Abstract
Purpose of the Review:
Osteoporosis is commonly diagnosed through the clinical assessment of bone quantity using bone mineral density, however the primary clinical concern is bone fragility. Bone fragility is determined by both bone quantity and bone quality. Over the past decade the gut microbiome has emerged as a factor that can regulate diseases throughout the body. This review discusses how microbial organisms and their genetic products that inhabit the gastrointestinal tract influence bone quantity, bone quality and bone strength.
Recent Findings:
Recent studies have shown that the gut microbiome regulates bone loss during estrogen depletion and glucocorticoid treatment. A series of studies has also shown that the gut microbiome influences whole bone strength by modifying bone tissue quality. The possible links between the gut microbiome and bone tissue quality are discussed focusing on the effects of microbiome-derived vitamin K.
Summary:
We provide a brief introduction to the gut microbiome and how modifications to the gut microbiome may lead to changes in bone. The gut microbiome is a promising target for new therapeutic approaches that address bone quality in ways not possible with current interventions.
Keywords: Gut Microbiome, Bone Quantity, Bone Quality, Bone Material Properties, Vitamin K
Introduction
Osteoporosis is characterized by bone loss and degradation of bone architecture leading to an increased risk of fracture during normal activities. Although low bone mineral density is the primary indicator of osteoporosis, fracture, not low bone density, is the primary clinical concern. Clinical fracture is the mechanical failure of the bone and is determined by the applied loads (e.g. a fall from standing height) as well as the ability of the bone to resist failure. Resistance to mechanical failure is determined by both the bone quantity and bone quality. Understanding the factors that contribute to mechanically relevant components of bone quantity and quality is necessary to reduce fracture risk below what is possible with current treatments [1•]. Bone quantity and quality are determined by genetic background, nutritional history, and hormonal status. The gut microbiome has recently emerged as a factor that can have a profound effect on bone quantity, quality and overall bone strength.
The gut microbiome consists of the community of microbial organisms that inhabit the gastrointestinal tract, including bacteria, archaea, single celled eukaryotes, viruses and their genetic components [2•]. The gut microbiome of a human typically includes more than 1000 distinct bacterial species that form a complex interacting community [3]. The complex microbial communities within the gut are examined using high throughput sequencing to determine which organisms are present (taxonomy) as well as the functional capacity (metagenomics). Studies of the gut microbiome have opened entirely new avenues for understanding disease pathogenesis and potential treatments. The gut microbiome influences human health and can contribute to diabetes, inflammatory arthritis, and Alzheimer’s disease as well as other conditions [2•]. Recent studies have linked the gut microbiome to bone and osteoporosis. The gut microbiome is an enticing target for osteoporosis treatments for a number of reasons: First, the gut microbiome has the potential to address aspects of bone quantity and quality that are not addressed by current pharmaceutical techniques. Second, existing microbiome-based therapeutics such as prebiotics and probiotics are innocuous and inexpensive, enabling widespread use [4]. Third, a well-balanced gut microbial community is self-sustaining and robust against transient disruptions [5–7]. Hence, an intervention that establishes a microbial community beneficial to bone may only need to be applied occasionally (for example at the time of screening colonoscopies), thereby avoiding daily or even monthly dosing typical of current osteoporosis therapeutics.
Here we review recent studies focusing on the effects of the gut microbiome on bone. Although the effects of the gut microbiome on bone quantity and bone loss are discussed, the review focuses on the effects of the gut microbiome on bone tissue failure properties because resistance to mechanical failure is the primary goal of therapeutics for osteoporosis [8, 9]. For more reviews focusing on molecular mechanisms linking the microbiome to osteoporosis we refer the reader to other articles [10–13].
Microbial Regulation of Bone
The constituents of the gut microbiome are influenced by host diet, genetics, and physiology [14, 15]. There are multiple mechanistic pathways through which modifications to the constituents of the gut microbiome influence organs distant from the gut. We have classified the mechanisms that link the microbiome to bone in three ways: 1) regulation of nutrient absorption, 2) the regulation of the immune system and 3) the translocation of microbial molecular components across the gut endothelial barrier [10].
