Abstract
Aim:
Binge drinking is common during college, and studies have shown that many college students drink in quantities that far exceed the standard binge drinking threshold. Previous research has noted personality differences in individuals who engage in binge drinking, but few studies have examined neurobiological differences in both standard bingers (4/5 drinks in two hours for females/males; sBinge) and extreme binge drinkers (8+/10+ drinks in two hours for females/males; eBinge).
Method:
The current study of 221 college students used functional magnetic resonance imaging (fMRI) to study neural activation on a stop signal task (SST) to assess behavioral inhibition and a monetary incentive delay (MID) task to assess activation to rewards and losses. Non-bingeing controls, sBinge, and eBinge freshmen and sophomores were recruited. In addition, because binge/extreme binge drinking is often associated with marijuana (MJ) use, MJ+sBinge and MJ+eBinge groups were also included.
Results:
All five groups showed strong activation in expected key cortical and striatal regions on both the SST and the MID. However, there were no significant differences between groups either at the whole-brain level or in specific regions of interest. Behavioral performance on the fMRI tasks also did not differ between groups.
Conclusions:
These results suggest that our sample of individuals who engage in binge or extreme binge drinking with or without MJ co-use do not differ in brain activity on reward and inhibitory tasks. Neural differences may be present on other cognitive tasks or may emerge later after more sustained use of alcohol, MJ, and other drugs.
Keywords: binge drinking, combined substance use, brain imaging, marijuana, inhibition, reward
1. Introduction
College students engage in greater alcohol use than high school students and similarly aged individuals who are not in college (Substance Abuse and Mental Health Services Administration, 2017). Many college students engage in “binge drinking” (consuming 4+/5+ drinks within a two-hour period for females/males). Binge drinking is problematic because it is associated with negative consequences such as blackouts, hangovers, accidents, and alcohol poisoning (Hermens and Lagopoulos, 2018; Kanny et al., 2015; Labhart et al., 2018; O’Leary et al., 2019) and is a leading cause of alcohol-attributable deaths (Stahre et al., 2014).
Personality and behavioral measures of behavioral disinhibition have been linked to binge drinking (Banca et al., 2016; Carbia et al., 2018; Carlson et al., 2010; Kazemi et al., 2011; Lees et al., 2019; Sanchez-Roige et al., 2014). Binge drinkers also show higher sensation seeking (O’Leary et al., 2019), a construct linked to reward sensitivity (Castellanos-Ryan et al., 2011). Consequently, there is significant interest in identifying neural correlates of inhibition and reward processing in binge drinkers.
To date, few consistent findings have emerged from functional magnetic resonance imaging (fMRI) tasks measuring behavioral inhibition. For instance, despite both assessing successful inhibition among young adult bingers, Ahmadi and colleagues (2013) reported decreased activation in fronto-parietal regions (supplementary motor area - SMA, anterior cingulate - ACC, superior parietal, precuneus), and subcortical regions (thalamus, putamen, hippocampus), whereas Ames and colleagues (2014) reported the reverse pattern, albeit in slightly different loci (dorsolateral prefrontal cortex, ACC, and insula). Whelan and colleagues (2014) also reported hyperactivation (left and right precuneus) during successful inhibition to be an important feature for classifying adolescent binge drinkers from non-bingers.
Studies assessing reward processing and reward-related decision-making are also inconsistent. For instance, Whelan and colleagues (2014) reported reduced activity (putamen and hippocampus) in adolescent binge drinkers on a monetary incentive delay (MID) task. Cservenka and colleagues also report decreased activation on a Wheel of Fortune task, although the regions showing reduced activation included the cerebellum (Cservenka et al., 2015) and portions of the fronto-parietal cortex (Jones et al., 2016). On the other hand, Crane and colleagues (2017) reported increased nucleus accumbens activation to reward (the Doors task) in young adult bingers, and Xiao and colleagues reported higher activity in amygdala and insula on the Iowa Gambling Task in adolescent binge drinkers (Xiao et al., 2013).
One possible factor contributing to these inconsistent findings is variable levels of binge drinking. Many adolescents and young adults commonly drink well beyond the standard bingeing threshold, meeting the extreme bingeing threshold of 8+/10+ drinks in two hours for females/males (Hingson and White, 2013; Naimi et al., 2010; Patrick et al., 2013; White et al., 2006). Compared to standard binge drinking, extreme binge drinking is particularly problematic, as it is linked to a greater number of alcohol-related consequences and greater risk for developing alcohol use disorder (Hingson and White, 2013; Linden-Carmichael et al., 2017; Read et al., 2008). While contextual factors such as sporting events, holidays, and 21st birthdays contribute to extreme bingeing (Neighbors et al., 2011; Rutledge et al., 2008), individual differences factors may also be important (O’Leary et al., 2019). Indeed, researchers have emphasized the need for differentiating between standard and extreme bingeing both in terms of their consequences, as well as their predictors, such as drinking motives, at-risk traits, and sociodemographic variables (O’Leary et al., 2019; Patrick, 2016; Patrick et al., 2013; White et al., 2016).
Furthermore, individuals who engage in binge drinking are also more likely to use other substances (Jones et al., 2001), especially marijuana (MJ; Jones et al., 2018; Pampati et al., 2018; Subbaraman and Kerr, 2015), highlighting the importance of studying binge drinking and combined substance use (CSU). CSU refers to either simultaneous use (consuming more than one substance at the same time/in close temporal proximity) or concurrent use (consuming more than one substance over a specific period of time (e.g., past 6 months), but not at the same time; Martin et al., 1992). Compared to using alcohol alone, CSU significantly increases the risk for binge drinking, drunk driving, adverse social consequences, and self-harm (Subbaraman and Kerr, 2015). fMRI studies comparing CSU to mono-substance use (MSU) on reward processing, however, are limited and inconsistent. For instance, Nestor and colleagues (2017) suggested synergistic effects of CSU as compared to MSU, while Karoly and colleagues (2015) suggested neuroprotective effects. Similarly, to our knowledge, there is only one study comparing CSU (abstinent polysubstance-dependent individuals) vs. MSU (abstinent alcoholics) during a behavioral inhibition task across different acute drug conditions (placebo vs. naltrexone; Nestor et al., 2019). For baseline inhibition (placebo), CSU did not significantly differ from MSU on either behavioral performance or neural activation.
