Abstract
Advances in medical science have led to diverse new therapeutic modalities, as well as enhanced understanding of the progression of various disease states. These findings facilitate the design and development of more customized and exquisite drug delivery systems that aim to improve therapeutic indices of drugs to treat a variety of conditions. Synthetic polymer-based drug carriers have often been the focus of such research. However, these structures suffer from challenges with heterogeneity of the starting material, limited chemical features, complex functionalization methods, and in some cases a lack of biocompatibility. Consequently, protein-based polymers have garnered much attention in recent years due to their monodisperse features, ease of production and functionalization, and biocompatibility. Genetic engineering techniques enable the advancement of protein-based drug delivery systems with finely tuned physicochemical properties, and thus an expanded level of customization unavailable with synthetic polymers. Of these genetically engineered proteins, elastin-like proteins (ELP), silk-like proteins (SLP), and silk-elastin-like proteins (SELP) provide a unique set of alternatives for designing drug delivery systems due to their inherent chemical and physical properties and ease of engineering afforded by recombinant DNA technologies. In this review we examine the advantages of genetically engineered drug delivery systems with emphasis on ELP and SLP constructions. Methods for fabrication and relevant biomedical applications will also be discussed.
Keywords: Recombinant protein, Genetic engineering, Silk-like proteins, Elastin-like proteins, Drug delivery, Gene delivery
Graphical Abstrct
1. Introduction
Natural and synthetic drugs have been used for centuries to treat health disorders and prolong lives. Unfortunately, many drugs have serious side effects because of their inherent toxicity and absence of specificity, which leads to harm to healthy organs and tissues. Such side effects limit the ability to create optimal treatments for many diseases such as cancers, neurodegenerative diseases, and infectious diseases. To circumvent these issues, research has focused on the development of efficient drug delivery systems (DDS). Such systems can help regulate the drug release rate, as well as the location of release, thereby improving therapeutic outcomes and reducing toxicity, and may also involve enhancing hydrophilicity, extending circulation times, and protecting the drug from undesired degradation [1]. The stability and chemical compositions of polymers make them great candidates for DDS. Fabrication formats (e.g., films and hydrogels) also improve utility of polymer-based systems. For example, the high stability and tunability of polymeric particles make them great candidate for drug delivery as they have good biocompatibility and can be functionalized for active or passive targeted therapy, while properties of hydrogels such as their high swelling ratio, their porosity and their soft consistency mimicking natural living tissue make them ideal candidates for the use in tissue engineering [2-6]. For this reason, various synthetic polymers such as polyesters, polyorthoesters, polyphosphoesters, and polyanhydrides have been utilized for the delivery of therapeutics. However, these systems can present significant challenges in terms of polymer heterogeneity, biocompatibility, bulk hydrolysis, and acidic degradation products, and often require additional processing and purification for utility in DDS [7]. Consequently, research focused on natural polymers such as silk fibroin, albumin, and alginate has been of interest to overcome the above limitations [8-12].
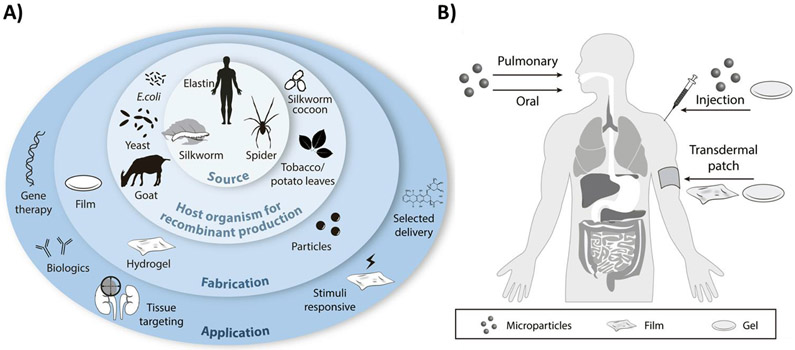
In contrast to synthetic polymers, protein-based polymers consisting of repetitive natural or engineered amino acid sequences, have advantages of homogeneity when generated via genetic engineering, biocompatibility, biodegradability without acidic degradation products, surface degradation due to enzymatic processes, aqueous and ambient processing of materials into delivery vehicles, and relative ease of scale-up and processing (Table 1) [7,13-17]. Additionally, as drugs become more complex and diverse, the control of drug delivery and release profiles becomes more demanding [1]. Thus, the properties necessary to match drug delivery demands require a level of customization that has not been attainable using synthetic polymer DDS [1,18]. The development of bioengineering methods facilitates the design and fabrication of biocompatible, responsive, and multi-faceted DDS (Figure 1A-B). First, genetic engineering enables control of sequence, protein size, and homogeneity (theoretically, a polydispersity of one), thus, enabling more precise control of DDS assembly with material functions accurately tuned and controlled. This aids in the production of new tailor-made polymeric biomaterials with improved properties for specific biomedical needs [1,19-21]. Moreover, when recombinant protein polymers are synthesized in a biological system, such as bacteria, the final isolated protein is homogeneous with little variability in sequence, composition, or size, which improves batch-to-batch reproducibility for the pharmaceutical industry [1,21,22]. At the same time, unwanted bacterial remnants must be removed that can otherwise result in inflammatory reactions, such as LPS, which can increase the cost of production [23-25]. Protein-based materials are biodegradable and can be modified so degradation rates match the specific application [1,21]. Another advantage in the use of recombinant proteins is the ability to combine various domains (e.g., amino acid sequence modules) to generate libraries differing in amino acid composition more precise control of structure–function relationships [1,21,22]. The use of biopolymer derived constructions further facilitates tailoring of the final DDS to include stimuli-responsive features, tissue targeting components, and selective release properties, all of which enhance the selectivity, specificity, and therapeutic index of the drug being delivered. The potential to effectively modify sequence elements within the protein, and thus the resulting structure and function, lends protein-based DDS to many diverse therapeutic applications, including the delivery of small molecule drugs and biologics, as well as gene therapies and related topics. Finally, the lower costs of larger-scale production in biological systems render recombinant protein polymers amenable to process scale-up. Industry favors recombinant protein expression systems that have a successful track record, in particular Chinese Hamster Ovarian (CHO) cells and Escherichia coli, usually with three goals: high quality, high yield, and low cost [26]. Although mammalian cells are favored for the production of complex proteins, prokaryotic cells are easier to handle and less expensive in terms of media requirements and for scale-up [27,28]. The development of efficient bioprocessing strategies is crucial for industrial production of recombinant proteins of therapeutic importance. Recent advances have been made in bioprocessing, including the use of high-throughput devices and of disposable systems, continuous upstream processing, continuous chromatography, integrated continuous bioprocessing and process analytical technologies to achieve quality products with higher yields [28].
Table 1.
Advantages of ELP and SLP as engineered protein for drug delivery.
| ELASTIN LIKE PROTEIN | SILK LIKE PROTEIN |
|---|---|
|
|
| COMMON ADVANTAGES | |
| |
Figure 1.
A) Recombinant proteins used as biomaterials for drug delivery applications. Recombinant proteins produced in host organisms for subsequent fabrications into a range of different material formats for a wide range of applications in the drug delivery field. B) Routes of administration of the different formats of DDS.
As a result, the interest and implementation of recombinant protein-based biopolymers for drug delivery has increased in recent years, with examples focused on the use of silk-like (SLP), silk-elastin-like (SELP), and elastin-like protein (ELP) polymers as delivery systems. In this review, we will discuss genetically engineered ELPs, and SLPs for the development of biopolymer-based DDS. Modifications to the original sequence, formulations, and current biomedical applications of these biomaterials will be reviewed, with emphasis on drug delivery.
2. Engineered Proteins
2.1. Silkworm silk-like-protein recombinant protein polymers.
Silks are naturally produced proteins, characterized as block copolymers with highly conserved repeats of short side-chain amino acids as hydrophobic blocks and short sequences of larger side-chain or charged amino acids as hydrophilic blocks [29-32]. Silk fibroin sequences derived from the cocoons of silkworm Bombyx mori are one the most studied recombinant sources of silk. Silk fibroin contains a heavy chain composed of glycine (G) and alanine (A) rich sequences of hexapeptides including: GAGAGS, GAGAGY, GAGAGA, or GAGYGA, where S is serine and Y is tyrosine. Additionally, domains based on the Anaphe panda silkworm silk utilize repeats of (AAG)6 or (AG)9 as their hydrophobic sequences [1,29,33-35].
One of the main features of silk proteins is the capability to self-assemble, due to the amphiphilic nature of the sequences, to form different structures. The self-assembly of SLPs into nanoparticles has for drug delivery is directly linked to the secondary structure. The secondary structure of silks allows SLPs to be produced to meet specific characteristics of solubility, mechanical strength, biodegradation rate, as well as drug release kinetics, and can be predesigned into the sequence [1].
Recombinant expression of SLPs for different applications, has been extensively reported in different hosts. Transgenic B. mori silkworms have been modified to produce full length silk fibroin with peptide fusions [33,36-38]. While transgenic species offer a unique approach to modifying protein structure and function, there are many drawbacks to harvesting from natural biomaterial sources, including impurities, batch-to-batch variation, or increased immune response [39]. To overcome these issues, SLPs expression and purification from bacterial sources has been optimized over the years, resulting in production levels of 500 mg/L in batch cultures and up to 12.8 g/L in fed-batch systems [34,40-45]. These examples demonstrate how scale up production can be used to increase the yield of recombinant SLPs for use in DDS, with the benefit to tight control of the protein polymer versus the use of naturally-derived silk proteins from B. mori.
A major modification to silk sequences used in the design of DDS has been the addition of cell-binding motifs. Many peptides from extracellular matrix (ECM) proteins have been added to full length and core peptide sequences of silks in SLPs, such as RGD (i.e. arginine-glycine-aspartic acid) or fibronectin (GAAVTGRGDSPASAAGYI) [34,36,40-42]. The main modification related to drug delivery applications is the addition of elastin domains to generate silk-elastin-like-proteins (SELPs). SELPs consist of blocks from the silkworm silk sequence [GAGAGS]n and mammalian tropoelastin sequence [GVGVP]n exploiting specific physicomechanical properties of each sequence. The silk-like block, from the core B. mori silk heavy chain sequence, tends to self-assemble into insoluble tightly packed secondary structures, β-sheets (crystals), to provide thermal and chemical stability, mechanical tunability and physical crosslinking sites for the SELP polymeric systems [21,32,46,47]. The elastin-like block undergoes reversible structural transitions upon exposure to specific environmental stimuli, providing dynamic functions to the SELPs [46-49]. The most attractive features of SELPs for drug delivery comes from the biological and physicochemical properties, which can be tuned by: 1) varying the silk-elastin ratio; 2) modifying the second residue in the elastin sequence; 3) modifying the molecular weight; and 4) adding peptides to expand functions [1,19,33,42,46,49-52]. By varying the silk-elastin ratio, the thermal responsive properties can be tuned, where an increase in the silk-elastin ratio leads to a higher inverse temperature transition (Tt) [47]. By modifying the hydrophobicity of the second residue in the elastin block, SELPs become further responsive to various stimuli including temperature, pH, ionic strength, redox, enzymes and electric fields (Table 2) [53]. Finally, the inverse transition temperature is inversely correlated with the molecular weight [54].
Table 2.
Stimuli responsive features of SELP-based dynamic proteins.
2.2. Spider silk-like-proteins recombinant protein polymers.
The other extensively studied source of silk is from spiders. The suborder Araneomorphea produce orb webs, which function as an extension of their sensory system, catching prey and providing protection [55-58]. These species produce at least 7 different kinds of silk by specialized glands in the spider abdomen.
