Abstract
Genetically engineered immune cells with chimeric antigen receptors (CAR) or modified T cell receptors (TCR) have demonstrated their potential as a potent class of new cancer therapeutic strategy. Despite the clinical success of autologous CD19 CAR T cells in hematological malignancies, allogeneic T cells exhibit many advantages over their autologous counterparts and have recently gathered widespread attention due to the emergence of multiplex genome editing techniques, particularly CRISPR/Cas systems. Furthermore, genetically engineered T cells face a host of major challenges in solid tumors that are not as significant for blood cancers such as T cell targeted delivery, target specificity, proliferation, persistence, and the immunosuppressive tumor microenvironment. We take this opportunity to analyze recent strategies to develop allogeneic T cells, specifically in consideration of CRISPR/Cas and its delivery systems for multiplex gene editing. Additionally, we discuss the current methods used to delivery CRISPR/Cas systems for immunotherapeutic applications, and the challenges to continued development of novel delivery systems. We also provide a comprehensive analysis of the major challenges that genetically engineered T cells face in solid tumors along with the most recent strategies to overcome these barriers, with an emphasis on CRISPR-based approaches. We illustrate the synergistic prospects for how the combination of synthetic biology and immune-oncology could pave the way for designing the next generation of precision cancer therapy.
Keywords: Adoptive Cell Therapy, Cancer Immunotherapy, CRISPR/Cas Delivery Systems, Chimeric Antigen Receptor, Allogeneic T Cells, Solid Tumor
1. Introduction
Recent developments in immunotherapy and gene therapy have sparked the next generation of cancer therapy. Cancer immunotherapy aims to redirect and boost the patient’s immune system to recognize proliferating cancer cells. Over the past few decades, several approaches have been tailored to address the immune-evasive behavior of various solid and blood cancers, including monoclonal antibodies, immune checkpoint inhibitors, anti-tumor vaccines, and adoptive cell-based therapies [1-4]. In particular, the recent development of antibody therapies targeting immune checkpoints such as programmed cell death protein 1 or its ligand (PD-1/PD-L1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) have seen clinical successes, enhancing T cell activation and thereby elevating anti-tumor responses [5].
Alternatively, the emergence of adoptive cell therapy, a therapeutic strategy that utilizes ex vivo genetically modified T cells isolated from patients to help the body fight and treat complex diseases, has also seen recent clinical success through autologous CD19-targeting CAR T cells in treating blood cancers [6]. This quickly evolving branch of immunotherapy has converged the recent evolution in synthetic biology and immune-oncology by using modern genome editing technologies like clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) and its delivery systems to offer innovative, combinatorial strategies that systematically engineer T cells to combat cancer. These T cells can be genetically modified to simultaneously target specific cancer antigens, by transducing either a tumor-specific chimeric antigen receptor (CAR) or a T cell receptor (TCR) specific for the tumor-associated antigen (TAA) of choice, and overcome various barriers presented by complex cancer tumors.
These recent advances in genome editing have provided new opportunities to generate a new class of off-the shelf allogeneic T cells that could address many of the limitations associated with autologous T cell therapies such as manufacture process, cost, and T cell dysfunction in cancer patients [7]. In particular, the precision, efficiency, and multiplex gene editing capabilities of CRISPR/Cas gene editing and its various delivery systems make it a powerful tool to design and develop allogeneic engineered T cells.
While the advent of CRISPR/Cas opened the door for convenient, efficient multiplex gene editing, the primary challenges preventing CRIPSR from reaching its full potential are the continued presence of off-target effects and the inability to target specific cells in vivo [8]. Both of these challenges can potentially be overcome by a combination of rational delivery system design, and advancements in the CRISPR gene editing technology itself. Current applications of CRISPR in immunotherapy rely primarily on physical delivery methods such as electroporation. This is not a major issue for immunotherapies of the current generation, since most are modified ex vivo anyway. However, next-gen therapeutics will depend on novel delivery systems to effectively target the intended cells and mitigate the occurrence of off-target effects.
Despite the success of CD19-targeting CAR T cells in blood cancers, the primary challenge in immune-oncology is to treat solid tumors safely and effectively, which has seen significantly reduced clinical success for adoptive cell therapy [9]. The fundamental reason behind this reduced clinical success rate is due to the host of major challenges in solid tumors that are not as significant in blood cancers, that must be simultaneously solved in the design of the engineered T cells.
Here, we discuss the therapeutic advantages and recent strategies to develop allogeneic T cells that overcome relevant challenges, in light of the maturation of CRISPR/Cas and its delivery systems for highly efficient multiplex gene editing. Furthermore, we provide a comprehensive evaluation of the major challenges that need to be overcome for the successful adoption of adoptive cell therapy to treat solid cancer tumors. The recent strategies that are being investigated to address these solid tumor-associated barriers are discussed in depth, with an emphasis on recent CRISPR-based approaches. The discussed strategies for the improvement of autologous adoptive cell therapy for solid tumors can also serve as an inspiration for the future potential to utilize allogeneic T cells for solid tumor cancer treatment. We demonstrate that the synergy between synthetic biology and immune-oncology could revolutionize the next generation of precision cancer therapy.
2. T Cell Receptors and Chimeric Antigen Receptors
Most T cell receptor (TCR) structures are heterodimers composed from an α chain encoded by the TCR α-gene (TRA) and a β chain encoded by the TCR β-gene (TRB). A small portion of T cells consist of TCR heterodimers composed from gamma and delta (γ/δ) chains, encoded by TRG and TRD, respectively. These TCRs form a complex with CD3 signaling molecules as a co-receptor for T cell activation along with a zeta chain (ζ-chain) to generate activation signals.
The activation of TCRs require binding to a peptide/major histocompatibility complex (MHC) complex to enable antigen specific recognition. TCRs can recognize peptides derived from cell surface or intracellular proteins as long as they complex with an MHC in a human leukocyte antigen (HLA)-dependent manner [10]. This MHC matching can be extremely complex due to an estimated > 20,000 different HLA alleles [11].
Chimeric antigen receptors (CARs) are receptor proteins that can be transduced into T cells after being engineered to target a user-specified antigen and to combine antigen-binding and T cell activation into a single receptor. These CAR T cells are unique and advantageous in that they enable MHC-independent activation and do not require HLA expression to recognize target molecules. Therefore, CAR T cells can be utilized for any HLA background, whereas the patient’s haplotype needs to be matched for TCRs [6].
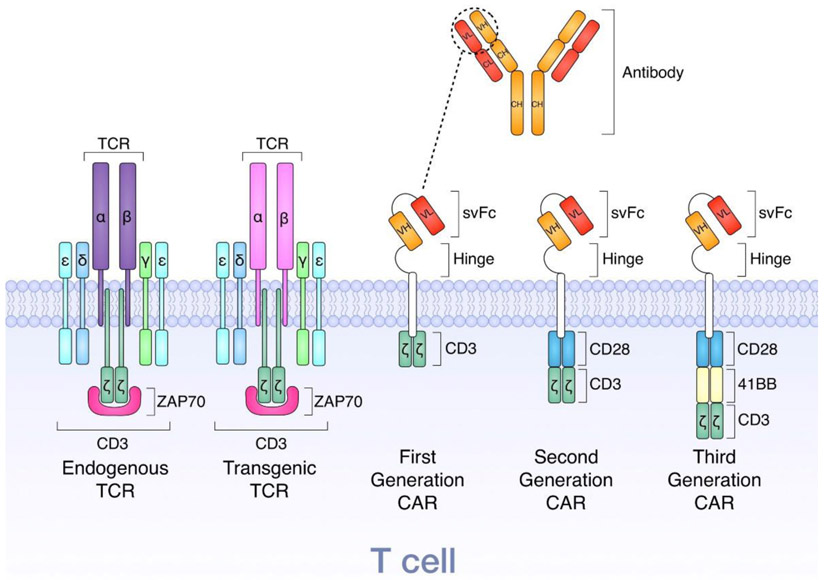
The first generation of CARs transduced into T cells are structurally composed of three components. The first component is an extracellular single-chain variable fragment (scFv) derived from a monoclonal antibody to recognize tumor specific antigens. The second component is responsible for anchoring the CAR to the T cell membrane using a transmembrane spacer and a hinge to enable the flexibility of the antigen specific scFv. Finally, the third component incorporates an intracellular TCR T3 zeta chain (CD3ζ) or CD247 submit that plays an important role in activating the signal-transduction pathways after antigen recognition. However, after some clinical trials where these first generation CAR T cells were unable to achieve appropriate therapeutic responses, the second generation of CAR T cells were developed by incorporating an intracellular co-stimulatory molecule, either CD28 or 4-1BB (CD137), with CD3ζ for improved proliferation and persistence. The third generation of CAR T cells further promote proliferation and persistence by including two co-stimulatory molecules as depicted in Figure 1 [12].
Figure 1.
Illustration depicting the structure of T cell receptors and chimeric antigen receptors. Reprinted from [12]. Copyright 2018, with permission from Elsevier.
These transduced T cells enable the targeting of a molecule of choice based on the scFv specificity, where successful binding triggers intracellular activating signals to mediate cytotoxic activity, proliferation, cytokine production, and persistence [13]. The CAR construct is typically encoded into the T cell genome using gene therapy viral vectors, most commonly lentivirus and gamma retroviruses [12]. One major limitation for CAR T cells is that they can only recognize cell surface antigens, thus limiting the possible tumor specific antigen targets for highly specific tumor targeting, whereas cancer-specific TCRs allow for the targeting of intracellular proteins and a wider array of antigen targets.
3. Autologous vs. Allogeneic T Cells
For adoptive cell therapy in a clinical setting, the primary source of the T cells currently is derived from the patients themselves. The autologous T cell therapy treatment involves collecting T cells from the patient’s blood, genetically engineering them to express CAR or a modified TCR, and finally readministering them back into the same patient. The use of autologous CAR T cells have produced both FDA approved CAR T cell therapies (Yescarta and Novartis’ Kymriah®), but there have been many limitations and constraints in the widespread adoption of these therapies [14]. The process of genetically modifying T cells ex vivo can be extremely inconsistent resulting in poor-quality modified T cells or insufficient T cell quantities, especially for newborn or elderly patients. Furthermore, the time and expense to modify autologous T cells can also limit the use of this treatment, especially for those patients in extremely critical conditions at late-stage cancer. Finally, there may also be limitations manufacturing enough functional T cells from patients who are lymphopenic due to prior chemotherapy or radiotherapy or other health condition [15].
An alternative to the use of autologous T cells is the development of allogeneic T cells by isolating T cells from healthy donors and genetically engineering them to target a patient’s tumor-associated antigens. This allogeneic approach can avoid many of the limitations described previously for autologous T cells because T cells from healthy donors are much more plentiful and can be carefully engineered in a controlled manufacturing process. However, the prospect of using off the shelf “universal” T cells from a foreign donor to treat patients poses several new risks and limitations. First there is a potential to induce graft versus host disease (GVHD), which is when the allogeneic T cells recognize the patient’s alloantigens as foreign, resulting in the allogeneic T cells attacking the body. Conversely, there is also a potential to induce host versus graft treatment rejection when the patient recognizes foreign HLA molecules on the donor T cells resulting in rapid rejection [10].
In order to address the GVHD complication imposed by allogeneic T cells, the general approach is to disrupt the donor T cell TCR-αβ expression by knocking out the constant region of the TRA (TRAC). On the other hand, the primary strategy to address host versus graft treatment rejection is the disruption of beta-2-microglobulin (B2M), which is an essential component for cell-surface expression of HLA-I heterodimers [7].
In order to generate these high quality universal allogeneic T cells, one extremely promising strategy to overcome these risks is through the use of genome editing to disrupt both the TCR and the HLA of the allogeneic universal T cell. CRISPR/Cas systems have proven to be an effective method to achieve highly efficient multiplex genomic editing, allowing for the option to simultaneously knockout multiple gene loci in T cells.
4. Multiplex Gene Editing
Genetic engineering can be a valuable component of immunotherapeutic strategies. Gene editing can be used to modulate receptor expression, induce the production of certain molecules, or even change cellular phenotypes. The three most common gene editing technologies are zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated (Cas) system as summarized in Table 1.
Table 1.
Comparison of modern gene-editing technologies.
| TALENS | ZFNS | CRISPR | |
|---|---|---|---|
|
Engineering simplicity [18] |
Moderate | Challenging; requires substantial protein engineering | Simple; sgRNA design based on complementary base pairing with target DNA |
| Multiplex genome editing capability [19] | Low | Low | High |
| Design Challenges [20] | Delivery of TALENs is challenging because of large size and repetition in TALENs sequence | Limited site selection; not all sequences are available for ZF binding | Target sequence must have an upstream PAM |
| Cost | Low | High | Lowest |
| Length of recognized DNA target | 30-40 bp | 9-18 bp | 20-bp guide sequence + PAM |
| Mechanism of target DNA recognition | DNA-protein interaction | DNA-protein interaction | DNA-RNA interaction |
| Mechanism of DNA cleavage and Repair | DSB induced by Fok1 | DSB induced by Fok1 | SSB/DSB induced by Cas9 |
To create double-stranded breaks at specific sites in the DNA, ZFNs and TALENs employ a non-specific FokI nuclease, as well as distinct DNA-binding protein domains: ZFNs use zinc finger proteins, and TALENs use transcription activator-like effectors derived from the plant pathogen Xanthomas. The CRISPR system relies on a target specific CRISPR RNA (crRNA), a target independent trans-activating RNA (tracrRNA), and a Cas nuclease [16]. The fusion of crRNA with tracrRNA into a single RNA duplex connected by a synthetic stem loop created an easily programmable genome engineering platform that can target different DNA sequences by a simple change in the 20-nucleotide crRNA sequence [17]. The question of which gene editing platform to use largely depends on a number of factors, including specificity in generating the intended modification, delivery efficiency, engineering simplicity, and affordability.
4.1. CRISPR/Cas Systems
Following the discovery of CRISPR and its role as an adaptive bacterial immune system, researchers sought to understand the mechanism by which this was accomplished. Rigorous sequence analyses of the CRISPR locus quickly led way to the observation that the spacer sequences corresponded to extrachromosomal DNA derived from phages and plasmids, consequently conferring resistance to those particular microbes [21-23]. Essentially, the spacer sequences served as genomic memory which could be used to identify and disable invading DNA.
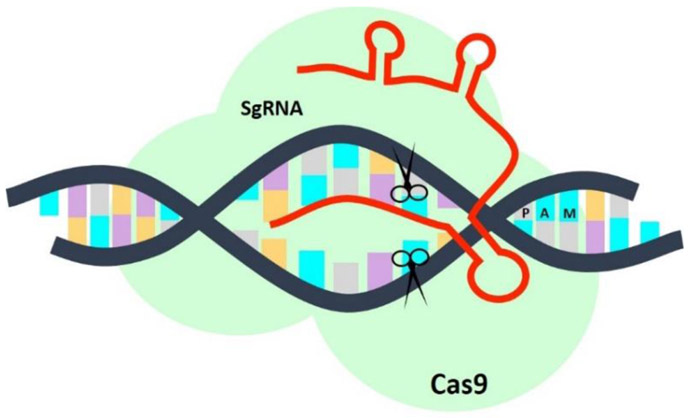
There are 2 classes of CRISPR/Cas systems and several subtypes, however, the class 2 type II system is the most routinely used. This system relies on a single Cas9 protein to induce double-stranded breaks in the genome, which then recruits DNA repair machinery to activate one of two competing pathways in the cell: the error-prone non-homologous end joining (NHEJ) or the template-driven homology-directed repair (HDR) pathway. In most organisms, the NHEJ pathway is the predominant DNA repair mechanism, which simply restores the integrity of the DNA by non-specifically rejoining broken ends, inducing frameshift mutations that lead to the successful knockout of a gene. Alternatively, the HDR pathway enables the insertion of precise genetic modifications by homologous recombination with exogenous repair templates [24,25]. Cas9 is a multi-domain protein with 2 nuclease domains, Ruvc and HNH, which generate site-specific nicks on opposite DNA strands. Mature crRNAs base pair with trans-activating RNA (tracrRNA) to form a single RNA complex that directs DNA cleavage by Cas9. As shown in Figure 2, DNA target recognition requires (1) site specific complementarity between the 20-nucleotide crRNA sequence and the target DNA and (2) the presence of an NGG protospacer adjacent motif (PAM) adjacent to the target sequence.
