Abstract
Background
Some types of beta-human papillomavirus (β-HPV) may be one of the probable causes of squamous cell carcinoma (SCC) in transplant recipients. β-HPVs are linked to SCC in the literature with small number of subjects.
Aim
Herein, the first meta-analysis was carried out on the association between β-HPVs and cutaneous SCC in immunosuppressed patients.
Methods
A systematic search was carried out in the PubMed and Scopus databases up to December 2018. The odds ratio (OR) were calculated by RevMan 5.3 software and the event rate (ER) by Comprehensive Meta-Analysis 2.0 software with a 95% confidence interval (CI).
Results
A total of 1250 records were identified through the two databases, but at last eleven studies were included in the meta-analysis that they were published from 1989 to 2018. The results showed a significantly high prevalence of β-HPVs in cutaneous SCC patients (ER = 69.1%; 95%CI: 58.7%, 77.8%). In addition, the prevalence of overall β-HPVs and β-HPVs of 5, 8, 9, 17, 49, 75, and 76 in immunosuppressed cutaneous SCC patients was significantly higher compared with controls.
Conclusions
The findings of the present meta-analysis support the hypothesis that β-HPV may play a role in cutaneous SCC development in immunosuppressed individuals.
Keywords: skin cancer, β-HPV, SCC, prevalence, immunosuppression, meta-analysis
1. Introduction
Among skin cancers, cutaneous SCC (cSCC) is a quite common malignant proliferation of the epithelial layers with an aggressive behavior and possible metastasis [1]. The risk factors for the SCC development are represented by fair skin, intense sun-exposure, history of sunburns, immunosuppression and beta-human papillomavirus (β-HPV) infection. Indeed, one of the probable causes of SCC is the HPV [2, 3]. HPVs are a great and various group of over 170 subsets with 5 main known HPV genera, including Alpha, Beta (β), Gamma, Mu and Nu papillomavirus [4, 5]. While other HPV genera contribute to the formation of verruca vulgaris, condyloma acuminate, and various types of anogenital cancers [6, 7], the β-genus appears to be involved in human cutaneous carcinogenesis and in promoting non-melanoma skin cancer development in immunosuppressed patients [8]. Among β-HPVs, HPVs 5 and 8 seem to have a potential role in warts that may culminate in SCC formation [9]. Due to the high incidence of SCC in organ transplant recipients in comparison with the general population as well as the similarity of their symptoms, clinical behavior and epidemiology with other virus-induced cancers (i.e. Kaposi's sarcoma), some studies have focused on their origin [10]. Among them, several studies have demonstrated that a further increase in the SCC appearance in transplant recipients is correlated with a significant degree of β-HPV detection [11, 12]. It was reported that 90% of the SCC lesions in Epidermodysplasia Verruciformis patients - a rare genodermatosis determined by multiple skin cancers on sun-exposed sites-were found to increase the likelihood of viral carcinogenicity [13]. Recently, the association between β-HPV and SCC in immunosuppressed patients has been studied in several epidemiological studies with controversial results [14, 15]. Considering the hypothesis that β-HPV may play a role in cSCC development in immunosuppressed individuals, this meta-analysis was carried out to explore an association between β-HPVs and cSCC in immunosuppressed patients.
2. Materials and Methods
2.1. Identification of studies
A systematic search was carried out in the PubMed and Scopus databases up to December 2018 without any restrictions. To retrieve the studies, one of authors (M.S) searched among two databases with the search strategy of (“human papillomavirus” or “HPV” or 00β-HPV” or “beta-HPV”) and (“cutaneous squamous cell carcinoma” or “cutaneous SCC” or “skin squamous cell carcinoma” or “skin SCC” or “cSCC” or “nonmelanoma skin neoplasms” or “nonmelanoma skin cancer” or “squamous cell cancer” or “squamous cell neoplasm”). In addition, the citations of the retrieved studies in relation to the topic of our meta-analysis were reviewed to ensure that no studies were missed. After that, other authors (M.R and F.B) assessed the relevant articles based on the titles and abstracts. Subsequently, the articles with the full-text meeting the criteria were screened. After screening, the reasons for exclusion were written for the studies removed and another author (A.A) resolved the disagreements between the authors.
