Abstract
COVID-19 pandemic has led to major changes in the delivery of medical care around the globe. Many investigations and elective procedures had to be rescheduled to decrease the risk of spreading the infection. Non-invasive cardiac imaging studies are requested to guide appropriate cardiac care in a variety of urgent, semi-urgent, and elective procedures. This position statement of the Cardiac Imaging Working Group of the Saudi Heart Association provides guidance into the protection of healthcare personnel, assessment of the indications of the imaging studies, and highlights consideration before, during, and after the study.
Keywords: COVID-19, cardiac CT, Cardiac MRI, cardiac imaging nuclear cardiology
1. Introduction
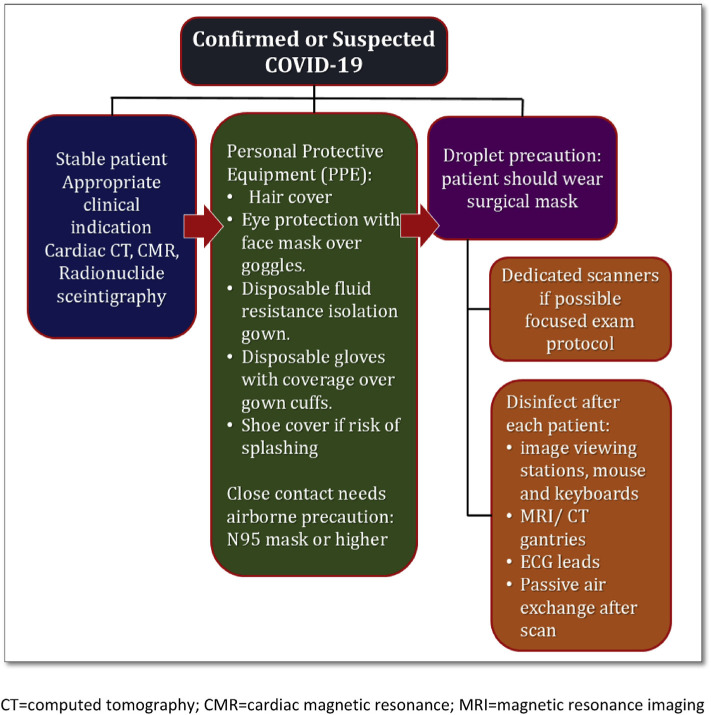
The declaration of Coronavirus disease 2019 (COVID-19) as a pandemic by the World Health Organization (WHO) has led to significant consequences on health care delivery all over the world. COVID-19 is highly infectious and has a widely variable presentation ranging from severe respiratory distress to no symptoms. Asymptomatic individuals, in particular, pose a high risk of infection to healthcare personnel [1]. The risk of COVID-19 transmission remains high, even within a distance of 1.8 m [2]. Therefore, it is imperative that non-urgent cases, where cardiac imaging does not have a direct impact on patient care, be postponed. Cases where cardiac imaging significantly impacts the management plan should be performed with necessary precautions to ensure the safety of the healthcare personnel (see Fig. 1).
Fig. 1.
Scheduling cardiac scan while considering the safety of the healthcare workers during the COVID-19 pandemic. CT = computed tomography; CMR = cardiac magnetic resonance; MRI = magnetic resonance imaging.
The purpose of this document is to ensure that appropriate and timely cardiac imaging studies are available to guide the management of all patients, whether or not they are COVID-19 infected, while ensuring the safety of the healthcare workers in particular technologists, nurses and imaging experts during image acquisition.
This document provides guidance into the protection of healthcare personnel, assessment of the indications of the imaging studies, and highlights consideration before, during, and after the study. It covers nuclear cardiac imaging, cardiac magnetic resonance (CMR), and cardiac computed tomography (CCT). These modalities have less direct contact with the patient compared to echocardiography making it more favorable during the pandemic to decrease the risk of infection to health care personnel.
2. Protection of Healthcare Personnel
The general principles of minimizing transmission of infection should be observed at all times. These recommendations include distancing, hand hygiene, and ensuring the availability of personal protective equipment (PPE). It is also recommended to avoid overcrowding at the workplace and to work remotely whenever feasible.
Healthcare personnel should follow institutional and local infection control recommendations. Healthcare personnel who perform imaging examinations and are in close or direct contact with the patient should use PPE as recommended by the WHO, including an isolation gown with fluid-resistant characteristics, a pair of gloves with coverage over gown cuffs, and eye protection with face shield [2]. The risk status of each patient should be evaluated according to the Saudi Center for Disease Prevention and Control (Saudi CDC) [3]. Airborne precautions, including N95/FFP2/FFP3 mask and goggles, should be used in cases with confirmed or high risk for COVID-19 infection. First and foremost, it is essential to consider the potential risk of transmission of the infection to patients when evaluating the indication for the imaging study.
