Abstract
Background:
Primary hyperparathyroidism (PHPT) is associated with substantial morbidity, including osteoporosis, nephrolithiasis, and chronic kidney disease (CKD). Parathyroidectomy (PTX) can prevent these sequelae but is poorly utilized in many practice settings.
Methods:
We performed a retrospective cohort study using the national Optum de-identified Clinformatics® Data Mart Database. We identified patients aged ≥35 with a first observed PHPT diagnosis from 2004–2016. Multivariable logistic regression was used to determine patient/provider characteristics associated with PTX.
Results:
Of 26,522 patients with PHPT, 10,101 (38.1%) underwent PTX. Of the 14,896 patients with any operative indication, 5,791 (38.9%) underwent PTX. Over time, there was a decreasing trend in the rate of PTX overall (2004: 54.4% to 2016: 32.4% p<.001) and among groups with and without an operative indication. On multivariable analysis, increasing age and comorbidities were strongly, inversely associated with PTX (age 75–84, OR 0.50[95%CI 0.45–0.55]; age ≥85, OR 0.21[95%CI 0.17–0.26] versus age 35–49; Charlson Comorbidity Index ≥2 vs 0 OR 0.62[95%CI 0.58–0.66]).
Conclusions:
The majority of U.S., privately insured patients with PHPT are not treated with PTX. Having an operative indication only modestly increases the likelihood of PTX. Further research is needed to address barriers to treatment and the gap between guidelines and clinical care in PHPT.
TOC Statement
Using the Optum de-identified Clinformatics Data Mart Database, we found that the majority of patients are not treated with parathyroidectomy and meeting the consensus criteria for surgery only modestly increases the likelihood of definitive surgical management. These results are important, because they suggest that focused efforts are needed to educate providers about appropriate, evidence-based guidelines for management of primary hyperparathyroidism and to identify barriers to surgical referral and utilization of parathyroidectomy.
INTRODUCTION
Primary hyperparathyroidism (PHPT) is a common endocrine disorder that is increasing in prevalence and is associated with morbidity related to osteoporotic fractures,1 nephrolithiasis,2 and chronic kidney disease (CKD).3 Parathyroidectomy (PTX) is the only definitive treatment for PHPT and is indicated for all patients with symptomatic disease. Evidence-based consensus guidelines for the operativemanagement of PHPT in seemingly asymptomatic patients have expanded over time, as the benefits of operative cure in additional populations have been documented.4, 5 Non-operative observation however, is an alternative management strategy pursued for some asymptomatic patients without PHPT-associated end organ damage or for those with presumed contraindications to operative intervention.
In patients with PHPT, PTX improves bone mineral density,6 decreases the risk of new fractures1 and nephrolithiasis,2, 6 and halts the deterioration of kidney function7 when compared with non-operative management. Despite these benefits, rates of PTX for the treatment of PHPT documented in regional, single-center, and Veteran populations in the U.S. are low.8–10 Up to 51% of patients with PHPT in the U.S. meet consensus criteria for operative management of their disease, but less than half of these patients undergo PTX.8, 9 Among patients 70 and older who meet consensus criteria for PTX, less than 24% undergo PTX.11 Age and comorbidity are two of the most important, independent predictors of non-operative management in academic and community settings,8, 9, 11 suggesting that PTX is not pursued due to concern for operative risks in older and sicker patients. Given the low rates of adherence to evidence-based guidelines, it is likely that PTX is not being optimally targeted to patients likely to benefit, especially among those who are older and with multimorbidity.12
There are few population-based data that document the management of PHPT in the U.S., which limits our ability to evaluate adherence to guidelines on a national scale. The aim of this study was to document the management of PHPT within a national, privately-insured patient population and determine which patient and provider characteristics are associated with PTX. We hypothesized that adherence to guidelines would be low overall and that older age and greater comorbidity burden would be the greatest predictors of non-operative management.
METHODS
Study Design
We performed a retrospective cohort study of patients with PHPT within the Optum’s de-identified Clinformatics® Data Mart Database from January 2004 to December 2016. The Optum Clinformatics® database contains deidentified private commercial insurance and Medicare advantage claims data from inpatient, outpatient, and pharmaceutical claims for approximately 12 million patients per year. The database includes insurance plan enrollment information, patient demographics (e.g. age, sex, race/ethnicity), socioeconomic data (e.g. highest level education, mean household income), geographic region of residence, medical claims data (related to hospital admissions, procedures andoperations, and outpatient visits), hospital and provider identifiers and information, pharmacy claims data (e.g. drug dispensed, dose, quantity), and limited lab results. The institutional review board of Stanford University determined this project to be exempt from review due to use of deidentified patient data.
Study Population
We identified a cohort of 57,860 patients based on the International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) diagnosis codes for PHPT (252.01, E21.0) on any claim from January 1, 2004 to December 31, 2016. We excluded 9,091 patients who: 1) had stages IV or V CKD; 2) were on hemodialysis; 3) had a documented prior kidney transplant or prior PTX and, therefore, were more likely to have secondary or tertiary hyperparathyroidism; 4) were <35 years of age and, therefore, more likely to have hereditary forms of PHPT (for which there is no consensus on appropriate timing of PTX); or 5) were missing age or sex (Figure 1). We then excluded all patients with less than 1 year of continuous enrollment before or after initial diagnosis of PHPT to ensure adequate time to identify comorbid conditions and evaluate rates of operativeversus medical management. Patients who underwent PTX within 1 year of diagnosis were identified based on ICD-9 and ICD-10 procedure codes (ICD-9: 0681, 0689, 0699; and ICD-10: 0GBLx, 0GBMx, 0GBNx, 0GBPx, 0GBQx, 0GBRx) and Current Procedural Terminology (CPT) codes 60500, 60502, and 60505.
