Abstract
BACKGROUND:
Functional brain connectivity is altered in children and adults with autism spectrum disorder (ASD). Functional disruption during infancy could provide earlier markers of ASD, thus providing a crucial opportunity to improve developmental outcomes. Using a whole-brain multivariate approach, here we asked whether electroencephalography (EEG) measures of neural connectivity at 3 months of age predict autism symptoms at 18 months.
METHODS:
Spontaneous EEG data were collected from 65 infants with and without familial risk for ASD at 3 months of age. Neural connectivity patterns were quantified using phase coherence in the alpha range (6–12Hz). Support vector regression (SVR) analysis was used to predict ASD symptoms at age 18 months, with ASD symptoms quantified by the Autism Diagnostic Observation Schedule-Toddler Module.
RESULTS:
ADOS scores predicted by SVR algorithms trained on 3-month EEG data correlated highly with ADOS scores measured at 18 months (r=0.76, p=0.02, root mean square error=2.38). Specifically, lower frontal connectivity and higher right temporo-parietal connectivity at 3 months predicted higher ASD symptoms at 18 months. The SVR model did not predict cognitive abilities at 18 months (r=0.15, p=0.36), suggesting specificity of these brain patterns to ASD.
CONCLUSIONS:
Using a data-driven, unbiased analytic approach, neural connectivity across frontal and temporo-parietal regions at 3 months predicted ASD symptoms at 18 months. Identifying early neural differences that precede an ASD diagnosis could promote closer monitoring of infants who show signs of neural risk and provide a crucial opportunity to mediate outcomes through early intervention.
Keywords: functional connectivity, autism spectrum disorder, infancy, prediction, electroencephalography, machine learning
Introduction
Autism is a disorder of early brain development that is diagnosed based on the presence of social-communication impairments and restricted, repetitive behaviors (1). The behavioral differences that define autism spectrum disorder (ASD) are typically identified after four years of age (2,3), preventing attempts to mediate outcomes before symptoms emerge. Mapping early markers of atypical brain development in ASD represents a promising opportunity to enhance objective and early detection. Identifying autism earlier in life would, in turn, allow interventions to target neurodevelopmental trajectories while they are most mutable, and before infant development is substantially impacted (4–7).
Neural differences in ASD affect how brain regions are structurally and functionally connected with one another (8–12). Social cognition and behavior depend on multiple distributed brain regions that interact in large-scale networks (13,14) by synchronizing their firing patterns (15–17). Microscopic neural changes in ASD are thought to disrupt the brain’s ability to generate and sustain coherent oscillatory activity, therefore impacting how information is communicated between neuronal populations. Evidence from postmortem studies supports the presence of fundamental network differences in ASD, demonstrated by changes in neuronal and axonal organization (18–22), myelination (23), and neurotransmitter receptor density (24,25). The large-scale oscillations that emerge from coherent neuronal activity can be measured non-invasively using EEG and fMRI. Direct (EEG) and indirect (fMRI) measures of oscillatory brain activity provide converging evidence that long-range functional connectivity is reduced across the lifespan in ASD (26–32).
Although the majority of connectivity differences in ASD have been studied after a diagnosis is made, emerging evidence suggests that they originate much earlier. The neuronal and synaptic building blocks that scaffold large-scale networks are established during very early brain development. Human neural stem cell models (33) and postmortem studies (34,35) demonstrate that the initial stages of neuronal maturation and organization are abnormal in ASD. ASD-associated genes are also found to converge upon molecular processes that govern neuronal differentiation and synaptic development (36,37). If these early network differences are present in infants who later go on to develop ASD, they could potentially be captured using measures of oscillatory brain activity.
Characterizing early functional connectivity patterns in vivo relies on prospective studies of infants who have a heightened risk of developing ASD. The younger siblings of children with ASD (familial-risk infants) have an ASD recurrence risk of nearly 20% (39) with another 30% showing other types of atypical development including the broader autism phenotype (38). Because these infants are identified based on family history, they can be studied from birth. MRI studies report early brain changes in familial-risk infants who later develop ASD. At 6 months of age, differences in structural brain development include atypical white matter integrity across distributed long-range tracts (40) and major tracts such as the corpus callosum (41,42). In the same sample of infants, fMRI patterns of atypical functional connectivity at 6 months are shown to predict later ASD diagnosis (43). However, as an indirect measure of neuronal activity, fMRI coactivation patterns cannot assay how specific neural communication mechanisms are altered. Direct measurement of patterns of functional connectivity using high temporal precision EEG can provide unique mechanistic insight, complementary to MRI techniques, into neural interactions in early infancy.
EEG is particularly well-suited to clinical screening, as it is portable, relatively low cost, and involves a lower testing burden than MRI (44). While EEG has been used to study early neural differences in ASD, there have been no multivariate studies that characterize cortex-wide functional connectivity patterns in infancy that are associated with ASD. Here we aim to address this gap, employing one type of multivariate pattern analysis method, support vector regression. Multivariate pattern analysis broadly refers to data analysis methods that analyze patterns of activity that are based on multiple input features. Multivariate approaches are sensitive to information that is provided by spatial distribution and therefore represents a powerful way to leverage the rich data that is provided by neural time series data.
This study maps functional connectivity patterns at 3 months of age that are associated with later ASD symptoms. Functional connectivity is quantified using the phase coherence of alpha oscillations (6–12Hz) (45,46), as alpha coherence is highly sensitive to early neural changes that occur in the context of both typical (47,48) and atypical brain development (49). Further, alpha oscillations are specifically associated with the structural (50,51) and functional (52) properties of long-range connections, and may therefore capture earlier markers of the long-range connectivity differences described in children and adults with ASD. Based on previous findings implicating distributed structural and functional connectivity disruptions infancy in ASD (41,42), we hypothesized that similar patterns of reduced long-range alpha coherence would predict a higher level of ASD symptoms at 18 months.
Methods and Materials
Sample
Participants in the present analyses were part of a larger ongoing study examining the development of infants with and without familial risk for ASD across the first 3 years of life. Exclusion criteria included evidence of a genetic condition or syndrome, gestational age<37 weeks, and prenatal/perinatal complications. Familial-risk infants (N=36) had at least one older sibling with a confirmed ASD diagnosis. Initial parent reports of sibling diagnoses were confirmed by a review of documented evidence. Low-risk infants (N=29) had no reported family history of ASD or other neurodevelopmental disorders within first degree relatives.
