Abstract
Introduction
Patients with advanced differentiated thyroid cancer (DTC) develop resistance to lenvatinib treatment from metabolic dysregulation. Heat shock protein 90 (Hsp90) is a molecular chaperone that plays an important role in glycolysis and metabolic pathway regulation. We hypothesize that lenvatinib-resistant (LvR) DTC cells will have increased dependency on glycolysis and that a novel C-terminal Hsp90 inhibitor (KU757) can effectively treat lenvatinib-resistant cells by targeting glycolysis.
Methods
IC50 values of thyroid cancer cells were determined by CellTiter-Glow assay. Glycolysis was measured through Seahorse experiments. RT-PCR and western blot (WB) evaluated glycolytic pathway genes/proteins. Exosomes were isolated/validated by nanoparticle tracking analysis and WB. Differentially expressed long non-coding RNAs (lncRNAs) in exosomes and cells were evaluated using qPCR.
Results
Extracellular Acidification Rate (ECAR) demonstrated >2-fold upregulation of glycolysis in LvR cells vs parent cells that was downregulated after KU757 treatment. LvR resistant cells showed increased expression of the glycolytic genes LDH,PKM1/2, and HK2. KU757 treatment resulted in downregulation of these genes and proteins. Several lncRNAs associated with glycolysis were significantly upregulated in WRO-LvR cells and exosomes, and downregulated following KU757 treatment.
Conclusion
Lenvatinib resistance leads to increased glycolysis, and KU757 effectively treats LvR cells and overcomes this increased glycolysis by targeting key glycolytic genes, proteins, and lncRNAs.
TOC Statement- 20-aaes-17
Lenvatinib parent and resistant cells response to glycolytic mechanism after treatment with C-terminal Hsp90 inhibitor KU757. The importance of this manuscript is the evaluation novel C-terminal Hsp90 inhibitor KU757 as potential strategy for overcoming lenvatinib resistance in differentiated thyroid cancer cells through targeting glycolysis.
Introduction
Thyroid cancer is the most common endocrine malignancy worldwide, and incidence rates have increased yearly for the past several decades; currently 52,890 new cases in the US are projected for 2020 (1). Overall, prognosis for patients with well-differentiated subtypes such as papillary or follicular cancers is good, with a 10-year survival rate of around 85% (2). However, 25% of thyroid cancer patients develop recurrence, with a third of these patients developing metastatic disease that does not respond to radioactive iodine (RAI) ablation (3). The 10-year survival rate for these patients is only 10% (2). Lenvatinib, a small molecule receptor tyrosine kinase (RTK) inhibitor, is one of the few FDA-approved treatments for patients with RAI-refractive differentiated thyroid cancer (RR-DTC). While lenvatinib increases progression-free survival in patients with advanced DTC, it has no significant impact on improving the overall survival rate for this disease (4). Therefore, one-half of patients with advanced DTC treated with lenvatinib will develop significant disease progression after an average of 18 months due to resistance mechanisms (5).
One of the key mechanisms driving chemo- and radiotherapy resistance in cancer cells is aberrant glucose metabolism (6). Malignant cells are able to shift from oxidative phosphorylation (OXPHOS) to glycolysis in response to changes in the tumor microenvironment from stress, nutrient deprivation, hypoxic conditions, and exposure to cytotoxic drugs. This metabolic regulation, also known as the Warburg effect, allows cancer cells to survive and progress despite these external stressors (6). Previous research has shown the critical importance of this glycolytic phenotype in thyroid cancer pathogenesis, with upregulation of glycolysis-related proteins including GLUT1, hexokinase (HK), and lactate dehydrogenase (LDH) (7–9). However, the precise mechanisms underlying aberrant glucose metabolism and its role in thyroid cancer resistance are unknown.
Evidence suggests that a potential mediator of altered glucose metabolism is exosomes in the tumor microenvironment. Exosomes present in patient’s body fluid or cell culture supernatant play an important role in driving cellular proliferation, invasiveness and angiogenesis. Exosomes are extracellular vesicles that contain proteins, DNAs, long non-coding RNAs (lncRNAs) and miRNAs from their constituent cells that serve as biomarkers of cancer pathogenesis and therapy response (10). LncRNAs are non-coding RNA transcripts greater than 200 nucleotides in length. LncRNAs play a key role in modulating metabolic signaling pathways such as the PI3K/Akt/mTOR pathway that lead to increased glycolysis in thyroid cancer (11). In addition to altering metabolic signaling pathways, lncRNAs can also directly bind to key glycolytic enzymes and regulate glycolysis. For example, LINC00092 binds to the glycolytic enzyme 6-phosphofructo-2-kinase/ fructose-2,6-biphosphatase 2 (PFKFB2) to promote metastasis by altering glycolysis and the tumor microenvironment, H19 and UCA1 are known to increase tumorigenesis by activating pyruvate kinase 2 (PKM2), and PVT1 and TUG1 are known to alter glucose metabolism, cell proliferation, and motility through HK2 (12,14). Although the role of several lncRNAs important in metabolism has been identified in thyroid cancer (1), few data exist on modulating lncRNA-mediated glycolytic metabolism as a potential mechanism to effectively treat drug-resistant tumor cells.
