircrew were Chinese. From February 8 to February 15, the pilgrim travelers visited various places, including Netanya, Tiberias, the Dead Sea, Beer Sheba, and Jerusalem. One local guide and 1 local bus driver accompanied the pilgrim travelers. The local guide and bus driver tested negative for SARS-CoV-2 in Israel.11,12 The pilgrim travelers left Israel on February 15 and arrived in Korea on February 16 (KE958). Two index COVID-19 cases (Case 08 and 15) in pilgrim travelers were reported on February 21. Another 37 pilgrim travelers were tested for SARS-CoV-2 from February 21 to February 22 regardless of symptoms. Contacts who experienced symptoms within 14 days of home quarantine were also tested for SARS-CoV-2. Then, confirmed COVID-19 cases were admitted to the hospitals.
Epidemic curve and primary case
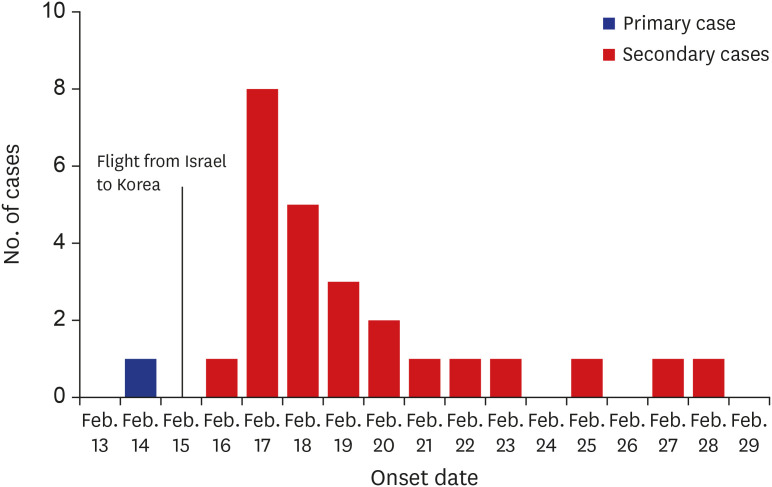
Out of 39 pilgrim travelers, 30 (77%) tested positive for SARS-CoV-2. The primary case occurred on February 14, and there was a peak of 8 secondary cases on February 17. The onset of secondary cases ranged from February 16 to February 28, within 2 weeks from the onset of the primary case (Fig. 1). None of the travelers wore masks during the pilgrimage because COVID-19 cases had not been reported in Israel.
Fig. 1. Epidemic curve of the outbreak in pilgrim travelers from Korea (symptomatic cases).
The date of symptom onset of two index cases was February 17 (Case 08) and February 18 (Case 15). During the interview, the primary case (Case 01) stated that his first symptom was myalgia on February 17, 2020. However, the tour guide informed us that someone else had tried to obtain cold medicine on February 14. One tourist witnessed that the primary case took cold medicine on February 14. Thus, the onset of the primary case was assumed to be on February 14. Among 39 pilgrim travelers and their families, only 1 person who was the primary case's son, was on the list of Shincheonji and tested positive for SARS-CoV-2 on February 26. Although the son stated that he did not visit the Shincheonji Church after 2019, he stayed at the Shincheonji Church in Daegu from January 31 to February 6, 2020, according to GPS records. On February 7, the son came home and met the primary case before departing for Israel.
Demographic and clinical characteristics
Thirty persons (77%) were women with a median age of 59.0 years (IQR, 55.0–65.0 years). Chi-square test showed that older individuals had a significant association with SARS-CoV-2 infections (P = 0.028), which were not significant using the Mann–Whitney U test (P = 0.155). The local bus in Israel consisted of 4 seats per row. The travelers placed in aisle seats, where the primary case had been seated, had a higher attack rate than those in the window seats, without statistical significance (Table 1).