O’Leary and colleagues (2019) reported personality differences among standard binge drinkers (sBinge), extreme binge drinkers (eBinge), standard and extreme binge drinkers who also use MJ (MJ+sBinge, MJ+eBinge), and control subjects with no past bingeing/MJ use. Groups were compared on the Barratt Impulsiveness Scale (BIS) and the Zuckerman Sensation Seeking Scale (SSS). When group differences on BIS and SSS subscales were tested separately, eBinge scored higher than sBinge on Thrill and Adventure Seeking. When considering all BIS and SSS subscales together in a logistic regression model, SSS Disinhibition showed group differences, controlling for other subscales, such that MJ+sBinge and MJ+eBinge scored higher than sBinge, and there was a trend toward MJ+sBinge scoring higher than MJ+eBinge.
Following up on O’leary et al.’s 2019 personality study, we sought to better understand the neurobiological differences linked to different patterns of alcohol and marijuana consumption. In this project, we again examined sBinge, eBinge, MJ+sBinge, MJ+eBinge, and control subjects, this time on two fMRI tasks, the Stop Signal Task (SST) and the MID, to assess behavioral inhibition and reward processing, respectively. We hypothesized that, compared to controls, sBinge, and to a greater extent, eBinge would show altered behavioral inhibition and reward sensitivity (without specifying direction of the difference due to inconsistencies in the literature). Comparisons between CSU groups and controls and MSU groups were also examined. However, given the limited and inconsistent findings, no specific hypotheses were formulated.
2. Materials and Methods
2.1. Participants
The criteria for the sBinge/eBinge groups were having 2+ sBinge/eBinge episodes, respectively, in the past 30 days or since the semester began and using MJ 3 times or less in the past month (no more than 30 occasions of lifetime MJ use). The CSU groups had to meet criteria for sBinge and eBinge outlined previously and have 4+ episodes of MJ use in the past month. The control group had to have no history of MJ or standard/extreme binge use, but alcohol use consisting of 1–2 drinks per occasion was allowed. In addition, all 4 substance use groups had to have limited lifetime use (<15 times) of other substances except nicotine. All subjects passed a breathalyzer test and had negative urine screens for all drugs except for MJ (due to its long half-life). Other exclusion criteria included: history of seizure disorders, head injury, neurologic, metabolic, or cardiovascular disease, cerebrovascular events, or meeting DSM-IV criteria for major psychiatric disorders (including substance use disorders other than alcohol use disorder for all binge groups, and MJ use disorder in the two MJ+Binge groups), based on the Mini International Neuropsychiatric Interview (Hergueta et al., 1998).
The final sample included 221 freshmen and sophomores (40 controls, 62 sBinge, 59 eBinge, 35 MJ+sBinge, and 25 MJ+eBinge). All participants were right-handed, and there was no group difference on age, sex, race, ethnicity, or parental occupation/highest education level.
2.2. Procedures
All procedures were approved by the University of Iowa Institutional Review Board. All participants signed informed consent forms, completed a brief medical history screening, web-based self-reported measures, and fMRI scans.
2.2.1. Substance Use
Participants reported total days of standard/extreme bingeing, MJ and tobacco use before and during college, which were combined to represent lifetime use. Recent use was calculated by dividing total days of use (current academic year) by the number of months each participant had been in school when they completed the study. Group membership was based on a screening questionnaire developed by our lab. Substance use was assessed again at the study visit, which sometimes occurred several weeks after the screening questionnaire. There were 14 subjects (4 sBinge, 3 eBinge, 2 MJ+sBinge, and 5 MJ+eBinge) who had inconsistent responses between the screen and study visit. Excluding these individuals resulted in largely unchanged group differences in self-reported substance use. Consequently, we report results based on the screening categorization.
2.2.2. Stop Signal Task
There were two SST runs, each consisting of 96 Go and 32 Stop trials (Rao et al., 2014). All trials started with a central fixation cross (500 ms) followed by the Go stimulus (left or right arrow). On Go trials, participants were instructed to indicate the arrow’s direction. On Stop trials, a pure tone (900 Hz; 500 ms) – the stop signal – was presented after the Go stimulus. Participants were asked to withhold responding when presented with the stop signal. The interval between the Go and the Stop stimuli is the stop signal duration, which changed according to participants’ performance (increased/decreased by 50ms for successful/unsuccessful inhibition).
2.2.3. Monetary Incentive Delay Task
The MID task was similar to the one used by Knutson and colleague (2003). There were three runs; each had 48 trials (24 gain, 24 loss), 6 trials for each level of gain/loss ($0.00, $0.20, $1.00, and $5.00). Within a trial, a cue signifying the valence (gain/loss) and level appeared (250 ms), followed by a delay period (2000-2500ms). Participants were then asked to respond to a target as quickly as possible. Afterward, there was another delay, after which participants saw feedback for their performance (1750 ms). Task difficulty was calibrated for each participant based on a practice performance in a mock scanner so that the success rate would be approximately 66%.