3. Consideration Before Patient Arrival to the Test
The indication of each test should be reviewed by the imaging expert and discussed with the treating physician by the appropriate use criteria of each modality [4–6]. Once the test is considered appropriate, then the level of urgency should be determined (Table 1). The indications are subdivided into two groups, urgent indications (where the test should be performed with a week) and semi-urgent indications (where the test should be performed within two weeks). As mentioned earlier, all the elective cases should be deferred until the pandemic resolves. The decision to perform the test should be based on the urgency, availability of the test, the possibility of deferring the test without affecting patient care, and finally, the time frame for performing the deferred tests. In COVID-19 confirmed cases and persons under investigation with a high index of suspicion, the indication of the test should be discussed with the main responsible physician (MRP), and the test should be performed if it is absolutely necessary and has an immediate impact on the management plan.
Table 1.
Urgency level of the indications for non-invasive cardiac imaging.
| Indication | Urgency Level | CCTA | CMR | Nuclear Imaging |
|---|---|---|---|---|
| Coronary Artery disease | Urgent (1–7 days)a | 1. Patient presenting to ED with chest pain or low-risk NSTEMI/UA 2. Quadruple rule out (CAD, PE, late iodine enhancement for myocarditis & pulmonary infiltrates) |
1. Stress CMR for patient presenting to ED with chest pain or low-risk NSTEMI/UA 2. Evaluation of viability in patients with ongoing symptoms undergoing possible CABG 3. Diagnosis of Myocarditis and MINOCA |
1. Patient presenting to ER with Chest pain or low-risk NSTEMI/UA 2. Evaluation of chest pain in moderate-high likelihood CAD 3. Pre-operative evaluation of patients with moderate to high- risk CAD 4. Evaluation of chest pain in patients with high risk for contract- induced nephropathy. 5. Evaluation of viability in patients with ongoing symptoms undergoing possible CABG |
| Elective (4–8 weeks) | 1. Evaluation of stable chest pain 2. Evaluation of congenital anomalies |
1. Evaluation of stable chest pain 2. Evaluation of ischemia after revascularization 3. Evaluation of medical therapy when revascularization is not urgent 4. Evaluation of viability in stable patients |
1. Evaluation of stable chest pain 2. Evaluation of ischemia after revascularization 3. Evaluation of medical therapy when revascularization is not urgent 4. Evaluation of viability in stable patients |
|
| Aortopathy | Urgent (1–7 days)a | Evaluation of acute aortic syndrome (Dissection/Aneurysm) | Evaluation of acute aortic syndrome (Dissection/Aneurysm) | |
| Elective (4–8 weeks) | Follow up of aortic aneurysm | Follow up of aortic aneurysm (CT is preferred) | Follow up of aortitis | |
| Valvular & structural heart diseases | Urgent (1–7 days)a | 1. Evaluation of thrombosed valve 2. Evaluation of endocarditis 3. Evaluation of the left atrial appendage 4. Evaluation for TAVR if AVR is urgent |
1. Evaluation of the degree of valvular regurgitations in cases requiring urgent intervention | 1. Evaluation of prosthetic valve endocarditis |
| Elective (4–8 weeks) | 1. Evaluation prior TAVR, TMVR, LAA closure 2. Pulmonary Vein assessment for AF ablation 3. Congenital Heart diseases |
1. Evaluation of complications of endocarditis 2. Congenital Heart diseases |
||
| Heart Failure | Urgent (1–7 days)a | 1. Evaluation of CAD in new CHF patient 2. Evaluation of LV thrombus 3. Evaluation of LVAD |
1. Stress CMR Evaluation new CHF with low- intermediate likelihood of CAD 2. Evaluation of LV thrombus 3. Evaluation of LVEF 4. Evaluation of the LVEF before chemotherapy or for follow up of chemotherapy |
1. Evaluation new CHF with low-intermediate likelihood of CAD 2. Evaluation of LV thrombus 3. Evaluation of LVEF 4. Evaluation of the LVEF before chemotherapy or for follow up of chemotherapy 5. Evaluation of LVAD/CEID endocarditis |
| Elective (4–8 weeks) | Follow up of LVEF | Follow up of the LVEF (continued on next page) | ||
| Arrhythmia and sudden cardiac death Cardiac masses |
Urgent (1–7 days)a Elective (4–8 weeks) Urgent (1–7 days)a Elective (4–8 weeks) |
Anomalous origin of coronary artery Evaluation of cardiac tumors/ thrombus Follow up of cardiac masses |
1. Evaluation of ARVC 2. Evaluation of sarcoidosis 1. Follow up of sarcoidosis 2. Follow up of HCM Evaluation of cardiac tumors/ thrombus Follow up of cardiac masses |
1. Evaluation of sarcoidosis 1. Follow up of sarcoidosis |
ED: emergency department, NSTEMI/UA: Non-ST-elevation myocardial infarction/unstable angina; CABG: coronary artery bypass grafting; MINOCA = myocardial infarction with non-obstructive coronary artery disease; CAD: coronary artery disease; PE: pulmonary embolism; TAVR: transcatheter aortic valve replacement; AVR: aortic valve replacement; TMVR: trans-catheter mitral valve replacement; LAA: Left atrial appendage; AF: atrial fibrillation; CHF: congestive heart failure; LV = left ventricle/ventricular; LVAD: left ventricular assist device; LVEF: left ventricular ejection fraction; CEID: cardiac implantable electronic device; ARVC: arrhythmogenic right ventricular cardiomyopathy; HCM: hypertrophic cardiomyopathy.