Table 1. Demographic characteristics of pilgrim travelers from Korea.
| Characteristics | All (n = 39) | SARS-CoV-2 test results | P valuea | ||
|---|---|---|---|---|---|
| Positive (n = 30) | Negative (n = 9) | ||||
| Age, yr | 59.0 (55.0–65.0) | 59.5 (58.0–66.0) | 58.0 (47.0–61.0) | 0.155 | |
| < 50 | 4 (10) | 1 (3) | 3 (33) | 0.028 | |
| 50–64 | 25 (64) | 20 (67) | 5 (57) | ||
| ≥ 65 | 10 (26) | 9 (30) | 1 (11) | ||
| Sex | 0.654 | ||||
| Male | 9 (23) | 8 (27) | 1 (11) | ||
| Female | 30 (77) | 22 (73) | 8 (89) | ||
| Current smoking | 2 (5) | 1 (3) | 1 (11) | 0.413 | |
| Any comorbidity | 11 (28) | 9 (30) | 2 (22) | 1.000 | |
| Diabetes mellitus | 1 (3) | 1 (3) | 0 (0) | 1.000 | |
| Hypertension | 7 (18) | 5 (17) | 2 (22) | 0.653 | |
| Cancer | 3 (8) | 3 (10) | 0 (0) | 1.000 | |
| Chronic hepatitis B | 2 (5) | 2 (7) | 0 (0) | 1.000 | |
| Chronic lung disease | 1 (3) | 1 (3) | 0 (0) | 1.000 | |
| Cardiovascular disease | 0 (0) | 0 (0) | 0 (0) | NA | |
| Room types in Israel | 0.556 | ||||
| Single | 3 (8) | 2 (7) | 1 (11) | ||
| Double | 36 (92) | 28 (93) | 8 (89) | ||
| Bus seats in Israel | 0.273 | ||||
| Window seats | 19 (49) | 13 (43) | 6 (67) | ||
| Aisle seats | 20 (51) | 17 (57) | 3 (33) | ||
Data are presented as median (interquartile rage) or number (%).
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, NA = not available.
aThe Mann–Whitney U test, χ2 test, or Fisher's exact test was applied.
Among 30 COVID-19 cases, 8 (27%) experienced no symptoms at the first sampling dates (Cases 22, 23, and 25–30) and 4 (13%) were asymptomatic (Cases 27–30). We could not obtain credible information on the specific symptoms of the primary case. Out of 29 cases, the most common symptoms at the first sampling dates, which was 3 days (median; IQR, 1–4 days) after symptom onset, were febrile/chilling sense (34%) and sore throat (34%). Upon admission, which was around 5 days (median; IQR, 3–6 days) from the onset of symptoms, the most common symptoms were cough (45%) and sputum (45%). The median hospital stay duration was 23 days (IQR, 20–29 days), and no fatal cases were present (Table 2). Out of 25 cases with symptoms, the most frequent first symptom was febrile/chilling sense (44%), followed by sore throat (40%), myalgia (32%), and cough (27%).
Table 2. Clinical characteristics of COVID-19 cases in pilgrim travelers.
| Characteristics | First sampling dates | Admission dates | Discharge dates | |
|---|---|---|---|---|
| Cases with symptomsa | 21/30 (70) | 24/30 (80) | 26/30 (87) | |
| Febrile/chilling sense | 10/29 (34) | 12/29 (41) | 16/29 (55) | |
| Fever, > 37.5°C | - | 2/29 (7) | 9/29 (31) | |
| Cough | 6/29 (21) | 13/29 (45) | 19/29 (66) | |
| Rhinorrhea | 5/29 (17) | 6/29 (21) | 7/29 (24) | |
| Sputum | 6/29 (21) | 13/29 (45) | 17/29 (59) | |
| Dyspnea | 0/29 (0) | 0/29 (0) | 2/29 (7) | |
| Sore throat | 10/29 (34) | 11/29 (38) | 14/29 (48) | |
| Myalgia | 9/29 (31) | 9/29 (31) | 11/29 (38) | |
| Headache | 4/29 (14) | 7/29 (24) | 11/29 (38) | |
| Diarrhea | 1/29 (3) | 1/29 (3) | 5/29 (17) | |
| Abdominal pain | 1/29 (3) | 2/29 (7) | 5/29 (17) | |
| Vomiting | 0/29 (0) | 0/29 (0) | 3/29 (10) | |
| Cases without symptoms | 9/30 (30) | 6/30 (20) | 4/30 (13) | |
Data are presented as number (%).
COVID-19 = coronavirus disease 2019.
aThe primary case experienced unknown symptoms before the first sampling date and did not include in the specific symptoms section considering the uncertainty of information.