Infants were recruited from the community through the UCLA Center for Autism Research and Treatment (CART). Sixty-five infants completed an EEG recording session at 3 months and underwent behavioral assessment at 18 months. Demographic data describing the final 65 participants are presented in Table 1. The study received ethical approval from the relevant institutional review board, and parents provided informed written consent on behalf of all infants in accordance with the Declaration of Helsinki.
Table 1.
Demographic participant details grouped by familial risk status, and ASD symptoms at 18 months.
| Familial Risk (N=36) | Low Risk (N=29) | P Valuea | ASD+ (N=14) | ASD− (N=51) | P Valuea | |
|---|---|---|---|---|---|---|
| Sex | 13 f | 11 f | .54 | 2 | 22 | 0.60 |
| n female (% female) | (36.1%) | (37.9%) | (14.3%) | (43.1%) | ||
| Race n (%) | ||||||
| White | 21 (58.3%) | 18 (62.1%) | .21 | 4 (28.6%) | 35 (68.6%) | .01* |
| More than one race | 9 (25%) | 10 (34.5%) | 6 (42.9%) | 13 (25.5%) | ||
| Asian/Black/Pacific Islander | 6 (16.7%) | 1 (3.4%) | 4 (28.6%) | 3 (5.9%) | ||
| Ethnicity n (%) | ||||||
| Hispanic | 16 (44.4%) | 4 (13.8%) | .01* | 5 (35.7%) | 15 (29.4%) | .65 |
| Non-Hispanic | 20 (55.6%) | 25 (86.2%) | 9 (64.3%) | 36 (70.6%) | ||
| Maternal Education n (%) | ||||||
| Some college or less | 2 (6.9%) | 2 (6.9%) | .756 | 2 (14.3%) | 2 (3.9%) | .20 |
| College Degree and above | 28 (77.8%) | 24 (82.8%) | 9 (64.3%) | 43 (84.3%) | ||
| Unreported | 6 (16.7%) | 3 (10.3%) | 3 (21.4%) | 6 (11.8%) | ||
|
Precise 3 Month EEG age Mean (SD), Range |
3.18 (0.35), 2.57–3.90 | 3.17 (0.32), 2.63–4.13 | .87 | 3.05 (.23), 2.57–3.43 | 3.21 (.36), 2.63–4.13 | .117 |
|
18 Month MSEL Verbal T-score Mean (SD), Range |
43.39 (9.61), 21–63.5 | 49.07 (10.63), 25–66 | .030* | 31.75 (5.82), 21–40.50 | 49.95 (7.43), 35.5–66 | <.001* |
|
18 Month MSEL Non-verbal T-score. Mean (SD), Range |
46.07 (7.51), 24.5–64 | 51.54 (9.08), 25–66 | .011* | 40.96 (9.70), 24.5–59.5 | 50.65 (7.02), 34–66 | <.001* |
|
18 Month ADOS-T Total Score Mean (SD), Range |
7.17 (5.12), 1–18 | 5.21 (4.44), 0–18 | .11 | 14.28 (2.67), 10–18 | 4.09 (2.46), 0–9 | <.001* |
|
18Month ADOS-T Total Score >10 n >10 (%>10) |
11 (30.6%) | 3 (10.3%) | .07 | |||
Group differences assessed using independent samples t-tests or chi-square analyses.
ASD Assessment
A trained clinician administered the Toddler Module of the Autism Diagnostic Observation Schedule-Second Edition (ADOS-T) at 18 months (53,54). The ADOS-T is an assessment tool used by clinicians and researchers to assess social-communication and repetitive behaviors associated with ASD (53) in children under 30 months of age (54). ASD symptoms were quantified using dimensional ADOS-T algorithm scores (total score ranging from 0–18). ADOS-T scores ≥ 10 indicate a clinically relevant level of symptoms at 18 months, and are highly indicative of ASD symptoms at later ages (measured using the ADOS) (55). Sample descriptions are provided according to both familial-risk status groupings and ADOS-T cut-off (ASD+ (ADOS-T ≥ 10) & ASD – (ADOS-T<10)) groupings. Of the infants considered ASD+ at 18 months, 11 had familial risk for ASD, and 3 were at low risk.
Cognitive Assessment
Cognitive function was also assessed, in order to distinguish EEG patterns associated with ASD symptoms, rather than general developmental level. The Mullen Scales of Early Learning (MSEL) (56) were administered by trained clinicians at 18 months. The MSEL is a standardized measure of developmental abilities that yields scores five subscales (visual reception, fine motor skills, gross motor skills, receptive language, and expressive language). Subscale t-scores (M=50, SD=10) were used in analyses. Receptive language and expressive language subscale t-scores were averaged to calculate verbal cognition, and the t-scores from the visual reception and fine motor subscales were averaged to calculate non-verbal cognition.
EEG Acquisition
Spontaneous EEG data were recorded using a 129-channel Hydrocel (Electrical Geodesic Net Inc., Eugene, OR), in a dimly lit, sound-attenuated room. EEG was sampled at 500 Hz and referenced to vertex (Cz) at the time of recording. Four electrodes positioned to record electrooculogram (EOG) (located below and lateral to the eyes) were removed from the net to increase comfort for infants. Net Station 4.4.5 software was used to record from a Net Amps 300 amplifier with a low-pass analog filter cutoff frequency of 6 KHz. Data were sampled at 500 Hz and referenced to vertex (Cz) at the time of recording. Electrode impedances were kept below 100 KΩ. Infants were held in a caregiver’s lap throughout the recording while bubbles were blown by an unseen experimenter, consistent with widely used spontaneous recording conditions in infant populations (57). EEG data were acquired for at least 3 minutes, with the recording session extended up to 5 minutes if the infant remained calm.
EEG Processing
All offline data processing and analyses were performed using EEGLAB (58) and in-house MATLAB scripts. The experimenter was blind to participant details (including risk status) throughout the data cleaning process. Data were high pass filtered to remove frequencies below 1 Hz and low pass filtered to remove frequencies above 90Hz, using a finite impulse response filter. Continuous data were then visually inspected, and any sections including excessive electromyogram or other non-stereotyped artifacts were removed. Artifact subspace reconstruction (ASR), a data cleaning method that uses sliding window principal component analysis, was then used to remove high amplitude artifacts, relative to artifact-free reference data (59,60). ASR is especially useful for retaining maximum data in infants (where the length of EEG recordings is limited), as it allows artifacts to be removed while retaining the co-occurring EEG activity that is ‘clean’. The eeglab function clean_RawData was used to implement ASR, with default parameters and rejection threshold k=8 (59).
