Abstract
Azaindole structural framework is an integral part of several biologically active natural and synthetic organic molecules; and several FDA approved drugs for various diseases. In the last decade, quite a number of literature reports appeared describing the pharmacology, biological activity and therapeutic applications of a variety of azaindole molecules. This prompted the organic and medicinal chemistry community to develop novel synthetic methods for various azaindoles and test them for a bioactivity against a variety of biological targets. Herein, we have summarized the biological activity of therapeutically advanced clinical candidates and several preclinical candidate drugs that contain azaindole structural moiety.
Keywords: Guitarrins, PLX4720, Pexidartinib, BMS626529, Fevipiprant and HIV treatment
Graphical Abstract
1. INTRODUCTION
Azaindoles are structurally bioisosteric chemical structures to ubiquitously appeared indoles in biological materials, natural products and pharmaceuticals.1–12 There are four structural isomers of azaindoles, named as 4-, 5-, 6-, and 7- azaindoles (Fig. 1). Interestingly, these four structural isomers show distinct physicochemical properties such as Log P (partition coefficient between octanol and water), total polar surface area (tPSA), and aqueous solubility (Log S) due to the presence of extra nitrogen atom within the six membered ring in comparison to indole structure (Fig. 1). Therefore, these azaindoles attracted medicinal chemistry community for the last decade to derive a number of pharmacological agents that showed enhancements in medicinal chemistry properties.13–15 Among these, 7-azaindole moiety has appeared in a plethora of biologically active molecules, relatively more in comparison to other three structural isomers, although all four seems to be represented as biologically active and important moieties. Notably, a majority of azaindole molecules appear to target a variety of kinases as inhibitors, in comparison to other targets.16 The biostructural analysis of azaindoles with kinase inhibitors revealed that two nitrogen atoms of azaindole form hydrogen bonds with the hinge region of the protein kinase, which is similar to the bonding pattern of adenine moiety of natural substrate ATP. Moreover, the introduction of N-atom in place of CH group in the molecules increases the aqueous solubility, results in favorable physicochemical, and adsorption, distribution, metabolism and excretion (DMPK), and pharmacokinetic (PK) properties, and molecular interactions as described in detail by Pennington and Moustakas.17 Moreover, their ultimate biological/pharmacological actions are beneficial to treat a variety of diseases, including but not limited to cancer, Alzheimer’s disease, diabetes, allergen-induced asthma, HIV, influenza infections and other diseases as detailed in below sections.18–31 Because of the biological significance witnessed by these azaindoles, various synthetic methods have also been developed to produce derivatives around these chemical structures.32, 33 While number of derivatives have been synthesized by the reported methods thus far, challenges remain to develop additional derivatives around these azaindoles as described elsewhere.31–33 As a part of our research on developing lead candidates towards Alzheimer’s disease, we have made several indole34–36 and azaindole derivatives. The literature survey of azaindoles offered significant therapeutic potential, which attracted us to review the recent developments in azaindole motifs. Herein, we have highlighted the pharmacological, biological properties of various isomeric azaindoles. The literature on these azaindoles biological activity appeared vast; therefore, we only surveyed and cited the literature from 2011-till now in this review article to stay focused on current developments.
Fig. 1:
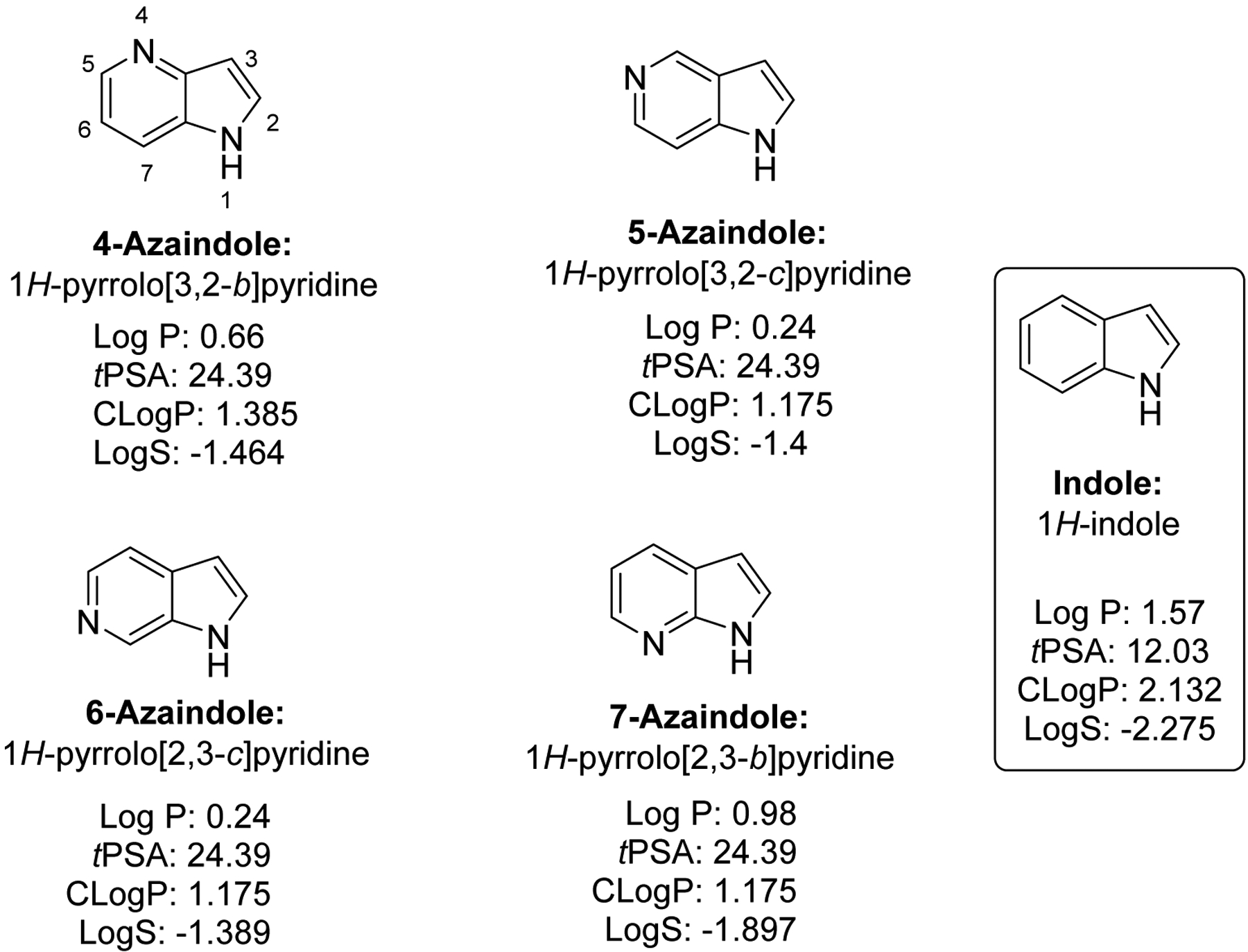
Azaindoles and their chemical names and biophysical properties (calculated using ChemDraw software 16.0).