Laboratory and radiologic findings
Routine blood tests and chest radiograph results showed no significant difference between cases with symptoms and cases without symptoms at admission. Specimens from the 29 confirmed cases were analyzed by the GGPIHE. The first sampling dates were ranged from February 20 to February 22, and majority of the first samples (27/29, 93.1%) were collected on February 21. Available Ct values from specimens of the lower respiratory tract were significantly lower in cases without symptoms (median, 22.8; IQR, 20.1–25.4) than in cases with symptoms at the first sampling dates (median, 27.9; IQR, 23.4–30.4) (Table 3). A lower Ct value indicates higher viral shedding.
Table 3. Laboratory and radiologic findings of COVID-19 cases in pilgrim travelers.
| Variables | All cases (n = 30) | Cases with symptoms at admission dates (n = 24) | Cases without symptoms at admission dates (n = 6) | P valuea | |
|---|---|---|---|---|---|
| White blood cell count, × 109/L | 4.2 (3.3–5.3) | 4.1 (3.3–5.0) | 5.1 (3.4–5.8) | 0.407 | |
| < 4 | 14 (47) | 12 (50) | 2 (33) | 0.657 | |
| 4–10 | 16 (53) | 12 (50) | 4 (67) | ||
| > 10 | 0 (0) | 0 (0) | 0 (0) | ||
| Platelet count, × 109/L | 199.5 (149.5–243.3) | 199.5 (158.5–244.5) | 185.0 (131.0–215.0) | 0.534 | |
| < 150 | 7 (23) | 5 (21) | 2 (33) | 0.603 | |
| ≥ 150 | 23 (77) | 19 (79) | 4 (67) | ||
| Hemoglobin, g/dL | 13.9 (13.2–14.6) | 13.9 (13.2–14.6) | 14.2 (13.8–14.6) | 0.483 | |
| Aspartate aminotransferase, U/L | 25.0 (21.8–34.3) | 25.0 (22.0–32.5) | 28.5 (21.0–35.0) | 0.795 | |
| Alanine aminotransferase, U/L | 20.5 (16.0–27.0) | 20.0 (17.0–25.0) | 23.0 (14.0–32.0) | 0.815 | |
| Total protein, g/dL | 7.2 (6.8–7.5) | 7.1 (6.8–7.4) | 7.3 (6.5–7.7) | 0.677 | |
| Albumin, g/dL | 4.1 (3.9–4.3) | 4.1 (4.0–4.3) | 4.1 (3.8–4.4) | 0.917 | |
| Sodium, mmol/L (n = 28) (22 and 6, respectively) | 139.0 (137.5–141.0) | 139.3 (138.0–141.0) | 138.3 (136.1–139.0) | 0.298 | |
| Potassium, mmol/L (n = 28) (22 and 6, respectively) | 4.0 (3.8–4.3) | 4.0 (3.7–4.3) | 3.9 (3.8–4.1) | 0.910 | |
| Creatinine, mg/dL (n = 29) (23 and 6, respectively) | 0.7 (0.6–0.9) | 0.7 (0.6–0.9) | 0.7 (0.6–0.7) | 0.548 | |
| C-reactive protein, mg/dL | 0.2 (0.1–1.0) | 0.3 (0.1–1.0) | 0.2 (0.1–0.4) | 0.755 | |
| Chest radiograph | 0.659 | ||||
| Bilateral pneumonia | 3 (10) | 2 (8) | 1 (17) | ||
| Unilateral pneumonia | 2 (7) | 2 (8) | 0 (0) | ||
| No abnormal density shadow | 25 (83) | 20 (83) | 5 (83) | ||
| Cycle threshold (first samples)b | |||||
| Upper respiratory tract (n = 23) (16 and 7, respectively) | 27.0 (22.1–32.0) | 27.0 (22.6–32.7) | 27.0 (21.2–29.6) | 0.423 | |
| Lower respiratory tract (n = 27) (19 and 8, respectively) | 26.4 (22.7–28.8) | 27.9 (23.4–30.4) | 22.8 (20.1–25.4) | 0.034 | |
Data are presented as median (interquartile range) or number (%).
