Abstract
Purpose:
To identify locus characteristics associated with locus-level sensitivity loss or improvement during N-acetylcysteine (NAC) treatment in retinitis pigmentosa (RP).
Design:
Retrospective analysis of prospectively collected data in the FIGHT RP clinical trial
Methods:
Patients (n=30) were treated with 600, 1200, or 1800 mg NAC twice/day for 3 months and then three times/day for 3 months. Microperimetry locus-level changes between baseline and month-6 were correlated with baseline locus characteristics using regression models. Main outcome measure was locus-level sensitivity change ≥ 6 dB.
Results:
Baseline mean sensitivity (3468 loci, 51 evaluable eyes) was 7.7 dB and for foveal, parafoveal, and perifoveal loci was 20.2, 11.8, and 5.8 dB. During treatment 287 (8.28%) loci increased ≥ 6 dB and 119 of 1613 loci with baseline sensitivity ≥ 6 dB decreased ≥ 6 dB (7.38%). Higher NAC dose was associated with lower likelihood of sensitivity loss ≥ 6 dB (p = 0.033). Loci with low baseline sensitivity were more likely to decrease ≥ 6 dB (p = 0.034), but also more likely to increase ≥ 6 dB (p < 0.001). Foveal versus perifoveal loci (p < 0.001) and superior versus inferior loci (p = 0.005) were more likely to increase ≥ 6 dB.
Conclusions:
Higher NAC dose reduces risk of macular loci sensitivity loss in RP. Greater sensitivity depression reversibility in the fovea during treatment suggests that high foveal cone density protects cones from irreversible loss of function in RP making them more likely to show improved function during NAC treatment.
Introduction
Retinitis pigmentosa (RP) is a group of diseases in which one of many different mutations results in degeneration of rod photoreceptor cells. The loss of rods makes it difficult to function in low light situations, but functioning is normal when illumination is adequate. However, after rod photoreceptors degenerate, cone photoreceptors begin to die in a stereotypical pattern. Cone cell death starts in the midperiphery resulting in midperipheral scotomata, and slowly progresses so that scotomata merge often resulting in a ring scotoma that separates central from peripheral field. Cone degeneration gradually progresses from the margins anteriorly and posteriorly. The gradual constriction of the posterior visual field is associated with significant visual disability because it compromises the ability to orient oneself in space and detect obstacles thereby limiting mobility. Over a period that usually spans decades there is progressive constriction of central visual fields further compromising mobility, but visual acuity remains relatively intact allowing reading and other tasks requiring good central vision. Eventually visual field constriction impinges on the fovea progressively reducing visual acuity ultimately resulting in blindness.
Oxidative stress is a contributing factor to cone degeneration after most rod photoreceptors are eliminated by mutation-induced cell death.1–3 Rod photoreceptors constitute 95% of the cells in the outer part of the human retina and after they are eliminated, oxygen consumption is reduced and tissue oxygen levels are increased4 resulting in generation of reactive oxygen species (ROS) and progressive oxidative damage in cones.5 Cone dysfunction and death are reduced by antioxidants that reduce oxidative stress in cones.2,3 A particularly effective antioxidant is N-acetylcysteine (NAC) which directly detoxifies ROS and is also a substrate for glutathione,6 a major component of the body’s antioxidant defense system. NAC is approved for acetaminophen overdose and when given in a timely fashion can markedly reduce oxidative damage in the liver from toxic metabolites of acetaminophen and is life-saving.7 In a mouse model of RP, oral NAC promotes cone survival and function.8
The FIGHT RP clinical trial was a phase I, dose escalation trial testing the effects of oral NAC in patients with RP. There were 3 cohorts of 10 patients each that received treatment for 6 months and were observed for 3 months after stopping treatment. Patients in cohort 1 received 600 mg NAC bid for 3 months and then tid for 3 months. Patients in cohort 2 received 1200 mg NAC bid for 3 months and then tid for 3 months. Patients in cohort 3 received 1800 mg NAC bid for 3 months and then tid for 3 months. NAC showed a good safety profile with a maximum tolerated dose of 1800 mg bid which resulted in good intraocular levels of NAC, as did 1200 mg bid. While the primary outcome was safety, secondary outcomes assessed visual functions including the best-corrected visual acuity (BCVA) and mean macular sensitivity (MMS) using microperimetry (MP). There was gradual, statistically significant improvement in BCVA in each cohort. The MMS provides a global assessment of central visual field calculated as the average of sensitivity thresholds of 68 test loci covering the central 20˚of the retina. Improvement in MMS was observed in all cohorts during the treatment period and was statistically significant for cohort 3. Additionally, qualitative assessments of serial MP tests during NAC treatment showed sensitivity loss at some loci and large improvements at others (see Figure 6 in reference 9). In this study, we analyzed sensitivity changes at individual loci to identify topographical characteristics associated with locus-level loss or gain of sensitivity during NAC treatment.
Methods
Study Design
The FIGHT RP trial was a single center, open-label clinical trial designed to test the safety and tolerability, pharmacokinetics, and effects on several parameters of visual function of several dosing regimens of NAC in patients with RP (ClinicalTrials.gov Identifier: NCT03063021). The protocol and consent form were approved by the Johns Hopkins Medicine IRB prior to study initiation. Informed consent was obtained for all participants. The first patient was enrolled in February 2017 and the last patient enrolled completed the final study visit in February 2019. A total of 30 patients were enrolled, 10 in each of 3 cohorts: (1) cohort 1, 600mg NAC BID × 12 weeks followed by 600mg NAC TID × 12 weeks, (2) cohort 2, 1200mg NAC BID × 12 weeks followed by 1200mg NAC TID × 12 weeks, and (3) cohort 3, 1800mg NAC BID × 12 weeks followed by 1800mg NAC TID × 12 weeks. NAC was provide as 600mg effervescent tablets (Zambon Pharmaceuticals, Milan, Italy). The trial was HIPAA compliant and adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the International Conference of Harmonisation E6 Guidelines for Good Clinical Practice.
