Abstract
Significant research and preclinical investment in cancer nanomedicine has produced several products, which have improved cancer care. Nevertheless, there exists a perception that cancer nanomedicine ‘has not lived up to its promise’ because the number of approved products and their clinical performance are modest. Many of these analyses do not consider the long clinical history and many clinical products developed from iron oxide nanoparticles. Iron oxide nanoparticles have enjoyed clinical use for about nine decades demonstrating safety, and considerable clinical utility and versatility. FDA-approved applications of iron oxide nanoparticles include cancer diagnosis, cancer hyperthermia therapy, and iron deficiency anemia. For cancer nanomedicine, this wealth of clinical experience is invaluable to provide key lessons and highlight pitfalls in the pursuit of nanotechnology-based cancer therapeutics. We review the clinical experience with systemic liposomal drug delivery and parenteral therapy of iron deficiency anemia (IDA) with iron oxide nanoparticles. We note that the clinical success of injectable iron exploits the inherent interaction between nanoparticles and the (innate) immune system, which designers of liposomal drug delivery seek to avoid. Magnetic fluid hyperthermia, a cancer therapy that harnesses magnetic hysteresis heating is approved for treating humans only with iron oxide nanoparticles. Despite its successful demonstration to enhance overall survival in clinical trials, this nanotechnology-based thermal medicine struggles to establish a clinical presence. We review the physical and biological attributes of this approach, and suggest reasons for barriers to its acceptance. Finally, despite the extensive clinical experience with iron oxide nanoparticles new and exciting research points to surprising immune-modulating potential. Recent data demonstrate the interactions between immune cells and iron oxide nanoparticles can induce anti-tumor immune responses. These present new and exciting opportunities to explore additional applications with this venerable technology. Clinical applications of iron oxide nanoparticles present poignant case studies of the opportunities, complexities, and challenges in cancer nanomedicine. They also illustrate the need for revised paradigms and multidisciplinary approaches to develop and translate nanomedicines into clinical cancer care.
Keywords: Nanomedicine, magnetic nanoparticle hyperthermia, iron oxide nanoparticles, cancer, immune therapy, iron deficiency anemia
1. Introduction
There’s plenty of room at the bottom.
Richard Feynman
In his address to the American Physical Society in 1959, Richard Feynman articulated the conceptual framework of nanotechnology [1]. Nanotechnology, defined by the National Nanotechnology Initiative (NNI), ‘…is the understanding and control of matter at the nanoscale, at dimensions between approximately 1 and 100 nanometers, where unique phenomena enable novel applications…’ [2]. At dimensions substantially greater than 100 nm, classical physics adequately describes most material properties; whereas quantum mechanical properties dominate at dimensions smaller than 1 nm. The nanoscale range is thus unique because neither classical physics nor quantum mechanics fully describes material behavior [1-3]. Nanomaterials are characterized by a high surface area-to-volume ratio with properties that are significantly influenced by both classical and quantum effects. Consequently, these materials have unique chemical, physical, and biological properties unlike their bulk (large dimensions) or atomic/molecular counterparts [1-3].
Nanotechnology has yielded various nano-structured materials to enhance performance of other technologies, e.g. computer memory, aerospace, food, fabrics and textiles, etc. Unique ‘nano-only’ technologies, such as ‘nanobots’ or ‘nanomachines’, while being explored, are not yet significant in consumer or medical products. Nevertheless, the global economic impact of nanotechnology is substantial, with revenues exceeding >US$4 trillion (million million) per year [4,5]. A smaller but still quite sizeable proportion of nanotechnology (>US$1 trillion projected for 2020) focuses on medical applications [4,5]. Nanomedicine is a growing research and manufacturing sector that is expanding the boundaries of knowledge in medicine, biology and materials science.
Given the significant investment made by the National Cancer Institute’s (NCI, USA) Cancer Nanotechnology program, some consider that relatively few cancer nanomedicine products have appeared in the clinic [6,7]. For those that have, expected successes have not materialized leading to an evaluation of discrepancies between preclinical predictions and clinical performance [7]. One impediment to progress has been the prevalence of paradigms for nanoparticle delivery based on oversimplified models of physiology and cancer biology, which emphasize passive processes for nanoparticle escape from blood into the tumor microenvironment. Another has been a reliance on data collected from immune deficient mouse models bearing cross-species tissue grafts that demonstrate significant efficacy but misrepresent the complex immuno-biology of spontaneous disease, or of nanoparticle interactions with host and tumor. In reality, nanoparticles interact with host biology and immune function in complex ways that affect both the performance of nanoparticle-based pharmaceuticals and the diseased host [8-12]. Expectations raised by early preclinical successes, which have proven to be unreliable predictors of clinical performance for cancer nanomedicine, remain unmet when measured against impact on patient survival [7]. Disconnect between expectations founded on preclinical data and realized clinical performance highlights critical gaps in knowledge.
For cancer therapies, clinical experience is the measure of success and serves to validate scientific paradigms used in preclinical product development. To date, approved cancer nanomedicines are liposomal drug delivery, and magnetic iron oxide nanoparticle-based magnetic fluid hyperthermia. Thus, clinical experience for therapeutic cancer nanomedicine may appear limited only to this subset of the preclinical cancer nanomedicine space, suggesting misalignment between paradigms and end-use. There is; however, a wealth of relevant information detailing clinical success with iron oxide nanoparticles for treating iron deficiency anemia, a condition that often presents with cancer [13,14]. Since the early 20th century, essentially predating the nanomedicine revolution, iron oxide nanoparticles have been in continuous medical use undergoing iterative improvements and refinement [13,14]. While iron oxide nanoparticles have demonstrated potential in preclinical settings for photothermal and photodynamic therapies, as well as magnetic nanoparticle hyperthermia, the only approved thermal nanomedicine is magnetic nanoparticle hyperthermia with magnetic iron oxide nanoparticles [3,6,15-17].
In this review, we evaluate available literature on nanotechnologies approved for hyperthermia, or approved nanotechnologies having relevance to cancer thermal medicine with iron oxide nanoparticles. Reviewed are the general nanomedicine paradigms, nanoparticle-immune interactions and magnetic hyperthermia concepts with a focus on iron oxide nanoparticles, the only approved thermal nanomedicine. For context and breadth, we contrast clinical experiences of the first liposomal drugs with iron oxide nanoparticle-based therapies for iron deficiency anemia. Both are systemic therapies administered intravenously; however, important differences emerge in clinical applications arising from designs based on current paradigms highlighting discrepancies between preclinical expectations and clinical end-use. Iron oxide nanoparticle formulations developed to treat anemia, by design exploit the very same immune-nanoparticle interactions that drug-delivery formulations attempt to avoid. Recent preclinical and clinical evidence shows (iron oxide) nanoparticle-immune interactions hitherto considered impediments for drug delivery, have potential to activate anti-tumor immune processes [10,18-20]. Other evidence indicates that, in the appropriate context, tissue heating with magnetic nanoparticles may further enhance immune function to benefit cancer treatment [21]. We also summarize the available literature on general hyperthermia to provide relevant background for the more specific discussion on magnetic fluid hyperthermia.
We acknowledge this review excludes numerous and intriguing concepts of magnetic nanoparticle hyperthermia or applications with iron oxide nanoparticles that have demonstrated promise in preclinical settings. Nevertheless, the history of cancer product development teaches it is difficult to predict from preclinical data, which product concepts will ultimately affect patient care and quality of life. Thus, it is important to evaluate critically the preclinical history with the clinical record of accomplishments to refine paradigms and identify new directions for cancer nanomedicine.
2. Nanomedicine
The performance of medical products depends upon biological effects deriving from their physical and chemical (physicochemical) attributes, making regulatory approval a requirement for commercial distribution [22]. A wide range of nanometer-sized tools incorporating diverse materials, and having varied shapes and sizes is available or under investigation for disease prevention, diagnosis, and treatment [4-6,23,24]. Depending on definition, one may count >50 nanotechnology-based devices or drug formulations approved by the US Food and Drug Administration (FDA) to diagnose and treat diseases [23,24].
2.1. What is a nanomedicine?
For nanotechnology-based medical products, the FDA provides guidance to industry that emphasizes the engineered aspect of the material or product dimensions (1 to 100 nm) and/or dimension-dependent properties; and, that dimensions can extend to 1 μm [22]. Nanomedicines are medical products developed from deliberate manipulation of both physical and chemical attributes to produce a dimension-dependent desired biological effect, where the dimension <1μm. By these criteria, we exclude (monoclonal) antibody- or other protein-drug conjugates as nanomedicines, which are ‘biologies’ [25]. Their nanometer size range is a natural feature and does not involve the ‘…deliberate and purposeful manipulation and control of dimensions to produce specific properties…’ [22,25]. We also consider that polymer-drug/peptide conjugates do not inherently involve the application of nanotechnology [22,26]. Thus, by these definitions the number of FDA approved nanomedicines is a more modest ~12 [6,22-27]. Certainly, nanomedicines may incorporate other agents making for more complex therapeutics, or combination products [22,25,26].
2.2. Nanomedicine delivery strategies: Do they work?
Except for iron oxide nanoparticles (Section 3), all approved anti-cancer nanomedicines are intravenous (i.v.) drugs (nanocarriers) designed to encapsulate a small molecule chemical agent, usually an established chemotherapeutic, as a ‘payload’ within a hollow ‘nano-shell’ for release in the tumor microenvironment or within cancer cells [6,7,23,24,27-31]. A brief review provides insights applicable to thermal nanomedicines. For drug delivery, nanocarriers are designed to accumulate in the tumor by either passive or active targeting via enhanced permeability and retention (EPR) following i.v. administration [6-9,27-33].
In its simplest form, the EPR paradigm asserts malignant tumors present with aberrant vascularity having fenestrations well-suited for passive extravasation (diffusion) of bloodborne nanoscale objects having size within a narrow range (enhanced permeability) which are then preferentially retained within the tumor by the aberrant vascularity and poor lymphatic draining (and retention) [34,35]. Passive targeting relies on tuning nanoparticle physicochemical properties to exploit biophysical features of tumors to optimize their extravasation from blood and retention in the tumor microenvironment [8,32]. Implicit in this strategy is the assumption that preclinical models and humans share similar tumor physical properties relevant to nanomedicine. In other words, the physicochemical properties of all approved cancer nanomedicines relying on passive targeting, were designed, developed and optimized in animal (usually mouse) models, before testing in humans.
Active targeting typically designates a strategy that aims to increase retention of the nanocarriers within the tumor microenvironment by chemical modification of the nanocarrier surface to have high affinity to some molecular target within the tumor [9,32,36-40]. Ligands that bind to proteins uniquely expressed or overexpressed on membrane(s) of cancer cells (or within the tumor microenvironment) are chemically bound to the surface of the nanoparticle. A ligand on the nanocarrier may be small molecule, protein or peptide, monoclonal antibody, or other molecule demonstrated to bind selectively as single agents to cancer cell membranes [38-40]. Once the nanoparticle-ligand enters the tumor microenvironment (assumed to occur via EPR), cell specific binding of the ligand will further enhance retention on the cell membrane or stimulate cell internalization and intracellular retention. Despite the misleading terminology, active targeting does not attempt to ‘target’ the nanoparticles to the tumor, per se, rather the strategy attempts to facilitate a more enhanced retention within the tumor microenvironment.
An issue often encountered with active targeting approaches is their unexpected complexity. Inevitably, the ligand possesses its own biological activity, pharmacokinetic (PK) properties, and biodistribution (BD). Its inclusion on the nanoparticle surface alters the physicochemical parameters, and biological performance of the nanocarrier-ligand construct [40]. Thus, as an active pharmaceutical ingredient (API) the nanocarrier-ligand conjugate becomes significantly more complex than either nanocarrier or ligand. Developing such complex combination products is significantly more challenging because characterization, achieving desired biological performance and activity, predicting or controlling cost, reliable manufacturing, demonstrating safety, and regulatory approval are rarely a linear combination of the components [22,25,26].
Two clinical attempts to deliver chemotherapeutics, docetaxel and doxorubicin, via active targeting, BIND-014 and MM-302, have not progressed beyond initial clinical trials [27,41,42]. This is due largely to failure of the product candidates to meet their clinical trial endpoints. There is no documented evidence of human clinical trials with active targeting for nanoparticle hyperthermia, or thermal nanomedicine. Indeed, documentation of ‘successful’ preclinical tests of the latter are relatively rare (Section 4) [39,43-45].
All approved cancer drug-delivery nanomedicines rely on passive targeting [23,24]. This fact is often used to validate the EPR paradigm and passive targeting; however, a close analysis of clinical performance reveals interesting nuances and has raised the question if the underlying assumptions, i.e. EPR, are flawed [7,10,18]. Within the EPR paradigm, the target PK design parameter considered important for both active and passive targeting strategies is long residence/circulation time of the nanocarriers in blood plasma, controlled through the physicochemical properties [6-9,23,24,32,38,45,46]. The prevailing assumption is that longer plasma half-life correlates with increased intratumor concentration of drug and improved disease control. Hence, preclinical optimization of cancer nanomedicines has focused on finetuning size and physicochemical properties to increase plasma circulation time [45,46]; however, evidence suggests the relationships among circulation time, nanoparticle retention in tumors, and patient benefit are considerably more complex [7,40].
2.3. Passive targeting: The Doxil® story
The first FDA approved cancer nanomedicine, Doxil®, is a liposomal formulation of doxorubicin approved in 1995 [23,47]. Liposomes are lipid-based nanocarriers comprising an aqueous core surrounded by phospholipid bilayer shell [6,24,47]. Doxil® was designed and developed using the following criteria [47]:
Nano-scale dimensions to enable extravasation from tumor vasculature into tumor tissue via EPR;
When in tumors, the drug concentration within the liposomes should be sufficient to achieve the desired efficacy;
The PK and BD profile of the doxorubicin (drug payload) should be controlled by the liposomal PK and BD; namely, the liposomal drug combination ‘should demonstrate a highly prolonged plasma circulation time’ to enable tumor accumulation; and,
Doxorubicin should become available to the tumor cells either by release from the liposomal carrier in the tumor microenvironment, or by ingestion/internalization of the liposomes by tumor cells.
The design strategy for Doxil® explicitly incorporated the EPR paradigm, thus directly constraining liposome size, drug loading, and imposing a requirement of prolonged circulation time. Through significant efforts, two novel technologies: a) drug loading into the nano-liposomes; and, 2) prolonging plasma circulation time (i.e. Stealth®), were developed by four independent teams working together to meet the above design criteria [47]. Considerations of drug loading and drug-release were necessary to ensure that doxorubicin PK and BD are determined by the liposome; and, that drug release occurs predominantly in the target. A consequence of the very small, i.e. nano-volume constraint is that a high dose of liposome-drug is needed to achieve therapeutic efficacy (~10 to 50 mg/m2 i.v. [47-54]).
Considerable effort, before and after the development of Doxil®, is devoted to understanding EPR and effects of nanoparticle physicochemical properties on circulation time [6-9,23,24,27-38,41,42,46-56]. Mechanistic hypotheses, correlating liposomal properties with increased circulation time led to inclusion of steric stabilization with addition of polyethylene glycol (PEG) to the liposome surface of Doxil® antecedents, with considerable success [47]. PEG-coated liposomes remain in circulation longer than their non-PEG coated counterparts [23,24,47,53-57]. Initial reports from early animal and (pilot) human data seemed to validate the design and optimization strategy by demonstrating increased tumor retention of doxorubicin with the increased circulation time [47,53-59]. Subsequent to its approval, however, preclinical reports and clinical data revealed a different picture.
Hong, et al. demonstrated in tumor-bearing BALB/c mice that a PEG-liposome showed a nearly two-fold increase of the plasma area under the concentration-time curve when compared with the bare liposome, but intratumor doxorubicin concentrations were more than two-fold higher when delivered with bare (non-PEGylated) liposomes [60]. Liposomal delivery increased intratumor doxorubicin concentrations when compared to free drug however the longer circulation time provided by PEG-coating reduced drug in the tumor. Recent results suggest that efficiency of EPR-driven drug delivery with liposomes depends on mouse strain, and that the PEG-layer may interact with tumor microenvironment to reduce retention. Song et al. report a 13-fold variation of PEG-liposomal doxorubicin clearance among 23 inbred strains of mouse showing considerable variation of EPR effect among mouse and tumor models [61].
Clinical experience with Doxil® and other liposome-drug products provides additional and extensive real-world economic and clinical, and clinical trials data [4-7,23,24,27-31,37,62]. Generally, liposomal drug formulations are less toxic than their conventional counterparts, often with more favorable pharmacological performance. The longer circulating PEG-liposomal drug (PLD) formulations do not generally increase drug in tumors or improve efficacy in patients when measured by progression free survival (PFS) or overall survival (OS) [7,63,64]. A recent meta-analysis of eight clinical trials comparing benefits of liposomal with conventional formulations of several drugs (anthracycline, cisplatin, paclitaxel, irinotecan) revealed no difference of efficacy, by OS in patients between liposomal and conventional formulations [63]. In contrast, when the authors conducted a meta-analysis of 11 preclinical studies comparing efficacy (OS) between PLD and conventional doxorubicin, they discovered a significantly increased survival in mice treated with PEG-liposomal doxorubicin over conventional doxorubicin [63]. The longer circulation time of PEG-liposomal doxorubicin also revealed unanticipated skin toxicity (hand-foot syndrome) in humans not observed with conventional doxorubicin or in mice [47,64].