COVID-19 = coronavirus disease 2019.
aThe Mann–Whitney U test, χ2 test, or Fisher's exact test was applied; bWe compared the cycle threshold values from the first samples between cases with symptoms (20) and cases without symptoms (9) at the first sampling dates, which were analyzed by the Gyeongsangbuk-do Government Public Institute of Health and Education.
Follow-up RT-PCR
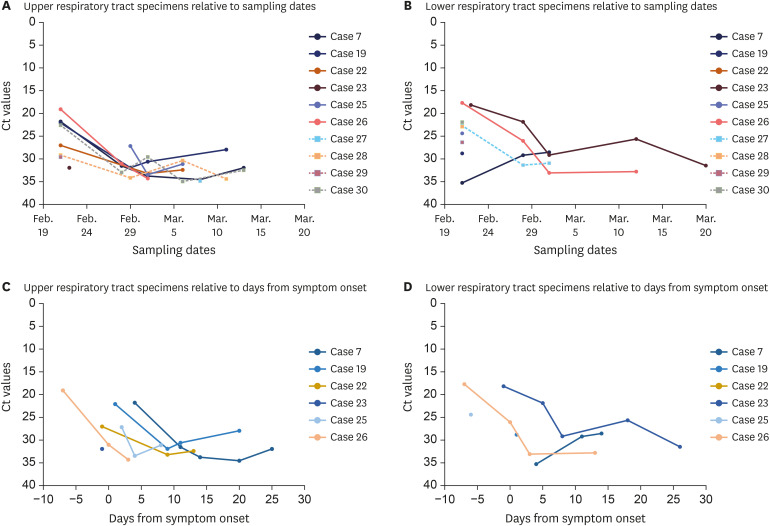
Fig. 2A (upper respiratory tract) and 2B (lower respiratory tract) show the available Ct values from 6 symptomatic cases (Cases 07, 19, 22, 23, 25, and 26) and 4 asymptomatic cases (Cases 27–30), relative to the sampling dates. The viral loads gradually decreased over time and were not different between symptomatic and asymptomatic cases. Fig. 2C (upper respiratory tract) and 2D (lower respiratory tract) show the available Ct values for 6 symptomatic cases relative to days from symptom onset; Cases 07 and 19 were postsymptomatic and Cases 22, 23, 25, and 26 were presymptomatic at the first sampling dates. The lowest Ct values from upper respiratory tract specimens (19.1) and lower respiratory tract specimens (17.7) were observed from the first samples of Case 26 collected 7 days before symptom onset.
Fig. 2. Follow-up viral loads relative to sampling dates and days from symptom onset.
Plots show the available Ct values relative to sampling dates for 10 cases from the upper respiratory tract specimens (nasopharyngeal plus oropharyngeal swabs; A) and 10 cases from the lower respiratory tract specimens (sputum; B). The available Ct values relative to days from symptom onset from the upper respiratory tract (C) and the lower respiratory tract (D) specimens for 6 symptomatic cases are shown. The circles are symptomatic cases and the squares are asymptomatic cases.
Ct = cycle threshold.
Contact tracing
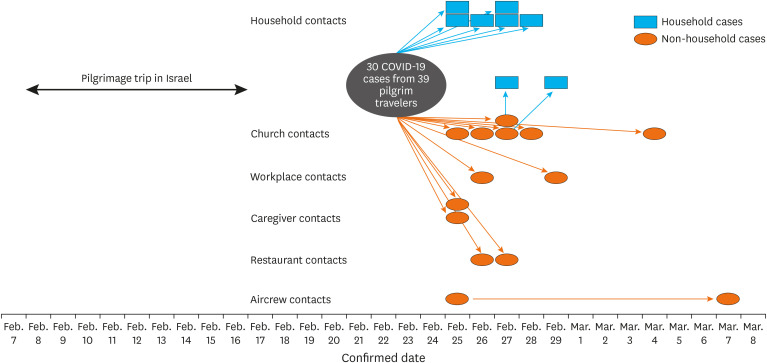
Out of 328 contacts related to the original COVID-19 cases in the pilgrim travelers, 22 additional cases (attack rate: 7%) were confirmed with SARS-CoV-2 infections (Table 4 and Fig. 3). The son of the primary case was excluded, considering the uncertainty of information on symptom onset. During transmission from secondary to tertiary cases, the attack rate was 7% (19/258) and that from tertiary to quaternary cases was 5% (3/60). No additional quinary cases occurred. The highest attack rate was observed from church contacts (19%, 6/31), followed by cohabiting family contacts (secondary attack rate: 18%, 8/44), caregiver contacts (13%, 2/15), and restaurant contacts (10%, 2/20). There were no confirmed cases out of the 19 clinic and pharmacy contacts. The tertiary case from the aircrew contacts was a stewardess who had boarded the airplane with the pilgrim travelers from Israel to Korea (KE958). The stewardess was in charge of the zone where 29 COVID-19 cases were seated. An additional quaternary case from the aircrew contacts was a stewardess who boarded the airplanes with the tertiary stewardess from Korea to the USA (KE017) and from the USA to Korea (KE012).