Figure 1.
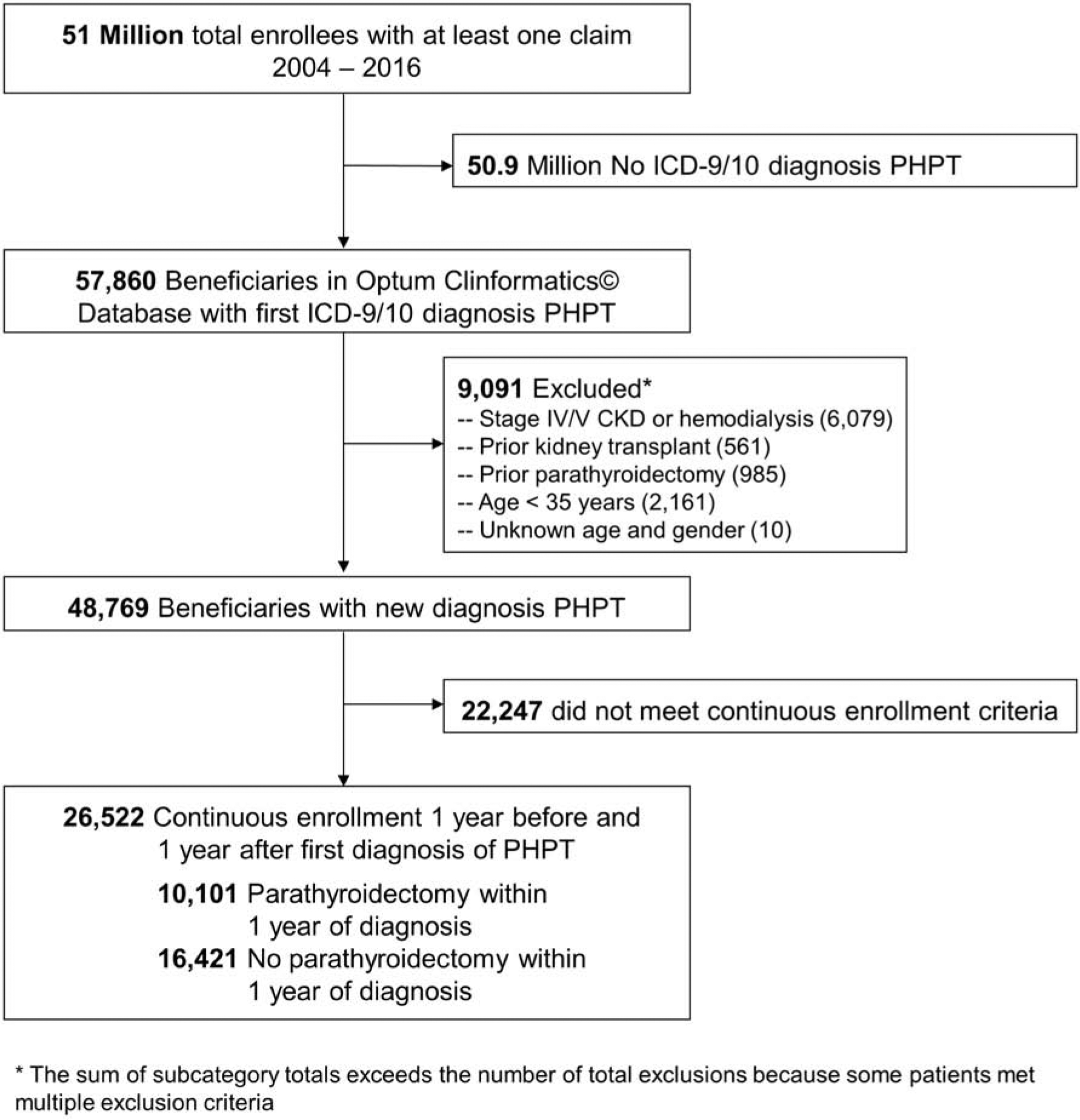
Cohort Enrollment Criteria
Covariates
Demographic characteristics of interest included age group (35–49, 50–64, 65–74, 75–84, ≥ 85 years), sex, and race/ethnicity (White, Black, Asian, Hispanic, or unknown). Socioeconomic data included highest level of education and mean household income. Patient comorbidities were identified using ICD-9 and ICD-10 codes from inpatient and outpatient claims from the 12-month period prior to index PHPT diagnosis. Charlson Comorbidity Index (CCI) was calculated as described previously .13 Patients were considered to have an operative indication for PTX if they met consensus criteria based on age <50, or diagnosis of osteoporosis, nephrolithiasis, or stage III CKD. Patients who were evaluated by an endocrinologist within 6 months before or after index diagnosis of PHPT were identified based on claims with: 1) a taxonomy or provider category code indicating subspeciality training in endocrinology; and 2) a diagnosis code related to PHPT or its associated sequelae (e.g. osteoporosis).