The primary effect of the gut microbiome on nutritional absorption is through regulation of inflammation at the gut lining. The gut microbiota can stimulate inflammatory responses in gut endothelial cells that lead to excessive inflammation that can hinder the absorption of key nutrients [16]. Inflammation at the gut endothelial barrier can influence gut permeability, enhancing the translocation of microbial molecules (see below). Additionally, microbes within the gut produce a number of molecules used by the host. The gut microbiota is a major source of vitamin B and vitamin K [17, 18]. These vitamins may have direct and/or indirect effects on bone biology and the bone matrix. For example, vitamin K is necessary to functionalize a number of proteins including osteocalcin, the most abundant non-collagenous protein in bone matrix (see below). The absence of matrix-bound osteocalcin in bone matrix makes the tissue more brittle and prone to fracture [19]. Additionally, vitamin K, by stimulating the xenobiotic receptor on osteoblasts can influence bone remodeling and bone mineralization processes [20].
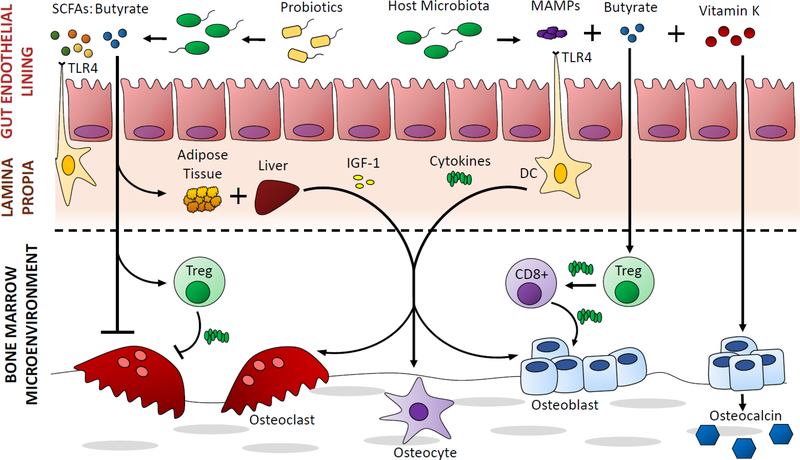
The gut microbiome provides a regular stimulus to the host immune system. Gut microbiota are in constant contact with dendritic cells and other immune cells at the endothelial boundary. Direct contact with host immune cells can trigger innate and adaptive immune responses that lead to the release of pro- and anti-inflammatory cytokines, and the stimulation of other resident immune cell populations. Additionally, immune cell populations stimulated at the gut lining may migrate to bone where they can influence bone remodeling [21] (Figure 1). For example, regulatory T cells (Tregs) activated at the gut lining can migrate to the bone marrow and have an effect on bone remodeling in the endosteal and trabecular envelopes [22, 23]. Pharmaceuticals that alter the interactions between the gut microbiota and the host [24, 25] also have the potential to influence bone loss and osteoporosis.
Figure 1.
A cartoon illustrating links between the gut microbiome and bone in some of the studies discussed in this review [12, 36, 26••, 66••]. Microbial short chain fatty acids, stimulation of the immune system and microbe-derived vitamin K have been proposed/shown to influence osteoclast and osteoblast activity.
Lastly, microbial molecular products can have a profound effect on the host. Microbial products including bacterial proteins (lipopolysaccharide, flagellin, etc.) are known collectively as microbe associated molecular patterns (MAMPs) and can initiate inflammatory responses in host cells by triggering innate immune receptors. Additionally, microbial metabolites released by the gut microbiota can influence the host. Short chain fatty acids released by the microbiota have a direct effect on host endothelial cells and are also distributed systemically. Butyrate is the most abundant short chain fatty acid produced by gut microbes and has a direct effect on epithelial cells that line the colon and also regulates Tregs that indirectly promote osteoblast activation and bone formation [26••]. Additionally, that butyrate can directly inhibit osteoclast differentiation [27] (Figure 1). Recent studies have suggested that probiotics have beneficial effects on the host primarily by stimulating increased butyrate production by commensal microbes [22, 23, 26••].