1.1. Therapeutically advanced naturally occurring and synthetic azaindoles
Azaindoles are widely represented in natural products, biologically active molecules and FDA approved drugs. For example, Guitarrins A-D (1–4, Fig. 2) are isolated from marine sponge Guitarra fimbriata, which exhibited a weak inhibition activity against alkaline phosphatase from the marine bacterium Cobetia marina. Variolin B (5) and deoxyvariolin B (6, aka, PM01218) were isolated from the rare access Antarctic sponge, Kirkpatrickis variolosa. Variolins 5 and 6 displayed potent cytotoxic activity against number of cancer cell lines including P388 murine leukemia cells.37 These derivatives are reported to inhibit various kinases such as cyclin dependent kinases (CDKs), glycogen synthase kinase-3 (GSK-3), cyclic nucleotide-dependent kinases, and casein kinase 1 (CK1), and subsequently show anti-proliferative effects. From these, deoxyvariolin 6 has been investigated in clinical trials as an anti-tumor agent. These natural products inspired the development of synthetic derivatives, Meriolin 1 (7) and Meriolin 3 (8), which showed improved potency, selectivity toward CDKs and exhibited better anti-proliferative and pro-apoptotic properties in cell cultures than their parent molecules. In particular, Meriolin 3 significantly inhibited tumor growth in two mouse xenograft models.37
Fig. 2:
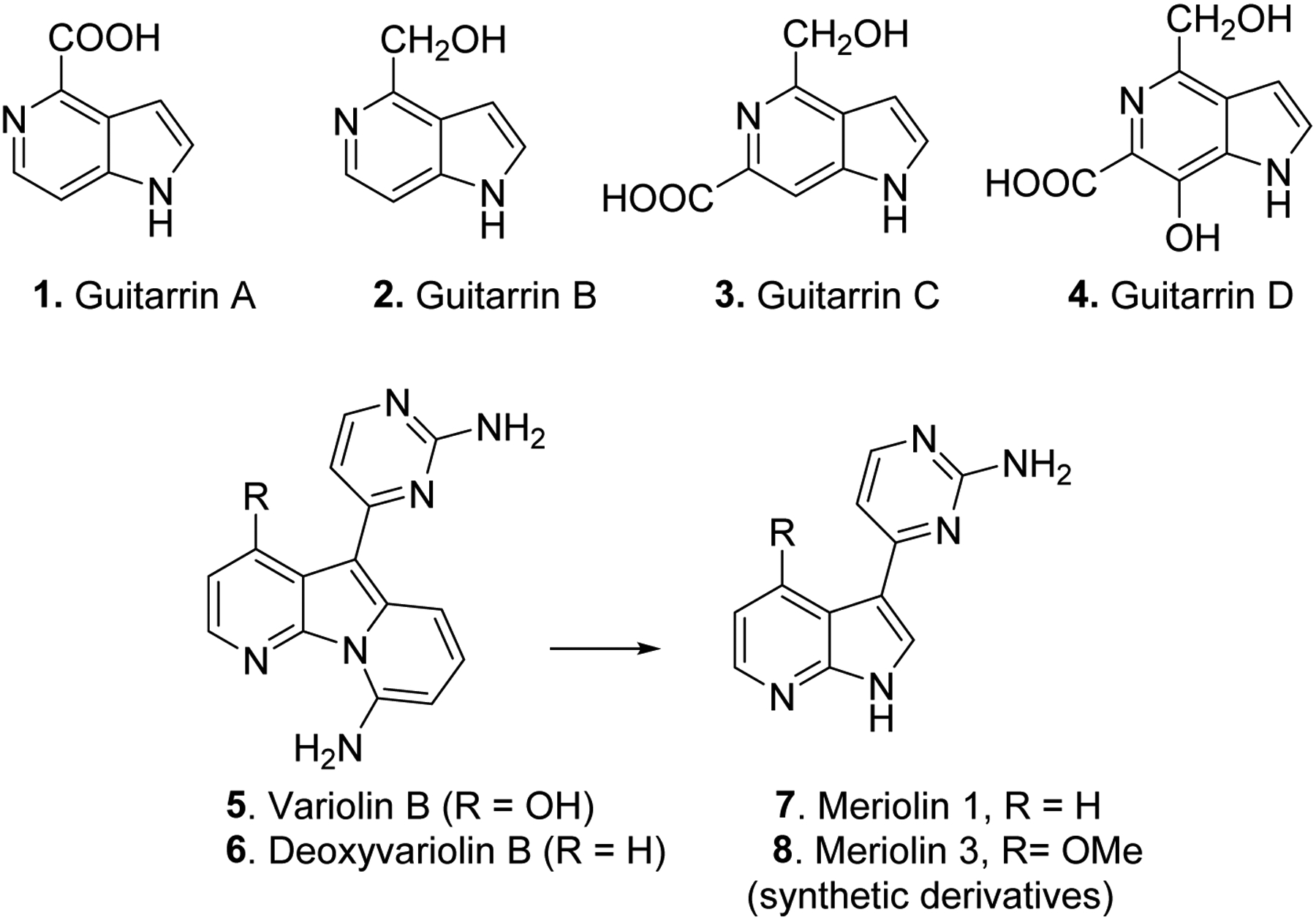
Azaindole natural products and synthetic derivatives.
Inspired by the above natural products, several biologically active azaindole clinical candidates and drugs have been developed thus far. For example, 7-azaindole derivative vemurafenib (9) (aka., PLX4720 and Zelboraf®) (Fig. 3), a potent protein kinase inhibitor that delays tumor growth, has received an FDA approval for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation.38 Likewise, a 7-azaindole derivative pexidartinib (10, Fig. 3) (Turalio®), a tyrosine kinase inhibitor, received a FDA approval for the treatment of adult patients with symptomatic tenosynovial giant cell tumors associated with severe morbidity.27, 39 A 6-azaindole derivative named Temsavir (aka, BMS-626529) (11a, Fig. 3) and its prodrug known as Fostemsavir 11b (aka., BMS-663068 (Fig. 3) have been shown to inhibit the attachment of viral gp120 thus preventing HIV entry. After 96 weeks of treatment, Fostemsavir has shown HIV viral suppression in 60% of the clinical study participants with HIV; therefore, it has been recently approved by the FDA for HIV treatment.26, 28 Another 7-azaindole clinical candidate named Fevipiprant (12, Fig. 3) is a prostanoid receptor DP2 antagonist, has been promoted for the treatment of asthma, however, it failed to meet clinical endpoints in the phase III trial, thus dropped from the clinic.22, 29
Fig. 3:
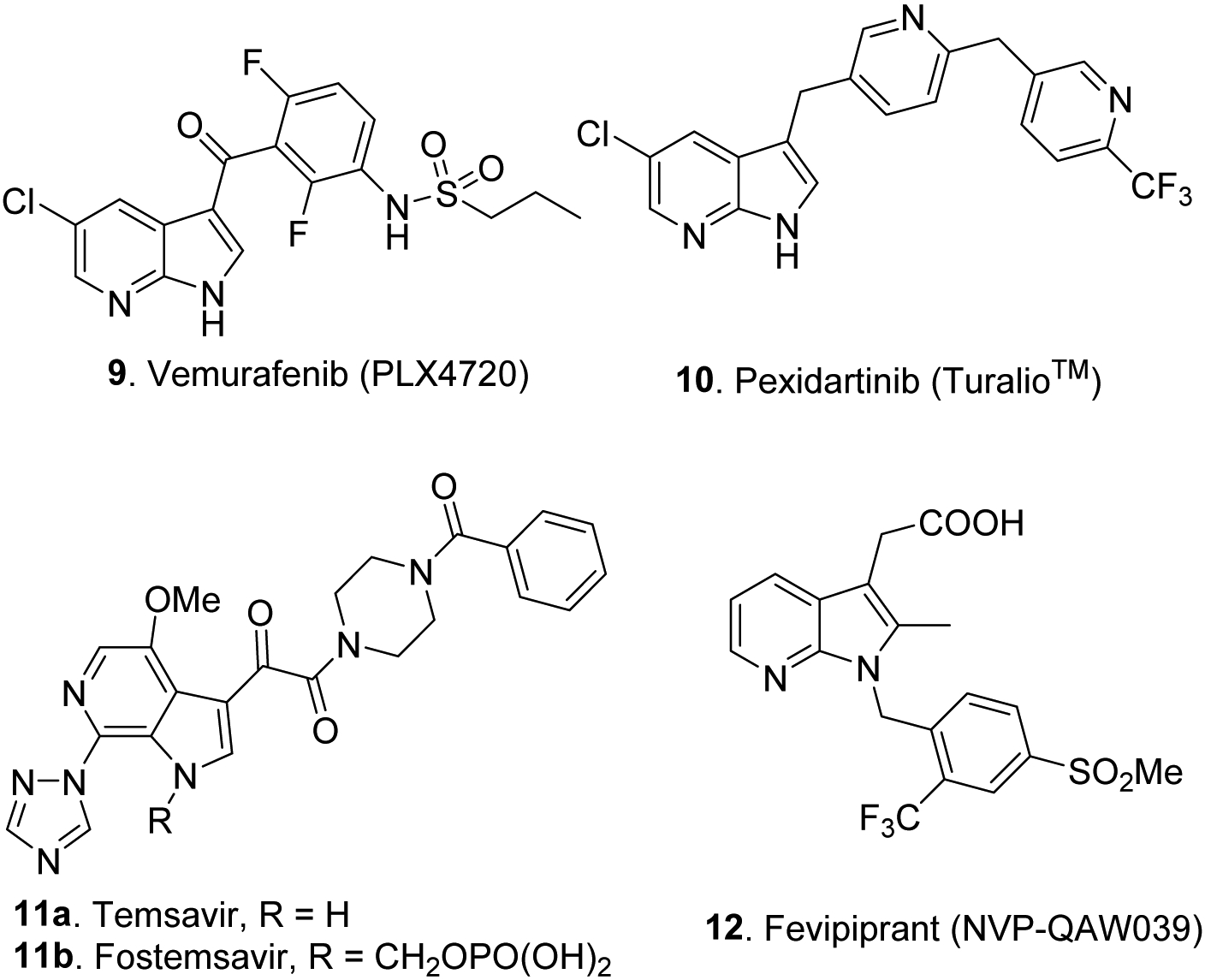
FDA approved drugs and clinical candidates with the azaindole core unit.