Study Protocol
The study population was comprised of men and women 18 years or older with a diagnosis of RP based on clinical phenotype and diagnostic testing. Subjects had screening and baseline visits and returned every 4 weeks for follow up study visits during which they had assessment of adverse events and change in concomitant medications, vital signs, measurement of BCVA, slit lamp examination, and indirect ophthalmoscopy. MAIA microperimetry (Centervue model, version 2.0.4US) and SDOCT were done at screening, baseline, and the week 12, 24, and 36 visits. Details of the study protocol and the results of the primary and secondary outcomes have been published.9
Statistical Analysis
The locus-level MP data at the baseline and month-6 visits were used to calculate the change during the treatment period for each locus. The predictor variables were the locus-level characteristics including: locus eccentricity categorized based on retinal anatomy as foveal region (i.e. the 4 loci of 2° eccentricity), parafoveal region (i.e. the 12 loci of 4° eccentricity) and perifoveal (i.e. the 52 loci on the rings of 6° to 10° eccentricity) (Figure 1)10; nasal vs. temporal field; superior vs. inferior hemisphere the locus’ own baseline-level sensitivity; and whether any neighboring locus was scotomatous (yes, if the minimum sensitivity of any neighboring loci was 0 dB; and no, if otherwise). Additionally, given that participants of different cohorts received different dosages of treatment, the predictor variable of participant cohort was included.
Figure 1. The microperimetry test pattern used in the Fight-RP study.
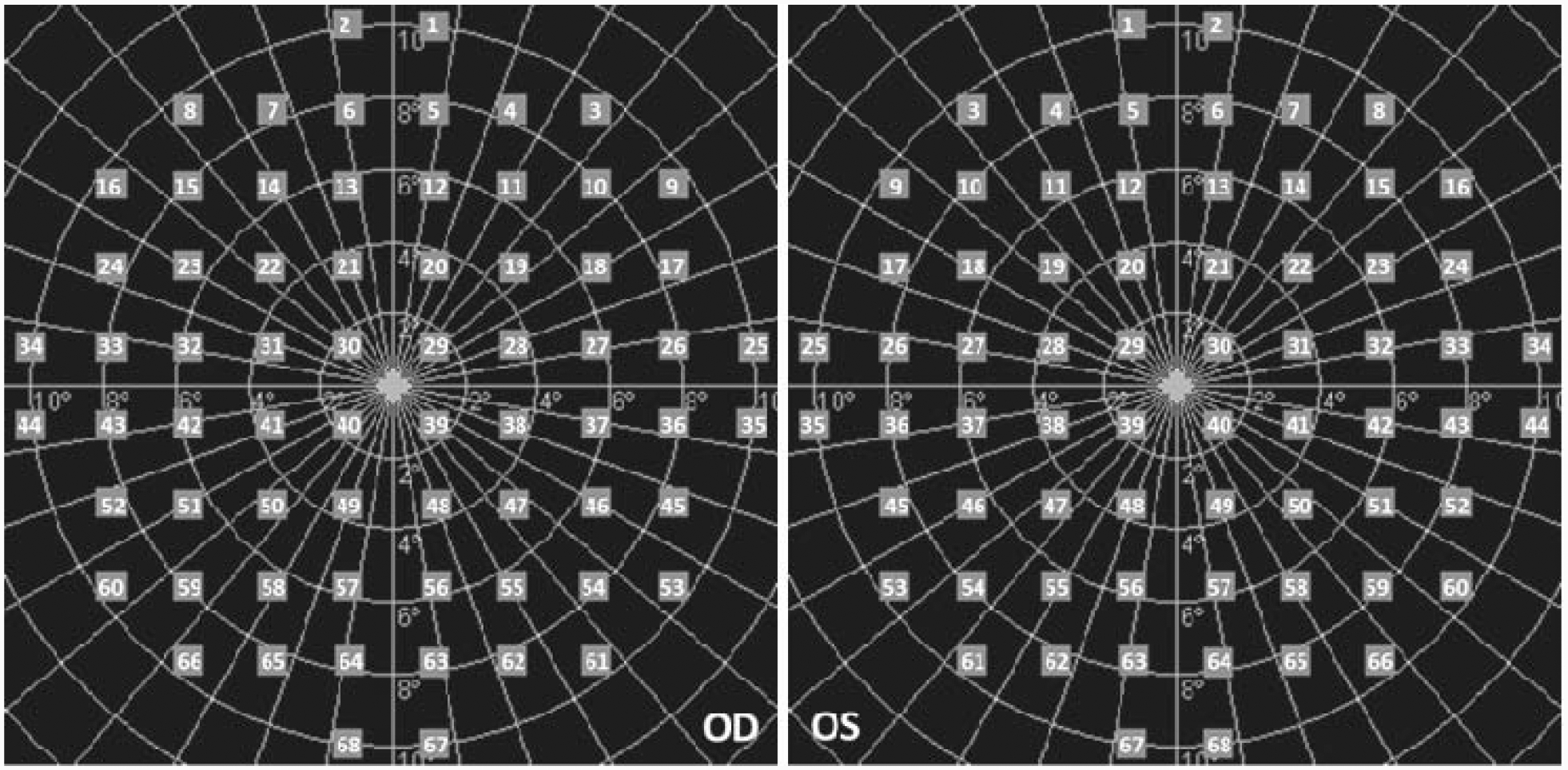
The pattern included 68 test loci covering the central 20° of the retina. Eccentricy of the test loci was categorized into 3 regions: Foveal region included 4 loci distributed on the ring of 2° eccentricity (Loci 29, 30, 39, and 40); Parafoveal region included 12 loci distributed on the ring of 4° eccentricity (Loci 19–22, 28, 31, 38, 41, 47–50); and Perifoveal region included 52 loci distributed on the rings of 6°−10° eccentricity (Loci 1–18, 23–27, 32–37, 42–46, 51–68).
For the analysis of identifying characteristics associated with positive treatment response, the outcome was operationally defined as whether a locus had a gain in sensitivity of ≥6 dB at month-6 compared to baseline. For the analysis of identifying characteristics associated with loss of sensitivity, the outcome was operationally defined as whether a locus had a loss in sensitivity of ≥ 6dB between baseline and month 6. The cut-off of 6 dB was used because it is about 1.96 times the standard deviations of the sensitivity changes between baseline and month 6 in the 3 cohorts. In particular, for the outcome of whether a locus had a loss in sensitivity ≥ 6dB, it was relevant only to those loci that had baseline sensitivity ≥ 6dB, and thus analyses for this outcome used the subset of locus-level data for which baseline sensitivity was ≥ 6dB.
For the analysis of each outcome, logistic regressions were used to model the probability of gain/loss in sensitivity, and generalized estimating equation (GEE) technique was used to account for intra- and inter-eye correlations using a compound symmetry structure. For each predictor variable representing a characteristic of locus or the variable of participant cohort, its univariate association with each outcome was estimated using bivariable logistic regression model with GEE. To estimate adjusted associations, a multivariate logistic regression model was built for each outcome and included predictor variables with p-values > 0.1 in bivariable regressions as well as the variable of participant cohort irrespective its univariate association with the outcome.