Outcomes
Our primary outcome was operative management with PTX within 1 year of PHPT diagnosis. We chose a 1-year time horizon for treatment decisions to understand patient and provider characteristics associated with an initial decision to pursue definitive operative management of PHPT. We performed a sensitivity analysis to determine whether using a time period greater than 1 year to identify medical versus operative treatment group had a clinically important effect on our results. We also identified patients managed non-operatively who had an operative consultation indicated by a claim for a visit with a general surgeon (which included those with subspeciality training in endocrine surgery and surgical oncology) or otolaryngologist and a diagnosis code related to PHPT or its associated sequelae.
Statistical Analysis
Demographic and clinical characteristics were compared for patients who underwent operation versus their medically managed counterparts using chi-square and student’s t-tests. We performed a Cochran-Armitage trend test to determine whether the rates of PTX changed consistently over time and Spearman rank correlations to assess yearly trends in age and CCI at the time of PHPT diagnosis. A multivariable logistic regression model was calculated to determine patient and provider characteristics independently associated with PTX. Covariates included in multivariable regression analyses included sex, age group, race/ethnicity, highest level of education, osteoporosis, nephrolithiasis, stage 3 CKD, CCI, geographic region, and evaluation by an endocrinologist within 6 months before or after PHPT diagnosis. The statistical significance level was set at P < 0.05, and all tests were two-tailed. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
After applying cohort selection criteria, we identified 26,522 patients with an incident diagnosis of PHPT from 2004 to 2016 (Figure 1). Patients in the study cohort were 76.7% female, 63.8% white, and had a mean age of 65.3 [SD 12.3]. Among the study cohort, 10,101 (38.1%) underwent PTX within one year of diagnosis and 16,421 (61.9%) were managed non-operatively (Table 1). Compared to those managed non-operatively, patients who underwent PTX were younger (mean age PTX 62.4 [SD 11.4] vs. non-operative management 67.1 [SD 12.4], p <.001) and had fewer comorbidities (mean CCI 0.95 [SD 1.38] vs. 1.49 [SD 1.8]; p <.001). Demographic disparities were observed by race/ethnicity and region. Patients who underwent PTX were somewhat more likely to be white (66.6% vs. non-operative management 62.1%; p<.001) and to reside in the Southern region (41.6% vs. 35.5%; p<.001) or Midwestern region (25.9% vs. 20.3%, p<.001).
Table 1:
Baseline characteristics of patients with PHPT in the Optum Clinformatics®* database 2004–2016.
| Characteristic | Parathyroidectomy within 1 year (n = 10,101) | No parathyroidectomy (n = 16,421) | P value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Demographics | |||||
| Sex | |||||
| Female | 7,715 | 76.4% | 12,636 | 77.0% | .28 |
| Male | 2,386 | 23.6% | 3,785 | 23.0% | |
| Age Group | |||||
| 35 – 49 | 1,393 | 13.8% | 1,631 | 9.9% | <.001 |
| 50 – 64 | 4,168 | 41.3% | 4,669 | 28.4% | |
| 65 – 74 | 2,904 | 28.7% | 4,877 | 29.7% | |
| 75 – 84 | 1,513 | 15.0% | 4,343 | 26.4% | |
| >= 85 | 123 | 1.2% | 901 | 5.5% | |
| Race | |||||
| White | 6,724 | 66.6% | 10,198 | 62.1% | <.001 |
| Black | 851 | 8.4% | 1,731 | 10.5% | |
| Asian | 180 | 1.8% | 341 | 2.1% | |
| Hispanic | 597 | 5.9% | 1,247 | 7.6% | |
| Unknown | 1,749 | 17.3% | 2,904 | 17.7% | |
| Highest Level Education | |||||
| High school diploma or less | 2,180 | 21.6% | 3,946 | 24.0% | <.001 |
| Some college | 5,514 | 54.6% | 8,739 | 53.2% | |
| Bachelor degree plus | 2,016 | 20.0% | 2,952 | 18.0% | |
| Unknown | 391 | 3.9% | 784 | 4.8% | |
| Geographic Region | |||||
| Midwest | 2,618 | 25.9% | 3,337 | 20.3% | <.001 |
| Northeast | 890 | 8.8% | 2,503 | 15.2% | |
| South | 4,206 | 41.6% | 5,827 | 35.5% | |
| West | 2,333 | 23.1% | 4,624 | 28.2% | |
| Unknown | 54 | 0.5% | 130 | 0.8% | |
| Operative Indications for Parathyroidectomy** | |||||
| Osteoporosis | 3,290 | 32.6% | 5,649 | 34.4% | .002 |
| Nephrolithiasis | 1,799 | 17.8% | 1,831 | 11.2% | <.001 |
| Stage III CKD (eGFR 30–59) | 497 | 4.9% | 1,738 | 10.6% | <.001 |
| Age < 50 years | 1,393 | 13.8% | 1,631 | 9.9% | <.001 |
| Preoperative Patient Characteristics | |||||
| Charlson Comorbidity Index | |||||
| 0 | 5,188 | 51.4% | 6,380 | 38.9% | <.001 |
| 1 | 2,626 | 26.0% | 4,088 | 24.9% | |
| >= 2 | 2,287 | 22.6% | 5,953 | 36.3% | |
| Endocrinologist care within 6 months | 6,105 | 60.4% | 8,842 | 53.8% | <.001 |
The Optum de-identified Clinformatics® Data Mart Database 2004–2016 is an adminsitraive database of privately insured patients in the United States
Serum calcium levels were unavailable for most patients in our cohort, therefore calcium >1.0 mg/dL above the upper limit of normal was not included as an operative indication in our analysis.