Currently, there is a paucity of effective therapeutics that can significantly improve overall survival in RR-DTC, and novel approaches are urgently needed. Heat shock protein 90 (Hsp90) is a molecular chaperone that plays an important role in the folding and maturation of several client proteins that contribute to the hallmarks of cancer (12). One crucial mechanism by which Hsp90 promotes tumorigenesis is through metabolic reprogramming and regulating the equilibrium between glycolysis and OXPHOS (13). Although 18 Hsp90 inhibitors have entered phase I/II clinical trials for the treatment of a variety of cancers, none have yet been FDA-approved as monotherapy for cancer treatment (14). All of the Hsp90 inhibitors evaluated in trials bind to the N-terminal ATP binding site of Hsp90 and induce the pro-survival heat shock response (HSR), increasing Hsp90 and Hsp70 levels at the same concentration that leads to client protein degradation (14). As a result, this leads to a need for dose escalation which ultimately results in dose-limiting gastrointestinal and hepatic toxicity (15). To overcome these limitations of N-terminal inhibitors, our group has developed several novel, potent, Hsp90 inhibitors that bind to the C-terminus of Hsp90. These inhibitors do not induce the pro-survival HSR but are equally potent in targeting several cancers including thyroid cancers both in vitro and in vivo (5, 14, 16–18).
In the present study, we will test our hypothesis that treatment of thyroid cancer cells with the novel C-terminal Hsp90 inhibitor KU757 will lead to the attenuation of glycolysis and alteration of lncRNAs targeting metabolism in order to effectively treat lenvatinib resistant DTC cells. To test our hypothesis, we will first confirm the role of the C-terminal Hsp90 inhibitor KU757 in altering glycolysis in thyroid cancer cells. Next, we will characterize how KU757 alters glycolysis in lenvatinib-resistant (LvR) cells. Finally, we will evaluate changes in lncRNA expression in both the cellular and tumor microenvironment before and after treatment of LvR thyroid cancer cells with KU757.
Methods
Cell lines and reagents
Validated thyroid cancer cell lines ACT1 (anaplastic) and WRO (follicular) were grown in DMEM (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% penicillin/streptomycin (Life Technologies) in a 37°C humidified atmosphere with 5% CO2. The lenvatinib-resistant line WRO-LvR was developed by culturing cells in increasing lenvatinib concentrations (from 10–45 μM) and selecting resistant colonies over a period of several months. The final colony of WRO-LvR resistant cells were maintained in 45 μM lenvatinib until cell growth was stable and resistant. The C-terminal Hsp90 inhibitor (KU757) was a gift from Dr. Brian S. J. Blagg (University of Notre Dame, IN).
Cell viability assays
Genetically confirmed thyroid cancer cell lines WRO, ACT1, and WRO-LvR were grown to 70–80% confluency, plated at 3000 cells/well in a 96-well plate, and incubated for 24 h at 37°C. Cells were treated with KU757 or lenvatinib for 72 h in triplicate. The percentage of viable cells was determined using the CellTiter-Glo luminescent cell viability assay (Promega; manufacturer’s protocol). IC50 values were defined by best-fit normalized dose-response curves (GraphPad Prism 7.0).
Glycolysis stress test
The extracellular acidification rates (ECAR) were measured using a Seahorse XFe96 analyzer (Agilent Technologies). Approximately 104 thyroid cancer cells (WRO, WRO-LvR, and ACT1) were plated in a 96-well plate. Cells were then treated with 2 μM KU757 for 48 h. Cells were then washed and equal numbers of viable cells plated for glycolysis stress test. After calibration of the analyzer, glucose, oligomycin A, and 2-DG were applied sequentially at 18, 36 and 54 min. Glycolysis was measured by evaluating the difference in maximum rate before oligomycin injection (36 min) and before glucose injection (18 min). Glycolytic capacity was measured by evaluating the difference between maximum rate after oligomycin injection (54 min) and before glucose injection (18 min). Glycolytic reserve was defined as the difference between glycolytic capacity and glycolysis.
RT-PCR for the analysis of glycolytic genes
WRO and WRO-LvR cells were treated with 2 μM KU757 for 48 h, and RNA was isolated using the miRNeasy kit according to the manufacturer’s protocol (Qiagen). RNA was quantified and its purity was validated by NanoDrop. cDNA was synthesized and genomic DNA was eliminated from the RNA samples using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-PCR was carried out using SYBR Green Real-time PCR Master Mix and the fold difference of target gene expression compared to that of the control group was calculated using the 2−ΔΔCT method. Actin and GAPDH were used as an internal control for RT-PCR.