Following interpolation to the international 10–20 system 25 channel montage (61), independent component analysis (ICA) was used to decompose data into maximally independent components (IC) (62), and the power spectral distribution (PSD), scalp topography and time course of each IC were visually examined. IC’s that represented non-neural activity (including EMG, EOG, heart artifact and line noise) were removed from the data.
ASR cleaning resulted in an average of 17 channels being removed, and 17.8% of data points being altered. The number of channels removed (P=.438), and data points changed (P=.398), did not vary between ASD outcome groups. The independent components removed during the second stage of cleaning averagely accounted for 24.7% of the EEG variance and did not vary between ASD outcome groups (P=.152). There was no difference between groups in the length of the EEG recording post-cleaning (M=139.54, P=.464).
Alpha Phase Coherence
Cleaned data were transformed to current source density (CSD) estimates, in order to mitigate the effects of volume conduction (46,63). Spherical spline Laplacian transforms were conducted using realistic head geometry, with head radius set at 7cm (representing the average head radius of 3-month-old infants), and flexibility constant m = 3. CSD data were separated into 3-second epochs to obtain coherence metrics. To retain consistent data length across all participants, the first 75 seconds of data were used in all further analyses (representing the minimum data length available across the sample). The newcrossf function provided by eeglab (58) was used to compute phase coherence (ERPCOH) from the aforementioned resting state epochs (for each frequency bin):
where represents the spectral estimate of channel a in epoch k at frequency f and time t. is the complex conjugate of (58). For each channel pair, ERPCOH was averaged across all frequency bins encompassed by alpha band (6–12Hz), resulting in 300 values that represented alpha phase coherence between every possible electrode pair.
Model Fitting
Prediction models were used to assess the relationship between 3-month coherence and 18-month ASD symptoms (ADOS-T total score), with all 300 alpha phase coherence values serving as the initial feature set. A “nested” leave-one-out cross validation (LOOCV) procedure was used to predict the ADOS score of each participant. A nested procedure includes an outer loop that is used to predict N=1, with the remaining N=64 being entered into an inner loop where predictive features are tuned using LOOCV (see Figure 1). Selecting features for each fold with the data of the test subject remaining entirely unseen ensured that feature model performance was not falsely inflated through circularity bias (64).
Figure 1.

A schematic representation of the machine learning approach used to predict ASD symptoms at 18 months.
Model Fitting: Feature Selection
A LOOCV regularized regression approach with an elastic net penalty was used to select a subset of functional connections within each fold. Elastic net regularization is a hybrid approach combining both the ℓ1 penalty of lasso, and the ℓ2 penalty of ridge regression (65,66), and it is well suited to remove redundant variables and prevent model overfitting for high dimensional data (67). There are two parameters that impact penalized regression, α and λ. α regulates the degree of mixing between ℓ1 and ℓ2 penalties, and effectively determines the compromise between lasso (least absolute shrinkage and selection operator) and ridge regression techniques. Here we implemented α=0.5 to represent an equal balance between ℓ1 and ℓ2 penalties. λ is the penalty term and defines the strength of regularization. A geometric sequence of λ values were trialed to determine the λ value that minimized model deviance (mean squared error; MSE), with the final values across all folds averaged to provide a consistent value (λ=1). The lasso function in MATLAB was used to implement the regression procedure, and all predictor variables were centered and standardized.
Model Fitting: Support Vector Regression
After conducting feature selection within each inner fold, linear-kernel support vector regression (SVR) models were trained using the default parameters of the fitrsvm function in MATLAB. In addition to the advantages of binary classification offered by traditional SVM, support vector machines for regression (SVR) offer an opportunity to assess the value of functional connections for predicting ASD behaviors dimensionally (68). The resulting model was used to estimate the ADOS-T score of the N=1 participant who was left out of the outer loop (validation sample). The procedure was then repeated N=65 times.
Predictive capabilities were examined through the relationship between observed and predicted ADOS-T score. The statistical significance of all LOOCV results was determined using a permutation testing approach (69,70). The null distribution of R2 was estimated by repeating the entire model fitting procedure (including feature selection within each fold) using 1000 surrogate datasets that were generated under the null hypothesis that there is no relation between 3-month EEG and 18-month ADOS. The final statistical significance of the model was determined by calculating the percentage of null-models that yielded symptom estimates better than the final model. The reported permutation p values therefore represent the probability of observing the reported R2 values by chance.
Predictive Model Features
A major benefit of multivariate pattern analysis is the ability to examine the features that drive the predictive capability of the SVR algorithm. We analyzed the final consensus feature set that consisted of 22 functional connections that had non-zero coefficients in 100% of folds (69,71), extracting the weight value assigned to each feature. Interpreting the weights from linear models in terms of neural activity patterns can be misleading (72,73). To allow neurophysiological interpretation of individual features in the model, SVR weights were transformed into activation patterns using the method described by Haufe and colleagues (73). Specifically, the activations are derived by,
where Σx denotes the covariance of the data, W represents the regression weights, and Σs−1 is the inverse covariance of the latent factor.
Results
Model Performance
Alpha phase coherence at 3 months predicted ADOS-T scores. Specifically, the SVR model estimated ADOS-T total scores that significantly correlated with actual ADOS-T scores measured at 18 months (Pearson’s r = 0.76; R2 = 0.58; p = 0.02; see Figure 2). Reported significance values were corrected to represent permutation testing (described in methods section). The average root mean square error across the sample was 2.38 (SD=2.08). Independent t-tests indicated that prediction errors did not vary according to familial-risk group (p = 0.20), ADOS outcome group (p=.19), or sex (p = 0.16).
Figure 2.

Correlation between actual ADOS-score (X axis), and the predicted ADOS score (Y axis) for each participant, with 95% confidence intervals.