Among 16,421 PHPT patients managed non-operatively, 1,857 (11.3%) had a claim for a visit with a general surgeon or otolaryngologist 6 months before or after their PHPT diagnosis with a visit diagnosis code related to PHPT, suggesting possible evaluation for PTX. When examining the number of patients treated with cinicalcet, a medication that decreases serum calcium levels but has not been shown to decrease the skeletal morbidity associated with PHPT,14 a total of 711 (4.3%) of patients were managed non-operatively compared to 77 (0.8%) patients after PTX (p<.001). Over time, there was a markedly decreasing trend in the rate of PTX(2004: 54.4% to 2016: 32.4%; p < .001 Figure 2;;). This coincided with an increase in the median age at PHPT diagnosis from 57 to 70 years and in the mean CCI score from 0.76 to 1.51 (both p < .001). The trend toward decreasing use of PTX was observed both among patients with and without an operative indication (with operative indication: 2004 60% to 2016 33.3%; with no indication: 2004 48.7% to 2016 31.3%; both p<0.001).
Figure 2.
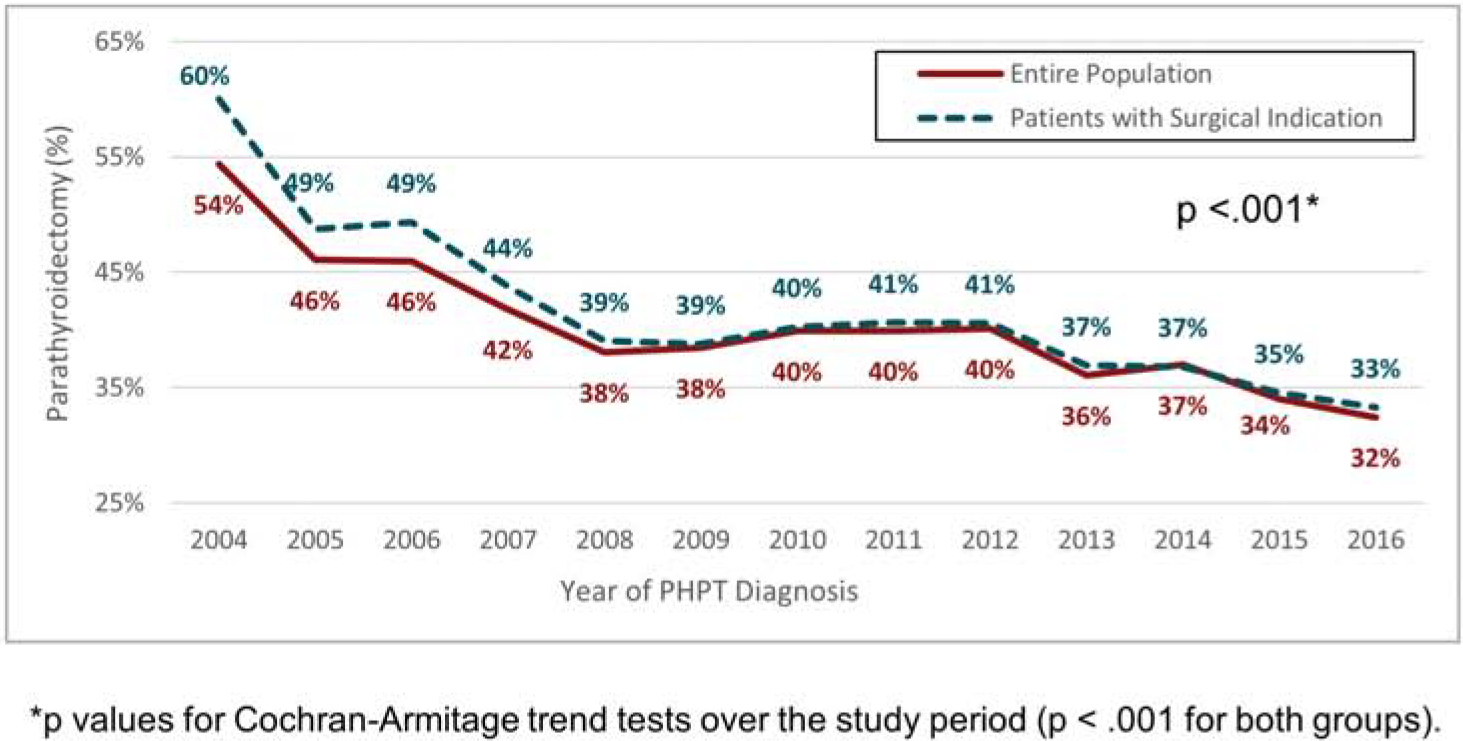
Rate of PTX according to year of PHPT diagnosis
Among the 14,896 patients who had at least one indication for PTX, only 5,791 (38.9%) were managed with PTX. Among those with at least one operative indication, patients with nephrolithiasis compared to those without nep[hrolithiasiswere most likely to undergo PTX(49.6% vs 36.3%: p<.001; Table 2). The unadjusted rate of PTX was similar for patients with and without a diagnosis of osteoporosis (36.8% vs. 38.7%, resp). Patients with stage 3 CKD were much less likely to undergo PTX compared to patients without documented kidney disease (22.2% vs. 39.5%,). With few exceptions, lesser proportions of PTX were observed with increasing age and morbidity (as measured by CCI), irrespective of an operative indication (Figure 3).
