Abstract
Purpose
To demonstrate a modified technique of fully automated direct perfluorocarbon liquid (PFCL)-silicone oil (SO) exchange.
Materials and Methods
This technique is indicated for cases that require direct PFCL-SO exchange as in giant retinal tear or large retinectomies to avoid retinal slippage. Pars plana vitrectomy (PPV) is carried out in standard fashion; then the dual active injection/extrusion mode is activated in the vitrectomy machine. The machine parameters are set at approximately 30–40 psi for SO injection and 150–250 mmHg for active PFCL aspiration. In this method, both the SO injection and the PFCL extrusion are simultaneously controlled by the foot pedal.
Results
We used this technique for a total of 24 cases: 6 cases of giant retinal tears and 18 retinectomies. We did not encounter any complications related to significant IOP spike during surgery or complete removal of the PFCL.
Conclusion
This automated technique for direct PFCL-SO exchange maintains a controlled balance between SO injection and PFCL aspiration, that mitigates the risk of intraoperative IOP spikes. It is safe, quick, and can be performed without the need of an assistant or extra chandelier light or high-pressure viscous tubing.
Keywords: giant retinal tear, retinectomy, perfluorocarbon liquid, PFCL, silicone oil, vitrectomy
Introduction
A major challenge in vitreoretinal surgery for giant retinal tear (GRT) or for large retinectomies is the risk of retinal slippage during the exchange of perfluorocarbon liquid (PFCL) for air or silicone oil (SO).1,2 Slippage refers to posterior displacement of residual aqueous fluid under the retina caused by an incoming bubble of endotamponade. Slippage has been shown to occur more frequently with PFCL-air exchange, compared to PFCL-SO exchange.3 This is because air has lower density that allows residual subretinal fluid to be displaced posteriorly. On the other hand, the hydrophobic nature of the SO and PFCL results in good contact between the two fluids, which shifts the aqueous layer above the SO layer thereby stabilizing the retina.4
In the conventional technique for direct PFCL-SO exchange, SO is actively injected with the foot pedal and PFCL is passively aspirated using a flute needle.5,6 Because passive aspiration of fluids is inherently slow, the inflow of SO during the exchange often exceeds the passive outflow of PFCL. As a result, the vitreous cavity becomes over-pressurized and intraocular pressure (IOP) rises. Significant elevation in IOP during surgery can lead to a dangerous situation resulting in corneal edema with impairment of the retinal view, SO migration into the anterior chamber, or iris prolapse through the corneal incision if cataract surgery was combined with the pars plana vitrectomy (PPV). Moreover, cases of scleral rupture have been reported.7
We herein demonstrate an alternative method for automated direct PFCL-SO exchange using active PFCL extrusion, that mitigates the high risk of IOP rise during the exchange.
Surgical Technique
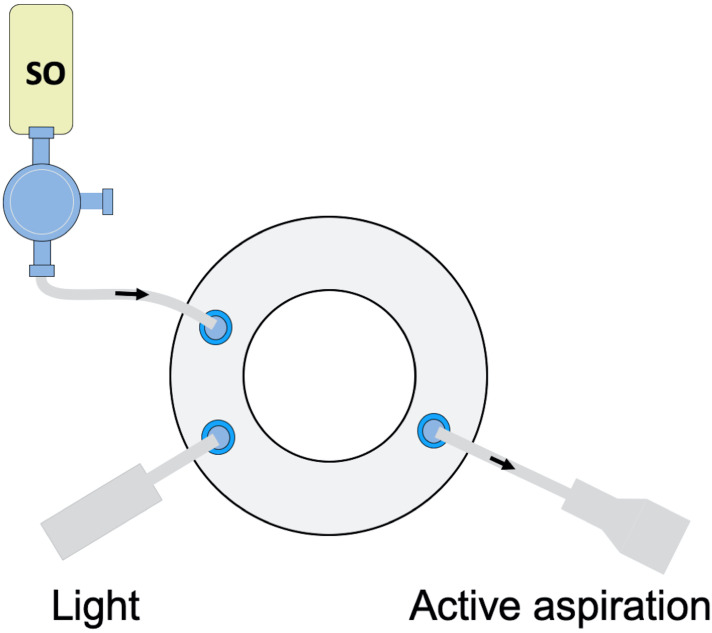
Three-port transconjunctival PPV is carried out in standard fashion and additional surgical steps are employed, as required, till the step of PFCL-SO exchange. At this point, while the vitreous cavity is filled with PFCL, we temporarily remove the infusion line from the vitrectomy canula, connect the SO filled syringe to the 3-way tap, and prime it with SO to remove any BSS from the infusion line before reconnecting it back to the eye. An aspiration cannula is connected to the active extrusion line of the vitrectomy machine and inserted via one of the superior trocars. The light pipe is placed through the other superior trocar (Figure 1).