2.2. Eligibility criteria
The authors used the mentioned criteria for selecting the studies. We included: 1) all types of studies; 2) inclusion of immunosuppressed patients of any age and gender; 3) diagnosis of cSCC established according to clinical and/or histologic criteria, and 4) studies reporting the prevalence of β-HPVs in cSCC patients. On the contrary, we excluded studies with irrelevant or unavailable data, studies including noncutaneous SCC, studies including unspecified nonmelanoma skin cancer, animal studies, duplicate studies, and conference papers, case reports, and reviews.
2.3. Data abstraction
The data of the studies entered to the analysis including first name of first author, publication year, research area, study design, number of individuals and β-HPV detection methods/subtypes, were independently extracted and analyzed by three authors (M.S, M.R and F.B).
2.4. Statistical analysis
The values of odds ratio (OR) were computed by Review Manager version 5.3 software and the event rate (ER) by Comprehensive Meta-Analysis-version 2.0 software with a 95% confidence interval (CI). To estimate the pooled OR significance, the Z test was applied with a p-value (2-sided) < 0.05. In addition, the I2 statistic was applied to estimate heterogeneity that if P > 0.1 (I2<50%), there was a significant heterogeneity and in this state, we used the fixed-effects model; otherwise, the random-effects model was used. The Funnel plots were analyzed with both Egger's and Begg's tests that P < 0.05 (two-sided) showed the significant publication bias. To estimate the stability of the pooled data, the sensitivity analyses (“cumulative analysis” and “the removal of one study”) were applied.
3. Results
3.1. Study selection
A total of 1250 records were identified through the two databases that after removing the duplicates, 775 records were screened, among which 748 irrelevant records were excluded (Fig. 1). Then, 27 full-texts were evaluated, from among which 16 full-texts were excluded with reasons (four reviews, two animal studies, four studies not reporting β-HPV types, one reporting HPV in organ transplant recipients not affected by squamous cell carcinoma, three reporting all HPV genera altogether (alpha, beta, and gamma), and two reporting different skin cancers altogether). At last, eleven studies were entered to the analysis.
Fig. 1.
Flow-chart of the study selection.
3.2. Features of studies
Table 1 is illustrated the features of eleven studies included in the meta-analysis published from 1989 to 2018. Four studies were presented from United Kingdom [16-19], two from the Netherlands [20,21], one from Scotland [22], one from the United States of America [23], one from Ireland [24], one from Germany [25] and one was a multicenter study (Queensland, Australia, and Italy) [26]. Six studies were uncontrolled [16,19,22-25], two were case-control [20,26] and three were cohort [17,18,21] studies. The detection methods of β-HPVs were the Polymerase Chain Reaction (PCR) for eight studies [16,18,19,22-26], serology for two studies [17,21] and PCR and serology together for two studies [20].
Table 1.
Characteristics of the studies included in the meta-analysis.
| First author, year publication | Country | Design/Population | β-HPV Detection Methods/Subtypes |
|---|---|---|---|
| Barr, 1989 [22] | Scotland | Uncontrolled | PCR |
| Case: 25 | HPV type 5/8 | ||
| Harwood, 2000 [16] | United Kingdom | Uncontrolled | PCR |
| Case: 26 | All beta HPV types | ||
| Arron, 2011 [23] | United States of America | Uncontrolled | PCR |
| Case: 14 | All beta HPV types | ||
| O'Connor, 2001 [24] | Ireland | Uncontrolled | PCR |
| Case: 9 | All beta HPV types | ||
| Stockfleth, 2004 [25] | Germany | Uncontrolled | PCR |
| Case: 16 | HPV types 5, 8 | ||
| Casabonne, 2009 [17] | United Kingdom | Cohort | Serology |
| Case: 119 | HPV types 5, 8, 9, 15, 17, 20, 23, 24, 36, 38, | ||
| Control: 425 | 49, 75, 76, 92, 93, 96 | ||
| Mackintosh, 2009 [18] | United Kingdom | Cohort | PCR |
| Case: 53 | HPV types 5, 8, 9, 12, 14, 15, 17, 19e25, 36 −38, 47, 49, 75, 76, 80, 92, 93 and 96 | ||
| Proby, 2011 [20] | The Netherlands | Case-control | PCR & Serology |
| Case: 204 | HPV types 5, 8, 9, 15, 17, 20, 23, 24, 36, 38, | ||
| Control: 377 | 49, 75, 76, 92, 93, 96 | ||
| Neale, 2013 [26] | Queensland, Australia, and Italy | Case-control | PCR |
| Case: 179 | HPV types 5, 8, 15, 20, 23, 24, 36, 38 | ||
| Control: 318 | |||
| Bouwes Bavinck, 2018 [21] | The Netherlands | Cohort | Serology |
| Case: 90 | All beta HPV types | ||
| Control: 536 | |||
| Purdie, 2018 [19] | United Kingdom | Uncontrolled | PCR |
| Case: 12 | All beta HPV types |
Abbreviations: PCR, Polymerase chain reaction; HPV, Human papillomavirus.