1.2. Azaindole’s biological activities
The above clinical candidates and drugs inspired many pharmaceutical companies and academic laboratories to develop various other azaindoles and investigate their biochemical and pharmacological properties in vitro and therapeutic efficacy in vivo models. For example, it has been reported that 7-azaindole derivative JNJ-63623872 (13, Fig. 4) as a potent influenza polymerase-B2 inhibitor with activity against multiple influenza strains such as H1N1 and H5N1.18,19, 40 Similarly, 7-azaindole 14 (Fig. 4) inhibit PI3K at molecular and cellular level and tumor cell proliferation,23 it is noteworthy to mention that the two nitrogen atoms of 7-azaindole form two hydrogen bonds with Val882 of PI3Kγ. Several compounds belongs to 7-azaindole represented by compound 15 are looking promising as anti-cancer agents via PIM2 (proviral integration site for moloney murine-2 Leukemia virus) kinase inhibition.21 Interestingly, 2-substituted-6-azaindoles represented by compounds 16 and 17 have shown promising glucocorticoid receptor agonist activity in vitro and they reduced collagen-induced arthritis, and prevented bone loss in mouse compared with traditional steroidal glucocorticoid agonists.20, 41 Another, 6-azaindole derivative named GNF2133 (18, Fig. 4) has been developed as DYRK1A inhibitor and has been shown to promote β-cell proliferation, glucose disposal capacity and insulin secretion in response to glucose potentiated arginine-induced insulin secretion challenged rats and mice, therefore they can be used for treatment of type 1 diabetes.15
Fig. 4:
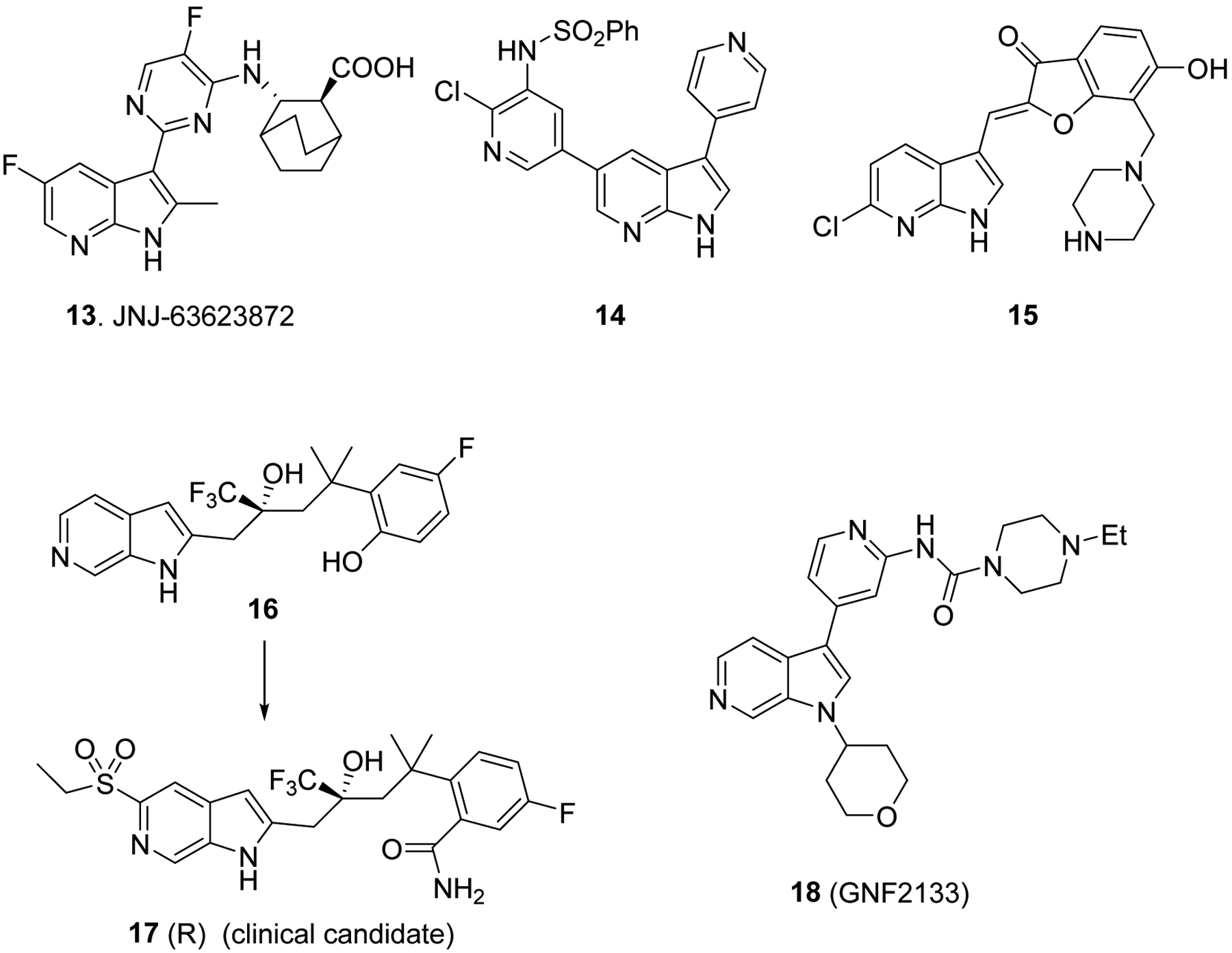
Clinical candidates and promising molecules of azaindole derivatives.