Figure 1.
A surgeon’s view diagram showing a 23-gauge pars plana vitrectomy system set up. A silicone oil (SO) filled syringe is connected to the infusion cannula. The light pipe is inserted through one of the superior ports. The active aspiration needle is used in the other hand to aspirate the perfluorocarbon liquid (PFCL). The exchange is automated, both the SO infusion and the PFCL aspiration controlled by the foot pedal.
The dual active injection/extrusion mode is activated in the vitrectomy machine. It is of note, that the dual function mode is available in most modern vitrectomy platforms (e.g. Viscous fluid control in Alcon Constellation [Alcon Laboratories Inc., Fort Worth, TX, USA] or DORC (Dutch Ophthalmic Research company, Zuidland, Netherlands) or the Dual Yaw/vacuum mode in Bausch and Lomb platform (Bausch and Lomb, Rochester, NY, USA). For 23-gauge PPV, we use 1000–2000 cs SO and set the machine parameters at approximately 30–40 psi for SO injection and 150–200 mmHg for active PFCL aspiration. The parameters can be refined according to the vitrectomy gauge and SO viscosity (e.g. for smaller 25-gauge PPV, we raise the vacuum to ~250–300 mmHg and if higher viscosity oil such as 5000 cs is used, we set the vacuum to ~150–200 mmHg, to adapt for the slower rate of the more viscous oil injection).
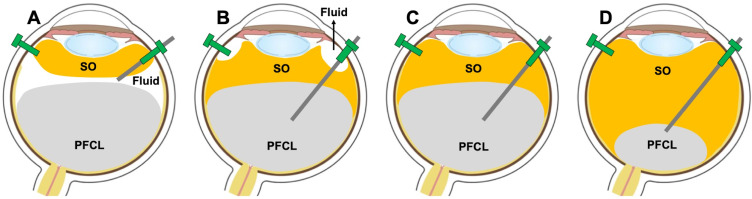
At the beginning of exchange, the aqueous layer on top of the PFCL layer is removed via active extrusion with metal-tipped cannula while injecting the SO (Figure 2A). While injecting the SO, gently pressing the SO trocar helps in establishing early contact between the oncoming SO and the PFCL bubble. Once the SO comes in contact with the PFCL bubble, they tend to form the interface at the expense of any residual aqueous, if any, which will be pushed away from the interface and can find its way out of the eye via the sclerotomy (Figure 2B). This is followed by further drying of any aqueous fluid at the edge of the giant retinal tear or the retinectomy, if required. Then, the active extrusion is dipped into the PFCL bubble and we follow the PFCL bubble toward the optic nerve to remove all the PFCL from the vitreous cavity (Figure 2C and D) with the aspiration cannula (Supplemental Video S1, an informed consent was obtained to publish video imagery).
Figure 2.
Steps of automated direct Perfluorocarbon liquid (PFCL) - Silicone oil (SO) exchange. (A) The silicone oil is injected while the foot pedal is pressed, meanwhile the fluid at the interface is removed via active extrusion. (B) Once the silicone oil comes in contact with the PFCL bubble, they form an interface at the expense of the remaining aqueous which will be pushed away from the interface and can find its way out of the eye via the sclerotomy. This is followed by further drying of any aqueous fluid at the edge of the giant retinal tear or the retinectomy. (C) Next, the active aspiration cannula is dipped into the PFCL bubble, and the PFCL is gradually removed as the silicone oil fills the vitreous cavity. (D) The extrusion cannula then follows the PFCL bubble toward the optic nerve for complete removal of the PFCL.
Since the extrusion is active, removal of the final PFCL droplets is usually straightforward. Caution must be taken to avoid the extrusion cannula slipping into the SO layer as this can block the cannula or any iatrogenic damage to the retina. It is preferred to use a metal-tipped extrusion cannula and to avoid silicone-tipped as they can be easily blocked. A further modification of the technique to help removal of the aqueous fluid out of the eye is to remove the valve of one of the superior trocars before the start of the exchange and to start by SO injection with the eye tipped down so that the opened trocar is placed at the highest point. This will result in the passive movement of the displaced aqueous layer towards this trocar and out of the eye.
The IOP is monitored during the procedure and the rate of injection/extrusion is continuously adjusted by the foot pedal. Indicators of high IOP are pallor of the disc and retinal arterial pulsations. Also, slightly pressing the light pipe and the extrusion cannula in the 2 superior trocars inward against the sclera also provides useful tactile feedback on the IOP status. Because the rate of automated aspiration of PFCL is often faster than the injection of SO, we often maintain the SO infusion at the preset maximum pressure and regulate the exchange mainly by controlling the rate of PFCL aspiration. This is important to avoid hypotony, which may itself be a potential reason for retinal slippage.
