Abstract
BACKGROUND:
Bleeding and venous thromboembolic disease are considered important sources of postoperative morbidity and mortality. Clinically, treatment of these 2 disorders is often competing. We sought to better understand the relative contributions of bleeding and venous thromboembolic disease to postoperative attributable mortality in a national cohort.
METHODS:
A retrospective analysis of the 2006–2017 American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) database was performed to assess the adjusted odds ratio and attributable mortality for postoperative bleeding and venous thromboembolism, adjusted by year.
RESULTS:
After adjustment for confounding variables, bleeding exhibited a high postoperative attributable mortality in every year studied. Venous thromboembolism appeared to contribute minimal attributable mortality.
CONCLUSIONS:
Bleeding complications are a consistent source of attributable mortality in surgical patients, while the contribution of venous thromboembolic disease appears to be minimal in this analysis. Further studies are warranted to better understand the etiology of this disparity.
Venous thromboembolism (VTE), consisting of deep vein thrombosis (DVT) and pulmonary embolism (PE), is a well-recognized cause of morbidity and mortality.1–3 Together, DVT and PE have been implicated in >100,000 deaths annually.1 Mortality rates for VTE have been estimated to be higher than mortality rates in breast cancer, stroke, and even myocardial infarction.4–6 Of those who survive, VTE frequently recurs with accompanying long-term complications, such as thrombophlebitis, postthrombotic syndrome, and pulmonary hypertension.7,8 This is particularly important in the surgical population, as patients are at increased risk for VTE in the postoperative period due to systemic inflammation in response to surgical tissue trauma and mechanical factors, such as immobility during the perioperative period.5,7 Additionally, perioperative VTE has been listed as one of the most preventable causes of death in hospitalized patients.2
There is a growing body of evidence that pharmacologic prophylaxis leads to a dramatically decreased incidence of VTE in surgical inpatients.9,10 In light of this evidence, more patients are receiving VTE prophylaxis.11 The surgeon general released a call to action to prevent VTE in 2008, and current guidelines from the American College of Chest Physicians recommend pharmacologic prophylaxis for all patients undergoing major surgical procedures, barring contra-indications.12 Compliance rates with these recommendations are currently tracked by the Joint Commission National Hospital Inpatient Quality Measures.13
Alternatively, while controversial,14 antithrombotic prophylaxis may be associated with an increased risk of postoperative bleeding,15 making them infrequently used in the early postoperative period.10 Perioperative bleeding can lead to a host of complications including hypovolemia, cardiac and renal injury, and the need for transfusion, which itself is associated with significant complications and increased mortality.16–18
Given this clinical trade-off, we sought to analyze a large, heterogeneous national cohort of patients undergoing noncardiac surgery to compare the rates, odds ratios (ORs) for mortality, and attributable mortality (AM) of thrombotic complications, as compared to bleeding complications, over time. We hypothesized that, during the past 12 years, mortality attributable to bleeding complications is higher than mortality attributable to thrombotic complications.
METHODS
The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were used in the preparation of this article.19
Data Source
This study was approved by the Vanderbilt University Medical Center Institutional Review Board (No. 171155). We performed a retrospective review of prospectively acquired data from the American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) database. ACS-NSQIP is a national database, which collects data on more than 150 variables, including preoperative risk factors, intraoperative variables, and 30-day postoperative mortality and morbidity outcomes for patients undergoing major surgical procedures in both the inpatient and outpatient setting. Complications are tracked in the ACS-NSQIP database using standardized definitions and with rigorous training to ensure high data quality.20 The ACS-NSQIP and participating hospitals are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Variables Screened
Data regarding bleeding and thrombotic complications in adult patients undergoing noncardiac surgery from 2006 to 2017 were obtained from the existing ACS-NSQIP registry for all noncardiac procedures by Current Procedural Terminology code and surgical specialty. Only patients ≥18 years of age were included in the analysis. Primary and secondary outcomes were defined using the ACS-NSQIP definitions (Supplemental Digital Content, Appendix 1, http://links.lww.com/AA/D117). Thrombotic events were defined as the composite of the occurrence of a deep venous thrombus or a PE. Bleeding complications were defined as bleeding requiring transfusion within 72 hours of surgery. There was a noteworthy change in the ACS-NSQIP definition of bleeding in 2010, which reduced the number of transfused units required from 4 to 1 and added transfusion during the intraoperative period to the definition.