Microbiome-Induced Alterations in Bone Quantity
The primary indicator of osteoporosis and a major determinate of bone strength is bone quantity. A number of clinical conditions associated with reduced bone mineral density and increased bone fragility are also associated with alterations in the constituents of the gut microbiota. However, the clinical studies necessary to link the gut microbiome to reduced bone mineral density in humans have not yet been reported. Here we review preclinical studies that have shown a relationship between the constituents of the gut microbiome and bone geometry and density.
Preclinical studies in rodents have indicated that the gut microbiome can have a profound effect on bone remodeling, bone geometry and whole bone mass [11, 12, 28]. The first report showing an effect of the gut microbiota on bone examined germ-free mice. Germ-free mice are raised in a completely sterile environment and are never exposed to a live microbe. The lack of a microbiome in young germ-free mice is associated with increases in overall bone mass and trabecular bone volume fraction [28]. Subsequent studies showed that differences in bone mass between germ-free mice and conventionally raised mice are related to animal age and the period of exposure to environmental microbes [29]. Both of these studies show that chronic oral dosing with a cocktail of broad spectrum antibiotics to decimate the gut microbiome (i.e. remove 99% of the microbes) can recapitulate the bone phenotype of germ-free mice [30–32].
Recent studies have shown that the presence of gut microbiota influences the amount of bone loss in models of osteopenia. Estrogen depletion results in osteopenia in mice, but in the absence of a gut microbiota (either using germ-free mice or using cocktails of broad spectrum antibiotics) estrogen depletion does not cause substantial bone loss [33]. Parathyroid hormone (PTH) dosing has long been known to influence bone, causing osteopenia when dosed continuously [34, 35] and causing increases in bone mass when provided intermittently [23]. However, in the absence of the gut microbiome, neither continuous nor intermittent PTH significantly alters trabecular bone volume fraction [22, 23]. Glucocorticoid-induced bone loss is a major cause of osteopenia and osteoporosis in humans, but when the gut microbiota is depleted in mice, glucocorticoid treatment has negligible effects on bone mass [30]. These studies clearly indicate that the microbiome can influence both mechanisms of bone loss as well as bone gain, although the components of the microbiome (microbial taxa or genes) that promote bone loss and gain are not yet known. Ongoing studies seek to determine the role of the immune system and/or changes in gut permeability (i.e. “gut leakiness”) that lead to microbiome-induced changes in bone. Additionally, these preclinical studies implicate the potential for microbiome-based therapeutics that can slow bone loss or even promote bone gain.
Probiotics consist of live microbes and are a widely available microbiome-based intervention [13, 28, 36, 37]. Although commonly referred to as “beneficial” microbes, recent studies have suggested that many probiotics do not have direct effects on the host but rather provide benefits by modifying the activity of other commensal organisms [11]. For example, in a study done in mice, an oral probiotic provided to the mice increased the production of butyrate in the gut leading to increases in bone formation. The probiotic itself was not a source of butyrate, but the presence of the probiotic promoted butyrate production by other components of the gut microbiome [26••]. The benefits of probiotics to bone in humans was recently demonstrated in a randomized control trial in which daily probiotic dosing was shown to reduce postmenopausal bone loss [38••]. Probiotics, however are only the most rudimentary microbiome-based therapeutic and their beneficial effects can vary based on probiotic species or strain as well as manufacturing quality (proportion of live microbes in an oral dose). More refined probiotic approaches are currently in development as are therapeutics that directly target the microbiome. The gut microbiome includes over 5 million microbial genes [39], many of which may be viable targets for therapeutics that are beneficial to bone mineral density.
Microbiome-Induced Alterations in Bone Tissue Strength
Although the studies reviewed so far have demonstrated an effect of the microbiome on bone quantity and/or bone mineral density, relatively little work has been done examining the effect of the microbiome on bone fragility. As mentioned above, the primary clinical concern for osteoporosis is bone fragility. While bone mineral density is a useful predictor of bone fragility, modifications to bone quality can alter bone fragility and fracture risk in ways that are not apparent from measures of bone quantity [40]. A number of clinical conditions are associated with greater risk of fracture than would be expected from measures of bone mineral density, including obesity [41, 42], type 2 diabetes [43, 44], and chronic kidney disease [45, 46] – each of these conditions is also associated with noticeable changes in the constituents of the gut microbiome [47, 48].