2.2.4. FMRI Acquisition
MRI scans were conducted on a Siemens Tim Trio 3T MRI scanner (Erlangen, Germany) equipped with a 12-channel phased array head coil. High-resolution T1-weighted MP-RAGE images (TR = 2,530 ms, TE = 2.8 ms, flip angle = 10 degrees, voxel size = 1.0 X 1.0 X 1.0 mm, series = interleaved) were collected. For the SST runs, a standard AC-PC acquisition was employed with in plane resolution of 2.0mm and the following parameters: TE = 30ms, TR = 2,800ms, Flip Angle = 80°, FOV = 220×220mm, Matrix = 128×128, slice thickness/gap = 4.0/.5mm, BW = 1954 Hz/pixel. For the MID runs, a 30-degree tipped acquisition sequence was used to recover signal in ventral PFC and VS (Deichmann et al., 2003). fMRI scans were acquired with in plane resolution of 3.4mm and the following parameters: TE = 30ms, TR = 2,000ms, Flip Angle = 77°, FOV = 220×220mm, Matrix = 64×64, slice thickness/gap = 3.5/0.875mm, BW = 2004 Hz/pixel.
2.3. FMRI Statistical Analysis
2.3.1. Preprocessing
Prior to all analysis, dummy TRs (the first five volumes of each SST run, and the first six volumes of each MID run) were discarded to remove artifacts associated with scanner disequilibrium. Afterward, all preprocessing steps for both tasks were run with fMRIPrep version 1.1.1 (Esteban et al., 2019). The following steps were applied to T1-weighted images: correction for intensity non-uniformity, skull stripping, spatial normalization to the ICBM 152 Nonlinear Asymmetrical template version 2009c using ANTs (Avants et al., 2009), and tissue segmentation using FSL (Smith et al., 2004). For fMRI data, the following steps were taken: slice time correction using AFNI (Cox, 1996), motion correction using FSL, ANTs “fieldmap-less” distortion correction, and co-registration using boundary-based registration.
2.3.2. Subject Level Analysis
For both tasks, each run was analyzed separately. After running fMRIPrep, the subject-level analysis was conducted with FSL FEAT with the following steps: spatial smoothing with a Gaussian kernel of 6-mm full width at half maximum, high-pass filtering with .01 Hz cutoff, and regression. For both tasks, nuisance regressors included the six motion regressors. Task regressors (described below) and their first temporal derivatives were also included.
Task regressors of the SST included Correct Go (pressing correct button on Go trials), Correct Stop (withholding response on Stop trials), Incorrect Stop (pressing correct button on Stop trials), Incorrect Go (pressing incorrect button on Go trials), Miss Go (failing to respond on Go trials), and Fail Stop (pressing incorrect button in Stop trials). We focused on the Correct Stop vs. Correct Go contrast.
Task regressors of the MID included hit and miss trials of both gain and loss conditions with varying levels of money at stake, allowing for modeling of reward/loss anticipation. We focused on Gain $5.00 vs. Gain $0.00 and Loss $5.00 vs. Loss $0.00 contrasts, as previous studies showed that the largest magnitude ($5.00) is more trait-like (Wu et al., 2014), showed the most robust effect (e.g., Knutson et al., 2003) and might be the most sensitive to group differences.
2.3.3. Region of Interest (ROI) Analysis
Group comparisons were done for each a priori ROI (Table S1) separately. Only significant F-tests (False Discovery Rate corrected) were followed up with pairwise comparison. For the SST, ROIs were brain areas activated during action cancellation reported in a meta-analysis (Zhang et al., 2017): Bilateral insula, opercular portion of the inferior frontal gyrus (IFG), SMA, pallidum, and supramarginal gyri. For the MID, ROIs included the ventral striatum, mPFC, and insula (Knutson et al., 2001a, 2001b, 2003; Sescousse et al., 2013; Wu et al., 2014). ROI locations were based on the work by Knutson and colleagues (Knutson et al., 2007). 6mm-radius spherical ROIs were created, from which median activation was extracted for each participant using FSL Featquery.
2.3.4. Whole-Brain Permutation Analysis
All whole-brain analyses were assessed using Randomise (5000 permutations of the data to build the null distribution). Prior to thresholding with the Threshold-Free Cluster Enhancement method, a gray matter mask was used.
3.1. Substance Use
Table 1 shows recent (days/month) and lifetime (days) substance use data for each group. The following group comparisons were reported for recent substance use using Kruskal-Wallis tests. Only significant Kruskal-Wallis tests were followed up with pairwise Wilcox tests. Controls were excluded for the group comparison on standard/extreme bingeing and MJ use.
Table 1.
Participant Characteristics
| Controls Mean (SD) | sBinge Mean (SD) | eBinge Mean (SD) | MJ+sBinge Mean (SD) | MJ+eBinge Mean (SD) | F/χ2 | p | |
|---|---|---|---|---|---|---|---|
| N | 40 | 62 | 59 | 35 | 25 | - | - |
| Males (% of N) | 20 (50%) | 33 (54%) | 29 (49%) | 19 (54%) | 11 (44%) | 0.85 | .93 |
| Age | 18.70 (0.61) | 18.65 (0.68) | 18.78 (0.72) | 18.60 (0.60) | 18.72 (0.68) | 0.51 | .73 |
| Recent Use (Days/Month) | |||||||
| sBinge | 0 | 2.42 (2.55) | 3.58 (3.74) | 3.83 (2.70) | 6.31 (6.67) | 15.12* | .002 |
| eBinge | 0 | 0.35 (0.66) | 2.04 (2.43) | 0.86 (1.17) | 4.25 (4.38) | 57.33* | <.001 |
| MJ | 0 | 0.33 (0.60) | 0.36 (0.61) | 8.68 (5.36) | 11.67 (9.41) | 125.13* | < .001 |
| Tobacco | 0.03 (0.19) | 1.02 (4.19) | 1.03 (3.99) | 2.33 (6.66) | 2.72 (5.89) | 37.98 | < .001 |
| Lifetime (Days) | |||||||
| sBinge | 0 | 17.76 (18.13) | 37.00 (40.25) | 48.37 (58.59) | 71.96 (69.51) | 29.05* | < .001 |
| eBinge | 0 | 2.19 (2.65) | 16.31 (20.02) | 9.17 (14.68) | 39.20 (57.31) | 68.47* | < .001 |
| MJ | 0 | 3.29 (6.47) | 3.22 (4.71) | 99.74 (87.86) | 113.92 (76.49) | 117.3 1* | < .001 |
| Tobacco | 0.28 (1.13) | 19.90 (81.68) | 10.31 (25.01) | 33.20 (122.56) | 24.76 (37.87) | 50.07 | < .001 |
Group differences in substance use were examined with Kruskal-Wallis tests. sBinge: Standard Bingeing, eBinge: Extreme Bingeing, MJ: marijuana.
denoted group comparison between the 4 substance using groups only.