Table 4. Contact tracing results of COVID-19 cases related to pilgrim travelers.
| Types of contacts | Secondary to tertiary cases | Tertiary to quaternary cases | Quaternary to quinary cases | Total |
|---|---|---|---|---|
| Cohabiting familya | 6/27 (22) | 2/14 (14) | 0/3 (0) | 8/44 (18) |
| Other family | 0/7 (0) | 0/0 (0) | 0/2 (0) | 0/9 (0) |
| Church | 6/31 (19) | 0/0 (0) | 0/0 (0) | 6/31 (19) |
| Workplace | 2/68 (3) | 0/7 (0) | 0/0 (0) | 2/75 (3) |
| Caregiver | 2/13 (15) | 0/0 (0) | 0/2 (0) | 2/15 (13) |
| Restaurant | 2/20 (10) | 0/0 (0) | 0/0 (0) | 2/20 (10) |
| Clinic/pharmacy | 0/15 (0) | 0/2 (0) | 0/2 (0) | 0/19 (0) |
| Aircrewb | 1/12 (8) | 1/23 (4) | 0/0 (0) | 2/35 (6) |
| Others | 0/65 (0) | 0/14 (0) | 0/1 (0) | 0/80 (0) |
| Total | 19/258 (7) | 3/60 (5) | 0/10 (0) | 22/328 (7) |
COVID-19 = coronavirus disease 2019.
aThe son of the primary case was not included in the result considering the uncertainty of information on symptom onset; bAirplane passengers contacts were not included in the result considering the limitation of foreigners contact tracing.
Fig. 3. Contact diagram of coronavirus disease 2019 cases related to pilgrim travelers.
The son of the primary case was not included in the result, considering the uncertainty of information on symptom onset.
One fatal tertiary case occurred, resulting in a total fatality rate of 2% (1/52) in this outbreak. Three tertiary cases were identified to be transmitted by presymptomatic secondary cases. An immobile cohabiting aunt of Case 26 was a tertiary case whose symptoms occurred 2 days before the symptom onset of Case 26 (February 26 for the aunt and February 28 for Case 26, respectively). Case 26 had cared for the aunt at home until hospital admission on February 28. Case 26, a woman in her 60s without any comorbidity, was positive with the highest viral loads from samples collected 7 days before her symptom onset (Fig. 2). Additionally, 2 tertiary cases were transmitted by the presymptomatic caregiver (Case 17), who had visited the home of the tertiary cases until 1 day before the symptom onset of Case 17.
DISCUSSION
Of 39 pilgrim travelers from Korea, 30 (77%) were confirmed with SARS-CoV-2 infections and 4 were asymptomatic cases (13%). Viral loads were the highest at the first sampling dates among both symptomatic and asymptomatic cases and decreased over time. After aggressive testing for SARS-CoV-2 and contact tracing, 22 additional cases related to the pilgrim travelers were confirmed. However, an explosive COVID-19 outbreak related to a religious group from Shincheonji in Korea was reported simultaneously.10
Considering the primary case experienced symptoms during the pilgrimage trip, he may have been infected before or during the travel in Korea. The primary case from the pilgrim travelers could have been infected by his son in Korea, who was related to the outbreak at Shincheonji. Utilization of novel technologies, including GPS records, could supplement both the investigation of the outbreak and contact tracing. However, the exact symptom onset date of the son was unknown. Older-aged individuals had a significantly higher risk of SARS-CoV-2 infection as revealed by the chi-square test, although it was not significant using the Mann–Whitney U test. According to a Chinese study performed in Guangzhou, the oldest age group (≥ 60 years) had a significantly higher risk of household infection.17 Although the higher attack rate among older-aged individuals might be possible, more studies are needed to determine which age may be a risk factor.