Western blot analysis
WRO and WRO-LvR cells (70–80% confluency) were treated with 2 μM KU757 for 48 h. Cells were lysed with lysis buffer and their proteins extracted as previously described (17). Protein concentrations of cells and exosomes were determined using BCA and Micro BCA protein quantification assay kits (ThermoFischer Scientific manufacturer’s protocol). Equal amounts of protein were electrophoresed, transferred to a nitrocellulose membrane (BioRad), blocked in 5% milk, and probed with the appropriate primary antibodies for glycolysis and GAPDH (Cell Signaling Technology). Membranes were incubated with an appropriate secondary antibody (Cell Signaling Technology), treated with SuperSignal West Pico PLUS (ThermoFisher), visualized by enhanced chemiluminescence, and captured on autoradiography film (Molecular Technologies) on a Konica Minolta SRX 101A developer. ImageJ software (NIH) was used to determine the relative density of protein bands.
Exosome isolation and nanoparticle tracking analysis
WRO and WRO-LvR cells were cultured to 70–80% confluency. The cells were washed in PBS and cultured in serum free media for 24 h, after which the cells were treated with 2 μM KU757 for 48 h, to avoid interference from exosomes from FBS. The media was spun at 2,000 g x 30 min, then the supernatant was spun at 12,000 g x 1 h and then spun again at 100,000 g x 1 h using PET thin walled tubes (Manufacturer part number 75000471) (Thermo Scientific). The exosome pellet was collected, washed with PBS, spun at 100,000 g for 1 h, and dissolved in PBS. A 1:1000 dilution of exosomes in nuclease-free water was loaded into a Nanosight NS300. Videos of each sample were collected (sCMOS camera and Blue 488 laser with a frame rate/fps of 24.9825) to determine size distribution and nanoparticles concentration.
LncRNA analysis of thyroid cancer cells and exosomes
RNA was isolated from cells and exosome samples using the Qiagen miRNeasy kit and the Norgen Biotek Corporation Single Cell RNA Purification Kit (manufacturer’s protocol). For drug treatment, the WRO and WRO-LvR cells were treated with 2 μM KU757 for 48 h. RNA was quantified and its purity validated by NanoDrop. cDNA was synthesized after the elimination of genomic DNA from 1 μg of RNA using the RT2 First Strand Kit (Qiagen). For exosomes, cDNA was synthesized after the elimination of genomic DNA from 50 ng of RNA using the RT2 PreAMP cDNA Synthesis Kit First (Qiagen; manufacturer’s protocol). cDNA target templates were then pre-amplified using the RT2 PreAMP cDNA Synthesis Kit First (Qiagen). Differentially expressed lncRNAs were evaluated using qPCR (Qiagen RT2 lncRNA PCR Array Human Cancer Pathway Finder) and analyzed using Qiagen GeneGlobe data analysis.
Statistical analysis
All cell-based experiments were performed in triplicate. Best-fit normalized dose-response curves for lenvatinib and KU757 were used to calculate IC50 values with 95% confidence intervals in GraphPad Prism 7.0. Significance (95%, p<0.05) was determined using a student’s two-tailed, unpaired t-test or one-way ANOVA as appropriate. Data for the viability assay and glycolysis are presented as mean values with error bars denoting standard error of the mean.
Results
KU757 is effective in reducing the proliferation of thyroid cancer cells by targeting glycolysis
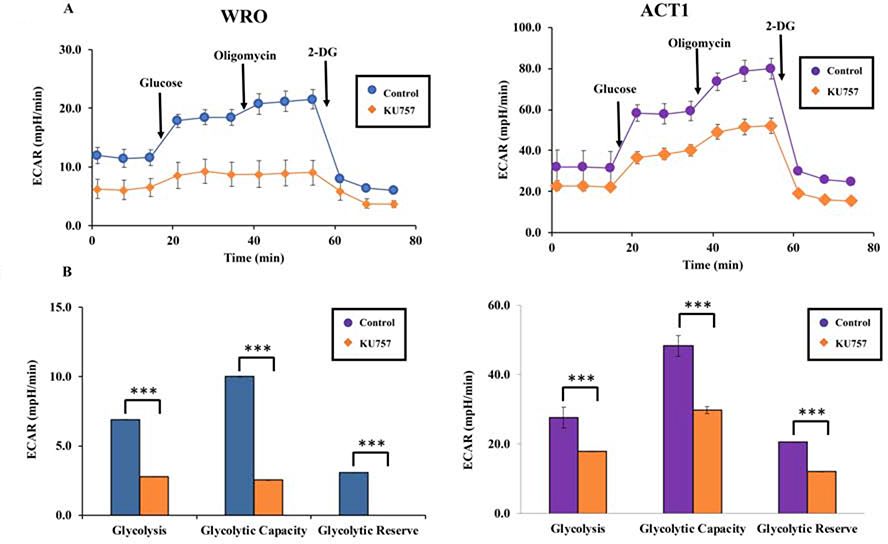
On cell viability assay, our novel C-terminal Hsp90 inhibitor KU757 was potent in two thyroid cancer cell lines, with a half-maximal inhibitory concentration (IC50) of 1.619 ± 0.18 μM for WRO and 1.806 ± 0.21μM for ACT1 (Figure 1), which is 4–10 fold less than IC50 in normal fibroblasts (17). KU757 treatment decreased glycolytic rate in WRO and ACT1 cells from 6.91 to 2.77 mpH/min (p<0.001) and from 27.66 to 17.80 mpH/min (p<0.001), respectively (Figure 2). Additionally, treatment with KU757 attenuated both glycolytic capacity (9.97 to 2.52 mpH/min in WRO cells and 48.31 to 29.79 mpH/min in ACT1 cells, p<0.001) and glycolytic reserve (3.06 to almost nil mpH/min in WRO cells and 20.65 to 11.99 mpH/min in ACT cells, p<0.001).