There was a significant group difference in standard bingeing (χ2(3) = 15.12, p = .002), with sBinge showing the lowest frequency (p’s < .05). The groups also differed in extreme bingeing (χ2(3) = 57.33, p < .001), such that sBinge showed the lowest frequency (p’s < .01), MJ+eBinge showed the highest (p’s < .02), and MJ+sBinge extreme binged less than eBinge (p = .002). Additionally, MJ use also showed a group effect (χ2(3) = 125.13, p < .001). Given the study criteria, it is not surprising that both MSU groups showed lower MJ use than the two CSU groups (p’s < .001); however, it is notable that there was no difference in MJ use between the two MSU groups, or between the two CSU groups (p’s > .30). Lastly, tobacco use also showed a significant group difference (χ2(4) = 37.98, p < .001). The Controls group showed the lowest use level (p’s < .05). Both MSU groups showed lower tobacco use than the two CSU groups (p’s < .05), but there was no difference between the MSU groups, or between the CSU groups (p’s > .20). Tobacco use was included as a covariate in all group analyses.
3.2. Stop Signal Task
3.2.1. Quality Control
For behavioral data, exclusionary criteria proposed by Congdon and colleagues (2012) were used, which involved meeting any of the following: Correct Stop < 25% or > 75%; Correct Go < 60%; Incorrect Go > 10%; negative Stop Signal Reaction Time (SSRT) or SSRT < 50 ms. One participant (MJ+sBinge) was excluded based on these criteria. For imaging data, participants with at least one frame-to-frame movement greater than 3mm in any direction were excluded, resulting in the removal of 18 participants (1 Control, 6 sBinge, 6 eBinge, and 5 MJ+sBinge). Analyses were run after excluding these 19 participants. There were no group differences in framewise displacement (FD).
3.2.2. Behavioral data
SSRT was calculated using the integration method, as recommended by Verbruggen and colleagues (2013). A one-way ANCOVA did not reveal any Group main effect on the log-transformed SSRT (F(4, 196) = 0.17, p = 0.95, partial η2 = 0.003), Percent Correct Stop (F(4, 196) = 0.47, p = 0.76, partial η2 = 0.01), or Go RT (F(4, 196) = 1.28, p = 0.28, partial η2 = 0.03; Table 2). Exploratory grouping schemes (Controls vs. MSU vs. CSU – grouping regardless of bingeing levels; and Controls vs. sBinge vs. eBinge – grouping regardless of mono- or combined-substance usage) were also run but revealed no significant differences.
Table 2.
Behavioral Performance of the Stop Signal Task
| Controls Mean (SD) | sBinge Mean (SD) | eBinge Mean (SD) | MJ+sBinge Mean (SD) | MJ+eBinge Mean (SD) | F | p | |
|---|---|---|---|---|---|---|---|
| N | 39 | 56 | 53 | 29 | 25 | - | - |
| SSRT | 203.40 (41.36) | 199.85 (43.76) | 208.42 (57.62) | 200.59 (36.72) | 203.42 (35.23) | 0.17 | 0.95 |
| Correct Stop (%) | 53.08 (5.91) | 51.14 (7.67) | 51.12 (8.70) | 51.13 (7.69) | 52.00 (7.40) | 0.47 | 0.76 |
| Go RT | 577.96 (105.76) | 577.38 (95.69) | 541.30 (90.41) | 554.15 (100.38) | 546.67 (110.52) | 1.28 | 0.28 |
Group differences in behavioral performance of the Stop Signal Task were assessed with one-way ANCOVAs with tobacco use as a covariate (details in the Results section). SSRT: Stop Signal Reaction Time (table showed original SSRT, but test statistics are from ANCOVA with the log transformed SSRT), RT: Reaction Time. All reported means are the original means, not the estimated marginal means from the ANCOVA models.
3.2.3. Imaging data
For each of the 5 groups, one-sample t-tests for the Correct Stop vs. Correct Go contrast showed strong activation in expected regions (IFG, anterior insula, ACC, precuneus, supramarginal; Figure S1). However, voxel-wise 5-group nonparametric analysis of the same contrast revealed no differences. For the ROI analyses, one-way ANCOVAs revealed no significant group differences in any ROIs (Table S2; see Figures 1A and 1B for left and right IFG). Exploratory whole-brain and ROI analyses for additional grouping schemes (Controls vs. MSU vs. CSU; Controls vs. sBinge vs. eBinge) revealed no group differences. Lastly, a whole-brain comparison between Controls vs. all Bingers combined also did not reveal any difference. Additionally, tobacco use was not significantly associated with any of the behavioral or imaging variables.
Figure 1.