Bone tissue material properties are a major contributor to bone strength and bone fragility and are not represented by measures of bone mineral density. The most drastic effects of bone tissue material properties on bone strength and fragility are seen in individuals with osteogenesis imperfecta, a disease in which alterations in the collagen I protein result in bone tissue that is substantially more brittle [49], leading to frequent fractures and associated morbidity. Additionally, bone tissue material properties may also be impaired by pharmaceuticals. Sodium fluoride, a treatment that increases bone mineral density, leads to impaired bone tissue mechanical properties [50, 51] and a corresponding increase in fracture risk in patients [52]. There are also findings suggesting that pharmaceuticals can improve bone tissue material properties. The anti-resorptive agent raloxifene, in addition to altering bone remodeling and bone quantity, can also improve bone tissue material properties [53, 54].
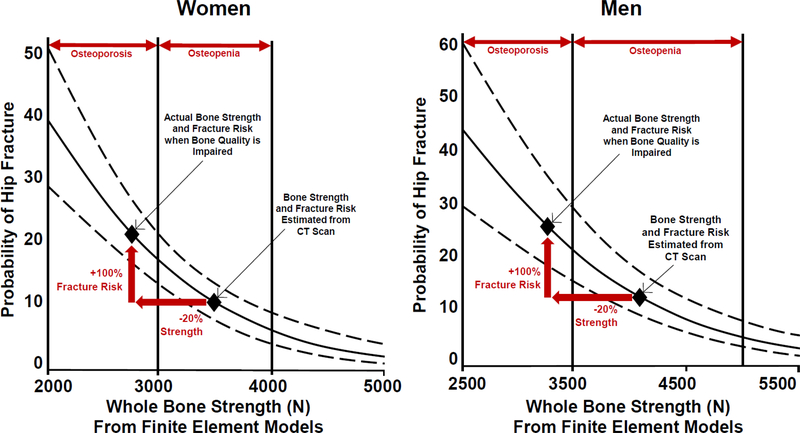
Modern biomechanical analyses make it possible to estimate whole bone strength from computed tomography scans that account for the effects of bone mineral density, whole bone geometry and the internal distribution of bone density [55]. More recently, these approaches have been shown in clinical studies to be closely associated with fracture risk [56]. These modeling approaches, while useful for predicting clinical fracture, are based on experimental examinations of bone from otherwise healthy individuals (i.e. individuals with “normal” bone tissue quality). However, whole bone strength is extremely sensitive to changes in tissue strength. Finite element models of whole bones suggest that extreme alterations in bone tissue brittleness can lead to reductions in whole bone strength as great as 50% [57]. While 50% reductions are a theoretical maximum, even reductions in whole bone strength by 20%, when caused by alterations in bone tissue strength, have the potential to greatly alter fracture risk in ways that would not be apparent from bone mineral density. For example, in the hip, the relationship between whole bone strength and probability of fracture follows a nonlinear relationship [56] (Figure 2). This relationship suggests that a 20% reduction in whole bone strength is capable of greatly altering the probability of fracture, especially in individuals with osteopenia (Figure 2 illustrates a doubling of fracture risk caused by a 20% reduction in whole bone strength). If the alteration in whole bone strength is caused by impaired tissue strength, the difference in probability of fracture would not be expected from densitometry. In this light, alterations in bone tissue strength that generate even modest changes in whole bone strength may influence fracture risk in ways that are not expected from bone mineral density.
Figure 2.
The relationship between probability of hip fracture and whole bone strength determined using participant specific finite element models from computed tomography images is shown for women and men [70]. The solid line indicates the age-adjusted logistic regression model expressing fracture probability v. whole bone strength estimated from finite element models assuming normal bone tissue quality (as reported by [56]). Dashed lines indicate the 95% confidence interval of the logistic regression. An impairment of bone tissue quality that reduces whole bone strength by 20% increases the probability of fracture by 100% (red single-headed arrows). The range of whole bone strength corresponding to individuals with osteoporosis and osteopenia as defined by BMD is indicated [56].