The predictor variable of locus’ own baseline sensitivity was first discretized into 10 categories including the group of 0 dB and 9 intervals each of which had a width of 4 dB in order to explore the shape of baseline sensitivity’s association with each outcome. Based upon the numbers of events of the outcomes in these categories (Figures 2 and 3), the baseline sensitivity was grouped as 0 dB, > 0 and ≤ 8 dB, > 8 and ≤ 16 dB, and > 16 dB for the analysis of the outcome of gaining sensitivity; and was grouped as > 6 to 8 dB, > 8 to ≤ 12 dB and > 12 dB for the analysis of outcome on loss of sensitivity. The predictor variable of participant cohort was modeled as a categorical variable to estimate differences between cohorts. Additionally, given the increasing dosage associated with cohort assignment, this variable was also modeled as continuous to assess whether there was a dose-response relationship with an outcome. All analyses used SAS 9.4. All p-values reported were based on two-sided Wald tests and without multiple testing adjustment. A significance level of 0.05 was used.
Figure 2. Percent of loci losing sensitivity by ≥6dB during the 6 months of treatment by baseline loci sensitivity level.
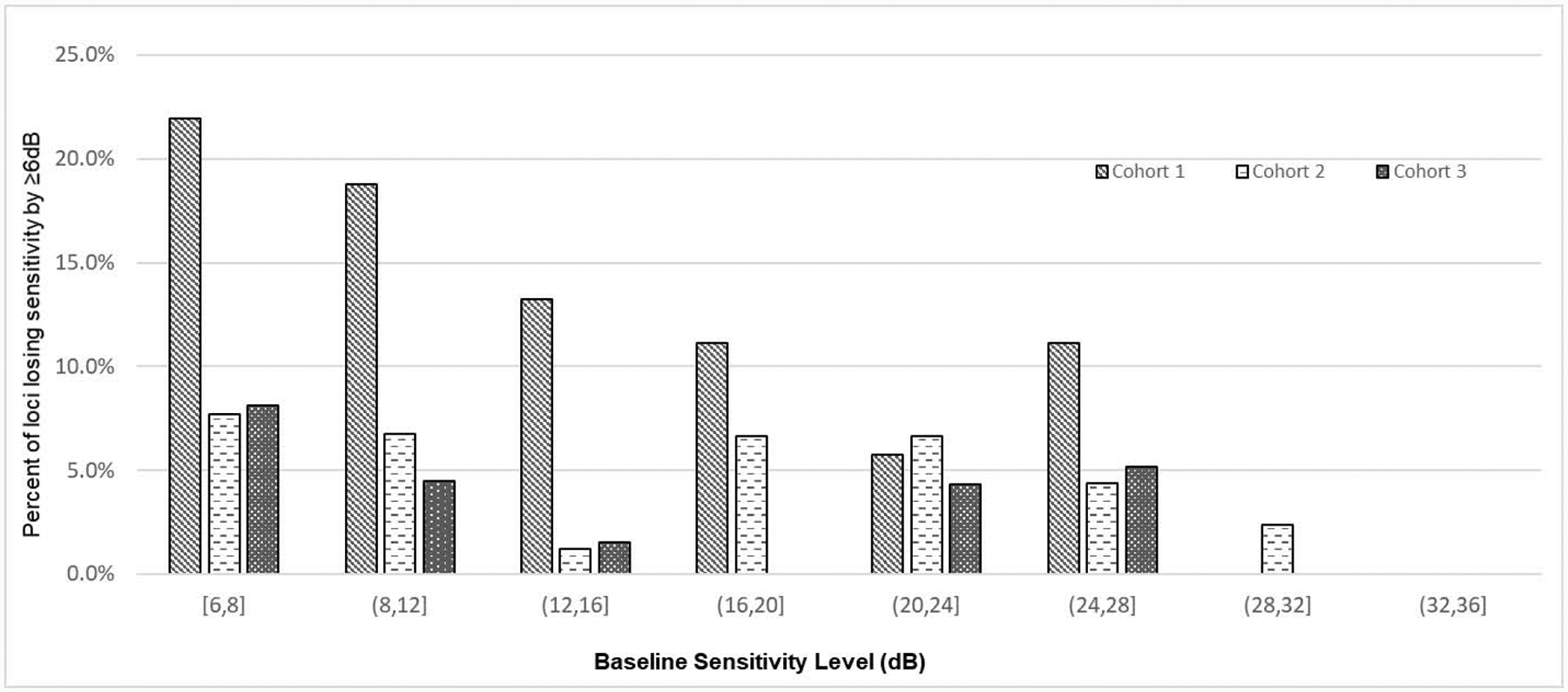
Bars show the percentage of loci with sensitivity loss ≥ 6 decibels (dB) during treatment as a function of baseline sensitivity. On the x-axis, parentheses beneath each group of 3 bars is the range of locus baseline sensitivity for that group: [6,8] = 6 to 8 dB, (8,12] = >8 to 12 dB, (12,16] = >12 to 16 dB, (16,20] = >16 to 20 dB, (20,24] = >20 to 24 dB, (24,28] = >24 to 28 dB, (28,32] = >28 to 32 dB, and (32,36] = >32 to 36 dB. The dose of NAC for each cohort was 600 mg bid for 3 months followed by 600 mg tid for 3 months for cohort 1, 1200 mg bid for 3 months followed by 1200 mg tid for 3 months for cohort 2, and 1800 mg bid for 3 months followed by 1800 mg tid for 3 months for cohort 3.
Figure 3. Percent of loci gaining sensitivity by ≥6dB during the 6 months of treatment by baseline loci sensitivity level.