3.3. Meta-analysis
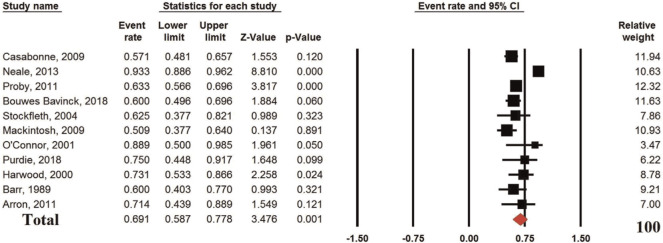
The pooled analysis of eleven studies reporting the prevalence of β-HPVs in immunosuppressed cSCC patients showed an ER of 69.1% (95%CI: 58.7%, 77.8%; p = 0.001; I2 87.7% (Ph or Pheterogeneity<0.0001) (Fig. 2). The results showed a significantly high prevalence of β-HPVs in cSCC in immunosuppressed patients.
Fig. 2.
Event rate of the prevalence of b-HPVs in immunosuppressed patients with cSCC.
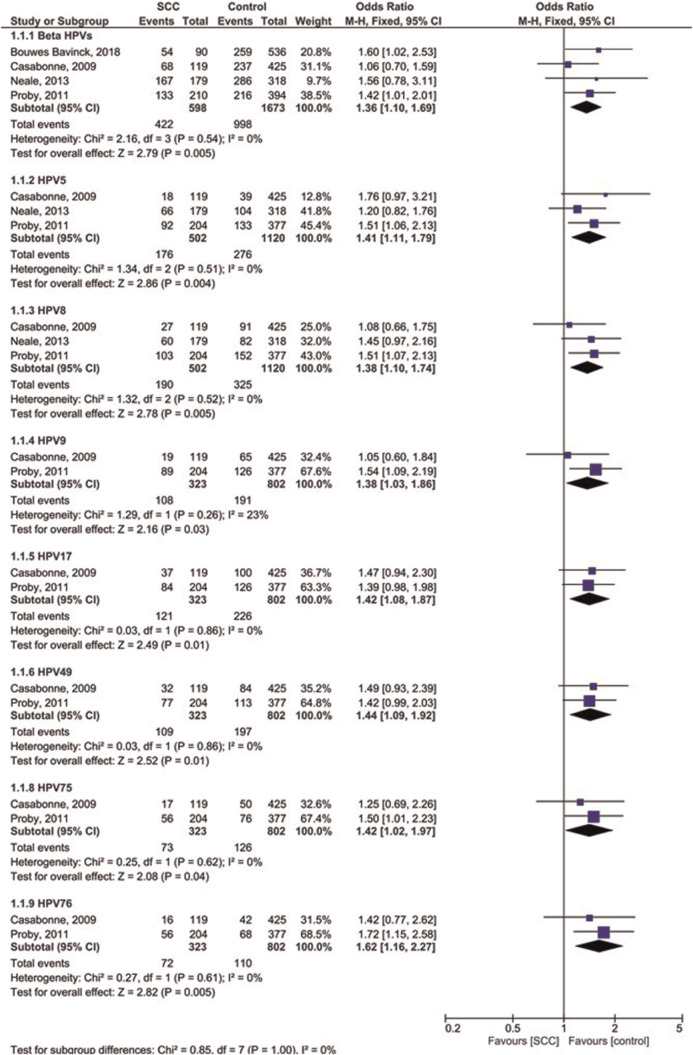
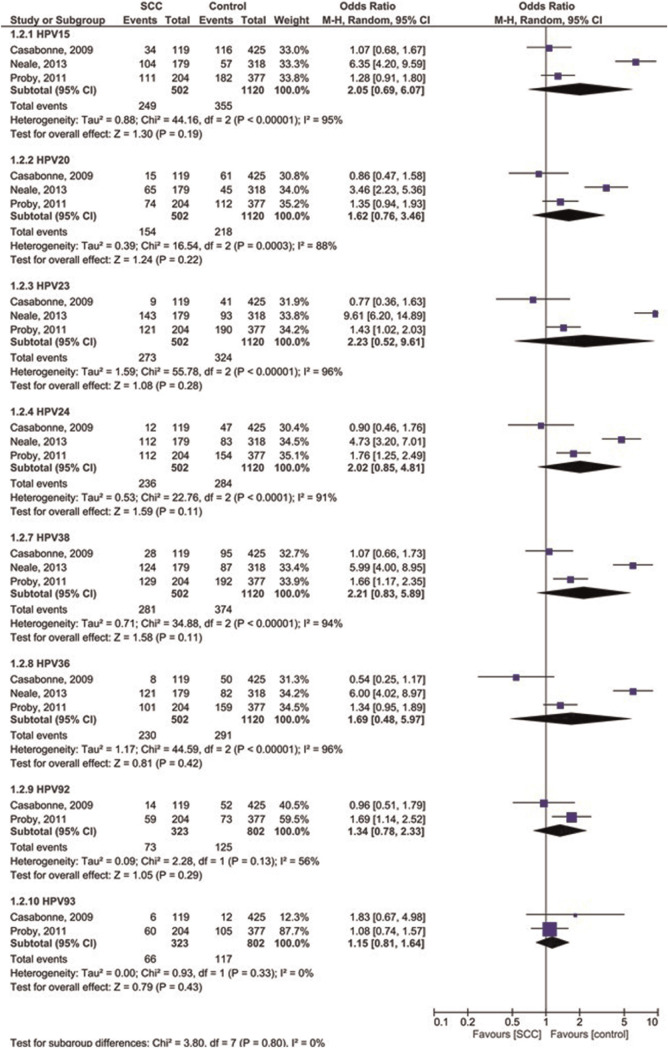
Four case-control studies were analyzed for OR of β-HPVs in immunosuppressed cutaneous patients affected by SCC compared to controls (Figs. 3 and 4). The OR was 1.36 for overall b-HPVs [95%CI: 1.10, 1.69; p = 0.005] without heterogeneity, 1.41 for HPV5 [95%CI: 1.11, 1.79; p = 0.004] without heterogeneity, 1.38 for HPV8 [95%CI: 1.10, 1.74; p = 0.005] without heterogeneity, 1.38 for HPV9 [95%CI: 1.03, 1.86; p = 0.03; I2 = 23% (Ph = 0.26)], 1.42 for HPV17 [95% CI: 1.08, 1.87; p = 0.01] without heterogeneity, 1.44 for HPV49 [95%CI: 1.09, 1.92; p = 0.01] without heterogeneity, 1.42 for HPV75 [95%CI: 1.02, 