Statistical Analysis
All patient data collected in the 2006–2017 ACS-NSQIP were included in the analysis. Data were analyzed separately by year. Patient demographics, clinic and hospital data, comorbidities, and complication variables were summarized with the median (25th and 75th percentiles) for continuous variables and percentages for categorical variables. For analysis, we included 79 potential confounding variables from ACS-NSQIP 2006–2017. These included demographics, laboratory values, surgery and anesthetic details, and complications. The 2000+ Current Procedural Terminology codes present in ACS-NSQIP were reclassified to 244 clinically meaningful categories using the Clinical Classification Software for Procedure Codes. For each laboratory variable, an indicator of whether the laboratory value was available, as well as the tested laboratory value (if available), was included in the model. The nonlinear effects of continuous variables were modeled using restricted cubic splines with 5 knots specified at the 5th, 25th, 50th, 75th, and 95th percentiles.
For each year of data, a 2-stage, relaxed least absolute shrinkage and selection operator (LASSO) logistic regression procedure21,22 was used to estimate the effects of bleeding and VTE on the odds of mortality, adjusting for potential confounders. In the first stage, potential confounders are selected from all potential confounders available in ACS-NSQIP, but excluding the bleeding and VTE complication variables, using the ordinary LASSO procedure. This method applies a penalty for complex models and can be used to eliminate potential confounders that do not substantially contribute to model fit. The penalty was selected by minimizing a 10-fold cross-validation estimate of model prediction error (ie, the binomial deviance); penalties that are too large result in inadequate model fit; and penalties that are too small result in overfitting. Using the ACS-NSQIP 2006 data as an example, 104 degrees of freedom (involving 31 predictors) were eliminated in the first-stage LASSO procedure. In the second stage, ordinary logistic regression was used to estimate the effects of bleeding and VTE on the odds of mortality, adjusting for the variables selected in the first stage. Finally, the bleeding and VTE AM was calculated using the population attributable fraction (PAF) method and normalized to 100,000 patients per year. AM is commonly used to describe the mortality that would have been prevented if a complication had not occurred, while adjusting for other complications or comorbidities.23–27 The effects of bleeding and VTE were quantified using the adjusted OR (95% confidence interval [CI]) and the AM (AM; 95% CI).
AM was calculated using the PAF method: AM = number of complications × (P(D|C+) − P(D|C−)), where P(D|C+) is the probability of death given a complication and P(D|C−) is the probability of death given that no complication occurs.23,28–30
Due to the uncertainty of the timing of postoperative complications relative to bleeding or VTE, a sensitivity analysis was conducted by omitting all postoperative complication variables from the regression procedures. A second sensitivity analysis was performed in the subgroup of patients undergoing vascular surgery, given concerns that this cohort might have a different risk/benefit ratio with regards to bleeding versus VTE.
All analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria), and source code has been included for reference and reproducibility (Supplemental Digital Content, Appendix 2, http://links.lww.com/AA/D117). A 2-sided significance level of 0.05 denoted statistical significance.
RESULTS
Patient demographics are summarized by year in Supplemental Digital Content, Table 1, http://links.lww.com/AA/D117. A total of 6,637,635 patients were included in the analysis, with sample size increasing annually from 2006 (N = 152,287) to 2017 (N = 1,028,731). Median patient age ranged from 54 to 58 years. Most patients were women (56.5%–58%) and Caucasian (79.4%–87.4%). The proportion of patients with American Society of Anesthesiologists (ASA) status III or IV varied between 44.4% and 47.2% (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D117). The highest fraction of inpatients (65.8%) was in 2008, and this decreased to 57% after 2011. Most patients were admitted from home (95.0%–96.7%) and had general anesthesia (88.7%–94.3%). The fraction of patients who underwent general surgery decreased from 85.2% in 2006 to 43.2% in 2017. The most common comorbidities were hypertension (43.3%–46.8%) and diabetes (13.7%–15.7%) (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D117).