Several other 7-azaindole derivatives that show promising biological activities are worth highlighting here. For example, 2-,5-disubstituted-7-azindole 19 has shown inhibition activity against multiple kinases (34 out of 104 kinases tested) and broad anti-proliferative activity against various cancer cells including PC-3, Caki-2, MDA-MB-231 and NCI-H1975.42 A study reports that substitution of one of the amino groups in anti-mitotic drug cisplatin with 1-methyl-7-azaindole moiety to afford compound 20 (cis-[PtCl2(NH3)(1-methyl-7-azaindole)], Fig. 5), increased efficiency and selectivity for tumor cells in cisplatin resistant cancer cells, and this was associated with increased level of cellular DNA platination by azaindole moiety.43 DYRK kinases, DYRK1A, DYRK1B and DYRK2, are implicated in progression of various cancers including glioblastoma. A number of 7-azaindole derivatives represented by compound 21 have shown to inhibit viability, survival, migration and invasion of glioblastoma with nanomolar potency against DYRK1B and DYRK2 kinases.44 Likewise, 7-azaindole sulfonamide 22 showed anti-proliferative activity against various cancer cell lines, anti-tumor activity in colorectal HCT116 xenografts via inhibition of HDAC6 enzyme with good selectivity against HDAC1 and HDAC2.8 A 7-azaindole clinical candidate AZD6738 (23, Fig. 5) is shown to inhibit growth of ataxia telangiectasia mutation (ATM) deficient xenografts via ataxia telangiectasia mutated and Rad3 related (ATR) kinase inhibition mechanism.6 Similarly, 7-azaindole 24 (Fig. 5) has shown promising activities against dengue virus both in vitro and in human primary dendritic cells and Ebola virus via adaptor-associated kinase 1 (AAK1) that functions as a key regulator of the trafficking of multiple unrelated RNA viruses.45 Likewise, 7-azaindole molecule named URMC-099 (25, Fig. 5) has been shown to display neuroprotective and anti-neuroinflammatory properties in vitro and in vivo models of HIV-1 associated neurocognitive disorders (HAND) via inhibition of mixed lineage kinase 3 (MLK3) and leucine-rich repeat kinase 2 (LRRK2).46 Similarly, 3,5-disubstituted-7-azaindoles such as 26 (Fig. 5) have shown anti-tumor activity in triple negative breast cancer mouse model, via inhibition of activity against cyclin-dependent kinases (CDK2 and CDK9).47
Fig. 5:
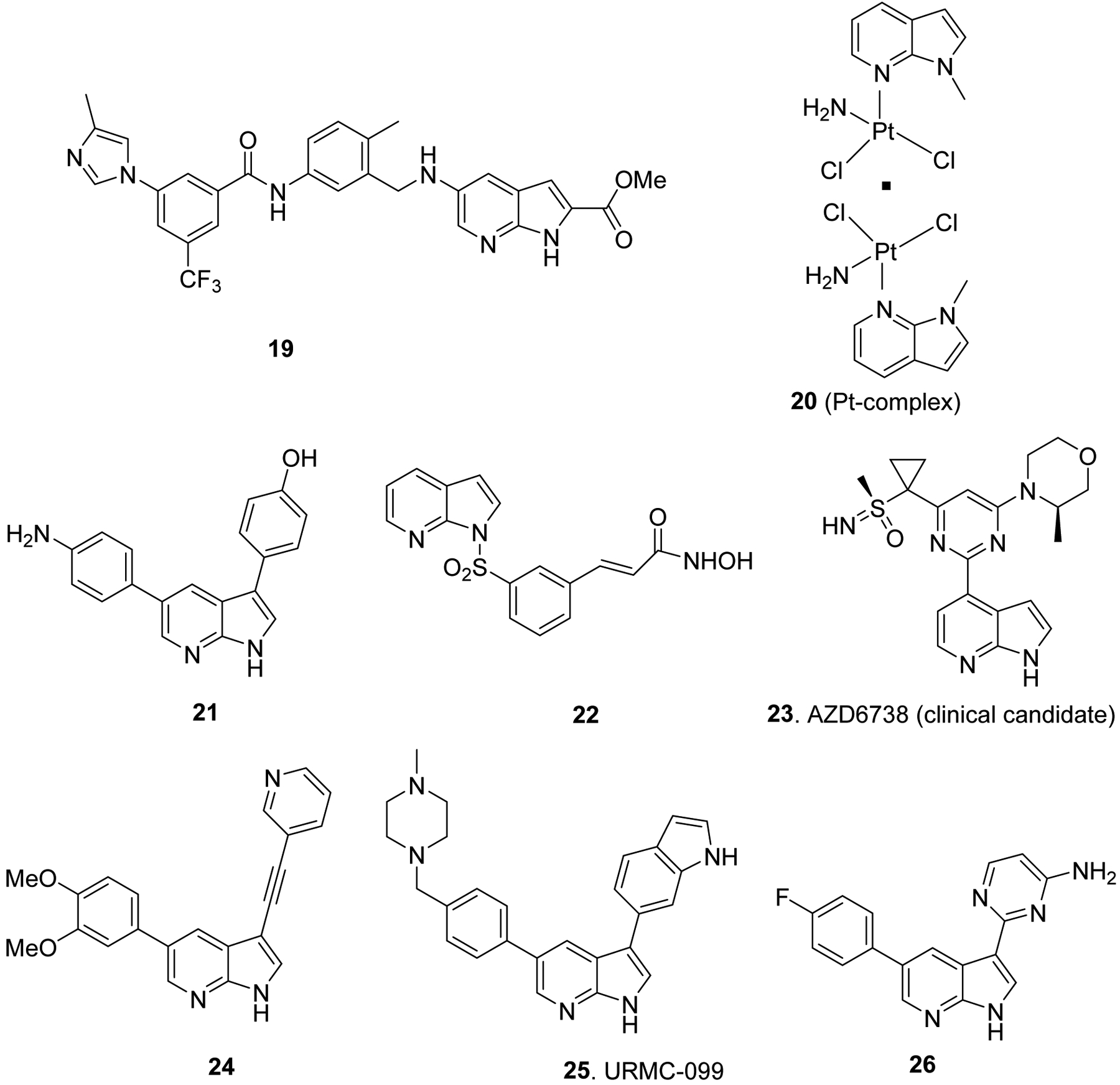
Azaindole derivatives that displayed promising biological activity.
Several compounds belongs to 1,3-disubstituted-4-azaindole such as PF-06764427 (27) and 28 (Fig. 6) have shown as M1 mAChR PAM agonists and potential treatment for Alzheimer’s disease.48 1,3-disubstituted-4-azaindoles (e.g. 29) kills Mycobacterium tuberculosis in vitro and efficacious in mouse TB model via non-covalent inhibition of decaprenylphosporyl-β-D-ribose2’-epimerase (DprE1), which plays a key role in synthesis of mycobacterial cell wall arabinan.10
Fig. 6:
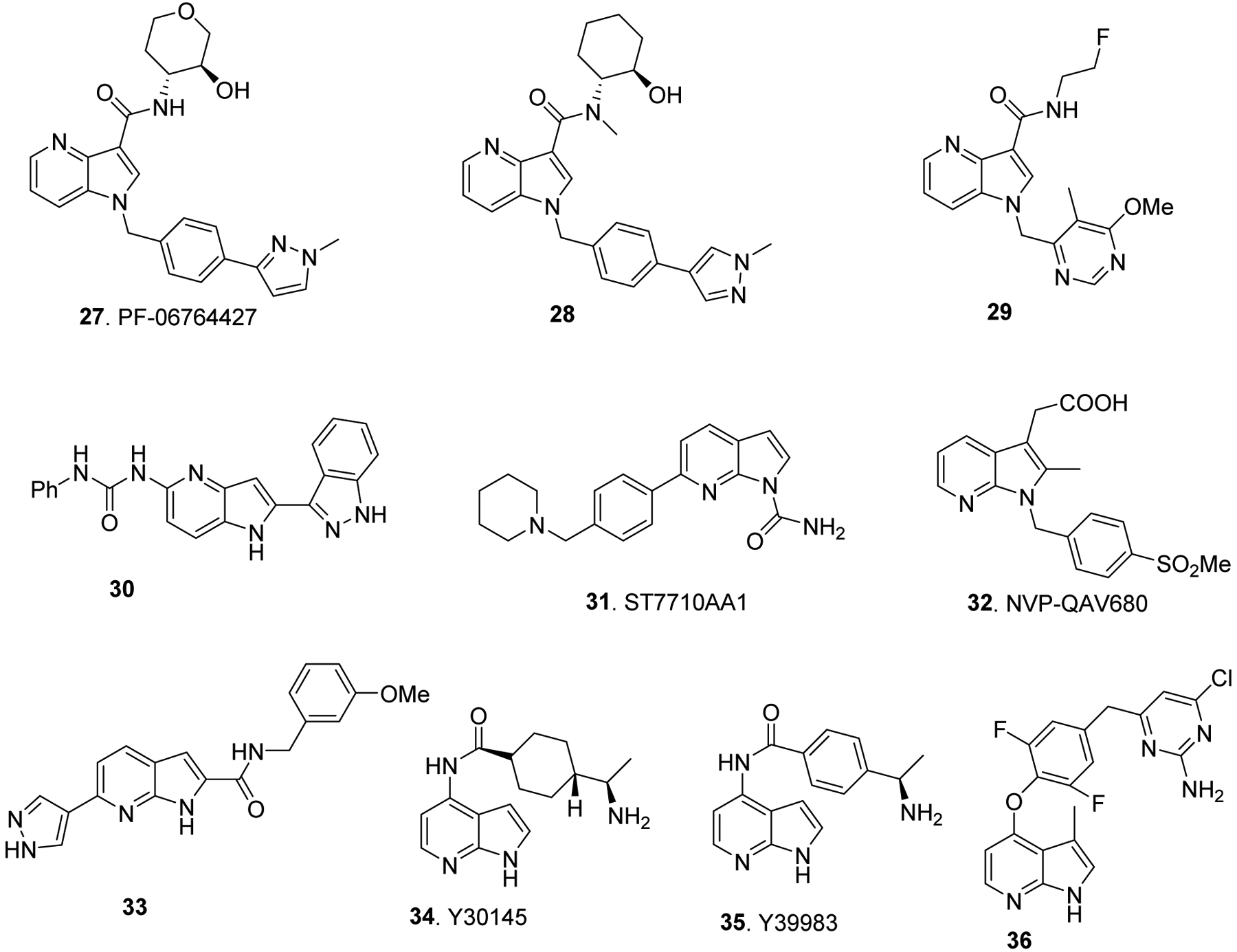
Bioactive azaindole derivatives.