1.97; p = 0.04] without heterogeneity, and 1.62 for HPV76 [95%CI: 1.16, 2.27; p = 0.005] without heterogeneity. In addition, the OR was 2.05 for HPV15 [95%CI: 0.69, 6.07; p = 0.19; I2 = 95% (Ph < 0.00001)], 2.23 for HPV23 [95%CI: 0.52, 9.61; p = 0.28; I2 = 96% (Ph < 0.00001)], 2.21 for HPV38 [95%CI: 0.83, 5.89; p = 0.11; I2 = 94% (Ph < 0.00001)], 1.34 for HPV92 [95%CI: 0.78, 2.33; p = 0.29; I2 = 56% (Ph = 0.13)], 1.62 for HPV20 [95%CI: 0.76, 3.46; p = 0.22; I2 = 88% (Ph = 0.0003)], 2.02 for HPV24 [95%CI: 0.85, 4.81; p = 0.11; I2 = 91% (Ph < 0.0001)], 1.69 for HPV36 [95% CI: 0.48, 5.97; p = 0.42; I2 = 96% (Ph < 0.00001)] and 1.14 for HPV93 [95%CI: 1.80, 1.63; p = 0.45] without heterogeneity. The results showed that the prevalence of overall β-HPVs and β-HPVs of 5, 8, 9, 17, 49, 75, and 76 was significantly higher in immunosuppressed SCC patients than in controls.
Fig. 3.
Forest plot of odds ratio of prevalence of some b-HPVs in immunosuppressed patients with SCC compared to healthy controls.
Fig. 4.
Forest plot of odds ratio of prevalence of other b-HPVs in immunosuppressed patients affected by SCC compared to healthy controls.