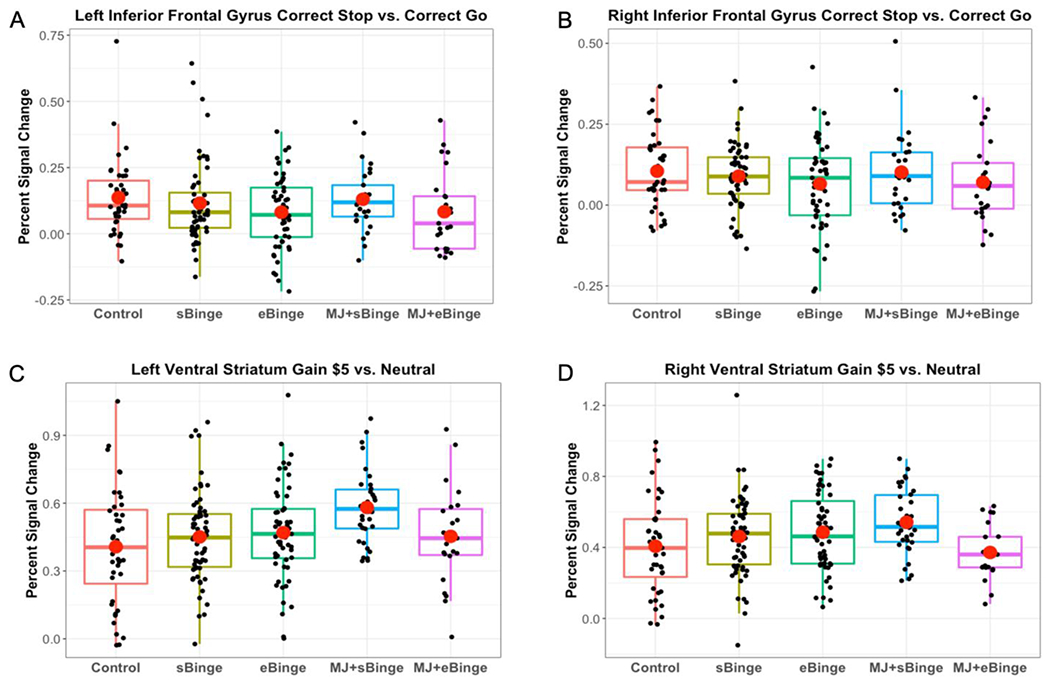
Regions of interest activation during the Stop Signal Task and the Monetary Incentive Delay task. Left (A) and right (B) Inferior Frontal Gyrus activation in response to Correct Stop relative to Correct Go. Left (C) and right (D) Ventral Striatum activation in response to Gain $5.00 relative to No Gain. Red circles represent group means.
3.3. Monetary Incentive Delay Task
3.3.1. Quality Control
The same exclusionary criteria due to excessive motion outlined in the SST section was used and resulted in the exclusion of 6 participants for the MID (3 eBinge, 1 MJ+sBinge, and 2 MJ+eBinge). FD did not show a group difference.
3.3.2. Behavioral data
Separate mixed Type (Reward, Loss) x Levels ($0.00, $0.20, $1.00, $5.00) x Group (Controls, sBinge, eBinge, MJ+sBinge, MJ+sBinge) ANCOVAs were run to detect group differences in RT and accuracy. There was no significant main effect of group, or significant group interaction for RT (all p’s > .05, Table 3). For accuracy, there was a significant Type x Group interaction (F(4, 209) = 2,51, p = 0.04, partial η2 = 0.05), but there was no significant pairwise group comparison for either Gain or Loss trial types (all p’s > .05). There was no other significant group interaction or group main effect for accuracy (all p’s > .05).
Table 3.
Behavioral Performance of the Monetary Incentive Delay Task
| Controls Mean (SD) | sBinge Mean (SD) | eBinge Mean (SD) | MJ+sBinge Mean (SD) | MJ+eBinge Mean (SD) | F | p | |
|---|---|---|---|---|---|---|---|
| N | 40 | 62 | 56 | 34 | 23 | - | - |
| Gain RT | 209.31 (16.34) | 204.41 (13.85) | 205.71 (15.68) | 208.23 (13.86) | 206.08 (14.31) | 0.60 | 0.67 |
| Loss RT | 210.86 (16.65) | 208.07 (16.02) | 208.02 (15.68) | 210.69 (14.24) | 206.73 (15.96) | 0.60 | 0.67 |
| Gain Accuracy (%) | 78.37 (6.62) | 78.92 (7.19) | 80.16 (6.70) | 79.25 (6.29) | 78.29 (6.54) | 0.06 | .993 |
| Loss Accuracy (%) | 78.02 (7.19) | 77.53 (9.28) | 76.56 (9.20) | 78.23 (7.08) | 79.35 (6.17) | 0.06 | .993 |
Group differences in behavioral performance of the Monetary Incentive Delay task were assessed with mixed ANCOVAs with tobacco use as a covariate (details in the Results section). All reported means are the original means, not the estimated marginal means from the ANCOVA models.
3.3.3. Imaging data
One-sample t-tests (Gain $5.00 vs. Gain $0.00) were run separately for each group and revealed activation in expected regions such as insula, dorsal and ventral striatum (Figure S2). Whole brain non-parametric (Gain $5.00 vs Gain $0.00 and Loss $5.00 vs Loss $0.00) with different grouping schemes (5 groups, Controls vs. MSU vs. CSU, Controls vs. sBinge vs. eBinge, and Controls vs. all Bingers combined) revealed no significant group differences. ROI analyses (5 groups, Controls vs. MSU vs. CSU, Controls vs. sBinge vs. eBinge) for these gain/loss contrasts also did not reveal any group differences (see Table S3 for the 5-group analysis). Figures 1C and 1D show percent signal change in left and right VS for Gain $5.00 vs. Gain $0.00. As with the SST, tobacco use was not significantly associated with the behavioral or imaging variables.
