Abstract
Background:
Hypertonic saline (HS) is commonly prescribed for children with cystic fibrosis (CF) despite the absence of strong data indicating clinical efficacy in a population with mild lung disease. We hypothesized that HS treatment would result in a sustained improvement in mucociliary clearance (MCC) in children with CF who had minimal lung disease, thus providing evidence for a biologically relevant effect that also may be associated with clinical improvements.
Methods:
We performed a randomized, placebo controlled, double blind study of 6% versus 0.12% sodium chloride, delivered three-times daily with an eFlow nebulizer for 4 weeks. MCC was measured using gamma scintigraphy at baseline, 2-hours after the first study treatment, and ~12-hours after the final dose (at day 28). Spirometry, respiratory symptoms (CFQ-R), and safety were also assessed.
Results:
Study treatments were generally well tolerated and safe. HS (6% sodium chloride) resulted in a significant, sustained improvement from baseline in whole lung clearance after 4 weeks of therapy (p = 0.014), despite absence of a prolonged single-dose effect after the initial dose. This sustained change (12 hrs after prior dose) was significantly greater when compared to placebo (0.12% sodium chloride) treatment (p = 0.016). Improvements in spirometry with HS did not reach statistical significance but correlated with MCC changes.
Conclusions:
The observed sustained improvement in MCC with HS suggests that this treatment may yield health benefits, even in relatively mildly affected children with CF. Highlighting this physiologic finding is important due to the lack of meaningful, validated endpoints in this population.
Keywords: cystic fibrosis, hypertonic saline, mucociliary clearance, clinical trials
1. INTRODUCTION
The absence of cystic fibrosis transmembrane regulator (CFTR) protein function results in dehydration of airway secretions in patients with CF(1). Over time, CF airway secretions progressively obstruct airway lumens and serve as the nidus of chronic airway infection. Strategies that reverse airway service liquid (ASL) dehydration and support mucus transport, therefore, address the primary pathogenesis of CF lung disease and are expected to improve clinical outcomes(2).
The simplest approach to hydrate mucus is the use of inhaled hyperosmolar solutions. Elkins and colleagues tested inhaled 7% sodium chloride given twice daily and demonstrated a large reduction in pulmonary exacerbation frequency and improved lung function(3). In separate studies, HS had durable (>4 hrs) effects on mucociliary clearance (MCC) in CF adults after a single dose(4); MCC effects lasting >8–12 hours were observed after 2 weeks of repeated use(5). It has been speculated that the prolonged improvement in MCC is responsible for the prevention of CF exacerbations.
Building on these data, recent studies evaluated HS in young children with CF. Large controlled studies evaluating HS in CF infants and toddlers, did not demonstrate reduced exacerbation frequency, but did demonstrate improvement in infant lung function and the lung clearance index (LCI), reflecting improved ventilation inhomogeneity(6, 7). A subsequent study in CF preschoolers validated these findings, reporting a moderate improvement in LCI but no impact on pulmonary exacerbations(8, 9). While there are multiple reasonable explanations for the apparent lack of effect on exacerbations in CF children, it raises the question whether HS efficacy depends upon patient age or disease severity. Further, it raises the question whether HS causes a sustained MCC improvement in these young patients, as is likely required to prevent disease exacerbations. Despite these uncertainties, HS is now prescribed for 75% of patients >6 years of age, and 50% of patients between 3–5 years of age(10). In this study, we tested the hypothesis that inhaled HS would have a sustained effect on MCC in CF children with mild lung disease, and would translate into improved clinical outcomes.
2. METHODS
2.1. Study Design
We conducted a randomized, placebo controlled, double-blind parallel group study to test our hypotheses. Subjects were recruited from the University of North Carolina Pediatric CF Clinic between October, 2009 and June, 2012. Inclusion criteria included: age ≥ 5 to <18 years; a confirmed CF diagnosis; and a FEV1 > 60% of predicted. Patients with unstable lung disease, who did not tolerate HS, or who were unable/unwilling to withhold prescribed HS for at least 2 weeks before the screening visit were excluded. The predefined primary outcome was the change in average whole lung MCC measured over 90 minutes (AveClr90) between baseline and 4 weeks of treatment. Secondary efficacy measures included the change in FEV1 and the CFQ-R respiratory symptom domain score. Exploratory outcomes included single dose MCC effects, and the effect of study treatments in lung subcompartments (e.g. central and peripheral lung) and other time domains (e.g. 24 hrs).
Study treatments consisted of either 6% or 0.12% sodium chloride (4ml) administered three times daily via an investigational eFlow nebulizer (Pari Respiratory Equipment, Inc, Midlothian, VA) for 4 weeks. The active treatment (6% NaCl) and delivery device were selected in an effort to balance the desire to maximize NaCl delivery to the lung against factors that could reduce treatment tolerability (e.g. higher salt concentration, faster aerosol output rate) or feasibility (e.g. frequency of dosing, treatment time). The placebo solution was selected to provide a low osmotic load while avoiding the deleterious effects that may occur after inhalation of distilled water(11). A randomization schedule was generated and maintained by the investigational drug service.