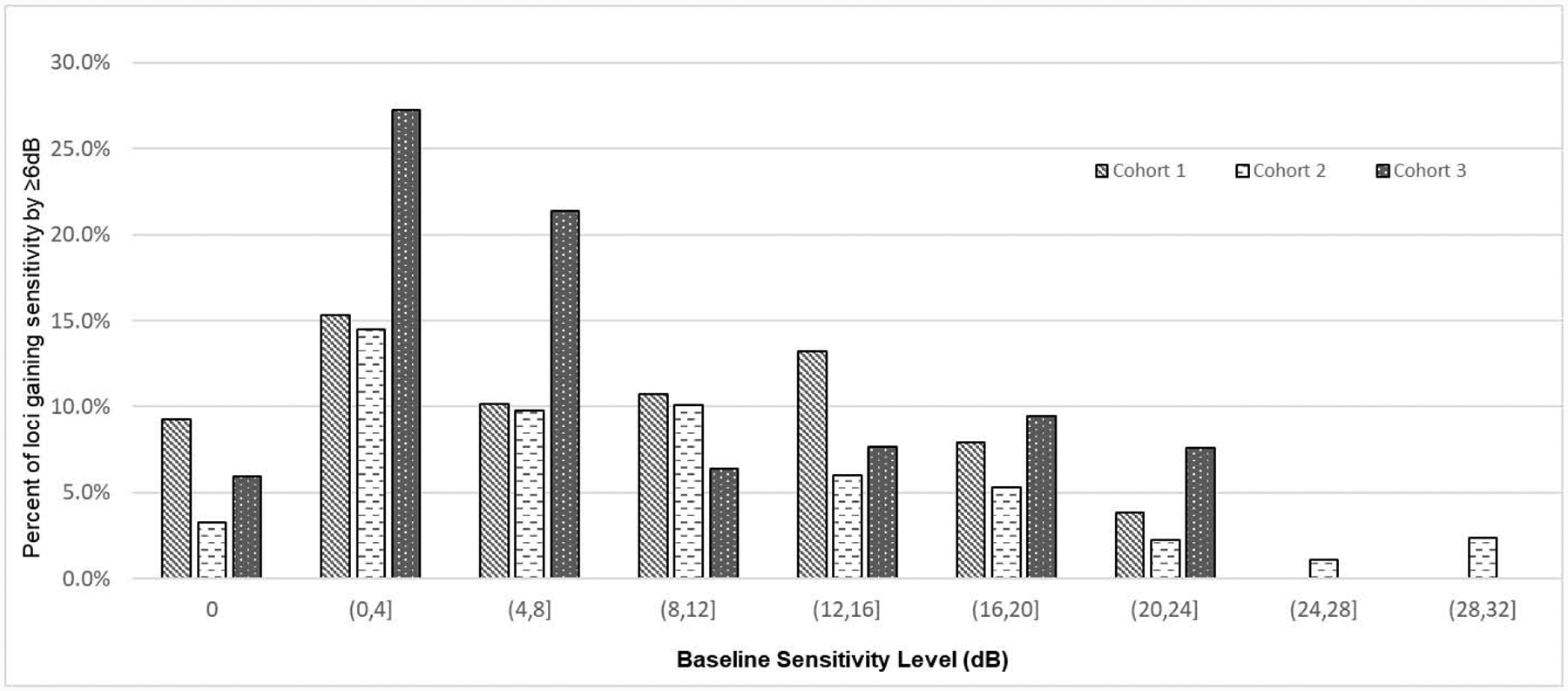
Bars show the percentage of loci with sensitivity gain ≥ 6 decibels (dB) during treatment as a function of baseline sensitivity. On the x-axis, parentheses beneath each group of 3 bars is the range of locus baseline sensitivity for that group: [0] = 0 dB, (0,4] = >0 to 4 dB, (4,8] = >4 to 8 dB, (8,12] = >8 to 12 dB, (12,16] = >12 to 16 dB, (16,20] = >16 to 20 dB, (20,24] = >20 to 24 dB, (24,28] = >24 to 28 dB, and (28,32] = >28 to 32 dB. The dose of NAC for each cohort was 600 mg bid for 3 months followed by 600 mg tid for 3 months for cohort 1, 1200 mg bid for 3 months followed by 1200 mg tid for 3 months for cohort 2, and 1800 mg bid for 3 months followed by 1800 mg tid for 3 months for cohort 3.
Results
Baseline Characteristics
The FIGHT-RP study enrolled 30 participants. Three of the 30 participants were unable to reliably perform MP testing, one participant was lost to follow-up, and one participant had one eligible eye; thus, 51 eyes of 26 participants were available for this study. Demographic and clinical characteristics are summarized in Table 1. The median age of the 26 participants was 54 (range 21 to 76) years, 46 % were female, and 73% were white. Median age of symptom onset was 21.5 (range 5 to 47) years, and the median duration of symptoms at baseline was 23.5 (range 3 to 52) years. The median BCVA of all eligible eyes at baseline was 78 ETDRS letters (20/33 Snellen equivalent). The mean sensitivity (MS) at baseline for all loci (N = 3468 for the 51 eyes) was 7.7 (SD = 9.0) dB. The MS of the foveal, parafoveal and perifoveal loci was 20.2 dB, 11.8 dB, and 5.8 dB, respectively. The MS was 7.7 dB for both the loci in the superior macula and those located in the inferior macula. The MS was 7.4 dB for loci in the nasal field and 8.1 dB for those in the temporal field (Table 1).
Table 1.
Baseline demographic and clinical characteristics and change in sensitivity in individual loci during the 6-months treatment period.
| No. of participants with reliable MP data and available at month-6 (Total N=26) | ||||
|---|---|---|---|---|
| Age at baseline visit (year) | Median | IQR | Range | |
| 54 | 33–60 | 21–76 | ||
| Sex | N | % | ||
| Female | 12 | 46.2 | ||
| Race | ||||
| White/Caucasian | 19 | 73.1 | ||
| Black/African Descendants | 4 | 15.4 | ||
| Other (Hispanic, Asian, Mid-East) | 3 | 11.5 | ||
| Age of symptom onset (years) | Median | IQR | Range | |
| 21.5 | 15–38 | 5–47 | ||
| Duration since symptom onset at the baseline visit (years) | 23.5 | 9–36 | 3–52 | |
| Baseline Eye-level Clinical Characteristics (Total number of study eyes=51) | ||||
| Median | IQR | Range | ||
| Best Corrected Visual Acuity | ETDRS letters | 78 | 70–82 | 37–90 |
| Snellen Equivalent | 20/33 | (20/40, 20/23) | (20/182, 20/16) | |
| Baseline Locus Sensitivity Summaries (Total number of loci=3468) | ||||
| Mean | SD | Range | ||
| Sensitivity of all loci (dB) | 7.72 | 9.00 | 0–3/3 | |
| Sensitivity of the fovea loci(dB) | 20.20 | 8.48 | 0–33 | |
| Sensitivity of the parafovea loci(dB) | 11.82 | 9.99 | 0–29 | |
| Sensitivity of the perifovea loci(dB) | 5.80 | 7.60 | 0–31 | |
| Sensitivity of superior loci(dB) | 7.72 | 9.08 | 0–33 | |
| Sensitivity of inferior loci(dB) | 7.73 | 8.93 | 0–31 | |
| Sensitivity of nasal loci(dB) | 7.39 | 9.04 | 0–33 | |
| Sensitivity of temporal loci(dB) | 8.06 | 8.96 | 0–32 | |
| Change of locus-level sensitivity during treatment (Month 6 - Baseline) (dB) (Total No. of points=3468) | Mean | SD | Range | |
| 0.69 | 3.50 | (−19, 27) | ||
| Points with increase ≥6dB | N | % | ||
| 287 | 8.28 | |||
| Points with decrease ≥6dB among the points with baseline sensitivity≥6dB (total No. of such points=1613) | 119 | 7.38 | ||
SD: standard deviation; IQR: interquartile range
ETDRS: Early Treatment Diabetic Retinopathy Study
Overall change in locus-level sensitivity during the 6-month treatment period
The mean change in locus-level sensitivity from baseline during treatment (month 6 minus baseline) was 0.69 (SD = 3.50) dB, ranging from −19 to 27 dB (Table 1). There were 287 loci (8.3%) for which sensitivity increased by at least 6 dB during the treatment period. At baseline, there were 1613 loci in 26 participants with baseline sensitivity ≥ 6 dB and thus had potential for sensitivity loss ≥ 6 dB, and 119 of these loci (7.4%) lost ≥ 6 dB during treatment.