Dragline silk has attracted attention due to its impressive mechanical properties and promising use in DDS. Spider dragline silk consists mainly of two high molecular weight proteins that exhibit a periodic pattern [56,58,59]. Most DDS based on spider silks have focused on the major ampullate gland silks from Nephila clavipes spiders (MaSp1 and MaSp2) and Araneus diadematus (ADF4, ADF3) [56,59-61]. A specific feature of the repeats in these spidroins is the hydrophobic poly-Ala (poly-A) domain consisting of 4 to 9 amino acid residues and a more hydrophilic Gly-enriched domain with GGX motifs for MaSp1 and GPGXX for MaSp2. Constructions mimicking the dragline core sequences from both MaSp and ADF have been studied as DDS using individual versions [19,62-64] or mixtures of spidroins [65]. Other DDS studies have also used other types of spider silks, including aciniform [50] and tubuliform [66].
The repetitive nature of the spider silk sequences, as well as the length, makes production and high yields in heterological expression systems challenging [56,58,60,67]. Spider silk expression in bacteria (E. coli), yeast Pichia pastoris [68] or Sacharomyces cerevisiae [69], animal cells [70], transgenic goats producing the proteins in their milk [71], plants (e.g. potato or tobacco), [72] or transgenic silkworms [52,73] have all been reported. Metabolically engineered E. coli generated the best yields, where the glycyl-tRNA pool was elevated [74,75].
An additional advantage of recombinant approaches to DDS is engineering in modifications to provide new properties to the recombinant proteins. An example is the addition of a glutamic acid to a silk variant based on core sequence of the MaSp2 protein from the spider N. clavipes; to modulate affinity of the engineered silk for drugs [76]. Another example demonstrated that engineering eADF4(C16) to incorporate a cysteine allowed the covalent coupling of peptides, enzymes or particles to the spider silk variant related to DDS [77]. Further modifications to eADF4(C16), replacing the negatively charged amino acids (glutamic acids) with positively charged amino acids (lysines), supported the sequestration of negatively charged, high-molecular-weight payloads, such as nucleic acids, and low-molecular-weight compounds [78]. Site specific modification with the replacement of methionine by L-azidohomoalanine (L-Aha) at the C-terminus of 4RepCT supported the chemical conjugation of different ligands (e.g., antibiotics, fluorophores) [79].
A critical challenge to efficacy of cancer chemotherapy concerns insufficient intracellular drug release. To improve cellular uptake and release, recombinant spider silk analogs were engineered to harbor poly-lysine/poly-arginine and cell penetrating peptides (CPPs) such as the Tat peptide (RKKRRQRRR) or the cell membrane-destabilizing peptide ppTG1 (GLFKALLKLLKSLWKLLLKA) [32,80-83]. Coupling peptides to poly-lysine variants ECM proteins (e.g. RGD or IKVAV) further improved cellular uptake by enhancing adhesion of the spider silk recombinant analogs to cells [83,84]. Although the CPPs facilitate cellular internalization, they lack cell specificity. To achieve greater selectivity, peptides that recognize cell surface features can be fused to the silk; such as to target specific cancer cells. The F3 tumor-homing peptide (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK) binds specifically to nucleoin, expressed on the surface of some tumor cells, and the CGKRK peptide to heparan sulfate present in tumor vessels. Both peptides were successfully fused to a silk protein functionalized with a poly-lysine peptide for nucleic acid binding [85]. In another study, the F3 peptide and Lypl peptide (CGNKRTRGC) that targets lymphatic vessels of certain tumors, has been used to bioengineer a MaSp1-poly-lysine monomer to form nanocomplexes with plasmid DNA [86]. Modification of silk proteins that target human epidermal growth factor receptor 2 (Her2), overexpressed in 20–30% of invasive breast carcinomas, was also accomplished [65,87]. Two variants of tumor-homing peptides, H2.1 (MYWGDSHWLQYWYE) and H2.2 (LTVSPWY), were evaluated as fusions at the N and C termini to functionalize both MaSp1 and MaSp2 constructs [65,87]. Functionalization to impart mucoadhesive properties by the addition of Human Galectin-3 Carbohydrate Recognition Domain (hGal3), which specifically binds the mucin glycans Galβ1-3GlcNAc and Galβ1-4GlcNAc, produced silk materials that showed enhanced mucin binding properties compared to the wild-type [88]. One of the most complicated modifications described involves the delivery of DNA to the nucleus of stem cells. Variants based on MaSp1 spidroin contained a poly-lysine sequence, the nuclear localization sequence (NLS) of the large tumor (T) antigen of the Simian virus 40 (SV40), an hMSC high affinity binding peptide (HAB), and a translocation motif (TLM) of the hepatitis-B virus surface protein (PreS2) [89]. Finally, modifications to optimize the electrostatic interaction of spheres for lysosomal drug delivery has also been pursued. Here, spider tubuliform silk proteins genetically engineered using a 5xHis Tag to modify the isoelectric point of the recombinant protein showed enhanced drug release [66].
2.3. Elastin-like-protein recombinant protein polymers.
Elastin is an extracellular matrix (ECM) protein found in almost all higher animals with domains in various conformations bound through crosslinking [48]. The basic unit studied for drug delivery from ELP is a pentapeptide sequence -(GXGVP), where ‘X’ can be any amino acid except proline- derived from the elastomeric domain of mammalian tropoelastin [90]. ELP sequences are generally tandem repeats of the pentapeptide which also contributes to the viscoelastic properties of elastin combined with dynamic properties depending on the amino acid in ‘X’ position. ELPs separate from aqueous solution above the Tt where the phase separation of the polymer occurs [48,90,91]. This Tt is dependent on ‘X’ as well as on molecular weight, where the Tt decreases with increasing molecular weight [92].
Successful expression and purification of ELPs has been demonstrated using P. pastoris [68,93], while bacteria (E. coli) is the preferred system [92,94-96].
Modifications to the pentapeptide at the ‘X’ position is most common, while amino acids at other positions have also been modified. For example, substitution of the glycine in the first position to alanine resulted in a change of mechanics from elastic to plastic [48]. In natural elastin, ‘X’ is frequently valine, alanine, or isoleucine. Replacing a valine residue with a residue containing a side chain with different properties introduces strong reactivity to specific stimuli, including to changes in pressure, salt, pH, and electrical current [92,97].
ELPs generation as multiblock elastin-like recombinant polymers have been utilized for drug delivery and medical applications [94,95,98]. These block copolymers have been constructed by genetically linking a hydrophobic and hydrophilic block (i.e., [VPGIG]n1-[VPGSG]n2) or by adding a third hydrophobic end block.
ELPs have been engineered to incorporate peptides such as RGD [99] for cell binding or CPPs [100,101] to enhance endocytic uptake. CPPs peptides SynB1 [101], penetratin (RQIKIWFQNRRMKWKK), Tat (YGRKKRRQRRR), and MTS (AAVALLPAVLLALLA) [100] were fused to the N-terminus of ELPs. The penetratin-ELP fused peptide was further modified with the addition of a peptide derived from the cyclin-dependent kinase inhibitor p21 (WPGSGGRKRRQTSMTDFYHSKRRLIFSKRKP) [100]. Other modifications aimed to control physicochemical properties of the polymers like reducing aggregation of ELPs by adding polyaspartic chains [102], or modification of the degradation rate by adding sequences recognized by matrix metalloproteinases (MMPs) [103]. Further modifications include fusions of therapeutic peptides such as humanin [104], Vascular Endothelial Growth Factor [105], Bone Morphogenetic factor 2 [106], peptides that promote insulin release from pancreatic β-cells (glucagon-like peptide 1, GLP-1) [107], neuropeptide that promotes heart contractility and induces coronary vasodilation with therapeutic applications against hypertension (Vasoactive intestinal peptide, VIP) [108], and even a single-chain variable fragment [109]. To generate target-specific systems, ELPs have been functionalized with tissue specific peptides [105].
3. Drug delivery system
3.1. Particle systems
Microparticles diameters usually range between 0.1 and 100 μm, while nanoparticle diameters usually range from 1 to 500 nm. Microparticle and nanoparticle systems have been widely used for controlled drug delivery owing to their large surface area, enhanced permeability and targeting ability [13,110]. Utilization of nanosized constructs suggest that poorly water-soluble drugs can be better administered via encapsulation, and diseased tissues can be targeted passively or actively[111,112]. Furthermore, with suitable targeting groups, macromolecular constructs can be delivered to intracellular sites of action. Targeting solid tumors using nanosized therapeutic constructs is often through the enhanced permeability and retention (EPR) effect. Due to the higher vascular density of tumor tissues and their lack of effective lymphatic drainage, macromolecular drugs can accumulate and be retained selectively without dispersing into healthy tissues [15,113-115]. The inverse temperature transition behavior of ELPs supports the retention of solubility in water under the critical transition temperature (Tt), while above the Tt, the polymeric chains hydrophobically fold and self-assemble into a more ordered structure suitable for drug delivery. Moreover, ELPs are biocompatible and do not generate immune responses as natural elastin analogs [116]. ELP nanoparticles were developed by thermo-responsive self-assembly for the sustained release of bone morphogenetic protein-2 (BMP-2) and bone morphogenetic protein-14 (BMP-14) over two weeks [117]. The nanoparticles were obtained by incubating the polymer solution at 37°C to yield particles ~238 nm diameter. The thermodynamically driven inverse phase transition of ELPs was used to design particles (300-400 nm) for the delivery of doxorubicin. During electrospraying, the solvent (water) rapidly evaporated to yield dehydrated ELP nanoparticles that could be controlled in size and morphology by adjusting the concentration and molecular weight of the protein [118]. ELP nanoparticles for drug delivery also present challenges, including the tendency to aggregate leading to larger structures, and they can have a critical transition temperature that is too low, factors that can lead to cell and organ damage. To prevent aggregation and increase the critical transition temperature, poly(aspartic acid) chains have been added to ELPs to obtain amphiphilic diblock peptides under 100 nm in diameter, with an critical transition temperature of ~37°C [102]. Following the same strategy, paclitaxel-loaded ELP-poly(aspartic acid) nanoparticles displaying EGF exhibiting active tumor-targeting capabilities were developed. The nanoparticles with a size of 30 nm successfully delivered the drug to HeLa cells, resulting in cell death[14]. To provide more control over the loading and the release of cargo, thermoresponsive crosslinked capsules were developed using microemulsion. The capsules, consisting of ELP and BSA, presented porous morphologies that could be tuned in terms of diameter and pore size by adjusting the ratio of ELP to BSA, with higher amounts of ELP leading to a more porous structures [119]. Porous structures of ELP microspheres were studied by adding albumin in different ratios and adjusting external stimuli; microspheres were obtained utilizing a water-in-oil emulsion and crosslinked with glutaraldehyde (Figure 2A) [120]. While the crosslinking provided control over the shape of the microspheres, the porosity of the structure was reversibly altered by changing the temperature and hence controlling the drug release profile. The size of the micropores was modified based on the mixing ratio of ELP. The thermo-responsiveness of the ELPs enabled pore opening and closing, whether the temperature was below or above its Tt, thus providing control over the release kinetics of drugs.
Figure 2.
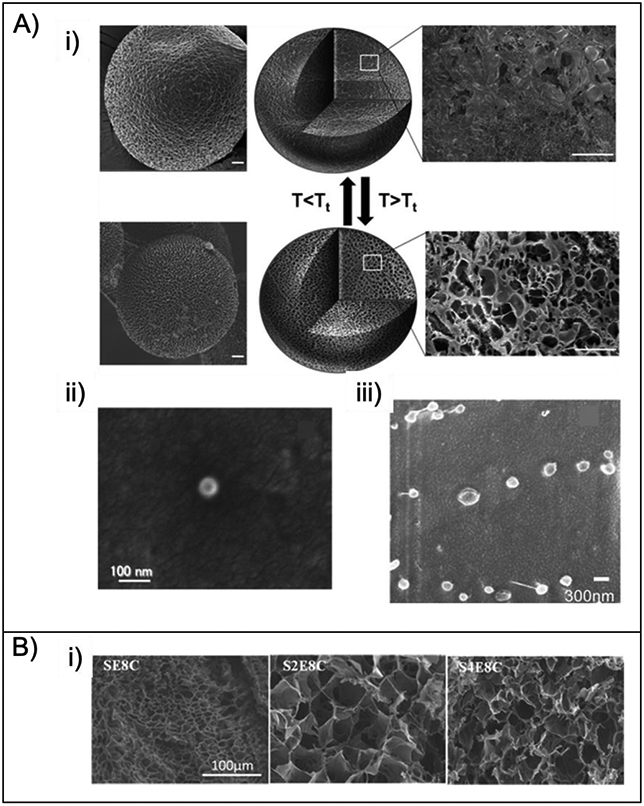
A) SEM of particles, i) ELP [120], ii) SLP [86], iii) Representative cryogenic scanning electron microscope (cryo-SEM) of Dox-loaded SE8Y nanoparticles [122]. B) SEM images of the lyophilized hydrogels fabricated with 4.05% (w/v) protein and 0.05% (w/v) H2O2 at 37 °C [124]. Figures reproduced with permission from the cited articles.