3.4. Sensitivity analysis
Two sensitivity analyses mentioned in methods were performed on the prevalence of β-HPVs in immunosuppressed patients with SCC. We found that the pooled ER did not alter; therefore, these results confirmed the stability of initial pooled data.
3.5. Publication bias
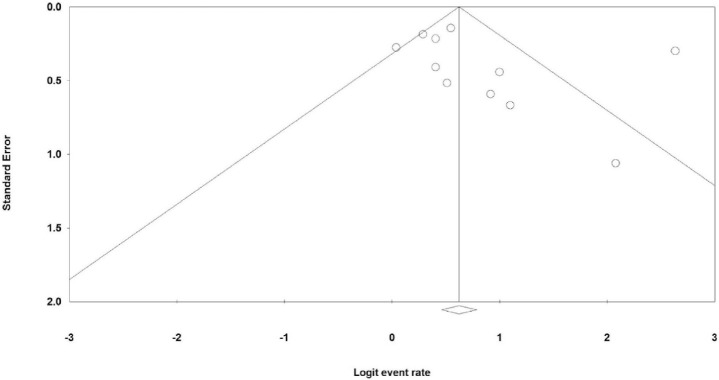
With regard to publication bias, Fig. 5 illustrates the funnel plot of the prevalence of β-HPVs in immunosuppressed patients affected by SCC. The Begg's test revealed a bias between the studies (p = 0.035), but no Egger's test (p = 0.340).
Fig. 5.
Funnel plot of the prevalence of b-HPVs in immunosuppressed patients with SCC.
4. Discussion
SCC is usually a very rapidly growing dangerous tumor with the proliferation of keratinocytes [27,28]. Renal transplant recipients have an elevated incidence of HPV-related cancers [29-33]. The beta genus comprises more than 50 β-HPVs [34]. Some studies have linked β-HPVs to SCC, but many studies have considered a small number of subjects and/or samples and others have been uncontrolled. The present meta-analysis evaluated the ER of β-HPVs in immunosuppressed patients with SCC and also the OR of β-HPVs among these patients in comparison to controls. The results showed that the prevalence of β-HPVs in such patients was significantly higher and also the OR of some β-HPV genotypes was significantly higher in patients than in controls (overall β-HPVs and β-HPVs of 5, 8, 9, 17, 49, 75, and 76).
The studies have shown a high detection of HPV in both precancerous lesions and SCC in renal transplant recipients (81% to 91%) [35,36]. The β-HPV prevalence in the studies included in the present meta-analysis was ranged from 50.9% to 93.3%; similarly, among uncontrolled studies, it varied from 50.9% to 88.9%. Among case-control [20,26] and cohort studies [17,21], including immunosuppressed individuals with SCC, one cohort study [21] based on serology and one case-control study [20] including the individuals with β-HPV antibodies showed a significantly increased risk. Two other studies [17,26] failed to illustrate significantly a difference between the patients and controls. The controversy between the results and the wide range of outcomes may be due to using different methods with different sensitivity and specificity.
A previous meta-analysis [37] showed that cSCCs were more probably to carry β-HPV genotypes compared to healthy skin, and there was an increase in β-HPV prevalence in tumors of immunosuppressed subjects in comparison to immunocompetent subjects. Concerning differences in β-HPV prevalence among different groups of patients, Harwood et al. [16] reported a different detection rate of 84% versus 27% between two groups of patients (immunosuppressed versus immunocompetent subjects), which was confirmed by Stockfleth et al. subsequently (75% vs. 37%) [18]. However, another study [20] did not detect differences (51% versus 52%) in viral detection among paraffin- embedded tumors from immunosuppressed and immunocompetent subjects. The younger age of immunosuppressed patients than immunocompetent individuals was suggested to explain such a result. Accordingly, several studies [23,38-40] reported that immunosuppression and older age had an association with the viral load and higher prevalence of β-HPV. In fact, it can be concluded that considering the age of the patient along with immune status is important in a correct interpretation of the results and predicting the outcome. Moreover, some studies have confirmed HPVs 5 and 8 to be more frequently detected in the SCC from organ transplant recipients [25,41,42]. Two studies [18,43] showed that β-HPV was detected in a greater proportion of frozen samples compared to paraffin-embedded specimens. A study [44] using Southern hybridization and type-specific PCR failed to detect any HPV 5 and 8 in 30 cSCC samples. Another study [45] did not identify any HPV-DNA in 28 non-genital SCCs from immunosuppressed renal allograft recipients. The methodical differences and the different clinical specimens can affect improved β-HPV detection [25]. Therefore, the used techniques (with their own different sensitivities) can explain the different results, and it is also mandatory to pay attention to such issues in β-HPV detection while analyzing the data. Another issue to be considered is the productive status of the virus versus the higher DNA (or even its small fragments) detection rate since this is of primary importance when investigating the role of β-HPVs in cutaneous carcinogenesis.