In short, PLD and other liposome-drug formulations have provided modest benefit to patients, primarily by increasing drug tolerability, thereby improving patient compliance and extending treatment duration. Doxil® reduces doxorubicin-associated cardiomyopathy by altering its BD, which enables patients to receive higher doses of drug for longer duration. Longer circulation times do not generally correspond to more drug in tumor or to improved efficacy in humans. Rather, prolonged circulation times alter BD and toxicity, which may lead to improved patient compliance enabling longer duration of treatment; but they also introduce new toxicities. Preclinical predictions of efficacy measured by PFS and OS have, however not been realized. Reasons for these discrepancies are the topic of ongoing debate questioning the significance and variability of EPR; and, more fundamentally, if nanoparticle retention in tumors results from active biological processes in the tumor microenvironment [10,18,19].
3. Iron oxide nanoparticles: A wealth of clinical experience
Although often not considered part of the nanotechnology revolution, parenteral iron (i.e. iron oxide nanoparticles) therapy for iron deficiency anemia (IDA) dates to the early 20th Century (ca. 1930) [13,65]. The earliest Fe formulations were associated with toxicities resulting from rapid release of bioactive Fe [13]. Presently, all approved i.v. iron formulations are iron oxide-carbohydrate complexes or colloids based on small spheroidal iron oxide-carbohydrate nanoparticles (i.e. nanoparticles). The carbohydrate shell stabilizes the Fe core to slow the release of Fe and maintains the iron oxide as a stable colloid in blood or biological media [13,65,66]. All i.v. Fe formulations share this basic structure but differ in size of nanoparticle core and type and density of carbohydrate coating. There currently exist about five i.v. iron formulations available in the USA [67]. Newer, ‘3rd generation’ iron comprise polysaccharides, such as carboxymaltose, that complex tightly with the iron oxide nanoparticles [64,65].
The rate of release of the bioactive Fe is inversely related to the strength of the Fecarbohydrate complex with stronger complexes providing slower release rates [60-62,68,69]. The slower release formulations have a lower potential to saturate transferrin with subsequent free Fe toxicity, as compared with weaker complexes, characteristic of many earlier formulations [13,66,69].
The mode of action is through uptake by the reticuloendothelial system (RES) (aka mononuclear phagocyte system, or MPS) and degradation of the carbohydrate shell by macrophages. Macrophages either store the Fe as ferritin or transport Fe out of the cell into circulation via ferroportin-1 (FPN1), the only known iron export protein [13,66,69-71]. Macrophages, such as red pulp macrophages in spleen and Kupffer cells in the liver, known to engulf the majority of systemically injected nanoparticles, are among the principle cells responsible for Fe homeostasis [13,65,66,68-75]. Thus, the clinical utility and success of parenteral iron oxide nanoparticle formulations exploits the very clearance mechanism(s) cancer nanomedicines attempt to avoid. Perhaps a deeper understanding of the biology of cancer-immune and nanoparticle-immune interactions will lead to more effective cancer nanomedicines.
Although the utility of parenteral Fe formulations in treating IDA had been reported, it was not until 1980 that the first prospective study of i.v. use of Fe appeared in the USA [13,76]. While all 471 patients with IDA in the trial responded with no deaths, three were considered to have had ‘anaphylactoid’ reactions, leading to a historical and inaccurate perception of risk [13]. The authors of the study concluded that i.v. iron should be reserved for those conditions in which oral Fe could not be used [76]. Since then, several other formulations of i.v. iron have become available and despite perceptions to the contrary, all are comparably safe and effective to treat absolute or functional iron deficiency. This success has prompted recommendations to use i.v. iron to enhance response in cancer patients who often present with anemia, either from the disease or from treatment [13,14,65,68-70,77-82].
Parenteral Fe therapy, with iron oxide nanoparticles, has become an important adjunct (with erythropoietin stimulating agents) to achieve and maintain hemoglobin levels in patients with end-stage renal and other diseases, including cancer [13,14,65,68,72,77-81]. Indeed, retrospective analyses of clinical data present clues that systemic exposure to iron oxide nanoparticles in the context of cancer therapy can enhance response to treatment, and improve survival in patients with metastatic disease [14,81,82]. Particularly intriguing is the connection between successful anemia treatment with parenteral iron and improved OS in patients with metastatic disease [82]. While the study authors attribute the effects to treating the anemia, one might pose the question whether the iron oxide nanoparticles and their interactions with immune cells via macrophage uptake, may have induced anti-tumor immune activity as recently observed in preclinical models [10,19].
A growing body of preclinical and clinical evidence suggests the possibility that nanoparticles, specifically iron oxide nanoparticles, and their interactions with cells of the host immune system can stimulate immune recognition of tumors to enhance therapy [10,14,19,70,82,82]. The mechanism(s) of this anti-tumor immune stimulation are unknown and complex, however early indications suggest that phagocyte ingestion of iron oxide nanoparticles may stimulate ‘pro-inflammatory’ immune cell phenotypes, similar to infection by pathogens that reverse cancer-induced immune suppression [10,19]. In their studies, Korangath, et al. documented a transient decline in T cell populations immediately following systemic exposure to starch-coated iron oxide nanoparticles. Within 7 days after exposure, they noted T cell infiltration into tumors that was associated with tumor growth suppression [10]. Similar experiments in T cell deficient (athymic nude) mice failed to produce tumor growth suppression. Zanganeh, et al. [19] recently revealed a hidden intrinsic therapeutic effect of ferumoxytol, an FDA-approved iron oxide nanoparticle compound, on tumors. Tumor cells mixed and co-injected with ferumoxytol into mice exhibited a markedly delayed growth rate compared with tumor cells injected without ferumoxytol. Further, they demonstrated that systemic exposure of T cell deficient mice to ferumoxytol before intravenous (i.v.) injection of small-cell lung cancer (SCLC) cells prevented formation of liver metastases [19]. They concluded that the intrinsic therapeutic effect of ferumoxytol on cancer growth arose from macrophage polarization into pro-inflammatory M1 phenotypes [19]. In other words, they demonstrated that innate immune cells in the tumor microenvironment responding to iron oxide nanoparticles were responsible for anti-tumor immune effects in their models and T cells were not necessary. In contrast to the results obtained by Zanganeh, et al., results from mouse models and analyses of human clinical trials data support the intriguing possibility that systemic exposure to iron oxide nanoparticles also can induce anti-tumor (T cell-mediated) immune effects [10,14,81,82].
Links between immune function and its role in cancer biology and response to treatment have become an established area of cancer research and drug development [83,84]. Iron homeostasis is intimately linked with immune function in the context of disease and infection [85,86]; and, nanoparticle-immune interactions, including iron oxide nanoparticles, are being explored as immune therapies or vaccines for infectious diseases and cancer [87-93]. Immune cell interactions with iron oxide nanoparticles have been reported to induce apoptosis with increased oxidative stress [11,94]; however, activating innate immune cell stress pathways can induce transformation to pro-inflammatory, anti-tumor phenotypes. The recent reports of systemic and local anti-cancer immune activation by iron oxide nanoparticles may indicate a more complex immune-biological process initiated by the nanoparticles or the Fe [10,19]. Korangath et al. observed that the time-dependent immune response following nanoparticle exposure potentially resembles immune responses to acute (non-lethal) infection by pathogens that has been associated with anti-tumor immune stimulation [10,19,95,96].
Given that recent evidence demonstrates potential that systemic exposure to iron oxide nanoparticles can induce anti-cancer immune effects, it is worthwhile to explore further reports of nanoparticle-immune interactions, especially for iron oxide nanoparticles (Section 3.4)
3.1. Nanoparticle-immune interactions
It is widely held that (surface) physicochemical properties of nanoparticles determine interactions with plasma proteins that produce a corona, altering the surface of the nanoparticle. It is also widely acknowledged that clearance from blood circulation of nanocarriers larger than ~15 nm diameter occurs via the RES, or MPS which comprises monocytes and macrophages [8,9,11,27,29-37,45-47,53-63]. In most studies, macrophages residing in liver (i.e. Kupffer cells) and spleen are observed to harbor high concentrations of nanoparticles after systemic delivery, leading to the conclusion that this system reacts to the total molecular signature presented by the nanocarrier + corona, causing ingestion by phagocytes. Thus, the organs containing high numbers of phagocytic cells such as macrophages/monocytes are the primary blood clearance agents for nanoparticles. Within the EPR paradigm, avoiding rapid clearance by this mechanism led to the development of ‘stealth’ technologies, which successfully reduce phagocytosis of nanoparticles and thus increase circulation times but do not improve efficacy.
The clinical success of parenteral iron therapies, unlike the experience with liposomal (and other) drug delivery formulations, relies on the general physiologic interaction between nanoparticles and macrophages, and the role of macrophages in iron homeostasis. Recently, the potential value of exploiting nanoparticle-phagocyte interactions has been proposed as an opportunity to redirect suppressed immune function for therapeutic benefit [10,12,13,14,19,20]. As described in the previous section, various groups have hypothesized that, depending on physicochemical properties, nanoparticles can either naturally or by deliberate engineering have ‘pathogen-like’ features that enhance immune-adjuvant properties or mimic some of the immune-stimulating properties of infectious agents [10,12,19,20,87-93]. An additional aspect unique to iron oxide nanoparticles, is the presence of elemental iron, which seemingly induces specific anti-cancer effects [19,20].
Evolutionarily conserved immune surveillance recognizes and reacts to pathogens through pathogen associated molecular patterns (PAMPs) receptors to protect the host. Initiation of immune responses is generally through phagocytes that recognize pathogens by their PAMPs and initiate immune response to pathogens, which ultimately determines the fate of both pathogen and host. A successful immune challenge to pathogen invasion resolves by clearing the agent effectively from the body and generally requires complex interactions between the adaptive immune cells (lymphocytes) and the innate immune cells (mostly phagocytes). The mammalian immune system has evolved such complex interrelationships to distinguish potential threats from ‘self and benign objects that enter the host, and thus may be predisposed to recognize nanoparticles as potential threats. Individual nanoparticles are within the size range of many viruses.
Strong interactions of nanoparticles with host immune systems were recognized early and cancer nanomedicine development has tried to minimize these interactions in order to enhance drug delivery to solid tumors [7,11,18,23,24,27-38,75,94]. Nanoparticles contaminated with endotoxins can induce immunotoxicity; thus, how much of immune reaction to nanoparticles is the result of bone vide nanoparticle effects is difficult to ascertain because appropriate tests are rarely conducted for preclinical studies [97]. FDA guidelines provide recommendations to test for endotoxins (LPS) in nanoparticle formulations destined for clinical use [98]. Iron oxide nanoparticles are the only approved metallic nanoparticles for clinical use, and often come with a warning for hypersensitivity, demonstrating that endotoxin free nanoparticles can interact with immune cells [99]. Modulating these interactions in the context of disease and the altered immune microenvironment is an interesting area of research. A complete understanding of nanoparticle interactions with immune cells remains a critical gap in knowledge impeding progress to develop effective cancer nanomedicines. Obtaining this complete understanding is a significant challenge since minor differences between nanoparticles have significant impact on immune interactions.
3.2. Cells of the immune system – the basics
The immune system comprises a collection of diverse and highly regulated cells that respond to complex stimulatory and inhibitory signals to protect the host while limiting associated immune damage to the host (autoimmunity). Innate and adaptive immune cells coordinate the function of immune surveillance, which protects the host from foreign invasion including nanoparticles. Lymphocytes are produced in the bone marrow. They mature and differentiate in secondary lymphoid organs such as the thymus, lymph nodes and spleen before entering circulation in the blood as effector cells. All lineages of blood cells come from a pluripotent hematopoietic stem cell. Neutrophils are the most common circulating immune cells and are very important, particularly for antibacterial responses. While all the leukocytes have specific roles in different types of immune responses, the antigen-presenting cells, monocytes, macrophages and dendritic cells are often considered the most important participants of the innate immune system because they specifically control activation of T cells through the process of antigen presentation. Lymphocytes form the adaptive immune response and produce genetically unique receptors to recognize specific targets unique to a specific pathogen. Eosinophils and basophils are generally responsible for allergy-like reactions. Although the types of cells are similar among many mammals, their number and specific function, often differ in important ways [100].
3.3. Factors determining nanoparticle clearance
By the virtue of their size, nanoparticles can present features recognized by host immune cells [10]. The general clearance mechanism for removal of nanoparticles from blood circulation is phagocytosis mediated by the cells of RES [101]. Physicochemical properties that determine clearance have been extensively studied (Sections 2.2 to 2.4) [8,9,11,32,33,38,45,102-104]. There is growing evidence that nanoparticle clearance from blood and factors affecting this are not easily generalized to nanoparticle properties, and that biological factors specific to the host account for much of the biological fate of nanoparticles. Jones et al. have demonstrated that nanoparticle clearance depends on T helper 1 and T helper 2 (Th1/Th2) type immune responses in normal C57BL/6 and BALB/c mouse models, respectively [105]. A host exhibiting Th1 type immunity will require more time for clearance of nanoparticle than will one that exhibits Th2 type immunity. Clearance mechanisms, including different organ distribution (i.e. lung instead of liver) for nanoparticles occur across species, e.g. primates, humans, or mice [100]. In other words, species- and individual-specific details of immune function/capacity determine host responses to nanoparticles, thus making predictions of in vivo nanoparticle fate in humans based on preclinical data difficult [7].
3.4. Immune reaction to iron oxide nanoparticles
An immune reaction to nanoparticles can depend considerably on their size, route of administration, dose, materials, coating, etc. The reactions can be hypersensitivity, inflammation, immunosuppression, immunostimulation, complement activation, or a combination [102-117]. Iron oxide nanoparticles elicit host immune responses that release cytokines and chemokines in the blood. In mouse models, magnetite (Fe3O4) nanoparticles having diameter 5-8 nm induced inflammatory reactions post intratracheal instillation measured by a dose-dependent increase of pro-inflammatory cytokines IL-1, TNF-α, and IL-6 in bronchoalveolar lavage fluid (BAL), and in blood [118]. Carboxydextran coated iron oxide nanoparticles (Resovist®) attenuated OVA-specific IgG1 and IgG2a and reduced IFN-γ and IL-4 production by splenocytes in OVA-sensitized BALB/C mice [119]. On the other hand, complement activation occurred with dextran-coated iron oxide nanoparticles [103]. Due to the inherent MRI contrast property of iron oxide many studies are conducted by imaging using these nanoparticles. It is particularly noteworthy that iron oxide nanoparticles can be useful to assess inflammatory disease progression, often with MRI [120]. In other words, depending on model and disease context, exposure to iron oxide nanoparticles can be immune-stimulating or immune-suppressing.
Unique physical and chemical properties arise from the high surface-to-volume ratio of nanometer-scale materials [1,2]. It is thus likely that this aspect of nanoparticles is also responsible for much of the nanoparticle-immune cell interactions, making the nanoparticle coating particularly important. Depending on coating, nanoparticles can present different features to immune cells, which generates different cellular responses, particularly when modified to include a protein or ligand for active targeting [12]. Korangath et al. demonstrate that a humanized monoclonal antibody on the surface of iron oxide nanoparticles led to significant retention in the tumor microenvironment via capture by resident (tumor-associated) innate immune cells [10]. The uptake of nanoparticles by host immune cells altered the tumor microenvironment leading to growth inhibition through T cell activation. In a series of elegant studies, Lo et al. demonstrated that anti-CD3 antibody-coated nanoparticles enhance T cell receptor crosslinking on effector T cells, which is an activation signal and has potential to improve efficacy of vaccines and immunotherapy [121]. Subsequently, Kosmides et al. demonstrated that antibodies conjugated to the surface of nanoparticles can activate CD8+ T cells [122]. Here they used an antibody against immunosuppressive PD-L1 antibody and a co-stimulatory agonist 4-1BB antibody conjugated to iron oxide dextran-coated nanoparticles and injected directly into tumors.
3.5. Other clinical and occupational health effects
Many occupational health studies demonstrate occupational (usually by inhalation) exposure to nanoparticles induces allergic responses in workers who routinely handle the nanoparticles without adequate protection [123,124]. Despite substantial contradictions on reported immunotoxicity of nanoparticles, in general, it is clear that nanoparticles have complex and profound effects on the immune system. These effects are specific to species and individuals, as well as to the nanoparticle physicochemical properties. Generalizations of specific immune responses to general nanoparticle features such as size or charge are unlikely to aid robust development of cancer nanomedicines. Further, the biological context of the host (e.g. diseased or healthy; young or old; male or female; etc.) present additional crucial factors that determine the nature of the interaction between nanoparticles and immune cells. Thus, depending on numerous factors, nanoparticle exposure can induce or suppress inflammatory responses, and either is potentially therapeutic or deleterious, depending on context [10,12,19,20].
Diseases alter the host immune system. For many chronic or inflammatory diseases, immune alterations can include IDA or functional anemia as a comorbidity that requires intervention. Iron oxide nanoparticles have enjoyed nearly one century of clinical use to exploit the nanoparticle-immune nexus as a way to resolve this condition; however, nanoparticles harbor potential to affect immune function significantly beyond the intended treatment. Clinical experience with parenteral iron oxide and other nanoparticles demonstrates that evaluations of each nanoparticle formulation must occur in the context of a wide range of biological scenarios. One size most certainly does not fit all.