The most common symptoms of COVID-19 cases experienced by the pilgrim travelers were cough, sputum, and febrile/chilling sense during the clinical course. A Chinese study reported that the most common symptom of COVID-19 was fever, followed by cough, fatigue, and sputum.4 The relatively low proportion of patients with febrile/chilling sense in this study may be influenced by the mild or asymptomatic cases, which could be close to the natural history of COVID-19. However, the most common symptoms at the first sampling dates were febrile/chilling sense and sore throat. To detect COVID-19 cases earlier, we need to place more emphasis on febrile/chilling sense and sore throat as symptoms rather than cough.
The proportion of asymptomatic COVID-19 cases in this study was 13%, which corresponded with the proportion of asymptomatic COVID-19 cases (13%, 2/23) in residents of a nursing facility in Washington.18 Additionally, this proportion is similar to the estimated proportion of asymptomatic COVID-19 cases (17.9%) among the confirmed cases on board the Diamond Princess.19 However, the asymptomatic proportion in our study was slightly higher than the reported 11% for the influenza virus infection,20 which might have been attributed to the COVID-19 outbreak around the world.
Follow-up RT-PCR tests revealed that higher viral loads were detected at the initial phase of COVID-19 and decreased over time in this study. Viral loads detected from upper and lower respiratory specimens in the asymptomatic group were similar to those in the symptomatic group. These results are comparable to a Chinese study investigating viral load in upper respiratory specimens of COVID-19 patients. They found that viral loads detected in symptomatic and asymptomatic patients were similar.21 During the presymptomatic period of COVID-19, viral loads were higher than during the postsymptomatic period, suggesting that there may be higher possibility of transmission before the onset of symptoms. To prevent further transmission of COVID-19, we need to focus on presymptomatic and asymptomatic cases rather than postsymptomatic cases. Therefore, massive testing for COVID-19 among exposed contacts without symptoms should be considered.
Viral loads from lower respiratory specimens in cases without symptoms (presymptomatic and asymptomatic cases) were significantly higher than those in cases with symptoms (postsymptomatic cases) at the first sampling dates. These results may be influenced by the higher viral loads of presymptomatic cases. Additionally, the minimum number of days from the first sampling date with positive results was 7 days before symptom onset. Another study performed in a nursing facility reported that the minimum number of days from possible infection to the first evidence of fever, cough, and shortness of breath was 7 days.22 Presymptomatic transmission has been reported from China, Germany, Singapore, and Taiwan.6,7 Out of 22 additional cases in this study, 3 tertiary cases were assumed to be transmitted before the onset of symptoms in the secondary cases and 1 tertiary case had onset of symptoms 2 days before that in the secondary case. Considering the minimum incubation period of 1 day,23 the transmission of COVID-19 may occur at least 3 days before symptom onset.
The highest attack rate was observed from church contacts through contact tracing of COVID-19 cases in pilgrim travelers. Out of 16 mass infections of COIVD-19 in Korea on March 6, 2020, 4 mass infections were related to the church, where many people gather in a closed and crowded environment.24 The secondary attack rate among cohabiting families in this outbreak was 18%, which was similar to 16.2% from the outbreak in call center in Korea.25 A Chinese study in Guangzhou reported 17.1% of the household secondary attack rate of COVID-19, while another study from Taiwan identified 4.6% among household contacts.7,17 Out of 19 contacts related to clinic/pharmacy, no additional case was detected. In Taiwan, the attack rate in health care settings was 0.9% and significantly lower than that among household contacts.7 Infection of the tertiary case of the stewardess was assumed to have happened during the flight of pilgrim travelers. Aircraft transmission of COVID-19 has been reported in 2 studies from China and France.26,27
Our study has several limitations. First, information on the date of symptom onset of the primary case was estimated based on statements from other pilgrim travelers. The symptom onset may have been earlier than what was reported. Second, follow-up Ct values were obtained from only 10 out of 30 cases owing to limited resources. Third, contact tracing in this study used the previous definition of contacts that was narrower. Additional cases who transmitted before symptom onset may be present.