Moreover, the studies reported that the sun-exposed samples showed higher β-HPV DNA prevalence in both immunosuppressed and immunocompetent individuals, which can help the theory of a powerful interaction between ultraviolet (UV) radiation and β-HPV presence [46-48]. Therefore, both immunosuppression and UV radiation may elevate the β-HPV activity that is able to promote the development of cancer [25,49].
The present meta-analysis suffers from several significant limitations. First, few studies reported. Second, most studies included a few number of patients. Third, different methods were used for the detection of β-HPVs. Fourth; there were differences in the detected β-HPV genotypes. These limitations and also the role of age, UV radiation, and geographical origin of cases could affect the obtained results in terms of β-HPV prevalence and also create a high heterogeneity across the included studies.
5. Conclusions
The findings of the present meta-analysis support the hypothesis that β-HPV may play a main role in the cSCC development in immunosuppressed subjects. The prevalence of β-HPVs in these patients was 69.1%, and several genotypes (overall β-HPVs and β-HPVs of 5, 8, 9, 17, 49, 75, 76, and 93) were associated with an elevated risk of developing cSCC in immunosuppressed subjects compared to healthy controls. Notwithstanding, it should be noted that demographic and environmental factors can affect the β-HPV prevalence. We believe that further studies are currently needed to include a great number of participants from different geographic areas. In order to confirm our results, it is also important to notice the β-HPV detection methods and considered genotypes.
Acknowledgment
This meta-analysis partially fulfills the requirements for the degree of specialty in Clinical and Surgical Pathology by Farideh Baharzadeh.
Funding
This research with a grant number of 980119 was funded by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran.
Conflict of interest
None.
References
- 1.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237–47. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 2.Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64:1359–68. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses in the causation of human cancersea brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Bernard HU, Burk RD, Chen Z, van Doorslaer K, zur Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Doorslaer K, Tan Q, Xirasagar S, Bandaru S, Gopalan V, Mohamoud Y, et al. The Papillomavirus Episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res. 2013;41(Database issue):D571–8. doi: 10.1093/nar/gks984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso JC, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Panonica Adriat. 2011;20:145–54. [PubMed] [Google Scholar]
- 7.Syrjanen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010;21:243–5. doi: 10.1093/annonc/mdq454. [DOI] [PubMed] [Google Scholar]
- 8.Rollison DE, Viarisio D, Amorrortu RP, Gheit T, Tommasino M. An emerging issue in oncogenic virology: the role of beta HPV types in development of cutaneous squamous cell carcinoma. J Virol. 2019;93:e01003–18. doi: 10.1128/JVI.01003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feltkamp MCW, Broer R, di Summa FM, Struijk L, van der Meijden E, Verlaan BP, et al. Seroreactivity to epidermodysplasia verruciformis-related human papilloma-virus types is associated with nonmelanoma skin cancer. Cancer Res. 2003;63:2695–700. [PubMed] [Google Scholar]
- 10.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 11.Reuschenbach M, Tran T, Faulstich F, Hartschuh W, Vinokurova S, Kloor M, et al. High-risk human papilloma-virus in non-melanoma skin lesions from renal allograft recipients and immunocompetent patients. Br J Cancer. 2011;104:1334–41. doi: 10.1038/bjc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipientsdwhere do we stand today? Am J Transplant. 2008;8:2192–8. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 13.Dell'Oste V, Azzimonti B, De Andrea M, Mondini M, Zavattaro E, Leigheb G, et al. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J Invest Dermatol. 2009;129:1026–34. doi: 10.1038/jid.2008.317. [DOI] [PubMed] [Google Scholar]