Recently our group found that alterations to the gut microbiome in mice during rapid bone acquisition (age 1–4 months) could lead to impaired whole bone strength caused by impaired bone tissue material properties[58•]. The alterations in bone tissue strength were not associated with gut inflammation or nutritional deficiencies (serum calcium was not modified nor was body mass or whole bone length), suggesting a more direct link between the gut microbiome and bone tissue. While there are many ways in which the gut microbiome may stimulate the host leading to changes in bone quantity (see above), relatively few are known to alter bone tissue quality [59]. Most discussion of bone tissue quality focuses on the chemical changes in bone matrix including collagen cross-links and collagen quality, mineral content, non-collagenous proteins, microdamage and osteocyte physiology. However, there is relatively little discussion of how these modifications to bone tissue quality come about in vivo. Genetic abnormalities (e.g. osteogenesis imperfecta) and the accumulation of matrix changes/damage associated with excessive tissue age (the length of time the tissue has been present in the body, not to be confused with the age of the individual) are well recognized means of modifying bone tissue [59]. Excessive tissue age is a likely contributor to bone tissue quality in humans, but is unlikely to explain the alterations in bone tissue quality observed in four month old mice.
Our recent work has implicated vitamins produced by the gut microbiota as a factor that influences bone matrix quality. Vitamin K is found in the diet but is also produced by the gut microbiota and has long been associated with bone health. Clinical studies have associated low vitamin K status with fracture risk but not with bone mineral density [60–62], an observation consistent with impaired bone tissue material properties. Vitamin K may influence bone matrix by regulating the presence of vitamin K dependent proteins in the bone matrix such as osteocalcin, the most abundant non-collagenous protein in bone. Vitamin K is necessary for the carboxylation of osteocalcin which allows proper binding of osteocalcin to bone mineral during bone formation. Insufficient vitamin K leads to an increase in the concentration of uncarboxylated osteocalcin in systemic circulation [63]. In the absence of osteocalcin, bone matrix is more brittle and less resistant to crack growth [19, 64]. In addition to regulating non-collagenous proteins such as osteocalcin, vitamin K can also directly stimulate the xenobiotic receptors in osteoblasts (SXR/PXR) and thereby alter the bone mineralization processes [20, 65]. Guss and colleagues recently associated microbiome-induced reductions to bone tissue strength in mice with reduced capacity of the gut microbiota to produce vitamin K, reduced vitamin K concentrations in the cecum, liver and kidneys, and reduced concentrations of osteocalcin in bone matrix [66••]. Further study is required to confirm the association between microbiome-derived vitamin K and bone tissue strength. Additionally, it remains possible that the gut microbiome may modulate bone matrix properties by stimulating the host immune system and/or the translocation of microbial molecular patterns, although mechanisms through which these factors would influence bone tissue quality, rather than simply altering tissue quantity, are not clear.
Conclusions and Open Questions
Although substantial attention has been paid to the role of the microbiome in health over the past 15 years (especially during the NIH Human Microbiome Project 2007–2016) the influence of the microbiome on host physiology has been known since the introduction of antibiotics in the early 20th century; the first animal studies of antibiotics showed that oral antibiotics influence animal growth including bone size [67–69], an effect attributed primarily to changes of the microbes resident in the gut. The idea that modifications to the gut microbiome could lead to changes in bone is therefore decades old.
The studies described in this review clearly demonstrate the effect of the microbiome on bone, but they raise many questions. First, most of the preclinical studies performed so far have looked at relatively young mice (less than 6 months of age) and it is unclear if modifications to the gut microbiome can regulate bone quantity and quality in aged animals. The ability of the gut microbiome to regulate bone quantity and quality late in life is particularly useful for the development of microbiome-based treatments for age-related osteoporosis. Second, the mechanisms that link the microbiome to bone remain to be determined, although the effects of immune cell populations [26••], circulating hormones [29] and microbe-derived vitamins [66••] suggest that there are multiple ways in which the gut microbiome may influence bone. Lastly, to our knowledge only a few peer-reviewed human studies have looked at the microbiome in the context of bone mineral density [38••]; however, none of these studies have reported the composition of the gut microbiota in participants, a key analysis that is necessary to identify microbial components that may influence bone. Lastly, there are no studies that have examined the microbiome in the context of fracture risk. Addressing these questions has the potential to lead to a new generation of microbiome-based interventions for osteoporosis that promise to address aspects of bone strength and fragility not well addressed by current therapies.