4. Hyperthermia
Hyperthermia is a cancer therapy having the objective to raise the local tumor temperature to either kill cancer cells or sensitize them to other treatments [125-127]. Tissue heating as a therapeutic modality was practiced in Egypt, India, and Greece [128,129]. Cytotoxic effects from heat exposure depend on dose, defined as time-at-temperature, maintained at between 41 – 45°C for a period of time [125-127,130,131]. For temperatures exceeding 45°C, thermal ablation ensues [127,127,131]. Treatments in the temperature range of 39 to 41 °C are mild hyperthermia [127,130,131].
The location and tissue volume (e.g. whole body or portion) of the target region and mode of energy application define the treatment [126,127,132]. For deep tumors, the heat source is within the tumor (interstitial hyperthermia), which is our current focus with brief mention of recent clinical results with magnetic nanoparticle ablation.
4.1. Fever, immune response, and hyperthermia
Carl Busch, a German surgeon, published the first scientific recognition of the relationship between temperature and cancer response in 1866 [127,133]. He reported that a patient's sarcoma underwent complete remission after infection with erysipelas [126,127,134]. Presumably, the disease induced an unspecified immune response leading to fever. Thus, Busch was the first to report that an elevated temperature can selectively kill tumor cells without adversely affecting surrounding cells, although in hindsight we now recognize that the hyperthermia did not directly mediate the killing of tumor cells [133]. This inspired Friedrich Fehleisen to infect cancer patients with bacteria in an attempt to treat tumors by causing erysipelas [127]. Intrigued by these reports, in the 1890s William Coley began treating inoperable cancer patients with bacterial extracts that later became known as Coley's toxins [126]. He recognized the treatment potential of the immune response associated with the induced fever.
Often overlooked in these and many early accounts focusing on elevated temperatures is the fact that the fever resulted from infection by a pathogen, which produced an immune response. It was the immune response and not the fever that affected the tumor; however, the history of hyperthermia links to these early cancer immune-therapies. The interconnections between immune response and its effects on cancer have become a recent focus in cancer medicine. From a historical perspective, Coley was a pioneer in cancer immunotherapy, who highlighted the ability to treat cancer with the immune system as well as the important relationships among immune function, cancer, and fever [126].
The first scientific attempt to induce hyperthermia directly, i.e. interventional and deliberate heating of a region of tissue, was by Frans Westermark in 1898 [124,130,131]. He treated inoperable carcinomas of the uterus by circulating water with a temperature between 42 and 44°C in a special metal coil [124,130]. In 1913, William Mayo observed that local hyperthermia treatment of cervical tumors before a vaginal hysterectomy increased the cure rate, if sufficient time transpired between the two treatments [123]. This hinted that perhaps activation of an immune response and/or reduction of transient thermal tolerance are required to enhance efficacy [123]. Fever-level hyperthermia is part of an immunologic cycle in which the temperature increase is generated by the immune response, but the increased temperature also stimulates the immune response. In effect, fever is a systemic immunological signaling mechanism between activated leukocytes and other cells.
Reports of clinical and fundamental biology studies in the 1950s revived hyperthermia [127,133]. In 1962, George Crile, Jr. reported that direct thermal damage at 42°C in mouse models correlated with an exponential increase of thermal damage at higher temperatures [133]. William Dewey et al. performed biology experiments with mammalian cells in 1971 demonstrating that hyperthermia can act as a radiation- and chemo-sensitizing agent [131,133,135]. The thermal dose concept and fundamental mechanisms of hyperthermia were developed in the 1970s and 1980s [131,133]. However, in the 1990s some clinical trials produced ambiguous results leading to dampened enthusiasm for hyperthermia in clinical practice [128]. More recently, favorable results obtained from carefully controlled clinical trials have led to a resurgence in hyperthermia research and clinical applications. Since then, additional details on the biological connections between hyperthermia and immunomodulation have been charted, with data from clinical trials supporting the rationale to combine hyperthermia with other therapies, taking care to administer appropriate thermal dose [128,135-138]. There is a continuous effort to achieve technological improvements with development of quality assurance standards to address the principle challenges of hyperthermia: precise energy delivery to (deep-seated) tumors with control to achieve a prescribed thermal dose [139].
4.2. Biological effects of hyperthermia
Hyperthermia causes local temperature elevation that can be non-lethal (39 to 42°C) or lethal (>42°C) [141]. Depending on the applied temperature and duration, various biological effects ensue. Heat is a pleiotropic damaging agent. Depending on dose (time-at-temperature), hyperthermia can inhibit or abrogate DNA damage repair, initiate apoptosis or other programmed cell death, or cause severe disruption of cell membranes leading to cell lysis [130,131,140,141]. Both the phenotype and genotype of cells determine sensitivity to thermal stresses. Even though clinical implementation of hyperthermia adds complexity, its use with radiation and other therapies often significantly improves response to treatment and overall survival [141]. The clinical benefits of hyperthermia stem from its ability to disrupt or denature DNA-damage repair proteins, reverse tumor hypoxia, increase metabolic rate and induce other physiologic changes; and, induce heat shock and immune responses. The individual effects depend on disease, mode of treatment, timing, and individual patient conditions. In many countries, including the USA, hyperthermia is indicated for use with radiation to treat recurrent, or refractory solid tumors, particularly in a re-irradiation setting [141,142].
4.3. Immunologic effects of hyperthermia
Tissue heating has unique immune-modulating properties. Temperature elevation between 1°C and 5°C above ambient body temperature is a universal response in vertebrates to infection that is both caused by and regulates the immune system [127,143], Thermoregulation is a major homeostatic system [127,143]. and accumulating evidence supports a critical role for the immune system to maintain tissue homeostasis [127,143].
There is a link between temperature and immune regulation maintained throughout evolution. In human cells, cytotoxic effects occur in vitro after heating to heat-shock temperatures between 42°C and 45°C [127,143]. Heating increases release of heat-shock proteins into the extracellular environment stimulating downstream immune activity. Heating also increases tumor cell-surface expression of major histocompatibility complex class I ligand [145]. Heating increases vascular perfusion and blood flow to the tumor through both thermoregulatory signals and changes in tumor metabolism, increasing expression of hypoxia-inducible factor-1 leading to increased production of reactive oxygen species and VEGF expression.
Fever-range hyperthermia modulates many aspects of the immune system. It increases trafficking and licensing of CD8+ T cells through heat-induced increases in E or P selectin, cytokine release, and intercellular adhesion molecule 1 on tumor blood vessels. With elevated temperature, there is also increased T cell receptor signaling and differentiation of naïve T cells to effector cells; upregulation of the receptor NKG2D on natural killer (NK) cells, increasing NK cell cytotoxic potential; and, increased functional activity of macrophages and dendritic cells [145-151]. Fever-range hyperthermia thus may be a broad-spectrum adjuvant that profoundly affects the tumor microenvironment with significant immune-modulating potential. Evidence that radiation therapy and hyperthermia independently modulate immunetumor interactions provides a compelling motivation to evaluate the immunologic consequences of combining these treatments [127,152-163].
5. Thermal nanomedicines
Most approved cancer nanomedicine products meeting the above criteria (Section 2.1) are liposomal drug delivery formulations, not indicated for hyperthermia [24]. Liposomes are colloidal nanoparticles comprising a hollow bilayer spherical shell made of synthetic or natural (phospho-)lipids [24,161]. The interior (lumen) of the liposomes is aqueous and encapsulates other molecules, usually chemotherapeutics [164].
One liposomal nanomedicine developed for hyperthermia (not yet approved) is ThermoDox®, a thermosensitive formulation that has undergone Phase III testing for advanced hepatocellular carcinoma with radiofrequency ablation (RFA) [165,166]. ThermoDox® releases its payload, doxorubicin, when heated by an external energy source [164]. The primary study endpoint of the most recently completed trial (OPTIMA) was to determine 5-year overall survival, but results are not yet published. An earlier multi-center Phase III trial (HEAT) included 701 patients [167]. ThermoDox® was administered i.v. as a 30-min infusion of 50 mg/m2 to patients in the treatment arm [164]. Authors of the HEAT study concluded that the combination of ThermoDox® with RFA was safe but that it did not increase PFS or OS [166]. Subsequently, a subgroup analysis determined that when RFA dwell time for a solitary lesion ≥45 min, both PFS and OS significantly increased prompting the more recent OPTIMA trial to include a minimum RFA dwell time of 45 min [164]. The clinical trials experience with ThermoDox® illustrates challenges with implementing thermal nanomedicine – formulating the nanocarriers to have suitable properties for drug delivery, choice of drug payload, and optimizing the combined performance of nanocarrier and release of its payload with device control [164].
Currently only one nanoparticle thermal therapy product, NanoTherm®, is approved for treatment of cancer [6,23,24]. It comprises magnetic iron oxide nanoparticles, which in various forms have enjoyed continuous clinical use to treat iron-deficient anemia for nearly one century (Section 3), and more recently as contrast agents for magnetic resonance imaging (MRI) [6,23,24,28].
5.1. Magnetic nanoparticle (fluid) hyperthermia with iron oxide nanoparticles
Gilchrist et al. first described the concept of heating with magnetic iron oxide particles, suspended in fluid and exposed to alternating magnetic fields in 1957 [167,168]. Magnetic fields interact with magnetic materials to generate heat, predominantly via magnetic hysteresis loss [169]. For therapy, the region containing the nanoparticles is exposed to an alternating magnetic field (AMF). Hysteresis heating forced by interactions of the magnetic moments of the nanoparticles with the AMF generates heat that transfers through the tissue by conduction and convection [169,170]. We refer the interested reader to Section 6 for a discussion of the physics of magnetic (iron oxide) nanoparticle heating.
The key feature for hyperthermia and magnetic resonance imaging of magnetic nanoparticles is their magnetic core, comprising one or multiple magnetic crystals (Section 6) [169]. The potential utility of other core materials, e.g. nickel or cobalt have been investigated, however clinical applications are likely to be limited to only iron oxide nanoparticles because of their demonstrated safety [3,169,170-172]. Hence also the focus on iron oxide nanoparticles in this review.
Iron oxide nanocrystals are hydrophobic, and their magnetic properties are sensitive to changes in surface making it necessary to coat them with a material that reduces toxicity (Section 3), preserves colloid stability in biological media, and preserves the integrity of the magnetic core (Section 6) [3,169,170,172,175,181]. Often, as with parenteral Fe agents, the biocompatible coating is a polysaccharide or carbohydrate introducing a hard-core, soft-shell structure.
The entire core-shell structure has a diameter typically between 10 and 120 nm [169,170,180,181]. The size of magnetic iron oxide nanoparticles is comparable to that of other nanomedicines; however, characterizing size and other properties, relating these to the magnetic and heating properties of such mixed core-shell structures is challenging. This is true for magnetic hyperthermia applications for which definitive characterization of size, magnetic properties, and heating is necessary because the therapeutic agent is heat generated when the nanoparticles are exposed to alternating magnetic fields [3,45,168,169]. Complexities associated with coupling among time-dependent magnetic responses [169,183,184], nanoparticle physical properties [183,184], their colloidal arrangements [185] and inter-particle interactions in fluids or tissues [169,170,183,185-190], and experimental conditions of measuring heating [191-196] ensure continued research effort because magnetic nanoparticle heating, driven primarily by magnetic hysteresis loss power, defies explanation with simple models (Section 6) [169,183-190,197-208]. The magnetic nature of magnetic iron oxide nanoparticles is thus simultaneously their singular advantage and presents their greatest challenge, often requiring technical demands that exceed resources of many researchers [168,169].
Despite these significant challenges, one thermal nanoparticle has already been approved for use in cancer therapy. NanoTherm® was approved in 2010 by the European Medicines Agency (EMA) for treatment of recurrent glioblastoma (GBM) with an AMF [6,23,24,28,171-175]. In February 2018, NanoTherm® received FDA approval for clinical testing in humans in the USA to treat prostate cancer [171,176-179]. NanoTherm® comprises a colloidal suspension of aminosilane-coated iron oxide nanoparticles delivered percutaneously into the tumor tissue [173-173]. Direct delivery avoids many issues associated with systemic nanomedicines; however, challenges remain to optimize therapy for individual patients [176]. For any nanoparticle construct, the intratumor distribution of nanoparticles is heterogeneous following direct delivery, with significant individual variations in total nanoparticle concentration and spatial distribution depending on individual tumor physical structure and injection characteristics [176,180].
5.1.1. Challenges for nanoparticle hyperthermia
The greatest challenge of hyperthermia is to obtain a precise energy delivery and control to the target, while avoiding non-specific heating of normal tissues. Hyperthermia treatments of deep-seated tumors therefore remain challenging. Magnetic iron oxide nanoparticles offer the potential to cause truly localized and precise thermal therapy. Furthermore, biological tissues do not attenuate magnetic fields. Summarized, magnetic nanoparticle hyperthermia offers the potential for precise dose control and true tissue specificity. However, new challenges arise.
The main challenges of magnetic nanoparticle hyperthermia concern the delivery and the energy deposition. Thermal nanomedicines are indeed combination products that include a drug-like injectable component that must be delivered to tumor (systemic or direct), which is then activated by an energy source to deposit localized heating in the treatment target (Figure 1). The heat can be used to activate release of drug (e.g. ThermoDox®), or it can be the active therapeutic agent itself (e.g. NanoTherm®). Nanoparticle delivery to tumor – whether systemic or local – involves numerous biological processes affected by the physicochemical properties of the nanoparticle, which can affect nanoparticle performance (Section 2).
Figure 1: Schematic illustration of the magnetic nanoparticle hyperthermia concept.
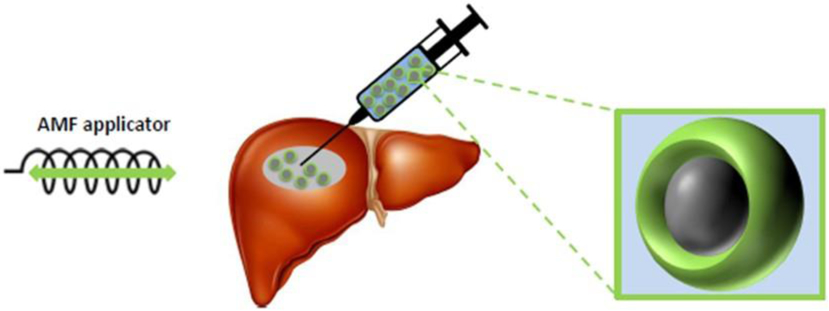
Magnetic nanoparticles comprising a magnetic core and a biocompatible coating suspended in liquid are directly injected into a liver tumor. An alternating magnetic field applicator generates an alternating magnetic field that interacts with the magnetic nanoparticles, generating local heat.
Systemic delivery is generally desirable because it is less invasive; however, systemic exposure carries risks to the patient, and the fate of the nanoparticle and its cargo depend on complex interactions with the patient’s biology (Section 2). Perhaps the most significant challenge with systemic delivery of magnetic iron oxide nanoparticles is achieving sufficient concentration in tumors to mediate hyperthermia in response to a clinically appropriate AMF. Direct approaches enable reliable delivery of nanomedicine to the tumor, but require more invasive procedure(s) with imaging support to inject tumors [173-177]. In addition, this mode of delivery does not directly address occult or widely metastatic disease. While dose of drug by direct delivery is controlled, distribution and disposition of the nanomedicine within the tumor, and escape from the tumor microenvironment are not [177,180].
Treatment with thermal therapy also requires directed energy deposition by a device, which introduces additional complexities for implementation, patient safety, manufacturing and cost, and regulatory approval [170-179]. The goal of magnetic nanoparticle hyperthermia is to achieve therapeutic temperatures in the tumor from heat generated by magnetic iron oxide nanoparticles embedded in the tumor. Power dissipated by magnetic iron oxide nanoparticles increases with increasing magnetic field amplitude (nonlinear) and frequency (linear) (Section 6). Therefore, one might consider increasing the AMF frequency and amplitude is useful to achieve therapeutic temperatures. According to Faraday's law of induction, interaction of AMFs with (diamagnetic) electrically conductive materials creates eddy currents, which deposit non-specific (Joule) heating into tissues [209-218]. This non-specific power deposition can initiate systemic thermoregulatory responses, creating complex thermal gradients throughout the body of a patient.
For magnetic hyperthermia, alternating magnetic frequencies (AMFs) range between ~100 to 300 kHz. Low-frequency AMFs (<10 MHz) are essentially not attenuated by tissues, but Joule heating from induced eddy currents presents safety constraints on coil design and operation. Depending on location of tumor in the patient and the specific loss power of nanoparticles, interaction of AMF with the volume of tissue exposed can potentially generate significant non-specific heating, even competing with the heat generated by the nanoparticles [219]. Such a scenario is clinically unacceptable, placing greater demands on nanoparticle development for enhanced specific loss power and efficient delivery, within clinical AMF design constraints [169,184,213].
Thermal nanomedicines, whether regulated as drug, device, or combination products are complex medical products that challenge clinical implementation. They may receive approval by demonstrating safety and efficacy comparable to, or slightly better than other treatments, however their complexity inhibits implementation by clinicians. Clinicians and their patients will likely choose treatments that are less complex, less expensive, and that have an established record unless convinced that the benefits far outweigh those offered by other options. This raises the bar for thermal nanomedicine products to demonstrate superior clinical benefits earlier in the development process. In other words, thermal nanomedicine must offer more for patient safety and disease management than competing products.