4. Discussion
The current study examined behavioral inhibition (SST) and reward processing (MID) in standard and extreme binge drinking individuals with and without regular MJ co-use. We found no evidence for group differences in behavioral performance or brain activation on either task. Although prior studies have reported brain activation differences in binge drinkers, sample sizes have been relatively small – most consisting of approximately 20 binge and 20 control subjects – and the results have been inconsistent (Cservenka and Brumback, 2017). Using this larger sample, we couldn’t replicate the previously reported findings: the voxel-wise analyses of both tasks did not reveal any difference between 181 binge drinkers and 40 non-bingeing controls. This discrepancy may reflect a bias in reporting positive findings, not only in brain imaging studies (Cservenka and Brumback, 2017) but also in non-imaging neuropsychological studies (Carbia et al., 2018; Lees et al., 2019). Specifically, a quantitative review with 18 non-imaging studies using behavioral inhibition tasks among youth aged 10-24 reported associations between binge drinking and inhibition deficit. However, there were significant concerns with risk of publication bias (significant Egger’s test), and with the inconsistency of results (Lees et al., 2019).
Alternatively, this inconsistency in the literature might partly be due to the possibility that bingers from different studies were at different stages of substance misuse, associated with different neural alterations. Researchers have argued that hypoactive frontoparietal responses during inhibition might be a predisposing factor of binge drinking initiation, whose neurotoxic effects can later shift the responses of those regions to hyperactivity (Jones et al., 2018; Padilla et al., 2017; Wetherill et al., 2013a, 2013b; Worhunsky et al., 2016; but see Whelan et al., 2014). Similarly, alterations in reward processing may only be prominent in later stages of substance abuse (Nees et al., 2012; Zilverstand et al., 2018). To our knowledge, there is only one paper comparing short-, medium-, and long-term bingers (Ruan et al., 2019); however, the authors employed resting state rather than task fMRI. Characterizing brain activation in binge drinkers during different stages of abuse will be important for future research.
Although additional work is needed, the current findings suggest that emerging adults without a long binge drinking history may not show any behavioral or neurophysiological differences from non-bingers. It’s worth noting that meaningful differences on personality measures of impulsivity and sensation seeking were reported in this sample (O’Leary et al., 2019). Personality traits may be better at discriminating between groups because they assess stable traits, whereas task performance and task-related activation are more state-dependent. This is consistent with a review by Padilla and colleagues (2017) in which increased trait (self-report) and choice impulsivity (choose immediate rather than delayed but larger rewards) showed consistent association with alcohol misuse, whereas the association between behavioral inhibition and alcohol misuse was mixed.
There are limitations that may affect the interpretation of the results. Firstly, all substance use data were self-reported, which may introduce deliberate or unintentional inaccuracies. Also, the sample was a group of relatively healthy young college students, which may limit the generalizability of the findings. Additionally, the study was initially designed to look at the effect of CSU regardless of the bingeing levels. However, we wanted to tease apart the effects of different bingeing levels in MSU and CSU, and thus the CSU group was further categorized into two groups with smaller sample sizes than other groups in the study. In addition, we didn’t have a marijuana only group, which is needed to better distinguish the effects of MSU (alcohol alone and MJ alone) and CSU (alcohol combined with MJ). This issue could be addressed in future studies by recruiting marijuana users with limited or no alcohol use. Lastly, we do not know how much of the reported CSU was simultaneous or concurrent. Simultaneous use has been shown to be more harmful than concurrent use (McCabe et al., 2006; Midanik et al., 2007). Future studies are needed to further test the interaction between simultaneous/concurrent alcohol bingeing and marijuana use.
In this study, we found no group differences in either performance or neural correlates of inhibition and reward. Longitudinal studies can investigate how and when chronic MSU and CSU take their toll on those measures over time. In addition, longitudinal data will also help compare people who have consistent substance use patterns with those who don’t. This will be extremely informative as we continue to study the characteristics of individuals with different substance use patterns, which is of great importance in developing treatments that fit individual needs.
Supplementary Material
Highlights.
Investigated inhibition and reward related brain activation in college students.
Focused on standard/extreme binge drinkers with and without marijuana use.
Groups did not differ from one another or in comparison to controls.
Functional brain differences may emerge after greater use of alcohol/marijuana.
Acknowledgements:
The work was carried out at University of Iowa Carver College of Medicine, Department of Psychiatry, Iowa City, IA.