During a screening visit, eligible subjects were randomized and received a test dose of the assigned study treatment after use of a bronchodilator (albuterol or levalbuterol; 2 puffs with spacer). Spirometry was performed pre/post bronchodilator and 15 minutes after completing inhalation of the assigned study treatment test dose. Subjects returned for baseline measures of spirometry and MCC after the screening visit. The first day of the treatment period occurred 7–14 days after screening. At this visit the CFQ-R score and spirometry were obtained prior to study medication administration. MCC was measured two hours after study medication to assess the durability of its effect after a single study treatment dose. Subjects were assessed either via phone or in clinic on day 14 to query for interval adverse events. On day 28, subjects returned to the clinic after holding study medications overnight. CFQ-R, spirometry and MCC were again performed. The timing between bronchodilator and MCC was constant at each visit. This study was reviewed and approved by the institutional human ethics committee at the University of North Carolina and the study was registered on ClinicalTrials.gov (NCT01031706). All subjects and parents provided signed informed consent and/or assent to participate in this study.
2.2. Spirometry
Spirometry was obtained using a KoKo hand-held spirometer (nSpire Health, Inc; Longmont, CO) by trained study staff according to ATS/ERS guidelines(12). Wang pediatric predicted equations were used for reference.
2.3. Respiratory symptoms
Respiratory symptoms were assessed with age and role-appropriate versions of the Cystic Fibrosis Questionnaire – Revised (CFQ-R)(13). Symptom scores ranged from 0 to 100, with higher values indicating fewer symptoms.
2.4. Measurement of MCC
Gamma scintigraphy was performed as previously described(14) at three separate visits to measure MCC at baseline, 2 hours after the first dose of study treatment, and 12 hours after the final dose of treatment. Briefly, a transmission image was obtained to outline lung borders and define regions of interest (ROIs) using a planar Co57 source. Nebulized Tc99m-sulfur colloid particles were inhaled with a defined breathing pattern: 30 breaths per minute; 1 second inspiratory time; and 300–500 ml/sec inspiratory flow rate according to the subject’s height; until a deposited dose of 20–40 uCi was achieved (typically, 2–3 min). Serial 2-minute gamma scintigraphy images tracked particle retention. During the first 64 minutes, subjects were asked to refrain from coughing to allow assessment of cilia-driven clearance. Subjects then performed 20 voluntary coughs through a peak-flow meter every ten minutes to assess cough-assisted clearance for an additional 30 minutes. Subjects returned 24 hours later to assess particle retention at this later time point. All images were corrected for background radiation and radioactive decay of Tc99m.
2.5. MCC Image Analyses
The initial pattern of particle deposition in the whole right lung was characterized by calculating the central:peripheral ratio of Tc99m activity, normalized to the central:peripheral Co57 activity ratio (transmission scan) to adjust for regional differences in lung thickness.
Percent particle clearance (100 - % retention) from the whole right lung and subregions (central and peripheral ROIs) were plotted over time. Clearance versus time curves were numerically represented by calculating average clearance rates using values at 10-minute intervals in each ROI. Cummulative clearance through 24-hrs was measured with a static image at that time point.
2.6. Statistical Analyses
A between treatment comparison of the change in AveClr90 in the whole right lung region, via an independent Student’s t-test, served as the primary study outcome. A paired t-test comparing AveClr90 at baseline and at day 28 was performed in each group to assess within treatment changes in MCC. Linear regressions on the change of AveClr90 explored the impact of potential covariates (i.e. C/P ratio, baseline FEV1, gender). Comparisons of the change in AveClr90 between baseline and 2 hrs after the first dose of each study treatment were performed to characterize the acute pharmacodynamic effect of HS with Student t-tests and linear models. The between and within group changes in CFQ-R scores, spirometry values, and secondary MCC outcomes were similarly analyzed. Correlations between endpoints of interest were calculated with the Pearson correlation. All results are reported as mean ± standard deviation. For calculated differences, mean and 95% confidence intervals were calculated.
A proposed sample size of 24 subjects was estimated to provide approximately 80% power at a two-sided significance level of 0.05 to detect a treatment difference of 6.6% (absolute change in MCC). A treatment difference of this magnitude approximated the sustained effect observed with HS treatment in older CF patients with more severe lung disease.
3. RESULTS
3.1. Subjects
Twenty four subjects were screened for the study, with one screen failure. Twenty-three subjects were enrolled and underwent randomization. Fourteen subjects were randomized to receive active treatment with 6% NaCl, and 9 to the placebo solution (0.12% NaCl). Baseline subject characteristics are shown in Table 1. On average, subjects (mean age 11.1 yrs) used relatively few chronic therapies and had normal FEV1 and FEF25–75 values, signifying mild lung disease. Expectorated sputum samples could only be collected from 3 of 23 subjects either before or after the treatment period, reflecting their mild disease.
