The severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) infection responsible for coronavirus disease 2019 (COVID-19) is associated with an alarming incidence of critical illness, thrombosis, and all-cause mortality.1–3 Myocardial injury, defined by elevated cardiac troponin (cTn) concentrations, is common in critical illness and has been reported during hospitalization in up to 36% of COVID-19 hospitalizations.1,4 We explored the frequency of myocardial injury at admission and during hospitalization and evaluated associations between myocardial injury and clinical outcomes in a large cohort of patients with COVID-19 from a high-volume health care system in New York.
The study was approved by the New York University School of Medicine Institutional Review Board with a waiver of informed consent. Identifiable data will not be made available to the public. We identified consecutive adults age ≥18 years with confirmed SARS CoV-2 infection admitted to the New York University Langone Health system between March 1, 2020 and April 16, 2020. Patients were eligible for inclusion if ≥1 cTn was measured during hospitalization. Routine cTn surveillance was performed in 95% of COVID-19 admissions because cTn assays were included in COVID-19–specific order sets in the electronic heath record. Non–high-sensitivity cTn assays were used (Siemens Dimension Vista Troponin I, Washington DC; Abbot Architect Troponin I, Chicago, IL). The initial and maximum cTnI measured during hospital admission were recorded. Myocardial injury was defined as cTn >99% upper reference limit for the cTn assay. Individuals were stratified into tertiles by initial and maximum cTn concentrations normalized to the assay-specific limit of detection. All-cause, in-hospital mortality was recorded for all patients. Critical illness was defined by treatment in an intensive care unit, need for mechanical ventilation, discharge to hospice, or death. Patient characteristics, laboratory data, and clinical outcomes were ascertained via automated extraction from the electronic health record. Categorical variables were compared by χ2 tests, and continuous variables were compared using Mann–Whitney tests. Logistic regression models were generated to estimate the odds of the study end points, adjusted for demographics, comorbidities, vital signs, laboratory findings, and baseline medications. Statistical tests are 2-sided, and P values <0.05 were considered statistically significant.
A total of 2163 consecutive adults with COVID-19 and ≥1 cTn measurement were identified. Myocardial injury was present at the initial cTn measurement in 665 (30.7%) cases. Patients with myocardial injury were older (71 versus 61 years; P<0.001) and more likely to be male (67.1% versus 61.5%; P=0.016). In-hospital mortality was higher in patients with myocardial injury at initial cTn measurement compared with those without myocardial injury (37.0% versus 15.4%; P<0.001; adjusted odds ratio [aOR], 2.04 [95% CI, 1.52–2.82]). Critical illness (54.1% versus 28.9%; P<0.001; aOR, 1.67 [95% CI, 1.23–2.07]) was also more frequent in patients with myocardial injury. When grouped by tertile of the initial cTn concentration, patients with the highest initial cTn concentrations were more likely to have in-hospital mortality and critical illness compared with patients in the lowest tertile of initial cTn (Figure).
Figure.
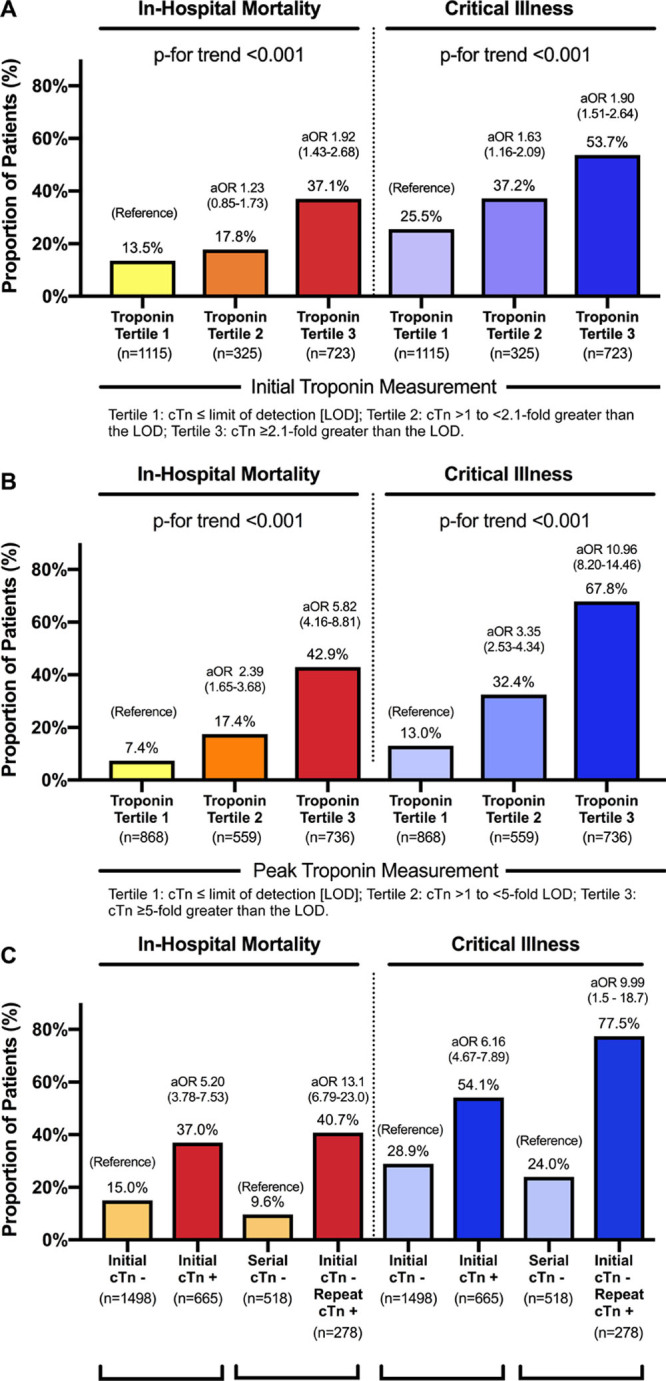