Figure 2.
A single guided RNA molecule (sgRNA), constructed by fusing the tracrRNA with the crRNA, can base pair with complementary regions of the DNA located next to a protospacer adjacent motif (PAM). With help of the Cas9 nuclease, the CRISPR/Cas9 system creates double-stranded breaks in the DNA, which can then be repaired using one of two pathways: non-homologous end joining or homology-directed repair.
4.2. CRISPR/Cas Delivery Systems
While CRISPR/Cas gene editing has opened up a wide variety of therapeutic avenues, one of the largest challenges related to its development is its ability to reliably and safely edit the intended target. CRISPR/Cas systems have a well-known propensity to exert off-target effects [26,27] and avoiding these off-target effects is critical in enabling CRISPR to be used in clinical settings. One of the primary means of increasing target specificity of gene editing systems is to utilize effective delivery systems. These delivery systems can increase the amount of CRISPR/Cas delivered into the relevant cells, reducing the payload needed, and in some cases can also specifically target the intended cells, reducing the chances of deleterious effects. Rational and strategic design of the appropriate delivery mechanism may allow gene editing technology to be used with fewer concerns related to potential toxicities. Currently, CRISPR/Cas delivery can be divided into two major categories: In vitro/ex vivo, and in vivo. Within these categories, there are non-viral and viral methods of delivering the gene editing technology to the cells, with some overlap in mechanisms between categories.
One important application of CRISPR/Cas gene editing is in immunotherapy. CRISPR/Cas can be used in CAR-T cell therapy, either in autologous therapy or in the development of allogeneic CAR-T cells. It can also be used to directly modulate immune cells in vivo, either downregulating aberrant gene expression [28], or enabling immune cells to recognize and destroy cancerous cells in the body.
4.2.1. Delivery of CRISPR DNA vs Ribonucleoprotein
One of the first decisions that must be made when deciding on a CRISPR/Cas delivery strategy is whether to use CRISPR nucleic acid or complexed ribonucleoprotein (RNP). The early CRISPR/Cas systems relied almost exclusively on CRISPR DNA delivery using either plasmids or viral vectors, since it was well-known and offered promising initial results, but there are significant problems with this delivery strategy. More specifically, delivery of CRIPSR DNA using plasmids or viral vectors results in long lasting expression of the Cas enzyme and associated guide RNA (gRNA). However, this can be disadvantageous. By increasing the time that the Cas enzyme is present in the nucleus, the chances of off-target effects (OTEs) are also significantly increased. In some cases, CRISPR DNA delivery has resulted in stable integration into the target cell genome [29]. Since OTEs are currently one of the major issues in CRISPR/Cas editing, it is essential to reduce that possibility. Another disadvantage of DNA delivery is that there is a lag period between transfection and expression. Once the DNA reaches the nucleus, the Cas mRNA and associated gRNA must be transcribed, then transported out of the nucleus for translation of the Cas-encoding mRNA. The Cas protein must then bind the gRNA and re-enter the nucleus before it can edit the cell genome.
In an alternative mechanism, the mRNA encoding the Cas protein can be synthesized and directly delivered alongside the gRNA. This mechanism avoids the issue of potential genome integration, and it also reduces the lag time between delivery and effect since the transcription step is skipped. Another advantage is that mRNA will be naturally degraded after a relatively short time, increasing the safety profde compared to DNA delivery. However, mRNA will still result in prolonged expression of the encoded Cas protein compared to directly delivered protein. Additionally, uncomplexed RNA is unstable and can be difficult to handle and store without degradation, potentially limiting its future applications.
In contrast, direct ribonucleoprotein delivery can often overcome these challenges. In this scheme, the Cas protein is synthesized and purified in vitro along with the gRNA, and the RNP complex is assembled prior to delivery. Once the RNP is delivered to the cell, it can immediately enter the nucleus to exhibit its gene editing effects. In this mechanism, there is no chance for error in the transcription and translation of plasmid DNA. More importantly, the RNP complex is cleared from the cell by natural degradation pathways much more quickly. If the time the RNP is active inside the nucleus can be reduced, then off-target effects can also be minimized. By using CRISPR RNP, there is no chance of unintentional incorporation of the Cas-encoding sequence into the host genome, which would be extremely dangerous in human applications. In a DNA-based system, the Cas protein and gRNA will continue to be expressed as long as the DNA is still being transcribed, which results in a delayed and prolonged response compared to the pre-assembled RNP method. In addition, the RNP system is significantly smaller than typical CRISPR plasmid DNA, potentially increasing transport efficiency and opening up delivery methods not available to plasmid DNA.
4.2.2. In Vitro/Ex Vivo Delivery Methods
The majority of CRISPR/Cas delivery systems are currently used in vitro or ex vivo. Because of the inherently controlled environment, edited cells can be evaluated after delivery to ensure that the intended gene edits have been made and that unintended edits are not present. Additionally, an in vitro environment makes it relatively easy to edit cells using physical delivery mechanisms, which makes it the most obvious choice for early clinical applications. All clinical applications of CRISPR/Cas are currently performed in vitro or ex vivo. Currently, the immunotherapeutic applications of CRISPR/Cas have primarily been limited to ex vivo manipulation of various immune cells, including T cells, B cells, dendritic cells, and NK cells [30-33]. Various non-viral and viral delivery methods are discussed in the following subsections and summarized in Table 2.
Table 2.
Summary of non-viral and viral CRISPR/Cas9 delivery strategies.
| Representative Methods |
Overview of Strategy |
Advantages | Disadvantages | |
|---|---|---|---|---|
| Non-viral Delivery Approach | Electroporation | Transient opening of pores in cell membrane via application of electric field | High transfection efficiency | Cell death, permanent membrane perforation |
| Nucleofection | Electroporation with specific voltage and nucleus-targeting reagents | High efficiency with lower collateral damage | Relatively recent, further trials needed to confirm efficacy | |
| Microinjection | Direct insertion of system into target nucleus (usually at embryonic stage) | Precise, effective gene editing for a single cell’s genome | Limited to single target, inefficient for large-scale editing | |
| Polymer/lipid systems | Engineered carriers, usually with specific targeting and release properties | Less invasive, potentially safer delivery mechanism | Few ongoing studies, limited applications in immunotherapy | |
| Viral Delivery Approach | Adenovirus | Non-integrating dSDNA vector with several cell receptor interactions | Broad cellular tropism, lower chance for OTEs | High immunogenicity, lower gene expression |
| Adeno-associated virus | Small ssDNA vector closely related to adenovirus, therapeutic strains remain episomal | High safety profile, low immunogenicity, sustained expression | Limited genome capacity, immune activation from high vector dosage | |
| Lentivirus/retrovirus | RNA virus with DNA intermediate, integration into host genome results in formation of provirus | Stable integration, targeting flexibility, produces heritable change | High safety concerns, potential for reverse mutations, integration can result in OTEs |
4.2.2.1. Non-Viral Methods
Electroporation is currently the most commonly used physical method for gene delivery. By applying an electrical field to the target cells, small pores are transiently opened in the cell membrane, allowing DNA or other molecules to be introduced to the cell [34]. Frequently, the CRISPR/Cas system is encoded into plasmid DNA, and that plasmid is then delivered to the cell to be expressed and perform the gene edits [35]. However, other methods such as viral vectors are also commonly used [33,36,37]. Recently, researchers have also been able to deliver the Cas protein or RNP directly to cells [32,38]. Electroporation is often preferred because it typically results in high transfection efficiency, however the technique often results in some degree of cell death due to the relatively harsh nature of the procedure [34]. Additionally, electroporation has been shown to lead to permanent permeabilization of the membrane in some cases [39].
In one interesting example, Su and coworkers showed that delivery of a plasmid encoding sgRNA and Cas9 by electroporation into human primary T cells was able to effectively knock out PD-1 expression [35], The researchers were able to show that even though PD-1 gene expression was reduced, the viability of the primary human T cells was not affected during prolonged in vitro culture. As a result of the gene modification, the cells showed up-regulated interferon-γ (IFN-γ) production and enhanced cytotoxicity. This direct modulation of human immune cells could show synergistic effects with current T-cell based therapeutic strategies.
In another example, Schumann and coworkers used electroporation to deliver Cas9 RNP to human primary CD4+ T cells [38]. By delivering the Cas9 RNP, the expression of CXCR4, which is one of the co-receptors used in HIV entry, could be reduced by about 40%. In addition to CXCR4 ablation, the authors were able to induce homology directed repair and knock-in targeted oligonucleotide replacements in both CXCR4 and PD-1, which regulates T cell exhaustion.
While electroporation is the most commonly used physical method for the introduction of gene editing systems, either in the form of plasmid DNA or RNP complexes, it has a number of limitations. Electroporation is a highly energetic technique, and often results in a significant amount of cell death [34]. Additionally, the efficiency of electroporation is limited in cells that do not divide rapidly, since the delivered DNA or RNP cannot access the nucleus of non-dividing cells once delivered to the cytosol. Nucleofection is one recent improvement over electroporation that combines electrical stimulation with specific reagents that instead allow the delivered material to access the nucleus of non-dividing cells [30,40]. This can result in significantly improved transfection efficiencies without the associated safety issues of viral delivery.
In one example using the nucleofection technique, Seki and Rutz were able to edit primary human and murine T cells with 80-90% efficiency, without prior TCR stimulation [41]. Such high efficiencies can reduce or eliminate the need to select and isolate targeted subpopulations post-editing. The authors were able to successfully knock out multiple cell surface receptors on human and murine CD4+ and CD8+ T cells, including CXCR4, PD-1, CTLA4, and TIGIT.
While the most common application of CRISPR/Cas in immunotherapy is T cell modification, there have also been reports of B cell, NK cell, and dendritic cell gene editing [30-32]. In one example, Hung et al. demonstrated that electroporation of CRISPR/Cas RNP into primary human B cells could effectively disrupt gene expression and alter plasma cell differentiation [31]. By co-delivering the CRISPR RNP with a single-stranded DNA oligonucleotide, plasma cells could be generated that secreted factor IX (FIX) or B cell activating factor (BAFF) at high levels. They further showed that these modified B cells could be engrafted into immunodeficient mice.
In another example, Kararoudi et al. showed that primary NK cells could be modified by electroporation of CRISPR/Cas9 RNPs [32]. Previous attempts to edit NK cells with CRISPR technology typically resulted in limited yields of genetically modified cells, often because the DNA mediated delivery schemes resulted in significant amounts of cell apoptosis, whether delivered by electroporation or viral vectors. In contrast, Kararoudi et al. achieved efficient knockdown of TGFBR2 and HPRT1 genes using RNP delivered by electroporation. In a cytotoxicity assay, it was shown that the genetically modified NK cells were less sensitive to TGF-β.
Another method of inducing gene editing is through the use of microinjection techniques. Microinjection is most often used in embryonic cell gene editing because the edit is limited to one cell at a time. Because of this, it is not an effective technique for editing a large number of cells in an efficient manner. However, microinjection is a highly effective and repeatable technique, making it valuable in the production of genetically modified animal models. In the future, it may be possible to discover and correct aberrant gene expression at the embryonic stage, allowing therapeutic correction at the embryonic level. However, ethical questions must be answered concerning the use of gene editing in embryos before such a strategy is viable.
Other non-viral delivery systems, including polymer and lipid systems, have been extensively investigated for CRISPR/Cas delivery both in vitro and in vivo [42-44]. however there are only a few known reports of these non-viral carriers being used in immunotherapeutic applications [28].
4.2.2.2. Viral Delivery
While in vitro delivery to cells can readily be achieved via electroporation, among other physical methods, a safer and more practical system is required for in vivo applications in human gene therapy. Viral vectors are a promising solution to this problem, as they naturally possess the ability to target specific cells and insert nucleotides into the cytoplasm. The main vectors currently being researched are adenoviruses, adeno-associated viruses, and lentiviruses. In all cases, upon binding to an extracellular receptor, the virus releases genetic material into the cell, which then enters the nucleus and may or may not integrate with the host genome. With viral vectors come the usual concerns of immunogenicity, potential for reverse mutations, and size limitations, and much care is needed in designing safe delivery vectors to prevent damage to the host [45]. Nevertheless, viral delivery of gene-editing technology may result in robust, personalized immunotherapy for a wide variety of diseases and improved patient outcomes.
The adenovirus, a non-enveloped, double-stranded DNA (dsDNA) animal virus with an icosahedral capsid, is capable of targeted delivery due to interactions with a large number of cell surface receptors on human cells. This broad tropism and ease of modification make this virus type attractive for gene delivery of CRISPR-Cas9 systems [45]. The genetic material does not integrate into the host genome, decreasing the chance for undesired mutations and giving adenoviruses a relatively high safety profile. Despite their advantages, concerns remain about their high immunogenicity and lack of integration into the genome, resulting in health risks to the host and lower levels of gene expression/deletion compared to other viral vectors [46]. Current research is focused on deleting a larger number of viral proteins while retaining function and isolating serotypes that have demonstrated lower immune activation in mammalian cells [47].
A recent study by Cheng et al utilized adenoviral delivery of a CRISPR/Cas9 system in adult mice to successfully demonstrate knockdown of specific liver genes, highlighting the system’s use beyond germline/embryonic cell applications [48]. Furthermore, another study investigated delivery of a CRISPR/Cas9 system via an adenovirus vector to knock down CCR5, a critical coreceptor used by the human immunodeficiency virus (HIV) to penetrate mammalian CD4+ T-cells. A chimeric Ad5/F35 adenoviral vector was used to transduce TZM-bl cells in vivo, where significant CCR5 knockdown was observed. Further assays confirmed that HIV infection was drastically reduced in these transfected cells, indicating that retroviral systems can be utilized to cleave genes for immunotherapeutic applications with high specificity and precision [49].
The adeno-associated virus (AAV) is derived from the parvovirus family and consists of a spherical capsid and a single stranded DNA (ssDNA) sequence. Modern recombinant AAVs used in therapeutic applications do not integrate into the genome and remain episomal, decreasing the chance of random mutation events and resulting in a relatively higher safety profile, low immunogenicity, and well as sustained expression within the body. However, the genome capacity of these vectors is approximately 4.5 kb, which is too small for many CRISPR/Cas9 systems. Additionally, high vector dosage and transgene products can cause a T-cell mediated response by the immune system [50]. Nevertheless, AAV is currently the most popular vector for CRISPR/Cas9 delivery due to its greatest potential use in humans, and despite its drawbacks, continues to be the standard of viral gene delivery and will certainly see clinical translation in the near future.
An increasingly popular approach to cancer immunotherapy involves enhancing the cells of the immune system to better target disease. Pomeroy et al increased the cytotoxicity of natural (NK) cells by using a CRISPR/Cas9 system packaged in recombinant adeno-associated virus serotype 6 (rAAV6) to reduce cell susceptibility to inhibitory signals from tumor and immune system sources. In an orthotopic mouse xenograft model of ovarian cancer, the treated NK cells resulted in significantly prolonged survival and decreased tumor burden. Tissue and ascites fluid analysis indicated that tumor cytotoxicity was a direct result of the inflammatory environment created by the enhanced NK cells, and that targeting was significantly improved by the presence of antibodies. This study demonstrates that coupling humoral immunity with gene-editing immunotherapy may yield novel treatment strategies for certain types of cancer [51]. Moreover, Dai et al investigated a strategy for designing modular CAR T cells with the addition of homology-directed-repair genes and knockout of immune checkpoint genes for more persistent tumor targeting. To transduce the gene, they utilized an AAV6 vector containing a CRISPRCas12a/Cpf1 system, a system closely related to CRISPR-Cas9. In vitro immunological assays of the dual-CAR knock-in and immune-checkpoint gene knockout (KIKO) cells demonstrated higher cytokine production, fewer exhaustion markers, and increased functionality against cognate cancer cells. This platform successfully generated transgenes in different gene loci and offers a promising solution for treating several different types of leukemia. Use of an AAV in this platform was particularly important for flexible multiplexed editing and offers advantages not available with lentiviruses or adenoviruses [52].