Table 1:
Subject Demographics
| Active (6% NaCl) (n=14) | Placebo (0.12% NaCl) (n=9) | All subjects (n = 23) | |
|---|---|---|---|
| Female, % | 8 (57%) | 4 (44%) | 12 (52%) |
| Age, years (SD) | 11.9 (3.3) | 9.8 (3.6) | 11.1 (3.5) |
| Post-BD FVC, % predicted (SD) | 101.4 (12.3) | 100.0 (10.9) | 100.8 (11.5) |
| Post-BD FEV1, % predicted (SD) | 97.0 (12.6) | 93.2 (13.4) | 95.5 (12.8) |
| Post-BD FEF25–75, % predicted (SD) | 91.1 (24.9) | 86.2 (37.8) | 89.7 (27.9) |
| Inhaled antibiotics (%) | 2 (14%) | 1 (11%) | 3 (13%) |
| Chronic HS (%) | 1 (7%) | 3 (33%) | 4 (17%) |
| Dornase Alfa (%) | 3 (36%) | 4 (44%) | 9 (39%) |
| Pancreatic Sufficient (%) | 0 (0%) | 1 (11%) | 1 (4%) |
Abbreviations: SD – Standard Deviation, BD – bronchodilator, FVC – forced vital capacity, FEV1 – Forced Expiratory Volume in 1 second, FEF25–75 - Forced expiratory flow 25–75%. No subjects used CFTR modulators during this study.
3.2. Safety
No subject met any objective withdrawal criteria following the test dose. Disposition of subjects is indicated in Figure 1. Frequency of adverse events were similar between groups (Supplement Table 1).
Figure 1:
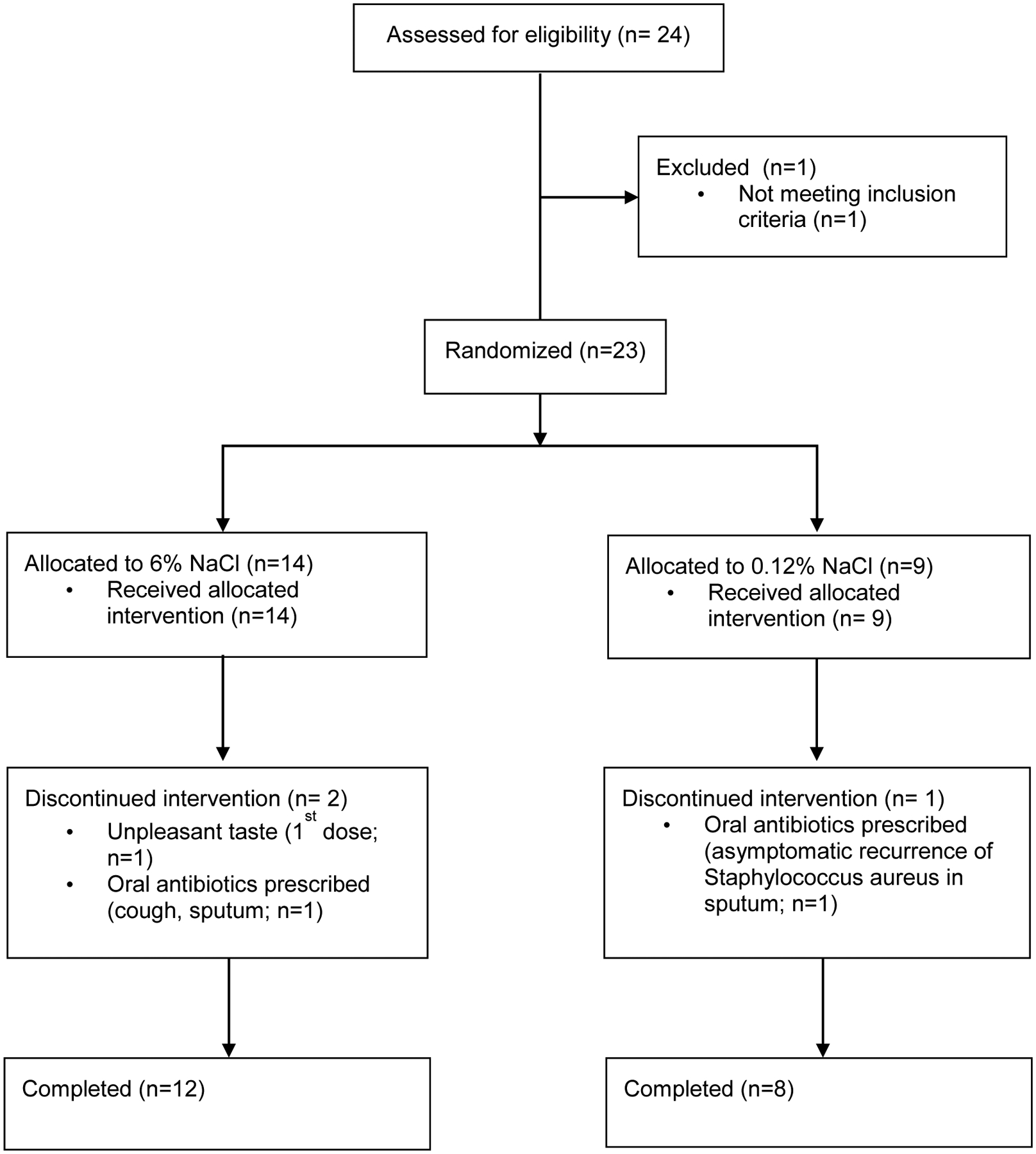
Study flow diagram
The acute effect of study treatments on spirometry was assessed 15 minutes after inhalation. At this early time point, a −6.4 ± 6.4% change in FEV1 was observed in the HS group, versus a −1.3 ± 4.5% change in the placebo group (p = 0.04). No subject exceeded the pre-defined 20% relative drop in FEV1 that would have triggered additional spirometric testing. None experienced oxygen desaturation, or had clinically apparent bronchospasm.