Figure 1.
C-terminal Hsp90 inhibitor KU757 is potent in targeting thyroid cancer cells. Genetically validated thyroid cancer cell lines WRO and ACT1 were treated with varying concentrations of KU757 for 72h. The percentage of viable cells was measured using the CellTiter Glo assay. IC50 values determined were determined by quantitative curve fitting in GraphPad prism 7.0.
Figure 2A and B.
Treatment of thyroid cancer cells WRO and ACT1with KU757 downregulates glycolysis. Glycolysis stress test was performed using the Seahorse XFe96 analyzer (Figure 2A). The x-axis in Figure 2A represents time since the start of the glycolysis stress test. Glucose was injected at 18 min, oligomycin was injected at 36 min and 2-DG was injected at 54 min. Data from Figure 2A was then used to calculate changes in glycolysis, glycolytic capacity, and glycolytic reserve shown in Figure 2B per the manufacturer protocol (Agilent Technologies) and represented with standard error bars.
Lenvatinib-resistant cells have increased glycolysis which is mitigated by treatment with KU757
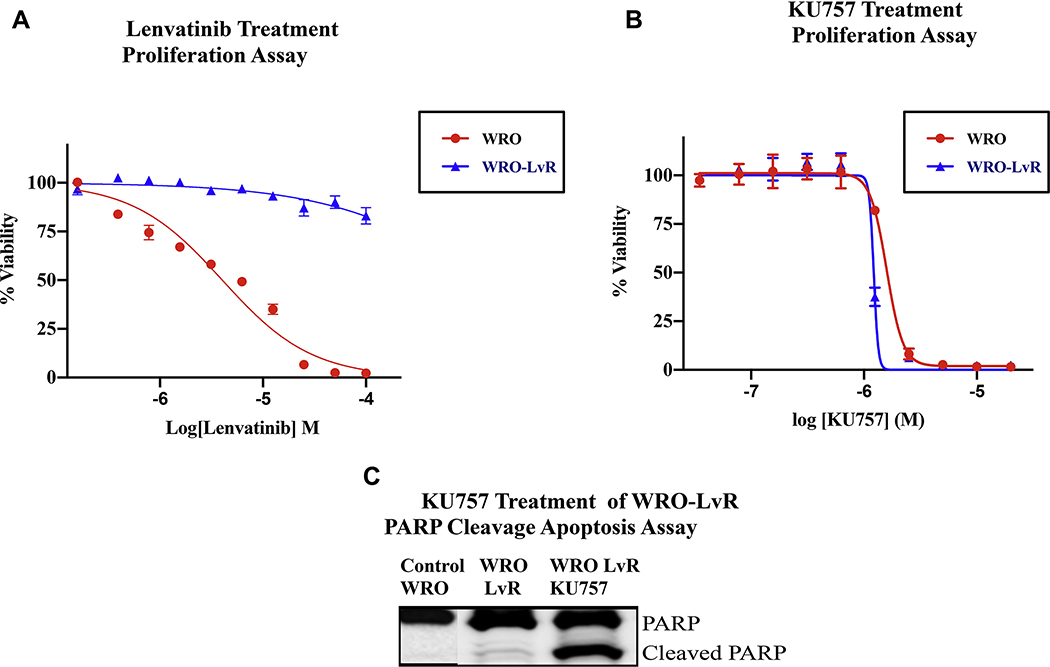
Since chemo- and radio-resistance is associated with aberrant glucose metabolism (6) and Hsp90 inhibition alters cancer glucose metabolism (14), we evaluated the effect of our C-terminal Hsp90 inhibitor on LvR thyroid cancer cells. Lv-resistant cells maintained viability at 50 μM lenvatinib, which is > 10-fold the normal drug IC50 level (4.032 μM) for the parent WRO cells (Figure 3A). KU757 treatment was able to potently and selectively inhibit the LvR cells, showing similar IC50 values between the WRO-LvR cells (1.619 ± 0.12 μM) and the parent WRO line (1.806 ± 0.17 μM) (Figure 3B) and this is almost 10-fold more potent than the effect previously observed in normal fibroblasts(17). In addition, KU757 treatment lead to increased cell death in the WRO-LvR cells as evidenced by PARP cleavage on WB (Figure 3C). This suggests that our novel C-terminal HSP90 inhibitor may be targeting the resistant pathways in the LvR cells that lead to increased growth of cancer after lenvatinib treatment. As HSP90 inhibition is known to alter glycolysis, which is one of the possible mechanism for lenvatinib resistant cancer growth, we then examined baseline glycolysis in WRO-LvR cells compared to the parent WRO cells and evaluated the effect of KU757 treatment on glycolysis in WRO-LvR cells. Drug resistance resulted in upregulation of glycolytic rate from 6.91 for WRO cells to 19.74 mpH/min for WRO-LvR cells. KU757 down-regulated glycolytic rate in WRO-LvR cells back to near baseline levels (8.62 mpH/min; p<0.001) (Figure 4). Consistent with an increase in glycolysis, WRO-LvR cells had higher levels of glycolytic capacity (30.34 vs. 9.97 in WRO; p<0.01) and glycolytic reserve (10.61 vs. 3.06 in WRO; p<0.01). Measurement of the glycolytic capacity and reserve also indicated a significant decrease (30.34 to 7.69 and 10.61 to almost zero, p<0.001) when the resistant WRO-LvR cells were treated with KU757.