The rate of bleeding complications was 0.4%–0.6% before the definition change in January 2010 and 4.5%–8.4% afterward (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D117). The rate of VTE complications ranged from 0.8% to 1.0%. The rates of other complications by year are shown in Supplemental Digital Content, Table 2, http://links.lww.com/AA/D117.
After adjustment for potential confounders, bleeding complications had a significant association with mortality in all 12 years. The ORs for mortality in patients with a bleeding complication ranged between 1.62 and 3.44 before the definition change and 1.19 and 1.42 afterward (all P values <.001). The ORs for mortality in patients with VTE ranged between 1.21 and 1.49 starting in 2012 (all P values <.001). There was no evidence that VTE was associated with mortality before 2012, except in 2008 (OR = 1.32 [1.07–1.64], P = .01) (Figure 1). AM from bleeding complications ranged from 6 [2–9] to 17 [12–21] per 100,000 patients before the definition change in 2010 and from 10 [6–13] to 52 [39–65] afterward (Figure 2). The highest AM due to VTE was 6 [1–10] per 100,000 in 2008 and ranged between 2 and 5 per 100,000 afterward.
Figure 1.
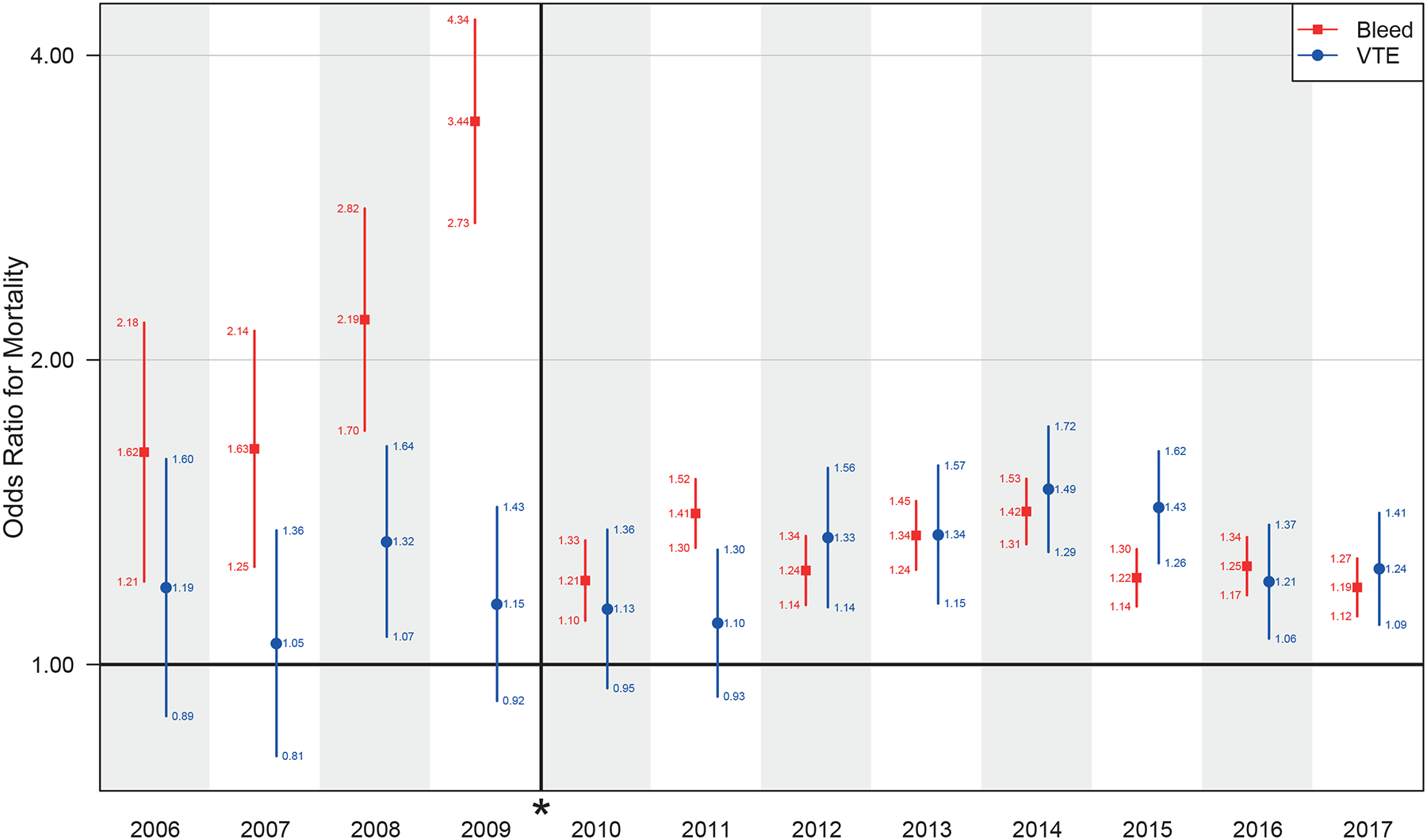
The adjusted odds ratio for mortality by year, with break and * denoting definition change for bleeding starting in 2010. VTE indicates venous thromboembolism.