3.3. Mucociliary Clearance
Mucociliary clearance was measured at baseline, 2 hours after the first dose, and approximately 12 hours after the final dose of study treatment. Results are shown in Table 3. Neither treatment was associated with a change in AveClr90 measured 2 hours after the first dose, suggesting that any acute effect on airway clearance was short-lived. However, the ″first dose″ of HS, but not placebo solution, was associated with a significant increase in 24-hr whole lung clearance. Because subjects initiated thrice daily treatment with study medications before returning for this 24-hr retention image, these additional treatments likely provided repeated, transient improvements in clearance that were detected at the 24-hr timepoint. Importantly, a modest increase in radioaerosol deposition index (i.e. C/P ratio) between baseline and this ″first dose″ study was observed in both treatment groups, which if anything would bias the result toward faster post-treatment clearance rates. The lack of improvement in AveClr90 in either arm, therefore, cannot be explained by a change in radioaerosol deposition.
Table 3:
Mucociliary Clearance and Deposition Data
| Baseline | First Dose | 4 weeks | Mean 4 week change | |
|---|---|---|---|---|
| Placebo | ||||
| Whole Lung | ||||
| AveClr60 (%) | 11.8 ± 11.2 | 10.2 ± 8.7 | 10.3 ± 8.3 | −3.1 ± 5.5 (CI: −7.8 – 1.5) |
| AveClr90 (%) | 13.9 ± 10.7 | 12.6 ± 9.8 | 13.3 ± 9.3 | −2.3 ± 5.2 (CI: −6.7 – 2.0) |
| 24-hr Clearance (%) | 33.4 ± 6.8 | 35.4 ± 5.9 | 34.8 ± 14.1 | 0.6 ± 11.9 (CI: −9.4 – 10.6) |
| C/P ratio | 1.83 ± 0.20 | 2.09 ± 0.14* | 1.92 ± 0.25 | 0.12 ± 0.23 (CI: −0.07 – 0.30) |
| Central Lung | ||||
| AveClr90 (%) | 22.0 ± 15.4 | 17.8 ± 15.7 | 21.0 ± 14.5 | −3.8 ± 9.3 (CI: −11.6 – 4.0) |
| Peripheral Lung | ||||
| AveClr90 (%) | 8.5 ± 10.4 | 8.9 ± 5.6 | 7.8 ± 5.8 | −1.7 ± 8.9 (CI: −9.2 – 5.7) |
| Active Treatment | ||||
| Whole Lung | ||||
| AveClr60 (%) | 7.5 ± 5.2 | 8.3 ± 5.2 | 9.9 ± 6.2 | 1.6 ± 4.1 (CI: −1.2 – 4.3)** |
| AveClr90 (%) | 9.1 ± 5.1 | 10.5 ± 5.9 | 12.6 ± 6.4* | 2.8 ± 3.2 (CI: 0.6 – 4.9)** |
| 24-hr Clearance (%) | 24.0 ± 8.8 | 38.6 ± 12.8* | 25.0 ± 8.0 | 1.6 ± 6.1 (CI: −2.3 – 5.5) |
| C/P ratio | 1.67 ± 0.32 | 1.87 ± 0.35* | 1.66 ± 0.26 | −0.01 ± 0.26 (CI: −0.18 – 0.15) |
| Central Lung | ||||
| AveClr90 (%) | 16.2 ± 13.6 | 16.8 ± 8.7 | 20.8 ± 9.2 | 2.6 ± 9.0 (CI: −3.5 – 8.6) |
| Peripheral Lung | ||||
| AveClr90 (%) | 6.1 ± 4.4 | 6.5 ± 4.8 | 7.3 ± 4.9 | 1.1 ± 4.7 (CI: −2.1 – 4.3) |
Clearance and deposition values at each time point, and calculated mean differences between baseline and 4 week visit.