SLPs seek to build upon the strength and physicochemical properties found naturally in silk and further control those properties to achieve desirable performance in drug delivery. The most widely studied engineered SLP comes from either the B. mori silkworm or the spider N. clavipes. Engineered spider SLP complexes were designed with poly(L-lysine) domains to interact with plasmid DNA and and RGD to enhance cell binding. The size of the nanocomplex was tuned by adjusting the ratio of polymer to pDNA or the molecular weight of the poly(L-lysine) domains [84]. Changing the length of the lysine domain changed the size of the nanoparticles from 310 to 435 nm [80]. Moreover, a higher content of tumor-homing peptide was utilized to increase specificity and efficiency to home to tumor cells (MDA-MB-435 and MDA-MB-231), and smaller complexes ~90 nm diam. were obtained by changing the core recombinant silk domain to improve gene delivery (Figure 2A) [86].
SELPs consisting of a series of silk- and elastin-like proteins consist of a blend of mechanical and biological properties of both silk and elastin. While ELP offers elasticity, SLPs provide robust stability due to the crystalline β-sheets. This unique bifunctional class of protein is very useful for drug delivery as it is biocompatible, biodegradable and highly tunable in terms of mechanical properties and degradation lifetime, by changing the ratio of silk and elastin blocks [46]. Silk blocks tend to self-assemble into the core of micellar-like SELP nanoparticles, and the radius can be tuned by adjusting the ratio of silk to elastin [121]. Three different SELPs were generated to form doxorubicin-loaded micellar-like nanoparticles with radii between 50 to 142 nm, and they were uptaken up by HeLa cells (Figure 2A) [122]. Furthermore, by modifying the primary sequence of the SELPs, micellar-like nanoparticles (73-206 nm) with enhanced mucoadhesive properties for transmucosal drug delivery were achieved [123].
3.2. Gel systems
Cross-linked, 3D hydrophilic polymeric networks or hydrogels are potential candidates in tissue engineering, drug delivery, and for implant materials [125-127]. Interest in hydrogels arises from their facile fabrication, potential for injectability for noninvasive delivery, and useful interactions with biological materials. Properties of hydrogels such as high swelling ratios, porosity and soft consistency render them similar to natural living tissue, and thus good candidates for biomedical applications [128]. While naturally derived biomaterials can produce inconsistent or unwanted biological responses, the use of bioengineered proteins allows for tuning of mechanical and stimuli responsive properties, along with high purity and consistent molecular weight, avoiding negative outcomes. The crosslinking of hydrogels ensures these scaffolds are self-supporting and provide similar properties as the extracellular matrix (ECM) environment to support cell adhesion and gene expression. Hydrogels that are chemically crosslinked offer more mechanically robust and materials. Further, physically crosslinked hydrogels provide stimuli-responsive materials sensitive to environmental changes, including temperature, pH, and ionic strength.
ELP hydrogels also display mechanical properties similar to natural elastin, thus useful for tissue engineering. Moreover, utilizing ELPs for the formation of hydrogels provides thermo-responsive materials with tunable drug release due to the changes in structure by cycling the temperature above and below the Tt of the hydrogel. For example, reversible chemically crosslinked ELP hydrogels were obtained by introducing cysteine residues in the sequence and adding oxidative agents (e.g. ,hydrogen peroxide) to initiate disulfide crosslinking [129]. Adjusting the amount of cysteine provided control over the structure and thermal properties of the gels by altering crosslinking density and the Tt. ELP hydrogels were also prepared using ultrasonication to induce physical crosslinking without the use of chemicals, and the release of doxycycline at two different temperatures was demonstrated [130]. A higher release could be observed at the higher temperature (37 °C) compared to the lower temperature (25 °C) due to the change in porosity of the scaffold.
SELPs are capable of transitioning from aqueous solution to a physically crosslinked hydrogel using increased temperature based on the ratio of silk to elastin. While some SELPs are liquid at room temperature, they can form into hydrogels at body temperature. The effect of shear stress on SELPs demonstrated that more robust gel networks formed when compared to those not subjected to shear stress [131]. The shear stress created disruptions in secondary and tertiary structure from decreased intramolecular interactions and favoring more intermolecular bonds, hence to a stronger scaffold [132]. SELP hydrogels sensitive to MMPs for enhanced degradation in tumor environments were also developed [103]. Degradation rate was dictated by the location of the MMP-responsive sequence, thus useful for localized gene delivery. Mild oxidative conditions can also be used for the formation of chemically crosslinked SELP hydrogels with cysteine residues in the elastin blocks and disulfide crosslinking in the presence of hydrogen peroxide (Figure 2B). The release kinetics of those gels were tuned by the addition of a reducing agent (dithiothreitol) [124].
3.2. Solid formats
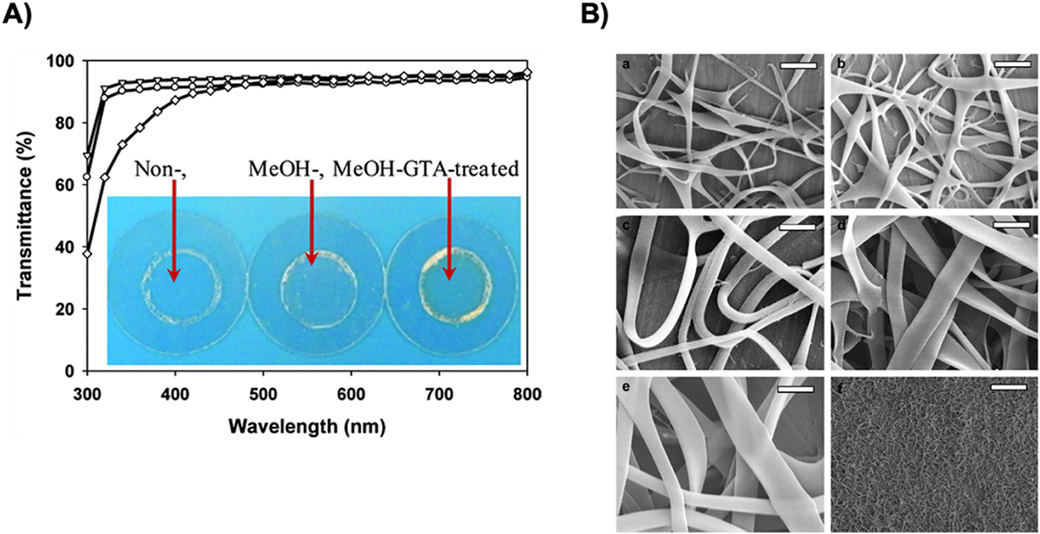
Solid carrier systems including films, wafers, reservoirs, foams and microneedles have been used for local and transdermal delivery due to the ease of modifications in terms of release kinetics, mechanical strength and size [18]. While bioengineered proteins are suitable starting materials for the fabrication of modular, solid delivery systems, limited use of protein-based materials has been developed to date. Thin films can be used on implanted devices to modify surface properties and facilitate integration with living tissues. For example, ELPs thin films containing sRGD were pH and thermo-responsive to provide tunable surface properties like wettability and topography [99]. Thin films are also ideal for optical applications for sustained drug delivery and bioavailability [133]. Chemically crosslinked films using glutaraldehyde were initially developed, but the transmittance of visible light was only 77%, while 95% was achieved with methanol induced physical crosslinking (Figure 3A) [134]. These SELP-based thin films were also used for the delivery of ciprofloxacin related to crystallization and kinetic release profiles. The films treated with methanol had slower release compared to ethanol due to the increased physical crosslinking density and enhanced stability [133].
Fig. 3.
A) Transmittance of non- (▽), MeOH- (○), and MeOH-GTA-treated (◊)SELP-47 Kdry films of 30 μm thickness. Measurements were done in triplicate. Insert: photo images of SELP- 47 K films cast on coverslips [131]., B) FESEM images of nanoribbons obtained by electrospinning from aqueous solution at various concentrations of SELP47K 6% (a), 9% (b), 12% (c), 15%(d), 18% (e) (w/w) and low magnification image at 15% (f), other electrospinning conditions were kept at a constant applied voltage (20 KV), collecting distance (15 cm) and flow rate (0.1 mL/h). (Scale bar – a–e: 1 μm, f: 25 μm) [134]. Figures were reproduced with permission from the cited articles.
Electrospun fibers are attractive for biomedical applications as they have similar morphological features as the ECM. The fibers can be used in wound dressings, as antibacterial materials and for drug delivery [135]. Using bioengineered proteins allows aqueous processing and simplifies the manufacturing while diminishing possible toxicity from residual solvent [136]. SELP-based fibers in aqueous solution without the addition of any surface modifying agents resulted in the formation of ribbon-like morphologies with self-standing and non-woven fiber meshes (Figure 3B) [137]. The diameter of the fibers ranged from 25 nm to 1.8 cm by varying the concentration of the SELP solution prior to electrospinning.
4. Biomedical Applications.
DDS that can improve the therapeutic index for a drug will ultimately lead to better patient outcomes. Recombinant proteins provide an excellent option for the development of DDS due to their precise molecular structure, tunability, and the range of physicochemical properties. SLPs [34,82,84,87,88,138,139], ELPs [140-146], and SELPs [51,147-152] have been utilized as drug carriers in many successful applications. The ability to finely tailor the protein structure and chemical composition makes them amenable for a variety of therapeutic options including small molecule drugs [153-157], biologics [158-162], and gene therapy systems [32,86,163-165].
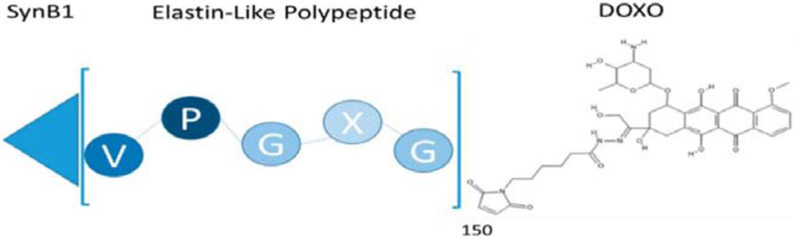
ELPs were used for treatment of glioblastomas, an especially aggressive form of cancer [101]. Despite numerous efforts to develop DDS targeted to this cancer, patient outcomes remain dire. ELP systems that killed glioblastoma cells selectively and effectively were developed using temperature-responsive properties for the aggregation and accumulation of the DDS in tumor cells at a specific temperature above physiological condition. Additionally, a cell penetrating peptide (CPP) was incorporated into the ELP to facilitate efficient uptake of the DDS into the cell. Finally, an acid sensitive linker was used to conjugate doxorubicin (Dox) to the carrier (Figure 4). This permits selective release of Dox once the DDS enters tumor cells and is exposed to decreased pH. The CPP was integrated into the ELP carrier via genetic fusion, removing the need for further functionalization or chemical conjugation. Additionally, the incorporation of the acid sensitive linker and the drug molecule was achieved in a highly selective and predictable manner by thiol-maleimide coupling to three cysteine residues on the ELP. This precise stoichiometric control enables a reliable and quantifiable measure of drug conjugation. A composite material formed from hyaluronic acid (HA) and dendritic ELP was also shown to release drug in a controlled fashion [16]. In this example, lysine terminated dendritic ELPs formed hydrogels via HA crosslinking using EDC coupling. The selective crosslinking sites provided by the ELPs allowed for control over crosslink density and enabled the controlled uptake and release of the model drug system dependent on hydrogel composition. SELP drug carriers were also designed using a block copolymer system that facilitated efficient micelle formation upon addition of a hydrophobic drug, while simultaneously enabling enhanced loading of the drug into the micelle core [166]. Using recombinantly produced constructs, varying SELP ratios were efficiently tested to determine the optimal composition for drug uptake and controlled micelle formation. Doxorubicin was used to trigger SELP micelle formation, and effective cell uptake and apoptosis was observed in HeLa cells indicating a promising system for tumor treatment and therapeutic delivery. Each of these examples demonstrates the advantages of recombinant polypeptide structures for drug carrier development.