Figure 2.
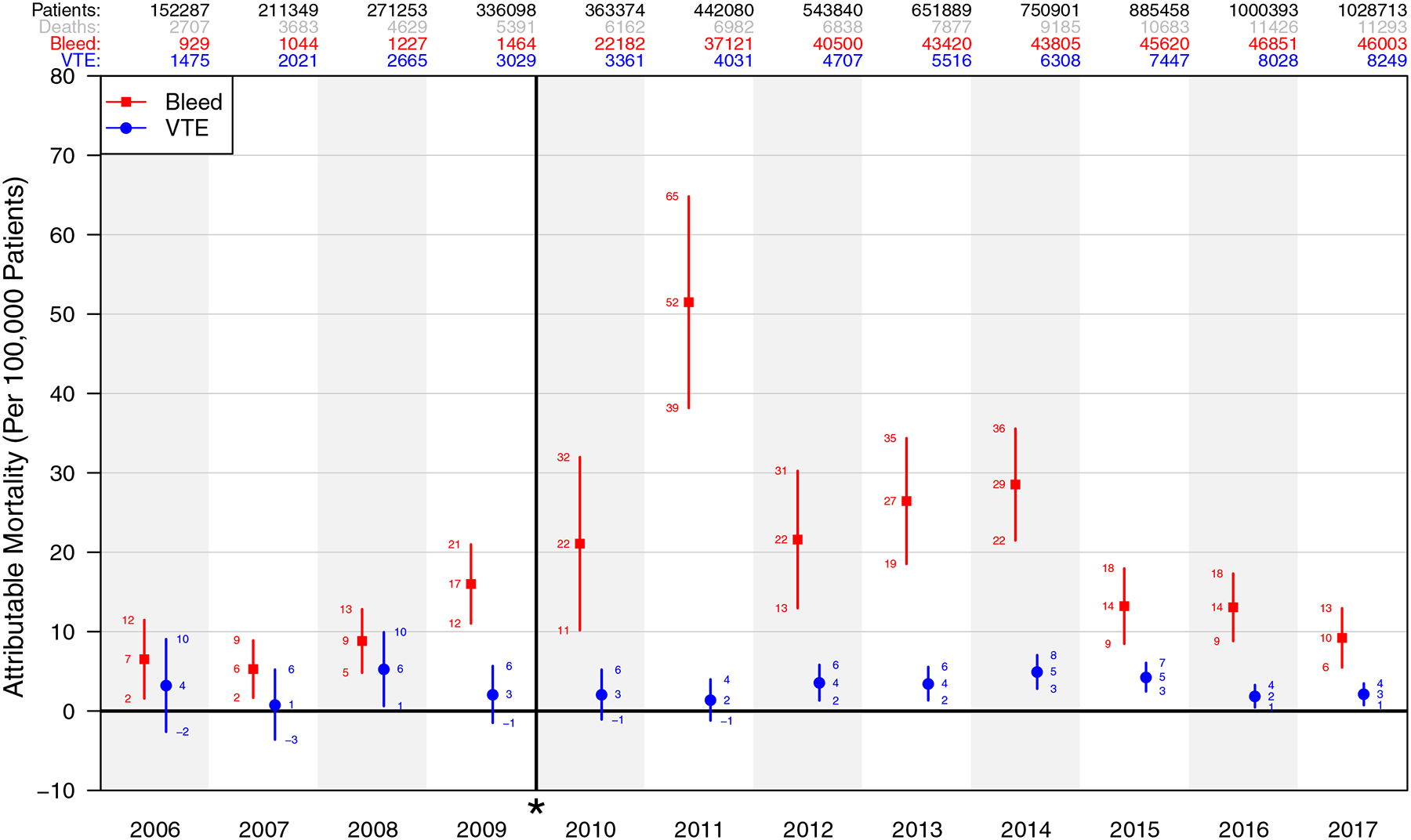
The attributable mortality by year, normalized per 100,000 patients, with break and * denoting definition change for bleeding starting in 2010. VTE indicates venous thromboembolism.