Significant difference (p < 0.05) from baseline via paired T-test.
Significant difference (p < 0.05) between groups via unpaired T-test.
In contrast, whole lung AveClr90 measured after 4 weeks of treatment was significantly faster than baseline in the HS group (p = 0.014), whereas no change from baseline was observed in the placebo group (Table 3, Figure 2). Comparison of the change from baseline between groups (p = 0.016) was also significant, favoring HS (Table 3, Figure 2). Using mixed linear models, we were unable to identify any significant clinical features (e.g. age, gender, baseline FEV1) or MCC covariates (C/P ratio) that influenced treatment responses.
Figure 2 –
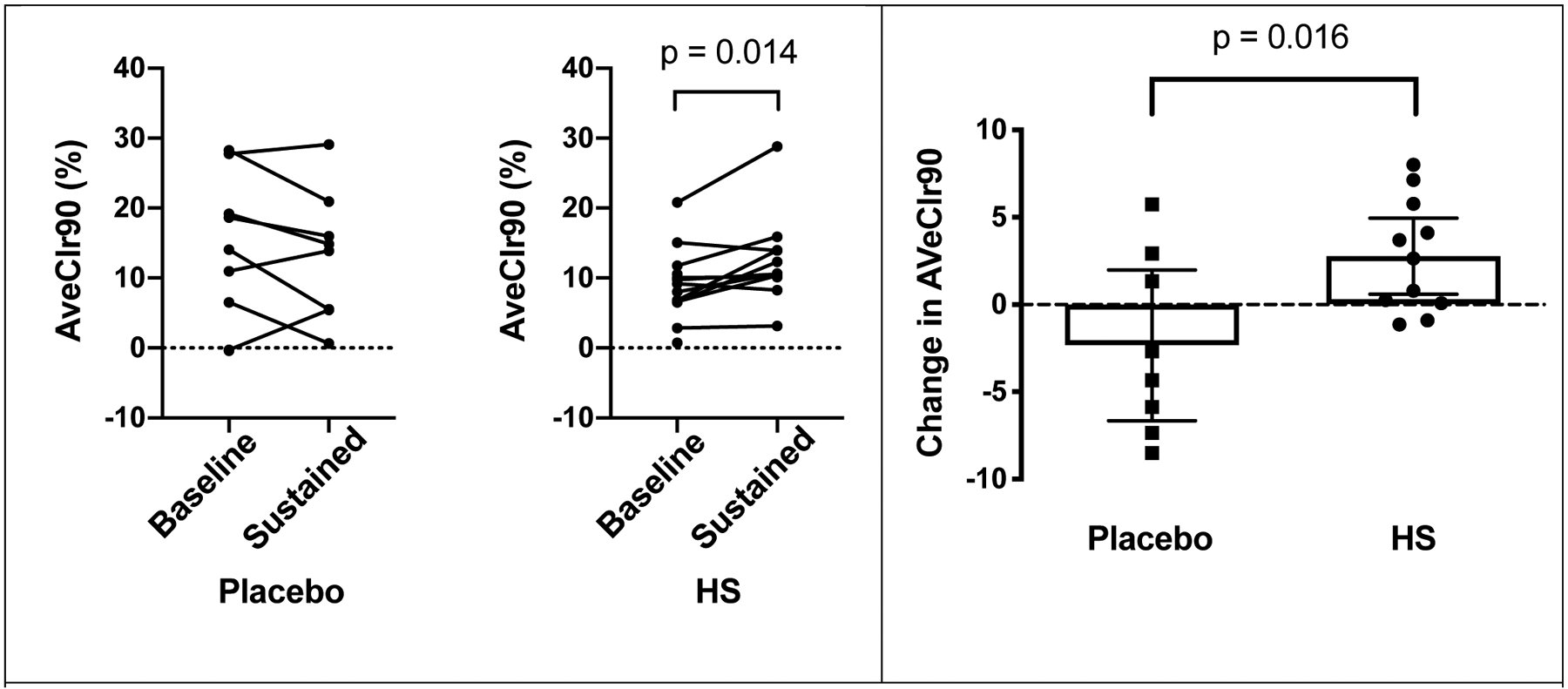
Spaghetti plots (left) showing individual AveClr90 values at baseline and after 4 weeks of treatment by treatment group. In right panel, scatter plots with mean and 95% CI demonstrate the mean change in AveClr90 between baseline and after 4 weeks of treatment in placebo and HS groups. Unpaired Student’s t-test revealed significant treatment effect (p = 0.016).