Viruses of the Lentivirus genus are a complex type of retrovirus that contain an additional set of genes for gene product processing and suppression of the host antiviral response. The viruses contain a glycoprotein capable of targeting cellular receptors; upon binding, the genetic material stably integrates with the host genome and a provirus is created, persisting in the body for a long period of time and capable of heritable change. As a CRISPR/Cas9 vector, lentiviruses are popular due to stable integration within the genome, their ability to infect both dividing and non-dividing cells, and broad tropism in immune cells, properties that allow for applications in a wide variety of diseases and flexibility of administration [20]. However, as the lentivirus is derived from highly infectious and deadly human pathogens such as HIV, there is a considerable safety concern when working with such vectors as there is potential for reverse mutation or rearrangement into a functioning, pathogenic virus. Additionally, lentiviral components integrate into the genome, posing a major health risk to the host as insertion in an incorrect location may disrupt normal functioning. These issues have been addressed by strategies that split essential integration genes or altogether removing infectious segments from the genome. Some lentivirus strains have also been engineered to be integrase-deficient to prevent genomic integration; though this eliminates the benefit of stable and heritable expression, it also results in a higher safety profile for the vector [53].
In CAR T cells, TCR and HLA-I surface proteins cause increased activation of PD-1 immune checkpoint receptor and downregulation of the immune response. Recently, a group investigated how disruption of these genetic loci could enhance CAR T therapy in tumor models. The modified CAR T cells were generated using lentiviral vectors containing a targeted CRISPR/Cas9 system to disrupt the genes and inserted into xenograft mouse models with leukemia, where quick, robust antitumor activity and low alloreactivity was observed relative to a control therapy as a direct result of decreased PD-1 activation. This study demonstrates how CRISPR/Cas9 is being utilized in conjunction with existing immunotherapy strategies to improve the overall functionality of anticancer therapeutics [53]. Another study by Zhang et al. further explored how genetic abrogation of PD-1 may result in improved outcomes against tumor activity, noting that current approaches utilizing systemic delivery of antibodies cause immune-related side effects by universal T cell activation. The researchers generated gene knockouts of the PD-1 pathway in antigen-specific cytotoxic T lymphocyte (CTL) cells using a replication-deficient lentiviral vector loaded with a CRISPR/Cas9 system. Researchers observed successful transduction of CTLs and efficient Cas9 mediated disruption of the PD-1 pathway. Additionally, sustained production of pro-inflammatory cytokines was observed in cell cultures, demonstrating successful establishment of a platform that can edit antigen-specific immune cells with a lentiviral gene-editing vector to bypass immunosuppressive checkpoints. This work carries major implications for cancer immunotherapy if successfully demonstrated in vivo [54].
4.2.3. In Vivo CRISPR/Cas Delivery
While in vitro/ex vivo methods are currently the de facto standard for gene editing in immunotherapy applications, there are advantages to developing systems capable of editing cells in vivo. If it can be accomplished safely and reliably, in vivo methods remove the need to extract cells from or re-introduce cells to the patient. Additionally, if autologous edits are required, in vivo editing reduces the time needed to complete treatment, since the gene editing process in vitro can take multiple weeks before edited cells can be re-introduced. Perhaps most important, effective in vivo editing could produce a higher percentage of successfully edited cells in the target population, resulting in greater treatment efficacy. However, there are a number of challenges that still need to be overcome, including the ability to selectively target the intended cells, efficiently transfect those cells, and avoid a systemic immune response to the delivery vehicle or gene editing system. As a result, there have been few reported cases of in vivo gene modification for immunotherapeutic applications thus far.
In one of the few cases, Zhang et al. showed that Cas9 mRNA combined with gRNA targeting CD40 could be effectively delivered in vitro and in vivo by cationic lipid-assisted nanoparticles (CLAN) to dendritic cells (DCs) [28]. By downregulating the expression of CD40 in DCs after intravenous injection, T cell activation was reduced in an acute mouse skin transplant model, which prolonged graft survival. In an indirect example, LaFleur et al. used an in vivo CRISPR delivery mechanism to screen for genes of interest in human innate and adaptive immune cells in their native environment [55].
5. Developing ‘Off-the-Shelf’ Universal Allogeneic T Cells
5.1. Allogeneic CAR T Cells
As previously discussed, viral vectors have been used as the primary delivery vehicle for CAR expression or genetically modified TCR delivery. Viral vector delivery has been shown to exhibit high gene-transfer efficiency and stable expression, however, the transgenic receptor will be semi-randomly inserted into the genome causing a certain level of unpredictability. This inaccurate delivery method can pose a potential safety hazard when integrated into the wrong genome location leading to the onset of other diseases and also requires a constitutively active promoter to drive the transgenic receptor expression [56].
The recent maturation of CRISPR/Cas technology has spurred an interest in non-viral vector insertion of large genes at specific genetic sites in T cells for both CAR T engineering and engineering αβ-TCR using HDR. Non-viral genome allows for the insertion of larger DNA sequences compared to viral vectors and also allows for correction of point mutations, in contrast to the unpredictability of viral vector delivery [57].
With the development of CRISPR/Cas and its ability to perform multiplex gene editing, Liu and coworkers generated universal and more potent allogeneic CAR T cells by disrupting the TRAC and B2M. They transduced the CD19 CAR with a lentiviral vector and confirmed that transducing CAR first followed by CRISPR/Cas9 electroporation for knockouts generated CAR T cells with higher gene editing efficiency and cell viability. They showed that these double knockout CAR T cells lost TCR and HLA-I expression on the cell surface, but exhibited comparable cytokine release to standard CD19 CAR T cells and did not show any compromise of their potent antitumor activity in vitro or in vivo [7].
Further success has been demonstrated in studies showing a targeted insertion of the CAR expression, while simultaneously knocking out the native TCR to generate universal allogeneic T cells. The T cells were electroporated to knockout TRAC encoding the native TCR, while also using an adeno-associated virus vector targeted to TRAC to transduce CAR expression by exploiting HDR CRISPR/Cas9 mechanisms that can knock in sequences to defined locations in the genome. The resulting allogeneic CAR T cell displayed reduced tonic CAR signaling and exhibited tight endogenous transcriptional regulation of CAR expression, delayed T cell exhaustion, and improved tumor eradication. The recent advancements in genome editing enabled sequence-specific gene delivery, thus resulting in this efficient 2-in-l TCR knockout and CAR knock in strategy. This strategy also further minimizes risks of insertional oncogenesis and TCR-induced autoimmunity for universal allogeneic T cells [58,59].
5.2. Allogeneic TCR Modified T Cells
Alternatively, CRISPR/Cas9 has also brought widespread attention to universal allogeneic TCR-modified T cells. Roth and coworkers showed in a recent study that the heterodimeric αβ-TCR can be integrated into the TRAC locus with sufficient efficiency, given that knock-in efficiency is lower for non-viral vectors compared to viral vectors. The non-viral genetically modified T cells successfully exhibited selective tumor antigen-specific killing of target cells expressing the NY-ESO-1 antigen in vitro. Furthermore, they demonstrated that ssDNA templates could significantly reduce off-target integration compared to dsDNA templates, while maintaining similar gene knock-in efficiency in T cells [57]. This shows that ssDNA is a more suitable HDR donor template format to reduce off-target integration for T cell modification through CRISPR-based gene insertion and replacement. Schober and coworkers also emphasized the importance of specific gene insertion using non-viral vectors by demonstrating that orthotopically placed TCRs enables physiological T cell regulation and provides promising results when combined with simultaneous knockout of both TCR α- and β-chains [60].
One major challenge associated with transducing tumor-specific TCRs for universal T cells is the presence of endogenous TCRs competing for CD3 association to form the TCR complex. Furthermore, transgenic TCRs are faced with the risk of forming mixed dimers, which occur from a mispairing of endogenous and transduced TCR chains. These mixed TCR dimers exhibit unpredictable target specificities leading to potentially dangerous autoimmunity or GVHD [61]. Affinity-enhanced TCRs have been used as a primary strategy to avoid complications associated with TCR competition and mispairing because only a small fraction of correct TCR paired molecules is sufficient to trigger a response, as shown in clinical trials targeting a variety of cancer-testis antigens [62,63]. However, these affinity-enhanced TCRs do not address mispairing directly, but rather promote another aspect to achieve preclinical and clinical success. In addition, these affinity-enhanced TCRs pose even greater risk for unanticipated crossreactivity causing fatal autoreactivity in patients [64].
With the development of genetic engineering, new strategies have been opened up to address mispairing and TCR competition directly. Legut and coworkers showed that cancer-specific TCRs that exhibit low functional activity due to weak competition with endogenous TCRs can still be utilized clinically when combined with a CRISPR knockout of endogenous constant regions of the TRB (TRBC). The results suggested that mispairing between the endogenous TCR-α chains and the transduced TCR-β chains is minimal because the resulting modified T cell showed equivalent antigen sensitivity to the starting T cell clone. This strategy showed that the knockout of the endogenous TCR-β chains led to an increase in surface expression of transgenic αβ and γδ TCRs for an improved therapeutic response without exacerbating T cell exhaustion [61]. However, Schober and coworkers showed that TCR mispairing is still prevalent following a TRAC knockout and residual mispairing could only be eliminated with a simultaneous knockout of TRBC. Simultaneously targeting both the α- and β-chains led to harmonized TCR surface expression and enhanced functional capacity [60]. It would be interesting to investigate why the single knockout of TRAC and the single knockout of TRBC results differ and the unbalanced affinity for endogenous α- and β-chains to create mispairings with transgenic α- and β-chains.
Another strategy investigated by Bethune and coworkers to prevent mispairing is to swap constant domains between the α- and β-chains of the transgenic TCR. This would still allow for the functional pairing and formation of domain swapped chains to assemble with CD3, while simultaneously preventing the proper assembly of the mispairing between domain swapped TCR chains and endogenous chains with CD3. They also show that this strategy can be used in complement with endogenous TCR knockdown for further improved TCR mispairing prevention and alleviation of some potential autoimmunity risk [65]. Other recent genetic modification strategies that have been proposed to prevent TCR mispairing include single-chain TCRs [66,67]. modified two-chain TCR linked to CD28 and CD3ε [68]. and RNA interference [69].
6. Challenges for Genetically Modified T Cells in Solid Tumors
The remarkable clinical success for autologous engineered T cells in blood cancers have prompted widespread optimism for engineered T cell cancer therapies to make transformative impacts on the next generation of cancer therapies. The logical next step aside from engineering previously discussed allogeneic T cells, is to transition to treatment of solid tumors, however, genetically engineered T cell therapies have largely failed against solid tumors in clinical settings. The poor clinical performance of CAR T cells in inducing anti-tumor responses against solid tumors can be attributed to the complex, multifaceted nature of cancer that requires treatments to simultaneously overcome multiple functional challenges. These major functional challenges can be categorized into targeted delivery of T cells through the solid tumor, recognizing tumor cells, avoiding on-target toxicities, overcoming the tumor suppressive microenvironment, and maintaining persistence and proliferation of tumor-targeted effector T cells. Here, we review the most recent CRISPR-based strategies to address each of these major functional challenges, as well as other promising genetic modification strategies or combination strategies.
6.1. T Cell Targeted Delivery and Tumor Infiltration
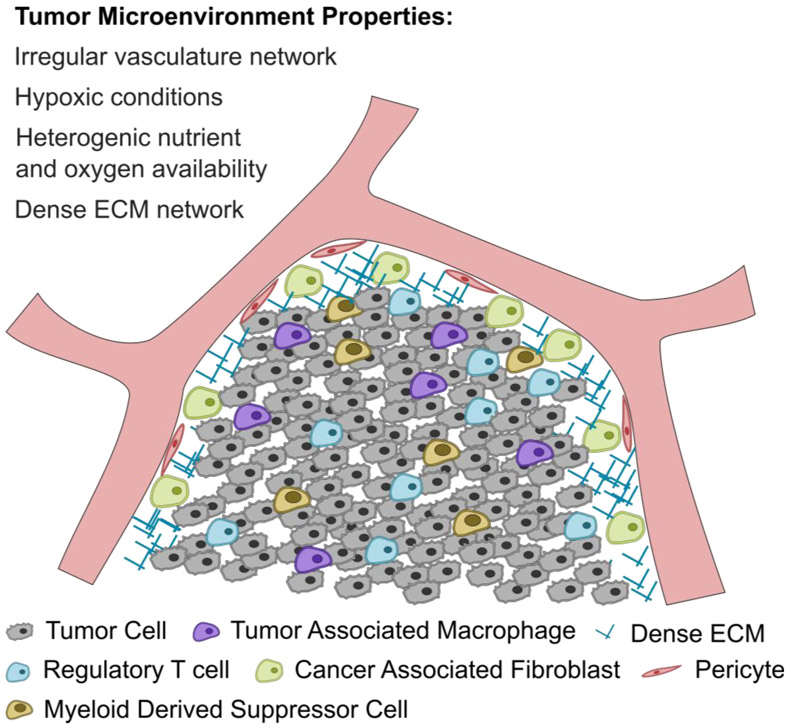
The tumor microenvironment (TME) exhibits a number of functional and physical barriers that disrupt lymphocyte infiltration and hinder genetically engineered T cell therapies including hypoxic conditions, irregular vasculature network, heterogenic nutrient and oxygen availability, dense ECM network, extensive stromal cells, and high levels of the FasL triggering FasL-induced apoptosis as shown in Figure 3 [70,71].
Figure 3.
Schematic of the tumor microenvironment and the major barriers associated with T cell infiltration and signaling.
6.1.1. CRISPR Modulation of Immunogenicity
One promising strategy to improve T cell targeted delivery is to increase the immunogenicity of tumor cells. Tumor cells are characterized by a number of mutated or amplified genes that can be immunogenic, but many may not be expressed at sufficient levels to trigger an effective immune response, especially in combination with downregulated antigen presentation in the TME, used to evade immune recognition. One group developed a new form of immunotherapy that elicits antitumor immunity through the multiplexed activation of endogenous genes in tumors (MAEGI). They directly amplified the in situ expression of endogenous genes using CRISPR activation (CRISPRa) to increase the presentation of tumor antigens [72]. CRISPRa can create non-permanent modifications in the DNA through targeted transcriptional regulation by relying on catalytically inactive Cas9 (dCas9), dCas9-transcriptional activators, and target-specific single guide RNAs to activate transcription by directly enhancing RNA polymerase binding [73,74]. An adeno-associated virus-CRISPRa vector was devised to drive the in vivo delivery of CRISPRa components into target tumors for precise targeting of mutated gene. MAGEI increased tumor gene expression and antigen presentation to enhance T cell infiltration and promote both local and systemic antitumor T cell responses [72].
6.1.2. Chemokine Signaling
The primary approach for the genetic engineering of T cells to improve targeted delivery of T cells involves transducing a chemokine receptor that matches the chemokine expressed by the tumor to enable a “homing” mechanism that enhances T cell infiltration into the tumor mass. A recent study by Liu and coworkers showed that CAR T cells modified to express CXCR2 displayed significantly accelerated in vitro migration and in vivo trafficking, while maintaining similar cytotoxicity for an improved anti-tumor effect due to overexpressed CXCR2 ligands (CXCL1) in human hepatocellular carcinoma tumor tissues and cell lines [75]. Another strategy involves transducing CCR4 on genetically modified T cells to mimic the CCR4 expressed on by regulatory T cells that are attracted to intratumoral CCL22. This strategy demonstrated improved homing and anti-tumor efficiency in vitro and in vivo for pancreatic tumors [76]. In contrast, Perera and coworkers also demonstrated a strategy that incorporated an anti-CCR4 antibody in CAR T cells to target the overexpressed CCR4 bearing regulatory T cells present in the TME. Not only does this strategy promote tumor trafficking, but can also be utilized as a CCR4-directed therapy to eliminate regulatory T cells that constitute as a barrier to host immune responses against the solid tumor [77]. The chemokine receptor trafficking strategy has also been utilized in tumor antigen-specific TCR modified T cells. A study showed that double transfected T cells to express WT1-specific TCR and CCR2 exhibited increased migration and anti-tumor activity in vitro and in vivo due to highly expressed CCL2 on the LK79 human lung cancer cell line [78]. Other genetic modification strategies to promote T cell trafficking include silencing Fc domains in T cell-engaging bispecific antibodies [79] and incorporating a “regulatory subunit I anchoring disruptori’ that inhibits the association of protein kinase A with ezrin [80].