To determine its specificity, we assessed the ability of the model to estimate cognitive function. Trained on the same input features, the SVR model was unable to predict verbal and non-verbal cognitive scores at 18 months. While there was a stronger relationship with verbal cognitive abilities (Pearson’s r =0.31, p =0.01, corrected p =0.91) than non-verbal cognitive abilities (Pearson’s r = 0.15; p =0.36, corrected p=0.99), neither of these relationships were significant.
Feature Activations
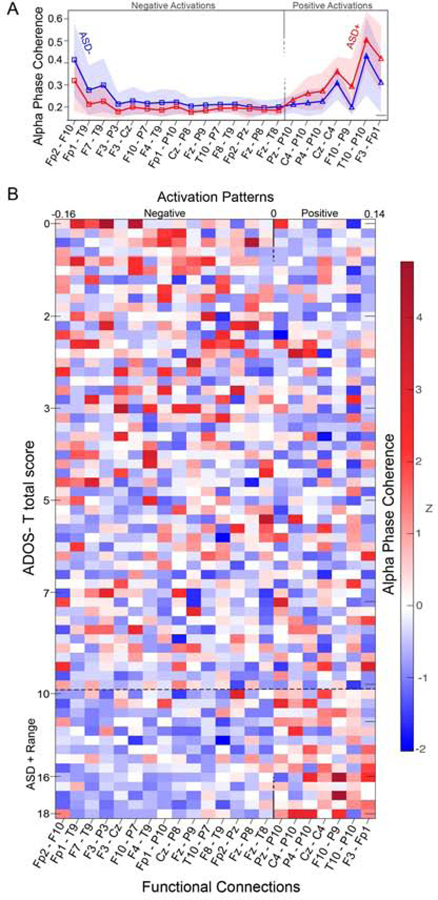
As described above, the contribution of individual functional connections to the SVR model was quantified using activation patterns, which are defined as transformed SVR weights that allow neurophysiological interpretation (but do not represent activation patterns as conventionally described in MRI work). Functional connections that contributed to the SVR model represented a mix of positive and negative features (See Figure 3 & 4).
Figure 3.
Mean feature activations for each of the 22 predictive function connections that defined the consensus feature set. Red lines represent a positive activation value (higher alpha phase coherence = higher ADOS-T score), and blue lines represent a negative activation value (lower alpha phase coherence = higher ADOS-T score). Wider lines indicating a larger contribution to the model (greater absolute activation strength). Graphical representations indicate the location of each measurement channel. White-gray shading of electrode labels indicates the anterior (white) – posterior (gray) location of each channel.
Figure 4.
(A) Mean alpha phase coherence for each of the 22 predictive function connections that defined the consensus feature set for ASD+ (red) and ASD- (blue) groups. Shaded regions represent SD. (B) Individual alpha phase coherence values (z scores) for each participant (arranged from low to high ADOS score) for each predictive function connection (with activation patterns arranged from negative to positive).
Discussion
The present study characterizes functional connectivity patterns during early infancy that predict individual differences in later ASD symptoms. Early connectivity differences that predicted ASD symptoms were multivariate, highlighting the importance of studying patterns of activity rather than specific functional connections. The regional distribution of predictive connections shows that decreased connectivity across frontal connections and increased connectivity across temporo-parietal areas are associated with a higher level of ASD symptoms at 18 months. Due to the limited spatial resolution of EEG, the precise cerebral structures driving these results cannot be determined. However, guided by an infant EEG-MRI localization study, we can consider general structures that underlie electrode locations (75).
Decreased frontal alpha phase coherence
Decreased alpha phase coherence across fronto-frontal, fronto-temporal and fronto-parietal connections predicted higher ASD symptoms. Early disruptions in frontal connectivity are particularly relevant, given the extensive previous literature that implicates frontal neuropathology in ASD. At a cellular level, postmortem studies show disruptions in neuronal (22,34,76), axonal (23), laminar (35), and minicolumn (18,77) organization in the frontal cortex of individuals with ASD. Differences in large scale frontal connectivity (often fronto-posterior hypoconnectivity) are also highly supported by EEG and fMRI studies of children and adults with ASD (30,78–83). We extend these findings to show that frontal disruptions occur prior to behavioral symptoms, suggesting that they represent core pathophysiology of the disorder, and not simply a consequence of ASD symptoms.
Frontal cortex may be particularly vulnerable to connectivity disruptions in ASD for several reasons, especially given its protracted development (84). For instance, ASD-associated risk genes are shown to converge upon co-expression networks in frontal cortex during fetal brain development (36). By disrupting key neurobiological processes (such as neuronal migration, synaptogenesis, and myelination) in frontal cortex, ASD-risk genes may particularly impact frontal functional connectivity (85). Further evidence linking ASD-risk genes to specific frontal disruptions comes from copy number variations and single gene disorders that confer susceptibility for ASD and are also associated with decreased fronto-temporal and fronto-parietal connectivity (86–91). The present data suggest that, in addition to the changes seen in syndromic ASD (91), early frontal dysconnectivity arising from familial risk may similarly predispose infants to the emergence of later ASD symptoms.
Increased temporo-parietal alpha phase coherence
Positively weighted predictors describe connections for which increased coherence is associated with higher levels of ASD symptoms at 18 months. These connections mainly bridged temporal and parietal areas in the right hemisphere and were localized above brain structures that subserve social information processing (13), including the superior temporal sulcus, as well as postcentral, supramarginal, temporal and angular gyri (75). These results implicate the right temporoparietal junction (rTPJ) (92), a social hub (93) that coordinates social information processing (94,95), and shows atypical function in ASD (96). Alpha phase coherence differences in these regions may reflect the network inefficiencies (40) and structural differences in temporal and parietal white matter tracts that have been identified at 6 months of age in ASD (41), especially given that white matter integrity is associated with alpha phase coherence (97).
In addition to revealing early connectivity differences during infancy, increased alpha phase coherence in temporal parietal areas may shed mechanistic insight into reports of hypoconnectivity that are observed following infancy in ASD. The deleterious effects of increased regional connectivity are well-described in neurocognitive disorders, where periods of increased connectivity are shown to precede decreased connectivity, a pathological process described as hub-overload (98). Increased alpha phase coherence may lead to hub-overload in ASD (99), and could underlie the transition from over- to under-connectivity that is seen in both alpha phase coherence and white matter integrity from around 2 years of age in ASD (41) (27), as well as widely described reductions in rTPJ activation and connectivity (31,96,100–106).