In the first sensitivity analysis, where postoperative complications were not considered in the model, the VTE and bleeding complication ORs for mortality and AM were elevated with respect to the primary analysis (Supplemental Digital Content, Appendices 3–4, http://links.lww.com/AA/D117). For example, in 2011, the AM of bleeding complications was 52 [39–65] and 88 [74–101] in the primary and sensitivity analyses, respectively. Nevertheless, the relative AM for VTE versus bleeding complications was similar in the primary and sensitivity analyses.
In the second sensitivity analysis of vascular surgery patients, results were consistent with the primary analysis, as well as the first sensitivity analysis, demonstrating a consistent AM of bleeding, with minimal AM of VTE (Supplemental Digital Content, Appendices 5–6, http://links.lww.com/AA/D117).
DISCUSSION
We demonstrate the AM of bleeding and VTE in the ACS-NSQIP database. While VTE had minimal AM, bleeding complications had a profound AM in every single year studied.
The perioperative literature consistently implicates VTE as having high morbidity and mortality.2,3,5,8,11 One review of perioperative VTE estimates that its mortality is higher than breast cancer, myocardial infarction, and stroke.10 In response to these and other findings, the Surgeon General of the United States released a call to action1 and the American College of Chest Physicians published 2 sets of guidelines, all highlighting the importance of preventing VTE.9,12
In recent years, VTE may be exhibiting a diminishing impact on mortality in recent years. A 2017 analysis of the National Inpatient Sample found that, despite increasing rates of VTE, in-hospital mortality decreased from 2005 to 2013.31 This may be due to effective prevention, as VTE prophylaxis may decrease mortality in perioperative patients.32 VTE chemoprophylaxis rates are a surrogate measure of hospital quality, assessed by the Joint Commission National Core Measures. In Washington state, VTE chemoprophylaxis rates for colorectal surgery increased between 2006 and 2011, without any resulting decrease in VTE. Perioperative transfusions, however, increased significantly.33
In our study, bleeding complications had a consistently high AM. Clinically, there is a trade-off between preventing VTE and bleeding in surgical patients. Previous perioperative studies have compared the rates of bleeding to VTE with varying results. All were small and single center, looking at specific surgical populations.34–37 While rates of VTE were higher than rates of bleeding in patients undergoing pancreatectomy and mastectomy,34,35 patients undergoing total thyroidectomy had an 8-fold higher risk of postoperative bleeding compared to VTE.36
In contrast, our study includes patients undergoing a broad range of operations and assesses AM, rather than unadjusted rates or ORs. We used robust, modern regression techniques in the analysis. Unlike ORs, which do not provide information on the number of patients experiencing a complication, AM is a combination of both the OR and frequency, providing a single measure of the effect of a complication on a population.
The ACS-NSQIP database is a large, national database and contains data from a wide variety of patient populations and practice settings, with rigorous data quality standards. This improves the generalizability of our results. Our large sample size allowed for greater statistical power that can be achieved in a single-center study. For example, the LASSO was applied separately for each year of data, requiring many degrees of freedom. Because the population included in the database is different each year, performing a separate regression for each year allowed us to adjust for the most important potential confounding variables in each year. Given the large size of the dataset and the high degree of complexity of the analysis, this was necessary, given computational restraints. Nevertheless, LASSO tended to select the same variables each year (Supplemental Digital Content, Appendix 7, http://links.lww.com/AA/D117).