5.2. Nanoparticle delivery
5.2.1. The direct approach
Direct injection into the tumor is the most common delivery mode of magnetic iron oxide nanoparticles (magnetic fluid) for preclinical studies, and is indicated for NanoTherm® [169-180,220,221]. Direct injection requires knowledge of tumor location which must be accessible, presenting challenges for some deep tissues, or tumors proximal to sensitive organs or tissues [170,180,220-224]. Nanoparticle physicochemical properties, injection parameters and tumor physical features determine the distribution and disposition within the tumor [175,177,180,223-225]. Consequently, direct injection often produces unpredictable and irreproducible nanoparticle distributions that affect quality assurance and therapeutic outcomes [173-177]. Both the injection rate and volume of injected material affect the resulting distribution [223,225]. Current consensus that the best method to deliver the nanoparticle suspensions is via a slow, i.e. convection-enhanced delivery (CED), multi-point percutaneous injection into the tumor [223]. Nevertheless, even with best efforts significant nanoparticle heterogeneity within an individual tumor and among patients is a reality, challenging quality assurance of treatment because thermal dose is unpredictable, lurther inhibiting clinical adoption [174-177,180]. Recent research however suggests that a carefully planned AMF amplitude modulation offers the potential to overcome some of these limitations [180,226]. The approach exploits non-linear responses of hysteresis loss power and temperature-dependent tissue cooling with appropriate tissue temperature feedback as input to a controller algorithm. Some benefit of the approach by improved responses in mouse models was demonstrated [180], with further optimization in computational phantoms [226]. Further development is needed in large animal models and companion animal trials before clinical implementation in humans.
5.2.2. Intracellular magnetic iron oxide nanoparticle hyperthermia
In 1979 Gordon, et al. proposed a significant advantage of magnetic nanoparticle hyperthermia is the possibility to cause intracellular heating [227]. They hypothesized that cell membranes act as thermal insulators, supporting an intracellular temperature gradient and that intracellular hyperthermia would be therapeutically superior to extracellular hyperthermia. Using thermal arguments and analytical expressions of heat transfer in aqueous media, Rabin concluded that, in the thermal sense, intracellular hyperthermia is unachievable with heating efficiencies of ~<10,000 W/g material. Few iron oxide nanoparticles are able to achieve >1,000 W/g material (a ten-fold less than minimum required), thus presenting a physical barrier to achieving this objective. In 2013, Hedayati et al. published experimental validation of Rabin’s theoretical argument by comparing effects of heating in vitro using pelleted (HCT116, human colorectal cancer) cell clusters containing varying amounts of iron oxide nanoparticles [229]. From clonogenic survival assays and thermometry, they demonstrated that Rabin’s predictions for heat transfer through the cell was essentially indistinguishable from that in the media. Nevertheless, examples of differential biological responses with measured bulk temperatures from in vitro experimental systems persist, leading to the conclusion that biological processes or cell stress responses, sensitive to transient heat transfer (i.e. non-equilibrium thermal effects), may be evident [230,231]. There has been no in vivo preclinical or clinical demonstration that such intracellular hyperthermia is clinically viable, much less superior – because it would require internalization of nanoparticles by all cancer cells. To the contrary, all preclinical and clinical evidence points to beneficial effects of macroscopic heat transfer (the dominant mechanism in aqueous or liquid environments) as necessary to achieve clinically relevant thermal doses [171-179,220].
5.3. Immune-mediated enhanced cancer therapy with magnetic hyperthermia – Abscopal effects and in situ vaccination
Magnetic hyperthermia to treat tumors has been investigated as a potential local tumor treatment and stimulator of systemic antitumor immune responses [12,21,126,152,163]. This builds on ideas first reported by Coley, in which injection of bacteria into one or a few tumors in a patient with metastatic cancer sometimes produced regression of untreated tumors. Radiation oncologists had published numerous case reports observing spontaneous (immune-mediated) regression of metastatic tumors outside the treatment field following irradiation of a patient. These radiation-induced effects on non-irradiated distant tumors became known as ‘abscopal effects’, and while they were considered to likely be immune mediated, their inconsistency generated limited interest until recently [232-236]. Currently the concept of ‘in situ vaccination’ (ISV) denotes a local tumor treatment that stimulates a local and systemic antitumor immune response [12,126]. The current understanding states that the local tumor is immunosuppressive; protecting the tumor from immune surveillance, but that local immune stimulation achieved by damaging the tumor can initiate systemic immune recognition of other tumors. Multiple therapeutic agents can achieve ISV, including heat or radiation as well as injection of reagents that directly stimulate the immune system.
There is considerable interest to develop strategies that reliably generate an abscopal effect with ISV, stimulating a rapid evolution in the field. The term should be limited to local tumor treatments that stimulate antitumor immunity but do not introduce new tumor antigens. All vaccines incorporate two components: 1) antigen, which is what the immune system learns to recognize; and, 2) immune adjuvant, which alerts the immune system to danger and stimulates response against the antigen. For ISV the adjuvant may be a wide range of agents, including hyperthermia, but the source of antigen is the tumor itself. Any antigen that can be useful in recognizing a tumor is in the tumor, whether tumor-associated (normal proteins expressed abnormally) or neoantigens (mutated proteins due to mutations in the tumor). While the concept is simple and attractive, optimal approaches and key pathways needed to achieve durable local and systemic antitumor immune responses remain unclear.
Magnetic hyperthermia shows promise for ISV [12,126,163,237-243]. Generally, to demonstrate a systemic immune response from local treatment, a two-tumor model is used in which one tumor is treated and the other monitored for response. Systemic antitumor immunity is demonstrated if the untreated tumor growth slows. Another method to demonstrate antitumor immunity is elimination of a tumor by heat, and then re-challenging the host by attempting to graft another tumor sometime after completing treatment. The control group receives surgical resection of the tumor. Early studies used hyperthermia alone [126,243] and more recently studies have utilized hyperthermia in combination with systemic checkpoint blockade antibody against PD-1 or CTLA4, since these are widely used immunotherapies [152,237-239].
One of the lessons learned from these studies is hyperthermia (lower temperature and long duration) is a generally more effective ISV strategy than ablation [126]. Although not fully understood, the reasons may include the reliance of successful ISV on stimulating two sets of immune recognition signals, one of which are PAMPs via exposure to the nanoparticles (Section 3). The second set of recognition signals, danger associated molecular patterns (DAMPs) occur by specific forms of cell death. Immunostimulatory cell death can occur by radiation or moderate hyperthermia [12,21,126,145,154-163,232-237] The most commonly recognized markers are an eclectic group that includes, extracellular ATP, extracellular HMGB1 (a nuclear protein), and calreticulin, a chaperone on the external surface of the cell. When an immune cell kills another cell it may or may not be immunogenic, depending on mode of death.
The immunogenic cell death paradigm explains the sporadic observations of abscopal effect with radiation, and the studies demonstrating potential for ISV with radiation or hyperthermia [244]. The recognition of appropriate molecular markers enables careful quantitative study of ISV. While there is currently no consensus, optimal ISV strategies seem to incorporate injection of immune adjuvant with induction of immunogenic cell death. Regardless, there is consensus that a combinatorial approach, using multiple immunotherapy approaches tailored for each patient, are likely to be most successful. Recent studies of ISV in clinical and preclinical settings with magnetic hyperthermia, immune adjuvant(s) and/or checkpoint inhibitors show promise [21,152,237,238,240,242]. Common to these approaches is enhanced T cell infiltration or function in tumors; however, mechanistic details and an understanding for optimizing ISV with magnetic hyperthermia remain unclear.
6. Physics of magnetic nanoparticle heating
For successful magnetic nanoparticle (fluid) hyperthermia (MFH), the nanoparticles must generate sufficient therapeutic heating at clinical AMF frequency and field combinations. This is particularly important given the limited concentrations achievable in tissues and the requirement to minimize off-target heating generated by interaction between tissues and AMF (Section 5) [169,170,179]. Therefore, the nanoparticles must possess key magnetic properties deriving from the physics of magnetic hysteresis heating [169,188,197,200-205,208]. Here, we introduce important concepts and highlight relevant gaps in knowledge and topics of research interest that may produce clinically meaningful results.
6.1. Magnetic materials
Magnetism arises from quantum mechanical interactions among orbital and spin motions of electrons in atoms [169,245]. These interactions can be strong or weak, and produce an atomic magnetic moment. Magnetic material classification is by their measured response (magnitude and direction of measured magnetic moment,) in an externally applied magnetic field, [169,245,246].
Some materials possess zero net magnetic moment, ∣M∣ = M = 0, in the absence of an external magnetic field (i.e. zero field, H = 0) and are therefore non-magnetic. Atoms in all materials have paired electrons and in zero field, the spin and orbital angular momenta of these paired electrons cancel giving rise to zero magnetic moment. When a magnetic field is applied, each electron opposes the applied magnetic field by changing its orbital angular momentum (Lenz's law) [169,245,246]. Consequently, the magnetic moment opposes the direction of the applied magnetic field, a property known as the diamagnetic response. On the other hand, unpaired (valence) electrons align themselves with the direction of the applied magnetic field, and thus generate a positive paramagnetic response. If the diamagnetic response in the material dominates, the material is diamagnetic. When the positive (paramagnetic) response dominates, the material’s classification is paramagnet. In the presence of an external magnetic field, paramagnets manifest a net magnetic moment aligned with the direction (vector) of the external field, but display a zero net magnetic moment at zero field [169]. Most materials, including biological tissues, are weakly diamagnetic.
Some materials inherently possess a nonzero net magnetic moment at zero external field. This is ferromagnetism and it is the underlying property of materials colloquially referred as ‘magnets’. Ferromagnetic materials possess a strong negative (inter-atomic) exchange interaction that dominates the diamagnetic response, producing a parallel alignment of the atomic magnetic moments when the material temperature is below a ‘magnetic’ transition temperature, known as the Curie temperature, TC [169,245,246]. At T < TC, the atomic magnetic moments align parallel to each other and to an external magnetic field. When the field is removed, H = 0, a ferromagnet will retain a nonzero magnetization, i.e. remanent magnetization or MR, having vector aligned with the (former) external field. Colloquially, the material is ‘magnetized’. Depending on material properties, its history, and experimental conditions (e.g. T), ∣MR∣ > 0 for a period of time that is characteristic of the internal magnetic properties. If T > TC, thermal energy overcomes the negative exchange interaction to disrupt the atomic magnetic correlations, with M → 0, giving rise to a paramagnet. Thus, TC is sometimes called the ferromagnetic-to-paramagnetic transition temperature.
Note that for ferro- (and ferri-)magnetic materials, measurement time and experimental temperature are important experimental variables that relate to magnetic hyperthermia. In magnetic nanoparticle hyperthermia, generally only ferro- (and ferri-)magnets (or magnets exhibiting borderline properties) are of interest. Magnetic iron oxides, e.g. magnetite (Fe3O4) and maghemite (γ-Fe2O3) are ferrimagnets having an inverse spinel crystalline structure with sub-lattices containing Fe having, respectively tetrahedral (A) and octahedral (B) coordination with O. The orientation of net M in each sub lattice opposes the other, but their relative magnitudes are unequal giving rise to ∣M∣ > 0. Most other forms of iron oxide, e.g. hematite (α-Fe2O3), are weakly diamagnetic at ambient conditions [169,245,246].
6.2. Magnetic domains
The collective behavior of atomic magnetic moments within a ferromagnet is determined by minimizing the total free energy [245]. The total free energy represents a balance of various energy contributions such as the exchange interaction, the Zeeman energy (i.e. the potential energy due to an external magnetic field), the magnetostatic energy due to dipole-dipole interactions (every magnetic moment experiences a demagnetizing field arising from the other magnetic moments) and the anisotropy energy. The latter contribution becomes important for heat generation. Many magnetic materials exhibit anisotropy, i.e. a preferred direction of the magnetic moment [169,245,246]. The anisotropy energy quantifies the energy needed to change the orientation of the magnetic moment with respect to the preferred orientation of the moment about the crystal axis.
Bulk magnetic materials exhibit magnetic multi-domain structure [245,246]. Each domain has a uniform magnetic moment, i.e. a parallel orientation of the atomic magnetic moments within the domain, because the exchange interaction is sufficient to overcome other ‘demagnetizing, influences, including thermal energy. While intra-domain moments are aligned, moments among domains have different orientations. Domain walls, i.e. a transition zone of moments changing direction, separate domains in order to minimize the total energy, which determines the length scale of the domains and thickness of domain walls [169,245,246]. When the size of the magnetic material is reduced, the size of the domains and the width of the domain walls are also reduced [246]. Below a critical dimension, creating a domain wall becomes energetically unfavorable, giving rise to a single-domain structure [169,246]. For magnetite, this critical dimension is of order of 50 nm [246]. Single-crystallite magnetic iron oxide nanoparticles are therefore often single-domain particles [169,246]. Even in the absence of a magnetic field, the individual atomic magnetic moments align within a single-domain magnetic material, giving rise to a single giant magnetic moment. This is the macrospin approximation because all atomic magnetic moments are parallel within the material and a change of particle moment orientation requires a coordinated change of all atomic magnetic moments, defining the energy required for moment reversal [247].
Single-domain magnets often exhibit interesting and specific properties, such as superparamagnetism, when compared to their bulk counterparts. The anisotropy energy of a single-domain particle is proportional to its volume [246]. With decreasing particle size, anisotropy energy decreases, eventually becoming comparable to thermal energy. In such a case, magnetization reversal is spontaneous and stochastic spin fluctuations occur creating zero net moment as observed in paramagnetic materials [169,246,248-250]. The nanoparticles however possess larger magnetic moments than do their bulk counterparts (paramagnets) and thus the magnetic behavior of an assembly of such ultrafine, independent single-domain magnetic nanoparticles is superparamagnetism [169].
Superparamagnetism has captured the imagination of the biomedical community, particularly for imaging, and appears frequently in magnetic nanoparticle hyperthermia literature [169]. All superparamagnets are single-domain magnets, but not all single-domain magnets are superparamagnetic [246]. Furthermore, superparamagnetism is neither a required nor a desirable property for magnetic hyperthermia. A misunderstanding of these facts in magnetic hyperthermia research confounds deeper insights of magnetic behavior of magnetic nanoparticles [169].
6.3. Hysteresis and magnetic nanoparticle heat generation
The characteristic timescale for thermally driven moment reversal, τR, of an isolated (i.e. not influence by other magnets) single-domain magnet depends on both anisotropy and thermal energies [169,246-250]. The actual magnetic behavior however also depends on the measurement time, τm, of the experiment [169,246]. If the characteristic reversal time, τR is much shorter than the measurement time, τm, the magnetic moment may spontaneously reverse during the measurement and ∣M∣ = 0 [169,246-250]. The ensemble of particles displays superparamagnetic properties. If, however τR is greater than τm, ∣M∣ > 0 and appears stable. The nanoparticles are in a blocked state. The blocking temperature TB is defined as the temperature at which τm = τR, and it separates the superparamagnetic and the blocked states [169,246].
Magnetic nanoparticles generate heat by transforming energy of an applied oscillating magnetic field by field-driven moment reversal and relaxation. Generating heat implies that the magnetization vector traces an irreversible path about an energy barrier, i.e. anisotropy (Figure 2) [169,201,208]. In other words, an associated (magnetic) energy transfer occurs in the form of (forced) hysteresis losses. Heat generation thus equates with area of the hysteresis loop [169,201,208]. Heating performance is typically reported as a mass normalized loss power. i.e. specific loss power (SLP), having units W/g material, which represents the electromagnetic energy that can be absorbed by the magnetic system and converted into heat. It is directly proportional to the hysteresis loop area (A) through SLP = A × f, where f is the frequency of AMF having maximum amplitude Hmax, HAC(t) = Hmax sin(2πft) (Figure 2) [169,201,208,252,253].
Figure 2: Schematic ol magnetic Hysteresis ana heating by torced hysteisis in an alternating magnetic field.
(Left) A diagram of an idealized hysteresis loop showing the characteristic parameters (MS, MR, HC, and Hsat) relevant for heat generation for magnetic hyperthermia. (Right) Illustration of the microscopic origin of the global field-dependent magnetization. M(H), of an idealized ensemble of non-interacting (single-domain) magnets (nanoparticle), some of which contain a representative cartoon of M(H) highlighting that the hysteresis loop of each panicle may significantly differ from that of other magnets. Differences depicted here arise from the relative orientations of each magnet’s moment (M) about its easy-axis orientation (represented by the yellow lines within each particle). Also depicted are the single-particle characteristic switching field (HS). which does not apply to the left average loop. The red vertical branches illustrate that heat is only dissipated on those irreversible portions.
In general, a combination of material properties and magnetic field parameters define the maximum (theoretical) SLP for any system. The maximum hysteresis corresponds to the square loop of area, A = 2Hmax × 2MS, with MS representing the saturation magnetization of the material. The shaded area in the left panel of Figure 2 illustrates this concept. Depending on the processes followed by the magnetization and AMF parameters, real systems can only access a fraction of maximum area, different for each nanoparticle and often defined by some combination that includes orientation of the easy axis, MR and coercive field (HC), leaving room for optimization (Figure 2) [169]. Models of basic heat-dissipation mechanisms of magnetic nanoparticles assume the macrospin approximation applies; and, for many magnetic materials, this approximation is valid to several tens of nanometers (Section 6.2).