Table 2.
Management of PHPT in patients with indications for parathyroidectomy (PTX) based on consensus criteria.
| Surgical Indication | Total Patients n | PTX n (%) |
|---|---|---|
| No Stage 3 Kidney Disease | 24,287 | 9,604 (39.5%) |
| Stage 3 Kidney Disease | 2,235 | 497 (22.2%) |
| No Osteoporosis | 17,583 | 6,811 (38.7%) |
| Osteoporosis | 8,939 | 3,290 (36.8%) |
| No Nephrolithiasis | 22,892 | 8,302 (36.3%) |
| Nephrolithiasis | 3,630 | 1,799 (49.6%) |
| Age ≥ 50 | 23,498 | 8,708 (37.1%) |
| Age < 50 | 3,024 | 1,393 (46.1%) |
Figure 3.
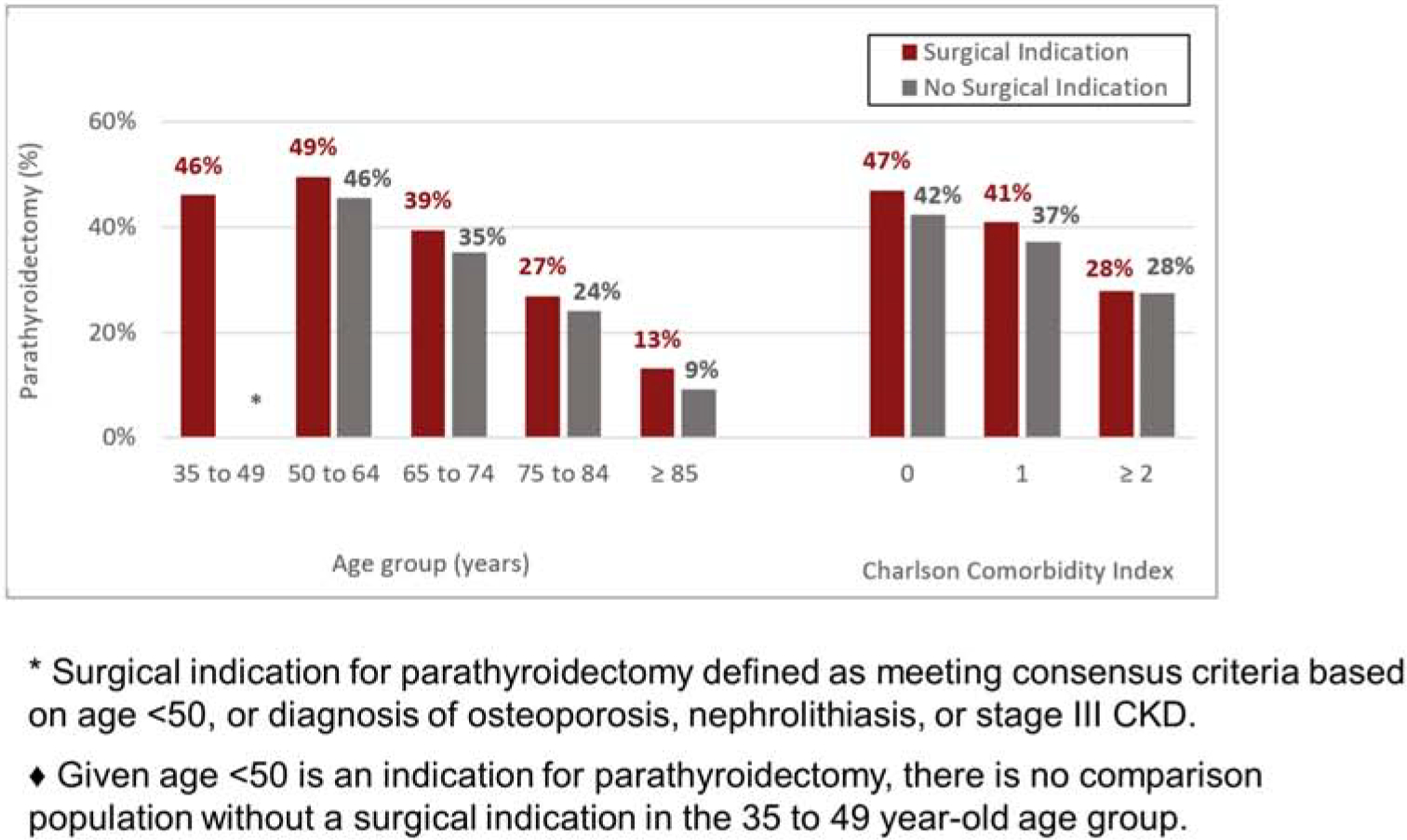
Percentage of patients undergoing parathyroidectomy according to age, comorbidity burden and indication for parathyroidectomy.*
Multivariable analysis demonstrated that patients who had an operative indication for PTX based on consensus criteria of nephrolithiasis and osteoporosis, increased the odds of PTX (ORs 1.62 [95% CI 1.51–1.75] and 1.15 [95%CI 1.08–1.22]), while having Stage 3 CKD decreased the odds of PTX (OR 0.79 [95% CI 0.71–0.89]) (Figure 4). Patients who were seen by an endocrinologist had a marginally increased odds of PTX compared to those managed by primary care or other providers (OR 1.13 [95% CI 1.07 – 1.19]). Increasing age and comorbidity burden were strongly inversely associated with PTX. Compared to patients 35–49 years of age, those aged 75–84 years at diagnosis had 50% lesser odds of undergoing PTX (OR 0.50 [95%CI 0.45–0.55]), and patients 85years of age and older had 79% lesser odds of PTX (OR 0.21 [95% CI 0.17–0.26]) compared to patients 35–49 years of age. Patients with a high comorbidity burden had 38% lesser odds of undergoing PTX (OR 0.62 [95%CI 0.58–0.66] for CCI ≥2 vs. 0). Black, Asian, and Hispanic patients had a statistically significantly decreased odds of PTX compared to white patients. When we performed a sensitivity analysis grouping patients who underwent PTX at any time during continuous enrollment in the PTX cohort, the overall rate of PTX increased slightly from 38.1% to 42.9%, and results of the multivariable analysis were clinically similar to the primary analysis.