Likewise, several 2-,5-disubstituted-4-azaindoles such as 30 (Fig. 6) have been shown as promising anti-proliferative agents against human cancer cell lines, via selective inhibition of Aurora A kinase with significant selectivity against CHK1, CDK2, and MEK1, GSK3β, BRAF, IKKβ and PKC.49 7-Azaindole-1-carboxamide named as ST7710AA1 (31, Fig. 6) displayed anti-proliferative activity against cancer cell lines in vitro, and antitumor activity against MX1 human breast cancer growth in nude mice via inhibition of poly(ADP-ribose)polymerase protein-1 (PARP-1), which is involved in DNA integrity and regulation of programmed cell death.24 Moreover, a 7- azaindole derivative NVP-QAV680 (32) has emerged as a clinical candidate for the treatment of allergic diseases such as Asthma via selective antagonism of CRTh2 receptor (also known as prostanoid receptor DP2).50 Several 7-azaindoles 33–36 (Fig. 6) are discovered as ROCK (Rho Kinase) inhibitors, which will find useful for the development of therapeutic agents for variety of disorders including hypertension, glaucoma, and erectile dysfunction.25, 51, 52
Likewise, 3,4-disubstituted-7-azaindole such as NPS-1034 (37, Fig. 7) was found to inhibit AXL (a receptor tyrosine kinase), which plays a key role in growth and proliferation of cancer cells.53 Other 7-azaindoles such as 38 has shown anti-HIV properties via non-nucleoside reverse transcriptase (NNRT) inhibition activity,54 and, 39 and 40 (Fig. 7) have been shown to inhibit CC-chemokine receptor-2 (CCR2), which is heavily implicated in various inflammatory pathological conditions such as asthma, atherosclerosis, rheumatoid arthritis and multiple sclerosis.55, 56 Additionally, tropomyosin-related kinase (Trk) is one of the important targets for development of therapeutics for the treatment of cancer and pain. A novel 7-azaindole derivative 41 was identified as Trk inhibitor through the structure-based design strategy and found to be selective inhibitor for TrkA over a panel of 30 other kinases (Fig. 7).57, 58 Finally, 7-azaindole derivative 42 (Fig. 7) showed anti-inflammatory activity, and inhibition of airway cell infiltration in bronchoalveolar lavage fluid (BALF) in ovalbumin induced rat model of allergic inflammation, via inhibition of Orai calcium channel.59 Furthermore, not only as therapeutic agents, azaindole moiety is appeared in unnatural amino acids such a 7-azatryptophan (43, Fig. 7), which is found to show pH sensitive vibrational frequencies and can be used as sensitive proton transfer markers in gated proton transfer reactions in photosystem II and other enzymes.60
Fig. 7:
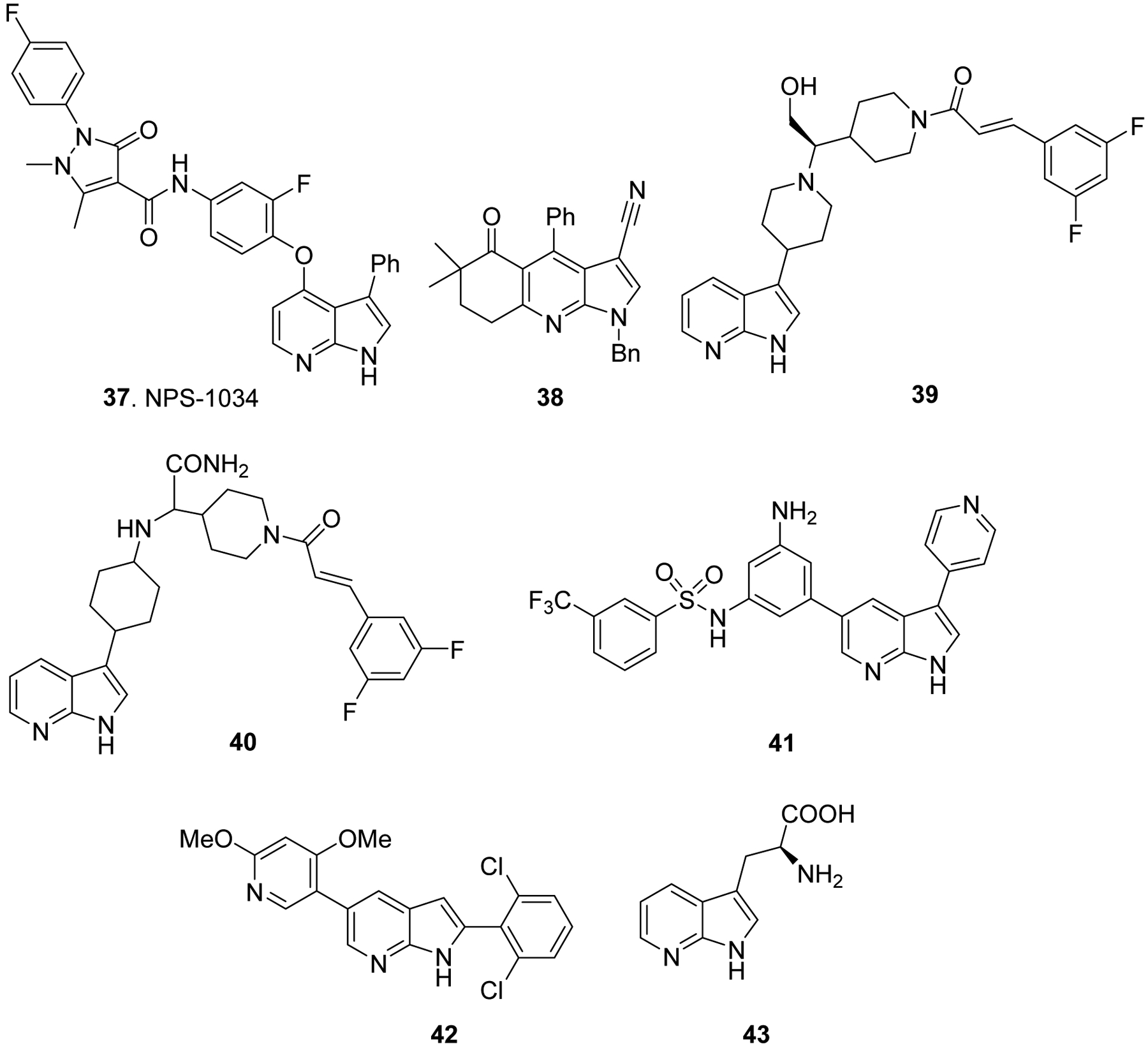
Additional biologically interesting azaindole derivatives.
The above listed compounds in Figures 4, 5, 6 and 7 are not comprehensive of various azaindole-containing biologically active molecules, however, these are the molecules reported with clear understanding on the molecular target, mechanism of action and animal proof-of-concept study; therefore, these may be useful for further development into therapeutically useful drugs.
2. CONCLUSIONS
From the above discussion, it is clear that azaindoles with a wide range of pharmacological and biological activities continue to attract and entice the medicinal chemistry community to develop them for therapeutic use. Interestingly, the biophysical and metabolic properties of azaindole framework are modulated by functionalization pattern and position of nitrogen atom in a six-membered ring (Fig. 1). Due to high biological significance, several laboratories have investigated syntheses for these isomeric azaindoles in the recent years, by many unconventional methods using simple building blocks and novel metal catalytic and metal-free conditions, which will further aid in design of diversely substituted novel azaindole therapeutics.