Locus characteristics associated with ≥ 6 dB sensitivity loss during treatment
Table 2 lists the observed number of loci with sensitivity loss ≥ 6 dB by each subgroup as well as the unadjusted and adjusted odds ratios (adjOR) associated with each predictor variable. Participant cohort was significantly associated with the risk of sensitivity loss (Figure 2). Eyes of participants in cohort 3 dosed with 1800 mg NAC bid/tid had the lowest percentage of loci with sensitivity loss ≥ 6 dB (3.69%), followed by eyes of participants in cohort 2 dosed with 1200 mg bid/tid (5.22%), and eyes of participants in cohort 1 dosed with 600 mg bid/tid (15.69%). Compared with cohort 1, the adjORs of sensitivity loss for cohorts 2 and 3 were 0.34 (95%CI: 0.14 to 0.79) and 0.33 (95%CI: 0.11, 1.02), respectively. Lower locus baseline sensitivity was significantly associated with higher risk of sensitivity loss (Figure 2). Compared with loci with baseline sensitivity > 12 dB, (4.9% lost ≥ 6 dB during treatment), there was greater risk of sensitivity loss for loci with baseline sensitivity between 6 and 8 dB (14.0% lost ≥ 6 dB, adjOR=1.56, 95%CI: 1.02 to 2.39) and for loci with baseline sensitivity between 8 and 12 dB (9.5% lost ≥ 6 dB, adjOR=1.62, 95%CI: 1.03 to 2.54).
Table 2.
Associations of the outcome of sensitivity loss ≥ 6dB during treatment with predictor variables including locus characteristics and participant cohort, using data of loci with baseline sensitivity ≥ 6 dB (total N = 1613 such loci)
| Observed No. of events/Total No. of loci (%) | Bivariable model | Multivariable model | |||
|---|---|---|---|---|---|
| Unadjusted ORs (95%CI) | p-value* | Adjusted ORs (95%CI) | p-value* | ||
| Participant cohort | .004 | .033 | |||
| C1 | 67/427 (15.69) | Ref | Ref | ||
| C2 | 28/536 (5.22) | 0.27 (0.13, 0.59) | 0.34 (0.14, 0.79) | ||
| C3 | 24/650 (3.69) | 0.28 (0.09, 0.86) | 0.33 (0.11, 1.02) | ||
| Locus own baseline sensitivity | .019 | .034 | |||
| >12 dB | 48/990 (4.85) | Ref | Ref | ||
| > 8 and ≤ 12dB | 34/358 (9.50) | 1.63 (1.15, 2.31) | 1.56 (1.02, 2.39) | ||
| >6 and ≤ 8dB | 37/265 (13.96) | 1.68 (1.08, 2.59) | 1.62 (1.03, 2.54) | ||
| Whether the locus neighbored a locus with 0 dB baseline sensitivity | .047 | .29 | |||
| No | 77/645 (11.94) | Ref | Ref | ||
| Yes | 42/968 (4.34) | 0.68 (0.46, 1.00) | 0.78 (0.49, 1.24) | ||
| Eccentricity of locus** | .18 | ||||
| Perifoveal | 71/1030 (6.89) | Ref | N/A | ||
| Parafoveal | 35/397 (8.82) | 0.60 (0.35, 1.04) | |||
| Foveal | 13/186 (6.99) | 0.93 (0.67, 1.30) | |||
| Hemisphere | .32 | ||||
| Superior | 53/811 (6.54) | Ref | N/A | ||
| Inferior | 66/802 (8.23) | 0.81 (0.53, 1.22) | |||
| Nasal vs. Temporal | .47 | ||||
| Nasal | 54/753 (7.17) | Ref | N/A | ||
| Temporal | 65/860 (7.56) | 1.09 (0.86, 1.39) |
N/A: not applicable because the variable was not included in the multivariable regression model since its univariate association had p>0.10.
the p-value is for testing whether there was any significant difference in the outcome event probability comparing the different groups. Wald test results were used.
Fovea: loci located on the ring of 2° eccentricity. Parafovea: loci located on the ring of 4° eccentricity. Perifovea: loci located on the rings of 6° to 10° eccentricity.
(OR: odds ratio. CI: confidence interval).
Among loci neighboring another locus with 0 dB sensitivity at baseline, 4.3% lost ≥ 6 dB during treatment; versus 11.9% of loci not neighboring any locus with 0 dB lost ≥ 6 dB. However, in the multivariable model, after adjusting for cohort and locus baseline sensitivity, loci bordering a locus with 0 dB sensitivity at baseline were not statistically significantly less likely to have a sensitivity loss ≥ 6 dB (adjOR 0.78, 95%CI: 0.49 to 1.24, p = 0.29). Other locus topographical characteristics including eccentricity, superior versus inferior location, or temporal versus nasal location were not significantly associated with the risk of loss ≥ 6 dB sensitivity (Table 2).
Participant cohort was also modeled as continuous to estimate the dose-response relationship. The unadjusted OR associated with increasing dosage level (1200 mg bid/tid versus 600 mg bid/tid or 1800 mg bid/tid versus 1200 mg bid/tid) was 0.49 (95%CI: 0.26 to 0.92, p=0.027). The corresponding adjOR from the multivariable model was 0.55 (95%CI: 0.30 to 1.02, p=.06).