Figure 4.
Schematic of ELP construction. An ELP of 60 kDa was fused to a cell penetrating peptide (SynB1) and chemically coupled to doxorubicin (DOXO) [101]. Figure is available for reproduction through MDPI open access policy.
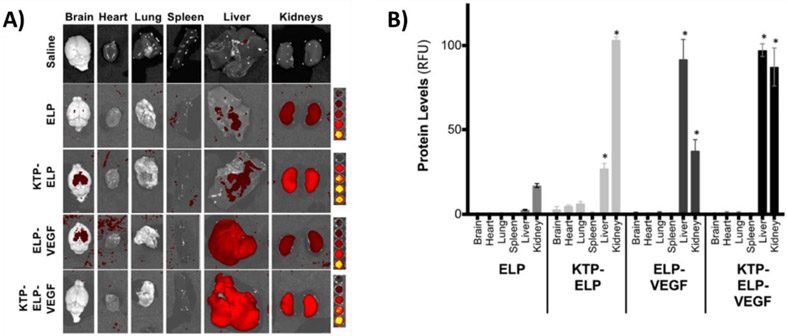
Proteins and peptide therapeutics often prove challenging to deliver due to their size and stability, complex structures, and susceptibility to enzymatic degradation. However, several examples of recombinant protein-based systems have been presented showing successful delivery of key protein therapeutics. For example, protein fusions for delivery of humanin, a crucial peptide required in the protection of human retinal pigment (RPE) cells in diseases such as macular degeneration, was demonstrated [104]. A temperature responsive ELP was fused to humanin to generate a stable DDS that showed binding to RPE cells under physiological conditions and demonstrated protection against oxidative stress that otherwise leads to apoptosis. In a similar fashion, a SELP fusion system was developed for the delivery of vascular endothelial growth factor (VEGF) a widely employed treatment of kidney disease [105]. To further functionalize the drug delivery system, a kidney targeting peptide was added, creating a chimeric protein to improve uptake in kidney tissue and reduce unwanted tissue accumulation. The DDS showed increased localization to the kidneys and decreased off target tissue deposition compared to the ELP construct without the targeting peptide (Figure 5A-B). This system successfully maintained the therapeutic efficacy of VEGF while facilitating renal tissue deposition. An ELP fusion delivery system was also generated for bone regeneration applications [106]. Bone morphogenic protein 2 (BMP2) was genetically incorporated into an ELP sequence resulting in a stable construct that retained the therapeutic efficacy of BMP2, as well as the temperature responsive features of the ELP. Testing of this ELP system in a mesenchymal stem cell model resulted in osteogenic differentiation, indicating the potential for use in bone healing. Nanoworm complexes consisting of an ELP domain functionalized with a single chain variable fragment (scFv) of the antibody therapeutic Rituximab were developed [109]. The use of the ELP fusion enabled an increase of therapeutic efficacy compared to the antibody alone due to the multivalent nanostructures which bind efficiently to CD20 receptors on two different B-cell lymphoma cell lines and induce apoptosis. In vivo experiments also showed an increase in efficacy of the protein polymer hybrid structures compared to the antibody drug alone. The utility of recombinant engineering is seen in this method, as the antibody structure was efficiently incorporated into the nanoparticle system without the need for typical bioconjugation strategies requiring additional chemistry and purification steps.
Figure 5.
Biodistribution analysis from mice following intravenous injection of ELP-VEGF constructs. A) Representative images from each treatment group showing uptake in each tissue. B) Quantified distribution via mean fluorescence intensity [105]. Figure is available for reproduction through MDPI open access policy.
Another rapidly evolving area of therapeutic development is gene therapy. Recombinant protein-base DDSs provide a novel approach to generate non-viral vectors for the delivery of genetic material. One such example can be seen in a nanoparticle delivery system developed with low immunogenicity to enable prolonged circulation and ultimately improve tissue uptake and therapeutic efficacy of plasmid DNA [167]. ELP constructs functionalized with a DNA condensing domain (RH3), which facilitates efficient packaging of plasmid molecules with the ELP chains, were applied to fabricated nanosized particles. When compared to pegylated delivery methods, the ELP nanoparticles showed significantly decreased immune responses and increased efficiency of plasmid delivery. This example highlights the biocompatibility of protein-based DDS, as well as the facile functionalization afforded by genetic engineering. In an elegant design of SELPs modified with matrix metalloproteinase (MMP) responsive sequences, the controlled delivery of viral particles for cancer therapy was validated [103]. Through addition of MMP cleavable domains along the protein backbone, degradation of the SELPs could be tightly controlled. Tunable degradation rates dependent on location of the MMP sites were demonstrated, and this feature was further illustrated in in vivo mouse models of head and neck squamous cell carcinoma where the SELP DDS significantly improved delivery of adenoviral vectors (Figure 6A-B). The usefulness of genetically engineered non-viral vectors was further established in a design of elastin-like recombiners (ELRs) for gene delivery [164]. ELR fusions incorporating penatratin and LAEL fusogenic peptide sequences (i.e. domains of Leu, Ala, Glu, and Leu) to facilitate cell uptake of the DDS and increased transfection levels of plasmid DNA were created using ELRs with cationic backbones for efficient complexation of plasmid DNA resulting in stable polyplexes. This was accomplished through modification of the elastin variable position to contain lysine residues. Peptide sequences were added using recursive ligation, which allows for control over the resulting polymer structure, size, and charge. In C6 rat glioma cells the constructions with the LAEL motif showed the highest transfection efficiency. Further application of SELPs as DNA carriers was also exemplified in SELP hydrogel systems [168]. Hydrogels fabricated from SELPs, generated with tunable degradation properties and temperature-responsive gelation at 37°C, displayed precise spatial and temporal control over adenovirus delivery indicating potential utility in head and neck cancer therapies. The SELP hydrogel demonstrated a 10-fold increase in gene expression upon intratumoral injection compared to the viral injection alone.
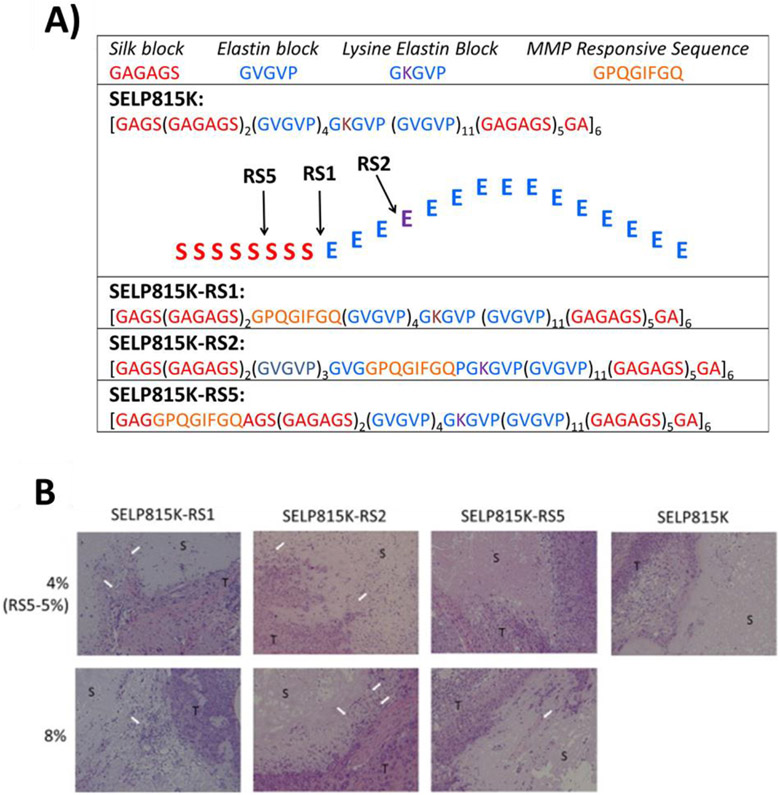
Figure 6.
In vivo evaluation of matrix metalloproteinase responsive SELPs. A) Amino acid sequences of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 showing insertion sites of matrix metalloproteinase responsive sequences for SELP815K monomer B) Post necropsy histological evaluation of SELP constructs by hematoxylin and eosin staining after 50 days of implantation. White arrows indicate vascular infiltration of the hydrogels. S: SELP hydrogel, T: tumor tissue. All images captured at 100 × magnification using a light microscope [103]. Figure reproduced with permission from Elsevier.
5. Conclusions
The need for new and refined therapeutics has led to the development of polymeric drug delivery systems to improve the therapeutic outcomes and diminish unwanted side effects. While much research has been focused on synthetic polymers, their limitations in terms of chemistry, tunable control of structure and mechanics, limited aqueous processing, bulk hydrolysis and production of acidic byproducts has drawn more attention toward natural polymers. Naturals proteins have been engineered to provide tunable features rendering them stimuli-responsive or allowing for the fabrication of disease-targeted DDS. ELP and SLP recombinant protein systems and their implementation in the field of drug delivery, as reviewed, are summarized in Table 3. Such bioengineered protein polymer systems offer precise tailoring and control that is useful in envisioning future needs with specialized or selective DDS. In particular, compatibility with complex proteins, peptides, and assemblies, tunable features related to targeting, degradation and stability, biocompatibility and safe degradation products, are some of the key values of these systems that can be achieved while maintaining the mechanical value of elasticity of natural elastin and robustness of natural silk. When combined with options to directly encode target sequences, therapeutics and related control points during design and fabrication of DDS, new avenues are realized for bioengineering protein polymers in the field of DDS. The high customization level and attractive physicochemical properties of such engineered proteins make them suitable candidates for drug and gene delivery. Despite the numerous advantages of engineered proteins, challenges remain with clinical translation. No ELP or SLP carrier has yet received clinical approval for drug delivery. Nevertheless, various ELP fusions have successfully completed phase I and II clinical trials: VIP-ELP (Vasomera™) for the treatment of pulmonary arterial hypertension, cardiomyopathies and cystic fibrosis completed phase I; and GLP1-ELP (Glymera™), for the treatment of type II diabetes completed phase IIB. These clinical trials demonstrate that ELPs are tolerated in humans and do not induce a significant immune response [169-171]. With the advent of personalized therapeutic modalities, recombinant protein systems, such as those presented in this review, should continue to grow in importance and implementation in the field, providing an advanced platform for modulating DDS to optimize patient outcomes.
Table 3.