For a magnetic iron oxide nanoparticle embedded in a viscous medium, not interacting with moments of other nanoparticles, and exposed to an external field, two main types of irreversible processes can occur: the particle itself rotates (Brown rotation) or the particle is fixed and its magnetization switches internally over the anisotropy energy barrier (Néel reversal) [169,201,208,251-254]. While both processes occur in an applied AMF, which of these dominates heating depends on the interrelationship of numerous nanoparticle and experimental parameters [169,208]. Among those considered important are the time scales of the processes with respect to the field frequency, and the magnetic anisotropy relative to field amplitude. The magnetic anisotropy links the magnetization to the crystal structure (through spin-orbit coupling), thus regulating the efficiency of both Néel and Brown processes. For efficient Brownian rotation, a very large anisotropy is required to achieve an effective torque [208]. On the other hand, efficient Néel reversal requires the anisotropy energy density constant, K, to be within specific limits to allow field-driven decoupling of the magnetization from the lattice for magnetization reversal through the anisotropy energy barrier. Other important parameters include symmetry of anisotropy, nanoparticle volume, magnetic domain volume, direction of easy axis, temperature, etc. While the exact relationship is more complex, a limit to K ensuring efficient Néel switching could be estimated as Kmax ~ HmaxMS/2 [259].
In the past, the competition between Néel and Brown relaxation times (τN and τB, respectively) influencing nanoparticle heating was interpreted within the framework of the linear response theory (LRT), which describes the heating process as dominated by the shortest relaxation time [254]. However, the LRT is only suitable for small particle sizes, low field amplitudes (relative to the anisotropy field of the particles, HK), and neglects interparticle magnetic (i.e. dipole-dipole) interactions. These conditions do not occur in clinical MFH scenarios, thus LRT and its modifications are unhelpful for MFH applications and its use in this context should be discontinued [169,208].
Magnetic nanoparticle heating by AMF results from a magnetic field-driven traversal of a materiafs magnetic moment through an irreversible path. To generate heat in an AMF, the magnetic material (ensemble of nanoparticles) must exhibit hysteresis at the conditions defined by environment and AMF. The microscopic mechanism(s) dominating heat generation, i.e. domain (Bloch) wall motion, Néel or Brownian relaxation, or combination may differ, but they are all manifestations of magnetic field-driven hysteresis losses. There is a growing consensus that Brown relaxation provides a negligible contribution to heating by nanoparticles embedded in cells or tissues [208,255,256]. To achieve significant heating from Brownian relaxation requires very large coupling of the magnetization to the lattice to generate adequate torque [257,258]. Serantes et al. demonstrated that heating by Brown rotation comparable to Néel reversal is achievable, but only with favorable conditions that require significantly lower frequencies (<<100 kHz) than currently used [263].
Significant impediments to developing MFH have been the absence of robust theoretical models to describe hysteresis loss power among ensembles of interacting particles embedded in viscous media under AC fields, and a reliance on LRT, which continues to hold appeal because of its simplicity. Recent efforts to describe magnetic heating have yielded improvements that incorporate nanoparticle polydispersity (i.e. size), dipole-dipole interactions, and other complexities encountered in biological systems [169,188-190,199-203,206-208,233,234,252-263]. An aspect of more recent attempts is a recognition that the (re-)orientation of the nanoparticles by Brownian motion alters the local energy relationship among interacting nanoparticles. This alteration inevitably changes the accessible area of the hysteresis loop for the given field conditions, thus creating a time-dependent loss power component [259]. Current models and techniques rely on short-timescale techniques such as Monte Carlo (MC) [188-190,203,260] or Landau-Lifshitz-Gilbert (LLG) [206,247] and thus are unable to predict long experimental timescales.
Another development is the departure from single-domain nanoparticles to synthesizing multi-domain, multi-crystallite (single core) magnetic iron oxide nanoparticles to exploit collective associations of magnetic moments of individual crystallites within the core to modify the amplitude dependence of hysteresis heating [169,181,183-185,189,252,253,264-266]. Bordelon et al. hypothesized that characterizing amplitude dependence of SLP revealed important clues to internal magnetic-structural properties [187]. Subsequently, Dennis et al. linked complex multi-domain micromagnetic intra-core structures to differences in time-dependent relaxation with the amplitude-dependent heat generation characterized in earlier studies with various magnetic and physical methods, including polarization-analyzed small angle neutron scattering [184]. Early developments exploring the utility of collective magnetic properties of assembled, multi-crystallite (i.e. aggregates of multiple single-domain magnets) have been extended more recently to develop magnetic nanoparticles exhibiting exotic micromagnetic domain structures optimized for magnetic particle imaging as well as magnetic hyperthermia [267].
7. Cancer nanomedicine: summary and perspectives
7.1. Nanomedicine paradigms versus clinical reality
Worldwide, most deaths from cancer result from treatment refractory disease recurrence and/or metastasis [84]. The historical reality of cancer research and development (R&D) is that the majority of product concepts fail to translate to the clinic. Development of smallmolecule cancer therapeutics has yet to overcome the challenges presented by metastatic disease, and this realization has produced increased demands of rigorous clinical validation of preclinical data.
By contrast, expectations for cancer nanomedicine remain high for breakthrough treatments. The notion that nanoengineering can produce devices or drug delivery vehicles able to distinguish tumor from normal tissue to improve efficacy, limit toxicity, and increase survival (even ‘cure’) is seductive and persists. The breakthroughs have not materialized. In reality, nearly 25 years after the approval of the first cancer nanomedicine (Doxil®), only about a dozen other nanomedicine formulations (most based on a variation of Doxil®) achieved approval, and none has yet recapitulated in humans the increased OS demonstrated in mice. Compared against expectations, and measured by the number of approved products and overall clinical performance, success is modest. Consequently, some question whether the return has justified the investment while others maintain that cancer nanomedicine is underappreciated and its successes downgraded by unrealistic expectations [7]. If the experience with iron oxide nanoparticle parenteral anemia therapies is a guide, several iterations or ‘generations’ of clinical products will be needed to acquire the relevant knowledge and sufficient experience with end-use requirements.
7.2. Oversold or underappreciated?
Technology advances by innovating, and optimizing successful designs because these embody an integration of viable solutions to numerous barriers. Failures in product development identify gaps between product design, based on defined specifications, and the intended end use. Product specifications, in turn, arise from the application of scientific paradigms and engineering principals, which if erroneous, inevitably lead to product failure. Thus, the perception that cancer nanomedicine has not lived up to expectations indicates that critical gaps in scientific knowledge exist, that paradigm(s) must be revised, and that the focus of development failed to incorporate realistic end-use considerations.
Perhaps the early (preclinical) successes of cancer drug-delivery nanomedicines were oversold, with promises that exceeded the pace of the science, prompting a range of reactions [7,9,27-32,37,268-274]. On the other hand, other successes are underappreciated – the wealth of clinical experience gained from nearly one century and ‘3 generations’ of successful iron oxide nanoparticle formulations is underappreciated, if only to demonstrate the time and effort required to achieve success. Even with this long clinical history, new discoveries of complex biological interactions with iron oxide nanoparticles demonstrate our inadequate understanding of complexities of cancer. Perhaps a lesson to be gained from iron oxide nanoparticles and magnetic hyperthermia experiences is that nanoparticle interactions with biological systems remain poorly understood, and that more significant potential exists to incorporate these interactions into the design and function of cancer nanomedicines than to engineer avoidance.
The reality of cancer research and product development is that preclinical data are not a universally reliable measure of clinical benefit. Cancer is a family of complex diseases that exhibits considerable heterogeneity among its subsets, among individuals within a disease subset, and within an individual patient [84,275,276]. Data also demonstrate that the tumor microenvironment is heterogeneous, dynamic, and complex [84]. Spontaneous tumors, such as those arising in patients, represent the culmination of complex biological developments taking place over many years or decades that incorporate complex genetic, cellular, and immune processes [275,276]. Evidence also demonstrates that behavioral and environmental exposure, along with a patient’s own microbiome interacting with the immune system can affect disease etiology, disease progression, and response to treatment [277-279].
7.3. Iron oxide magnetic nanoparticle hyperthermia: a wealth of information and opportunity
Much of ‘cancer nanomedicine’ has focused on developing drug delivery platforms that attempt to evade the host (patient) immune system. After nearly one century of clinical experience and successes with parenteral iron therapies, designed to interact with the host immune system to achieve their intended therapeutic objective, it is surprising that cancer nanomedicine development has persisted so long on its present course. Preclinical and clinical results of iron oxide nanoparticles clearly indicate that nanoparticle-immune interactions are complex, depend on host disposition as well as nanoparticle properties, and that they are likely unavoidable. Further, a significant body of literature demonstrates that if understood properly, nanoparticle engineering can exploit nanoparticle-immune interactions to enhance cancer (immune) therapies.
Iron oxide nanoparticles are also clinically approved agents for magnetic nanoparticle hyperthermia. The concept of heating magnetic iron oxide particles suspended in fluid and exposed to alternating magnetic fields was first described in 1957. Magnetic fields interact with the iron oxide nanoparticles to generate heat, predominantly via magnetic hysteresis. The heat transfers through the tissue by conduction and convection. While the physics of hysteresis and heat is reliable and generally understood, complexities introduced by the fluidic and individual nature of magnetic iron oxide nanoparticles suspended in a carrier fluid and interacting with each other and with a living biological system create complexities and opportunities.
Hyperthermia in general is a cancer therapy having the objective to raise the local tumor temperature to either kill cancer cells or sensitize them to other treatments. The history of hyperthermia is deeply intertwined with the early cancer immune therapies, indicating the important relationship between immune function, cancer and fever. Heat is a pleiotropic damaging agent, depending on applied temperature and duration, various biological effects are triggered: inhibition or abrogation of DNA repair mechanisms, initiation of apoptosis or other cell death mechanisms, cell lysis etc. The clinical benefits of hyperthermia originate from its ability to disrupt or denature DNA-damage repair proteins, reverse tumor hypoxia, increase metabolic rate and induce other physiologic changes; as well as to induce heat shock and immune responses. Fever-range hyperthermia modulates the immune system: increased trafficking and licensing of CD8+ T cells, cytokine release, increased T cell receptor signaling and differentiation of naïve T cells to effector cells, increased NK cell cytotoxicity, and increased macrophage and dendritic cell activity. Fever-range hyperthermia may therefore be a broad-spectrum adjuvant that influences the tumor microenvironment with significant immune-modulating potential.
The greatest challenge of hyperthermia is to achieve precise energy delivery and control to the tumor region, while avoiding non-specific heating of normal tissues. Hyperthermia treatments of deep-seated tumors therefore remain challenging. Magnetic iron oxide nanoparticle hyperthermia offers the potential for precise dose control and true tissue specificity.
Magnetic nanoparticle hyperthermia results in the same thermal and immune features as general hyperthermia. Additionally, the aforementioned nanoparticle-immune interactions can be exploited or modulated to enhance cancer immune therapy. Iron oxide nanoparticles are consequently true agents of thermal and immune cancer therapies.
Magnetic nanoparticles embedded in the tumor need generate sufficient heat to achieve therapeutic temperatures in the tumor. Therefore, the main challenges of magnetic nanoparticle hyperthermia are the delivery and energy deposition. Systemic deliveries are generally desirable, but might not result in a sufficient nanoparticle concentration in tumors. Direct deliveries enable reliable delivery of nanoparticles to the tumor, but they require more invasive procedures and offer no direct therapy for metastatic disease. To deliver enough energy, one might consider simply increasing magnetic field amplitude and frequency. However, due to Faraday’s law of induction, the interaction between AMFs and electrically conductive materials (such as biological tissues) increases non-specific heating. Active research is devoted to balance magnetic iron oxide nanoparticle properties to achieve the desired energy deposition. Finally, the intricate interplay between iron oxide nanoparticles, the tumor microenvironment and the immune system will continue to challenge scientists to exploit the unique features of iron oxide nanoparticles.
It is clear that magnetic iron oxide nanoparticle hyperthermia offers considerable potential as thermal and immune therapies. The therapy is however inherently interdisciplinary and complex. Clinicians and patients will continue to choose less complex treatments having established records of accomplishment, unless magnetic iron oxide nanoparticle hyperthermia demonstrates distinct advantages. This raises the bar for thermal and immune nanomedicine products to demonstrate superior clinical benefits earlier in the development process.
7.4. Perspectives
In general, the stage at which a product concept fails identifies the flaws in product design and development – but it does not necessarily invalidate the innovation or the idea. In other words, testing with models can only validate the product design to the limitations of the model, making it impossible to predict whether the concept or product design will ultimately perform as conceived. The corollary is that optimizing a product to demonstrate satisfactory performance in a chosen model can only lead to heightened expectations and probable failure in the intended end use, unless the model is an accurate representation of the intended end use. The wealth of clinical data, accumulated across multiple nanomedicine products provides ample material to revise the approach and to discard obsolete or erroneous paradigms.
Certainly, the data demonstrate a need for greater care in selection of preclinical animal models and judicious evaluation of preclinical results. Nanoparticles harbor incredible potential to interact and modify host immune-biology in the context of disease to affect treatment outcome. A deeper understanding of the biology of cancer-immune and nanoparticle-immune interactions should lead to more effective cancer nanomedicines. Iron oxide nanoparticles have proven to be beneficial and versatile for medial applications. There is significant potential to advance cancer nanomedicine, however with a different perspective and a multi-disciplinary approach.
Acknowledgments
Declaration of interest
R.I. is an inventor on nanoparticle patents, and all these patents are assigned to either The Johns Hopkins University or Aduro BioTech, Inc. R.I. consults for Imagion Biosystems, a company developing imaging with magnetic iron oxide nanoparticles. All other authors report no conflicts of interest. R.I., P.K., and F.S. received partial funding from the Jayne Koskinas and Ted Giovanis Foundation for Health and Policy (JKTGF); R.I. and P.K. also received partial funding from the National Cancer Institute (5R01CA194574-02 and 5R01CA247290). D.S acknowledges Xunta de Galicia for financial support under the I2C Plan and the Strategic Grouping in Materials (AeMAT; grant No. ED431E2018/08). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins University, JKTGF, NIH, other funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feynman RP RP, There’s plenty of room at the bottom. Engineering and Science 23 (1960) 22–36. [Google Scholar]
- 2.NNI, https://www.nano.gov/nanotech-101/what; Searched January 02, 2020.