Role of Funding Source: This work was supported by the National Institutes of Health (NIH; grant number 5R01AA021165, PI: O’Leary, Daniel S., “The Relationship of Adolescent Binge Drinking to Measures of Brain and Behavior”). In addition, this work was conducted on an MRI instrument funded by 1S10OD025025-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Conflict of Interest: No conflict declared
Conflict of interest statement
Tien T. Tong: Conflicts of interest: none
Jatin G. Vaidya: Conflicts of interest: none
John R. Kramer: Conflicts of interest: none
Samuel Kuperman: Conflicts of interest: none
Douglas R. Langbehn: Conflicts of interest: none
Daniel S. O’Leary: Conflicts of interest: none
References
- Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, Wood RM, Stevens MC, 2013. Influence of alcohol use on neural response to Go/No-Go task in college drinkers. Neuropsychopharmacology 38, 2197–2208. 10.1038/npp.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW, 2014. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav. Brain Res 274, 382–389. 10.1016/j.bbr.2014.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G, 2009. Advanced normalization tools (ANTS). Insight J 2, 1–35. [Google Scholar]
- Banca P, Lange I, Worbe Y, Howell NA, Irvine M, Harrison NA, Moutoussis M, Voon V, 2016. Reflection impulsivity in binge drinking: Behavioural and volumetric correlates. Addict. Biol 21, 504–515. 10.llll/adb.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C, López-Caneda E, Corral M, Cadaveira E, 2018. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci. Biobehav. Rev 90, 332–349. 10.1016/j.neubiorev.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Carlson SR, Johnson SC, Jacobs PC., 2010. Disinhibited characteristics and binge drinking among university student drinkers. Addict. Behav 35, 242–251. 10.1016/j.addbeh.2009.10.020 [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, Conrod PJ, 2011. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol. Clin. Exp. Res 35, 140–155. 10.1111/j.1530-0277.2010.01331.x [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA, 2012. Measurement and reliability of response inhibition. Front. Psychol 3. 10.3389/fpsyg.2012.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL, 2017. Preliminary evidence for disrupted nucleus accumbens reactivity and connectivity to reward in binge drinkers. Alcohol Alcohol. 52, 647–654. 10.1093/alcalc/agx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Brumback T, 2017. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front. Psychol 8. 10.3389/fpsyg.2017.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ, 2015. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev. Cogn. Neurosci., Substance Use and the Adolescent Brain: Developmental Impacts, Interventions, and Longitudinal Outcomes 16, 110–120. 10.1016/j.dcn.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R, 2003. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19, 430–441. [DOI] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ, 2019. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, 2233. [PubMed] [Google Scholar]
- Hermens DF, Lagopoulos J, 2018. Binge drinking and the young brain: A mini review of the neurobiological underpinnings of alcohol-induced blackout. Front. Psychol 9. 10.3389/fpsyg.2018.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, White A, 2013. Trends in extreme binge drinking among US high school seniors. JAMA Pediatr. 167, 996–998. 10.1001/jamapediatrics.2013.3083 [DOI] [PubMed] [Google Scholar]
- Jones J, Nicole Jones K, Peil J, 2018. The impact of the legalization of recreational marijuana on college students. Addict. Behav 77, 255–259. 10.1016/j.addbeh.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Jones SA, Cservenka A, Nagel BJ, 2016. Binge drinking impacts dorsal striatal response during decision making in adolescents. Neuroimage 129, 378–388. 10.1016/j.neuroimage.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Lueras JM, Nagel BJ, 2018. Effects of binge drinking on the developing brain. Alcohol Res. Curr. Rev 39, 87–96. [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Oeltmann J, Wilson TW, Brener ND, Hill CV, 2001. Binge drinking among undergraduate college students in the United States: Implications for other substance use. J. Am. Coll. Health 50, 33–38. 10.1080/07448480109595709 [DOI] [PubMed] [Google Scholar]
- Kanny D, Brewer RD, Mesnick JB, Paulozzi LJ, Naimi TS, Lu H, 2015. Vital signs: Alcohol poisoning deaths—United States, 2010–2012. MMWR Morb. Mortal. Wkly. Rep 63, 1238. [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Ewing SWF, 2015. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev. Cogn. Neurosci 16, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi DM, Wagenfeld M, Van Horn RK, Levine MJ, Dmochowski J, 2011. Binge drinking among underage college students: Role of impulsivity and the transtheoretical model. J. Addict. Nurs 22, 193. 10.3109/10884602.2011.616605 [DOI] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D, 2001a. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci 21, RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001b. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D, 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G, 2007. Neural predictors of purchases. Neuron 53, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart F, Livingston M, Engels R, Kuntsche E, 2018. After how many drinks does someone experience acute consequences—determining thresholds for binge drinking based on two event-level studies. Addiction 113, 2235–2244. 10.1111/add.14370 [DOI] [PubMed] [Google Scholar]
- Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M, 2019. Neurobiological and cognitive profile of young binge drinkers: A systematic review and meta-analysis. Neuropsychol. Rev 10.1007/sll065-019-09411-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Vasilenko SA, Lanza ST, Maggs JL, 2017. High-intensity drinking versus heavy episodic drinking: Prevalence rates and relative odds of alcohol use disorder across adulthood. Alcohol. Clin. Exp. Res 41, 1754–1759. 10.1111/acer.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Clifford PR, Clapper RL, 1992. Patterns and predictors of simultaneous and concurrent use of alcohol, tobacco, marijuana, and hallucinogens in first-year college students. J. Subst. Abuse 4, 319–326. 10.1016/0899-3289(92)90039-Z [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Morales M, Young A, 2006. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: Prevalence, correlates, and consequences. J. Stud. Alcohol 67, 529–537. 10.15288/jsa.2006.67.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C, 2007. Concurrent and simultaneous drug and alcohol use: Results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 90, 72–80. 10.1016/j.drugalcdep.2007.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE, Brewer RD, 2010. The intensity of binge alcohol consumption among U.S. adults. Am. J. Prev. Med 38, 201–207. 10.1016/j.amepre.2009.09.039 [DOI] [PubMed] [Google Scholar]