Example of SLP and ELP sequences, structures, formulations, and applications.
| Structure | Sequence Modifications | Material Format | Applications | Reference |
|---|---|---|---|---|
| Silkworm silk | ||||
| GAGAGS | Elastin GXGVP X is any amino acid except Pro |
Nanoparticles | Drug delivery (doxorubicin) | [140,161,162] |
| Elastin GXGVP X is Valine |
Hydrogels | Gene delivery | [160] | |
| Spider silk | Poly-lys | Particles (pDNA complexes) | Gene delivery | [20,73] |
| ECM peptides (RGD) Cell penetrating peptides |
Particles (pDNA complexes) | Gene delivery | [77] | |
| Tumor-homing peptides | Particles | Gene delivery | [78,79] | |
| Nuclear localization sequence and translocation motifs | Nanoparticles | Delivery of Cy-5-labeled pDNA | [82] | |
| Elastin | ||||
| GXGVP | X is Valine | Nanoparticles | Delivery of bone morphogenic proteins | [108] |
| X is Valine | Hydrogels | Delivery of antibiotics and proteins | [120] | |
| X is any amino acid except proline | Nanoparticles, microgels | Delivery of Doxorubicin, rhodamine | [109,111] | |
| X is Lysine | Microspheres | Delivery of BSA and prednisone acetate | [112] | |
| X is polyaspartic acid | Particles | Delivery of hydrophobic fluorescent molecules | [95] | |
| Bioengineered with polyaspartic acid | Nanoparticles | Delivery of Paclitaxel | [110] | |
| X is Valine Bioengineered with VEGF and kidney-targeting peptide |
Soluble protein | Delivery of VEGF in kidney | [98] | |
| X is Alanine | Nanoparticles | Delivery of antibody Rituximab | [100] |
Acknowledgements
The authors thank Dr. Francisco Carrillo-Salinas for his contribution in the graphical abstract and Figure 1.
Funding: This work was supported by the National Institutes of Health [P41EB027062, R01NS094218, U01EB014976], the Air Force Office of Scientific Research (FA9550-17-1-0333) and the Army Research Office (W911NF-17-1-0384). Special thanks to Ralph Parchment, Ralph regarding helpful discussions in translational aspects of the proteins with respect to the NIH NCI (contract HHSN261200800001E).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Frandsen JL, Ghandehari H, Recombinant protein-based polymers for advanced drug delivery, Chem. Soc. Rev 41 (2012) 2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- [2].Xiong M-H, Bao Y, Yang X-Z, Zhu Y-H, Wang J, Delivery of antibiotics with polymeric particles, Adv. Drug Deliv. Rev 78 (2014) 63–76. doi: 10.1016/j.addr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [3].Sepantafar M, Maheronnaghsh R, Mohammadi H, Radmanesh F, Hasani-Sadrabadi MM, Ebrahimi M, Baharvand H, Engineered Hydrogels in Cancer Therapy and Diagnosis., Trends Biotechnol. 35 (2017) 1074–1087. doi: 10.1016/j.tibtech.2017.06.015. [DOI] [PubMed] [Google Scholar]
- [4].Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP, Basic concepts and recent advances in nanogels as carriers for medical applications, Drug Deliv. 24 (2017) 539–557. doi: 10.1080/10717544.2016.1276232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li J, Mooney DJ, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater 1 (2016) 16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stevanović M, Polymeric micro- and nanoparticles for controlled and targeted drug delivery, Nanostructures Drug Deliv. (2017) 355–378. doi: 10.1016/B978-0-323-46143-6.00011-7. [DOI] [Google Scholar]
- [7].Yucel T, Lovett ML, Kaplan DL, Silk-based biomaterials for sustained drug delivery, J. Control. Release 190 (2014) 381–397. doi: 10.1016/j.jconrel.2014.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yavuz B, Chambre L, Kaplan DL, Extended release formulations using silk proteins for controlled delivery of therapeutics, Expert Opin. Drug Deliv 16 (2019) 1–16. doi: 10.1080/17425247.2019.1635116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’souza AA, Shegokar R, Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications, Expert Opin. Drug Deliv 13 (2016) 1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- [10].Mohammadi-Samani S, Taghipour B, PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches, Pharm. Dev. Technol 20 (2015) 385–393. doi: 10.3109/10837450.2014.882940. [DOI] [PubMed] [Google Scholar]
- [11].Duncan R, The dawning era of polymer therapeutics, Nat. Rev. Drug Discov 2 (2003) 347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- [12].Neuse EW, Synthetic polymers as drug-delivery vehicles in medicine., Met. Based. Drugs 2008 (2008) 469531. doi: 10.1155/2008/469531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wagner V, Dullaart A, Bock A-K, Zweck A, The emerging nanomedicine landscape, Nat. Biotechnol 24 (2006) 1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- [14].Assal Y, Mizuguchi Y, Mie M, Kobatake E, Growth Factor Tethering to Protein Nanoparticles via Coiled-Coil Formation for Targeted Drug Delivery, Bioconjug. Chem 26 (2015) 1672–1677. doi: 10.1021/acs.bioconjchem.5b00266. [DOI] [PubMed] [Google Scholar]
- [15].Lee Y, Thompson DH, Stimuli-responsive liposomes for drug delivery, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 9 (2017) e1450. doi: 10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shmidov Y, Zhou M, Yosefi G, Bitton R, Matson JB, Hydrogels composed of hyaluronic acid and dendritic ELPs: Hierarchical structure and physical properties, Soft Matter. 15 (2019) 917–925. doi: 10.1039/c8sm02450b. [DOI] [PubMed] [Google Scholar]
- [17].Nair LS, Laurencin CT, Biodegradable polymers as biomaterials, Prog. Polym. Sci 32 (2007) 762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- [18].Jao D, Xue Y, Medina J, Hu X, Protein-based drug-delivery materials, Materials (Basel). 10(2017). doi: 10.3390/ma10050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aigner TB, DeSimone E, Scheibel T, Biomedical Applications of Recombinant Silk-Based Materials, Adv. Mater 30 (2018) 1704636. doi: 10.1002/adma.201704636. [DOI] [PubMed] [Google Scholar]
- [20].Numata K, Kaplan DL, Silk-based delivery systems of bioactive molecules, Adv. Drug Deliv. Rev 62 (2010) 1497–1508. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang W, Rollett A, Kaplan DL, Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics, Expert Opin. Drug Deliv 12 (2015) 779–791. doi: 10.1517/17425247.2015.989830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Price R, Poursaid A, Ghandehari H, Controlled release from recombinant polymers, J. Control. Release 190 (2014) 304–313. doi: 10.1016/j.jconrel.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gorbet MB, Sefton MV, Endotoxin: The uninvited guest, Biomaterials. 26 (2005) 6811–6817. doi: 10.1016/j.biomaterials.2005.04.063. [DOI] [PubMed] [Google Scholar]
- [24].Beenken-Rothkopf LN, Karfeld-Sulzer LS, Zhang X, Kissler H, Michie SA, Kaufman DB, Fontaine MJ, Barron AE, Protein polymer hydrogels: Effects of endotoxin on biocompatibility, (n.d.). doi: 10.1177/0885328212454555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lieder R, Petersen PH, Sigurjónsson ÓE, Endotoxins—the Invisible Companion in Biomaterials Research, Tissue Eng. Part B Rev 19 (2013) 391–402. doi: 10.1089/ten.teb.2012.0636. [DOI] [PubMed] [Google Scholar]
- [26].Schillberg S, Raven N, Spiegel H, Rasche S, Buntru M, Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins, Front. Plant Sci 10 (2019) 720. doi: 10.3389/fpls.2019.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cardoso VM, Campani G, Santos MP, Silva GG, Pires MC, Gonçalves VM, de C. Giordano R, Sargo CR, Horta ACL, Zangirolami TC, Cost analysis based on bioreactor cultivation conditions: Production of a soluble recombinant protein using Escherichia coli BL21(DE3), Biotechnol. Reports 26 (2020) e00441. doi: 10.1016/j.btre.2020.e00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tripathi NK, Shrivastava A, Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development, Front. Bioeng. Biotechnol 7 (2019) 420. doi: 10.3389/fbioe.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vepari C, Kaplan DL, Silk as a biomaterial, Prog. Polym. Sci 32 (2007) 991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Katashima T, Malay AD, Numata K, Chemical modification and biosynthesis of silk-like polymers, Curr. Opin. Chem. Eng 24 (2019) 61–68. doi: 10.1016/j.coche.2019.01.005. [DOI] [Google Scholar]
- [31].Shi P, Gustafson JA, Andrew MacKay J, Genetically engineered nanocarriers for drug delivery, Int. J. Nanomedicine 9 (2014) 1617–1626. doi: 10.2147/IJN.S53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Numata K, Kaplan DL, Silk-based gene carriers with cell membrane destabilizing peptides, Biomacromolecules. 11 (2010)3189–3195. doi: 10.1021/bm101055m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yanagisawa S, Zhu Z, Kobayashi I, Uchino K, Tamada Y, Tamura T, Asakura T, Improving cell-adhesive properties of recombinanant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms, Biomacromolecules. 8 (2007) 3487–3492. doi: 10.1021/bm700646f. [DOI] [PubMed] [Google Scholar]
- [34].Tanaka C, Asakura T, Synthesis and characterization of cell-adhesive silk-like proteins constructed from the sequences of anaphe silk fibroin and fibronectin, in: Biomacromolecules, American Chemical Society, 2009: pp. 923–928. doi: 10.1021/bm801439t. [DOI] [PubMed] [Google Scholar]
- [35].Huang J, Wong Po Foo C, Kaplan DL, Biosynthesis and applications of silk-like and collagen-like proteins, Polym. Rev 47 (2007) 29–62. doi: 10.1080/15583720601109560. [DOI] [Google Scholar]
- [36].Kambe Y, Yamamoto K, Kojima K, Tamada Y, Tomita N, Effects of RGDS sequence genetically interfused in the silk fibroin light chain protein on chondrocyte adhesion and cartilage synthesis, Biomaterials. 31 (2010) 7503–7511. doi: 10.1016/j.biomaterials.2010.06.045. [DOI] [PubMed] [Google Scholar]
- [37].Nagano A, Tanioka Y, Sakurai N, Sezutsu H, Kuboyama N, Kiba H, Tanimoto Y, Nishiyama N, Asakura T, Regeneration of the femoral epicondyle on calcium-binding silk scaffolds developed using transgenic silk fibroin produced by transgenic silkworm, Acta Biomater. 7 (2011) 1192–1201. doi: 10.1016/j.actbio.2010.10.032. [DOI] [PubMed] [Google Scholar]
- [38].Zhu Z, Kikuchi Y, Kojima K, Tamura T, Kuwabara N, Nakamura T, Asakura T, Mechanical properties of regenerated Bombyx mori silk fibers and recombinant silk fibers produced by transgenic silkworms., J. Biomater. Sci. Polym. Ed 21 (2010) 395–411. doi: 10.1163/156856209X423126. [DOI] [PubMed] [Google Scholar]
- [39].Aamodt JM, Grainger DW, Extracellular matrix-based biomaterial scaffolds and the host response, Biomaterials. 86 (2016) 68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Asakura T, Suzuki Y, Nagano A, Knight D, Kamiya M, Demura M, Synthesis and characterization of water-soluble silk peptides and recombinant silk protein containing polyalanine, the integrin binding site, and two glutamic acids at each terminal site as a possible candidate for use in bone repair materials, Biomacromolecules. 14 (2013) 3731–3741. doi: 10.1021/bm401118m. [DOI] [PubMed] [Google Scholar]