- 3.Cole AJ, Yand VC, David AE, Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends in Biotechnology 29 (2011) 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafarizadeh-Malmiri H, Sayyar Z, Anarjan N, Berenjian A. Commercialization Consideration, in: Nanobiotechnology in Food: Concepts, applications and perspectives. Springer Nature AG, Cham, Switzerland, 2019, pp 137–151. [Google Scholar]
- 5.Hobson DW, Commercialization of nanotechnology. WIREs Nanomed. Nanobiotechnol 1 (2009) 189–202. [DOI] [PubMed] [Google Scholar]
- 6.Marchal S, El Hor A, Millard M, Gillon V, Bezdetnaya L, Anticancer drug delivery: an update on clinically applied nanotherapeutics. Drugs 75 (2015) 1601–1611. [DOI] [PubMed] [Google Scholar]
- 7.van der Meel R, Lammers T, Hennik WE, Cancer nanomedicines: Oversold or underappreciated? Expert Opinion on Drug Delivery 14 (2017) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand N, et al. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics, Nat. Comm 8 (2017) 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm S, Tavares AJ, Dai D, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 1 (2016)1–12. [Google Scholar]
- 10.Korangath P, Barnett JD, Sharma A, Henderson ET, Stewart J, Yu S-H, Kandala SK, Yang C-T, Hedayati M, Armstrong T, Jaffee E, Gruettner C, Zhou XC, Fu W, Hu C, Sukumar S, Simons BW, Ivkov R, Nanoparticle interactions with immune cells dominate tumor retention and induce T-cell mediated tumor suppression in breast cancer. Science Advances, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrovolskaia MA, Shurin M, Shvedova AA, Current understanding between nanoparticles and the immune system. Toxicol. App. Pharmacol 299 (2015) 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheen MR, Lizotte PH, Toraya-Brown S, Fiering S, Stimulating antitumor immunity with nanoparticles. Wires Nanomed. Nanobiotechnol 6 (2014) 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerbach M, Ballard H, Clinical use of intravenous iron: Administration, efficacy, and safety. Hematology-Am. Soc. Hematol. Educ. Program, 2010 (2010) 338–347. [DOI] [PubMed] [Google Scholar]
- 14.Lebrun F, Klastersky J, Levacq D, Wissam Y, Paesmans M, Intravenous iron therapy for anemic cancer patients: A review of recently published clinical studies. Support. Care Cancer 25 (2017) 2313–2319. [DOI] [PubMed] [Google Scholar]
- 15.Jordan A, Scholz R, Wust P, Fahling H, Feliz R, Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Mag. Magn. Mat 201 (1999) 413–419. [Google Scholar]
- 16.Pedrigo EA, Hemery G, Sandre O, Orega D, Garaio E, Plazaola F, Teran FJ, Fundamentals and advances in magnetic hyperthermia. Appl. Phys Rev 2 (2015) 041302. [Google Scholar]
- 17.Nuzhina JV, StU AA, Prilepskii AY, Vinogradov VV, Preclinical evaluation and clinical translation of magnetite-based nanomedicines. J. Drug Del. Sci. Tech 54 (2019) 101282. [Google Scholar]
- 18.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Sothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyan B, Lin Z, Wang L, Egeblad M, Chan WCW. The entry of nanoparticles into solid tumours. Nat. Mater (2020) 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 19.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, H.E. Daldrup-Link. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol 11 (2016) 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, et al. Nanomaterial-induced ferroptosis for cancer specific therapy. Coord. Chem. Rev 382 (2019) 160–180. [Google Scholar]
- 21.Grauer O, Jaber M, Hess K, Weckesser M, Schwindt W, Maring S, Wőlfer J, Stummer W, Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neuro-Onc 141 (2019) 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office of the Commissioner, Guidance for Industry: Considering whether an FDAregulated produce involves the application of nanotechnology. US Department of Health and Human Services, Food and Drug Administration, June 2014. (https//www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-guidance-documents) [Google Scholar]
- 23.Choi YH, Han H-K, Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharmac. Invest 48 (2018) 43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevastre A-S, Horescu C, Baloi SC, Cioc CE, Batu BI, Tuta C, Artene SA, Danciulescu MM, Tudorache S, Dricu A, Benefits of nanomedicine for therapeutic intervention in malignant diseases. Coatings 9 (2019) 628 (17 pages). [Google Scholar]
- 25.Chemistry, manufacturing, and controls changes to an approved application: Certain biological products (Draft Guidance for Industry), 21CFR 32(e); US Department of Health and Human Services, Food and Drug Administration, December 2017. (https//www.fda.gov/media/109615/download). [Google Scholar]
- 26.FYs 2013-2017 regulatory science report: Complex mixtures and peptides. Report by US Food and Drug Administration, US Department of Health and Human Services; (https//www.fda.gov/media/112368/download). [Google Scholar]
- 27.Harshom CM, Bradbury MS, Lanza GM, Nel AE, Rao J, Wang AZ, Weisner UB, Yang L, Grodzinksi P, Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 12 (2018) 24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res 33 (2016) 2373–2387. [DOI] [PubMed] [Google Scholar]
- 29.Grodzinski P, Kircher M, Goldberg M, Gabison A, Integrating nanotechnology into cancer care. ACS Nano 13 (2019) 7370–7376. [DOI] [PubMed] [Google Scholar]
- 30.Tran S, DeGiovanni P-J, Piel B, Rai P, Cancer nanomedicine: a review of recent success in drug delivery. Clin. Trans. Med 6 (2017) 44 (21 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J, Nanomedicine in cancer therapy. J. Contr. Rel 200 (2015) 138–157. [DOI] [PubMed] [Google Scholar]
- 32.Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotech 33 (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BYS, Rutka JT, Chan WCW, Nanomedicine: Current Concepts. N. Engl. J. Med 363 (2010) 2434–2443. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs, Cancer Res. 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- 35.Maeda H, Bharate GY, Daruwalla J, Polymeric drugs for efficient tumor targeted drug delivery based on EPR-effect, Eur. J. Pharm. Biopharm 71 (2009) 409–419. [DOI] [PubMed] [Google Scholar]
- 36.Albanese A, Tang PS, Chan WCW, The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng 14 (2012)1–16. [DOI] [PubMed] [Google Scholar]
- 37.Duncan R, Gaspar R, Nanomedicine(s) under the microscope. Mol. Pharm, 8 (2011) 2101–2141. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand N, Wu J, Xu X, Kamly N, Farokhzad OC, Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug. Del. Rev 66 (2014) 2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Adamson GN, Gruettner C, Ivkov R, Development of tumor targeting bioprobes (111In-chimeric L6 monoclonal antibody nanoparticles) for alternating magnetic field cancer therapy, Clin. Cancer Res 11 (2005) 7087s–7092s. [DOI] [PubMed] [Google Scholar]
- 40.Natarajan A, Gruettner C, Ivkov R, DeNardo GL, Mirick G, Yuan A, Foreman A, DeNardo SJ, NanoFerrite particle based radioimmunonanoparticles: Binding affinity and in vivo pharmacokinetics, Bioconjugate Chem. 19 (2008) 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, Hart LL, Milowsky MI, Posadas EM, Ryan CJ, Graf RP, Dittamore R, Schreiber NA, Summa JM, Youssoufian H, Morris MJ, Scher HI, Safety and Efficacy of BIND-014, a Docetaxel Nanoparticle Targeting Prostate-Specific Membrane Antigen for Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 4 (2018) 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, Riahi K, Reynolds JG, Wickham TJ, Molnar I, Yardley D, HERMIONE: A Randomized Phase 2 Trial of MM-302 Plus Trastuzumab Versus Chemotherapy of Physician’s Choice Plus Trastuzumab in Patients with Previously Treated, Anthracycline Naive, HER2-Positive, Locally Advanced/Metastatic Breast Cancer. BMC Cancer 16 (2016) 352 (11 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R, Thermal dosimetry predictive of efficacy of 111In-Ch L6 nanoparticle AMF induced therapy for human breast cancer in mice, J. Nucl. Med 48 (2007) 437–444. [PubMed] [Google Scholar]
- 44.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T, Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosd. Bioeng 96 (2003) 364. [DOI] [PubMed] [Google Scholar]
- 45.Kozissnik B, Bohorquez AC, Dobson J, Rinaldi C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperthermia 29 (2013) 706–714. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, et al. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol. Pharm 11(2011)1250–1258. [DOI] [PubMed] [Google Scholar]
- 47.Barenholz Y, Doxil® - The first FDA-approved nano-drug: Lessons learned. J. Control. Release 160 (2012) 117–134. [DOI] [PubMed] [Google Scholar]
- 48.Weiss RB, The anthracyclines: will we ever find a better doxorubicin? Semin. Oncol. 19(1992) 670–686. [PubMed] [Google Scholar]
- 49.Skeel RT, Handbook of Cancer Chemotherapy, Lippincott Williams & Wilkins, Philadelphia, 1999. [Google Scholar]
- 50.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L, Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity, Pharmacol. Rev 56 (2004) 185–229. [DOI] [PubMed] [Google Scholar]
- 51.Kenyon J, Chemotherapy and cardiac toxicity — the lesser of two evils, Doctors Lounge Website, 2010. [Google Scholar]
- 52.Takimoto CH, Calvo E, Principles of oncologic pharmacotherapy, in: Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds.), Cancer Management: a Multidisciplinary Approach 11th ed., 2008. [Google Scholar]
- 53.Barenholz Y, Cohen R, Rational design of amphiphile-based drug carriers and sterically stabilized carriers, J. Liposome Res 5 (1995) 905–932. [Google Scholar]
- 54.Barenholz Y, Relevancy of drug loading to liposomal formulation therapeutic efficacy, J. Liposome Res 13 (2003) 1–8. [DOI] [PubMed] [Google Scholar]
- 55.Lasic DD, Martin F (Eds.), Stealth Liposomes, CRC Press, Boca Raton, FL: 1995. [Google Scholar]
- 56.Gabizon A, Catane R, Uziely B, Kaufman B, Saffa T, Cohen R, Martin F, Huang A Barenholz Y, Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes, Cancer Res. 54 (1994) 987–992. [PubMed] [Google Scholar]
- 57.Gabizon A, Shmeeda H, Barenholz Y, Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies, Clin. Pharmacokinet 42 (2003) 419–436. [DOI] [PubMed] [Google Scholar]
- 58.Symon Z, Peyser A, Tzemach D, Lyass O, Sucher E, Shezen E, Gabizon A, Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes, Cancer 86 (1999) 72–78. [PubMed] [Google Scholar]
- 59.Druckmann S, Gabizon A, Barenholz Y, Separation of liposome-associated doxorubicin from non-liposome-associated doxorubicin in human plasma: implications for pharmacokinetic studies, Biochim. Biophys. Acta 980 (1989) 381–384. [DOI] [PubMed] [Google Scholar]
- 60.Hong R-L Huang C-J Tseng Y-L Pang VF Chen S-T, Liu J-J, Chang F-H Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: Is surface coating with polyethylene glycol beneficial? Clin. Cancer Res 5 (1999) 3645–3652. [PubMed] [Google Scholar]
- 61.Song G Suzuki OT, Santos CM, Lucas AT, Wiltshire T, Zamboni WC, Gulpl is associated with the pharmacokinitecs of PEGylated liposomal doxorubicin (PLD) in inbred mouse strains. Nanomedicine: Nanotech, Biol, Med 12 (2016) 2007–2014. [DOI] [PubMed] [Google Scholar]
- 62.Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Femández JB,Vallejo-Cardona A, Nanomedicine review: Clinical developments in liposomal applications, 10 (2019) 11 (40 pages). [Google Scholar]
- 63.Petersen GH Alzghari SK, Chee W, Sankari SS, La-Beck NM, Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Drug Release 22 (2016) 255–264. [DOI] [PubMed] [Google Scholar]
- 64.Doxil®, product insert.
- 65.Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am. J. Hematol 83 (2008) 580–588. [DOI] [PubMed] [Google Scholar]
- 66.Danielson BG BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J. Am. Soc. Nephrolo 15 (2004) (Suppl 5)S93–S98. [DOI] [PubMed] [Google Scholar]
- 67.Search conducted on DailyMed, US National Library of Medicine, NIH (https://dailymed.nlm.nih.gov/dailymed/). Search terms: ‘iron’ (active ingredient) AND ‘injection’ (dosage and administration) AND ‘anemia’ (indications and usage) OR’iron deficiency’ (indications and usage) performed 2020-02-03.
- 68.Bhandari S, Pereira DIA, Chappell HF, Drakesmith H, Intravenous irons: From basic science to clinical practice. Pharmaceuticals 11 (2018) 82 (20 pages) doi: 10.3390/phl1030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Auerbach M, Deloughery T, Single-dose intravenous iron for iron deficiency: A new paradigm. Hematology Am. Soc. Hematol. Educ. Program 2016 (2016) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfeifhofer-Obermair C, Tymoszuk P, Petzer V, Weiss G, Nairz M, Iron in the tumor microenvironment – connecting the dots. Frontiers in Oncology, 8 (2018) 549 (pages 1-24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science 306 (2004) 2090–2093. [DOI] [PubMed] [Google Scholar]
- 72.Moneim KA, Bhandari S, Tolerability and efficacy of parenteral iron therapy in hemodialysis patients, a comparison of preparations. Transfus. Alternat. Transfus. Med 9 (2007) 37–42. [Google Scholar]
- 73.Varde KN, Treatment of 300 cases of iron deficiency of pregnancy by total dose infusion of iron-dextran complex. J Obstet Gynaecol Br Comm. 71 (1964) 919–922. [DOI] [PubMed] [Google Scholar]
- 74.Sav T, Tokgoz B, Sipahioglu MH, et al. Is there a difference between the allergic potencies of the iron sucrose and low molecular weight iron dextran? Ren Fail. 29 (2007) 423–426. [DOI] [PubMed] [Google Scholar]
- 75.Zolnik BS, Gonzalez-Femandez A, Sadrieh N, Dobrovolskaia MA, Nanoparticles and the immune system. Endocrinology. 151 (2010) 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamstra RD, Block MH, Schocket AL, Intravenous iron dextran in clinical medicine. JAMA 243 (1980) 1726–1731. [PubMed] [Google Scholar]
- 77.Auerbach M, Ballard H, Trout JR, Mcllwain M, Ackerman A, Bahrain H, Balan S, Baker L, Rana J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J. Clin. Oncol 22 (2004) 1301–1307. [DOI] [PubMed] [Google Scholar]
- 78.Auerbach M, Should intravenous iron be the standard of care in oncology? J. Clin. Oncol 26(2008) 1579–1581. [DOI] [PubMed] [Google Scholar]
- 79.Aapro M, Beguin Y, Bokemeyer C, Dicato M, Gascon P, Glaspy J, Hofmann A, Link H, Littlewood T, Ludwig H, Ősterborg A, Pronzato P, Santini V, Schrijvers D, Stauder R, Jordan K, Herrstedt J, Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann. Oncol 29 2018. (Supplement 4) iv96–iv110. [DOI] [PubMed] [Google Scholar]
- 80.Gafter-Gvili A, Steensma DP, Auerbach M, Should the ASCO/ASH guidelines for the use of intravenous iron in cancer- and chemotherapy-induced anemia be updated? JNCCN 12 (2014) 657–664. [DOI] [PubMed] [Google Scholar]
- 81.Baribeault D, Auerbach M, Iron replacement therapy in cancer-related anemia. Am. J. Health-Syst. Pharm 68 (2011) Suppl1 S4–S14. [DOI] [PubMed] [Google Scholar]
- 82.Gemici C, Yetmen O, Yaprak G, Ozden S, et al. , Is there a role of intravenous iron for the treatment of anemia in cancer? BMC Cancer 16 (2016) 661 (9 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G, The anticancer immune response: Indispensable for therapeutic success?, .J. Clin. Investigation 118 (2008) 1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 14 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- 85.Nairz M, Haschka D, Demetz E, Weiss G, Iron at the interface of immunity and infection. Frontiers in Pharmacology, 5 (2014) 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nairz M, Schroll A, Sonnweer T, Weiss G, The struggle for iron – a metal at the host-pathogen interface. Cell. Microbiol 12 (2010) 1691–1702. [DOI] [PubMed] [Google Scholar]
- 87.Neto LMM, Zufelato N, de Sousa-Junior AA, Trentini MM, da Costa AC, et al. Specific T cell induction using iron oxide based nanoparticles as subunit vaccine adjuvant. Human Vaccines & Immunotherapeutics, early online DOI: 10.1080/21645515.2018.1489192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baumann D, Homann D, Nullmeier S, et al. Complex encounters: nanoparticles in whole blood and their uptake into different types of white blood cells. Nanomedicine-UK 8 (2013) 699–713. [DOI] [PubMed] [Google Scholar]