Locus characteristics associated with ≥ 6 dB sensitivity gain during treatment
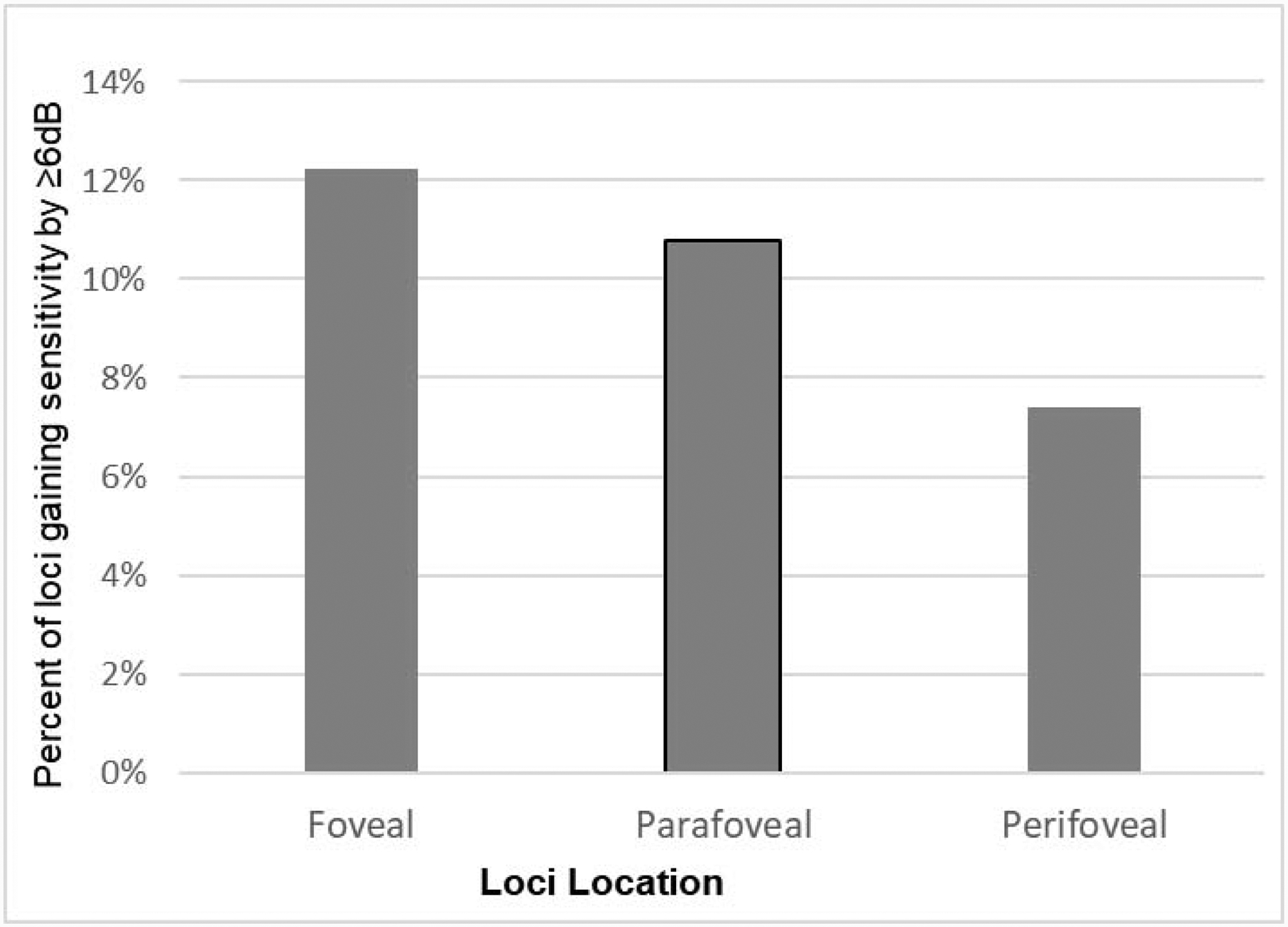
Table 3 lists the observed number of loci with sensitivity improvement ≥ 6 dB by each subgroup as well as the unadjusted OR and adjOR associated with each predictor variable. The odds of sensitivity gain ≥ 6 dB was not significantly different among the three dosage cohorts. The baseline sensitivity of a locus was significantly associated with its odds of sensitivity gain ≥ 6 dB (Figure 3). It was least likely for loci with baseline sensitivity > 16 dB (4.4%) and most likely for loci with baseline sensitivity between 0 and 8dB (16.2%). Compared with loci with baseline sensitivity > 16 dB, the adjOR was 4.31 (95%CI: 1.01 to 18.38) for loci with baseline sensitivity of 0 dB, 7.32 (95%CI: 2.53 to 19.47) for loci with baseline sensitivity between 0 and 8 dB, and 2.22 (95%CI: 1.0 to 4.68) for loci with baseline sensitivity between 8 and 16 dB. With respect to locus eccentricity, foveal loci were most likely to improve ≥ 6 dB sensitivity (12.3%), followed by parafoveal loci (10.8%) and perifoveal loci (7.4%. Figure 4). Compared to perifoveal loci, the adjOR for foveal loci was 7.37 (95%CI: 3.65 to 14.86) and for parafoveal loci the adjOR was 2.48 (95%CI: 1.73 to 3.56). Additionally, loci located inferior to the horizontal meridian were more likely to gain ≥ 6 dB than superiorly located loci (adjOR=1.41, 95%CI: 1.11 to 1.80). Nasal versus temporal location or neighboring a locus with 0 dB sensitivity at baseline did not significantly associate with the likelihood of ≥ 6 dB sensitivity improvement (Table 3).
Table 3.
Associations of the outcome of ≥ 6 dB sensitivity gain during treatment with predictor variables including locus characteristics and participant cohort.
| Observed No. of events/Total No. of loci (%) | Bivariable model | Multivariable model | |||
|---|---|---|---|---|---|
| Unadjusted ORs (95%CI) | p-value* | Adjusted ORs (95%CI) | p-value* | ||
| Participant cohort | .40 | .44 | |||
| C1 | 122/1224 (9.97) | Ref | Ref | ||
| C2 | 53/1020 (5.20) | 0.56 (0.20, 1.55) | 0.56 (0.10, 3.13) | ||
| C3 | 112/1224 (9.15) | 0.91 (0.36, 2.33) | 1.40 (0.27, 7.19) | ||
| Locus own baseline sensitivity | <.001 | <.001 | |||
| >16dB | 31/709 (4.37) | Ref | Ref | ||
| >8 and ≤16dB | 55/639 (8.61) | 2.22 (1.06, 4.68) | 4.87 (2.30, 10.32) | ||
| >0 and ≤8dB | 101/624 (16.19) | 7.02 (2.53, 19.47) | 17.68 (6.64,47.07) | ||
| 0dB | 100/1496 (6.68) | 4.31 (1.01, 18.38) | 13.51 (3.88, 47.06) | ||
| Whether the locus neighbored a locus with 0 dB baseline sensitivity | .22 | N/A | |||
| No | 193/2387 (8.09) | Ref | |||
| Yes | 94/1081 (8.70) | 1.46 (0.80, 2.68) | |||
| Eccentricity of locus** | .049 | <.001 | |||
| Perifoveal | 196/2652 (7.39) | Ref | Ref | ||
| Parafoveal | 66/612 (10.78) | 1.74 (0.97, 3.12) | 2.48 (1.73, 3.56) | ||
| Foveal | 25/204 (12.25) | 1.51 (1.05, 2.18) | 7.37 (3.65, 14.86) | ||
| Hemisphere | .013 | .005 | |||
| Superior | 120/1734 (6.92) | Ref | Ref | ||
| Inferior | 167/1734 (9.63) | 1.43 (1.08, 1.89) | 1.41 (1.11, 1.80) | ||
| Nasal vs. Temporal | 0.81 | N/A | |||
| Nasal | 146/1734 (8.42) | Ref | |||
| Temporal | 141/1734 (8.13) | 1.04 (0.77, 1.41) |
N/A: not applicable because the variable was not included in the multivariable regression model since its univariate association had p>0.10.