6.1.3. Tumor Vasculature Targeting
An alternative strategy to improve trafficking of genetically engineered T cells is to target the tumor vasculature as opposed to overexpressed targets on heterogenic tumor cells. Targets on the tumor vasculature are more readily available and can also help avoid the immunosuppressive TME within the solid tumor. A recent study presented a CAR T cell that targets the EIIIB+ fibronectin splice variant, which has been reported to be expressed on the neovasculature. Most solid tumors require angiogenesis to provide nutrients for survival and targeting the neovasculature may compromise the blood supply for the tumor and improve tumor accessibility for other potential therapies used in combination [81]. Another target that is highly expressed on tumor epithelium is the epithelial-specific integrin, αvβ6, which was targeted by a CAR developed by Whilding and coworkers to elicit potent therapeutic activity against solid tumors [82].
Another component of the tumor vasculature is the cancer-associated stromal cells that provide a variety of signals for tumor cell growth and metastasis. Fibroblast activation protein (FAP) is a marker that is present on a majority of stromal cells in the TME, therefore CAR T cells that target FAP were developed for improved delivery towards the solid tumor. In addition, the adoptive transfer of FAP-targeted CAR T cells decreased tumor vascular density and restricted tumor growth in lung and pancreatic cancer in vivo models [83].
Other promising tumor vasculature targets include the vascular endothelial growth factor receptor 1 (VEGFR1) [84], vascular endothelial growth factor receptor 2 (VEGFR2) [85], prostate specific membrane antigen [86], and tumor endothelial marker-8 [87].
6.1.4. Combination Therapies
An additional approach to improve T cell trafficking is to combine adoptive cell therapy with another treatment that increases T cell infiltration in solid tumors. Enhanced cellular infiltration in solid tumors for improved CAR T cell activity and sensitivity has already been achieved using traditional cancer treatment methods such as radiotherapy [88] and chemotherapy [89]. Another example of a combination treatment utilizes an extracellular matrix (ECM) degrading enzyme, heparanase, that is co-expressed on engineered CAR T cells to enable simultaneous degradation of the ECM without compromising their viability, resulting in promoted T cell tumor infiltration and enhanced CAR T cell antitumor efficacy [90].
6.2. Tumor-Specific Recognition
The fundamental purpose of adoptive cell therapy in cancer is the ability to redirect T cell recognition to user-specified tumor-specific cells. One essential requirement for identification of new antigens for T cell interaction is the ability to distinguish between tumor-specific cells and bystander non-cancer cells to avoid cross-reaction toxicities and lethal side effects. The first type of cross-reaction occurs when the genetically modified T cell recognizes the targeted tumor antigen that is also expressed on non-cancer cells, known as on-target off-tumor cross-reaction. The other type of cross-reaction occurs when the transduced T cell attacks an unexpected antigen other than the intended target on bystander cells, known as off-target cross-reaction. Many studies have looked into identifying the ideal tumor-unique “magic bullet” antigen to avoid cross-reaction toxicities, but many of the identified antigens are still expressed at lower levels on normal tissues or only expressed on a subpopulation of tumor cells. For this purpose, genetically engineered TCRs may have a higher potential due to the ability to recognize a broader range of intracellular antigen targets as opposed to the limited surface antigens for CAR T cells.
6.2.1. Boolean Logic Gate CAR Circuits
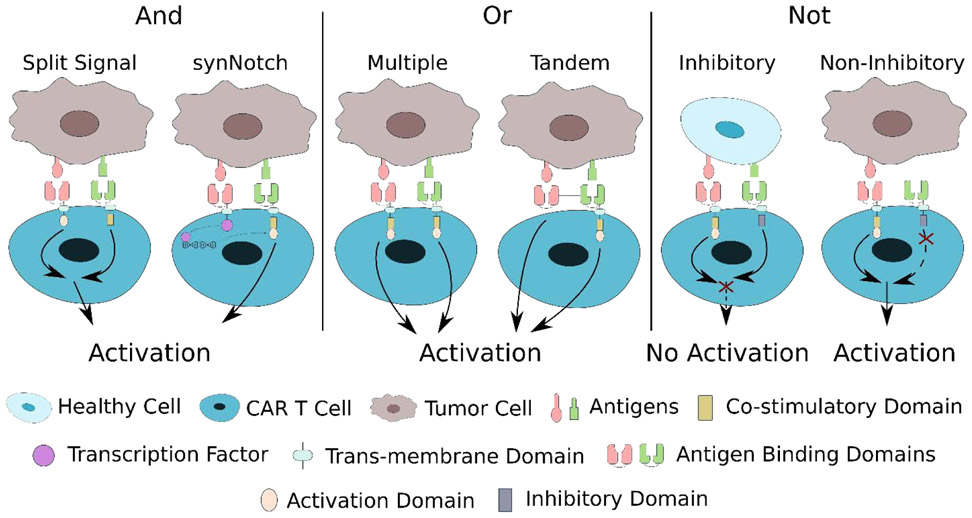
Because this ideal tumor-unique “magic bullet” antigen has not yet been identified, the most promising approach to improve the tumor specificity and minimize cross-reaction of genetically modified T cells involve the use of combinatorial antigen recognition through “OR”, “AND”, and “NOT” Boolean logic gates as shown in Figure 4. The integration of multiple target antigens would significantly enhance the capability of tumor-specific recognition.
Figure 4.
Schematic of various strategies utilizing “AND”, “OR”, and “NOT” Boolean logic gates to enhance tumor specificity of adoptive cell therapy.
The “OR” logic gate implies that the genetically modified T cells can be activated in the presence of any single or combination of target antigens. This “OR” logic gate strategy is particularly useful to overcome the development of immunotherapeutic resistance through antigen escape. Researchers have utilized this “OR” gate using multiple modified T cells each for different targets or multiple specific antigen targets incorporated on a single modified T cell. Feng and coworkers performed a cocktail CAR T treatment with successive infusions of CAR T cells targeting epidermal growth factor receptor (EGFR) and CD133 for a patient with advanced cholangiocarcinoma. This cocktail treatment provided partial remission, demonstrating the feasibility to treat solid tumors, but was hampered by a series of known and unknown toxicities that require the further safety investigation for this type of treatment [91]. Moreover, researchers have investigated the impact of T cells transduced with multiple distinctly targeted receptors or T cells that have multiple distinct antigen-binding domains on a single receptor. Ruella and coworkers showed that a dual CAR-expressing T cell that combined CD19 and CD123 activation provided superior in vivo antitumor effect compared to single CAR-expressing T cells and pooled combination of CART cells [92]. Spear and coworkers demonstrated similar capabilities from dual-reactive T cells that recognized two distinct antigens after transducing HCV1406 TCR to T cells stimulated with the CMVpp65:495-503 peptide. These engineered T cells that express both TCRs may be effective against immune escape variants of patients with hepatitis C virus-associated diseases, such as hepatocellular carcinoma [93]. Some researchers have attempted utilizing CAR T cells that simultaneously target three antigens, as demonstrated by Bielamowicz and coworkers who simultaneously targeted human epidermal growth factor receptor 2 (HER2), interleukin-13 receptor subunit alpha-2, and ephrin-A2 to overcome interpatient variability in glioblastoma patients [94]. Finally, many studies have investigated modifying T cells to coexpress distinct antigen-binding domains in tandem to exhibit a distinct T cell response to each antigen and demonstrating synergistic efficacy when simultaneously encountering both antigens [95,96].
The “AND” logic gate indicates that the genetically modified T cells can only be activated in the presence of multiple antigens simultaneously. This “AND” logic gate can be achieved through two general approaches. The first approach separates the signaling chain from the co-stimulatory motif present in second and third generation CAR T cells into separate CARs that are targeting different antigens. This way, the only way to exhibit long-term persistence and enhanced antitumor efficacy from later generations of CAR T cells is to simultaneously encounter both antigens to trigger the activation of both CARs, similar to endogenous TCR activation with separate co-stimulatory signaling [97]. A recent study by Sukumaran and coworkers utilized this same concept in three separate CARs that targeted prostate stem cell antigen, transforming growth factor-β (TGF-β), and interleukin 4 (IL-4) paired with signaling domains, co-stimulatory motifs, and cytokine support, respectively. This trivalent CAR T cell takes advantage of the T cell immunosuppressive cytokines present in the TME as tumor-specific targets to develop a highly selective modified T cell for pancreatic cancer [98].
The second approach utilizes a synthetic Notch (synNotch) receptor system that can regulate the transcription and expression of a second CAR or TCR that mediates the cytotoxic behavior. Only when the synNotch receptor is activated through the binding of the first targeted antigen will it activate transcription and expression of the second receptor that will fully activate the T cell once exposed to the second targeted antigen. Tests using this synNotch receptor confirmed that the modified T cells were only activated by tumor cells that expressed both CD19 and mesothelin and tumors cells that expressed either single antigen did not activate the modified T cells [99-101]. This conditional expression of the cytotoxic CAR helps regulate on-target off-tumor toxicities and reliably discriminates tumors by achieving a highly specific combinatorial recognition of tumor-specific cells.
The “NOT” logic gate takes advantage of antigens that are largely expressed on bystander cells and not expressed on cancer cells to distinguish between target and non-target cells. This approach utilizes a combination of a CAR targeted towards a TAA with an inhibitory CAR (iCAR) that dampens the CAR signaling if activated. The inhibitory CAR utilizes the signaling domains from immune inhibitory receptors like CTLA-4 or PD-1 as the intracellular domain to limit T cell responsiveness upon activation [102]. These iCARs will require further optimization and investigation in terms of the optimal ratio of CAR/iCAR and antigen selection that is selectively present on non-cancer cells, but they provide a promising proof of concept that could promote tumor specificity by distinguishing non-cancer signals.
6.2.2. Tumor Microenvironment Sensitive Recognition
Another approach for improving tumor recognition specificity is to take advantage of specific properties in the TME. For example, Juillerat and coworkers demonstrated a strategy that takes advantage of the hypoxic tumor microenvironment to develop oxygen sensitive CAR T cells by integrating oxygen sensitive subdomains of human hypoxia-inducible factors 1 -alpha to modulate surface expression of the engineered CARs. This strategy minimizes on-target off-tumor toxicity effects by adding a TME-specific property as an additional activation requirement [103]. This strategy that makes engineered T cells sensitive to TME-specific properties, enables the expansion of the potential TAA targets available by adding rigorous requirements for T cell activation. Another example utilizes the mechanism of immune evasion in the tumor microenvironment, specifically the production of the inhibitory cytokine IL-4, as a signal to distinguish tumor-specific cells. Mohammed and coworkers reversed the inhibitory effects of IL-4 by fusing the exodomain of the IL-4 receptor to the signaling endodomain of the IL7 receptor, a Th1 cytokine receptor, in tumor-directed T cells. The resulting engineered T cells required the exposure of both the tumor antigen and tumor-derived IL-4 to activate the chimeric cytokine receptor and support the maintenance of a Thl phenotype in effector cells to augment cell proliferation and antitumor function [104].
6.2.3. Antigen Density Sensitivity
Due to the difficulty of identifying TAA targets that are exclusively expressed on cancer cells, another approach that distinguishes cancer from normal cells relies on using CAR T cells that are activated upon reaching a threshold antigen density. This method requires the modification of the selected co-stimulatory motif or the affinity of the scFv within a CAR to be at a certain threshold where a high density of the TAA expressed on cancer cells would still be able to bind to the CAR, but a lower density of the TAA expressed on normal cells would not be able to bind to the CAR. Modifications to the CAR affinity based on antigen density has been performed for a variety of different targets including EGFR [105,106], HER2 [107], and CD19 [108].
6.3. Avoiding On-Target Toxicities
In addition to cross-reaction toxicity, on-target toxicity can also occur where the activated T cells trigger excessive cytokine release resulting in cytokine release syndrome (CRS) or other serious adverse effects. CRS is the most common acute toxicity for adoptive cell therapy by triggering a positive feedback loop where activated immune cells release inflammatory cytokines, which can in turn activate more immune cells, eventually leading to high fever, multiple organ dysfunction, or other serious toxicities.
6.3.1. User-Controlled OFF-Switch
Many researchers have investigated user-controlled regulatory mechanisms for controlling T cell responses to mitigate these potentially lethal toxicities and serious adverse effects. One effective strategy that places the treatment under user controls is the incorporation of a suicide gene. Cells that express the suicide gene will trigger selective destruction through apoptosis when activated via administration of a nontoxic prodrug or the administration of rimiducid for the novel inducible caspase 9 (iCasp9) suicide switch. Genetically modified T cells that also express iCasp9 can be specifically eliminated by physicians to prevent the occurrence of on-target toxicities during treatment, but this strategy would also eliminate the therapeutic potential of adoptive cell therapy [109,110]. A more recent study that incorporated the rimiducid-induced iCasp9 suicide switch demonstrated that rimiducid titration allows for partial elimination of iCasp9-expressing CAR T cells for more precise user control and preservation of long-term antitumor efficacy [111].
Alternatively, the therapeutic T cells could also be modified to express some antibody-recognizable target cell surface to allow for selective elimination of the T cells by administration of the cognate antibody. This has been demonstrated using EGFR [112], CD20 [113], and CD52 [114] as elimination markers and administrating cetuximab, rituximab, and alemtuzumab, respectively, for elimination. A more recent study by Koristka and coworkers has improved upon this strategy by incorporating a targetable short peptide epitope (E-tag) into the CAR architecture. This tag can be recognized and targeted by aE-tag CAR T cells to allow for a specific killing of E-tagged CAR T cells in vitro and in vivo. This strategy bypasses many drawbacks associated with using antibodies for elimination such as immunogenicity, on-target side effects, and decline of pharmacological drug therapeutic effect due to their short half-life [115]. These kill switches have proven to be effective for enhanced safety in the cases of unintended toxicities, but they do not provide precise control over T cell activation and the effects of the kill switch are irreversible.
6.3.2. User-Controlled Reversible ON-Switch
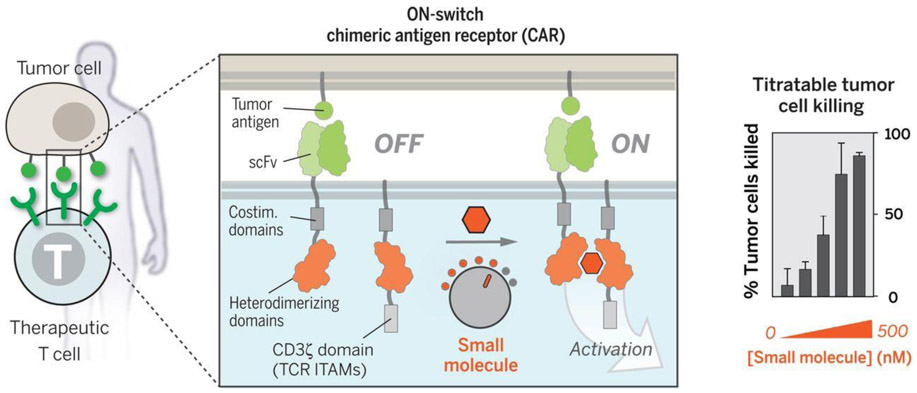
Another approach to allow precise user control for safer therapeutic cells is to incorporate an “ON-switch” that enables the CAR function only in the presence of a heterodimerizing small molecule as shown in Figure 5. This strategy separates the extracellular recognition scFv and the intracellular signaling domains onto separate heterodimerizing proteins, namely the FK506 binding protein (FKBP) domain and the FKBP-rapamycin binding (FRB) domain, which will heterodimerize in the presence of the rapamycin analog AP21967. This process is titratable and reversible, which allows for precise remote control of timing, location, and dosage of CAR T cell activity [116]. A similar strategy by Juillerat and coworkers utilized the same heterodimizering domains as a trigger, but incorporated them into the hinge region of the CAR to separate the scFv from the cell membrane. The addition of the heterodimerizing small molecule would shorten the distance between the scFv and the cell membrane to switch on the CAR expression, allowing for selective control of “transient” CAR T cells [117]. A more recent study demonstrated a combination of the rapamycin-directed dimerization with the previously discussed iCasp9 to generate a rapamycin-induced, caspase-9 safety switch because the classic use of rimiducid as a trigger was being used in a separate switch to induce MyD88 and CD40 for controlled costimulation of CAR T cells. This approach generated a dual-switch CAR T cell that could regulate costimulation to drive CAR T cell proliferation and activity, while retaining a user-controlled switch to ensure safety [118].