We have used this technique for a total of 24 cases, operated by 3 vitreoretinal surgeons (ABS, AAS and AAE). Those included 6 cases of giant retinal tears and 18 retinectomies. We used 23-gauge vitrectomy system in 21 cases and 25-gauge in 3 cases. Viscosity of SO used included: 1000 cs in 6 cases, 2000 cs in 8 cases, and 5000 cs in 10 cases. We did not encounter any complications related to significant IOP spike during surgery or any case of intraoperative retinal slippage. The average time for exchange ~5–6 minutes and was slightly longer if 25-gauge PPV or 5000 cs SO were used. This study was approved by the Institutional Review Board and Ethics Committee of Suez Canal University, which adhered to the tenets of the Declaration of Helsinki. Written informed consent for each participant was obtained.
Discussion
Direct PFCL-SO exchange in the context of GRT repair or a large relaxing retinectomy has several advantages as compared to indirect exchange of PFCL for air and air for SO. It is easier to visualize the PFCL-SO interface than the PFCL-air interface; this makes PFCL removal more simple and complete. More importantly, there is a significantly decreased risk of retinal slippage with direct PFCL-SO exchange. However, the conventional method for performing the direct exchange relies on passive aspiration of PFCL and is associated with an inherent risk of significant IOP rise. As a result, many surgeons are not comfortable performing direct PFCL-SO exchange, and the technique is not widely taught.
The active extrusion technique described here allows for automated, precise control of both SO injection and PFCL removal, using the foot pedal. As opposed to passive aspiration, active extrusion of PFCL allows for removal of the PFCL at a rate that matches the SO injection. This helps prevent over-pressurization of the vitreous cavity and maintains a safe level of IOP. The surgeon sets the machine parameters specified for injection and extrusion and these can be further refined during the procedure as needed to maintain the balance between inflow and outflow of fluids. The technique of automated direct exchange does not require the use of an additional chandelier or high viscosity fluid infusion cannula that are sometimes required during the passive exchange technique. It is of note, that the technology of controlling both the extrusion of PFCL and the injection of SO using the foot pedal is available on most of the modern vitrectomy platforms.
In conclusion, the use of an active technique for direct PFCL-SO exchange provides an efficient and safe method to avoid retinal slippage. The technique maintains good balance between SO injection and PFCL aspiration, and mitigates the risk of intraoperative IOP spikes encountered with the passive exchange technique.
Acknowledgment
The abstract of this paper was presented at the 19th EVRS congress (Lisbon, Portugal) as an oral presentation with interim findings.
Funding Statement
There is no funding to report.
Disclosure
All authors of this manuscript have no commercial financial relationships to disclose and report no conflicts of interest for this work.
References
- 1.Mathis A, Pagot V, Gazagne C, Malecaze F. Giant retinal tears. Surgical techniques and results using perfluorodecalin and silicone oil tamponade. Retina. 1992;12(3 Suppl):S7–S10. doi: 10.1097/00006982-199212031-00003 [DOI] [PubMed] [Google Scholar]
- 2.Han DP, Rychwalski PJ, Mieler WF, Abrams GW. Management of complex retinal detachment with combined relaxing retinotomy and intravitreal perfluoro-n-octane injection. Am J Ophthalmol. 1994;118(1):24–32. doi: 10.1016/S0002-9394(14)72838-7 [DOI] [PubMed] [Google Scholar]
- 3.Wong D, Williams RL, German MJ. Exchange of perfluorodecalin for gas or oil: a model for avoiding slippage. Graefes Arch Clin Exp Ophthalmol. 1998;236(3):234–237. doi: 10.1007/s004170050070 [DOI] [PubMed] [Google Scholar]
- 4.Wong D. Slippage of the retina: what causes it and how can it be prevented? In: Kirchhof B, Wong D, editors. Essentials in Ophthalmology. Vitreo-Retinal Surgery. Berlin, Ney York: Springer-Verlag; 2007:41–51. [Google Scholar]
- 5.Astir S, Shroff DN, Gupta C, Shroff CM, Saha I, Bimanual DR. 25-gauge chandelier technique for direct perfluorocarbon liquid-silicone oil exchange in retinal detachments associated with giant retinal tear. Indian J Ophthalmol. 2018;66(12):1849–1851. doi: 10.4103/ijo.IJO_440_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrocal MH, Chenworth ML, Acaba LA. Management of giant retinal tear detachments. J Ophthalmic Vis Res. 2017;12(1):93–97. doi: 10.4103/2008-322X.200158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal L, Tarun Sharma PS, Bhende MB. Giant retinal tear In: Schachat AP, editor. Ryan’s Retina. Sixth ed. Elsevier Inc; 2018:2072–2080. [Google Scholar]