- 89.Hubbell JA, Thomas SN, Swartz MA, Materials engineering for immunomodulation. Nature 462 (2009) 449–460. [DOI] [PubMed] [Google Scholar]
- 90.Cho N-H, Cheong T-C, Min JH, Wu JH, et al. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 6 (2011) 675–682. [DOI] [PubMed] [Google Scholar]
- 91.Mi Y, Smith CC, Yang F, Qi Y, Roche KC, et al. A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy, Adv. Mat 30 (2018) 1706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy, Nat. Nanotechnol 12 (2017) 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao L, Seth A, Wibowo N, et al. Nanoparticle vaccines. Vaccine 32 (2014) 327–337. [DOI] [PubMed] [Google Scholar]
- 94.Dobrovolskaia MA, R Germolec D, Weaver JL, Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol 4 (2009) 411–414. [DOI] [PubMed] [Google Scholar]
- 95.Wood LM, Paterson Y, Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front. Cell. Infect. Microbiol 4 (2014) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang J, Lau LL, Shen H, Selective depletion of nonspecific T cells during the early stages of immune responses to infection. J. Immunology 171 (2003) 4352–4358. [DOI] [PubMed] [Google Scholar]
- 97.Vallhov H. et al. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 6 (2006) 1682–1686. [DOI] [PubMed] [Google Scholar]
- 98.US Food and Drug Administration Report of the FDA Nanotechnology Task Force (FDA, 2007); available at: <http://tinyurl.com/n6c2zl>. [Google Scholar]
- 99.Ventola CL (2017). Progress in Nanomedicine: Approved and Investigational Nanodrugs. P & T : a peer-reviewed journal for formulary management, 42 (2017) 742–755. [PMC free article] [PubMed] [Google Scholar]
- 100.Zamboni WC, Szebeni J, Kozlov SV, Lucas AT, Piscitelli JA, & Dobrovolskaia MA, Animal models for analysis of immunological responses to nanomaterials: challenges and considerations. Adv. DrugDeliv. Rev 136 (2018) 82–96. [DOI] [PubMed] [Google Scholar]
- 101.Cataldi M, Vigliotti C, Mosca T, Cammarota M, Capone D. Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes. Int. J. Mol. Sci 18 (2017) 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoshyar N, et al. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (London, England) 11 (2016) 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pedersen MB, Zhou X, Larsen EKU, Sørensen US, Kjems J, Nygaard JV, Nyengaard JR, Meyer RL, Boesen T, and Vorup-Jensen T. Curvature of synthetic and natural surfaces is an important target feature in classical pathway complement activation. J. Immunol 184 (2010) 1931–1945. [DOI] [PubMed] [Google Scholar]
- 104.Kim C-S, et al. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. International journal of nanomedicine 9 (2014) Suppl 2, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones SW, et al. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. The Journal of clinical investigation 123 (2013) 3061–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mocan T, Matea CT, Iancu C, Agoston-Coldea L, Mocan L, and Orasan R, Hypersensitivity and nanoparticles: update and research trends. Clujul Medical, 89 (2016) 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kharazian B, Lohse SE, Ghasemi F, Raoufi M, Saei AA, Hashemi F, Farvadi F, Alimohamadi R, Jalali SA, Shokrgozar MA, Hadipour NL, Bare surface of gold nanoparticle induces inflammation through unfolding of plasma fibrinogen. Sci. Rep 8 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deng Z Jin J, Wang Z Wang Y Gao Q, Zhao J, The metal nanoparticle-induced inflammatory response is regulated by SIRT1 through NF-κB deacetylation in aseptic loosening. Int. J. Nanomed 12 (2017) 3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ilinskaya AN, and Dobrovolskaia MA, 2016. Immunosuppressive and antiinflammatory properties of engineered nanomaterials. In HANDBOOK OF IMMUNOLOGICAL PROPERTIES OF ENGINEERED NANOMATERIALS: Volume 3: Engineered Nanomaterials and the Immune Cell Function (pp. 139–163). [Google Scholar]
- 110.Ngobili TA, and Daniele MA, Nanoparticles and direct immunosuppression. Exp. Biol. Med 241 (2016) 1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei M, Chen N, Li J, Yin M, Liang L He Y, Song H Fan C, and Huang Q Polyvalent immunostimulatory nanoagents with self-assembled CpG oligonucleotide- conjugated gold nanoparticles. Angew. Chem 51 (2012) 1202–1206. [DOI] [PubMed] [Google Scholar]
- 112.Quach QH, Kong RLX, and Kah JCY, Complement activation by PEGylated gold nanoparticles. Bioconjugate Chem. 29 (2018) 976–981. [DOI] [PubMed] [Google Scholar]
- 113.Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, and Hunter AC, Material properties in complement activation. Adv. Drug. Deliv. Rev 63 (2011) 1000–1007. [DOI] [PubMed] [Google Scholar]
- 114.Park E-J, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, and Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. toxicol. Pharmacol 30 (2010) 162–168. [DOI] [PubMed] [Google Scholar]
- 115.De Jong WH, Van Der Ven LTM, Sleijffers A, Park MVDZ, Jansen EHJM, Van Loveren H, Vandebriel RJ. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 34 (2013), 8333–8343. [DOI] [PubMed] [Google Scholar]
- 116.Hussain S, Vanoirbeek JA, Luyts K , De Vooght V, Verbeken E, Thomassen LC, Martens JA, Dinsdale D, Boland S, Marano F, Nemery B, Hoet PH. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur. Respir. J 37 (2011) 299–309. [DOI] [PubMed] [Google Scholar]
- 117.Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K, & Takano H, Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol. Pathol 34 (2006) 958–965. [DOI] [PubMed] [Google Scholar]
- 118.Park E, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 275 (2010) 65–71. [DOI] [PubMed] [Google Scholar]
- 119.Shen C-C, Wang C-C, Liao M-H, and Jan T-R. A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice. Int. J. Nanomed 6 (2011) 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirschbaum K, Sonner JK, Zeller MW, Deumelandt K, Bode J, Sharma R, Kruwel T et al. In vivo nanoparticle imaging of innate immune cells can serve as a marker of disease severity in a model of multiple sclerosis. Proc. Nat. Acad. Sci 113 (2016) 13227–13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lo Y-C, Edidin MA, Powell JD. Selective activation of antigen-experienced T cells by anti-CD3 constrained on nanoparticles. J. Immunol 191 (3013) 5107–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kosmides AK, Sidhom JW, Fraser A, Bessell CA, & Schneck JP, Dual targeting nanoparticle stimulates the immune system to inhibit tumor growth. ACS Nano, 11 (2017) 5417–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Journeay W, Shane W, and Goldman RH. "Occupational handling of nickel nanoparticles: a case report." Am. J. Ind. Med 57 (2014) 1073–1076. [DOI] [PubMed] [Google Scholar]
- 124.Liao HY, Chung YT, Lai CH, Lin MH, & Liou SH, Sneezing and allergic dermatitis were increased in engineered nanomaterial handling workers. Ind. Health 52 (2014) 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falk MH, Issels RD, Hyperthermia in oncology. Int. J. Hyperthermia 17 (2001) 1–18. [DOI] [PubMed] [Google Scholar]
- 126.Toraya-Brown S, Fiering S, Local tumour hyperthermia as immunotherapy for metastatic cancer. Int. J. Hyperthermia 30 (2014) 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Tempel N, Horsman MR, Kanaar R, Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. .J. Hyperthermia 32 (2016) 446–454. [DOI] [PubMed] [Google Scholar]
- 128.Datta N, Gómez Ordóñez S, Gaipl U, Paulides M, Crezee H, Gellermann J, Marder D, Puric E, Bodis S, Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treatment Rev. 41 (2015) 742–753. [DOI] [PubMed] [Google Scholar]
- 129.Singh BB, Hyperthermia: An ancient science in India. Int. J. Hyperthermia 7 (1991) 1–6. [DOI] [PubMed] [Google Scholar]
- 130.Dewhirst MW, VigUanti BL, Lora-Michiels M, Hanson M, Hoopes PJ, Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperthermia 28 (2012) 509–517. [DOI] [PubMed] [Google Scholar]
- 131.Dewhirst M, Das S, Stauffer P, Craciunescu O, Vujaskovic Z, Thrall D, Hyperthermia Clinical Radiation Oncology. 3 ed: Elsevier Health Sciences; 2011. p. 385–403. [Google Scholar]
- 132.Bettaieb A, Wrzal PK, Averill-Bates DA, Cancer Treatment – Conventional and Innovative Approaches InTech, 2013, Ch. Hyperthermia: Cancer Treatment and Beyond, pp 257–283. [Google Scholar]
- 133.Gas P, Essential facts on the history of hyperthermia and their connections with electromedicine. Przeglad Elektrotechniczny 87 (2011) 37–40. [Google Scholar]
- 134.Sardari D, Verga N, Current Cancer Treatment – Novel Beyond Conventional Approaches InTech, 2013, Ch. Hyperthermia: Cancer Treatment and Beyond, pp 455–474. [Google Scholar]
- 135.Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin. Oncol 19(2007) 418–426. [DOI] [PubMed] [Google Scholar]
- 136.Bakker A, van der Zee J, van Teinhoven G, Kok MP, Rasch CRN, Crezee H, Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: a systematic review. Int. J. Hyperthermia 36 (2019) 1023–1038. [DOI] [PubMed] [Google Scholar]
- 137.Elming PB, Sørensen BS, Oei AL, Franken NAP, Crezee J, Overgaard J, Horsman MR, Hyperthermia: The optimal treatment to overcome radiation resistant hypoxia. Cancers 11 (2019) 60 (20 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mace TA, Zhong L, Kokolus KM, Repasky EA, Effector CD8+ T cell IFN-γ production and cytotoxicity are enhance by mild hyperthermia; Int. J. Hyperthermia 28 (2012) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dobšiček Trefná H, Schmidt M, van Rhoon GC, Kok HP, Gordeyev SS, Lamprecht U, Marder D, Nadobny J, Ghadjar P, Abdel-Rahman S, Kukileka AM, Stmad V, Hurwitz MD, Vujaskovic Z, Diederich CJ, R Stauffer P, Crezee J. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperthermia 36 (2019) 276–293. [DOI] [PubMed] [Google Scholar]
- 140.Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM, Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat. Oncol 10 (2015) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Issels R, Kampmann E, Kanaar R, Lindner LH, Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperthermia 32 (2016) 89–95. [DOI] [PubMed] [Google Scholar]
- 142.Crezee H, van Leeuwen CM, Oei AL, Stalpers LJA, Bel A, Franken NA, Kok HP, Thermoradiotherapy planning: Integration in routine clinical practice. Int. J. Hyperthermia 32 (2015) 41–49. [DOI] [PubMed] [Google Scholar]
- 143.Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists, Cancer Immunol. Res 1 (2013) 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Senovilla L, Galluzzi L, Zitvogel L, Kroemer G, Immunosurveillance as a regulator of tissue homeostatis. Trends Immunol. 34 (2013) 471–478. [DOI] [PubMed] [Google Scholar]
- 145.Repasky E, Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. ; Old and new facts about hyperthermia-induced modulations of the immune system; Int. J. Hyperthermia 28 (2012) 528–542. [DOI] [PubMed] [Google Scholar]
- 146.Dewhirst MW, Jones E, Samulski T, Vujaskovic Z, Li C, Prosnitz L, Hyperthermia In Kufe DW, Pollock RE, Weichselbaum RE, Bast RC, Gansler TS. Cancer Medicine 6th Ed, Hamilton:BC Decker; 2003, pp. 623–636. [Google Scholar]
- 147.Knippertz I, Stein MF, Dorrie J, Schaft N, Muller I, Deinzer A, et al. ; Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies, Int. J. Hyperthermia 27 (2012) 591–603. [DOI] [PubMed] [Google Scholar]
- 148.Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, Formstecher P, et al. ; Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines; J. Leukocyte Biol 81 (2007) 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Zhang W, Geng C, Lin T, Wang X, Zhao L, Thermal ablation versus conventional regional hyperthermia has greater anti-tumor activity against melanoma in mice by upregulating CD4+ cells and enhancing IL-2 secretion, Int. J. Hyperthermia 28(2012)528–542.22690925 [Google Scholar]
- 150.Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA, 107 (2010) 20477–20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clin. Invest 121 (2011) 3846–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oei AL, Korangath P, Mulka K, Helenius M, Coulter JB, Stewart J, Velarde E, Crezee J, Simons B, Stalpers LJA, Kok HP, Gabrielson K, Franken NAP, Ivkov R, Enhancing the abscopal effect of radiation and immune checkpoint inhibitor therapies with magnetic nanoparticle hyperthermia in a model of metastatic breast cancer. Int. J. Hyperthermia 39 (2019) 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang HY, Fu JC, Lee YC, et al. Hyperthermia stress activates heat shock protein expression via propyl isomerase 1 regulation with heat shock factor 1. Mol Cell Biol. 33(2013)4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Basu S, Binder RJ, Suto R, et al. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 12 (2000) 1539–1546. [DOI] [PubMed] [Google Scholar]
- 155.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med 191 (2000) 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Knippertz I, Stein MF, Dome J, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia. 27 (2012) 591–603. [DOI] [PubMed] [Google Scholar]
- 157.Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperthermia 28 (2012) 528–542. [DOI] [PubMed] [Google Scholar]
- 158.Mace TA, Zhong L, Kokolus KM, et al. Effector CD8+ T-cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int. J. Hyperthermia 28 (2012) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hatzfeld-Charbonnier AS, Lasek A, Castera L, et al. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J. Leukocyte Biol 81 (2007) 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Verbrugge I, Hagekyriakou J, Shapr LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72 (2012) 3163–3174. [DOI] [PubMed] [Google Scholar]
- 161.Higgins JP, Berstein MB, Hodge JW JW. Enhancing immune responses to tumor-associated antigens. Cancer Biol Ther. 8 (2009) 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Drake CG, Combination immunotherapy approaches. Ann Oncol. 23 (2012) 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S, Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine. 10 (2014) 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dou Y, Hynynen K, Allen C, To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Controlled Rel 249 (2017) 63–73. [DOI] [PubMed] [Google Scholar]
- 165.ClinicalTrials.gov, Study of ThermoDox with Standardized Radiofrequency Ablation (RFA) for Treatment of Hepatocellular Carcinoma (HCC) (OPTIMA). In: https://clinicaltrials.gov/ct2/show/NCT02112656?term=optima+thermodox&rank=1
- 166.Tak WY, Lin S-M, Wang Y, Zhen J, Vecchione A, Pak SY, Chen MH, Wong S, Xu R, Pen C-Y, Chiou Y-Y, Huang G-T, Cai J, Abdullah BJJH, Lee JS, Lee JY, Choi J-Y, Gopez-Cervantes J, Sherman M, Finn RS, Omata M, O’Neal M, Makris L, Borys N, Poon R, Lencioni R, Phase III HEAT study adding lysothermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin. Cancer Res 24 (2018)73–83. [DOI] [PubMed] [Google Scholar]
- 167.Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB, Selective Inductive Heating of Lymph Nodes. Annals of Surgery. 146 (1957) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ivkov R, Magnetic nanoparticle hyperthermia: A new frontier in biology and medicine? Int. J. Hyperther 29 (2013) 703–5. [DOI] [PubMed] [Google Scholar]
- 169.Dennis CL, Ivkov R, Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperther. 29 (2013) 715–29. [DOI] [PubMed] [Google Scholar]
- 170.Etheridge ML, Bischof JC, Jordan A. Magnetic nanoparticles for cancer therapy; In: Physics of Thermal Therapy: Fundamentals and Clinical Applications Ed. Moros EG; Series in: Imaging in medical diagnosis and therapy (Series Ed. Hendee WR) CRC Press, Taylor&Francis Group, Boca Raton, FL USA: (Chapter 17; pp 293–318). [Google Scholar]
- 171.Announcement on company site (https//www.magforce.com/en/home/about_magforce/#highlights).