In conclusion, SARS-CoV-2 was detected up to 7 days before symptom onset and viral loads may be greater during the presymptomatic period of infection. The proportion of asymptomatic cases was 13% and the viral loads in asymptomatic cases were similar to that in symptomatic cases. Massive testing for the detection of presymptomatic and asymptomatic cases is required to control outbreaks of COVID-19. Considering the case of transmission at least 3 days before symptom onset, the definition of contacts who were exposed to a COVID-19 case from 2 days before8 may be insufficient. More research about presymptomatic transmission over 2 days before symptom onset is required.
ACKNOWLEDGMENTS
We express our gratitude to our colleagues in the public health centers, the Gyeongsangbuk-do Provincial Government, the Gyeongsangbuk-do Medical Association, and the KCDC.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park JH.
- Data curation: Park JH, Jang J.
- Formal analysis: Park JH, Jang J.
- Investigation: Park JH, Jang J, Lee K, Yoo SJ, Shin H.
- Methodology: Park JH, Jang J.
- Writing - original draft: Park JH, Jang J.
- Writing - review & editing: Lee K, Yoo SJ.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) weekly operational update 9 October 2020. [Updated 2020]. [Accessed October 19, 2020]. http://www.who.int/publications/m/item/weekly-update-on-covid-19-9-october-2020.
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 – studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26(7):e201595. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH, et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Contact tracing in the context of COVID-19. [Updated 2020]. [Accessed July 20, 2020]. http://www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19.
- 9.Kim SE, Jeong HS, Yu Y, Shin SU, Kim S, Oh TH, et al. Viral kinetics of SARS-CoV-2 in asymptomatic carriers and presymptomatic patients. Int J Infect Dis. 2020;95:441–443. doi: 10.1016/j.ijid.2020.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of Coronavirus Disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health - State of Israel. Coronavirus patients summary announcement. [Updated 2020]. [Accessed July 20, 2020]. http://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/28022020_4.aspx.
- 12.Ministry of Health - State of Israel. Investigation findings update on patient no. 7 from Italy. [Updated 2020]. [Accessed July 20, 2020]. http://www.health.gov.il/English/News_and_Events/Spokespersons_Messages/Pages/29022020_2.aspx.
- 13.Central Disease Control Headquarters. Coronavirus disease 2019 response guidelines for local governments in South Korea, edition 6. [Updated 2020]. [Accessed September 17, 2020]. http://www.gidcc.or.kr/epvbr/코로나바이러스감염증-19covid-19.
- 14.Central Disease Control Headquarters. Coronavirus disease 2019 response guidelines for local governments in South Korea, edition 7-4. [Updated 2020]. [Accessed September 17, 2020]. http://www.gidcc.or.kr/epvbr/코로나바이러스감염증-19covid-19.
- 15.Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40(5):351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Central Disease Control Headquarters. Coronavirus disease 2019 response guidelines for local governments in South Korea, edition 7. [Updated 2020]. [Accessed September 17, 2020]. http://www.gidcc.or.kr/epvbr/코로나바이러스감염증-19covid-19.
- 17.Jing QL, Liu MJ, Zhang ZB, Fang LQ, Yuan J, Zhang AR, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141–1150. doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimball A, Hatfield KM, Arons M, James A, Taylor J, Spicer K, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):200180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip DK, Lau LL, Leung NH, Fang VJ, Chan KH, Chu DK, et al. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infection in the community. Clin Infect Dis. 2017;64(6):736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in the upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang YJ. Lessons learned from cases of COVID-19 infection in South Korea. Disaster Med Public Health Prep. 2020:1–8. doi: 10.1017/dmp.2020.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SY, Kim YM, Yi S, Lee S, Na BJ, Kim CB, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian GQ, Yang NB, Ding F, Ma AH, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldin C, Lagier JC, Mailhe M, Gautret P. Probable aircraft transmission of Covid-19 in-flight from the Central African Republic to France. Travel Med Infect Dis. 2020;35:101643. doi: 10.1016/j.tmaid.2020.101643. [DOI] [PMC free article] [PubMed] [Google Scholar]