MCC responses in the central and peripheral lung regions of interest revealed similar trends toward improvements in MCC after HS but the placebo treatment did not reach statistical significance (Table 3).
3.4. CFQ-R Respiratory Domain
The treatment effect of HS versus placebo on the CFQ-R respiratory symptom domain between baseline and at the end of the assigned treatment was +7.3 ± 5.1. Although greater than the accepted minimum clinically important difference (MCID) of 4 for this instrument, it did not reach statistical significance (p = 0.17). Similarly, a non-significant within group improvement in the CFQ-R respiratory domain score was observed in the HS group (+5.8 ± 12.6; p = 0.18), but not in the placebo group (−1.6 ± 9.8).
3.5. Lung Function
The treatment difference in FEV1 % predicted (absolute change) between the active and placebo groups following 4 weeks of treatment was 1.9 ± 3.3% (p = NS). The within group improvement in FEV1 % predicted after HS (3.2 ± 7.0%) was also not significant.
3.6. Correlation Between Endpoints
Correlations between various endpoints were calculated to explore the role that MCC measurements might play in this population. At baseline, no relationships between baseline MCC rates and subject age, gender, or baseline FEV1 were noted. Correlation between AveClr90 values at baseline and after 4 weeks of treatment were highly significant, indicating that repeated MCC measurements in individual subjects are reliable (R2=0.75, p=0.0005 for active treatment, R2 = 0.74, p = 0.007 for placebo). Interestingly, the correlation between changes in AveClr90 and FEV1 revealed a highly significant relationship in subjects who were randomized to HS (R2=0.67, p=0.002; Fig 3), suggesting that sustained improvement in MCC might predict improvements in lung function, even in this mildly affected population. No other factors correlated with the observed change in FEV1, and no relationship between changes in MCC and respiratory symptoms were observed.
Figure 3:
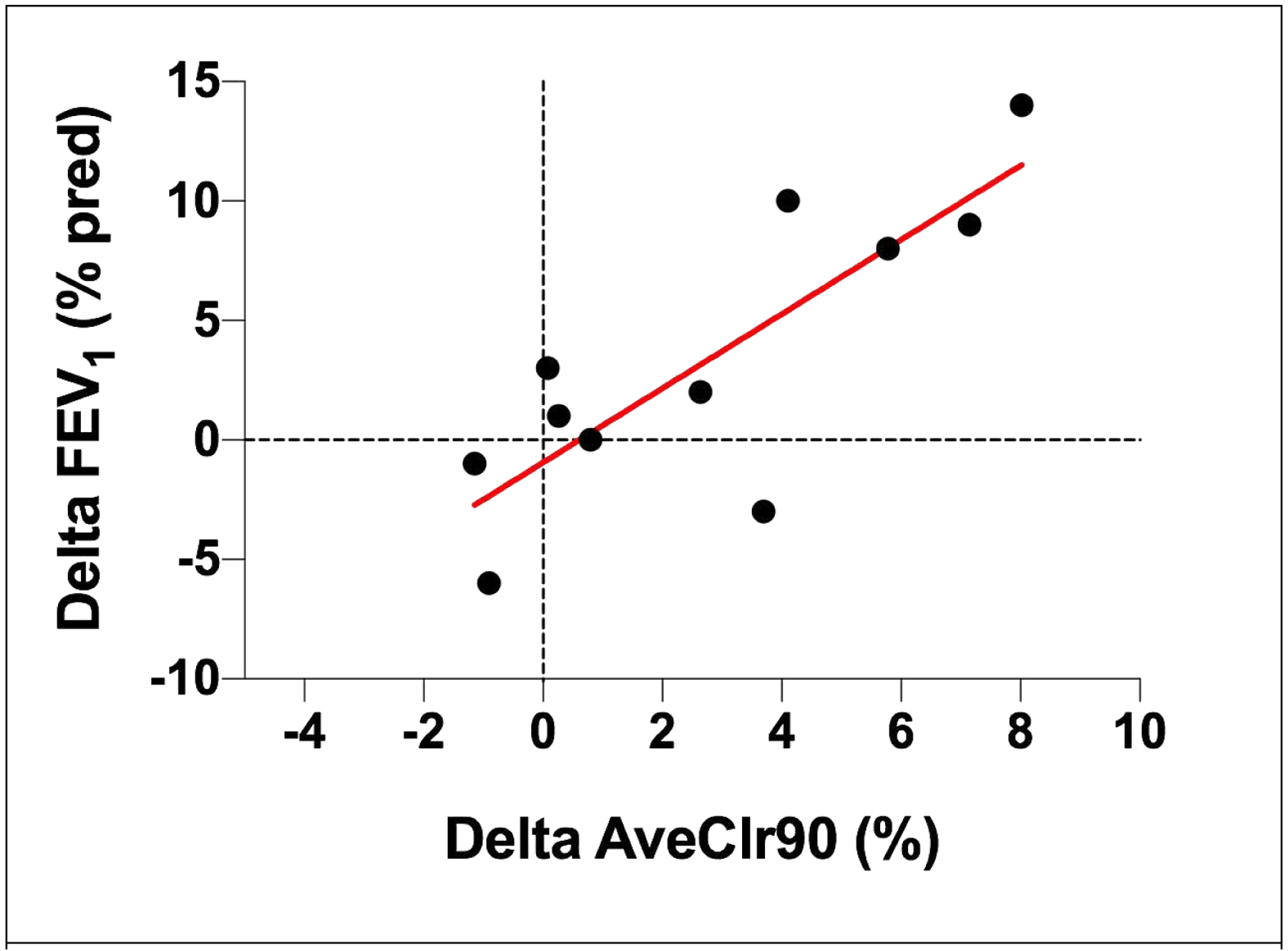
Correlation between change in MCC (AveClr90) and FEV1 % predicted after 4 weeks of HS treatement. Pearson R2 = 0.67; p = 0.002
4. DISCUSSION
The demonstration of treatment efficacy in mildly affected CF is a growing challenge. This is particularly problematic in pediatric populations where the median FEV1 currently remains >90% of predicted until early adolescence(10), and where other outcome measures may not be well suited to younger patients. The identification of endpoints that are physiologically relevant, feasible in young populations, sensitive, repeatable, and not limited by ceiling effects are necessary to guide drug development and inform treatment decisions in young CF populations.