Figure 3A,B and C.
KU757 treatment is effective in treating lenvatinib-resistant cells. WRO-LvR cells were treated with varying concentrations of (A) Lenvatinib and (B) KU757. Cell viability was determined by cellTiter Glo assay. IC50 values indicated the development of lenvatinib resistance (>50 μM for WRO-LvR cells compared to 4.03μM for the parent WRO cells) and the sensitivity of KU757 (1.396 μM for WRO-LvR and 1.589 μM for WRO) in targeting the resistant cells. (C) Immunoblot analysis of PARP cleavage after treatment of WRO (Control) and WRO-LvR cells (middle column) with solvent DMSO, and WRO-LvR cells with 2μM KU757 to evaluate cell death by measuring for PARP-cleavage. KU757 demonstrated increased cell death with PARP-cleavage in the WRO-LvR cells.
Figure 4.
Lenvatinib resistance increases glycolysis and KU757 treatment attenuates glycolysis. Using the Seahorse XFe96 analyzer, glycolysis stress test was performed on WRO-LvR and WRO cells. Results indicate the upregulation of total glycolysis, glycolysis capacity, and glycolytic reserve in WRO-LvR cells compared to WRO cells that is reduced after treatment with KU757. The x-axis represents time since the start of the glycolysis stress test. Glucose was injected at 18 min, oligomycin was injected at 36 min and 2-DG was injected at 54 min.
Treatment of WRO-LvR cells with KU757 attenuates the expression of glycolytic genes and proteins
Noting downregulation of glycolysis with KU757 treatment, we further evaluated the expression levels of glycolytic genes and proteins by RT-PCR and western blot (WB) analysis. WRO-LvR cells had significantly increased expression of lactate dehydrogenase (LDH) (p<0.0001), pyruvate kinase 2 (PKM2) (p<0.0001), and hexokinase 2 (HK2) (p=0.0098) genes compared WRO cells (Figure 5A). KU757 treatment down regulates glycolytic gene expression; there is significantly decreased expression of LDH (p<0.0001), PKM2 (p<0.0001), and HK2 (p=0.0028) in treated compared to untreated WRO-LvR cells. This suggests that Hsp90 inhibition is effective at mitigating LvR-upregulated glycolysis. To evaluate changes in protein levels of glycolytic enzymes and to validate glycolysis gene level expression, we performed WB analysis (Figure 5B) of WRO and WRO-LvR cells with and without KU757 treatment. Similar to our findings at the gene-level, WRO-LvR cells treated with KU757 had a 46.3%, 66.5%, and 41.2% decrease in LDHA, HK2, and PKM1/2 protein levels, respectively, when compared to untreated control WRO or WRO-LvR cells. Levels of glycolytic proteins HK1 and PFDH did not show any appreciable changes after treatment with KU757 (data not shown). Overall, WRO-LvR cells showed increased expression of HK2, PKM1/2 and LDH which was attenuated after KU757 treatment.
Figure 5A and B.
RNA and protein expression in parent WRO and WRO-LvR cells were evaluated with or without treatment with 2 μM KU757 for 48 h. Analysis of glycolytic genes (Figure 5A) and proteins (Figure 5B) by RT-PCR and western blot indicate that glycolytic genes and proteins are upregulated in WRO-LvR cells compared to parent WRO cells. Treatment of WRO-LvR cells with KU757 results in downregulation of glycolytic enzymes.