- 172.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, Pinkemelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol 78 (2006) 7–14. [DOI] [PubMed] [Google Scholar]
- 173.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol 81 (2007)53–60. [DOI] [PubMed] [Google Scholar]
- 174.Maier-Hauff K; Ulich F, Nestler D, Niehofl H; Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol 103 (2011) 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy – feasibility, tolerance, and achieved temperatures. Int. J. Hyperthermia 22 (2006) 673–685. [DOI] [PubMed] [Google Scholar]
- 176.Johannsen M, Gneueckow U, Thiesen B, Taymoorian K, Cho CH, Waldofner N, et al. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 52 (2007) 1653–1662. [DOI] [PubMed] [Google Scholar]
- 177.Johannsen M, Gneueckow U, Taymoorian K, Thiesen B, Waldofner N, Scholz R, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperther 23 (2007) 315–23. [DOI] [PubMed] [Google Scholar]
- 178.Johannsen M, Thiesen B, Wust P, Jordan A, Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperthermia 26 (2010) 790–5. [DOI] [PubMed] [Google Scholar]
- 179.Johannsen M, Gneueckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperthermia 21 (2005) 637–47. [DOI] [PubMed] [Google Scholar]
- 180.Attaluri A, Kandala SK, Zhou H, Cornejo C, Armour M, Hedayati M, Zhang Y, DeWeese TL, Herman C, Ivkov R, Magnetic nanoparticle hyperthermia enhances radiation therapy: A study in mouse models of human prostate cancer, Int.J. Hyperthermia 31 (2015) 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grüettner C, Muller K, Teller J, Westphal F, Foreman AR, Ivkov R, Synthesis and antibody conjugation of magnetic nanoparticles with improved specific power absorption rates for alternating magnetic field cancer therapy, J. Magn. Magn. Mater 311 (2007) 181–186. [Google Scholar]
- 182.Gruttner C, Muller K, Teller J, Westphal F. Synthesis and functionalization of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperthermia 29 (2013) 777–789. [DOI] [PubMed] [Google Scholar]
- 183.Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge RR, Foreman AR, van Lierop J, Gruttner C, Ivkov R, Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia, Nanotechnology 20 (2009) 395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dennis CL, Krycka KL, Borchers JA, Desutels RD, van Lierop J, Huls NF, Jackson AJ, Gruttner C, Ivkov R, Internal magnetic structure of nanoparticles dominates time-dependent relaxation processes in a magnetic field, Adv. Funct. Mater 25 (2015) 4300–4311. [Google Scholar]
- 185.Dennis CL, Jackson JA, Borchers JA, Gruettner C, Ivkov R, Correlation between physical structure and magnetic anisotropy of a magnetic nanoparticle colloid, Nanotechnology, 29 (2018) 215705. [DOI] [PubMed] [Google Scholar]
- 186.Jordan A, Wust P, Scholz R, Tesche B, Fahling H, Mitrovics T, Vogl T, Cervos-Navarro J, Felix R. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int. J. Hyperthermia 12 (1996) 705–722. [DOI] [PubMed] [Google Scholar]
- 187.Bordelon D, Cornejo C, Gruettner C, DeWeese TL, Ivkov R, Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with a wide ranging and high amplitude alternating magnetic field, J. Appl. Phys 109 (2011) 124904. [Google Scholar]
- 188.Serantes D et al. , Influence of dipolar interactions on hyperthermia properties of ferromagnetic particles. J. Appl Phys 108 (2010) 073918. [Google Scholar]
- 189.Martínez-Boubeta C et al. Adjustable Hyperthermia Response of Self-Assembled Ferromagnetic Fe-MgO Core–Shell Nanoparticles by Tuning Dipole–Dipole Interactions. Adv. Funct. Mater 22 (2012) 3737. [Google Scholar]
- 190.Branquinho LC, Carriao MS, Costa AS, Zufelato N, Sousa MH, Miotto R, Ivkov R, Bakuzis AF, Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia, Sci. Rep 3 (2013) 2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Soetaert F, Kandala SK, Bakuzis A, Ivkov R, Experimental estimation and analysis of variance of the measured loss power of magnetic nanoparticles, Sci. Rep 7 (2017) 6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Andreu I, Natividad E, Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. Int. J. Hyperthermia 29 (2013) 739–751. [DOI] [PubMed] [Google Scholar]
- 193.Lahiri BB, Ranoo S, Philip J, Uncertainties in the estimation of specific absorption rate during radiofrequency alternating magnetic field induced non-adiabatic heating of ferrofluids. J. Phys. D: Appl. Phys 50 (2017) 455005. [Google Scholar]
- 194.Makridis A, et al. , A standardization protocol for accurate evaluation of specific loss power in magnetic hyperthermia. J. Phys. D: Appl. Phys 52 (2019) 255001. [Google Scholar]
- 195.Natividad EM, Castro A, and Mediano A, Accurate measurement of the specific absorption rate using a suitable adiabatic magnetothermal setup. Appl. Phys. Lett 92 (2008) 093116. [Google Scholar]
- 196.Skumiel A, et al. , Uses and limitation of different thermometers for measuring heating efficiency of magnetic fluids. Appl. Therm. Eng 100 (2016) 1308–1318. [Google Scholar]
- 197.Salas G, Veintemillas-Verdaguer S, del Puerto Morales M, Relationship between physico-chemical properties of magnetic fluids and their heating capacity. Int. J. Hyperthermia 29 (2013) 768–776. [DOI] [PubMed] [Google Scholar]
- 198.Dutz S, Hergt R, Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations for tumour therapy. Int. J. Hyperthermia 29 (2013) 790–800. [DOI] [PubMed] [Google Scholar]
- 199.Tong S et al. , Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 11 (2017) 6808–6816. [DOI] [PubMed] [Google Scholar]
- 200.Suriyanto E, Ng EYK and Kumar SD, Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: a review. Biomed. Eng. Online 16 (2017) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carrey J, Mehdaoui B, Respaud M. Simple models for dynamic hysteresis loop calculations of magnetic singl-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys 109 (2011) 1–17. [Google Scholar]
- 202.Wu K et al. , Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology 30 (2019) 502003. [DOI] [PubMed] [Google Scholar]
- 203.Ruta S, Chantrell R, Hovorka O. Unified model of hyperthermia via hysteresis heating in systems of interacting magnetic nanoparticles. Sci. Rep 5 (2015) 9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dutz S and Hergt R. Magnetic particle hyperthermia – a promising tumour therapy? Nanotechnology 25 (2014) 452001. [DOI] [PubMed] [Google Scholar]
- 205.Tebaldi ML et al. , Biomedical nanoparticle carriers with combined thermal and magnetic response: Current preclinical investigations”, J. Magn. Magn. Mater 461 (2018) 116. [Google Scholar]
- 206.Haase C and Nowak U, Role of dipole-dipole interactions for hyperthermia heating of magnetic nanoparticle ensembles. Phys. Rev. B 85 (2012) 045435. [Google Scholar]
- 207.Usov NA, Low frequency hysteresis loops of superparamagnetic nanoparticles with uniaxial anisotropy. J. Appl. Phys 107 (2010) 123909. [Google Scholar]
- 208.Engelmann UM, Shasha C, Teeman E, Slabu I, Krishnan KM, Predicting size-dependent heating efficiency of magnetic nanoparticles from experiment and stochastic Néel-Brown Lengevin simulation. J. Magn. Magn. Mat 471 (2019) 450–456. [Google Scholar]
- 209.Barnes FS, Greenebaum B, Biological and Medical Aspects of Electromagnetic Fields: Taylor & Francis; 2006. [Google Scholar]
- 210.Greenebaum B, Bames FS, Bioengineering and Biophysical Aspects of Electromagnetic Fields: Taylor & Francis; 2006. [Google Scholar]
- 211.Baronzio GF, Hager ED, Hyperthermia In Cancer Treatment: A Primer: A Primer: Landes Bioscience; 2008. [Google Scholar]
- 212.Dughiero F, Corazza S, Numerical simulation of thermal deposition with induction heating used for oncological hyperthermia treatment, Med. Biol. Eng. Comput 43 (2006) 40–46. [DOI] [PubMed] [Google Scholar]
- 213.Atkinson WJ, Brezovich IA, Chakraborty DP, Usable frequencies in hyperthermia with thermal seeds, IEEE Trans. Biomed. Eng 31 (1984) 70–75. [DOI] [PubMed] [Google Scholar]
- 214.Nyenhuis JA, Mouchawar GA, Bourland JD, Geddes LA, Energy considerations in the magnetic (eddy-current) stimulation of tissues, IEEE Trans. Magn 27 (1991) 680–687. [Google Scholar]
- 215.Lin JC, Bemardi P, Computational methods for predicting field intensity and temperature change In Bames FS, Greenebaum B, eds. Bioengineering and biophysical aspects of electromagnetic fields, Third Edition. Boca Raton:CRC Press, Taylor & Francis Group; 2007, pp. 293–380. [Google Scholar]
- 216.Liu F, Zhao H, Crazier S, On the induced electric field gradients in the human body for magnetic stimulation by gradient coils in MRI. IEEE Trans. Biomed. Eng 50 (2003) 804–815. [DOI] [PubMed] [Google Scholar]
- 217.Black DR, Thermoregulation in the presence of radio frequency fields In Barnes FS, Greenebaum B, eds. Biological and Medical Aspects of Electromagnetic Fields, 3rd Edition. Boca Raton:CRC Press, Taylor & Francis Group; 2006, pp. 215–226. [Google Scholar]
- 218.Polk C, Introduction In Barnes FS, Greenebaum B, eds. Biological and Medical Aspects of Electromagnetic Fields, 3rd Edition. Boca Raton:CRC Press, Taylor & Francis Group; 2006, pp. xiii–xxvi. [Google Scholar]
- 219.Attaluri A, Jackowski J, Sharma A, Kandala SK, Nemkov V, Yakey C, DeWeese TL, Kumar A, Goldstein RC, Ivkov R R, Design and construction of a Maxwell-type inductor coil for magnetic nanoparticle hyperthermia. Int. J. Hyperthermia 37 (2020) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hilger I, In vivo applications of magnetic nanoparticle hyperthermia. Int. J. Hyperthermia 29 (2013) 828–834. [DOI] [PubMed] [Google Scholar]
- 221.Etheridge ML, and Bischof JC, Optimizing Magnetic Nanoparticle Based Thermal Therapies Within the Physical Limits of Heating. Ann. Biomed. Eng 41 (2013) 78–88. [DOI] [PubMed] [Google Scholar]
- 222.Lanier OL, Korotych OI, Monsalve AG, Wable D, Savliwala S, Grooms NWF, Nacea C, Tuitt OR, Dobson J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperthermia 36 (2019) 686–700. [DOI] [PubMed] [Google Scholar]
- 223.Mahmoudi K, Bouras A, Bozec D, Ivkov R, Hadjipanayis C, Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy, and application in humans, Int. J. Hyperthermia 34 (2018) 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Salloum M, Ma R, Zhu L, An in vivo experimental study of temperature elevations in animal tissue during magnetic nanoparticle hyperthermia. Int. J. Hyperthermia 24 (2008) 589–601. [DOI] [PubMed] [Google Scholar]
- 225.Golneshan AA, Lahonian M, Diffusion of magnetic nanoparticles in a multi-site injection process within a biological tissue during magnetic fluid hyperthermia using lattice Boltzmann method. Mech. Res. Comm 38 (2011) 425–430. [Google Scholar]
- 226.Kandala SK, Liapi E, Whitcomb LL, Attaluri A, Ivkov R, Temperature-controlled power modulation compensates for heterogeneous nanoparticle distributions: A computational optimization analysis for magnetic hyperthermia, Int. J. Hyperthermia 36(2019) 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gordon RT, Hines JR, Gordon D, Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular termperature and biophysical alterations, Med. Hypotheses 5 (1979) 83–102. [DOI] [PubMed] [Google Scholar]
- 228.Rabin Y, Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int. J. Hyperthermia 18 (2002) 194–202. [DOI] [PubMed] [Google Scholar]
- 229.Hedayati M, Thomas Ο, Abubaker-Sharif Β; Zhou H, Cornejo C, Zhang Y et al. The effect of cell cluster size on intracellular nanoparticle-mediated hyperthermia: Is it possible to treat microscopic tumors? Nanomedicine, UK 8 (2013) 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C, EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 5 (2011) 7124–7129. [DOI] [PubMed] [Google Scholar]
- 231.Mukherjee A , Castanares M, Hedayati M, Wabler M, Trock B, Kulkami P, et al. Monitoring nanoparticle mediated cellular hyperthermia with a high sensitivity biosensor, Nanomedicine-London, 9 (2014) 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. ; Immunologic correlates of the abscopal effect in a patient with melanoma; N. Engl. J. Med 366 (2012) 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hodge JW, Sharp HJ, Gameiro SR, Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Bioth. Radiopharm 27 (2012) 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dewan ZM, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. ; Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody, Clin. Cancer Res 15 (2009) 5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen H-R, Getnet D, Bruno TC, Goldberg MV Pardoll DM, DeWeese TL, Drake CG Radiotherapy augments the immune response to prostate cancer in a time-dependent manner; The Prostate 68 (2008) 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, et al. ; γ-radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo; PLoS ONE 6 (2011) e28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chao Y, Chen G, Lian C, Xu J, Don Z, Han X, Wang C Liu Z, Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Lett. 19 (2019) 4287–4296. [DOI] [PubMed] [Google Scholar]
- 238.Pan J, Hu P, Guo Y, Hao J, Ni D, Xu Y, Bao Q, Yao H, Wei C, Wu Q, Shi J, Combined Magnetic Hyperthermia and Immune Therapy for Primary and Metastatic Tumor Treatments. ACS Nano 14 (2020) 1033–1044. [DOI] [PubMed] [Google Scholar]
- 239.Wang Z Zhang F Shao D, Chang Z Wang L Hu H, Zheng X Li X, Chen F, Tu Z, LI M, Sun W, Chen L, Dong WF Janus Nanobullets Combine Photodynamic Therapy and Magnetic Hyperthermia to Potentiate Synergetic Anti-Metastatic Immunotherapy. Adv Sci (Weinh) 6 (2019) 1901690 (10 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hoopes PJ, Mazur CM, Osterberg B Song A Gladstone DJ, Steinmetz NF, Veliz FA, Bursey AA, Wagner RJ, Fiering SN Hypo-fractionated Radiation, Magnetic Nanoparticle Hyperthermia and a Viral Immunotherapy Treatment of Spontaneous Canine Cancer. Proc SPIE Int Soc Opt Eng. 2017. Jan-Feb;10066 pii: 1006605. doi: 10.1117/12.2256213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ito A, Matsuoka F, Honda H, Kobayashi T, Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Ther. 10 (2003) 918–25. [DOI] [PubMed] [Google Scholar]
- 242.Hoopes PJ, Mazur CM, Osterberg B Song A Gladstone DJ, Steinmetz NF, Veliz FA, Bursey AA, Wagner RJ, Fiering SN Effect of intratumoral magnetic nanoparticle hyperthermia and viral nanoparticle immunogenicity on primary and metastatic cancer. Proc SPIE Int Soc Opt Eng. (2017) 10066G. doi: 10.1117/12.2256062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Suzuki M, Shinkai M, Honda H, Kobayashi T, Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res. 13 (2003) 129–35. [DOI] [PubMed] [Google Scholar]
- 244.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G, Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol 17 (2017) 97–111. [DOI] [PubMed] [Google Scholar]
- 245.Coey JMD, Magnetism and magnetic materials. Cambridge University Press, 2010, ISBN:978–0-5218–1614-4. [Google Scholar]
- 246.Dormann JL, Fiorani D, Tronc E, Magnetic relaxation in fine-particle systems In: Prigogine I, Rice SA eds. Advances in Chemical Physics, Volume XCVIII, New York: John Wiley & Sons, Inc., 1997, pp. 283–494. [Google Scholar]
- 247.Carriao MS et al. , Giant-spin nonlinear response theory of magnetic nanoparticle hyperthermia: A field dependence study. J. Appl. Phys 111 (2012) 043901. [Google Scholar]
- 248.Néel L, Théorie du trainage magnétique des ferromagnétiques en grains fins avec applications aux terres cuites. Ann Géophys 5 (1949) 99–136. [Google Scholar]
- 249.Néel L, Influence of thermal fluctuations on the magnetization of ferromagnetic small particles, C.R Acad. Sci 228 (1949) 664–668. [Google Scholar]
- 250.Coffey WT, Kalmykov YP. Thermal fluctuations of magnetic nanoparticles: Fifty years after Brown. J. Appl. Phys 112 (2012) 121301. [Google Scholar]
- 251.Tong S Zhu H, and Bao G, Magnetic iron oxide nanoparticles for disease detection and therapy, Mater. Today 31 (2019) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Martinez-Boubeta C et al. , Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep 3 (2013) 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Serantes D et al. , Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C 118 (2014) 5927. [Google Scholar]
- 254.Rosensweig RE Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater 252 (2002) 370. [Google Scholar]
- 255.di Corato R et al. , Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 35 (2014) 6400. [DOI] [PubMed] [Google Scholar]
- 256.Soukup D et al. , In Situ Measurement of Magnetization Relaxation of Internalized Nanoparticles in Live Cells. ACS Nano 9 (2015) 231. [DOI] [PubMed] [Google Scholar]
- 257.Zhao Z and Rinaldi C, Magnetization Dynamics and Energy Dissipation of Interacting Magnetic Nanoparticles in Alternating Magnetic Fields with and without a Static Bias Field. J. Phys. Chem. C 122 (2018) 21018. [Google Scholar]
- 258.Suzuki S and Satoh A, Influence of the cluster formation in a magnetic particle suspension on heat production effect in an alternating magnetic field. Colloid Polymer Sci. 297 (2019)1265. [Google Scholar]
- 259.Simeonidis K, Morales MP, Marciello M, Angelakeris M, de la Presa P, Lazaro-Carrillo A, Tabero A, Villanueva A, Chubykalo-Fesenko O, Serantes D, In-situ particles reorientation during magnetic hyperthermia application: Shape matters twice. Sci. Rep 6 (2016) 38382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Conde-Leboran I et al. , A Single Picture Explains Diversity of Hyperthermia Response of Magnetic Nanoparticles. J. Phys. Chem. C 119 (2015) 15698. [Google Scholar]
- 261.Munoz-Menendez C et al. , Towards improved magnetic fluid hyperthermia: majorloops to diminish variations in local heating. Phys. Chem. Chem. Phys 19 (2017) 14527. [DOI] [PubMed] [Google Scholar]
- 262.Conde-Leboran I, Serantes D and Baldomir D, Orientation of the magnetization easy axes of interacting nanoparticles: Influence on the hyperthermia properties, J. Magn. Magn. Mater 380 (2015) 321. [Google Scholar]
- 263.Serantes D et al. , Anisotropic magnetic nanoparticles for biomedicine: bridging frequency separated AC-field controlled domains of actuation. Phys. Chem. Chem. Phys 20 (2018) 30445. [DOI] [PubMed] [Google Scholar]
- 264.Ivkov R, Magnetic nanoscale particle compositions, and therapeutic methods related thereto. US Patent 7,731,648.
- 265.Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Lau JW, Goernitz E, Gruettner C, The influence of collective behavior on the magnetic and heating properties of iron oxide nanoparticles, J. of Appl. Phys 103 (2008) 07A319. [Google Scholar]
- 266.Ivkov R, Process for making iron oxide nanoparticle preparations for cancer hyperthermia, US Patent 10,406,228 (2019).
- 267.Gavilán H, Kowalski A, Heinke D, Sugunan A, Sommertune J, Varón M, Bogar LK, Posth O, Zeng L, González-Alonso D, Balceris C, Fock J, Wetterskog E, Frandsen C, Gehrke N, Grüttner C, Fornara A, Ludwig F, Veintemillas-Verdaguer S, Johansson C, Morales MP. Colloidal flower-shaped iron oxide nanoparticles: Synthesis strategies and coatings. Part. Part. Syst. Charact 34 (2017) 1700094 (12 pages). [Google Scholar]
- 268.DuRoss AN, Neufeld MJ, Rana S, Thomas CR Jr., Sun C, Integrating nanomedicine into clinical radiotherapy regimens. Adv. Drug. Del. Rev 144 (2019) 35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hong E Dobrovolskaia MA, Addressing barriers to effective cancer immunotherapy with nanotechnology: Achievements, challenges, and roadmap to the next generation of nanoimmunotherapeutics. Adv. Drug Del. Rev 141 (2019) 3–22. [DOI] [PubMed] [Google Scholar]
- 270.Bulbake U, Doppalapudi S, Kommineni N, Khan W, Liposomal formulations in clinical use: An updated review. Pharmaceutics 9 (2017) 12 (33 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wolfram J, Ferrari M, Clinical cancer nanomedicine. Nano Today 25 (2019) 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jian W, Yuan H, Chan CK, von Roemeling CA Yan Z, Weissman IL, Kim BYS, Lessons from immuno-oncology: a new era for cancer nanomedicine? Nat. Rev. Drug Disc 16 (2017) 369–370. [DOI] [PubMed] [Google Scholar]
- 273.Editorial, The two directions of cancer nanomedicine. Nat. Nanotechnol 14 (2019) 1083. [DOI] [PubMed] [Google Scholar]
- 274.Nardecchia S, Sánchez-Moreno P, de Vicente J, Marchal JA, Boulaiz H, Clinical trials of thermosensitive nanomaterials: An overview. Nanomaterials 9 (2019) 191 (23 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bunz F, Principles of Cancer Genetics, 2nd Ed. Springer, 2016. ISBN:978-94-017-7482-6. [Google Scholar]
- 276.Tomasetti C , Bogelstein B, Variatino in cancer risk among tissuescn be explained by the number of stem cel divisions. Science 347 (215) 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Song MY Chan AT, Sun J, Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 158 (2020) 322–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schwabe RF, Greten TF, Gut microbiome in HCC – Mechanisms, diagnosis, and therapy. J. Hepatology 72 (2020) 230–238. [DOI] [PubMed] [Google Scholar]
- 279.Pryor R, Martinez-Martinez D, Quintaneiro L, Cabreiro F, The role of the microbiome in drug response. Ann. Rev. of Pharmacol. Toxicol 60 (2020) 417–435. [DOI] [PubMed] [Google Scholar]