Scalability
EEG measures of early brain network function can serve as scalable and clinically actionable predictors of ASD in early infancy, at a time when behavioral signs of atypical development remain unclear. The portability, relatively low cost and low testing burden of EEG renders it practical for community screening in large populations (44). To translate laboratory-based EEG studies to community settings, a neural signal of interest must be accurately measured under task-free conditions in less controlled environments. Alpha phase coherence, in particular, represents a highly scalable metric. Alpha oscillations are dominant in spontaneous brain activity and are less susceptible to biological and environmental artifacts (107,108), thus facilitating its measurement in larger, clinical or community samples (109).
Early Identification & Intervention
Behavioral features that can consistently predict later ASD diagnosis have not been identified in the first year of life, and predominantly emerge after 12 months of age (110–115). Although EEG is not intended to replace behavioral assessment of ASD, EEG markers are uniquely positioned to elucidate individual differences that confer neural risk for ASD. By examining dimensional risk (rather that binary diagnostic labels), the present study highlights that early network disruptions in ASD occur along a continuum. This approach will facilitate the identification of neural risk associated with milder/borderline ASD symptoms, a clinical group that elude early behavioral identification (3), but may be particularly responsive to prompt intervention (116,117).
Early disruptions in brain activity may also impact how an infant responds to their environment, causing a cascading brain-behavior-environment interaction (76,118–122) that will further impact brain development (123,124). Identifying individuals using objective EEG markers will facilitate a shift from reactionary interventions that focus on modifying established behaviors, towards preemptive interventions that may mitigate the effects of early disruptions (125,126).
Strengths, Limitations & Future Directions
The present study leveraged the benefits of machine learning to model multivariate data. However, in order to retain interpretable links between neurobiology and behavior (127,128), we employed a hypothesis-driven modelling approach that reflects our prioritization of interpretability over prediction. For instance, although the inclusion of additional EEG features may capture interactions leading to better model prediction, by focusing on one neurobiologically- and clinically-relevant EEG metric (alpha phase coherence), we retain the ability to map predictive model features back on to EEG data (129). These links were also preserved through the use of linear modelling, as well as forward modelling transformations (73,130). These steps allow us to understand very early brain differences that precede ASD, and ultimately optimize the translatable clinical utility of machine learning methods in ASD.
As with many prior EEG studies of familial-risk infants, a relatively small sample size and lack of independent validation limits the generalizability of this study. To determine if alpha phase coherence patterns can provide a clinically applicable biological marker of risk, we need studies in diverse participant samples representing wider etiological factors beyond familial risk, such as infants with known genetic syndromes or preterm infants, as well as a community screened cohort. Although the patterns of functional connectivity described here were not associated with later verbal cognition, it may be the case that they are predictors of general language delays that are not specific to ASD. In order to ascertain the specificity of the present findings to ASD, future studies will examine social communication impairments and restricted repetitive behaviors using separate assessments. Disentangling ASD symptom domains will elucidate whether the patterns described here are equally predictive of restricted and repetitive behaviors and social communication impairments.
The present study also focused on one measurement technique. Since EEG and fMRI provide complementary information about brain function (131), a recently initiated study by our group integrates both methods to examine how the timing of structural and functional brain changes are related to one another during the first year of life in ASD. Finally, longitudinal monitoring of behavior, environment, and brain development will broaden our understanding of dynamic early changes in ASD and inform decisions around the exact timing and targets of preventative interventions to ultimately improve developmental outcomes.
Acknowledgements
The authors wish to thank all of the infants and families who generously participated in this study. The present research was supported by the National Institute of Child Health and Human Development (2P50HD055784–08), and the UCLA Brain Research Institute (Postdoctoral Award to A.D). The authors are also grateful to Rujuta B. Wilson for her helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
S.S.J. serves as a consultant for Roche Pharmaceuticals. The remaining authors (A.D, M.D, A.M, B.G, M.D, & N.M.M) report no biomedical financial interests or potential conflicts of interest.
References
- 1.Association AP (2013): Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 2.Hall-Lande J, Esler AN, Hewitt A, Gunty AL (2018): Age of Initial Identification of Autism Spectrum Disorder in a Diverse Urban Sample. J Autism Dev Disord. 10.1007/s10803-018-3763-y [DOI] [PubMed]
- 3.Sheldrick RC, Maye MP, Carter AS (2017): Age at First Identification of Autism Spectrum Disorder: An Analysis of Two US Surveys. J Am Acad Child Adolesc Psychiatry 56: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb SJ, Jones EJH, Kelly J, Dawson G (2014): The motivation for very early intervention for infants at high risk for autism spectrum disorders. International journal of speech-language pathology 16: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yirmiya N, Charman T (2010): The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics: Autism prodrome. Journal of Child Psychology and Psychiatry 51: 432–458. [DOI] [PubMed] [Google Scholar]
- 6.Shattuck PT, Grosse SD (2007): Issues related to the diagnosis and treatment of autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews 13: 129–135. [DOI] [PubMed] [Google Scholar]
- 7.Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, et al. (2015): Early Intervention for Children With Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research. Pediatrics 136: S60–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ (2004): Autism and Abnormal Development of Brain Connectivity. Journal of Neuroscience 24: 9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgeron T (2015): From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nature Reviews Neuroscience 16: 551–563. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind DH, Levitt P (2007): Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology 17: 103–111. [DOI] [PubMed] [Google Scholar]
- 11.Parikshak NN, Gandal MJ, Geschwind DH (2015): Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nature Reviews Genetics 16: 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Port RG, Gandal MJ, Roberts TPL, Siegel SJ, Carlson GC (2014): Convergence of circuit dysfunction in ASD: a common bridge between diverse genetic and environmental risk factors and common clinical electrophysiology. Frontiers in cellular neuroscience 8: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolphs R (2009): The Social Brain: Neural Basis of Social Knowledge. Annu Rev Psychol 60: 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossmann T, Johnson MH (2007): The development of the social brain in human infancy. European Journal of Neuroscience 25: 909–919. [DOI] [PubMed] [Google Scholar]