Treatment with KU757 results in differential regulation of oncogenic, tumor suppressor and glycolysis related lncRNAs in both thyroid cancer cells and exosomes
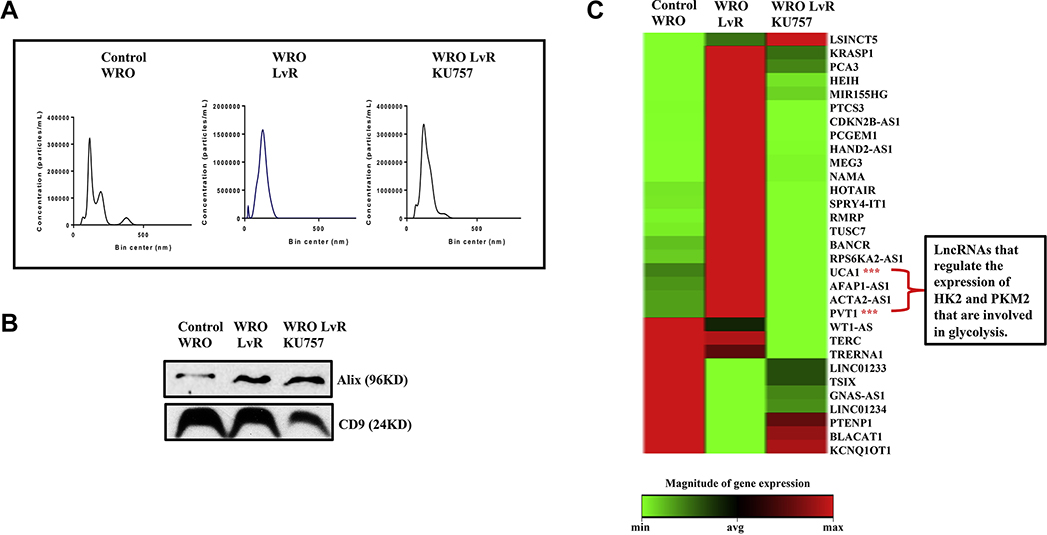
As lncRNA dysregulation plays a role in thyroid cancer growth, migration, and invasiveness, qRT-PCR was used to study how lncRNA expression in WRO-LvR cells (compared to control WRO cells) is altered after treatment with KU757. Several oncogenic lncRNAs involved in multidrug resistance and metastasis were upregulated in WRO-LvR cells compared to control WRO cells (expression heat map in Figure 6, p<0.05 for each). Treatment of WRO-LvR cells with KU757 significantly down-regulated these oncogenic lncRNAs and upregulated several tumor suppressor lncRNAs (Figure 6, p<0.05). Additionally, lncRNAs that are associated with glycolysis (LINC00963, LINC00152, PVT1, and TUG1) were upregulated in WRO-LvR cells, and KU757 treatment of lenvatinib resistant cells downregulated these lncRNAs (marked with “***” in Figure 6). To better understand the changes in the tumor microenvironment (TME) in lenvatinib-resistant thyroid cancers cells and to evaluate how KU757 alters the TME, we evaluated changes in exosomal lncRNAs. Cellular exosomes were isolated, purified, and verified by WB for ALIX, CD9, and absence of calnexin as well as by NTA (Figure 7A and B). Absence of endoplasmic reticulum marker protein, calnexin in our exosomes indicated that the exosomes were not contaminated with vesicles (data not shown). Exosomal lncRNAs were evaluated by qPCR (Figure 7C). A number of lncRNAs that play an important role in glycolysis, such as PVT1 and UCA1, were significantly upregulated in exosomes isolated from WRO-LvR cells when compared to WRO cells (p<0.05) and significantly down regulated after KU757 treatment of WRO-LvR cells (p<0.05).
Figure 6.
Heat map of differentially expressed lncRNAs in thyroid cancer cells. Analysis of lncRNA expression indicated statistically significant upregulation of oncogenic lncRNAs in WRO-LvR cells compared to WRO cells that was downregulated after 2 μM KU757 treatment for 48 h (greater than 2-fold change, p<0.05). LncRNAs marked with (***) contribute to increased glycolysis. These lncRNAs up regulated in WRO-LvR cells were downregulated after treatment of these cells with KU757.
Figure 7A, B and C.
KU757 treatment of WRO-LvR downregulates the glycolytic and oncogenic lncRNAs present in exosomes. Exosomes were isolated by standard ultracentrifugation method. Nanoparticle analysis by NTA (Figure 7A) and Western Blot for Alix, CD9 (Figure 7B) show the purity of exosomes. LncRNA analysis of exosomes was performed by RT-PCR to evaluate differentially expressed lncRNAs (Figure 7C). TUG1 and UCA1 (marked with (***)) regulate the expression of HK2 and PKM2 that are involved in glycolysis.