Figure 5.
Schematic of the titratable and reversible “ON-switch” mechanism through a heterodimerizing small molecule to allow for precise remote control. From [116]. Reprinted with permission from AAAS.
Another target that has been studied as a potential on/off switch is the zeta-chain associated protein kinase 70 (ZAP70), a tyrosine kinase that is involved in the signal transduction of T cell activation. Wong and coworkers developed a dual-gated ZAP70 signaling switch to regulate T cell activity by fusing ZAP70 to the ligand binding domain of the estrogen receptor, ERT2. The ZAP70 activity can be turned ON using 4-hydroxy-tamoxifen, and turned OFF using an ATP analogue, 3-MB-PP1. The concentrations of the activator and inhibitor can be varied to modulate the strength of T cell signaling on the time scale of minutes [119]. Another recent study utilizes dasatinib, a tyrosine kinase inhibitor that can inhibit the phosphorylation of ZAP70, as an OFF switch to halt cytolytic activity, proliferation, and cytokine production from CAR T cells. Similarly, the dose of dasatinib can be titrated for partial inhibition of CAR T cell function and the inhibition can be completely reversed upon discontinuation of dasatinib as an ON-switch [120].
6.3.3. Adaptor Molecule Trigger Switches
An alternative strategy to control T cell activation towards cancer cells is through the use of intermediate adaptor molecules as a trigger switch. Adaptor molecules will mediate the formation of the immunological synapse between the CAR T cell and the cancer cell. The CAR T cells will be engineered to recognize the adaptor molecule and the adaptor molecule will be responsible for specifically targeting the cancer cells. Examples of adaptor molecules include peptide neo-epitope (PNE) antibody fragments (Fab) [121], FITC-anti-CD19 Fab [122], FITC-anti-CD22 Fab [122], FITC-folate [123,124], and other FITC-tumor-specific ligand combinations [125]. This strategy provides an advantage in targeting flexibility based on the variety of adaptor molecules that can be developed for numerous tumor-specific targets. Similar to the adaptor molecule strategy, a recent study integrated a leucin zipper domain to both the extracellular component of the CAR and a separate TAA-specific scFv to form a bridge that can control T cell activation. This study demonstrated the universal capability of a CAR system that can be easily modulated to target different antigens, regulate different signaling pathways, and precisely tune T cell activation [126].
6.3.4. Gene Knockout Mitigation of CRS
Furthermore, one recent promising strategy by Sterner and coworkers demonstrated that CRISPR/Cas9 disruption of granulocyte-macrophage colony-stimulating factor (GM-CSF) in CAR T cells resulted in a significant reduction in neuroinflammation and prevention of CRS because GM-CSF has been shown to be a key CRS-promoting protein. The knockout of GM-CSF did not impact CAR T cell function and displayed promising antitumor activity in vivo, while minimizing risks of CRS and neuroinflammation [127].
6.4. Immunosuppressive Tumor Microenvironment
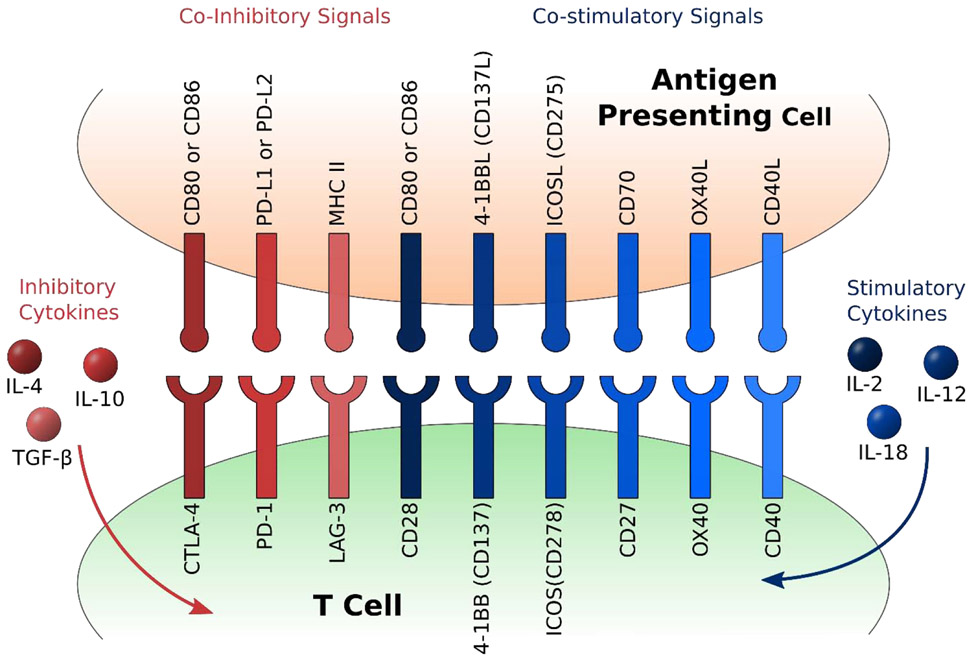
The tumor microenvironment produces an immunosuppressive environment that plays a significant role in directly downregulating T cells. The tumor microenvironment is extremely complex and composed of a diverse population of immunosuppressive cell types such as cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and many others. These cell types secrete soluble factors such as vascular endothelial growth factor (VEGF), TGF-β, IL-4, and IL-10. Furthermore, tumor cells will upregulate ligands such as PD-L1 as an immune checkpoint and other coinhibitory signals that further hinders the activation of T cells as shown in Figure 6. The progress and success of using genetically modified T cells as a therapeutic treatment hinges on the ability of these T cells to overcome the immunosuppressive properties of the tumor microenvironment and exhibit a clinically relevant response.
Figure 6.
Schematic of various costimulatory and coinhibitory signals for T cell activation and related pro-inflammatory and anti-inflammatory cytokines.
6.4.1. PD-1 Modulation Combination Therapy
The other major breakthrough in immunotherapy, checkpoint inhibitors, can be utilized as a combination therapy with adoptive cell therapy to assist in overcoming the immunosuppressive checkpoints. Many ongoing clinical trials exploring the combination therapy of checkpoint inhibitor antibodies and CAR T cells have yielded promising results [128,129]. However, the use of checkpoint inhibitor antibodies also exhibit some limitations including multiple administrations, systemic nontargeted therapy, and short half-life of the antibodies.
Other combination strategies between PD-1 and CAR T cells include the transduction of a dominant negative receptor for PD-1 consisting of only the extracellular domain into CAR T cells to outcompete endogenous PD-1 receptor binding for PD-L1/PD-L2 [130]. Another approach flips the regulatory property of PD-1 to actually provide costimulation on binding to PD-L1 by replacing the native intracellular signaling domain with the intracellular domains of CD28 [131]. Finally, Rafiq and coworkers have investigated a strategy that modifies CAR T cells to secrete PD-1-blocking scFv, which would allow local delivery of these scFv to alleviate the toxicities associated with systemic administration of immune checkpoint blockade, while enhancing the antitumor efficacy in vivo [132].
6.4.2. CRISPR Knockouts of Inhibitory Signal
With the development of genome editing technology, another combination therapy approach where T cells are genetically modified to induce sustained resistance to immune checkpoints has achieved widespread attention. Many studies have incorporated checkpoint inhibitor signaling through gene editing to improve the efficacy of genetically modified T cells. Rupp and coworkers developed a CRISPR/Cas9-based strategy to generate PD-1 deficient CAR T cells that augmented tumor cell killing by CAR T cells in vitro and displayed improved therapeutic efficacy for PD-L1+ tumor xenografts in vivo [133], Liu and coworkers generated triple knockout CAR T cells by targeting B2M, TRAC, and PD-1. These triple knockout CAR T cells released more IFN-γ and showed a higher efficiency of cytotoxicity to tumor cells compared to unmodified CAR T cells and B2M/TRAC double knockout CAR T cells [7]. Similarly, Ren and coworkers also demonstrated that the triple ablation of TCR, B2M, and PD-1 of CAR T cells enhanced the efficacy of allogenic CAR therapy in tumor models through a quicker and more robust antitumor response [53]. This PD-1 knockout genome-editing strategy is currently being investigated in many clinical trials (NCT03081715, NCT02867332, NCT02867345, and NCT02793856). Many other groups are investigating the effect of CRISPR/Cas9 disruption of other inhibitory receptors on CAR T cells for various cancer applications [134,135].
Outside of PD-1, there are also many other promising knockout targets that would help the genetically modified T cells overcome the immunosuppressive tumor microenvironment. Jung and coworkers chose to knockout diacylglycerol kinase (DGK) to increase CD3 signaling, which in turn increased TCR signaling for augmented effector functions. Furthermore, DGK knockout rendered the CAR T cells resistant to TGF-β and prostaglandin E2, both soluble immunosuppressive factors produced in the tumor microenvironment. The enhanced effector functions were evident both in vitro and in vivo by causing significant regression of glioblastoma tumors in a xenograft mouse model [136]. Another knockout target is the Fas ligand because Fas-FasL-dependent activation induced cell death (AICD) have been reported to attenuate CAR T cell activity. Ren and coworkers developed a one-shot CRISPR protocol to generate Fas-resistant universal CAR T cells through a triple knockout of endogenous TCR, HLA-I, and FAS. These triple gene disrupted CAR T cells exhibited elevated AICD resistance and prolonged survival in vitro and in vivo. Ren and coworkers also attempted a quadruple gene disruption to generate allogeneic universal CAR T cells deficient of both PD-1 and CTLA-4. These immune checkpoints can attenuate CAR T cell activation and accelerate T cell exhaustion and blocking them have shown promising clinical results. Despite the lower efficiency for the quadruple gene disrupted T cells, the potential can be seen for CRISPR/Cas9 multiplex genome-editing to advance immunotherapy and develop the next generation of genetically modified T cells [137].
6.4.3. Armored Engineered T Cells
Another general strategy to overcome the immunosuppressive microenvironment is to engineer “armored” CAR T cells, sometimes referred to as fourth generation CAR T cells, that are designed to secrete immune stimulatory cytokines or express ligands that improve CAR T cell efficacy. One promising pro-inflammatory cytokine candidate is interleukin-12 (IL-12) for its role in enhancing the cytotoxic activity of CD8+ T cells, recruiting macrophages, and reprogramming MDSCs. Yeku and coworkers have designed a CAR T cell that can constitutively secrete IL-12 to decrease apoptosis, enhance cytotoxicity, promote proliferation, and resist PD-L1 inhibition in vitro and in vivo. Furthermore, the constitutive secretion of IL-12 has also demonstrated the capability of remodeling the tumor microenvironment through increased levels of IFN-γ and TNF-α and decreased levels of TAMs [138]. Another pro-inflammatory cytokine that is being investigated is interleukin-18 (IL-18) for its role in stimulating IFN-γ secretion. CAR T cells that have been modified to secrete IL-18 exhibit enhanced expansion and significantly increased long-term survival in vivo. Similarly, IL-18 secretion can also remodel the tumor microenvironment by recruiting endogenous anti-tumor immune effector cells such as CD8 T cells, Ml macrophages, and DCs [139]. An alternative armored CAR T cell was developed by Kuhn and coworkers by constitutively expressing the CD40 ligand (CD40L), an immune-stimulatory molecule. CD40 is expressed on antigen-presenting cells and certain epithelial tumor cells, therefore, constitutive expression of CD40L not only is cytotoxic towards CD40-expressing tumor cells, but also leads to activation of dendritic cells to secrete pro-inflammatory cytokines and support CD4+/CD8+ T cells. This strategy sustains an endogenous immune response through recruitment of immune effectors, while maintaining superior antitumor efficacy [140].
6.5. Proliferation and Persistence
The ability for adoptive cell therapies to maintain proliferation and persistence has been one of the most important success markers for effective antitumor efficacy. In order for adoptive cell therapy to successfully eliminate the solid cancer tumor, the genetically modified T cells need to meet the required effector cell:target cell threshold ratio as well as persist for the necessary amount of time to eliminate a tumor.
6.5.1. Costimulatory Domains
Researchers quickly identified that the first generation of CAR T cells without any costimulatory domain expression exhibited poor proliferation and were unable to persist in the highly immunosuppressive tumor microenvironment [141]. Therefore, second and third generation CAR T cells were engineered to express one and two costimulatory signaling domains, respectively. The primary incorporated costimulatory domain is CD28 or 4-1BB, both well characterized costimulatory molecules expressed by T cells, to increase IL-2 secretion and promote T cell activation. Comparison studies have identified that CD28 CAR T cells elicit robust proliferative response and yield effector memory cells, whereas 4-1BB promoted central memory T cells to induce a progressive response and enhance long-term persistence [142].
Many other costimulatory domains besides CD28 and 4-1BB have been incorporated into CAR T cells to further enhance T cell proliferation and persistence as shown in Figure 6. Inducible T cell co-stimulator (ICOS) is another costimulatory domain that has been incorporated in CAR T cells and demonstrated superior T cell persistence compared to CD28. Furthermore, Guedan and coworkers engineered a third generation CAR T cell that incorporated both ICOS and 4-1BB costimulatory domains, resulting in superior antitumor effects and enhanced persistence in vivo. Interestingly, they also demonstrated that the order and location of the incorporated costimulatory intracellular domains had differential effects on the CAR T cell, where in this case, the optimal orientation is to position the ICOS intracellular signaling domain closer to the cell membrane than the 4-1BB intracellular signaling domain [143]. Other potential costimulatory domains that can incorporated in CAR T cells to promote proliferation and persistence include CD27 [144,145], OX40 [145,146]. and the Toll/IL-1 receptor domain of Toll-like receptor 2 [147].
Another recent novel strategy by Foster and coworkers demonstrated an activation of TLR and CD40 signaling in T cells using inducible MyD88/CD40 (iMC) to provide potent costimulation to CAR T cells. The transduced iMC signal induces a series of pro-survival cellular pathways and provides sufficient costimulatory signaling to drive T cell proliferation and promote antitumor efficacy [111,118,148].
6.5.2. Fratricidal Resistance
Another aspect to maintain proliferation and persistence of CAR T cell therapy is to prevent the selfkilling of CAR T cells through self-targeting. These fratricidal effects have been observed in CAR T cells that are transduced to target CD7 expressed by many T cell malignancies, but also expressed by T cells themselves. Cooper and coworkers developed “off-the-shelf’ CD7-targeting CAR T cells that were fratricide-resistant by using multiplex CRISPR/Cas9 gene editing to disrupt TRAC and the surface expression of CD7. This gene-editing strategy efficiently prevented fratricidal effects and potentiated antitumor efficacy in vitro and in vivo [149]. Similar fratricidal resistant effects through CRISPR/Cas9 gene editing were observed in CD5-targeting CAR T cells [150].
6.5.3. Cytokine Engagement
An alternative approach to improve T cell activation and proliferation involves the emphasis of cytokine engagement as a third signal outside of TCR engagement and costimulation. Kagoya and coworkers constructed a CAR transgene that induces cytokine signaling after antigen stimulation by encoding a truncated intracellular domain from the IL-2 receptor β-chain and a STAT3-binding (YXXQ) motif with the CD247 and CD28 domains. This newly modified CAR T cell will activate JAK kinase, STAT3, and STAT5 signaling pathways upon antigen recognition to promote proliferation and persistence for a superior antitumor effect in vitro and in vivo compared to CAR T cells without this third signal [151]. Further emphasis on the importance of cytokine engagement is demonstrated by the enhanced in vivo expansion and persistence of CAR T cells that have been modified to secrete IL-18 [139] or express intrinsic IL-15Rα signaling [152].