- [41].Anderson JP, Cappello J, Martin DC, Morphology and primary crystal structure of a silk-like protein polymer synthesized by genetically engineered Escherichia coli bacteria, Biopolymers. 34 (1994) 1049–1058. doi: 10.1002/bip.360340808. [DOI] [PubMed] [Google Scholar]
- [42].Yang M, Tanaka C, Yamauchi K, Ohgo K, Kurokawa M, Asakura T, Silklike materials constructed from sequences of Bombyx mori silk fibroin, fibronectin, and elastin., J. Biomed. Mater. Res. A 84 (2008) 353–63. doi: 10.1002/jbm.a.31348. [DOI] [PubMed] [Google Scholar]
- [43].Barroca M, Rodrigues P, Sobral R, Costa MMR, Chaves SR, MacHado R, Casal M, Collins T, Antibiotic free selection for the high level biosynthesis of a silk-elastin-like protein, Sci. Rep 6 (2016). doi: 10.1038/srep39329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Collins T, Azevedo-Silva J, da Costa A, Branca F, Machado R, Casal M, Batch production of a silk-elastin-like protein in E. coli BL21(DE3): Key parameters for optimisation, Microb. Cell Fact 12 (2013). doi: 10.1186/1475-2859-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Collins T, Barroca M, Branca F, Padrão J, Machado R, Casal M, High level biosynthesis of a silk-elastin-like protein in E. coli, Biomacromolecules. 15 (2014) 2701–2708. doi: 10.1021/bm5005564. [DOI] [PubMed] [Google Scholar]
- [46].Megeed Z, Cappello J, Ghandehari H, Genetically engineered silk-elastinlike protein polymers for controlled drug delivery, Adv. Drug Deliv. Rev 54 (2002) 1075–1091. doi: 10.1016/S0169-409X(02)00063-7. [DOI] [PubMed] [Google Scholar]
- [47].Xia X-X, Xu Q, Hu X, Qin G, Kaplan DL, Tunable self-assembly of genetically engineered silk--elastin-like protein polymers., Biomacromolecules. 12 (2011) 3844–50. doi: 10.1021/bm201165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, Safavy A, Temperature of Polypeptide Inverse Temperature Transition Depends on Mean Residue Hydrophobicity, J. Am. Chem. Soc 113 (1991)4346–4348. doi: 10.1021/ja00011a057. [DOI] [Google Scholar]
- [49].Aghaei-Ghareh-Bolagh B, Mithieux SM, Weiss AS, Elastic proteins and elastomeric protein alloys, Curr. Opin. Biotechnol 39 (2016) 56–60. doi: 10.1016/j.copbio.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xu L, Tremblay ML, Orrell KE, Leclerc J, Meng Q, Liu XQ, Rainey JK, Nanoparticle self-assembly by a highly stable recombinant spider wrapping silk protein subunit, FEBS Lett. 587 (2013) 3273–3280. doi: 10.1016/j.febslet.2013.08.024. [DOI] [PubMed] [Google Scholar]
- [51].Zhou M-L, Qian Z-G, Chen L, Kaplan DL, Xia X-X, Rationally Designed Redox-Sensitive Protein Hydrogels with Tunable Mechanical Properties., Biomacromolecules. 17 (2016) 3508–3515. doi: 10.1021/acs.biomac.6b00973. [DOI] [PubMed] [Google Scholar]
- [52].Xu J, Dong Q, Yu Y, Niu B, Ji D, Li M, Huang Y, Chen X, Tan A, Mass spider silk production through targeted gene replacement in Bombyx mori, Proc. Natl. Acad. Sci. U. S. A 115 (2018) 8757–8762. doi: 10.1073/pnas.1806805115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang Q, Xia X, Huang W, Lin Y, Xu Q, Kaplan DL, High throughput screening of dynamic silk-elastin-like protein biomaterials, Adv. Funct. Mater 24 (2014) 4303–4310. doi: 10.1002/adfm.201304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nagarsekar A, Crissman J, Crissman M, Ferrari F, Cappello J, Ghandehari H, Genetic engineering of stimuli-sensitive silkelastin-like protein block copolymers, Biomacromolecules. 4 (2003) 602–607. doi: 10.102l/bm0201082. [DOI] [PubMed] [Google Scholar]
- [55].Sutherland TD, Young JH, Weisman S, Hayashi CY, Merritt DJ, Insect silk: one name, many materials., Annu. Rev. Entomol 55 (2010) 171–88. doi: 10.1146/annurev-ento-112408-085401. [DOI] [PubMed] [Google Scholar]
- [56].Humenik M, Pawar K, Scheibel T, Nanostructured, Self-Assembled Spider Silk Materials for Biomedical Applications, in: Adv. Exp. Med. Biol, Springer, 2019: pp. 187–221. doi: 10.1007/978-981-13-9791-2_6. [DOI] [PubMed] [Google Scholar]
- [57].Widhe M, Johansson J, Hedhammar M, Rising A, Current progress and limitations of spider silk for biomedical applications, Biopolymers. 97 (2012) 468–478. doi: 10.1002/bip.21715. [DOI] [PubMed] [Google Scholar]
- [58].Tokareva O, Jacobsen M, Buehler M, Wong J, Kaplan DL, Structure-function-property-design interplay in biopolymers: Spider silk, Acta Biomater. 10 (2014) 1612–1626. doi: 10.1016/j.actbio.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Beckwitt R, Arcidiacono S, Stote R, Evolution of repetitive proteins: spider silks from Nephila clavipes (Tetragnathidae) and Araneus bicentenarius (Araneidae)., Insect Biochem. Mol. Biol 28 (1998) 121–30. doi: 10.1016/s0965-1748(97)00083-0. [DOI] [PubMed] [Google Scholar]
- [60].Rising A, Widhe M, Johansson J, Hedhammar M, Spider silk proteins: Recent advances in recombinant production, structure-function relationships and biomedical applications, Cell. Mol. Life Sci 68 (2011) 169–184. doi: 10.1007/s00018-010-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ayoub NA, Garb JE, Tinghitella RM, Collin MA, Hayashi CY, Blueprint for a High-Performance Biomaterial: Full-Length Spider Dragline Silk Genes, PLoS One. 2 (2007). doi: 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jastrzebska K, Kucharczyk K, Florczak A, Dondajewska E, Mackiewicz A, Dams-Kozlowska H, Silk as an innovative biomaterial for cancer therapy, Reports Pract. Oncol. Radiother 20 (2015) 87–98. doi: 10.1016/j.rpor.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hofer M, Winter G, Myschik J, Recombinant spider silk particles for controlled delivery of protein drugs, Biomaterials. 33 (2012) 1554–1562. doi: 10.1016/j.biomaterials.2011.10.053. [DOI] [PubMed] [Google Scholar]
- [64].Lammel A, Schwab M, Hofer M, Winter G, Scheibel T, Recombinant spider silk particles as drug delivery vehicles, Biomaterials. 32 (2011) 2233–2240. doi: 10.1016/j.biomaterials.2010.11.060. [DOI] [PubMed] [Google Scholar]
- [65].Florczak A, Jastrzebska K, Mackiewicz A, Dams-Kozlowska H, Blending two bioengineered spider silks to develop cancer targeting spheres, J. Mater. Chem. B 5 (2017) 3000–3011. doi: 10.1039/c7tb00233e. [DOI] [PubMed] [Google Scholar]
- [66].Chen J, Hu J, Zuo P, Su X, Liu Z, Yang M, Tailor-made spider-eggcase-silk spheres for efficient lysosomal drug delivery, RSC Adv. 8 (2018) 9394–9401. doi: 10.1039/c8ra00232k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schacht K, Scheibel T, Processing of recombinant spider silk proteins into tailor-made materials for biomaterials applications, Curr. Opin. Biotechnol 29 (2014) 62–69. doi: 10.1016/j.copbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- [68].Werten MWT, Eggink G, Cohen Stuart MA, de Wolf FA, Production of protein-based polymers in Pichia pastoris, Biotechnol. Adv 37 (2019) 642–666. doi: 10.1016/j.biotechadv.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sidoruk KV, Davydova LI, Kozlov DG, Gubaidullin DG, Glazunov AV, Bogush VG, Debabov VG, Fermentation optimization of a Saccharomyces cerevisiae strain producing 1F9 recombinant spidroin, Appl. Biochem. Microbiol 51 (2015) 766–773. doi: 10.1134/S0003683815070066. [DOI] [Google Scholar]
- [70].Lazaris A, Arcidiacono S, Huang Y, Zhou JF, Duguay F, Chretien N, Welsh EA, Soares JW, Karatzas CN, Spider silk fibers spun from soluble recombinant silk produced in mammalian cells, Science (80-. ). 295 (2002) 472–476. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- [71].Williams D, Sows’ Ears, Silk Purses and Goats’ Milk: New Production Methods and Medical Applications for Silk - PubMed, Med Device Technol. 14 (2003) 9–11. https://pubmed.ncbi.nlm.nih.gov/12852112/?from_single_result=silk+purses+and+goats’+milk%3A+new+production+methods+and+811+medical+application+for+silk. (accessed June 4, 2020). [PubMed] [Google Scholar]
- [72].Scheller J, Gührs KH, Grosse F, Conrad U, Production of spider silk proteins in tobacco and potato, Nat. Biotechnol 19 (2001) 573–577. doi: 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- [73].Wen H, Lan X, Zhang Y, Zhao T, Wang Y, Kajiura Z, Nakagaki M, Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons., Mol. Biol. Rep 37 (2010) 1815–21. doi: 10.1007/s11033-009-9615-2. [DOI] [PubMed] [Google Scholar]
- [74].Xia XX, Qian ZG, Ki CS, Park YH, Kaplan DL, Lee SY, Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 14059–14063. doi: 10.1073/pnas.1003366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bowen CH, Dai B, Sargent CJ, Bai W, Ladiwala P, Feng H, Huang W, Kaplan DL, Galazka JM, Zhang F, Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk, Biomacromolecules. 19 (2018) 3853–3860. doi: 10.1021/acs.biomac.8b00980. [DOI] [PubMed] [Google Scholar]
- [76].Kucharczyk K, Weiss M, Jastrzebska K, Luczak M, Ptak A, Kozak M, Mackiewicz A, Dams-Kozlowska H, Bioengineering the spider silk sequence to modify its affinity for drugs, Int. J. Nanomedicine 13 (2018) 4247–4261. doi: 10.2147/IJN.S168081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Spieß K, Wohlrab S, Scheibel T, Structural characterization and functionalization of engineered spider silk films, Soft Matter. 6 (2010) 4168–4174. doi: 10.1039/b927267d. [DOI] [Google Scholar]
- [78].Doblhofer E, Scheibel T, Engineering of recombinant spider silk proteins allows defined uptake and release of substances, J. Pharm. Sci 104 (2015) 988–994. doi: 10.1002/jps.24300. [DOI] [PubMed] [Google Scholar]
- [79].Harvey D, Bardelang P, Goodacre SL, Cockayne A, Thomas NR, Antibiotic Spider Silk: Site-Specific Functionalization of Recombinant Spider SilkUsing “Click” Chemistry, Adv. Mater 29 (2017). doi: 10.1002/adma.201604245. [DOI] [PubMed] [Google Scholar]
- [80].Numata K, Subramanian B, Currie HA, Kaplan DL, Bioengineered silk protein-based gene delivery systems, Biomaterials. 30 (2009) 5775–5784. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kozlowska AK, Florczak A, Smialek M, Dondajewska E, Mackiewicz A, Kortylewski M, Dams-Kozlowska H, Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment, Acta Biomater. 59 (2017) 221–233. doi: 10.1016/j.actbio.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Elsner MB, Herold HM, Müller-Herrmann S, Bargel H, Scheibel T, Enhanced cellular uptake of engineered spider silk particles, Biomater. Sci 3 (2015) 543–551. doi: 10.1039/c4bm00401a. [DOI] [PubMed] [Google Scholar]
- [83].Schierling MB, Doblhofer E, Scheibel T, Cellular uptake of drug loaded spider silk particles, Biomater. Sci 4 (2016) 1515–1523. doi: 10.1039/c6bm00435k. [DOI] [PubMed] [Google Scholar]