the p-value is for testing whether there was a significant difference in the outcome event probability comparing the different groups. Wald test results were used.
Fovea: loci located on the ring of 2° eccentricity. Parafovea: loci located on the ring of 4° eccentricity. Perifovea: loci located on the rings of 6° to 10° eccentricity.
(OR: odds ratio. CI: confidence interval).
Discussion
In animal models of RP, NAC and other antioxidants reduce oxidative damage in cones, slow the decline of cone function assessed by electroretinography, and slow cone cell death.2,3,8 This led to the FIGHT RP trial,9 which had primary aims of assessing the safety and tolerability of oral NAC and determining intraocular levels after different oral doses in patients with RP. Secondary aims were to assess parameters of visual function. Microperimetry provides a particular informative tool to measure photoreceptor function, because it combines visual field (VF) testing with real time fundus imaging, allowing greater monitoring and control over patient fixation during testing and precise localization of functional defects superimposed on a fundus image. Using the MP1 microperimeter (Nidek Technologies, Gamagori, Japan), a first generation instrument with somewhat limited dynamic range, a retrospective study of serial MP1 tests in 75 eyes of 39 RP patients over a follow up period of 1–4 years showed regional reductions in sensitivity over a 1-year period despite lack of any change in visual acuity or fixation stability.11 In the natural history of RP, the greatest loss of function and death of cones occurs at the outside border of remaining cones resulting in gradual constriction of remaining VF, but decline in sensitivity was observed in the central macula as well as eccentrically.11 The MAIA microperimeter (Centervue, Padova, Italy), which has a larger dynamic range (0–36 dB) than the MP1 microperimeter, was used in the FIGHT RP clinical trial. In normal individuals, MS with the MAIA microperimeter decreases slightly with age ranging from 33.6 dB in indiviuals 21–30 years old to 30.6 dB in individuals 61–70 years old.12 The MS of 7.72 dB in 51 eyes of the RP patients in this study is a large reduction from normal and although the reduction was greatest for perifoveal loci (5.80 dB), there was also substantial reduction for foveal loci (20.20 dB). This indicates that despite excellent mean baseline BCVA, sensitivity of foveal cones was substantially reduced in this study population similar to what was observed in previous studies.11,13
In order to identify loci that continued to decrease in sensitivity due to disease progression, a 6 dB threshold was used. This threshold is well above the test-retest variability of 4 dB14 and is approximately 1.96 (i.e. the 2.5% percentile of the standard normal distribution) times the standard deviation of sensitivity change for all loci during treatment. This means that it would be unlikely to observe a gain or loss of 6 dB or more if the difference between baseline and month-6 was purely due to random variation. The percentage of loci with a decrease in sensitivity ≥ 6 dB was significantly greater in cohort 1 versus cohorts 2 and 3, indicating a NAC dose effect. Increasing dose was associated with ~45% lower odds of sensitivity loss of ≥ 6 dB. This inverse dose-response relationship of sensitivity loss with NAC dosage was statistically significant in the bivariable model, and had a p-value of 0.06 in the multivariable model most likely because of the limited power given the relatively small sample size. These data provide support for the hypothesis that reduction of oxidative stress in cones by NAC treatment slows loss of function in cones and also supports the use of the maximum tolerated dose, 1800 mg bid, in future trials to test the efficacy of NAC in slowing the loss of visual function in RP.
Unlike the situation regarding loci that lost ≥ 6 dB, there was not a NAC dose effect for the 287 loci that showed a sensitivity increase ≥ 6 dB. This suggests that with a treatment period of 6 months, cones that are capable of functional improvement are just as likely to improve with a NAC dose of 600 mg bid/tid as a dose of 1800 mg bid/tid. This may mean that even a modest reduction in oxidative stress is sufficient to allow functional improvement in cones that are still capable of improvement, while a large reduction in oxidative stress is needed to prevent continued loss of sensitivity in susceptible cones. Cones more susceptible to sensitivity loss are more likely to reside in loci with low baseline sensitivity, because such loci were more likely to lose ≥ 6 dB. This suggests that some cones with severely compromised function may be so severely damaged that they continue to lose function despite reduction in oxidative stress. However, other cones with profound sensitivity loss may still be salvageable, because loci with lower baseline sensitivity were also more likely to gain ≥ 6 dB. In fact, even loci with 0 dB sensitivity at baseline were more likely to improve than loci with higher sensitivity. This is encouraging because it means that macular loci that fail to respond to the maximum MAIA stimulus luminance of 1000 apostilbs may still contain viable cones that are capable of improving function. Thus loci with low sensitivity contain mixed populations of cones, some that are moribund and continue to lose function despite reduction in oxidative stress and others that despite low baseline function are able to respond to a reduction in oxidative stress by improving function. The relative proportions of these two cone populations in a particular locus with low sensitivity, is likely to determine whether the locus improves or deteriorates during treatment.