6.5.4. Modulation of T Cell Exhaustion
Another important aspect of maintaining T cell proliferation and persistence is the ability to avoid or rescue exhausted T cells. T cell exhaustion describes effector T cells that are unable to perform functional effector activities due to chronic antigen exposure and stimulation [153]. Some of the previously mentioned costimulatory strategies also help with T cell exhaustion. Comparison studies have shown that CD28 costimulation augments T cell exhaustion from persistent CAR signaling, whereas 4-1BB costimulation reduces T cell exhaustion, suggesting that 4-1BB costimulatory CAR T cells may be more appropriate for beneficial clinical performance [154,155]. In addition, CD40 signaling has also been shown to play a crucial role in rescuing T cells from exhaustion, further demonstrating the clinical potential of iMC costimulatory signaling [156]. A promising alternative approach by Eyquem and coworkers demonstrated that incorporation of the CAR gene directly into the TRAC locus using sequence-specific targeting techniques in CRISPR/Cas9 enabled transcriptional regulation of CAR similar to endogenous TCR. This endogenous regulation of CAR expression resulted in sustained T cell function and delayed T cell exhaustion [59].
Researchers have shown that PD-1 expression is upregulated during T cell exhaustion and has been identified as a marker for exhausted T cells. Many successful studies have shown that blockade of the PD-1 pathway with anti-PD-1 antibody checkpoint blockade treatment can reinvigorate exhausted T cells [130]. However, the genetic disruption of PD-1 did not eliminate the induction of T cell exhaustion indicating that PD-1 does not directly cause or induce T cell exhaustion [133,157]. Further studies will need to be performed to investigate the molecular mechanisms associated with PD-1 regulation of T cell exhaustion and the specific genetic modifications necessary to PD-1 to trigger T cell recovery.
7. Perspectives and Conclusion
In the last few years, we have seen extraordinary growth and expansion in cancer immunotherapy, particularly in the use of genetically engineered immune cells and their interface with fast developing genome editing techniques. The recent engineering capabilities associated with using T cells as a therapeutic for solid cancer tumors has demonstrated substantial progress in preclinical studies, but still faces many challenges that obstruct success in clinical trials. Major steps need to be taken to address the targeted delivery of T cells to the tumor site, minimizing toxicities and adverse side effects from nontumor-specific recognition, overcoming the immunosuppressive tumor microenvironment, and sustaining proliferation and persistence once the modified T cell is on site. Only the precise balance between efficacy and toxicity will prompt the emergence of adoptive cell therapy to become a consistent, safe, and effective platform for treating cancer.
The safety of adoptive cell therapy treatment methods for cancer should always be the first priority during evaluation. As discussed earlier in this review, many regulatory strategies to avoid toxicity are user-controlled and require the physician to intervene in some manner. It would be extremely powerful if we could engineer an autonomous feedback system into the genetically modified T cells to automatically respond to signs of toxicity. The feedback circuit could automatically prevent the activation of the therapeutic T cells in the case of unexpected toxicities and recover the therapeutic capabilities once the toxicity signs have subsided. This strategy would open up the freedom to not require user interference to ensure safety for a potentially longer-lasting therapeutic treatment.
The majority of the current research and aforementioned strategies have been applied to CAR T cell therapy, however, CAR T cells are limited to the recognition of surface antigens for activation. Research has shown that only 1% of total cellular proteins are expressed on the cell surface, thus severely limiting the potential antigens for CAR T cells to target. For this reason, many more studies could look at the modification strategies that help CAR T cells overcome the various challenges associated with adoptive cell therapy for cancer treatment, and apply them to TCR modified T cells with the ability to target a larger pool of peptides from cellular protein degradation. Furthermore, some groups have started to investigate the potential for hybrid receptors that utilize advantages from both CAR and TCR, while avoiding the limitations associated with each [158,159].
Cancer is an extremely complex and multifaceted problem that requires simultaneous and highly flexible solutions from multiple aspects for the effective treatment of cancer tumors that are highly heterogenic within the tumor microenvironment, variable between patients with the same cancer type, and unique for different cancer types. Fortunately, the advancement of immunotherapy coincides with the development of multiplex genome editing techniques and synthetic biology. These powerful genome editing tools and strategies such as CRISPR/Cas9 have driven the recent advances in adoptive cell therapy for improvements to the current ex vivo electroporation T cell modification strategy used in many clinical trials, which can be extremely costly, inconsistent, and can damage the T cell. Therefore, it is essential to explore alternative gene editing strategies that are more efficient and safely delivered using novel viral vectors or non-viral vectors to generate genetically modified T cells with both high quality and yield. Moreover, the recent developments for in vivo CRISPR/Cas delivery systems have provided an extremely promising alternative opportunity to genetically modify T cells for adoptive cell therapy in vivo and overcome many challenges associated with ex vivo engineering of autologous immune cells.
Although there has been significant progress over the last few years towards clinical translation of the CRISPR/Cas9 gene editing system, there are still many challenges that need to be addressed. A major shortcoming of the CRISPR/Cas9 system is the off-target activity of Cas9, arising largely in part from binding of the guide RNA to sequences of imperfect complementarity. One strategy to overcome the off-target activity of Cas9 is to modify the sgRNA used to target a gene of interest. For example, because Cas9 is able to tolerate up to 10 mismatches between the target sequence and sgRNA, truncation of the sgRNA at the 5’ end has been shown to increase the specificity of sgRNA without sacrificing on-target editing [160]. Furthermore, there is a need for analytical tools that can predict the target efficiency and specificity of gRNA’s. Another strategy is to optimize the specificity of Cas9 by using Cas9 variants that require higher specificity, including paired nickase and the Fok1 endonuclease’s catalytic domain fused to a catalytically deactivated Cas9 protein (dCas9) [161,162]. Additionally, the use of Cas9 orthologs as well as the use of Cas nucleases from other systems offer attractive, higher fidelity alternatives to the Cas9 nuclease [163].
Some other challenges associated with the therapeutic use of CRISPR/Cas9 include large genomic deletions and rearrangements at target sites, as well as limited homology directed repair and delivery efficiencies of gene editing components. One specific challenge in using CRISPR/Cas9 gene editing to engineer T cells is that CRISPR-mediated gene editing can induce apoptosis and delay growth through p53 function in the engineered T cells [164]. Thorough characterization of genetically modified cells and the development of new approaches that address these various challenges are required to assess the practicality of gene editing techniques within immunotherapy.
The recent growth in genetic engineering has spurred the development of off-the-shelf universal allogeneic T cells that are readily available for patient treatment to significantly increase the potential and availability of adoptive cell therapy. Future work should better utilize highly efficient approaches such as the 2-in-1 strategy to knockout TRAC and knock in a CAR gene at the same location to develop off-the-shelf CAR T cells [59]. Overall, the recent widespread popularity of immunotherapy, gene therapy, and the combination of the two for cancer treatment is justifiable, and rational design of genetically modified T cells has great potential to revolutionize the next generation of cancer treatment.
Acknowledgements
The authors gratefully recognize financial support from the UT-Portugal Collaborative Research Program (CoLAB), the Cockrell Family Chair Foundation, and the National Institutes of Health (NIH) (EB022025 to NAP). MM would like to acknowledge support by a National Science Foundation Graduate Research Fellowship under Grant No. 1610403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Van Der Burg SH, Arens R, Ossendorp F, Van Hall T, Melief CJM, Vaccines for established cancer: Overcoming the challenges posed by immune evasion, Nat. Rev. Cancer 16 (2016) 219–233. 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- [2].Guevara ML, Persano F, Persano S, Nano-immunotherapy: Overcoming tumour immune evasion, Semin. Cancer Biol (2020). 10.1016/j.semcancer.2019.11.010. [DOI] [PubMed] [Google Scholar]
- [3].Bates JP, Derakhshandeh R, Jones L, Webb TJ, Mechanisms of immune evasion in breast cancer, BMC Cancer. 18 (2018) 1–14. 10.1186/s12885-018-4441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kobayashi Y, Lim SO, Yamaguchi H, Oncogenic signaling pathways associated with immune evasion and resistance to immune checkpoint inhibitors in cancer, Semin. Cancer Biol (2020). 10.1016/j.semcancer.2019.11.011. [DOI] [PubMed] [Google Scholar]
- [5].Topalian SL, Drake CG, Pardoll DM, Immune checkpoint blockade: A common denominator approach to cancer therapy, Cancer Cell. 27 (2015) 450–461. 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lim WA, June CH, The Principles of Engineering Immune Cells to Treat Cancer, Cell. 168 (2017) 724–740. 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, Xia C, Wei X, Liu X, Wang H, CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells, Cell Res. 27 (2017) 154–157. 10.1038/cr.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kimberland ML, Hou W, Alfonso-Pecchio A, Wilson S, Rao Y, Zhang S, Lu Q, Strategies for controlling CRISPR/Cas9 off-target effects and biological variations in mammalian genome editing experiments, J. Biotechnol 284 (2018) 91–101. 10.1016/j.jbiotec.2018.08.007. [DOI] [PubMed] [Google Scholar]
- [9].Jindal V, Arora E, Gupta S, Challenges and prospects of chimeric antigen receptor T cell therapy in solid tumors, Med. Oncol 35 (2018) 87 10.1007/s12032-018-1149-9. [DOI] [PubMed] [Google Scholar]
- [10].Liu X, Zhao Y, CRISPR/Cas9 genome editing: Fueling the revolution in cancer immunotherapy, Curr. Res. Transl. Med 66 (2018) 39–42. 10.1016/j.retram.2018.04.003. [DOI] [PubMed] [Google Scholar]
- [11].Nilofer C, Arumugam M, Kangueane P, Modem Developments in Short Peptide Viral Vaccine Design, in: Glob. Virol. III Virol. 21st Century, Springer International Publishing, 2019: pp. 131–147. 10.1007/978-3-030-29022-1_7. [DOI] [Google Scholar]
- [12].Borne AE, Maleki Vareki S, T Lymphocyte–Based Cancer Immunotherapeutics, Int. Rev. Cell Mol. Biol 341 (2018) 201–276. 10.1016/bs.ircmb.2018.05.010. [DOI] [PubMed] [Google Scholar]
- [13].Madduri D, Dhodapkar ΜV, Lonial S, Jagannath S, Cho HJ, SOHO State of the Art Updates and Next Questions: T-Cell–Directed Immune Therapies for Multiple Myeloma: Chimeric Antigen Receptor–Modified T Cells and Bispecific T-Cell–Engaging Agents, Clin. Lymphoma, MyelomaLeuk 19 (2019) 537–544. 10.1016/j.clml.2019.08.002. [DOI] [PubMed] [Google Scholar]
- [14].Zhou X, Empowering chimeric antigen receptor T-cell therapy with CRISPR, Biotechniques. 68 (2020) 169–171. 10.2144/btn-2019-0107. [DOI] [PubMed] [Google Scholar]
- [15].Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L, Off-the-shelf allogeneic CART cells: development and challenges, Nat. Rev. Drug Discov 19 (2020) 185–199. 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- [16].Koo T, Lee J, Kim JS, Measuring and reducing off-target activities of programmable nucleases including CRISPR-Cas9, Mol. Cells 38 (2015) 475–481. 10.14348/molcells.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E, A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity, Science (80-.). 337 (2012) 816–821. 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cox DBT, Platt RJ, Zhang F, Therapeutic genome editing: Prospects and challenges, Nat. Med 21 (2015) 121–131. 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khan SH, Genome-Editing Technologies: Concept, Pros, and Cons ofVarious Genome-Editing Techniques and Bioethical Concerns for Clinical Application, Mol. Ther. - Nucleic Acids 16 (2019) 326–334. 10.1016/j.omtn.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lino CA, Harper JC, Carney JP, Timlin JA, Delivering crispr: A review of the challenges and approaches, Drug Deliv. 25 (2018) 1234–1257. 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bolotin A, Quinquis B, Sorokin A, Dusko Ehrlich S, Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin, Microbiology. 151 (2005) 2551–2561. 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- [22].Pourcel C, Salvignol G, Vergnaud G, CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies, Microbiology. 151 (2005) 653–663. 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- [23].Mojica FJM, Díez-Villasenor C, García-Martinez J, Soria E, Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements, J. Mol. Evol 60 (2005) 174–182. 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- [24].Doudna JA, Charpentier E, The new frontier of genome engineering with CRISPR-Cas9, Science (80-. ). 346 (2014) 1258096 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- [25].Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kühn R, Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells, Nat. Biotechnol 33 (2015) 543–548. 10.1038/nbt.3198. [DOI] [PubMed] [Google Scholar]
- [26].Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD, High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells, Nat. Biotechnol 31 (2013) 822–826. 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim JS, Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases, Genome Res. 24 (2014) 132–141. 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y, Shen S, Zhao G, Xu CF, Zhang HB, Luo YL, Cao ZT, Shi J, Bin Zhao Z, Lian ZX, Wang J, In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance, Biomaterials. 217 (2019) 119302 10.1016/j.biomaterials.2019.119302. [DOI] [PubMed] [Google Scholar]
- [29].Chakraborty S, Sequencing data from Massachusetts General Hospital shows Cas9 integration into the genome, highlighting a serious hazard in gene-editing therapeutics, F1000Research. 8 (2019) 1846 10.12688/f1000research.20744.1. [DOI] [Google Scholar]
- [30].Lenz P, Bacot SM, Frazier-Jessen MR, Feldman GM, Nucleoporation of dendritic cells: Efficient gene transfer by electroporation into human monocyte-derived dendritic cells, FEBS Lett. 538 (2003) 149–154. 10.1016/S0014-5793(03)00169-8. [DOI] [PubMed] [Google Scholar]
- [31].Hung KL, Meitlis I, Hale M, Chen CY, Singh S, Jackson SW, Miao CH, Khan IF, Rawlings DJ, James RG, Engineering Protein-Secreting Plasma Cells by Homology-Directed Repair in Primary Human B Cells, Mol. Ther 26 (2018) 456–467. 10.1016/j.ymthe.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kararoudi MN, Dolatshad H, Trikha P, Hussain SRA, Elmas E, Foltz JA, Moseman JE, Thakkar A, Nakkula RJ, Lamb M, Chakravarti N, McLaughlin KJ, Lee DA, Generation of knock-out primary and expanded human nk cells using cas9 ribonucleoproteins, J. Vis. Exp 2018 (2018) e58237 10.3791/58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li C, Guan X, Du T, Jin W, Wu B, Liu Y, Wang P, Hu B, Griffin GE, Shattock RJ, Hu Q, Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirusdelivered CRISPR/Cas9, J. Gen. Virol 96 (2015) 2381–2393. 10.1099/vir.0.000139. [DOI] [PubMed] [Google Scholar]
- [34].Rols MP, Parameters affecting cell viability following electroporation in vitro, in: Handb. Electroporation, Springer International Publishing, 2017: pp. 1449–1465. 10.1007/978-3-319-32886-7_149. [DOI] [Google Scholar]