Figure 4.
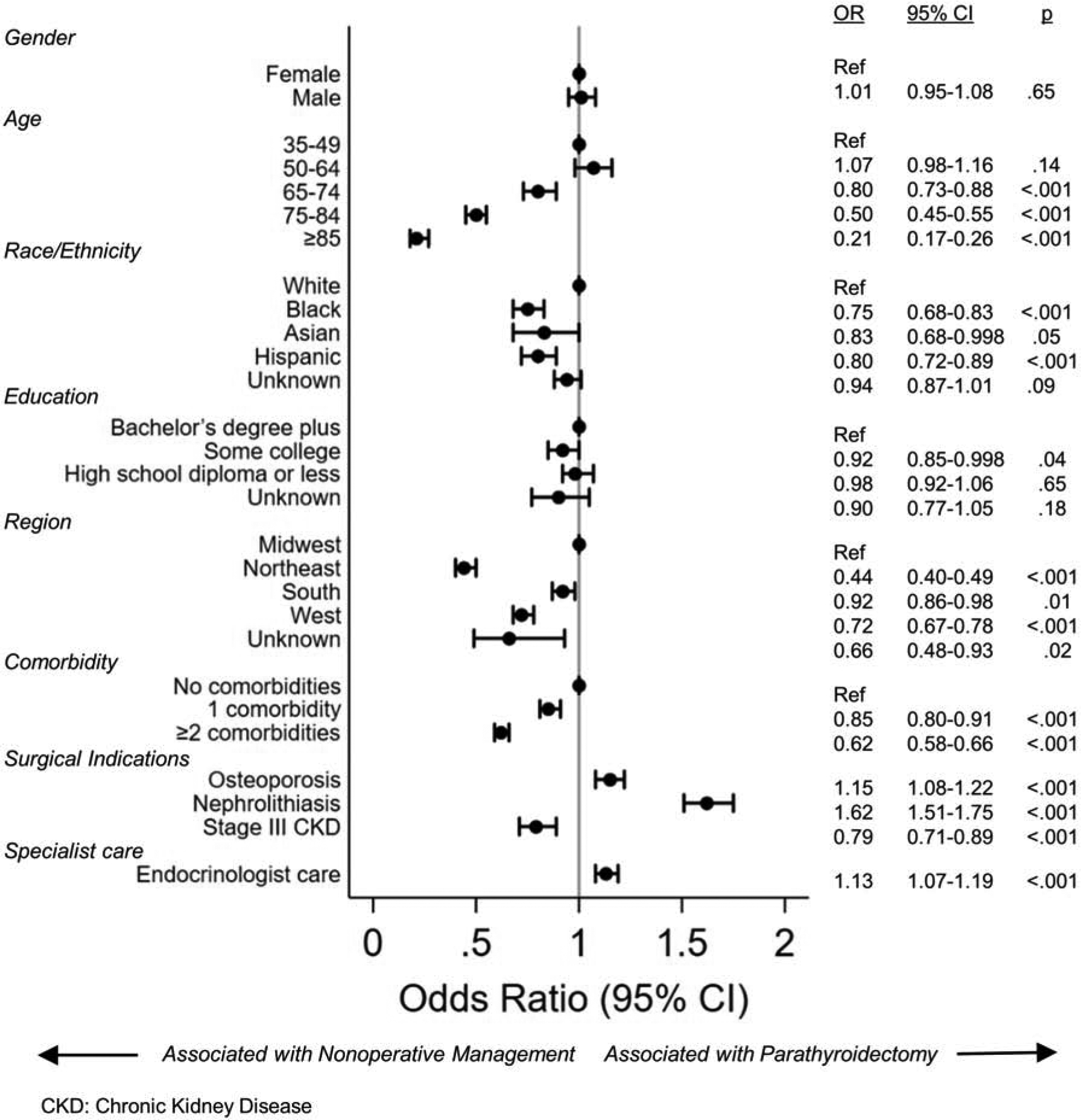
Variables associated with parathyroidectomy for patients with PHPT based on multivariable logistic regression.