- 14.Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J Clin Virol. 2008;43:353–60. doi: 10.1016/j.jcv.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin-Drubin ME. Human papillomaviruses and non-melanoma skin cancer. Semin Oncol. 2015;42:284–90. doi: 10.1053/j.seminoncol.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Casabonne D, Lally A, Mitchell L, Michael KM, Waterboer T, Pawlita M, et al. A case-control study of cutaneous squamous cell carcinoma among Caucasian organ transplant recipients: the role of antibodies against human papillomavirus and other risk factors. Int J Cancer. 2009;125:1935–45. doi: 10.1002/ijc.24511. [DOI] [PubMed] [Google Scholar]
- 18.Mackintosh LJ, de Koning MN, Quint WG, Ter Schegget J, Morgan IM, Herd RM, et al. Presence of beta human papillomaviruses in nonmelanoma skin cancer from organ transplant recipients and immunocompetent patients in the West of Scotland. Br J Dermatol. 2009;161:56–62. doi: 10.1111/j.1365-2133.2009.09146.x. [DOI] [PubMed] [Google Scholar]
- 19.Purdie KJ, Proby CM, Rizvi H, Griffin H, Doorbar J, Sommerlad M, et al. The Role of Human Papillomaviruses and Polyomaviruses in BRAF-Inhibitor Induced Cutaneous Squamous Cell Carcinoma and Benign Squamoproliferative Lesions. Front Microbiol. 2018;1806;9 doi: 10.3389/fmicb.2018.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proby CM, Harwood CA, Neale RE, Green AC, Euvrard S, Naldi L, et al. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am J Transplant. 2011;11:1498–508. doi: 10.1111/j.1600-6143.2011.03589.x. [DOI] [PubMed] [Google Scholar]
- 21.Bouwes Bavinck JN, Feltkamp MCW, Green AC, Fiocco M, Euvrard S, Harwood CA, et al. Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: A multicenter, prospective cohort study. Am J Transplant. 2018;18:1220–30. doi: 10.1111/ajt.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr BB, Benton EC, McLaren K, Bunney MH, Smith IW, Blessing K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124–9. doi: 10.1016/s0140-6736(89)91143-4. [DOI] [PubMed] [Google Scholar]
- 23.Arron ST, Ruby JG, Dybbro E, Ganem D, Derisi JL. Tran-scriptome sequencing demonstrates that human papilloma-virus is not active in cutaneous squamous cell carcinoma. J Invest Dermatol. 2011;131:1745–53. doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor DP, Kay EW, Leader M, Atkins GJ, Murphy GM, Mabruk MJ. p53 codon 72 polymorphism and human papillomavirus associated skin cancer. J Clin Pathol. 2001;54:539–42. doi: 10.1136/jcp.54.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockfleth E, Nindl I, Sterry W, Ulrich C, Schmook T, Meyer T. Human papillomaviruses in transplant-associated skin cancers. Dermatol Surg. 2004;30:604–9. doi: 10.1111/j.1524-4725.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 26.Neale RE, Weissenborn S, Abeni D, Bavinck JN, Euvrard S, Feltkamp MC, et al. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:719–27. doi: 10.1158/1055-9965.EPI-12-0917-T. [DOI] [PubMed] [Google Scholar]
- 27.Gordon LG, Scuffham PA, van der Pols JC, McBride P, Williams GM, Green AC. Regular sunscreen use is a cost-effective approach to skin cancer prevention in subtropical settings. J Invest Dermatol. 2009;129:2766–71. doi: 10.1038/jid.2009.141. [DOI] [PubMed] [Google Scholar]
- 28.Gordon R. Skin cancer: An overview of epidemilogy and risk factors. Semin Oncol Nursing. 2013;29:160–9. doi: 10.1016/j.soncn.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Grulich AE, Van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 30.Sillman FH, Sentovich S, Shaffer D. Anogenital neoplasia in renal transplant patients. Ann Transplant. 1997;2:59–66. [PubMed] [Google Scholar]
- 31.Tornesello ML, Loquercio G, Tagliamonte M, Rossano F, Buonaguro L, Buonaguro FM. Human papillomavirus infection in urine samples from male renal transplant patients. J Med Virol. 2010;82:1179–85. doi: 10.1002/jmv.21784. [DOI] [PubMed] [Google Scholar]
- 32.Besarani D, Cranston D. Urological malignancy after renal transplantation. BJU Int. 2007;100:502–5. doi: 10.1111/j.1464-410X.2007.07049.x. [DOI] [PubMed] [Google Scholar]