- [35].Su S, Hu B, Shao J, Shen B, Du J, Du Y, Zhou J, Yu L, Zhang L, Chen F, Sha H, Cheng L, Meng F, Zou Z, Huang X, Liu B, CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients, Sci. Rep 6 (2016) 1–14. 10.1038/srep20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dai X, Park JJ, Du Y, Kim HR, Wang G, Errami Y, Chen S, One-step generation of modular CAR-T cells with AAV–Cpf1, Nat. Methods 16 (2019) 247–254. 10.1038/s41592-019-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pomeroy EJ, Hunzeker JT, Kluesner MG, Lahr WS, Smeester BA, Crosby MR, lin Lonetree C, Yamamoto K, Bendzick L, Miller JS, Geller MA, Walcheck B, Felices M, Webber BR, Starr TK, Moriarity BS, A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy, Mol. Ther 28 (2019) 52–63. 10.1016/j.ymthe.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, Marson A, Generation of knock-in primary human T cells using Cas9 ribonucleoproteins, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 10437–10442. 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hui SW, Overview of drug delivery and alternative methods to electroporation., Methods Mol. Biol 423 (2008) 91–107. 10.1007/978-1-59745-194-9_6. [DOI] [PubMed] [Google Scholar]
- [40].Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK, Efficient transfection method for primary cells, Tissue Eng. 8 (2002) 235–245. 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- [41].Seki A, Rutz S, Optimized RNP transfection for highly efficient CRI SPR/Cas9-mediated gene knockout in primary T cells, J. Exp. Med 215 (2018) 985–997. 10.1084/jem.20171626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, Zhu H, Siegwart DJ, Non-Viral CRISPR/Cas Gene Editing In Vitro and In Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 mRNA and sgRNA, Angew. Chemie - Int. Ed 56 (2017) 1059–1063. 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Timin AS, Muslimov AR, Lepik KV, Epifanovskaya OS, Shakirova AI, Mock U, Riecken K, Okilova ΜV, Sergeev VS, Afanasyev BV, Fehse B, Sukhorukov GB, Efficient gene editing via non-viral delivery of CRISPR–Cas9 system using polymeric and hybrid microcarriers, Nanomedicine Nanotechnology, Biol. Med 14 (2018) 97–108. 10.1016/j.nano.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [44].Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG, Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo, Nat. Biotechnol 34 (2016) 328–333. 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu CL, Ruan MZC, Mahajan VB, Tsang SH, Viral delivery systems for crispr, Viruses. 11 (2019). 10.3390/v11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tasca F, Wang Q, Gonsalves MAFV, Adenoviral Vectors Meet Gene Editing: A Rising Partnership for the Genomic Engineering of Human Stem Cells and Their Progeny, Cells. 9 (2020) 953 10.3390/cells9040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M, Viral vectors: a look back and ahead on gene transfer technology, 2013. www.wiley.co.uk/genmed/clinical (accessed May 30, 2020). [PubMed] [Google Scholar]
- [48].Cheng R, Peng J, Yan Y, Cao P, Wang J, Qiu C, Tang L, Liu D, Tang L, Jin J, Huang X, He F, Zhang P, Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9, FEBS Lett. 588 (2014) 3954–3958. 10.1016/j.febslet.2014.09.008. [DOI] [PubMed] [Google Scholar]
- [49].Li C, Guan X, Du T, Jin W, Wu B, Liu Y, Wang P, Hu B, Griffin GE, Shattock RJ, Hu Q, Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirusdelivered CRISPR/Cas9, J. Gen. Virol 96 (2015) 2381–2393. 10.1099/vir.0.000139. [DOI] [PubMed] [Google Scholar]
- [50].Colella P, Ronzitti G, Mingozzi F, Emerging Issues in AAV-Mediated In Vivo Gene Therapy, Mol. Ther. - Methods Clin. Dev 8 (2018) 87–104. 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pomeroy EJ, Hunzeker JT, Kluesner MG, Lahr WS, Smeester BA, Crosby MR, lin Lonetree C, Yamamoto K, Bendzick L, Miller JS, Geller MA, Walcheck B, Felices M, Webber BR, Starr TK, Moriarity BS, A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy, Mol. Ther 28 (2019) 52–63. 10.1016/j.ymthe.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dai X, Park JJ, Du Y, Kim HR, Wang G, Errami Y, Chen S, One-step generation of modular CAR-T cells with AAV–Cpfl, Nat. Methods 16 (2019) 247–254. 10.1038/s41592-019-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y, Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition, Clin. Cancer Res 23 (2017) 2255–2266. 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang C, Peng Y, Hublitz P, Zhang H, Dong T, Genetic abrogation of immune checkpoints in antigen-specific cytotoxic T-lymphocyte as a potential alternative to blockade immunotherapy, Sci. Rep 8 (2018) 1–13. 10.1038/s41598-018-23803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].LaFleur MW, Nguyen TH, Coxe MA, Yates KB, Trombley JD, Weiss SA, Brown FD, Gillis JE, Coxe DJ, Doench JG, Haining WN, Sharpe AH, A CRISPR-Cas9 delivery system for in vivo screening of genes in the immune system, Nat. Commun 10 (2019) 1–10. 10.1038/s41467-019-09656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu J, Zhou G, Zhang L, Zhao Q, Building potent chimeric antigen receptor T cells with CRISPR genome editing, Front. Immunol 10 (2019) 456 10.3389/fimmu.2019.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Roth TL, Puig-Saus C, Yu R, Shifrut E, Camevale J, Li PJ, Hiatt J, Saco J, Krystofinski P, Li H, Tobin V, Nguyen DN, Lee MR, Putnam AL, Ferris AL, Chen JW, Schickel JN, Pellerin L, Carmody D, Alkorta-Aranburu G, Del Gaudio D, Matsumoto H, Morell M, Mao Y, Cho M, Quadras RM, Gurumurthy CB, Smith B, Haugwitz M, Hughes SH, Weissman JS, Schumann K, Esensten JH, May AP, Ashworth A, Kupfer GM, Greeley SAW, Bacchetta R, Meffre E, Roncarolo MG, Romberg N, Herald KC, Ribas A, Leonetti MD, Marson A, Reprogramming human T cell function and specificity with non-viral genome targeting, Nature. 559 (2018) 405–409. 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].MacLeod DT, Antony J, Martin AJ, Moser RJ, Hekele A, Wetzel KJ, Brown AE, Triggiano MA, Hux JA, Pham CD, Bartsevich VV, Turner CA, Lape J, Kirkland S, Beard CW, Smith J, Hirsch ML, Nicholson MG, Jantz D, McCreedy B, Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells, Mol. Ther 25 (2017) 949–961. 10.1016/j.ymthe.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eyquem J, Mansilla-Soto J, Giavridis T, Van Der Stegen SJC, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M, Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection, Nature. 543 (2017) 113–117. 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Schober K, Müller TR, Gokmen F, Grassmann S, Effenberger M, Poltorak M, Stemberger C, Schumann K, Roth TL, Marson A, Busch DH, Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function, Nat. Biomed. Eng 3 (2019) 974–984. 10.1038/s41551-019-0409-0. [DOI] [PubMed] [Google Scholar]
- [61].Legut M, Dolton G, Mian AA, Ottmann OG, Sewell AK, CRISPR-mediated TCR replacement generates superior anticancer transgenic t cells, Blood. 131 (2018) 311–322. 10.1182/blood-2017-05-787598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM, Directed evolution of human T-cell receptors with picomolar affinities by phage display, Nat. Biotechnol 23 (2005) 349–354. 10.1038/nbtl070. [DOI] [PubMed] [Google Scholar]
- [63].Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, Williams D, Tayton-Martin HK, Ribeiro L, Holdich T, Yanovich S, Hardy N, Yared J, Kerr N, Philip S, Westphal S, Siegel DL, Levine BL, Jakobsen BK, Kalos M, June CH, NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma, Nat. Med 21 (2015) 914–921. 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK, Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells, Sci. Transl. Med 5 (2013) 197ra103–197ra103. 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bethune MT, Gee MH, Bunse M, Lee MS, Gschweng EH, Pagadala MS, Zhou J, Cheng D, Heath JR, Kohn DB, Kuhns MS, Uckert W, Baltimore D, Domain-swapped t cell receptors improve the safety of TCR gene therapy, Elife. 5 (2016). 10.7554/eLife.19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xue SA, Chen Y, Voss RH, Kisan V, Wang B, Chen KK, He FQ, Cheng XX, Scolamiero L, Holler A, Gao L, Morris E, Stauss HJ, Enhancing the expression and function of an EBV-TCR on engineered T cells by combining Sc-TCR design with CRISPR editing to prevent mispairing, Cell. Mol. Immunol (2020) 1–3. 10.1038/s41423-020-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Knies D, Klobuch S, Xue SA, Birtel M, Echchannaoui H, Yildiz O, Omokoko T, Guillaume P, Romero P, Stauss H, Sahin U, Herr W, Theobald M, Thomas S, Voss RH, An optimized single chain TCR scaffold relying on the assembly with the native CD3-complex prevents residual mispairing with endogenous TCRs in human T-cells, Oncotarget. 7 (2016) 21199–21221. 10.18632/oncotarget.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Govers C, Sebestyén Z, Roszik J, van Brakel M, Berrevoets C, Szöőr Á, Panoutsopoulou K, Broertjes M, Van T, Vereb G, Szollosi J, Debets R, TCRs Genetically Linked to CD28 and CD3ε Do Not Mispair with Endogenous TCR Chains and Mediate Enhanced T Cell Persistence and Anti-Melanoma Activity, J. Immunol 193 (2014) 5315–5326. 10.4049/jimmunol.1302074. [DOI] [PubMed] [Google Scholar]
- [69].Campillo-Davo D, Fujiki F, Van den Bergh JMJ, De Reu H, Smits ELJM, Goossens H, Sugiyama H, Lion E, Bememan ZN, Van Tendeloo V, Efficient and Non-genotoxic RNA-Based Engineering of Human T Cells Using Tumor-Specific T Cell Receptors With Minimal TCR Mispairing, Front. Immunol 9 (2018) 2503 10.3389/fimmu.2018.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Anderson KG, Stromnes IM, Greenberg PD, Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies, Cancer Cell. 31 (2017) 311–325. 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cantor JM, Homing Improvement: Boosting T Cell Trafficking for Cancer Immunotherapy, in: Springer, Cham, 2016: pp. 127–161. 10.1007/978-3-319-42223-7_6. [DOI] [Google Scholar]
- [72].Wang G, Chow RD, Bai Z, Zhu L, Errami Y, Dai X, Dong MB, Ye L, Zhang X, Renauer PA, Park JJ, Shen L, Ye H, Fuchs CS, Chen S, Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity, Nat. Immunol 20 (2019) 1494–1505. 10.1038/s41590-019-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Braun CJ, Bruno PM, Horlbeck MA, Gilbert LA, Weissman JS, Hemann MT, Zender L, Versatile in vivo regulation of tumor phenotypes by dCas9-mediated transcriptional perturbation, (n.d.). 10.1073/pnas.1600582113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS, Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation, Cell. 159 (2014) 647–661. 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu G, Rui W, Zheng H, Huang D, Yu F, Zhang Y, Dong J, Zhao X, Lin X, CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma, Eur. J. Immunol 50 (2020) 712–724. 10.1002/eji.201948457. [DOI] [PubMed] [Google Scholar]
- [76].Moritz R, Simon G, Stefan E, David A, Sebastian K, ITOC2 – 025. Transduction with C-Cchemokine receptor type 4 (CCR4) enhances tumour-specific migration of adoptively transferred T cells in a model of pancreatic cancer, Eur. J. Cancer 51 (2015) S9 10.1016/j.ejca.2015.01.039. [DOI] [Google Scholar]
- [77].Perera LP, Zhang M, Nakagawa M, Petrus MN, Maeda M, Kadin ME, Waldmann TA, Perera P-Y, Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies, Am. J. Hematol 92 (2017) 892–901. 10.1002/ajh.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Asai H, Fujiwara H, An J, Ochi T, Miyazaki Y, Nagai K, Okamoto S, Mineno J, Kuzushima K, Shiku H, Inoue H, Yasukawa M, Co-Introduced Functional CCR2 Potentiates In Vivo Anti-Lung Cancer Functionality Mediated by T Cells Double Gene-Modified to Express WT1-Specific T-Cell Receptor, PLoS One. 8 (2013). 10.1371/journal.pone.0056820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang L, Shahabuddin Hoseini S, Xu H, Ponomarev V, V Cheung N-K, Author C, Silencing Fc domains in T Cell-Engaging Bispecific Antibodies Improves T-Cell Trafficking and Antitumor Potency, (2019). 10.1158/2326-6066.CIR-19-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Newick K, O’brien S, Sun J, Kapoor V, Maceyko S, Lo A, Pur E, Moon E, Albelda SM, Augmentation of CAR T-cell Trafficking and Antitumor Efficacy by Blocking Protein Kinase A Localization, (2016). 10.1158/2326-6066.CIR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO, Ploegh H, Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 7624–7631. 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Whilding LM, Parente-Pereira AC, Zabinski T, Davies DM, Petrovic RMG, Kao YV, Saxena SA, Romain A, Costa-Guerra JA, Violette S, Itamochi H, Ghaem-Maghami S, Vallath S, Marshall JF, Maher J, Targeting of Aberrant αvβ6 Integrin Expression in Solid Tumors Using Chimeric Antigen Receptor-Engineered T Cells, Mol. Ther 25 (2017) 259–273. 10.1016/j.ymthe.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lo A, Wang LCS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C, Durham AC, Buza EL, Vonderheide RH, June CH, Albelda SM, Pure E, Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells, Cancer Res. 75 (2015) 2800–2810. 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo FC, Zhang J, Li D, Mo BH, Wen F, Liu T, Liu YT, Wang YS, Wei YQ, Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency, Gene Ther. 20 (2013) 970–978. 10.1038/gt.2013.19. [DOI] [PubMed] [Google Scholar]
- [85].Hajari Taheri F, Hassani M, Sharifzadeh Z, Behdani M, Arashkia A, Abolhassani M, T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy, IUBMB Life. 71 (2019) 1259–1267. 10.1002/iub.2019. [DOI] [PubMed] [Google Scholar]
- [86].Wernicke AG, Kim S, Liu H, Bander NH, Pirog EC, Prostate-specific Membrane Antigen (PSMA) Expression in the Neovasculature of Gynecologic Malignancies: Implications for PSMAtargeted Therapy, Appl. Immunohistochem. Mol. Morphol 25 (2017) 271–276. 10.1097/PAI.0000000000000297. [DOI] [PubMed] [Google Scholar]
- [87].Byrd TT, Fousek K, Pignata A, Szot C, Samaha H, Seaman S, Dobrolecki L, Salsman VS, Zami Oo H, Bielamowicz K, Landi D, Rainusso N, Hicks J, Powell S, Baker ML, Weis WS, Koch J, Sorensen PH, Deneen B, Ellis MJ, Lewis MT, Hegde M, Fletcher BS, St Croix B, Ahmed N, Translational Science TEM8/ANTXR1-Specific CAR T Cells as a Targeted Therapy for Triple-Negative Breast Cancer, (2018). 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, Sadelain M, Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape, Mol. Ther 26 (2018) 2542–2552. 10.1016/j.ymthe.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J, Fisher C, Lefkowitz RA, Panageas KS, Pulitzer M, Vignali M, Emerson R, Tipton C, Robins H, Merghoub T, Yuan J, Jungbluth A, Blando J, Sharma P, Rudensky AY, Wolchok JD, Allison JP, Robust Antitumor Responses Result from Local Chemotherapy and CTLA-4 Blockade, Cancer Immunol Res. 6 (2018). 10.1158/2326-6066.CIR-17-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Doth G, Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes, Nat. Med 21 (2015) 524–529. 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].chao Feng K, lei Guo Y, Liu Y, ren Dai H, Wang Y, yan Lv H, hua Huang J, ming Yang Q, dong Han W, Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma, J. Hematol. Oncol 10 (2017) 1–11. 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, Scholler J, Lacey SF, Melenhorst JJ, Morrissette JJD, Christian DA, Hunter CA, Kalos M, Porter DL, June CH, Grupp SA, Gill S, Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies, J. Clin. Invest 126 (2016) 3814–3826. 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC, Murray DC, Scurti GM, Li M, Thomas JT, Langerman A, Garrett-Mayer E, Zhang Y, Nishimura MI, TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors, Cancer Immunol. Immunother 65 (2016) 293–304. 10.1007/s00262-016-1800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukheqee M, Aware N, Wu M-F, Orange JS, Sumazin P, Man T-K, Joseph SK, Hegde M, Ahmed N, Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma, Neuro. Oncol 20 (2017) 506–518. 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KKH, Brawley VS, Byrd TT, Krebs S, Gottschalk S, Weis WS, Baker ML, Dotti G, Mamonkin M, Brenner MK, Orange JS, Ahmed N, Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape, J. Clin. Invest 126 (2016) 3036–3052. 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zah E, Lin MY, Anne SB, Jensen MC, Chen YY, T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells, Cancer Immunol. Res 4 (2016) 498–508. 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ, Chimeric Antigen Receptor T Cells with Dissociated Signaling Domains Exhibit Focused Antitumor Activity with Reduced Potential for Toxicity In Vivo, (2013). 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sukumaran S, Watanabe N, Bajgain P, Raja K, Mohammed S, Fisher WE, Brenner MK, Leen AM, Vera JF, Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment, (2018). 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA, Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors, Cell. 164 (2016) 780–791. 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, Walker WJ, McNally KA, Lim WA, Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors, Cell. 167 (2016) 419–432.e16. 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA, Precision Tumor Recognition by T Cells with Combinatorial Antigen-Sensing Circuits, Cell. 164 (2016) 770–779. 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fedorov VD, Themeli M, Sadelain M, PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses, Sci. Transl. Med 5 (2013) 215ra172–215ra172. 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Juillerat A, Marechal A, Filhol JM, Valogne Y, Valton J, Duclert A, Duchateau P, Poirot L, An oxygen sensitive self-decision making engineered CAR T-cell, Sci. Rep 7 (2017) 1–8. 10.1038/srep39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, Brenner MK, Fisher WE, Leen AM, Vera JF, Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer, Mol. Ther 25 (2017) 249–258. 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ravanpay AC, Gust J, Johnson AJ, Rolczynski LS, Cecchini M, Chang CA, Hoglund VJ, Mukheqee R, Vitanza NA, Orentas RJ, Jensen MC, EGFR806-CART cells selectively target a tumor-restricted EGFR epitope in glioblastoma, Oncotarget. 10 (2019) 7080–7095. 10.18632/oncotarget.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Caruso HG, V Hurton L, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, Lee DA, Heimberger AB, Champlin RE, Cooper LJN, Microenvironment and Immunology Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity, (2015). 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, Zhou L, Loew A, Young RM, June CH, Zhao Y, Therapeutics, Targets, and Chemical Biology Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice, Cancer Res. 75 (2015). 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, Myklebust JH, Kadapakkam M, Weber EW, Tousley AM, Richards RM, Heitzeneder S, Nguyen SM, Wiebking V, Theruvath J, Lynn RC, Xu P, Dunn AR, Vale RD, Mackall CL, Tuning the Antigen Density Requirement for CAR T-cell Activity, (2020). 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Minagawa K, Al-Obaidi M, Di Stasi A, Generation of suicide gene-modified chimeric antigen receptor-redirected T-cells for cancer immunotherapy, in: Methods Mol. Biol, Humana Press Inc, 2019: pp. 57–73. 10.1007/978-1-4939-8922-5_5. [DOI] [PubMed] [Google Scholar]