DISCUSSION
This study showed that less than 40% of privately insured patients with PHPT in the U.S. were managed with PTX and that there was a negligible difference in operative management for patients with at least one indication for PTX versus those without. Increasing age and comorbidity burden were the strongest predictors of non-operative management, and receiving specialist care from an endocrinologist only marginally increased the likelihood of PTX. Disparities in care based on race and ethnicity were evident: Black, Asian, and Hispanic patients were statistically significantly less likely to undergo PTX than white patients. As guidelines for the operative management of PHPT have expanded, utilization of PTX has decreased, including in patients with an objective,evidence-based indication for PTX. Collectively, these results suggest that these evidence-based guidelines for PHPT are not followed closely and that PTX is underutilized as definitive treatment for PHPT.
Our results from a national, privately insured patient population are consistent with prior regional, single-center, and Veterans Health Administration studies showing low rates of operative management for PHPT in the U.S.8,9,10 It is notable that rates of PTX for the management of PHPT remain still low in a privately insured population, given that patients with private health insurance are more likely to: (1) have access to regular heath evaluations; (2) be diagnosed at earlier stages of disease; and (3) receive therapeutic interventions, compared to patients who are uninsured, underinsured, or covered by Medicaid.15–18 Fewer barriers to operative management in privately-insured patients may account for the minimal impact of having an operative indication on the rate of PTX. In addition, our claims analysis did not capture patients with unrecognized PHPT, which results in an underestimation of the population managed non-operatively without a deliberate treatment decision and actually an overestimation in the rate of appropriate operartive management. This finding suggests an even larger gap between care based on acdepted,evidence-based guidelines and the clinical management of patients with PHPT in the U.S. than is documented in this study. Therefore, focused efforts are needed to educate providers about guidelines for the management of PHPT and identify barriers to operative referral and utilization.
The fact that age and comorbidity are two of the most important independent predictors of non-operative management in our patient population and prior studies8, 9, 11 suggests that PTX is avoided due to concern that the operative risks for older adults outweigh the potential benefits related to bone fracture, kidney stone formation, and CKD risk reduction. These associations may be in part responsible for the trend toward decreasing utilization of PTX over time, given the median age at diagnosis increased by more than a decade during the study period, coincident with increasing comorbidity burden and are likely related to aging of the U.S. population and screening in older adults with osteoporosis. The absolute risk of perioperative complications associated with PTX is low (1.1%), but age 75 and older and patient frailty are independently associated with an increased risk for serious complications.19, 20 Limited survey studies of clinicians who care for patients with PHPT have found that endocrinologists tend to harbor concerns about medical contraindications for and the anticipated complexity of PTX, while endocrine surgeons are more likely to deem operative management indicated for mild or subjective symptoms.21–24 Out study shows that more detailed qualitative research is needed to further examine the nuances of treatment decisions in PHPT and understand the gap between guidelines and clinical care, especially in older adults. In addition, education on patient characteristics associated with an increased operative risk and improved tools for individualized risk-prediction are needed and would possibly be indicated to improve decision-making in older, multimorbid patient populations with the goal of increasing the appropriateutilization of PTX in those likely to benefit.
Our study has limitations related to the use of administrative claims database. Given prior studies have shown low rates of documentation of a PHPT diagnosis in patients with a biochemical diagnosis in the electronic health record, the use of ICD-9/ICD-10 codes to identify our cohort likely results in under-identification of patients with PHPT within the Optum database.10 As a result, the rate of PTX that we report is limited to patients with PHPT that is recognized and documented by physicians in medical claims, and, therefore, the rate of PTX we reported is greater than would be expected if all patients with biochemical disease were included., We purposely chose to use only the most specific diagnosis codes to identify patients with PHPT and applied stringent exclusion criteria of the cohorts to prevent cohort contamination from patients with secondary or tertiary hyperparathyroidism; this approach allowed us to evaluate factors associated with treatment decisions once the PHPT diagnosis was made and, therefore, identify where to focus efforts to improve appropriate operative utilizationof PTX. Calcium and parathyroid hormone levels were found to be missing from the majority of patients in the Optum database, which also does not include data on bone mineral density; therefore, we were unable to incorporate PHPT disease severity in our models. High enrollment turnover in private insurance programs may limit our ability to identify patients with an incident diagnosis of PHPT, although we ensured 1 year of continuous enrollment without a PHPT diagnosis prior to the index PHPT diagnosis. Lastly, patients with private insurance may differ from patients who are uninsured, part of integrated health care systems, or enrolled in government-sponsored insurance programs, so our results may not be generalizable to all patients with PHPT. We would expect health care utilization and therapeutic interventions to be greater in this privately insured population,;16 but,whether utilization of PTX remains low and comparable to those in managed care settings is still unknown..
This report confirms the results of prior regional and single-center studies showing that the majority of patients with PHPT and with an operative indication for PTX are not treated with PTX, indeed Ptientss who meet consensus criteria or receive endocrinology care only modestly increases the likelihood of definitive appropriate operative management. Despite expanding operative guidelines, the utilization of PTX has continued to decrese over time recently. Increasing patient age and comorbidity are the most evidentpatient factors associated with non-operative management of PHPT. Further research and educatin of medical providers is needed to address this gap between guidelines and appropriate evidence-based clinical management in patients with PHPT, especially in older and multimorbid populations, with the goal of individualizing treatment decisions and targeting PTX to those who will benefit.