There are limitations to this study. Complications were modeled independently, and clusters or sequences of complications were not studied.38 A full examination of this would be nearly impossible, given the large number of possible sequences of complications. Importantly, this study cannot establish causality between the studied complications and mortality. It is important to note, however, that these same inferential techniques are widely used throughout epidemiology to identify—and intervene on—important public health hazards.
As with other observational studies of existing data, unmeasured complications and covariates may have introduced confounding. Each year was studied independently, mitigating the impact of definition changes and demographic shifts in the ACS-NSQIP population. This precludes a direct analysis of temporal trends. Intraoperative complications and postoperative complications are included in the same analyses and, while bleeding complications are tracked for 72 postoperative hours, all other complications and outcomes were followed for 30 days. While this may introduce some degree of censoring, it is important to note that these are largely noncompeting. Our first sensitivity analysis, excluding all postoperative complications, revealed results congruent with our primary analysis, allaying some concern about the impact of this bias.
Due to the high frequency of missingness for laboratory values, we used the “missingness indicator” method. This may introduce bias. Multiple imputation, which is an alternative recommended method, cannot be used directly in combination with LASSO for variable selection, because the selected variables will differ across rounds of imputation. Missing laboratory values are often missing not at random; the value is often related to the likelihood of missingness (eg, troponin is typically measured only when there is suspicion of cardiac injury). In this situation, even multiple imputation strategies do not mitigate bias.
Similarly, the reported incidence of VTE depends on clinical suspicion and methods available for diagnosis. VTE rates are often underreported due to delayed or missed diagnoses, with limited screening, and some cases go undiagnosed until autopsy. In our study, the overall incidence of VTE remained constant.
Our results may underestimate mortality attributable to bleeding, as only patients requiring transfusion are captured using the NSQIP definition. Other clinically significant bleeding events, such as epidural hematoma, are not included. Interestingly, the results appear to be applicable to vascular surgery patients, in whom there may be a higher tolerance for bleeding to prevent thrombosis.
The ACS-NSQIP database does not provide information regarding the use, nonuse, or type of VTE prophylaxis; thus, we cannot assess the impact of VTE prophylaxis. This may bias our findings. Studies have shown, however, that prophylaxis is nearly universal.11 Even small doses of anticoagulation may be associated with increased bleeding risk.10 As pharmacologic VTE prophylaxis may lead to an increased risk of bleeding and has been widely used in the perioperative period, this is a possible explanation for our findings, but one that we are unable to assess using these data.15,36,39 In fact, the AM of VTE may be negligible because of widespread use of effective treatment.
In conclusion, there is a high AM of perioperative bleeding compared to VTE in the ACS-NSQIP database. Additional study is needed to better understand the underlying etiology of this phenomenon.
Supplementary Material
KEY POINTS.
Question: Does the attributable mortality from bleeding exceed the attributable mortality from venous thromboembolic disease in surgical patients?
Findings: A retrospective epidemiologic analysis of the American College of Surgeons’ National Surgical Quality Improvement Program (ACS-NSQIP) 2006–2017 retrospective, observational database revealed that, after adjustment for confounding variables, bleeding exhibited consistently higher attributable mortality than venous thromboembolism.
Meaning: Bleeding complications are a consistent source of attributable mortality in surgical patients, while the contribution of venous thromboembolic disease is minimal; further studies are warranted to better understand the etiology of this disparity.
Funding:
R.E.F. was supported by an National Center for Advancing Translational Sciences (NCATS) KL2 TR002245 Grant and also receives grant support from National Heart Lung and Blood Institute (NHLBI) 1K23HL148640. C.G.H. is supported by NHLBI R01HL111111 and National Institute of General Medical Sciences (NIGMS) R01GM120484 as well as funding from Dr Franz Kohler Chemie GmbH. The other authors are supported by institutional and/or departmental funding.