- [110].Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK, Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy A bs t r ac t, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Collinson-Pautz MR, Chang WC, Lu A, Khalil M, Crisostomo JW, Lin PY, Mahendravada A, Shinners NP, Brandt ME, Zhang M, Duong ML, Bayle JH, Slawin KM, Spencer DM, Foster AE, Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies, Leukemia. 33 (2019) 2195–2207. 10.1038/s41375-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Paszkiewicz PJ, FraBlc SP. Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, Busch DH, Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia, J. Clin. Invest 126 (2016) 4262–4272. 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K, Flutter B, Marin V, Marafioti T, Chakraverty R, Linch D, Quezada SA, Peggs KS, Pule M, A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy, Blood. 124 (2014) 1277–1287. 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- [114].Ma G, Shen J, Pinz K, Wada M, Park J, Kim S, Togano T, Tse W, Targeting T Cell Malignancies Using CD4CAR T-Cells and Implementing a Natural Safety Switch, Stem Cell Rev. Reports 15 (2019) 443–447. 10.1007/s12015-019-09876-5. [DOI] [PubMed] [Google Scholar]
- [115].Koristka S, Ziller-Walter P, Bergmann R, Amdt C, Feldmann A, Kegler A, Cartellieri M, Ehninger A, Ehninger G, Bomhauser M, Bachmann MP, Anti-CAR-engineered T cells for epitope-based elimination of autologous CAR T cells, Cancer Immunol. Immunother 68 (2019) 1401–1415. 10.1007/s00262-019-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wu C-Y, Roybal KT, Puchner EM, Onuffer J, Lim WA, Remote control of therapeutic T cells through a small molecule-gated chimeric receptor, Science (80-.). 350 (2015) aab4077–aab4077. 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Juillerat A, Marechal A, Filhol JM, Valton J, Duclert A, Poirot L, Duchateau P, Design of chimeric antigen receptors with integrated controllable transient functions, Sci. Rep 6 (2016) 1–7. 10.1038/srep18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Duong MLT, Collinson-Pautz MR, Morschl E, Lu A, Szymanski SP, Zhang M, Brandt ME, Chang WC, Sharp KL, Toler SM, Slawin KM, Foster AE, Spencer DM, Bayle JH, Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches, Mol. Ther. - Oncolytics 12 (2019) 124–137. 10.1016/j.omto.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wong NML, Wong WW, Engineering a Dual Small Molecule Gated ZAP70 Switch in T Cells, ACS Synth. Biol 7 (2018) 969–977. 10.1021/acssynbio.7b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek Μ, The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CART cells, Sci. Transl. Med 11 (2019). 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy ER, Schulman A, Du J, Wang F, Singer O, Ma J, Nunez V, Shen J, Woods AK, Wright TM, Schultz PG, Kim CH, Young TS, Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies, (n.d.). 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ma JSY, Kim JY, Kazane SA, Choi S-H, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, Fonslow BR, Kochenderfer JN, Wright TM, Schultz PG, Young TS,Kim CH, Cao Y, June CH, Shokat KM, Versatile strategy for controlling the specificity and activity of engineered T cells, (n.d.). 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lee YG, Chu H, Lu Y, Leamon CP, Srinivasarao M, Putt KS, Low PS, Regulation of CAR T cell-mediated cytokine release syndrome-like toxicity using low molecular weight adapters, Nat. Commun 10 (2019) 1–11. 10.1038/s41467-019-10565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lu YJ, Chu H, Wheeler LW, Nelson M, Westrick E, Matthaei JF, Cardie LI, Johnson A, Gustafson J, Parker N, Vetzel M, Xu LC, Wang EZ, Jensen MC, Klein PJ, Low PS, Leamon CP, Preclinical evaluation of bispecific adaptor molecule controlled folate receptor CAR-T cell therapy with special focus on pediatric malignancies, Front. Oncol 9 (2019). 10.3389/fonc.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lee YG, Marks I, Srinivasarao M, Kumar Kanduluru A, Mahalingam SM, Liu X, Chu H, Low PS, Tumor Biology and Immunology Use of a Single CAR T Cell and Several Bispecific Adapters Facilitates Eradication of Multiple Antigenically Different Solid Tumors, (2019). 10.1158/0008-5472.CAN-18-1834. [DOI] [PubMed] [Google Scholar]
- [126].Cho JH, Collins JJ, Wong WW, Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses, Cell. 173 (2018) 1426–1438.e11. 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, Schick KJ, Walters DK, Ahmed O, Chappell D, Sahmoud T, Durrant C, Nevala WK, Patnaik MM, Pease LR, Hedin KE, Kay NE, Johnson AJ, Kenderian SS, GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts, Blood. 133 (2019) 697–709. 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].CART-EGFRvIII + Pembrolizumab in GBM - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT03726515 (accessed May 29, 2020).
- [129].A Safety and Efficacy Trial of JCAR017 Combinations in Subjects With Relapsed/Refractory B-cell Malignancies (PLATFORM) - Full Text View - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT03310619 (accessed May 29, 2020).
- [130].Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS, Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumormediated inhibition, J. Clin. Invest 126 (2016) 3130–3144. 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Fiu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, Newick K, Fo A, June CH, Zhao Y, Moon EK, A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors, Cancer Res. 76 (2016) 1578–1590. 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Feeuwen DG, Drakes DJ, Song M, Miele MM, Fi Z, Wang P, Yan S, Xiang J, Ma X, Seshan VE, Hendrickson RC, Fiu C, Brentjens RJ, Targeted delivery of a PD-1-blocking scFV by CAR-T cells enhances anti-tumor efficacy in vivo, Nat. Biotechnol 36 (2018) 847–858. 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Fim WA, Marson A, CRISPR/Cas9-mediated PD-1 disruption enhances anti-Tumor efficacy of human chimeric antigen receptor T cells, Sci. Rep 7 (2017) 1–10. 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Su S, Zou Z, Chen F, Ding N, Du J, Shao J, Fi F, Fu Y, Hu B, Yang Y, Sha H, Meng F, Wei J, Huang X, Fiu B, CRISPR-cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer, Oncoimmunology. 6 (2017). 10.1080/2162402X.2016.1249558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zhang Y, Zhang X, Cheng C, Mu W, Fiu X, Fi N, Wei X, Fiu X, Xia C, Wang H, CRISPR-Cas9 mediated FAG-3 disruption in CAR-T cells, Front. Med 11 (2017) 554–562. 10.1007/s11684-017-0543-6. [DOI] [PubMed] [Google Scholar]
- [136].Jung IY, Kim YY, Yu HS, Fee M, Kim S, Fee J, CRISPR/Cas9-Mediated Knockout of DGK Improves Antitumor Activities of Human T Cells, Cancer Res. 78 (2018) 4692–4703. 10.1158/0008-5472.CAN-18-0030. [DOI] [PubMed] [Google Scholar]
- [137].Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y, A versatile system for rapid multiplex genome-edited CAR T cell generation, Oncotarget. 8 (2017) 17002–17011. 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yeku OO, Purdon TJ, Koneru M, Spriggs D, Brentjens RJ, Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment, Sci. Rep 7 (2017) 1–14. 10.1038/s41598-017-10940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Avanzi MP, Yeku O, Li X, Wijewamasuriya DP, van Leeuwen DG, Cheung K, Park H, Purdon TJ, Daniyan AF, Spitzer MH, Brentjens RJ, Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System, Cell Rep. 23 (2018) 2130–2141. 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kuhn NF, Purdon TJ, van Leeuwen DG, Lopez AV, Curran KJ, Daniyan AF, Brentjens RJ, CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response, Cancer Cell. 35 (2019) 473–488.e6. 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Thistlethwaite FC, Gilham DE, Guest RD, Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW, Sharma SK, Chester KA, Westwood NB, Halford SER, Nabarro S, Wan S, Austin E, Hawkins RE, The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioningdependent respiratory toxicity, Cancer Immunol. Immunother 66 (2017) 1425–1436. 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Patel PR, Guedan S, Scholler J, Keith B, Snyder N, Blair I, Milone MC, June CH, Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CART Cells, Immunity. 44 (2016) 380–390. 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- [143].Guedan S, Posey AD, Shaw C, Wing A, Da T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M, Song D, Melenhorst JJ, Lacey SF, Scholler J, Keith B, Young RM, June CH, Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation, JCI Insight. 3 (2018). 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ, CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo, Blood. 119 (2012) 696–706. 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- [145].Buchan SL, Rogel A, Al-Shamkhani A, The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy, Blood. 131 (2018) 39–48. 10.1182/blood-2017-07-741025. [DOI] [PubMed] [Google Scholar]
- [146].Guercio M, Orlando D, Di Cecca S, Sinibaldi M, Boffa I, Caruso S, Abbaszadeh Z, Camera A, Cembrola B, Bovetti K, Manni S, Caruana I, Ciccone R, Del Bufalo F, Merli P, Vinti L, Girardi K, Ruggeri A, De Stefanis C, Pezzullo M, Giorda E, Scarsella M, De Vito R, Barresi S, Ciolfi A, Tartaglia M, Moretta L, Locatelli F, Quintarelli C, De Angelis B, CD28.OX40 co-stimulatory combination is associated with long in vivo persistence and high activity of CAR.CD30 T-cells, Haematologica. (2020) haematol.2019.231183. 10.3324/haematol.2019.231183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Weng J, Lai P, Qin L, Lai Y, Jiang Z, Luo C, Huang X, Wu S, Shao D, Deng C, Huang L, Lu Z, Zhou M, Zeng L, Chen D, Wang Y, Chen X, Geng S, Robert W, Tang Z, He C, Li P, Du X, A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia, J. Hematol. Oncol 11 (2018) 1–12. 10.1186/s13045-018-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Foster AE, Mahendravada A, Shinners NP, Chang WC, Crisostomo J, Lu A, Khalil M, Morschl E, Shaw JL, Saha S, Duong MLT, Collinson-Pautz MR, Torres DL, Rodriguez T, Pentcheva-Hoang T, Bayle JH, Slawin KM, Spencer DM, Regulated Expansion and Survival of Chimeric Antigen Receptor-Modified T Cells Using Small Molecule-Dependent Inducible MyD88/CD40, Mol. Ther 25 (2017) 2176–2188. 10.1016/j.ymthe.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, Rettig MP, Wang B, Eissenberg LG, Ghobadi A, Gehrs LN, Prior JL, Achilefu S, Miller CA, Fronick CC, O’Neal J, Gao F, Weinstock DM, Gutierrez A, Fulton RS, DiPersio JF, An “off-the-shelf’ fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies, Leukemia. 32 (2018) 1970–1983. 10.1038/s41375-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Fleischer LC, Raikar SS, Moot R, Knight KA, Doering CB, Spencer HT, Engineering CD5-Targeted Chimeric Antigen Receptors and Edited T Cells for the Treatment of T-Cell Leukemia, Blood. 130 (2017) 1914–1914. 10.1182/BLOOD.V130.SUPPL_1.1914.1914. [DOI] [Google Scholar]
- [151].Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N, A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects, Nat. Med 24 (2018) 352–359. 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Nair S, Wang J-B, Tsao S-T, Liu Y, Zhu W, Slayton WB, Moreb JS, Dong L, Chang L-J, Functional Improvement of Chimeric Antigen Receptor Through Intrinsic Interleukin-15Rα Signaling, Curr. Gene Ther 19 (2019) 40–53. 10.2174/1566523218666181116093857. [DOI] [PubMed] [Google Scholar]
- [153].Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D, Defining ‘T cell exhaustion,’ Nat. Rev. Immunol 19 (2019) 665–674. 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ying Z, He T, Wang X, Zheng W, Lin N, Tu M, Xie Y, Ping L, Zhang C, Liu W, Deng L, Qi F, Ding Y, an Lu X, Song Y, Zhu J, Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin’s Lymphoma, Mol. Ther. - Oncolytics 15 (2019) 60–68. 10.1016/j.omto.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Long AH, Haso WM, Shem JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ, Mackall CL, 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors, Nat. Med 21 (2015) 581–590. 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Bhadra R, Gigley JP, Khan IA, Cutting Edge: CD40–CD40 Ligand Pathway Plays a Critical CD8-Intrinsic and -Extrinsic Role during Rescue of Exhausted CD8 T Cells, J. Immunol 187 (2011) 4421–4425. 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Odorizzi PM, Pauken KE, Paley MA, Sharpe A, John Wherry E, Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells, J. Exp. Med 212 (2015) 1125–1137. 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kisielow J, Obermair FJ, Kopf M, Deciphering CD4+ T cell specificity using novel MHC-TCR chimeric receptors, Nat. Immunol 20 (2019) 652–662. 10.1038/s41590-019-0335-z. [DOI] [PubMed] [Google Scholar]
- [159].Walseng E, Koksal H, Sektioglu IM, Fane A, Skorstad G, Kvalheim G, Gaudemack G, Inderberg EM, Walchli S, A TCR-based Chimeric Antigen Receptor, Sci. Rep 7 (2017) 1–10. 10.1038/s41598-017-11126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK, Improving CRISPR-Cas nuclease specificity using truncated guide RNAs, Nat. Biotechnol 32 (2014) 279–284. 10.1038/nbt.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wyvekens N, Topkar VV, Khayter C, Joung JK, Tsai SQ, Dimeric CRISPR RNA-Guided FokI-dCas9 Nucleases Directed by Truncated gRNAs for Highly Specific Genome Editing, Hum. Gene Ther 26 (2015) 425–431. 10.1089/hum.2015.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mali P, Aach J, Stranges PB, Esvelt KM, Moosbumer M, Kosuri S, Yang L, Church GM, CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering, Nat. Biotechnol 31 (2013) 833–838. 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Swarts DC, Jinek M, Cas9 versus Cas12a/Cpf1: Structure-function comparisons and implications for genome editing, Wiley Interdiscip. Rev. RNA 9 (2018) e1481 10.1002/wrna.1481. [DOI] [PubMed] [Google Scholar]
- [164].Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J, CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response, Nat. Med 24 (2018) 927–930. 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]