Timing of performing the test should be individualized based on the acuity of each indication.
If the test is deemed urgent, it should be performed with the appropriate precautions based on the patient status. Likewise, a semi-urgent test can be scheduled within two weeks. The patient should be called before the day of the test, and his/her risk should be reassessed according to the most updated Saudi CDC definitions.
All routine requests should be reviewed and tri-aged according to how long it can be deferred without affecting patient outcome. The rationale behind deferring the tests should be documented, and a priority list should be made to reschedule the deferred cases in a timely fashion.
4. Consideration During the Test
The patient should be interviewed again upon arrival to the imaging suite for exposure and symptoms of COVID-19. If feasible, a temperature check by a non-contact thermometer is encouraged before the patient enters the test location. Surgical face mask should be worn by all patients in the waiting areas and in the test location. Appropriate distancing of around 2 m should always be observed. Measures should be taken to minimize the number of technologists and nurses in direct contact with each patient. Whenever possible, patients who have been tested positive should bypass the waiting or receiving areas and enter the examination room immediately, avoiding contact with any other patient.
Short protocols are encouraged to minimize contact with the patient. The protocol should be tailored to answer the question of the referring physician. For scheduled stress tests, vasodilator stress testing is preferred over those employing exercise to decrease the chance of droplet and airborne exposure. Short vasodilator protocols such as regadenson should be utilized when possible.
A separate machine should be dedicated to COVID-19 positive patients if feasible. Otherwise, COVID-19 patients should be booked at the end of the day. In studies conducted for COVID-19 positive patients, at least one healthcare worker (preferably a technologist) should have full PPE to position and set up the patient in the scanner. Another technologist should remain in the control room and configure the acquisition protocol. The computer workstation should not be accessed by anyone who has been in the scanning room and in contact with the patient. The imaging expert should remain in the control room or remotely to monitor and interpret the study.
5. Consideration After the Test
Reading and reporting of the test should be performed through a remote system whenever possible. The reporting should be done by the assigned imaging expert. If multiple physicians are required (trainees), then distancing should be encouraged with telephone discussion whenever possible. If a second opinion is required from another expert colleague or the MRP, that should be conducted remotely as well.
6. Terminal Cleaning of the Machine
The machine, blood pressure cuffs, ECG cables, and reporting workstations, including the mouse and keyboard, should be cleaned and disinfected after each use. Doorknobs, table surfaces, and floors should also be cleaned after each use.
After scanning a COVID-19 patient, the machine should be cleaned per the local infection control recommendations. The machine should be closed for at least 1 h to allow for passive ventilation. Thereafter, the room cab terminally cleaned and closed for another hour before it can be used again. Meticulous decontamination of all the equipment that came in contact with a COVID-19 positive patient per the manufacturer's recommendations is advised.
7. Conclusions
This document is a general statement about the best practices for Carduac CT, Cardiac MRI and nuclear cardiac imaging during the COVID-19 pandemic. The choice of imaging including the consideration of echocardiography should be considered based on the availability of the modality and expertise in individual hospitals. It is also essential to be mindful that there may be a shortage of some of the required material, such as radioisotopes.
These recommendations are designed to assist in management decisions only and do not replace clinical judgment. Individualized case by case decisions are strongly advised, especially for elderly and immunocompromised patients. Urgent cases of COVID-19 confirmed, or highly suspected cases must be discussed with the imaging expert beforehand to determine the most suitable imaging modality and expedite reporting to facilitate patient disposition.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Kooraki S, et al. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol. 2020;17(4):447–51. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Saudi Center for Disease Control and Prevention Information about Coronavirus disease COVID-19. 2020 https://covid19.cdc.gov.sa. [Accessed 18 April 2020]
- 4.Doherty JU, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multi-modality imaging in the assessment of cardiac Structure and function in nonvalvular heart disease: a report of the American College of Cardiology appropriate use criteria Task Force, American Association for Thoracic Surgery, American heart Association, American Society of echocardiography, American Society of nuclear Cardiology, heart Rhythm Society, Society for Cardiovascular Angiography and interventions, Society of Cardiovascular computed tomography, Society for Cardiovascular magnetic resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2019;73(4):488–516. doi: 10.1016/j.jacc.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography A report of the American College of Cardiology Foundation appropriate use criteria Task Force, the Society of Cardiovascular computed tomography, the American College of Radiology, the American heart Association, the American Society of echocardiography, the American Society of nuclear Cardiology, the North American Society for Cardiovascular imaging, the Society for Cardiovascular Angiography and interventions, and the Society for Cardiovascular magnetic resonance. J Am Coll Cardiol. 2010;56(22):1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Hendel RC, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac Radionuclide imaging: a report of the American College of Cardiology Foundation appropriate use criteria Task Force, the American Society of nuclear Cardiology, the American College of Radiology, the American heart Association, the American Society of echocardiography, the Society of Cardiovascular computed tomography, the Society for Cardiovascular magnetic resonance, and the Society of nuclear medicine. J Am Coll Cardiol. 2009;53(23):2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]