- 15.Buzsáki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- 16.Fries P (2005): A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences. Retrieved from http://www.sciencedirect.com/science/article/pii/S1364661305002421 [DOI] [PubMed]
- 17.Varela F, Lachaux J-P, Rodriguez E, Martinerie J (2001): The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience 2: 229–239. [DOI] [PubMed] [Google Scholar]
- 18.Casanova MF, Van Kooten I, Switala AE, Van Engeland H, Heinsen H, Steinbusch HWM, et al. (2006): Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clinical Neuroscience Research 6: 127–133. [Google Scholar]
- 19.Casanova MF (2006): Neuropathological and genetic findings in autism: the significance of a putative minicolumnopathy. Neuroscientist 12: 435–441. [DOI] [PubMed] [Google Scholar]
- 20.Casanova MF, van Kooten IAJ, Switala AE, van Engeland H, Heinsen H, Steinbusch HWM, et al. (2006): Minicolumnar abnormalities in autism. Acta Neuropathologica 112: 287–303. [DOI] [PubMed] [Google Scholar]
- 21.McKavanagh R, Buckley E, Chance SA (2015): Wider minicolumns in autism: A neural basis for altered processing? Brain 138: 2034–2045. [DOI] [PubMed] [Google Scholar]
- 22.Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP (2012): Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Research 1456: 72–81. [DOI] [PubMed] [Google Scholar]
- 23.Zikopoulos B, Barbas H (2010): Changes in prefrontal axons may disrupt the network in autism. J Neurosci 30: 14595–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD (2009): Expression of GABAB Receptors Is Altered in Brains of Subjects with Autism. Cerebellum 8: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD (2009): GABAA receptor downregulation in brains of subjects with autism. Journal of Autism and Developmental Disorders 39: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson AM, Salowitz NM, Scheidt RA, Dolan BK, Van Hecke AV (2014): Electroencephalogram coherence in children with and without autism spectrum disorders: decreased interhemispheric connectivity in autism. Autism Research 7: 334–343. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson A, DiStefano C, Lin YY, Scheffler AW, Senturk D, Jeste SS (2018): Interhemispheric alpha-band hypoconnectivity in children with autism spectrum disorder. Behav Brain Res 348: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2006): Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral cortex 17: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Reilly C, Lewis JD, Elsabbagh M (2017): Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE 12: e0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rane P, Cochran D, Hodge SM, Haselgrove C, Kennedy D, Frazier JA (2015): Connectivity in autism: a review of MRI connectivity studies. Harvard review of psychiatry 23: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von dem Hagen EAH, Stoyanova RS, Baron-Cohen S, Calder AJ (2013): Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci 8: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wass S (2011): Distortions and disconnections: Disrupted brain connectivity in autism. Brain and Cognition 75: 18–28. [DOI] [PubMed] [Google Scholar]
- 33.Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, Pena M, et al. (2019): Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nature Neuroscience 22: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. (2011): Neuron number and size in prefrontal cortex of children with autism. Jama 306: 2001–2010. [DOI] [PubMed] [Google Scholar]
- 35.Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. (2014): Patches of Disorganization in the Neocortex of Children with Autism. New England Journal of Medicine 370: 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, et al. (2013): Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, et al. (2013): Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155: 1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charman T, Young GS, Brian J, Carter A, Carver LJ, Chawarska K, et al. (2017): Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A baby siblings research consortium (BSRC) study. Autism Research 10: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. (2011): Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics 128 10.1542/peds.2010-2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis JD, Evans AC, Pruett JR, Botteron KN, McKinstry RC, Zwaigenbaum L, et al. (2017): The Emergence of Network Inefficiencies in Infants With Autism Spectrum Disorder. Biol Psychiatry 82: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. (2012): Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. American Journal of Psychiatry 169: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, et al. (2015): Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 138: 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emerson R, Adams C, Nishino T (2017): Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Science. Retrieved from http://stm.sciencemag.org/content/9/393/eaag2882.abstract [DOI] [PMC free article] [PubMed]
- 44.Debener S, Minow F, Emkes R, Gandras K, Vos M de (2012): How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology 49: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 45.Thatcher RW (2012): Coherence, phase differences, phase shift, and phase lock in EEG/ERP analyses. Developmental neuropsychology 37: 476–496. [DOI] [PubMed] [Google Scholar]
- 46.Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ (1997): EEG coherency: I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalography and clinical neurophysiology 103: 499–515. [DOI] [PubMed] [Google Scholar]
- 47.Smit DJA, Boersma M, Schnack HG, Micheloyannis S, Boomsma DI, Hulshoff Pol HE, et al. (2012): The Brain Matures with Stronger Functional Connectivity and Decreased Randomness of Its Network ((Valdes-Sosa PA, editor)). PLoS ONE 7: e36896–e36896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vakorin VA, Lippé S, McIntosh AR (2011): Variability of Brain Signals Processed Locally Transforms into Higher Connectivity with Brain Development. J Neurosci 31: 6405–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinkley LBN, Vinogradov S, Guggisberg AG, Fisher M, Findlay AM, Nagarajan SS (2011): Clinical Symptoms and Alpha Band Resting-State Functional Connectivity Imaging in Patients With Schizophrenia: Implications for Novel Approaches to Treatment. Biological Psychiatry 70: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bells S, Lefebvre J, Prescott SA, Dockstader C, Bouffet E, Skocic J, et al. (2017): Changes in White Matter Microstructure Impact Cognition by Disrupting the Ability of Neural Assemblies to Synchronize. J Neurosci 37: 8227–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinkley LBN, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, et al. (2012): The role of corpus callosum development in functional connectivity and cognitive processing. PLoS ONE 7: e39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Stein A, Sarnthein J (2000): Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38: 301–313. [DOI] [PubMed] [Google Scholar]
- 53.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000): The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders 30: 205–23. [PubMed] [Google Scholar]