GLOSSARY
- ACS-NSQIP
American College of Surgeons’ National Surgical Quality Improvement Program
- AM
attributable mortality
- ASA
American Society of Anesthesiologists
- CI
confidence interval
- DVT
deep vein thrombosis
- LASSO
least absolute shrinkage and selection operator
- OR
odds ratio
- PAF
population attributable fraction
- PE
pulmonary embolism
- VTE
venous thromboembolism
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Presented at the International Anesthesia Research Society (IARS)/ Association of University Anesthesiologists (AUA)/Society of Critical Care Anesthesiologists (SOCCA) 2019 Annual Meeting, Montreal, Canada, May 16–19, 2019.
DISCLOSURES
Name: Melissa L. Bellomy, MD.
Contribution: This author helped conceptualize and design the study, acquire and interpret the data, draft and revise the manuscript for important intellectual contents, and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Milo C. Engoren, MD.
Contribution: This author helped conceptualize and design the study, acquire and interpret the data, revise the manuscript for important intellectual contents, and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Barbara J. Martin, RN, MBA.
Contribution: This author helped conceptualize and design the study, acquire and interpret the data, revise the manuscript for important intellectual contents, and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Yaping Shi, MS.
Contribution: This author helped acquire, analyze, and interpret the data for this study; critically revise the manuscript for important intellectual contents; and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Matthew S. Shotwell, PhD.
Contribution: This author helped acquire, analyze, and interpret the data for this study; critically revise the manuscript for important intellectual contents; and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Christopher G. Hughes, MD, MS.
Contribution: This author helped analyze and interpret the data for this study, revise the manuscript for important intellectual contents, and review and approve the final version of the manuscript.
Conflicts of Interest: None.
Name: Robert E. Freundlich, MD, MS, MSCI.
Contribution: This author helped conceptualize and design the study, acquire and interpret the data, revise the manuscript for important intellectual contents, and review and approve the final version of the manuscript.
Conflicts of Interest: R. E. Freundlich has received grant support and consulting fees from Medtronic and owns stock in 3M and Johnson and Johnson.
This manuscript was handled by: Roman M. Sniecinski, MD.
REFERENCES
- 1.Office of the Surgeon General; National Heart, Lung, and Blood Institute. Publications and Reports of the Surgeon General The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General (US); 2008. [PubMed] [Google Scholar]
- 2.Buesing KL, Mullapudi B, Flowers KA. Deep venous thrombosis and venous thromboembolism prophylaxis. Surg Clin North Am. 2015;95:285–300. [DOI] [PubMed] [Google Scholar]
- 3.Raskob GE, Angchaisuksiri P, Blanco AN, et al. ; ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38:S502–S509. [DOI] [PubMed] [Google Scholar]
- 7.Kyrle PA, Eichinger S. Deep vein thrombosis. Lancet. 2005;365:1163–1174. [DOI] [PubMed] [Google Scholar]
- 8.Heit JA. Estimating the incidence of symptomatic postoperative venous thromboembolism: the importance of perspective. JAMA. 2012;307:306–307. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. [DOI] [PubMed] [Google Scholar]
- 10.Gordon RJ, Lombard FW. Perioperative venous thromboembolism: a review. Anesth Analg. 2017;125:403–412. [DOI] [PubMed] [Google Scholar]
- 11.Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailey KR. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e185S–e194S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakazawa KR, Egorova NN, Power JR, Faries PL, Vouyouka AG. The impact of surgical care improvement project measures on in-hospital outcomes following elective vascular procedures. Ann Vasc Surg. 2017;38:17–28. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141:790–797. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:405–414. [DOI] [PubMed] [Google Scholar]
- 16.Bennett S, Baker LK, Martel G, et al. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford). 2017;19:321–330. [DOI] [PubMed] [Google Scholar]