- [84].Numata K, Hamasaki J, Subramanian B, Kaplan DL, Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs, J. Control. Release 146 (2010) 136–143. doi: 10.1016/j.jconrel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Numata K, Reagan MR, Goldstein RH, Rosenblatt M, Kaplan DL, Spider silk-based gene carriers for tumor cell-specific delivery, Bioconjug. Chem 22 (2011) 1605–1610. doi: 10.1021/bc200170u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Numata K, Mieszawska-Czajkowska AJ, Kvenvold LA, Kaplan DL, Silk-Based Nanocomplexes with Tumor-Homing Peptides for Tumor-Specific Gene Delivery, Macromol. Biosci 12 (2012) 75–82. doi: 10.1002/mabi.201100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Florczak A, Mackiewicz A, Dams-Kozlowska H, Functionalized spider silk spheres as drug carriers for targeted cancer therapy, Biomacromolecules. 15 (2014) 2971–2981. doi: 10.1021/bm500591p. [DOI] [PubMed] [Google Scholar]
- [88].Petrou G, Jansson R, Högqvist M, Erlandsson J, Wågberg L, Hedhammar M, Crouzier T, Genetically Engineered Mucoadhesive Spider Silk, Biomacromolecules. 19 (2018) 3268–3279. doi: 10.1021/acs.biomac.8b00578. [DOI] [PubMed] [Google Scholar]
- [89].Tokareva OS, Glettig DL, Abbott RD, Kaplan DL, Multifunctional spider silk polymers for gene delivery to human mesenchymal stem cells, J. Biomed. Mater. Res. Part B Appl. Biomater 103 (2015) 1390–1401. doi: 10.1002/jbm.b.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Urry DW, Trapane TL, Prasad KU, Phase-structure transitions of the elastin polypentapeptide–water system within the framework of composition–temperature studies, Biopolymers. 24 (1985) 2345–2356. doi: 10.1002/bip.360241212. [DOI] [PubMed] [Google Scholar]
- [91].Le DHT, Sugawara-Narutaki A, Elastin-like polypeptides as building motifs toward designing functional nanobiomaterials, Mol. Syst. Des. Eng 4 (2019) 545–565. doi: 10.1039/c9me00002j. [DOI] [Google Scholar]
- [92].Meyer DE, Chilkoti A, Quantification of the effects of chain length and concentration on the thermal behavior of elastin-like polypeptides, Biomacromolecules. 5 (2004) 846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- [93].Schipperus R, Eggink G, de Wolf FA, Secretion of elastin-like polypeptides with different transition temperatures by Pichia pastoris, Biotechnol. Prog 28 (2012) 242–247. doi: 10.1002/btpr.717. [DOI] [PubMed] [Google Scholar]
- [94].Kim W, Chaikof EL, Recombinant elastin-mimetic biomaterials: Emerging applications in medicine, Adv. Drug Deliv. Rev 62 (2010) 1468–1478. doi: 10.1016/j.addr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL, Elastin-mimetic protein polymers capable of physical and chemical crosslinking, Biomaterials. 30 (2009) 409–422. doi: 10.1016/j.biomaterials.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Nagapudi K, Brinkman WT, Leisen J, Thomas BS, Wright ER, Haller C, Wu X, Apkarian RP, Conticello VP, Chaikof EL, Protein-based thermoplastic elastomers, Macromolecules. 38 (2005) 345–354. doi: 10.1021/ma0491199. [DOI] [Google Scholar]
- [97].Urry DW, Urry KD, Szaflarski W, Nowicki M, Elastic-contractile model proteins: Physical chemistry, protein function and drug design and delivery, Adv. Drug Deliv. Rev 62 (2010) 1404–1455. doi: 10.1016/j.addr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- [98].Janib SM, Pastuszka MF, Aluri S, Folchman-Wagner Z, Hsueh PY, Shi P, Lin YA, Cui H, Mackay JA, A quantitative recipe for engineering protein polymer nanoparticles, Polym. Chem 5 (2014) 1614–1625. doi: 10.1039/c3py00537b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Costa RR, Custódio CA, Testera AM, Arias FJ, Rodríguez-Cabello JC, Alves NM, Mano JF, Stimuli-Responsive Thin Coatings Using Elastin-Like Polymers for Biomedical Applications, Adv. Funct. Mater 19 (2009) 3210–3218. doi: 10.1002/adfm.200900568. [DOI] [Google Scholar]
- [100].Massodi I, Bidwell GL, Raucher D, Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery, J. Control. Release 108 (2005) 396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- [101].Dragojevic S, Mackey R, Raucher D, Evaluation of elastin-like polypeptides for tumor targeted delivery of doxorubicin to glioblastoma, Molecules. 24 (2019). doi: 10.3390/molecules24183242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fujita Y, Mie M, Kobatake E, Construction of nanoscale protein particle using temperature-sensitive elastin-like peptide and polyaspartic acid chain, Biomaterials. 30 (2009) 3450–3457. doi: 10.1016/J.BIOMATERIALS.2009.03.012. [DOI] [PubMed] [Google Scholar]
- [103].Price R, Poursaid A, Cappello J, Ghandehari H, In vivo evaluation of matrix metalloproteinase responsive silk-elastinlike protein polymers for cancer gene therapy, J. Control. Release 213 (2015) 96–102. doi: 10.1016/j.jconrel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li Z, Sreekumar PG, Peddi S, Hinton DR, Kannan R, Mackay JA, Mackay JA, The humanin peptide mediates ELP nanoassembly and protects human retinal pigment epithelial cells from oxidative stress, Nanotechnology, Biol. Med 24 (2020) 102111. doi: 10.1016/j.nano.2019.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mahdi F, Chade AR, Bidwell GL, Utilizing a kidney-targeting peptide to improve renal deposition of a pro-angiogenic protein biopolymer, Pharmaceutics. 11 (2019). doi: 10.3390/pharmaceutics11100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McCarthy B, Yuan Y, Koria P, Elastin-like-polypeptide based fusion proteins for osteogenic factor delivery in bone healing, Biotechnol. Prog 32 (2016) 1029–1037. doi: 10.1002/btpr.2269. [DOI] [PubMed] [Google Scholar]
- [107].Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A, A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection, J. Control. Release 172 (2013) 144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].del Rio RL, Carlos L; Youngblood Bradley; Yeh Steve T; Georgopoulos Lynne; Arnold Sue; Wallery Jeff; Hamlin, Evaluation of Vasomera™, A Novel VPAC2-selective Vasoactive Intestinal Peptide Agonist, in Rats with Doxorubicin-Induced Cardiomyopathy: Evidence for Chronic Cardio-Protection, Circulation. (2012) 126:A18796. doi: 10.1161/circ.126.suppl_21.a18796 (accessed September 29, 2020). [DOI] [Google Scholar]
- [109].Aluri SR, Shi P, Gustafson JA, Wang W, Lin Y-A, Cui H, Liu S, Conti PS, Li Z, Hu P, Epstein AL, Mackay JA, A Hybrid ProteinAPolymer Nanoworm Potentiates Apoptosis Better than a Monoclonal Antibody, ACS Nano. 8 (2014) 2064–2076. doi: 10.1021/nn403973g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Whitesides GM, The “right” size in nanobiotechnology, Nat. Biotechnol 21 (2003) 1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- [111].Li Z, Tan S, Li S, Shen Q, Wang K, Cancer drug delivery in the nano era: An overview and perspectives, Oncol. Rep 38 (2017) 611–624. doi: 10.3892/or.2017.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Xin Y, Yin M, Zhao L, Meng F, Luo L, Recent progress on nanoparticle-based drug delivery systems for cancer therapy, Cancer Biol. Med 14 (2017) 228. doi: 10.20892/J.ISSN.2095-3941.2017.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Maeda H, Tsukigawa K, Fang J, A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy-Problems, Solutions, and Prospects, Microcirculation. 23 (2016) 173–182. doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- [114].Maeda H, The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting., Adv. Enzyme Regul. 41 (2001) 189–207. [DOI] [PubMed] [Google Scholar]
- [115].Farokhzad OC, Langer R, Impact of Nanotechnology on Drug Delivery, ACS Nano. 3 (2009) 16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- [116].Le DHT, Sugawara-Narutaki A, Elastin-like polypeptides as building motifs toward designing functional nanobiomaterials, Mol. Syst. Des. Eng 4 (2019) 545–565. doi: 10.1039/C9ME00002J. [DOI] [Google Scholar]
- [117].Bessa PC, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha AM, Rodríguez-Cabello JC, Redl H, van Griensven M, Reis RL, Casal M, Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs, J. Control. Release 142 (2010)312–318. doi: 10.1016/J.JCONREL.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [118].Wu Y, MacKay JA, McDaniel JR, Chilkoti A, Clark RL, Fabrication of Elastin-Like Polypeptide Nanoparticles for Drug Delivery by Electrospraying, Biomacromolecules. 10 (2009) 19–24. doi: 10.1021/bm801033f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cheng J, Park M, Lim DW, Hyun J, Polypeptide microgel capsules as drug carriers, Macromol. Res 21 (2013) 1163–1166. doi: 10.1007/s13233-013-1167-6. [DOI] [Google Scholar]
- [120].Na K, Jung J, Lee J, Hyun J, Thermoresponsive Pore Structure of Biopolymer Microspheres for a Smart Drug Carrier, Langmuir. 26 (2010) 11165–11169. doi: 10.1021/la1013285. [DOI] [PubMed] [Google Scholar]
- [121].Xia X-X, Xu Q, Hu X, Qin G, Kaplan DL, Tunable Self-Assembly of Genetically Engineered Silk–Elastin-like Protein Polymers, Biomacromolecules. 12 (2011) 3844–3850. doi: 10.1021/bm201165h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kaplan X-XX, Ming W, Yinan L, Qiaobing X, David L, Hydrophobic Drug-Triggered Self-Assembly of Nanoparticles from Silk-Elastin-Like Protein Polymers for Drug Delivery, Biomacromolecules. 15 (2014) 908–914. doi: 10.1021/bm4017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Parker, Wu, McKay, Xu, Kaplan, Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties, J. Funct. Biomater 10 (2019) 49. doi: 10.3390/jfb10040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhou M-L, Qian Z-G, Chen L, Kaplan DL, Xia X-X, Rationally Designed Redox-Sensitive Protein Hydrogels with Tunable Mechanical Properties, Biomacromolecules. 17 (2016) 3508–3515. doi: 10.1021/acs.biomac.6b00973. [DOI] [PubMed] [Google Scholar]
- [125].Ahadian S, Sadeghian RB, Salehi S, Ostrovidov S, Bae H, Ramalingam M, Khademhosseini A, Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine, Bioconjug. Chem 26 (2015) 1984–2001. doi: 10.1021/acs.bioconjchem.5b00360. [DOI] [PubMed] [Google Scholar]
- [126].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology, Adv. Mater 18 (2006) 1345–1360. doi: 10.1002/adma.200501612. [DOI] [Google Scholar]
- [127].Hoffman AS, Hydrogels for biomedical applications., Adv. Drug Deliv. Rev 54 (2002) 3–12. [DOI] [PubMed] [Google Scholar]
- [128].Hoare TR, Kohane DS, Hydrogels in drug delivery: Progress and challenges, Polymer (Guildf). 49 (2008) 1993–2007. doi: 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- [129].Asai D, Xu D, Liu W, Garcia Quiroz F, Callahan DJ, Zalutsky MR, Craig SL, Chilkoti A, Protein polymer hydrogels by in situ, rapid and reversible self-gelation, Biomaterials. 33 (2012) 5451–5458. doi: 10.1016/J.BIOMATERIALS.2012.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Amruthwar SS, Janorkar AV, Preparation and characterization of elastin-like polypeptide scaffolds for local delivery of antibiotics and proteins, J. Mater. Sci. Mater. Med 23 (2012) 2903–2912. doi: 10.1007/s10856-012-4749-5. [DOI] [PubMed] [Google Scholar]