- 54.Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. (2009): The Autism Diagnostic Observation Schedule – Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord 39: 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guthrie W, Swineford LB, Nottke C, Wetherby AM (2013): Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of child psychology and psychiatry, and allied disciplines 54: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullen EM (1995): Mullen Scales of Early Learning. Retrieved from http://www.v-psyche.com/doc/special-cases/Mullen Scales of Early Learning.docx
- 57.Levin AR, Varcin KJ, O’Leary HM, Tager-Flusberg H, Nelson CA (2017): EEG power at 3 months in infants at high familial risk for autism. J Neurodev Disord 9 10.1186/s11689-017-9214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delorme A, Makeig S (2004): EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods 134: 9–21. [DOI] [PubMed] [Google Scholar]
- 59.Chang C, Hsu S, Pion-Tonachini L, Jung T (2018): Evaluation of Artifact Subspace Reconstruction for Automatic EEG Artifact Removal. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1242–1245. [DOI] [PubMed] [Google Scholar]
- 60.Mullen TR, Kothe CAE, Chi M, Ojeda A, Kerth T, Makeig S, et al. (2015): Real-time Neuroimaging and Cognitive Monitoring Using Wearable Dry EEG. IEEE Trans Biomed Eng 62: 2553–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jasper H (1958): The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neuroph siology 10: 371–375. [PubMed] [Google Scholar]
- 62.Onton J, Westerfield M, Townsend J, Makeig S (2006): Imaging human EEG dynamics using independent component analysis. Neuroscience & biobehavioral reviews 30: 808–822. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan R, Winter WR, Ding J, Nunez PL (2007): EEG and MEG coherence: measures of functional connectivity at distinct spatial scales of neocortical dynamics. Journal of neuroscience methods 166: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira F, Mitchell T, Botvinick M (2009): Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 45: S199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Mol C, Mosci S, Traskine M, Verri A (2009): A regularized method for selecting nested groups of relevant genes from microarray data. Journal of Computational Biology 16: 677–690. [DOI] [PubMed] [Google Scholar]
- 66.Zou H, Hastie T (2005): Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 67: 301–320. [Google Scholar]
- 67.Teipel SJ, Kurth J, Krause B, Grothe MJ (2015): The relative importance of imaging markers for the prediction of Alzheimer’s disease dementia in mild cognitive impairment — Beyond classical regression. Neuroimage Clin 8: 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vapnik V, Golowich SE, Smola AJ (1997): Support Vector Method for Function Approximation, Regression Estimation and Signal Processing In: Mozer MC, Jordan MI, Petsche T, editors. Advances in Neural Information Processing Systems 9 MIT Press, pp 281–287. [Google Scholar]
- 69.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. (2010): Prediction of individual brain maturity using fMRI. Science 329: 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golland P, Fischl B (2003): Permutation Tests for Classification: Towards Statistical Significance in Image-Based Studies In: Taylor C, Noble JA, editors. Information Processing in Medical Imaging. Springer Berlin Heidelberg, pp 330–341. [DOI] [PubMed] [Google Scholar]
- 71.Zeng L-L, Shen H, Liu L, Wang L, Li B, Fang P, et al. (2012): Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135: 1498–1507. [DOI] [PubMed] [Google Scholar]
- 72.Gaonkar B, Davatzikos C (2013): Analytic estimation of statistical significance maps for support vector machine based multi-variate image analysis and classification. Neuroimage 78: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haufe S, Meinecke F, Görgen K, Dähne S, Haynes J-D, Blankertz B, Bießmann F (2014): On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage 87: 96–110. [DOI] [PubMed] [Google Scholar]
- 74.Joseph RM, Tager-Flusberg H, Lord C (2002): Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry 43: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabdebon C, Leroy F, Simmonet H, Perrot M, Dubois J, Dehaene-Lambertz G (2014): Anatomical correlations of the international 10–20 sensor placement system in infants. NeuroImage 99: 342–356. [DOI] [PubMed] [Google Scholar]
- 76.Courchesne E, Pierce K (2005): Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology 15: 225–230. [DOI] [PubMed] [Google Scholar]
- 77.Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E (2006): Reduced minicolumns in the frontal cortex of patients with autism. Neuropathology and applied neurobiology 32: 483–491. [DOI] [PubMed] [Google Scholar]
- 78.Cherkassky VL, Kana RK, Keller TA, Just MA (2006): Functional connectivity in a baseline resting-state network in autism. Neuroreport 17: 1687–1690. [DOI] [PubMed] [Google Scholar]
- 79.Just MA, Cherkassky VL, Keller TA, Minshew NJ (2004): Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127: 1811–1821. [DOI] [PubMed] [Google Scholar]
- 80.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2006): Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral cortex 17: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Just MA, Keller TA, Malave VL, Kana RK, Varma S (2012): Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews 36: 1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shukla DK, Keehn B, Smylie DM, Müller R-A (2011): Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia 49: 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller R-A (2005): Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage 25: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rapoport JL, Gogtay N (2008): Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology 33: 181. [DOI] [PubMed] [Google Scholar]
- 85.Sestan N, State MW (2018): Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron 100: 406–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lazaro MT, Taxidis J, Shuman T, Bachmutsky I, Ikrar T, Santos R, et al. (2019): Reduced prefrontal synaptic connectivity and disturbed oscillatory population dynamics in the CNTNAP2 model of autism. Cell reports 27: 2567–2578. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liska A, Bertero A, Gomolka R, Sabbioni M, Galbusera A, Barsotti N, et al. (2017): Homozygous loss of autism-risk gene CNTNAP2 results in reduced local and long-range prefrontal functional connectivity. Cerebral cortex 28: 1141–1153. [DOI] [PubMed] [Google Scholar]
- 88.Pagani M, Bertero A, Liska A, Galbusera A, Sabbioni M, Barsotti N, et al. (2019): Deletion of autism risk gene Shank3 disrupts prefrontal connectivity. Journal of Neuroscience 2529–18. [DOI] [PMC free article] [PubMed]
- 89.Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, et al. (2010): Altered Functional Connectivity in Frontal Lobe Circuits Is Associated with Variation in the Autism Risk Gene CNTNAP2. Science Translational Medicine 2: 56ra80–56ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bertero A, Liska A, Pagani M, Parolisi R, Masferrer ME, Gritti M, et al. (2018): Autism-associated 16p11.2 microdeletion impairs prefrontal functional connectivity in mouse and human. Brain 141: 2055–2065. [DOI] [PubMed] [Google Scholar]