- 17.Zhang LM, Li R, Zhao XC, Zhang Q, Luo XL. Increased transfusion of fresh frozen plasma is associated with mortality or worse functional outcomes after severe traumatic brain injury: a retrospective study. World Neurosurg. 2017;104:381–389. [DOI] [PubMed] [Google Scholar]
- 18.Roshanov PS, Eikelboom JW, Crowther M, et al. ; Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Investigators. Bleeding impacting mortality after noncardiac surgery: a protocol to establish diagnostic criteria, estimate prognostic importance, and develop and validate a prediction guide in an international prospective cohort study. CMAJ Open. 2017;5:E594–E603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 20.Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 21.Hastie T, Tibshirani R, Tibshirani RJ. Extended comparisons of best subset selection, forward stepwise selection, and the lasso. arXiv:1707.08692v2 [stat.ME] 29 July 2017. [Google Scholar]
- 22.Meinshausen NJCS, Analysis D. Relaxed lasso. Comput. Stat. Data Anal 2007;52:374–393. [Google Scholar]
- 23.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. [DOI] [PubMed] [Google Scholar]
- 24.Nonnemaker J, Rostron B, Hall P, MacMonegle A, Apelberg B. Mortality and economic costs from regular cigar use in the United States, 2010. Am J Public Health. 2014;104:e86–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmet S, Rehm J, Gmel G, Frick H. Alcohol-attributable mortality in Switzerland in 2011--age-specific causes of death and impact of heavy versus non-heavy drinking. Swiss Med Wkly. 2014;144:w13947. [DOI] [PubMed] [Google Scholar]
- 26.Vaara ST, Pettilä V, Kaukonen KM, et al. ; Finnish Acute Kidney Injury Study Group. The attributable mortality of acute kidney injury: a sequentially matched analysis*. Crit Care Med. 2014;42:878–885. [DOI] [PubMed] [Google Scholar]
- 27.Muscedere JG, Martin CM, Heyland DK. The impact of ventilator-associated pneumonia on the Canadian health care system. J Crit Care. 2008;23:5–10. [DOI] [PubMed] [Google Scholar]
- 28.Melsen WG, Rovers MM, Bonten MJ. Attributable mortality of ventilator-associated pneumonia - authors’ reply. Lancet Infect Dis. 2013;13:1015. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher M, Wangler M, Wolkewitz M, Beyersmann J. Attributable mortality due to nosocomial infections. A simple and useful application of multistate models. Methods Inf Med. 2007;46:595–600. [PubMed] [Google Scholar]
- 30.Freundlich RE, Maile MD, Sferra JJ, Jewell ES, Kheterpal S, Engoren M. Complications associated with mortality in the National Surgical Quality Improvement Program Database. Anesth Analg. 2018;127:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smilowitz NR, Gupta N, Guo Y, et al. Trends in perioperative venous thromboembolism associated with major noncardiac surgery. TH Open. 2017;1:e82–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon S, Meissner M, Symons R, et al. ; Surgical Care and Outcomes Assessment Program Collaborative. Perioperative pharmacologic prophylaxis for venous thromboembolism in colorectal surgery. J Am Coll Surg. 2011;213:596–603, 603.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson DW, Simianu VV, Bastawrous AL, et al. Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg. 2015;150:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzeng CW, Katz MH, Lee JE, et al. Predicting the risks of venous thromboembolism versus post-pancreatectomy haemorrhage: analysis of 13,771 NSQIP patients. HPB (Oxford). 2014;16:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovely JK, Nehring SA, Boughey JC, et al. Balancing venous thromboembolism and hematoma after breast surgery. Ann Surg Oncol. 2012;19:3230–3235. [DOI] [PubMed] [Google Scholar]
- 36.Limongelli P, Tolone S, Gubitosi A, et al. Relationship between postoperative venous thromboembolism and hemorrhage in patients undergoing total thyroidectomy without preoperative prophylaxis. Int J Surg. 2014;12(Suppl 1):S198–S201. [DOI] [PubMed] [Google Scholar]
- 37.Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: an updated systematic review and meta-analysis. J Neurosurg. 2018;129:906–915. [DOI] [PubMed] [Google Scholar]
- 38.Silber JH, Rosenbaum PR, Trudeau ME, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43:122–131. [DOI] [PubMed] [Google Scholar]
- 39.Roy M, Rajamanickam V, Chen H, Sippel R. Is DVT prophylaxis necessary for thyroidectomy and parathyroidectomy? Surgery. 2010;148:1163–1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