The use of HS provides a clear example of this dilemma. Whereas trials of HS in older CF populations provided fairly clear signals of clinical efficacy (3, 5), similar large studies in toddlers and preschoolers were less definitive. Although LCI measurements demonstrated clear HS treatment effects(6, 8), the lack of improvement in exacerbation frequency raises uncertainty over the clinical benefit of HS in this population. In parallel, marked differences in MCC responses to HS in healthy(15) and CF adult populations(4) also raise concerns that a mildly affected CF patient might benefit less from HS than a more severely affected CF adult. Indeed, a single dose HS study by Laube et al failed to show overall improvement in MCC in CF children(16).
In this trial, we observed a significant, sustained improvement in MCC after 4 weeks of dosing. The average relative improvement in MCC over baseline (~33%) was nearly identical to that reported in our earlier study of HS in older CF subjects(5), suggesting that a similar, sustained physiologic effect was achieved despite the differences in disease severity in these studies. In contrast, we did not observe a prolonged acute effect on MCC (measured at 2 hrs post-dose) after a single HS dose in these CF children, whereas in CF adults this effect appeared to last >4 hours(4). Together these data suggest that repeated treatment with a relatively short-acting agent has the potential to influence MCC in meaningful ways after repetitive dosing. We further speculate that the more prolonged pharmacodynamic effect of a single HS dose in CF adults might relate to their higher mucus burden and its ability to prolong ASL volume responses to HS, as previously shown by in vitro studies(17).
We view these study results as complimentary to prior studies of HS in children where physiologic (LCI) improvements were demonstrated. Together, they increase our confidence that the demonstrated improvements in airways obstruction and mucus clearance will lead to clinical benefits, despite the challenges of demonstrating this directly in young, mildly affected CF patients. Although we view the mechanisms of dornase alfa and HS as being quite distinct, the emergence of CFTR modulators makes the future role of HS in treatment regimens for CF children uncertain. We have demonstrated that highly effective CFTR modulators markedly improve MCC, likely through similar mechanisms as HS (i.e. restoration of mucus properties that facilitate clearance). Until highly effective modulators are available for all children, HS appears to be a useful part of the maintenance regimen.
The role that MCC imaging studies might play in drug development in mildly affected CF children is highlighted by two factors. First, we showed a clear improvement in MCC in a small group where ceiling effects on FEV1 and CFQ-R likely played a role in the failure to observe a treatment difference. Second, the strong correlation between changes in MCC and lung function strengthens the clinical meaningfulness of the MCC endpoint. Of note, similar correlations between MCC and lung function changes were also seen in studies of ivacaftor in CF patients with the G551D-CFTR mutation(18). MCC may, therefore, be well suited to small trials where FEV1 ceiling effects and variability may be limiting. It is also intriguing to consider use of MCC measurements as a way of assessing whether discontinuation or addition of a standard therapy such as HS might be appropriate in the context of CFTR modulator use.
This study has several limitations. First, although protection against pulmonary exacerbations was the dominant clinical benefit in older CF populations, we don’t know whether improvements in MCC will predict this outcome. Uncertainties in how to define a CF exacerbation in young populations further complicate this dilemma. If we knew that improved MCC actually does predict protection against pulmonary exacerbations, use of this endpoint could help to circumvent this issue of exacerbation definitions in the young, mildly affected populations.