- 91.Rowley PA, Guerrero-Gonzalez J, Alexander AL, John-Paul JY (2019): Convergent microstructural brain changes across genetic models of autism spectrum disorder—A pilot study. Psychiatry Research: Neuroimaging 283: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geng JJ, Vossel S (2013): Re-evaluating the role of TPJ in attentional control: contextual updating? Neuroscience & Biobehavioral Reviews 37: 2608–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lahnakoski JM, Glerean E, Salmi J, Jääskeläinen IP, Sams M, Hari R, Nummenmaa L (2012): Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Frontiers in human neuroscience 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saxe R, Kanwisher N (2003): People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind.” Neuroimage 19: 1835–1842. [DOI] [PubMed] [Google Scholar]
- 95.Deen B, Koldewyn K, Kanwisher N, Saxe R (2015): Functional organization of social perception and cognition in the superior temporal sulcus. Cerebral Cortex 25: 4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lombardo MV, Chakrabarti B, Bullmore ET, MRC AIMS Consortium, Baron-Cohen S (2011): Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage 56: 1832–1838. [DOI] [PubMed] [Google Scholar]
- 97.Teipel SJ, Pogarell O, Meindl T, Dietrich O, Sydykova D, Hunklinger U, et al. (2009): Regional networks underlying interhemispheric connectivity: an EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Hum Brain Mapp 30: 2098–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ (2012): Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS computational biology 8: e1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stam CJ (2014): Modern network science of neurological disorders. Nature Reviews Neuroscience 15: 683–695. [DOI] [PubMed] [Google Scholar]
- 100.Murdaugh DL, Nadendla KD, Kana RK (2014): Differential role of temporoparietal junction and medial prefrontal cortex in causal inference in autism: An independent component analysis. Neuroscience letters 568: 50–55. [DOI] [PubMed] [Google Scholar]
- 101.Abu-Akel AM, Wood SJ, Hansen PC, Apperly IA (2015): Perspective-taking abilities in the balance between autism tendencies and psychosis proneness. Proceedings of the Royal Society B: Biological Sciences 282: 20150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A (2006): Neural mechanisms of imitation and ‘mirror neuron’functioning in autistic spectrum disorder.Neuropsychologia 44: 610–621. [DOI] [PubMed] [Google Scholar]
- 103.Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S (2011): Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PloS one 6: e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kana RK, Maximo JO, Williams DL, Keller TA, Schipul SE, Cherkassky VL, et al. (2015): Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Molecular autism 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Igelström KM, Webb TW, Graziano MS (2016): Functional connectivity between the temporoparietal cortex and cerebellum in autism spectrum disorder. Cerebral Cortex 27: 2617–2627. [DOI] [PubMed] [Google Scholar]
- 106.Pantelis PC, Byrge L, Tyszka JM, Adolphs R, Kennedy DP (2015): A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Social cognitive and affective neuroscience 10: 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, et al. (2007): Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol 118: 1877–1888. [DOI] [PubMed] [Google Scholar]
- 108.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY (2008): Transient Induced Gamma-Band Response in EEG as a Manifestation of Miniature Saccades. Neuron 58: 429–441. [DOI] [PubMed] [Google Scholar]
- 109.DiStefano C, Dickinson A, Baker E, Jeste SS (2019): EEG data collection in children with ASD: The role of state in data quality and spectral power. Research in Autism Spectrum Disorders 57: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estes A, Zwaigenbaum L, Gu H, John T St., Paterson S, Elison JT, et al. (2015): Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flanagan JE, Landa R, Bhat A, Bauman M (2012): Head Lag in Infants at Risk for Autism: A Preliminary Study. Am J Occup Ther 66: 577–585. [DOI] [PubMed] [Google Scholar]
- 112.Iverson JM, Shic F, Wall CA, Chawarska K, Curtin S, Estes A, et al. (2019): Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. Journal of Abnormal Psychology 128: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH (2014): Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews 39: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones W, Klin A (2013): Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504: 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.LeBarton ES, Landa RJ (2018): Infant motor skill predicts later expressive language and autism spectrum disorder diagnosis. Infant Behav Dev 54: 37–47. [DOI] [PubMed] [Google Scholar]
- 116.Ben Itzchak E, Zachor DA (2011): Who benefits from early intervention in autism spectrum disorders? Research in Autism Spectrum Disorders 5: 345–350. [Google Scholar]
- 117.Ben-Itzchak E, Zachor DA (2007): The effects of intellectual functioning and autism severity on outcome of early behavioral intervention for children with autism. Research in Developmental Disabilities 28: 287–303. [DOI] [PubMed] [Google Scholar]
- 118.Karmiloff-Smith A, D’Souza D, Dekker TM, Herwegen JV, Xu F, Rodic M, Ansari D (2012): Genetic and environmental vulnerabilities in children with neurodevelopmental disorders. PNAS 109: 17261–17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004): Organization, development and function of complex brain networks. Trends in cognitive sciences 8: 418–425. [DOI] [PubMed] [Google Scholar]
- 120.Bell MA, Fox NA (1992): The relations between frontal brain electrical activity and cognitive development during infancy. Child development 63: 1142–1163. [PubMed] [Google Scholar]
- 121.Hodel AS (2018): Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review 48: 113–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller EK, Cohen JD (2001): An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience 24: 167–202. [DOI] [PubMed] [Google Scholar]
- 123.Hebb DO (1949): The Organization of Behavior; a Neuropsychological Theory. Oxford, England: Wiley. [Google Scholar]
- 124.Hensch TK (2005): Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience 6: 877–888. [DOI] [PubMed] [Google Scholar]
- 125.Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, et al. (2015): Parent-mediated intervention versus no intervention for infants at high risk of autism: a parallel, single-blind, randomised trial. The Lancet Psychiatry 2: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jones EJH, Dawson G, Kelly J, Estes A, Jane Webb S (2017): Parent-delivered early intervention in infants at risk for ASD: Effects on electrophysiological and habituation measures of social attention. Autism Res 10: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Athey S (2017): Beyond prediction: Using big data for policy problems. Science 355: 483–485. [DOI] [PubMed] [Google Scholar]
- 128.Price WN (2018): Big data and black-box medical algorithms. Science translational medicine 10: eaao5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scheinost D, Noble S, Horien C, Greene AS, Lake EMR, Salehi M, et al. (2019): Ten simple rules for predictive modeling of individual differences in neuroimaging. NeuroImage 193: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X, Constable RT (2017): Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc 12: 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wirsich J, Ridley B, Besson P, Jirsa V, Bénar C, Ranjeva J-P, Guye M (2017): Complementary contributions of concurrent EEG and fMRI connectivity for predicting structural connectivity. Neuroimage 161: 251–260. [DOI] [PubMed] [Google Scholar]