- 33.Roka S, Rasoul-Rockenschaub S, Roka J, Kirnbauer R, Mühlbacher F, Salat A. Prevalence of anal HPV infection in solid-organ transplant patients prior to immunosuppression. Transpl Int. 2004;17:366–9. doi: 10.1007/s00147-004-0738-z. [DOI] [PubMed] [Google Scholar]
- 34.Tommasino M. The biology of beta human papillomaviruses. Virus Res. 2017;231:128–38. doi: 10.1016/j.virusres.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 35.de Jong-Tieben LM, Berkhout RJ, ter Schegget J, Vermeer BJ, de Fijter JW, Bruijn JA, et al. The prevalence of human papillomavirus DNA in benign keratotic skin lesions of renal transplant recipients with and without a history of skin cancer is equally high: a clinical study to assess risk factors for keratotic skin lesions and skin cancer. Transplantation. 2000;69:44–9. [PubMed] [Google Scholar]
- 36.deVilliers EM, Lavergne D, McLaren K, Benton EC. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int J Cancer. 1997;73:356–61. doi: 10.1002/(sici)1097-0215(19971104)73:3<356::aid-ijc9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol. 2014;70:621–9. doi: 10.1016/j.jaad.2014.01.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boxman IL, Russell A, Mulder LH, Bavinck JN, ter Schegget J, Green A. Association between epi-dermodysplasia verruciformis-associated human papillo-mavirus DNA in plucked eyebrow hair and solar keratoses. J Invest Dermatol. 2001;117:1108–12. doi: 10.1046/j.1523-1747.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- 39.de Koning MN, Weissenborn SJ, Abeni D, Bouwes Bavinck JN, Euvrard S, Green AC, et al. Prevalence and associated factors of betapapillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol. 2009;90:1611–21. doi: 10.1099/vir.0.010017-0. [DOI] [PubMed] [Google Scholar]
- 40.Struijk L, Bouwes Bavinck JN, Wanningen P, van der Meijden E, Westendorp RG, Ter Schegget J, et al. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J Invest Dermatol. 2003;121:1531–5. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- 41.Meyer T, Arndt R, Nindl I, Ulrich C, Christophers E, Stockfleth E. Association of human papillomavirus infections with cutaneous tumors in immunosuppressed patients. Transpl Int. 2003;16:146–53. doi: 10.1007/s00147-002-0525-7. [DOI] [PubMed] [Google Scholar]
- 42.Aldabagh B, Angeles JG, Cardones AR, Arron ST. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg. 2013;39:1–23. doi: 10.1111/j.1524-4725.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfister H, Fuchs PG, Majewski S, Jablonska S, Pniewska I, Malejczyk M. High prevalence of epidermodysplasia verru-ciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent population. Arch Dermatol Res. 2003;295:273–9. doi: 10.1007/s00403-003-0435-2. [DOI] [PubMed] [Google Scholar]
- 44.Stark LA, Arends MJ, McLaren KM, Benton EC, Shahidullah H, Hunter JA, et al. Prevalence of human papillomavirus DNA in cutaneous neoplasms from renal allograft recipients supports a possible viral role in tumour promotion. Br J Cancer. 1994;69:222–9. doi: 10.1038/bjc.1994.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SE, Davis IC, Leshin B, Fleischer AB, White WL, Feldman SR. Absence of Human Papillomavirus in Squamous-Cell Carcinomas of Nongenital Skin from Immunocompromised Renal-Transplant Patients. Arch Dermatol. 1993;129:1585–8. [PubMed] [Google Scholar]
- 46.Bouwes Bavinck JN, Neale RE, Abeni D, Euvrard S, Green AC, Harwood CA, et al. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res. 2010;70:9777–86. doi: 10.1158/0008-5472.CAN-10-0352. [DOI] [PubMed] [Google Scholar]
- 47.Bernat-García J, Morales Suarez-Varela M, Vilata-Corell JJ, Marquina-Vila A. Detection of human papillomavirus in nonmelanoma skin cancer lesions and healthy perilesional skin in kidney transplant recipients and immunocompetent patients. Actas Dermosifiliogr. 2014;105:286–94. doi: 10.1016/j.adengl.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Uberoi A, Yoshida S, Frazer IH, Pitot HC, Lambert PF. Role of Ultraviolet Radiation in Papillomavirus-Induced Disease. PLoS Pathog. 2016;12:e1005664. doi: 10.1371/journal.ppat.1005664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363–74. doi: 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]