- Nees E, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, Banaschewski T, Barker GJ, Biichel C, Conrod PJ, Garavan H, Heinz A, Gallinat J, Lathrop M, Mann K, Artiges E, Paus T, Poline J-B, Robbins TW, Rietschel M, Smolka MN, Spanagel R, Struve M, Loth E, Schumann G, Flor H, 2012. Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology 37, 986–995. 10.1038/npp.2011.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C, Atkins DC, Lewis MA, Lee CM, Kaysen D, Mittmann A, Fossos N, Rodriguez LM, 2011. Event specific drinking among college students. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav 25, 702–707. 10.1037/a0024051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Paterson LM, Smith D, Bullmore ET, Ersche KD, Suckling J, Tait R, Elliott R, Deakin B, Rabiner I, Lingford-Hughes A, Nutt DJ, Sahakian B, Robbins TW, 2017. Acute naltrexone does not remediate fronto-striatal disturbances in alcoholic and alcoholic poly sub stance-dependent populations during a monetary incentive delay task. Addict. Biol 22, 1576–1589. 10.1111/adb.12444 [DOI] [PubMed] [Google Scholar]
- Nestor LJ, Paterson LM, Murphy A, McGonigle J, Orban C, Reed L, Taylor E, Flechais R, Smith D, Bullmore ET, Ersche KD, Suckling J, Elliott R, Deakin B, Rabiner I, Hughes AL, Sahakian BJ, Robbins TW, Nutt DJ, 2019. Naltrexone differentially modulates the neural correlates of motor impulse control in abstinent alcohol-dependent and polysubstance-dependent individuals. Eur. J. Neurosci 50, 2311— 2321. 10.1111/ejn.14262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Langbehn DR, Kramer JR, Kuperman S, Fuhrmeister LA, Vaidya JG, 2019. Personality traits and negative consequences associated with binge drinking and marijuana use in college students. Am. J. Drug Alcohol Abuse 45, 400–409. 10.1080/00952990.2019.1601200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla M, O’Halloran L, Bennett M, Cao Z, Whelan R, 2017. Impulsivity and reward processing endophenotypes in youth alcohol misuse. Curr. Addict. Rep 4, 350–363. 10.1007/s40429-017-0167-6 [DOI] [Google Scholar]
- Pampati S, Buu A, Hu Y-H, Mendes de Leon CF, Lin H-C, 2018. Effects of alcohol and cigarette use on the initiation, reinitiation, and persistence of cannabis use from adolescence to emerging adulthood. Addict. Behav 79, 144–150. 10.1016/j.addbeh.2017.12.019 [DOI] [PubMed] [Google Scholar]
- Patrick ME, 2016. A call for research on high-intensity alcohol use. Alcohol. Clin. Exp. Res 40, 256–259. 10.1111/acer.12945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD, 2013. Extreme binge drinking among 12th-grade students in the United States: Prevalence and predictors. JAMA Pediatr. 167, 1019–1025. 10.1001/jamapediatrics.2013.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JA, Harrington DL, Durgerian S, Reece C, Mourany L, Koenig K, Lowe MJ, Magnotta VA, Long JD, Johnson HJ, Paulsen JS, Rao SM, 2014. Disruption of response inhibition circuits in prodromal Huntington disease. Cortex 58, 72–85. 10.1016/j.cortex.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Beattie M, Chamberlain R, Merrill JE, 2008. Beyond the “binge” threshold: Heavy drinking patterns and their association with alcohol involvement indices in college students. Addict. Behav 33, 225–234. 10.1016/j.addbeh.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Ruan H, Zhou Y, Luo Q, Robert GH, Desrivieres S, Quinlan EB, Liu Z, Banaschewski T, Bokde ALW, Bromberg U, Biichel C, Flor H, Frouin Y, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot J-L, Martinot M-LP, Nees F, Orfanos DP, Poustka L, Hohmann S, Frohner JH, Smolka MN, Walter H, Whelan R, Li E, Schumann G, Feng J, 2019. Adolescent binge drinking disrupts normal trajectories of brain functional organization and personality maturation. Neuroimage Clin. 22, 101804. 10.1016/j.nicl.2019.101804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge PC, Park A, Sher KJ, 2008. 21st birthday drinking: Extremely extreme. J. Consult. Clin. Psychol 76, 511–516. 10.1037/0022-006X.76.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Baro Y, Trick L, Peña-Oliver Y, Stephens DN, Duka T, 2014. Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice. Neuropsychopharmacology 39, 2919–2927. 10.1038/npp.2014.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldd X, Segura B, Dreher J-C, 2013. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev 37, 681–696. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR., De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, Mathematics in Brain Imaging 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X, 2014. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic. Dis 11. 10.5888/pcdll.130293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraman MS, Kerr WC, 2015. Simultaneous versus concurrent use of alcohol and cannabis in the national alcohol survey. Alcohol. Clin. Exp. Res 39, 872–879. 10.1111/acer.12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2017. National Survey on Drug Use and Health. Accessed at https://www.samhsa.gov/data/report/2017-nsduh-annual-national-report on 31 October 2019.
- Verbruggen E, Chambers CD, Logan GD, 2013. Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychol. Sci 24, 352–362. 10.1177/0956797612457390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF, 2013a. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 128, 243–249. 10.1016/j.drugalcdep.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF, 2013b. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berk) 230, 663–671. 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde ALW, Biiche C, Carvalho FM, Conrod PJ, Flor H, Fauth-Biihler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot J-L, Nees F, Ortiz N, Paillere-Martinot M-L, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Strohle A, Schumann G, Garavan H, 2014. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature 512, 185–189. 10.1038/naturel3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Kraus CL, Swartzwelder HS, 2006. Many college freshmen drink at levels far beyond the binge threshold. Alcohol. Clin. Exp. Res 30, 1006–1010. 10.1111/j.1530-0277.2006.00122.X [DOI] [PubMed] [Google Scholar]
- White HR, Anderson KG, Ray AE, Mun E-Y, 2016. Do drinking motives distinguish extreme drinking college students from their peers? Addict. Behav 60, 213–218. 10.1016/j.addbeh.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Dager AD, Meda SA, Khadka S, Stevens MC, Austad CS, Raskin SA, Tennen EL, Wood RM, Fallahi CR, Potenza MN, Pearlson GD, 2016. A preliminary prospective study of an escalation in ‘maximum daily drinks’, fronto-parietal circuitry and impulsivity-related domains in young adult drinkers. Neuropsychopharmacology 41, 1637–1647. 10.1038/npp.2015.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Samanez-Larkin GR, Katovich K, Knutson B, 2014. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage 84, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu Z-L, Palmer P, Wei Y, Jia Y, Johnson CA, 2013. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav 27, 443–454. 10.1037/a0027892 [DOI] [PubMed] [Google Scholar]
- Zhang R, Geng X, Lee TMC, 2017. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct. Funct 222, 3973–3990. 10.1007/s00429-017-1443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ, 2018. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: A systematic review. Neuron 98, 886–903. 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