Discussion
The RTK inhibitor lenvatinib is a mainstay of treatment for advanced, RIA-resistant recurrent or metastatic thyroid cancers. However, most patients experience adverse side effects and develop resistance to lenvatinib therapy. One of the possible mechanisms through which resistance to TKIs occurs is the Warburg effect, a pro-survival mechanism by cancer cells to increase metabolism through glycolysis (13). It is known that Hsp90 participates in metabolic rewiring either by directly changing the conformation, stability, and function of metabolic pathway enzymes or by indirectly regulating the signaling pathways that alter the expression of proteins involved in the metabolic network. We therefore expect that inhibition of Hsp90 using our novel C-terminal Hsp90 inhibitor, KU757, may be effective at targeting glycolysis and TKI resistance. This is supported by our finding that KU757 was equally effective in inhibiting proliferation in both the parent WRO and WRO-LvR cancer cells. Additionally, we observed an increase in glycolytic capacity and glycolytic reserve of LvR thyroid cancer cells after the development of drug resistance, which was attenuated after treatment with KU757. Similar to WRO-LvR cells with high glycolytic capacity, the ACT1 cells that have a much higher glycolytic rate were also sensitive to KU757 treatment. To further test differences in glycolytic activation between WRO and WRO-LvR cells, RT-PCR and WB for glycolytic genes and proteins were performed. This analysis indicated upregulation of three glycolytic enzymes (LDH, PKM1/2, and HK2) in WRO-LvR cells compared to WRO cells. Consistent with our glycolysis stress test, these results support the claim that lenvatinib-resistant DTC cells have increased expression and activation of glycolytic genes. Furthermore, we found that our C-terminal Hsp90 inhibitor, KU757, significantly reduced the expression and abundance of the glycolytic genes and proteins LDH, PKM2, and HK2, which are normally upregulated in LvR cells. These results indicate that KU757 effectively treats lenvatinib-resistant thyroid cancer cells and inhibits the increased expression/activation of glycolytic genes seen in LvR cells.
Because lncRNA dysregulation has been shown to play a role in thyroid cancer growth, migration, and invasiveness, we predicted that our C-terminal Hsp90 inhibitor could affect lncRNA expression. To test this prediction, qRT-PCR was used to study how KU757 modulates lncRNA expression in WRO and WRO-LvR cells. Results demonstrated that several oncogenic lncRNAs involved in multidrug resistance and metastasis are upregulated in WRO-LvR cells compared to untreated WRO cells, whereas treatment with KU757 significantly downregulated most of these while upregulating several tumor suppressor lncRNAs (Figure 6). LncRNAs that are known to accelerate thyroid cancer growth, migration, and invasion such as NEAT1, HOTAIR, PVT1, and SPRY4-IT1 are significantly upregulated in WRO-LvR cells, but were significantly downregulated back to near baseline levels with KU757 (p<0.005). Additionally, lncRNAs such as LINC00963, LINC00152, PVT1, MALAT1, and TUG1 (marked with “***”, Figure 6) that contribute to tumorigenesis by targeting glycolysis are upregulated in WRO-LvR cells, but again are downregulated after treatment with KU757. Together, these results demonstrating downregulation of the lncRNA PVT1 and glycolytic enzyme HK2 are consistent with prior findings in which PVT1 altered glycolysis through the miR-497/HK2 pathway in osteosarcoma (19).
The exosomal process is abnormal in cancer cells, and exosomes have been shown to play a role in tumor initiation, growth, progression, and drug resistance (20, 21). To evaluate this further, we sought to investigate what changes in exosomal lncRNAs occur in the tumor microenvironment of WRO-LvR cells compared to WRO cells and how our C-terminal Hsp90 inhibitor treatment might affect that TME. Exosomal lncRNA analysis allowed for the comparison of lncRNA expression profiles in cells (Figure 6) and within exosomes (Figure 7). Several oncogenic lncRNAs, such as PCA3, PVT1, and UCA1, were significantly upregulated in exosomes isolated from WRO-LvR cells when compared to exosomes from WRO cells. Additionally, many tumor suppressor lncRNAs such as PTENP1, and GNAS-AS1 were significantly downregulated in exosomes isolated from WRO-LvR cells compared to WRO cells. Importantly, exosomes from WRO-LvR cells treated with KU757 downregulated oncogenic lncRNAs and upregulated tumor suppressor lncRNAs. Furthermore, our results shown in Figures 6 and 7 indicate that cells and their released exosomes have similar, but distinct lncRNA expression profiles. In both cells and exosomes, however, our C-terminal Hsp90 inhibitor KU757 was able to alter lncRNA expression changes that are associated with lenvatinib resistance. Further investigation is necessary to evaluate the significance of differential lncRNA expression between cells and exosomes and why certain lncRNAs are expressed in cells but not exosomes and vice versa. What is clear, however, is that KU757 targets and down-regulates several oncogenic lncRNAs and upregulates several tumor suppressor lncRNAs both in cells and within the exosomes of the TME. In summary, these alterations in lncRNA expression levels by KU757 contribute to its ability to overcome lenvatinib resistance.
Together, our results suggest that the novel C-terminal Hsp90 inhibitor, KU757 can effectively inhibit lenvatinib-resistant thyroid cancer cells in vitro. KU757 both inhibits the activation of many glycolytic enzymes known to be upregulated in LvR thyroid cancer, and targets and down-regulates several oncogenic lncRNAs upregulated both in the LvR cells and their released exosomes. C-terminal Hsp90 inhibition may represent a potential novel strategy for patients with radioiodine-refractory differentiated thyroid cancer that is resistant to lenvatinib. Limitations of the present study include (1) the usage of single lenvatinib resistant thyroid cancer cell line, (2) exosomes were taken from tumor cells in cultured media, and (3) upregulated glycolysis is just one mechanism of lenvatinib resistance. Therefore, further evaluation of additional resistance pathways in multiple lenvatinib resistant thyroid cancer cell lines and in vivo investigation as well as evaluation of patient-derived exosomes is warranted to determine the translatability of these important in vitro findings to better elucidate the clinical potential of this novel therapeutic for patients with advanced TKI-resistant thyroid cancer.