Another limitation of our study is the use of a non-standard HS formulation (6% NaCl), treatment frequency and delivery device. This decreases our ability to extrapolate our results to those that might be achieved with more typical clinical regimens (i.e. 7% NaCl, twice daily via jet nebulizer). The desire to optimally test the hypothesis that inhaled osmotic agents will benefit mild CF lung disease, balanced against feasibility and tolerability concerns, led to the selected study design. Taste masking of study solutions was also not attempted in this study, increasing the risk that subjects might have been aware of their treatment assignment. However, only 3 of 23 subjects had used HS at the time of enrollment, reducing this risk.
5. CONCLUSIONS
HS is frequently prescribed to young CF patients despite a relative paucity of data supporting its clinical efficacy. In this study, HS was well tolerated and safe. We demonstrated a sustained improvement in a biologically relevant physiologic process (i.e. MCC), and this change correlated with those in FEV1. Despite the acute effects of HS on MCC being short-lived (<2 hrs), repeated dosing yielded sustained improvements (>12 hrs). This observation suggests that potent, but short-acting, therapeutics designed to improve MCC might still achieve efficacy with repeated use. In total, observations from this study could influence the pre-clinical goals of CF drug development (i.e. required pharmacodynamic profile) and how they are tested in early phase clinical trials. Future work is needed to define the relationship between MCC responses and the risk of pulmonary exacerbations, as well as studies to test the utility of osmotic hydrators across various CF populations after the initiation of highly active CFTR modulators.
Supplementary Material
HIGHLIGHTS.
Hypertonic saline (HS) via eFlow nebulizer was well tolerated in CF children
HS inhalation for 4 weeks increased mucociliary clearance in a sustained fashion
Sustained changes in mucociliary clearance correlated with FEV1 changes
Funding source:
This work was supported by the National Institutes of Health (NHLBI, P50 HL084934; NIDDK, P30DK065988), who played no role in in study design, data collection, analysis, or writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov registration number: NCT01031706
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
6. REFERENCES
- 1.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J InternMed. 2007;261(1):5–16. [DOI] [PubMed] [Google Scholar]
- 2.Tarran R, Donaldson S, Boucher RC. Rationale for hypertonic saline therapy for cystic fibrosis lung disease. SeminRespirCrit Care Med. 2007;28(3):295–302. [DOI] [PubMed] [Google Scholar]
- 3.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. [DOI] [PubMed] [Google Scholar]
- 4.Trimble AT, Whitney Brown A, Laube BL, Lechtzin N, Zeman KL, Wu J, et al. Hypertonic saline has a prolonged effect on mucociliary clearance in adults with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus Clearance and Lung Function in Cystic Fibrosis with Hypertonic Saline. The New England Journal of Medicine. 2006;354(3):241–50. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, et al. Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA. 2012;307(21):2269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl M, Wielputz MO, Ricklefs I, Dopfer C, Barth S, Schlegtendal A, et al. Preventive Inhalation of Hypertonic Saline in Infants with Cystic Fibrosis (PRESIS). A Randomized, Double-Blind, Controlled Study. Am J Respir Crit Care Med. 2019;199(10):1238–48. [DOI] [PubMed] [Google Scholar]
- 8.Ratjen F, Davis SD, Stanojevic S, Kronmal RA, Hinckley Stukovsky KD, Jorgensen N, et al. Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. The Lancet Respiratory medicine. 2019;7(9):802–9. [DOI] [PubMed] [Google Scholar]
- 9.Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, et al. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med. 2013;188(4):456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2017.
- 11.Bascom R, Bleecker ER. Bronchoconstriction induced by distilled water. Sensitivity in asthmatics and relationship to exercise-induced bronchospasm. Am Rev Respir Dis. 1986;134(2):248–53. [DOI] [PubMed] [Google Scholar]
- 12.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J RespirCrit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 13.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol. 2003;28(8):535–45. [DOI] [PubMed] [Google Scholar]
- 14.Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, et al. Multisite comparison of mucociliary and cough clearance measures using standardized methods. Journal of aerosol medicine and pulmonary drug delivery. 2013;26(3):157–64. [DOI] [PubMed] [Google Scholar]
- 15.Bennett WD, Wu J, Fuller F, Balcazar JR, Zeman KL, Duckworth H, et al. Duration of action of hypertonic saline on mucociliary clearance in the normal lung. Journal of applied physiology. 2015;118(12):1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laube BL, Sharpless G, Carson KA, Kelly A, Mogayzel PJ Jr., Acute inhalation of hypertonic saline does not improve mucociliary clearance in all children with cystic fibrosis. BMC pulmonary medicine. 2011;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goralski JL, Wu D, Thelin WR, Boucher RC, Button B. The in vitro effect of nebulised hypertonic saline on human bronchial epithelium. Eur Respir J. 2018;51(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson SH, Laube BL, Corcoran TE, Bhambhvani P, Zeman K, Ceppe A, et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight. 2018;3(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