ACKNOWLEDGEMENTS
This work was supported by NIH/NIA grant U01 AG052460 (T.G.) and NINDS grant R21 NS101167 (T.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
There are no conflicts to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES:
- 1.Walker SR, Carter EJ, Huff BC, Morris JC, Variolins and Related Alkaloids, Chem. Rev, 109 (2009) 3080–3098. [DOI] [PubMed] [Google Scholar]
- 2.Zhao S-B, Wang S, Luminescence and Reactivity of 7-Azaindole Derivatives and Complexes, Chem. Soc. Rev, 39 (2010) 3142–3156. [DOI] [PubMed] [Google Scholar]
- 3.Merour J-Y, Joseph B, Synthesis and Reactivity of 7-Azaindoles (1H-pyrrolo[2,3-b]pyridines), Curr. Org. Chem, 5 (2001) 471–506. [Google Scholar]
- 4.Echalier A, Bettayeb K, Ferandin Y, Lozach O, Clelment M, Valette A, Liger F, Marquet B, Morris JC, Endicott JA, Joseph B, Meijer L, Meriolins (3-(Pyrimidin-4-yl)-7-azaindoles): Synthesis, Kinase Inhibitory Activity, Cellular Effects, and Structure of a CDK2/Cyclin A/Meriolin Complex, J. Med. Chem, 51 (2008) 737–751. [DOI] [PubMed] [Google Scholar]
- 5.Ermoli A, Bargiotti A, Brasca MG, Ciavolella A, Colombo N, Fachin G, Isacchi A, Menichincheri M, Molinari A, Montagnoli A, Pillan A, Rainoldi S, Sirtori FR, Sola F, Thieffine S, Tibolla M, Valsasina B, Volpi D, Santocanale C, Vanotti E, Cell Division Cycle 7 Kinase Inhibitors: 1H-Pyrrolo[2,3-b]pyridines, Synthesis and Structure-Activity Relationships, J. Med. Chem, 52 (2009) 4380–4390. [DOI] [PubMed] [Google Scholar]
- 6.Foote KM, Nissink JWM, McGuire T, Turner P, Guichard S, Yates JWT, Lau A, Blades K, Heathcote D, Odedra R, Wilkinson G, Wilson Z, Wood CM, Jewsbury PJ, Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent, J. Med. Chem, 61 (2018) 9889–9907. [DOI] [PubMed] [Google Scholar]
- 7.Ganser C, Lauermann E, Maderer A, Stauder T, Kramb J-P, Plutizki S, Kindler T, Moehler M, Dannhardt G, Novel 3-Azaindolyl-4-arylmaleimides Exhibiting Potent Antiangiogenic Efficacy, Protein Kinase Inhibition, and Antiproliferative Activity, J. Med. Chem, 55 (2012) 9531–9540. [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Tsai AC, Chen MC, Shen PJ, Cheng YC, Kuo CC, Pan SL, Liu YM, Liu JF, Yeh TK, Wang JC, Chang CY, Chang JY, Liou JP, Azaindolylsulfonamides, with a more Selective Inhibitory Effect on Histone Deacetylase 6 Activity, Exhibit Antitumor Activity in Colorectal Cancer HCT116 cells, J. Med. Chem, 57 (2014) 4009–4022. [DOI] [PubMed] [Google Scholar]
- 9.Meanwell NA, Krystal MR, Nowicka-Sans B, Langley DR, Conlon DA, Eastgate MD, Grasela DM, Timmins P, Wang T, Kadow JF, Inhibitors of HIV-1 Attachment: The Discovery and Development of Temsavir and its Prodrug Fostemsavir, J. Med. Chem, 61 (2018) 62–80. [DOI] [PubMed] [Google Scholar]
- 10.Shirude PS, Shandil R, Sadler C, Naik M, Hosagrahara V, Hameed S, Shinde V, Bathula C, Humnabadkar V, Kumar N, Reddy J, Panduga V, Sharma S, Ambady A, Hegde N, Whiteaker J, McLaughlin RE, Gardner H, Madhavapeddi P, Ramachandran V, Kaur P, Narayan A, Guptha S, Awasthy D, Narayan C, Mahadevaswamy J, Vishwas KG, Ahuja V, Srivastava A, Prabhakar KR, Bharath S, Kale R, Ramaiah M, Choudhury NR, Sambandamurthy VK, Solapure S, Iyer PS, Narayanan S, Chatterji M, Azaindoles: Noncovalent DprE1 Inhibitors from Scaffold Morphing Efforts, Kill Mycobacterium Tuberculosis and are Efficacious in vivo, J. Med. Chem, 56 (2013) 9701–9708. [DOI] [PubMed] [Google Scholar]
- 11.Cash MT, Schreiner PR, Phillips RS, Excited State Tautomerization of Azaindole, Org. Biomol. Chem, 3 (2005) 3701–3706. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y-P, You Y, Zhao J-Q, Zhou X-J, Zhang X-M, Xu X-Y, Yuan W-C, A AgOAc/quinine-derived Aminophosphine Complex As An Efficient Catalyst For Diastereo-And Enantioselective 1,3-Dipolar Cycloaddition of A,B-Unsaturated 7-Azaindoline Amides and Azomethine Ylides, Org. Chem. Front, 6 (2019) 1879–1884. [Google Scholar]
- 13.Takayuki I, Masaaki S, 7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors, Chem. Pharm. Bull 66, (2018) 29–36. [DOI] [PubMed] [Google Scholar]
- 14.Kim KS, Zhang L, Schmidt R, Cai Z-W, Wei D, Williams DK, Lombardo LJ, Trainor GL, Xie D, Zhang Y, An Y, Sack JS, Tokarski JS, Darienzo C, Kamath A, Marathe P, Zhang Y, Lippy J, Jeyaseelan R, Wautlet B, Henley B, Gullo-Brown J, Manne V, Hunt JT, Fargnoli J, Borzilleri RM, Discovery of Pyrrolopyridine-Pyridone Based Inhibitors of Met Kinase: Synthesis, X-ray Crystallographic Analysis, and Biological Activities, J. Med. Chem, 51 (2008) 5330–5341. [DOI] [PubMed] [Google Scholar]
- 15.Liu YA, Jin Q, Zou Y, Ding Q, Yan S, Wang Z, Hao X, Nguyen B, Zhang X, Pan J, Mo T, Jacobsen K, Lam T, Wu TY, Petrassi HM, Bursulaya B, DiDonato M, Gordon WP, Liu B, Baaten J, Hill R, Nguyen-Tran V, Qiu M, Zhang YQ, Kamireddy A, Espinola S, Deaton L, Ha S, Harb G, Jia Y, Li J, Shen W, Schumacher AM, Colman K, Glynne R, Pan S, McNamara P, Laffitte B, Meeusen S, Molteni V, Loren J, Selective DYRK1A Inhibitor for the Treatment of Type 1 Diabetes: Discovery of 6-Azaindole Derivative GNF2133, J. Med. Chem, 63 (2020) 2958–2973. [DOI] [PubMed] [Google Scholar]
- 16.Merour J-Y, Buron F, Ple K, Bonnet P, Routier S, The Azaindole Framework in the Design of Kinase Inhibitors, Molecules, 19 (2014) 19935–19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennington LD, Moustakas DT, The Necessary Nitrogen Atom: A Versatile High-Impact Design Element for Multiparameter Optimization, J. Med. Chem, 60 (2017) 3552–3579. [DOI] [PubMed] [Google Scholar]