Table 1.
List of glycolysis related primer sequences used for the RT-PCR analysis.
| Gene ID | Primer (5’-3’) |
|---|---|
| LDH-FP | ATT CAG CCC GAT TCC GTT AC |
| LDH-RP | GAC ACC AGC AAC ATT CAT TCC |
| Pkm2-FP | TGTCTGGAGAAACAGCCAAG |
| Pkm2-RP | TCCTCGAATAGCTGCAAGTG |
| HK2-FP | CGGCCGTGCTACAATAGG |
| HK2-RP | CTCGGGATCATGTGAGGG |
| β-actin-FP | TGACAGCAGTCGGTTGGA |
| β-actin-RP | CAAAGTCCTCGGCCACAT |
Acknowledgments
Funding/Support:
This work was funded in part by the National Institutes of Health [T32 CA009672 (TW), R01 CA173292 and R01 CA216919 (MSC and BSJB), 3U01 CA120458 (MSC and BSJB)], Coller Surgical Society Research Fellowship (TW), the University of Michigan Comprehensive Cancer Center Support Grant P30-CA-046592, and the University of Michigan Department of Surgery.
Footnotes
COI/Disclosures: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sui F, Ji M, Hou P. Long non-coding RNAs in thyroid cancer: Biological functions and clinical significance. Mol Cell Endocrinol. 2018;469:11–22. [DOI] [PubMed] [Google Scholar]
- 2.Worden F Treatment strategies for radioactive iodine-refractory differentiated thyroid cancer. Ther Adv Med Oncol. 2014;6(6):267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–9. [DOI] [PubMed] [Google Scholar]
- 4.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–30. [DOI] [PubMed] [Google Scholar]
- 5.White PT, Subramanian C, Zhu Q, Zhang H, Zhao H, Gallagher R, et al. Novel HSP90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery. 2016;159(1):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J, Xia L, Liang J, Han Y, Wang H, Oyang L, et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J Exp Clin Cancer Res. 2019;38(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciavardelli D, Bellomo M, Consalvo A, Crescimanno C, Vella V. Metabolic Alterations of Thyroid Cancer as Potential Therapeutic Targets. Biomed Res Int. 2017;2017:2545031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho RG, Fortunato RS, Carvalho DP. Metabolic Reprogramming in Thyroid Carcinoma. Front Oncol. 2018;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahm JH, Kim HM, Koo JS. Glycolysis-related protein expression in thyroid cancer. Tumour Biol. 2017;39(3):1010428317695922. [DOI] [PubMed] [Google Scholar]
- 10.Goran Ronquist K Extracellular vesicles and energy metabolism. Clin Chim Acta. 2019;488:116–21. [DOI] [PubMed] [Google Scholar]
- 11.Fan C, Tang Y, Wang J, Xiong F, Guo C, Wang Y, et al. Role of long non-coding RNAs in glucose metabolism in cancer. Mol Cancer. 2017;16(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 13.Condelli V, Crispo F, Pietrafesa M, Lettini G, Matassa DS, Esposito F, et al. HSP90 Molecular Chaperones, Metabolic Rewiring, and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells. 2019;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd KM, Subramanian C, Sanchez J, Motiwala HF, Liu W, Cohen MS, et al. Synthesis and Biological Evaluation of Novobiocin Core Analogues as Hsp90 Inhibitors. Chemistry. 2016;22(20):6921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, et al. KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol Pharmacol. 2009;76(6):1314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Zhao H, Hall JA, Brown D, Brandes E, Bazzill J, et al. Triazole Containing Novobiocin and Biphenyl Amides as Hsp90 C-Terminal Inhibitors. Medchemcomm. 2014;5(9):1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian C, Kovatch KJ, Sim MW, Wang G, Prince ME, Carey TE, et al. Novel C-Terminal Heat Shock Protein 90 Inhibitors (KU711 and Ku757) Are Effective in Targeting Head and Neck Squamous Cell Carcinoma Cancer Stem cells. Neoplasia. 2017;19(12):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SM, Mukerji R, Samadi AK, Zhang X, Zhao H, Blagg BS, et al. Novel C-terminal Hsp90 inhibitor for head and neck squamous cell cancer (HNSCC) with in vivo efficacy and improved toxicity profiles compared with standard agents. Ann Surg Oncol. 2012;19 Suppl 3:S483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han X, Yang Y, Sun Y, Qin L, Yang Y. LncRNA TUG1 affects cell viability by regulating glycolysis in osteosarcoma cells. Gene. 2018;674:87–92. [DOI] [PubMed] [Google Scholar]
- 20.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–94. [DOI] [PubMed] [Google Scholar]
- 21.de la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T, Kussmann M. “Exosomics”-A Review of Biophysics, Biology and Biochemistry of Exosomes With a Focus on Human Breast Milk. Front Genet. 2018;9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]