Interestingly and fortuitously, foveal loci appear more likely to contain a greater proportion of cones that are able to respond to a reduction in oxidative stress by improving function (Figure 4). In fact, the more centrally a locus was located, the more likely it was to show improvement ≥ 6 dB during NAC treatment regardless of baseline sensitivity. After controlling for baseline sensitivity in the multivariable model, compared with perifoveal loci, which are most peripheral, foveal and parafoveal loci had > 7 times and ~2.5 times higher odds of gaining ≥ 6 dB in sensitivity, respectively. Cone density is highest in the fovea and decreases with eccentricity.15 In RP, loss of neighboring cells reduces oxygen consumption and increases tissue oxygen, which is the source of oxidative stress.16 Thus a possible explanation for the greater likelihood of sensitivity improvement in the fovea during NAC treatment is that due to the high density of foveal cones the level of chronic oxidative stress in RP retinas is less in the fovea compared to more eccentrically, resulting in a higher proportion of cones that have the potential for improved sensitivity. In addition, the greater number of cones within a foveal locus that can contribute to improvement may play a role. The improvement in sensitivity in foveal loci explains the statistically significant improvement in BCVA that occurred during NAC treatment.9 The greater likelihood of sensitivity improvement ≥ 6 dB for inferior versus superior loci is more difficult to understand and it remains to be seen if this observation is replicated in future studies before considering possible explanations.
In summary, changes in sensitivity at individual loci during NAC treatment in patients with RP provide new insights. Foveal loci are substantially depressed in patients with RP who still have as much as 20°or 30° of remaining VF, and these loci are more likely to show sensitivity improvement compared to more peripheral loci during NAC treatment. This suggests that that the depression in foveal sensitivity is due at least in part to oxidative stress and to some extent is recoverable by reduction of oxidative stress. This is encouraging since foveal function is most important for performance of visual tasks. It is also notable that while loci with low baseline sensitivity are most likely to continue to lose sensitivity during NAC treatment, they are also most likely to improve. Thus, some loci with low sensitivity contain cones that may not be salvageable despite NAC treatment, at least over a 6 months period, and cones at other loci with similarly low sensitivity are capable to show functional improvement soon after treatment initiation. We hypothesize that these functional improvements predict improved cone survival and maintenance of visual function with long term NAC treatment, thereby preventing progressive visual disability and blindness in patients with RP. A randomized, double-masked, placebo-controlled, multicenter trial is necessary to test this hypothesis.
Supplementary Material
Acknowledgements/Disclosures
Funding/Support: The study was supported by a grant from the National Institutes of Health (NIH) (R34EY031429). Drs. Kong and Campochiaro had full access to all of the data and take responsibility for the data integrity and accuracy of data analysis.
Biography
Xiangrong Kong is an associate professor in the Dana Center for Preventive Ophthalmology at the Wilmer Eye Institute of the Johns Hopkins University School of Medicine. She is also associate professor in Biostatistics, Epidemiology, and Health Behavior and Society at the Johns Hopkins Bloomberg School of Public Health. Her ophthalmic research focuses on natural history studies and clinical trials of inherited retinal degeneration. Her methodological research focuses on correlated data analysis and clinical trial design.
Peter A. Campochiaro, is the Eccles Professor of Ophthalmology and Neuroscience at the Wilmer Eye Institute of the Johns Hopkins University School of Medicine. He directs a laboratory focused on elucidating the molecular pathogenesis of retinal diseases and development of new treatments. He conducts early phase and late phase clinical trials and has helped to develop new treatments for retinal/choroidal vascular diseases with a more recent focus on inherited retinal degenerations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None of the JHU authors have any conflict of interest related to this study. However, Johns Hopkins University has a licensing agreement concerning N-acetylcysteine amide with a company, Nacuity Pharmaceuticals, Inc (Nacuity), under which the University is entitled to royalty distributions and has equity in Nacuity. While this manuscript does not deal with N-acetylcysteine amide, it was judged by the Conflict of Interest Committee of Johns Hopkins University, that there is enough overlap between the drugs that reported results with N-acetylcysteine might have an effect on the value of N-acetylcysteine amide, and therefore we are declaring an institutional conflict of interest.
PAC has potential conflicts of interest unrelated to the subject of the manuscript: grants and personal fees from AERPIO Pharmaceuticals, personal fees and equity from Allegro, personal fees from Applied Genetic Technologies Corporation, personal fees from Asclepix Therapeutics, personal fees from Baucsh and Lomb, personal fees from Curevac, personal fees from Exonate Ltd, grants and personal fees from Genentech/Roche Inc, grants and personal fees from Sanofi Genzyme, grants, personal fees, and equity from Graybug Vision, personal fees from Merck & Co, Inc, personal fees from NOVARTIS Pharmaceuticals Corporation, grants and personal fees from Oxford Biomedica, grants and personal fees from Regeneron Pharmaceuticals, Inc, grants and personal fees from Regenxbio, Inc, personal fees from Wave Life Sciences.
None of the other authors have any conflicts of interest.
References
- 1.Shen J, Yan X, Dong A, et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203(3):457–464. [DOI] [PubMed] [Google Scholar]
- 2.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213(3):809–815. [DOI] [PubMed] [Google Scholar]
- 3.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103(38):11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 5.Usui S, Oveson BC, Lee SY, et al. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa J Neurochem 2009;110 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldini G, Altomare A, Baron G, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52(7):751–762. [DOI] [PubMed] [Google Scholar]
- 7.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–434. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Usui S, Zafar AB, et al. N-acetylcysteine promotes long term survival of cones in a model of retinitis pigmentosa. j Cell Physiol. 2011;226:1843–1849 [DOI] [PubMed] [Google Scholar]
- 9.Campochiaro PA, Iftikhar M, Hafiz G, et al. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase 1 trial. J Clin Invest. 2019;pii: 132990. doi: 10.1172/JCI132990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schubert HD. Retina and Vitreous In: Yanoff M, Duker JS, eds. Ophthalmology. 5th ed. Edinburgh, London, New York, Oxford, Philadelphia, St Louis, Sydney: Elsevier; 2019:421. [Google Scholar]
- 11.Iftikhar M, Kherani S, Kaur R, et al. Progression of retinitis pigmentosa as measured on microperimetry: the PREP-1 study. Ophthalmology Retina. 2018;2(5):502–507. [DOI] [PubMed] [Google Scholar]
- 12.Molina-Martin A, Pinero DP, Perez-Cambrodi RJ. Normal values for microperimetery with the MAIA micorperimeter: sensitivity and fixation analysis in healthy adults and children. Eur J Ophthalmol. 2017;27(5):607–613. [DOI] [PubMed] [Google Scholar]
- 13.Birch DG, Wen Y, Locke K, Hood DC. Rod sensitivity, cone sensitivity, and photoreceptor layer thickness in retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2011;52(10):7141–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Ayton LN, Guymer R, Luu CD. Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7378–7385. [DOI] [PubMed] [Google Scholar]
- 15.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. [DOI] [PubMed] [Google Scholar]
- 16.Campochiaro PA, Mir TA. The mechanism of cone cell death in retinitis pigmentosa. Prog Retin Eye Res. 2018;62(1):24–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