- 18.Bandarage UK, Clark MP, Perola E, Gao H, Jacobs MD, Tsai A, Gillespie J, Kennedy JM, Maltais F, Ledeboer MW, Davies I, Gu W, Byrn RA, Nti Addae K, Bennett H, Leeman JR, Jones SM, O’Brien C, Memmott C, Bennani Y, Charifson PS, Novel 2-Substituted 7-Azaindole and 7-Azaindazole Analogues as Potential Antiviral Agents for the Treatment of Influenza, ACS Med. Chem. Lett, 8 (2017) 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer LJ, Clark MP, Boyd MJ, Perola E, Jones SM, Tsai A, Jacobs MD, Bandarage UK, Ledeboer MW, Wang T, Deng H, Ledford B, Gu W, Duffy JP, Bethiel RS, Shannon D, Byrn RA, Leeman JR, Rijnbrand R, Bennett HB, O’Brien C, Memmott C, Nti-Addae K, Bennani YL, Charifson PS, Discovery of Novel, Orally Bioavailable beta-Amino Acid Azaindole Inhibitors of Influenza PB2, ACS Med. Chem. Lett, 8 (2017) 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harcken C, Riether D, Liu P, Razavi H, Patel U, Lee T, Bosanac T, Ward Y, Ralph M, Chen Z, Souza D, Nelson RM, Kukulka A, Fadra-Khan TN, Zuvela-Jelaska L, Patel M, Thomson DS, Nabozny GH, Optimization of Drug-Like Properties of Nonsteroidal Glucocorticoid Mimetics and Identification of a Clinical Candidate, ACS Med. Chem. Lett, 5 (2014) 1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano H, Hasegawa T, Kojima H, Okabe T, Nagano T, Design and Synthesis of Potent and Selective PIM Kinase Inhibitors by Targeting Unique Structure of ATP-Binding Pocket, ACS Med. Chem. Lett, 8 (2017) 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandham DA, Barker L, Brown L, Brown Z, Budd D, Charlton SJ, Chatterjee D, Cox B, Dubois G, Duggan N, Hall E, Hatto J, Maas J, Manini J, Profit R, Riddy D, Ritchie C, Sohal B, Shaw D, Stringer R, Sykes DA, Thomas M, Turner KL, Watson SJ, West R, Willard E, Williams G, Willis J, Discovery of Fevipiprant (NVP-QAW039), a Potent and Selective DP2 Receptor Antagonist for Treatment of Asthma, ACS Med. Chem. Lett, 8 (2017) 582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C, Zhang X, Wang Y, Yang Y, Liu X, Deng M, Jia Y, Ling Y, Meng LH, Zhou Y, Discovery of a Novel Series of 7-Azaindole Scaffold Derivatives as PI3K Inhibitors with Potent Activity, ACS Med. Chem. Lett, 8 (2017) 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cincinelli R, Musso L, Merlini L, Giannini G, Vesci L, Milazzo FM, Carenini N, Perego P, Penco S, Artali R, Zunino F, Pisano C, Dallavalle S, 7-Azaindole-1-carboxamides as a New Class of PARP-1 Inhibitors, Bioorg. Med. Chem, 22 (2014) 1089–1103. [DOI] [PubMed] [Google Scholar]
- 25.Bandarage UK, Cao J, Come JH, Court JJ, Gao H, Jacobs MD, Marhefka C, Nanthakumar S, Green J, ROCK inhibitors 3: Design, Synthesis and Structure-Activity Relationships of 7-Azaindole-Based Rho Kinase (ROCK) Inhibitors, Bioorg. Med. Chem. Lett, 28 (2018) 2622–2626. [DOI] [PubMed] [Google Scholar]
- 26.Regueiro-Ren A, Xue QM, Swidorski JJ, Gong YF, Mathew M, Parker DD, Yang Z, Eggers B, D’Arienzo C, Sun Y, Malinowski J, Gao Q, Wu D, Langley DR, Colonno RJ, Chien C, Grasela DM, Zheng M, Lin PF, Meanwell NA, Kadow JF, Inhibitors of Human Immunodeficiency Virus Type 1 (HIV-1) Attachment. 12. Structure-Activity Relationships Associated with 4-Fluoro-6-Azaindole Derivatives Leading to the Identification of 1-(4-Benzoylpiperazin-1-yl)-2-(4-fluoro-7-[1,2,3]triazol-1-yl-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione (BMS-585248), J. Med. Chem, 56 (2013) 1656–1669. [DOI] [PubMed] [Google Scholar]
- 27.Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, Alcindor T, Ganjoo K, Martin-Broto J, Ryan CW, Thomas DM, Peterfy C, Healey JH, van de Sande M, Gelhorn HL, Shuster DE, Wang Q, Yver A, Hsu HH, Lin PS, Tong-Starksen S, Stacchiotti S, Wagner AJ, investigators E, Pexidartinib Versus Placebo for Advanced Tenosynovial Giant Cell Tumour (ENLIVEN): A Randomised Phase 3 Trial, Lancet, 394 (2019) 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalezari JP, Latiff GH, Brinson C, Echevarria J, Trevino-Perez S, Bogner JR, Thompson M, Fourie J, Sussmann Pena OA, Mendo Urbina FC, Martins M, Diaconescu IG, Stock DA, Joshi SR, Hanna GJ, Lataillade M, A.I.s. Team, Safety and Efficacy of The HIV-1 Attachment Inhibitor Prodrug BMS-663068 In Treatment-Experienced Individuals: 24 Week Results of AI438011, a Phase 2b, Randomised Controlled Trial, Lancet HIV, 2 (2015) e427–437. [DOI] [PubMed] [Google Scholar]
- 29.Gonem S, Berair R, Singapuri A, Hartley R, Laurencin MFM, Bacher G, Holzhauer B, Bourne M, Mistry V, Pavord ID, Mansur AH, Wardlaw AJ, Siddiqui SH, Kay RA, Brightling CE, Fevipiprant, A Prostaglandin D2 Receptor 2 Antagonist, in Patients with Persistent Eosinophilic Asthma: A Single-centre, Randomised, Double-blind, Parallel-group, Placebo-controlled Trial, Lancet Respir Med, 4 (2016) 699–707. [DOI] [PubMed] [Google Scholar]
- 30.Neha S, Anurag, 7-Azaindole Analogues as Bioactive Agents and Recent Results, Mini-Rev. Med. Chem, 19 (2019) 727–736. [DOI] [PubMed] [Google Scholar]
- 31.Sureyya O, Recent Development of New Substituted Indole and Azaindole Derivatives as Anti-HIV Agents, Mini-Rev. Med. Chem, 13 (2013) 1700–1708. [DOI] [PubMed] [Google Scholar]
- 32.Prokopov AA, Yakhontov LN, Chemistry of the Azaindoles (review), Pharm. Chem. J, 28 (1994) 471–506. [Google Scholar]
- 33.Santos AS, Mortinho AC, Marques MMB, Metal-Catalyzed Cross-Coupling Reactions on Azaindole Synthesis and Functionalization, Molecules, 23 (2018) 2673–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaradhi R, Banik A, Mohammed S, Patro V, Rojas A, Wang W, Motati DR, Dingledine R, Ganesh T, Potent, Selective, Water Soluble, Brain-Permeable EP2 Receptor Antagonist for Use in Central Nervous System Disease Models, J. Med. Chem, 63 (2020) 1032–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganesh T, Banik A, Dingledine R, Wang W, Amaradhi R, Peripherally Restricted, Highly Potent, Selective, Aqueous-Soluble EP2 Antagonist with Anti-Inflammatory Properties, Mol Pharm, 15 (2018) 5809–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang C, Amaradhi R, Ganesh T, Dingledine R, An Agonist Dependent Allosteric Antagonist of Prostaglandin EP2 Receptors, ACS Chem. Neurosci 11, (2020) 1436–1446. [DOI] [PubMed] [Google Scholar]
- 37.Bettayeb K, Tirado OM, Marionneau-Lambot S, Ferandin Y, Lozach O, Morris JC, Mateo-Lozano S, Drueckes P, Schachtele C, Kubbutat MH, Liger F, Marquet B, Joseph B, Echalier A, Endicott JA, Notario V, Meijer L, Meriolins, a New Class of Cell Death Inducing Kinase Inhibitors with Enhanced Selectivity for Cyclin-dependent Kinases, Cancer Res, 67 (2007) 8325–8334. [DOI] [PubMed] [Google Scholar]
- 38.Kim G, McKee AE, Ning YM, Hazarika M, Theoret M, Johnson JR, Xu QC, Tang S, Sridhara R, Jiang X, He K, Roscoe D, McGuinn WD, Helms WS, Russell AM, Miksinski SP, Zirkelbach JF, Earp J, Liu Q, Ibrahim A, Justice R, Pazdur R, FDA Approval Summary: Vemurafenib for Treatment of Unresectable or Metastatic Melanoma with the BRAFV600E Mutation, Clin Cancer Res, 20 (2014) 4994–5000. [DOI] [PubMed] [Google Scholar]
- 39.FDA Approves Pexidartinib for Tenosynovial Giant Cell Tumor, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pexidartinib-tenosynovial-giant-cell-tumor.