Acknowledgements
Research reported in the article was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under the National Institutes of Health award numbers R21AR068061, R21AR073454, and R21AR071534.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hernandez CJ, van der Meulen MC (2017) Understanding Bone Strength Is Not Enough. J Bone Miner Res 32:1157–1162•This review, intended for non-engineers, explains the effects of tissue material properties on whole bone strength
- 2.Knight R, Callewaert C, Marotz C, Hyde ER, Debelius JW, McDonald D, Sogin ML (2017) The Microbiome and Human Biology. Annu Rev Genom Hum Genet 18:65–86•This review motivates the need for further investigation into the microbiome in human health and disease.
- 3.Rajilić-Stojanović M, de Vos WM (2014) The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38:996–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimee M, Citorik RJ, Lu TK (2016) Microbiome therapeutics — Advances and challenges. Adv Drug Deliv Rev 105:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faith JJ, Guruge JL, Charbonneau M, et al. (2013) The Long-Term Stability of the Human Gut Microbiota. Science 341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozupone CA, Stombaugh J, Gonzalez A, Ackermann G, Wendel D, Vazquez-Baeza Y, Jansson JK, Gordon JI, Knight R (2013) Meta-analyses of studies of the human microbiota. Genome Res 23:1704–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaiss CA, Elinav E (2017) The remedy within: will the microbiome fulfill its therapeutic promise? J Mol Med 95:1021–1027 [DOI] [PubMed] [Google Scholar]
- 8.Einhorn TA (1992) Bone strength: The bottom line. Calcif Tiss Int 51:331–339 [DOI] [PubMed] [Google Scholar]
- 9.Järvinen TL, Sievänen H, Jokihaara J, Einhorn TA (2005) Revival of Bone Strength: The Bottom Line. J Bone Miner Res 20:717–720 [DOI] [PubMed] [Google Scholar]
- 10.Hernandez CJ, Guss JD, Luna M, Goldring SR (2016) Links Between the Microbiome and Bone. J Bone Miner Res 31:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacifici R (2018) Bone Remodeling and the Microbiome. Cold Spring Harb Perspect Med 10.1101/cshperspect.a031203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan J, Charles JF (2017) Gut Microbiome and Bone: to Build, Destroy, or Both? Curr Osteoporos Rep 15:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe L, Britton RA, Parameswaran N (2015) Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr Osteoporos Rep 13:363–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ (2014) Host lifestyle affects human microbiota on daily timescales. Genome Biol 15:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodrich JK, Waters JL, Poole AC, et al. (2014) Human Genetics Shape the Gut Microbiome. Cell 159:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu TK, Donaldson D (2003) Intestinal absorption in health and disease: micronutrients. Best Pract Res Cl Ga 17:957–979 [DOI] [PubMed] [Google Scholar]
- 17.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI (2011) Human nutrition, the gut microbiome and the immune system. Nature 474:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yatsunenko T, Rey FE, Manary MJ, et al. (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D (2012) Dilatational band formation in bone. Proceedings of the National Academy of Sciences 109:19178–19183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM (2009) Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol 297:C1358–C1367 [DOI] [PubMed] [Google Scholar]
- 21.Tsukasaki M, Takayanagi H (2019) Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nat Rev Immunol 19:626–642 [DOI] [PubMed] [Google Scholar]
- 22.Li J-Y, Yu M, Pal S, Tyagi AM, Dar H, Adams J, Weitzmann MN, Jones RM, Pacifici R (2020) Parathyroid hormone–dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest 130:1767–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Malik Tyagi A, Li J-Y, Adams J, Denning TL, Weitzmann MN, Jones RM, Pacifici R (2020) PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat Commun 11:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vich Vila A, Collij V, Sanna S, et al. (2020) Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun 11:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier L, Pruteanu M, Kuhn M, et al. (2018) Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555:623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi AM, Yu M, Darby TM, et al. (2018) The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 49:1116–1131.e7•• This study provides a link between the microbial metabolite butyrate and bone.