Frequency and adjusted odds of in-hospital mortality and critical illness in patients with COVID-19. Shown are frequencies and odds of in-hospital mortality and critical illness by tertile of initial cardiac troponin (cTn) concentration (A) and peak cTn concentration (B), and based on the timing of myocardial injury (C). Adjusted odds associated with each tertile of cTn in A and B are compared with COVID-19 patients in the lowest tertile of cTn measured. Odds ratios are adjusted for age, sex, race, body mass index, tobacco use, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, previous myocardial infarction, previous heart failure, atrial fibrillation or malignancy, temperature at presentation, pulse oximetry at presentation, outpatient prescriptions for antiplatelets, statin and β-blocker use, C-reactive protein, creatinine, D-dimer, absolute lymphocyte count, and platelet count.
Among 1498 COVID-19 patients without myocardial injury at the time of initial assessment, serial cTn measurements were available in 796 (53%). Among these, 278 developed acute myocardial injury in-hospital that was first detected after a median 3.6 (0.9–7.6) days. Associations between cTn and outcomes were consistent when myocardial injury was present at initial cTn measurement, when new myocardial injury occurred in-hospital (Figure), and in patients without prevalent coronary artery disease or chronic kidney disease (data not shown).
In total, 943 patients (43.6%) were diagnosed with myocardial injury based on the peak cTn measured during their COVID-19 hospitalization. Patients with myocardial injury based on the peak cTn had higher in-hospital mortality (39.1% versus 9.7%; P<0.001; aOR 5.20 [95% CI, 3.78–7.53]) and more frequent critical illness (61.0% versus 17.9%; P<0.001; aOR 6.16 [95% CI, 4.67–7.89]) than patients without myocardial injury. Individuals with the highest peak cTn concentrations also had the greatest risk of critical illness and death (Figure).
We report that nearly one-third of patients hospitalized with COVID-19 had myocardial injury at presentation, and nearly half had injury detected during the course of their hospitalization. Regardless of when it was first detected, myocardial injury was associated with increased odds of mortality and critical illness, with higher cTn measurements associated with worse outcomes. The mechanism of myocardial injury is unknown.1,4 Among decedents with COVID-19, megakaryocytes associated with fibrin microthrombi were identified within the cardiac, pulmonary, renal, and hepatic microvasculature.5 Thus, thrombosis may contribute to multi-organ failure in patients with fatal COVID-19 infections and may underlie myocardial injury during the course of disease.
This study was retrospective, observational, and only in-hospital outcomes were recorded. Still, this is the first study to demonstrate robust associations between the magnitude and timing of cTn elevation, measured at presentation and during admission, and subsequent critical illness and mortality. The optimal management of patients with myocardial injury in COVID-19 has not been defined and requires further study.
Disclosures
Dr Smilowitz is supported, in part, by the National Heart, Lung, And Blood Institute of the National Institutes of Health (K23HL150315); and reports consulting for Abbott Vascular. Dr Fishman is funded, in part, by the National Heart Lung and Blood Institute of the National Institute of Health (R01HL105983, R01HL142498, and R01HL146107). Dr Berger is funded, in part, by the National Heart and Lung Blood Institute of the National Institute of Health (R01HL139909 and R35HL144993). The other authors report no conflicts.
Footnotes
Data sharing: Identifiable data will not be made available to the public.
References
- 1.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966 doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434 doi: 10.1016/j.eclinm.2020.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]