FUNDING/SUPPORT:
The authors acknowledge funding support from the National Institutes of Health, National Institute on Aging by awards R03AG060097 (CDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI/DISCLOSURES: None
Accepted to be presented at the American Association of Endocrine Surgeons (AAES) Annual Meeting, Birmingham, Alabama April 4–6, 2020 (Cancelled due to COVID-19)
REFERENCES
- 1.Yeh MW, et al. , The Relationship of Parathyroidectomy and Bisphosphonates With Fracture Risk in Primary Hyperparathyroidism: An Observational Study. Annals of Internal Medicine, 2016. 164(11): p. 715–723. [DOI] [PubMed] [Google Scholar]
- 2.Mollerup CL, et al. , Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. Bmj, 2002. 325(7368): p. 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assadipour Y, et al. , End-organ effects of primary hyperparathyroidism: A population-based study. Surgery, 2019. 165(1): p. 99–104. [DOI] [PubMed] [Google Scholar]
- 4.Bilezikian JP, et al. , Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary Statement from the Fourth International Workshop. The Journal of Clinical Endocrinology & Metabolism, 2014. 99(10): p. 3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, et al. , The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA surgery, 2016. 151(10): p. 959–968. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg SJ, et al. , A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. New England Journal of Medicine, 1999. 341(17): p. 1249–1255. [DOI] [PubMed] [Google Scholar]
- 7.Tassone F, et al. , Parathyroidectomy halts the deterioration of renal function in primary hyperparathyroidism. The Journal of Clinical Endocrinology & Metabolism, 2015. 100(8): p. 3069–3073. [DOI] [PubMed] [Google Scholar]
- 8.Yeh MW, et al. , Surgery for primary hyperparathyroidism: are the consensus guidelines being followed? Annals of surgery, 2012. 255(6): p. 1179–1183. [DOI] [PubMed] [Google Scholar]
- 9.Kuo EJ, et al. , Surgery for primary hyperparathyroidism: adherence to consensus guidelines in an academic health system. Annals of surgery, 2019. 269(1): p. 158–162. [DOI] [PubMed] [Google Scholar]
- 10.Alore EA, et al. , Diagnosis and Management of Primary Hyperparathyroidism Across the Veterans Affairs Health Care System. JAMA Internal Medicine, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu B, et al. , Underutilization of parathyroidectomy in elderly patients with primary hyperparathyroidism. The Journal of Clinical Endocrinology & Metabolism, 2010. 95(9): p. 4324–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wentworth K and Shoback D, Applying the Guidelines for Primary Hyperparathyroidism: The Path Not Taken. JAMA Internal Medicine, 2019. 179(9): p. 1227–1229. [DOI] [PubMed] [Google Scholar]
- 13.Quan H, et al. , Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care, 2005: p. 1130–1139. [DOI] [PubMed] [Google Scholar]
- 14.Peacock M, et al. , Cinacalcet Treatment of Primary Hyperparathyroidism: Biochemical and Bone Densitometric Outcomes in a Five-Year Study. The Journal of Clinical Endocrinology & Metabolism, 2009. 94(12): p. 4860–4867. [DOI] [PubMed] [Google Scholar]
- 15.Franks P, Clancy CM, and Gold MR, Health insurance and mortality: evidence from a national cohort. Jama, 1993. 270(6): p. 737–741. [PubMed] [Google Scholar]
- 16.Hadley J, Sicker and poorer—The consequences of being uninsured: A review of the research on the relationship between health insurance, medical care use, health, work, and income. Medical Care Research and Review, 2003. 60(2_suppl): p. 3S–75S. [DOI] [PubMed] [Google Scholar]
- 17.Lemaire A, et al. , The impact of race and insurance type on the outcome of endovascular abdominal aortic aneurysm (AAA) repair. Journal of vascular surgery, 2008. 47(6): p. 1172–1180. [DOI] [PubMed] [Google Scholar]
- 18.Boxer LK, et al. , Payer status is related to differences in access and outcomes of abdominal aortic aneurysm repair in the United States. Surgery, 2003. 134(2): p. 142–145. [DOI] [PubMed] [Google Scholar]
- 19.Seib CD, et al. , Patient Frailty Should Be Used to Individualize Treatment Decisions in Primary Hyperparathyroidism. World Journal of Surgery, 2018. 42(10): p. 3215–3222. [DOI] [PubMed] [Google Scholar]
- 20.Seib CD, et al. , Association of Patient Frailty With Increased Morbidity After Common Ambulatory General Surgery Operations. JAMA Surgery, 2018. 153(2): p. 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouvaraki MA, et al. , Indications for operative intervention in patients with asymptomatic primary hyperparathyroidism: practice patterns of endocrine surgery. Surgery, 2006. 139(4): p. 527–534. [DOI] [PubMed] [Google Scholar]
- 22.Torres MM, et al. , Results from a national survey on the management of primary hyperparathyroidism. J Endocrinol Invest, 2012. 35: p. 957–963. [DOI] [PubMed] [Google Scholar]
- 23.Sosa JA, et al. , Thresholds for surgery and surgical outcomes for patients with primary hyperparathyroidism: a national survey of endocrine surgeons. The Journal of Clinical Endocrinology & Metabolism, 1998. 83(8): p. 2658–2665. [DOI] [PubMed] [Google Scholar]
- 24.Clerici T, et al. , National survey on the management of primary hyperparathyroidism by Swiss endocrinologists. Langenbeck’s Archives of Surgery, 2007. 392(5): p. 611–615. [DOI] [PubMed] [Google Scholar]