- 40.Clark MP, Ledeboer MW, Davies I, Byrn RA, Jones SM, Perola E, Tsai A, Jacobs M, Nti-Addae K, Bandarage UK, Boyd MJ, Bethiel RS, Court JJ, Deng H, Duffy JP, Dorsch WA, Farmer LJ, Gao H, Gu W, Jackson K, Jacobs DH, Kennedy JM, Ledford B, Liang J, Maltais F, Murcko M, Wang T, Wannamaker MW, Bennett HB, Leeman JR, McNeil C, Taylor WP, Memmott C, Jiang M, Rijnbrand R, Bral C, Germann U, Nezami A, Zhang Y, Salituro FG, Bennani YL, Charifson PS, Discovery of a Novel, First-in-class, Orally Bioavailable Azaindole Inhibitor (VX-787) of Influenza PB2, J. Med. Chem, 57 (2014) 6668–6678. [DOI] [PubMed] [Google Scholar]
- 41.Harcken C, Riether D, Kuzmich D, Liu P, Betageri R, Ralph M, Emmanuel M, Reeves JT, Berry A, Souza D, Nelson RM, Kukulka A, Fadra TN, Zuvela-Jelaska L, Dinallo R, Bentzien J, Nabozny GH, Thomson DS, Identification of Highly Efficacious Glucocorticoid Receptor Agonists with a Potential for Reduced Clinical Bone Side Effects, J. Med. Chem, 57 (2014) 1583–1598. [DOI] [PubMed] [Google Scholar]
- 42.Dayde-Cazals B, Fauvel B, Singer M, Feneyrolles C, Bestgen B, Gassiot F, Spenlinhauer A, Warnault P, Van Hijfte N, Borjini N, Cheve G, Yasri A, Rational Design, Synthesis, and Biological Evaluation of 7-Azaindole Derivatives as Potent Focused Multi-Targeted Kinase Inhibitors, J. Med. Chem, 59 (2016) 3886–3905. [DOI] [PubMed] [Google Scholar]
- 43.Pracharova J, Saltarella T, Radosova Muchova T, Scintilla S, Novohradsky V, Novakova O, Intini FP, Pacifico C, Natile G, Ilik P, Brabec V, Kasparkova J, Novel Antitumor Cisplatin and Transplatin Derivatives Containing 1-Methyl-7-azaindole: Synthesis, Characterization, and Cellular Responses, J. Med. Chem, 58 (2015) 847–859. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Phoa AF, Abbassi RH, Hoque M, Reekie TA, Font JS, Ryan RM, Stringer BW, Day BW, Johns TG, Munoz L, Kassiou M, Structural Optimization and Pharmacological Evaluation of Inhibitors Targeting Dual-Specificity Tyrosine Phosphorylation-Regulated Kinases (DYRK) and CDC-like Kinases (CLK) in Glioblastoma, J. Med. Chem, 60 (2017) 2052–2070. [DOI] [PubMed] [Google Scholar]
- 45.Verdonck S, Pu SY, Sorrell FJ, Elkins JM, Froeyen M, Gao LJ, Prugar LI, Dorosky DE, Brannan JM, Barouch-Bentov R, Knapp S, Dye JM, Herdewijn P, Einav S, De Jonghe S, Synthesis and Structure-Activity Relationships of 3,5-Disubstituted-pyrrolo[2,3-b]pyridines as Inhibitors of Adaptor-Associated Kinase 1 with Antiviral Activity, J. Med. Chem, 62 (2019) 5810–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodfellow VS, Loweth CJ, Ravula SB, Wiemann T, Nguyen T, Xu Y, Todd DE, Sheppard D, Pollack S, Polesskaya O, Marker DF, Dewhurst S, Gelbard HA, Discovery, Synthesis, and Characterization of an Orally Bioavailable, Brain Penetrant Inhibitor of Mixed Lineage Kinase 3, J. Med. Chem, 56 (2013) 8032–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh U, Chashoo G, Khan SU, Mahajan P, Nargotra A, Mahajan G, Singh A, Sharma A, Mintoo MJ, Guru SK, Aruri H, Thatikonda T, Sahu P, Chibber P, Kumar V, Mir SA, Bharate SS, Madishetti S, Nandi U, Singh G, Mondhe DM, Bhushan S, Malik F, Mignani S, Vishwakarma RA, Singh PP, Design of Novel 3-Pyrimidinylazaindole CDK2/9 Inhibitors with Potent In Vitro and In Vivo Antitumor Efficacy in a Triple-Negative Breast Cancer Model, J. Med. Chem, 60 (2017) 9470–9489. [DOI] [PubMed] [Google Scholar]
- 48.Dallagnol JCC, Khajehali E, van der Westhuizen ET, Jorg M, Valant C, Goncalves AG, Capuano B, Christopoulos A, Scammells PJ, Synthesis and Pharmacological Evaluation of Heterocyclic Carboxamides: Positive Allosteric Modulators of the M1 Muscarinic Acetylcholine Receptor with Weak Agonist Activity and Diverse Modulatory Profiles, J. Med. Chem, 61 (2018) 2875–2894. [DOI] [PubMed] [Google Scholar]
- 49.Song P, Chen M, Ma X, Xu L, Liu T, Zhou Y, Hu Y, Identification of novel inhibitors of Aurora A with a 3-(Pyrrolopyridin-2-yl)indazole Scaffold, Bioorg. Med. Chem, 23 (2015) 1858–1868. [DOI] [PubMed] [Google Scholar]
- 50.Sandham DA, Arnold N, Aschauer H, Bala K, Barker L, Brown L, Brown Z, Budd D, Cox B, Docx C, Dubois G, Duggan N, England K, Everatt B, Furegati M, Hall E, Kalthoff F, King A, Leblanc CJ, Manini J, Meingassner J, Profit R, Schmidt A, Simmons J, Sohal B, Stringer R, Thomas M, Turner KL, Walker C, Watson SJ, Westwick J, Willis J, Williams G, Wilson C, Discovery and Characterization of NVP-QAV680, A Potent and Selective Crth2 Receptor Antagonist Suitable for Clinical Testing in Allergic Diseases, Bioorg. Med. Chem, 21 (2013) 6582–6591. [DOI] [PubMed] [Google Scholar]
- 51.Chowdhury S, Sessions EH, Pocas JR, Grant W, Schroter T, Lin L, Ruiz C, Cameron MD, Schurer S, LoGrasso P, Bannister TD, Feng Y, Discovery and Optimization of Indoles and 7-Azaindoles as Rho kinase (ROCK) Inhibitors (part-I), Bioorg. Med. Chem. Lett, 21 (2011) 7107–7112. [DOI] [PubMed] [Google Scholar]
- 52.Sessions EH, Chowdhury S, Yin Y, Pocas JR, Grant W, Schroter T, Lin L, Ruiz C, Cameron MD, LoGrasso P, Bannister TD, Feng Y, Discovery and Optimization of Indole and 7-Azaindoles as Rho kinase (ROCK) Inhibitors (part-II), Bioorg. Med. Chem. Lett, 21 (2011) 7113–7118. [DOI] [PubMed] [Google Scholar]
- 53.Feneyrolles C, Guiet L, Singer M, Van Hijfte N, Dayde-Cazals B, Fauvel B, Cheve G, Yasri A, Discovering Novel 7-Azaindole-based Series as Potent AXL Kinase Inhibitors, Bioorg. Med. Chem. Lett, 27 (2017) 862–866. [DOI] [PubMed] [Google Scholar]
- 54.Stanton RA, Lu X, Detorio M, Montero C, Hammond ET, Ehteshami M, Domaoal RA, Nettles JH, Feraud M, Schinazi RF, Discovery, Characterization, and Lead Optimization of 7-Azaindole Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors, Bioorg. Med. Chem. Lett, 26 (2016) 4101–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia M, Hou C, DeMong D, Pollack S, Pan M, Singer M, Matheis M, Murray W, Cavender D, Wachter M, Synthesis and Structure-Activity Relationship of 7-Azaindole Piperidine Derivatives as CCR2 Antagonists, Bioorg. Med. Chem. Lett, 18 (2008) 6468–6470. [DOI] [PubMed] [Google Scholar]
- 56.Lanter JC, Markotan TP, Zhang X, Subasinghe N, Kang FA, Hou C, Singer M, Opas E, McKenney S, Crysler C, Johnson D, Molloy CJ, Sui Z, The Discovery of Novel Cyclohexylamide CCR2 Antagonists, Bioorg. Med. Chem. Lett, 21 (2011) 7496–7501. [DOI] [PubMed] [Google Scholar]
- 57.Hong S, Kim J, Seo JH, Jung KH, Hong S-S, Hong S, Design, Synthesis, and Evaluation of 3,5-Disubstituted 7-Azaindoles as Trk Inhibitors with Anticancer and Antiangiogenic Activities, J. Med. Chem, 55 (2012) 5337–5349. [DOI] [PubMed] [Google Scholar]
- 58.Yan W, Lakkaniga NR, Carlomagno F, Santoro M, McDonald NQ, Lv F, Gunaganti N, Frett B, Li H.-y., Insights into Current Tropomyosin Receptor Kinase (TRK) Inhibitors: Development and Clinical Application, J. Med. Chem, 62 (2019) 1731–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esteve C, Gonzalez J, Gual S, Vidal L, Alzina S, Sentellas S, Jover I, Horrillo R, De Alba J, Miralpeix M, Tarrason G, Vidal B, Discovery of 7-Azaindole Derivatives as Potent Orai Inhibitors Showing Efficacy in a Preclinical Model of Asthma, Bioorg. Med. Chem. Lett, 25 (2015) 1217–1222. [DOI] [PubMed] [Google Scholar]
- 60.Offenbacher AR, Pagba CV, Polander BC, Brahmachari U, Barry BA, First Site-specific Incorporation of a Noncanonical Amino Acid into the Photosynthetic Oxygen-evolving Complex, ACS Chem. Biol, 9 (2014) 891–896. [DOI] [PubMed] [Google Scholar]