- 27.Yan J, Takakura A, Zandi-Nejad K, Charles JF (2018) Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes 9:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA 113:E7554–E7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schepper JD, Collins F, Rios‐Arce ND, et al. (2020) Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid‐Induced Osteoporosis. J Bone Miner Res 35:801–820 [DOI] [PubMed] [Google Scholar]
- 31.Rios-Arce ND, Schepper JD, Dagenais A, Schaefer L, Daly-Seiler CS, Gardinier JD, Britton RA, McCabe LR, Parameswaran N (2020) Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone 134:115269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schepper JD, Collins FL, Rios-Arce ND, Raehtz S, Schaefer L, Gardinier JD, Britton RA, Parameswaran N, McCabe LR (2019) Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J Bone Miner Res 34:681–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J-Y, Chassaing B, Tyagi AM, et al. (2016) Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. Journal of Clinical Investigation 126:2049–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilezikian JP (2018) Primary Hyperparathyroidism. J Clin Endocrinol Metab 103:3993–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iida-Klein A (2005) Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol 186:549–557 [DOI] [PubMed] [Google Scholar]
- 36.Zaiss MM, Jones RM, Schett G, Pacifici R (2019) The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest 129:3018–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ericsson AC, Franklin CL (2015) Manipulating the Gut Microbiota: Methods and Challenges: Figure 1. ILAR J 56:205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansson P-A, Curiac D, Lazou Ahrén I, Hansson F, Martinsson Niskanen T, Sjögren K, Ohlsson C (2019) Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet Rheumatology 1:e154–e162•• This is the first randomized control trial in which daily that showed that probiotic dosing reduced postmenopausal bone loss. This reveals the potential to lead to a new generation of microbiome-based interventions for osteoporosis.
- 39.Cully M (2019) Microbiome therapeutics go small molecule. Nat Rev Drug Discov 18:569–572 [DOI] [PubMed] [Google Scholar]
- 40.Hernandez CJ, Keaveny TM (2006) A biomechanical perspective on bone quality. Bone 39:1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ionova-Martin SS, Do SH, Barth HD, Szadkowska M, Porter AE, Ager JW, Ager JW, Alliston T, Vaisse C, Ritchie RO (2010) Reduced size-independent mechanical properties of cortical bone in high-fat diet-induced obesity. Bone 46:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslam MN, Jepsen KJ, Khoury B, Graf KH, Varani J (2016) Bone structure and function in male C57BL/6 mice: Effects of a high-fat Western-style diet with or without trace minerals. Bone Reports 5:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creecy A, Uppuganti S, Merkel AR, O’Neal D, Makowski AJ, Granke M, Voziyan P, Nyman JS (2016) Changes in the Fracture Resistance of Bone with the Progression of Type 2 Diabetes in the ZDSD Rat. Calcif Tissue Int 99:289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furst JR, Bandeira LC, Fan W-W, Agarwal S, Nishiyama KK, McMahon DJ, Dworakowski E, Jiang H, Silverberg SJ, Rubin MR (2016) Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J Clin Endocrinol Metab 101:2502–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM (2016) Hip Fracture in Patients With Non-Dialysis-Requiring Chronic Kidney Disease. J Bone Miner Res 31:1803–1809 [DOI] [PubMed] [Google Scholar]
- 46.Matias PJ, Laranjinha I, Azevedo A, et al. (2020) Bone fracture risk factors in prevalent hemodialysis patients. J Bone Miner Metab 38:205–212 [DOI] [PubMed] [Google Scholar]
- 47.Ramezani A, Raj DS (2014) The Gut Microbiome, Kidney Disease, and Targeted Interventions. JASN 25:657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad A, Yang W, Chen G, Shafiq M, Javed S, Ali Zaidi SS, Shahid R, Liu C, Bokhari H (2019) Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS ONE 14:e0226372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bishop N (2016) Bone Material Properties in Osteogenesis Imperfecta. J Bone Miner Res 31:699–708 [DOI] [PubMed] [Google Scholar]