- [131].Price R, Poursaid A, Cappello J, Ghandehari H, Effect of shear on physicochemical properties of matrix metalloproteinase responsive silk-elastinlike hydrogels, J. Control. Release 195 (2014) 92–98. doi: 10.1016/J.JCONREL.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Poursaid A, Price R, Tiede A, Olson E, Huo E, McGill L, Ghandehari H, Cappello J, In situ gelling silk-elastinlike protein polymer for transarterial chemoembolization, Biomaterials. 57 (2015) 142–152. doi: 10.1016/J.BIOMATERIALS.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Teng W, Cappello J, Wu X, Physical crosslinking modulates sustained drug release from recombinant silk-elastinlike protein polymer for ophthalmic applications, J. Control. Release 156(2011) 186–194. doi: 10.1016/J.JCONREL.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Teng W, Huang Y, Cappello J, Wu X, Optically Transparent Recombinant Silk-Elastinlike Protein Polymer Films, J. Phys. Chem. B 115 (2011) 1608–1615. doi: 10.1021/jp109764f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sill TJ, von Recum HA, Electrospinning: Applications in drug delivery and tissue engineering, Biomaterials. 29 (2008) 1989–2006. doi: 10.1016/J.BIOMATERIALS.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [136].Khadka DB, Haynie DT, Protein- and peptide-based electrospun nanofibers in medical biomaterials, Nanomedicine Nanotechnology, Biol. Med 8 (2012) 1242–1262. doi: 10.1016/J.NANO.2012.02.013. [DOI] [PubMed] [Google Scholar]
- [137].Ner Y, Stuart JA, Whited G, Sotzing GA, Electrospinning nanoribbons of a bioengineered silk-elastin-like protein (SELP) from water, Polymer (Guildf). 50 (2009) 5828–5836. doi: 10.1016/J.POLYMER.2009.09.017. [DOI] [Google Scholar]
- [138].Lucke M, Mottas I, Herbst T, Hotz C, Römer L, Schierling M, Herold HM, Slotta U, Spinetti T, Scheibel T, Winter G, Bourquin C, Engert J, Engineered hybrid spider silk particles as delivery system for peptide vaccines, Biomaterials. 172 (2018) 105–115. doi: 10.1016/j.biomaterials.2018.04.008. [DOI] [PubMed] [Google Scholar]
- [139].Luo F, Qian Z-G, Xia X-X, Responsive Protein Hydrogels Assembled from Spider Silk Carboxyl-Terminal Domain and Resilin Copolymers, Polymers (Basel). 10 (2018) 915. doi: 10.3390/polym10080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bidwell GL, Davis AN, Raucher D, Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides, J. Control. Release 135 (2009) 2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- [141].Dhandhukia JP, Shi P, Peddi S, Li Z, Aluri S, Ju Y, Brill D, Wang W, Janib SM, Lin Y-A, Liu S, Cui H, MacKay JA, Bifunctional Elastin-like Polypeptide Nanoparticles Bind Rapamycin and Integrins and Suppress Tumor Growth in Vivo, Bioconjug. Chem 28 (2017) 2715–2728. doi: 10.1021/acs.bioconjchem.7b00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Luo T, David MA, Dunshee LC, Scott RA, Urello MA, Price C, Kiick KL, Thermoresponsive Elastin-b-Collagen-Like Peptide Bioconjugate Nanovesicles for Targeted Drug Delivery to Collagen-Containing Matrices, Biomacromolecules. 18 (2017) 2539–2551. doi: 10.1021/acs.biomac.7b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Santos M, Gonzalez-Obeso C, Orbanic D, del Rio E, Arias FJ, Advanced Systems for Controlled Drug Delivery from Chemically Modified Elastin-like Recombinamers, Curr. Org. Chem 21 (2017)21–33. doi: 10.2174/1385272820666160511120925. [DOI] [Google Scholar]
- [144].Gonzalez-Valdivieso J, Borrego B, Girotti A, Moreno S, Brun A, Bermejo-Martin JF, Javier Arias F, A DNA Vaccine Delivery Platform Based on Elastin-Like Recombinamer Nanosystems for Rift Valley Fever Virus, Mol. Pharm 17 (2020) 1608–1620. doi: 10.1021/acs.molpharmaceut.0c00054. [DOI] [PubMed] [Google Scholar]
- [145].Jung S-H, Lee J-Y, Lee S-H, Kwon M-H, Han E-T, Park WS, Hong S-H, Kim Y-M, Ha K-S, Preventive Effects of Thermosensitive Biopolymer-Conjugated C-Peptide against High Glucose-Induced Endothelial Cell Dysfunction, Macromol. Biosci 19 (2019). doi: 10.1002/mabi.201900129. [DOI] [PubMed] [Google Scholar]
- [146].Zhang W, Garg S, Eldi P, Zhou FH, Johnson IRD, Brooks DA, Lam F, Rychkov G, Hayball J, Albrecht H, Targeting prostate cancer cells with genetically engineered polypeptide-based micelles displaying gastrin-releasing peptide, Int. J. Pharm 513 (2016) 270–279. doi: 10.1016/j.ijpharm.2016.09.039. [DOI] [PubMed] [Google Scholar]
- [147].Von A, Cresce W, Dandu R, Burger A, Cappello J, Ghandehari H, Characterization and real-time imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels, Mol Pharm. 5 (2008) 891–897. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Fernández-Colino A, Quinteros DA, Allemandi DA, Girotti A, Palma SD, Arias FJ, Self-Assembling Elastin-Like Hydrogels for Timolol Delivery: Development of an Ophthalmic Formulation Against Glaucoma, Mol. Pharm. 14 (2017) 4498–4508. doi: 10.1021/acs.molpharmaceut.7b00615. [DOI] [PubMed] [Google Scholar]
- [149].Jensen MM, Jia W, Schults AJ, Isaacson KJ, Steinhauff D, Green B, Zachary B, Cappello J, Ghandehari H, Oottamasathien S, Temperature-responsive silk-elastinlike protein polymer enhancement of intravesical drug delivery of a therapeutic glycosaminoglycan for treatment of interstitial cystitis/painful bladder syndrome, Biomaterials. 217 (2019). doi: 10.1016/j.biomaterials.2019.119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Parker RN, Wu WA, McKay TB, Xu Q, Kaplan DL, Design of Silk-Elastin-Like Protein Nanoparticle Systems with Mucoadhesive Properties., J. Funct. Biomater 10 (2019). doi: 10.3390/jfb10040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jeon H-Y, Jung S-H, Jung YM, Kim Y-M, Ghandehari H, Ha K-S, Array-Based High-Throughput Analysis of Silk-Elastinlike Protein Polymer Degradation and C-Peptide Release by Proteases, Anal. Chem 88 (2016) 5398–5405. doi: 10.1021/acs.analchem.6b00739. [DOI] [PubMed] [Google Scholar]
- [152].Poursaid A, Jensen MM, Nourbakhsh I, Weisenberger M, Hellgeth JW, Sampath S, Cappello J, Ghandehari H, Silk-Elastinlike Protein Polymer Liquid Chemoembolic for Localized Release of Doxorubicin and Sorafenib, Mol. Pharm 13 (2016) 2736–2748. doi: 10.1021/acs.molpharmaceut.6b00325. [DOI] [PubMed] [Google Scholar]
- [153].Andrew MacKay J, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A, Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection, Nat. Mater 8 (2009) 993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Moktan S, Perkins E, Kratz F, Raucher D, Thermal targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo, Mol. Cancer Ther 11 (2012) 1547–1556. doi: 10.1158/1535-7163.MCT-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hill LK, Frezzo JA, Katyal P, Hoang DM, Ben Z, Gironda Y, Xu C, Xie X, Delgado-Fukushima E, Wadghiri YZ, Montclare JK, Protein-Engineered Nanoscale Micelles for Dynamic 19 F Magnetic Resonance and Therapeutic Drug Delivery Article, ACS Nano. 13 (2019) 50. doi: 10.1021/acsnano.8b07481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Dai M, JA F, Engineered Protein Polymer-Gold Nanoparticle Hybrid Materials for Small Molecule Delivery, J. Nanomed. Nanotechnol 07 (2016). doi: 10.4172/2157-7439.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Isaacson KJ, Jensen MM, Steinhauff DB, Kirklow JE, Mohammadpour R, Grunberger JW, Cappello J, Ghandehari H, Location of Stimuli-Responsive Peptide Sequences within Silk-Elastinlike Protein-Based Polymers Affects Nanostructure Assembly and Drug-Polymer Interactions, J. Drug Target (2020) 1–47. doi: 10.1080/1061186X.2020.1757099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bidwell GL, Perkins E, Raucher D, A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth, Cancer Lett. 319 (2012) 136–143. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gonzalez-Valdivieso J, Girotti A, Munoz R, Carlos Rodriguez-Cabello J, Javier Arias F, Self-Assembling ELR-Based Nanoparticles as Smart Drug-Delivery Systems Modulating Cellular Growth via Akt, Biomacromolecules. 20 (2019) 1996–2007. doi: 10.1021/acs.biomac.9b00206. [DOI] [PubMed] [Google Scholar]
- [160].Serrano-Ducar S, Girotti A, Rodriguez-Cabello JC, Arias FJ, Synthesis and characterization of new recombinant Elastin-Like Recombinamers fused to scFv for the formation of targeted polyplex, N. Biotechnol 44 (2018) S25. doi: 10.1016/j.nbt.2018.05.141. [DOI] [Google Scholar]
- [161].Gomes S, Gallego-Llamas J, Leonor IB, Mano JF, Reis RL, Kaplan DL, Biological responses to spider silk-antibiotic fusion protein, J. Tissue Eng. Regen. Med 6 (2012) 356–368. doi: 10.1002/term.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jensen MM, Jia W, Schults AJ, Isaacson KJ, Steinhauff D, Green B, Zachary B, Cappello J, Ghandehari H, Oottamasathien S, Temperature-responsive silk-elastinlike protein polymer enhancement of intravesical drug delivery of a therapeutic glycosaminoglycan for treatment of interstitial cystitis/painful bladder syndrome, Biomaterials. 217 (2019). doi: 10.1016/j.biomaterials.2019.119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Pina MJ, Girotti A, Santos M, Carlos Rodriguez-Cabello J, Javier Arias F, Biocompatible ELR-Based Polyplexes Coated with MUC1 Specific Aptamers and Targeted for Breast Cancer Gene Therapy, Mol. Pharm 13 (2016) 795–808. doi: 10.1021/acs.molpharmaceut.5b00712. [DOI] [PubMed] [Google Scholar]
- [164].Pina MJ, Alex SM, Arias FJ, Santos M, Rodriguez-Cabello JC, Ramesan RM, Sharma CP, Elastin-like recombinamers with acquired functionalities for gene-delivery applications, J. Biomed. Mater. Res. PART A 103 (2015) 3166–3178. doi: 10.1002/jbm.a.35455. [DOI] [PubMed] [Google Scholar]
- [165].Greish K, Frandsen J, Scharff S, Gustafson J, Cappello J, Li D, O’Malley BW Jr., Ghandehari H, Silk-elastinlike protein polymers improve the efficacy of adenovirus thymidine kinase enzyme prodrug therapy of head and neck tumors, J. Gene Med 12 (2010) 572–579. doi: 10.1002/jgm.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Xia XX, Wang M, Lin Y, Xu Q, Kaplan DL, Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery, Biomacromolecules. 15 (2014) 908–914. doi: 10.1021/bm4017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Nouri FS, Wang X, Chen X, Hatefi A, Reducing the Visibility of the Vector/DNA Nanocomplexes to the Immune System by Elastin-Like Peptides, Pharm. Res 32 (2015) 3018–3028. doi: 10.1007/s11095-015-1683-5. [DOI] [PubMed] [Google Scholar]
- [168].Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H, Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression, J Control Release. 140 (2009) 256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].P. P., Phase 2b Multicenter, Randomized, Double-Blind, Placebo- and Active-Controlled. Parallel-Group Study to Assess the PD Response and Safety of Three Dose Levels of Glymera Injection Following 20 Weeks of Weekly SC Dosing in Adults With T2DM, (2012). [Google Scholar]
- [170].Pharmaceutics P., Phase 1, Randomized, Double-Blind, Placebo-Controlled Exploratory Study That Will Assess the Safety, Tolerability. Pharmacokinetics and Hemodynamic Response to a Single 30 Minute Intravenous Infusion of Vasomera™ (PB1046) in Adult Subjects With Stage 1 or, (2013). [Google Scholar]
- [171].Pharmaceutics P., VIP-ELP fusion molecules PB1120 and PB1046 correct F508del-CFTR Dysfunction, (2015). [Google Scholar]