- 50.Lafage MH, Balena R, Battle MA, Shea M, Seedor JG, Klein H, Hayes WC, Rodan GA (1995) Comparison of alendronate and sodium fluoride effects on cancellous and cortical bone in minipigs. A one-year study. J Clin Invest 95:2127–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chachra D, Turner CH, Dunipace AJ, Grynpas MD (1999) The Effect of Fluoride Treatment on Bone Mineral in Rabbits. Calcif Tissue Int 64:345–351 [DOI] [PubMed] [Google Scholar]
- 52.Riggs L, Hodgson S, O’Fallen M, Chao E, Wahner H, Muhs J, Cedel S, Melton J III (1990) Effect of Flouride Treatment on the Fracture Rate in Postmenopausal Women with Osteoporosis. N Engl J Med [DOI] [PubMed] [Google Scholar]
- 53.Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, Almer JD, Stock SR, Allen MR, Burr DB (2014) Bone cell-independent benefits of raloxifene on the skeleton: A novel mechanism for improving bone material properties. Bone 61:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell KM, Brown AP, Skaggs CG, Pulliam AN, Berman AG, Deosthale P, Plotkin LI, Allen MR, Williams DR, Wallace JM (2020) 6′-Methoxy Raloxifene-analog enhances mouse bone properties with reduced estrogen receptor binding. Bone Rep 12:100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keaveny TM (2010) Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans: BCT and bone strength. An NY Acad Sci 1192:57–65 [DOI] [PubMed] [Google Scholar]
- 56.Kopperdahl DL, Aspelund T, Hoffmann PF, Sigurdsson S, Siggeirsdottir K, Harris TB, Gudnason V, Keaveny TM (2014) Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res 29:570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nawathe S, Yang H, Fields AJ, Bouxsein ML, Keaveny TM (2015) Theoretical effects of fully ductile versus fully brittle behaviors of bone tissue on the strength of the human proximal femur and vertebral body. J Biomech 48:1264–1269 [DOI] [PubMed] [Google Scholar]
- 58.Guss JD, Horsfield MW, Fontenele FF, et al. (2017) Alterations to the Gut Microbiome Impair Bone Strength and Tissue Material Properties. J Bone Miner Res 32:1343–1353• This is the first study to demonstrate an effect of the gut microbiome on bone strength.
- 59.Alliston T (2014) Biological Regulation of Bone Quality. Curr Osteoporos Rep 12:366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knapen MHJ, Schurgers LJ, Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rønn SH, Harsløf T, Pedersen SB, Langdahl BL (2016) Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol 175:541–549 [DOI] [PubMed] [Google Scholar]
- 62.Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GKR, Salomonsen L, Fønnebø V (2010) Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 21:1731–1740 [DOI] [PubMed] [Google Scholar]
- 63.Division D, Mills J-O Low-Dose Daily Intake of Vitamin K2 (Menaquinone-7) Improves Osteocalcin g-Carboxylation: A Double-Blind, Randomized Controlled Trials. J Nutr Sci Vitaminol 61:471–480 [DOI] [PubMed] [Google Scholar]
- 64.Nikel O, Poundarik AA, Bailey S, Vashishth D (2018) Structural role of osteocalcin and osteopontin in energy dissipation in bone. J Biomech 80:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S (2006) Steroid and Xenobiotic Receptor SXR Mediates Vitamin K 2 -activated Transcription of Extracellular Matrix-related Genes and Collagen Accumulation in Osteoblastic Cells. J Biol Chem 281:16927–16934 [DOI] [PubMed] [Google Scholar]
- 66.Guss JD, Taylor E, Rouse Z, et al. (2019) The microbial metagenome and bone tissue composition in mice with microbiome-induced reductions in bone strength. Bone 127:146–154•• This is the first paper to associate the functional capacity of the gut microbiome to bone phenotypes in mice.
- 67.Jukes TH, Williams WL (1953) Nutritional effects of antibiotics. Pharmacol Rev 5:381–420 [PubMed] [Google Scholar]
- 68.Rusoff LL, Fussell JM, Hyde CE, Crown RM, Gall LS (1954) Parenteral Administration of Aureomycin to Young Calves with a Note on Mode of Action. J Dairy Sci 37:488–497 [Google Scholar]
- 69.Ross E, Yacowitz H (1954) Effect of Penicillin on Growth and Bone Ash of Chicks Fed Different Levels of Vitamin-D and Phosphorus. Poultry Sci 33:262–265 [Google Scholar]
- 70.Harris TB, Launer LJ, Eiriksdottir G, et al. (2007) Age, Gene/Environment Susceptibility-Reykjavik Study: Multidisciplinary Applied Phenomics. Am J Epidemiol 165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]