Abstract
Animal behavior can be modulated by multiple interacting factors. In rodents such as rats, these factors include, among others, the female estrous cycle, exposure to a novel environment, and light. Here, we used the open field test to disassociate differences in behavior resulting from each of these factors by testing the hypothesis that locomotor and anxiety-related behaviors differ between estrous cycle phases in female rats and that novelty and light exposure concurrently influence these behaviors in both female and male rats. Adult female rats were tested twice under red or white light in estrus and diestrus estrous cycle phases. Adult male rats were also tested twice under either red or white light. In females, an interaction between novelty and estrous cycle phase influenced locomotor and anxiety-related behaviors. In males, novelty influenced locomotor and anxiety-related behaviors differentially under red and white light. Light exposure increased anxiety-related behaviors in both males and females, but reduced locomotor behavior only in females. These findings reveal the complexities of behavioral testing and highlight the importance of factors such as the estrous cycle, novelty, and light exposure.
Keywords: Estrous cycle, locomotor and anxiety-related behavior, open-field, novelty, light, sex differences
Introduction
Animal behavior is influenced by concurrent and interacting factors which are often kept constant in the laboratory to produce a well-controlled experiment focused on one variable. However, it is possible that the selection of one factor of interest will obscure or otherwise impact the influence of another factor. Here we disassociate differences in rat locomotor and anxiety-related behaviors resulting from three external factors known to be of importance to behavior: the female estrous cycle, exploration of a novel environment, and the absence or presence of light. In adult females, fluctuating ovarian hormones play a large role in not only the reproductive system, but also in modulating behavior. In particular, behaviors related to locomotion and anxiety fluctuate naturally across the estrous cycle in rodents and the menstrual cycle in humans [1-3]. In female rats, estrous cycle-induced locomotor and anxiety-related behavioral changes are studied using the open field test among other behavioral tests including the elevated plus maze, voluntary wheel running, and passive avoidance task [2, 4-8]. When used to assess the role of the estrous cycle on female locomotor behavior, the open field test may detect either differential or null effects [7-10]. Additionally, anxiety measurements using the open field test often do not detect robust differences across the estrous cycle, but using a testing method such as the elevated plus maze to assess anxiety-related behaviors reveals more consistent estrous cycle-induced changes [2, 6, 11, 12]. These differences in the magnitude of estrous cycle effects on behavior suggest that other factors may be influencing behavioral output within the open field. Not surprisingly, specific methods of the open field often vary widely in regard to factors such as the length of the test, habituation periods, and the presence and intensity of light. This encourages consideration of other factors that influence open field behavior and the potential obstacles they present when assessing variables such as estrous cycle effects.
Two of these other factors are exposure to a novel environment and the presence of light. Both of these factors induce a physiological stress response and influence locomotor activity in rats [13, 14]. Novelty typically induces increased locomotor and exploratory behaviors, while light exposure has been documented to decrease locomotor behavior [13, 15, 16]. Despite the behavioral changes caused by novelty and light-induced stress in the open field, this test is widely used in the assessment of anxiety-related behaviors in a variety of contexts. Often, the effect from experimental variables are sufficiently robust to detect changes in open field behavior and this method of testing is still a well-respected assessment of locomotor and anxiety-related behaviors [17]. Although both novelty and light exposure are factors in modulating behavior, the magnitude of their influence is not well-understood, especially in the context of the estrous cycle. This interaction may potentially contribute to the misinterpretation of less robust behavioral changes and thus the conflicting results across behavioral studies assessing estrous cycle effects. This is a significant oversight, as understanding additional factors of influence on open field behaviors will lead to a more accurate evaluation of results.
To address this gap in knowledge, we tested the hypothesis that locomotor and anxiety-related behaviors differ between estrous cycle phases in female rats and that novelty and light exposure concurrently influence these behaviors in both female and male rats in the open field test. We performed four different experiments which test specific aspects of this hypothesis. In experiment 1, we tested the effects of novelty and two estrous cycle phases on female rat open field behavior by testing each rat twice under red light in the diestrus and estrus phase. In experiment 2, we tested the effect of white light exposure, novelty, and two estrous cycle phases on female rat open field behavior by testing each rat twice under white light in the diestrus and estrus phase. In experiment 3, we tested the effect of novelty on female rat open field behavior by testing each rat twice under red light in either the diestrus or estrus phase. Experiment 4 tested the effect of novelty and light exposure on male rat open field behavior by testing each rat twice under either red or white light.
Methods
Animals:
All animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at North Carolina State University. Male and female Sprague-Dawley rats were born from timed-pregnant females purchased from Charles River Laboratories and housed at the Biological Resource Facility at North Carolina State University. Rats were housed in pairs after weaning at P21 through adulthood. Ten days before behavioral testing (P102±4), rats were single housed. All rats were handled daily five days prior to experimental assessment. Cages were BPA free and filled with bedding manufactured from virgin hardwood chips (Beta Chip; NEPCO, Warrensburg, NY) to avoid endocrine disruptors present in corncob bedding [18-20]. Soy protein-free rodent chow (2020X; Teklad, Madison, WI) and glass water bottles were provided ad libitum. Rats were housed in a temperature (23°C, 40% humidity) and light controlled room on a 12:12 hour light:dark cycle with lights turning off at 9:00 am.
Estrous Cycle Assessment:
Female estrous cycle assessment began at least five days prior to behavioral testing. Males were similarly handled five days prior to behavioral testing. Rats in diestrus and estrus were employed for behavioral testing. Females were vaginally swabbed with potassium phosphate buffer solution daily between 9:00 am and 10:00 am. Swabs were prepared on a wet mount slide and epithelial cell morphology was visualized under a microscope as previously described [21]. This vaginal cytology method has been verified by our laboratory to be a valid assessment of estrous cycle phases, as phases identified through cytology correspond with expected hormone levels [22, 23]. For the purpose of this study, diestrus phase rats were employed during the second day after the completion of estrus, exhibiting vaginal cytology of predominately leukocytes and few nucleated cells. Estrus phase rats were employed during the first day of estrus or behavioral estrus as noted in the literature [24], during the first few hours of the dark cycle where vaginal cytology exhibited predominately clumped, cornified cells with few nucleated circular cells.
Behavioral Testing and Data Analysis:
All behavioral testing occurred within the first three hours of the dark cycle, between 10:00 am and 12:00 pm. During each test, rats were individually placed into an open field arena (60cm X 60cm X 60cm; Cleversys Inc, Reston, VA). Following previously documented protocols [16, 25, 26], activity was recorded for 30 minutes with a video camera located above the open field. Behaviors were analyzed both as the total activity during the 30 minute testing period as well as activity during the first 10 minutes of the test to allow the comparison of novelty-induced behavior occurring early in the test to general locomotor and anxiety-related behaviors represented over the longer test period [25-28]. Following each test, the open field was thoroughly cleaned with 70% isopropyl alcohol. In all experiments, locomotion was determined by measuring the total distance traveled in the open field and anxiety-related behaviors were evaluated using the time spent in the center of the open field, number of entries into the center, and latency to enter the center. For statistical analysis, rats that did not enter the center were assigned a latency of 1800 seconds, which is the total duration of the test. All activities were analyzed blind to treatment using TopScan software version 3.0 (Cleversys Inc., Reston, VA).
Experiment 1:
Behavioral testing of female rats was conducted under red light (0.5±0.5 lux). Each rat was tested in both diestrus and estrus phases but separated into two cycle sequence groups, either diestrus first (n=7) or estrus first (n=8) (Figure 1A). Rats experienced at least one full estrous cycle between the first and second behavioral test (12±2 days).
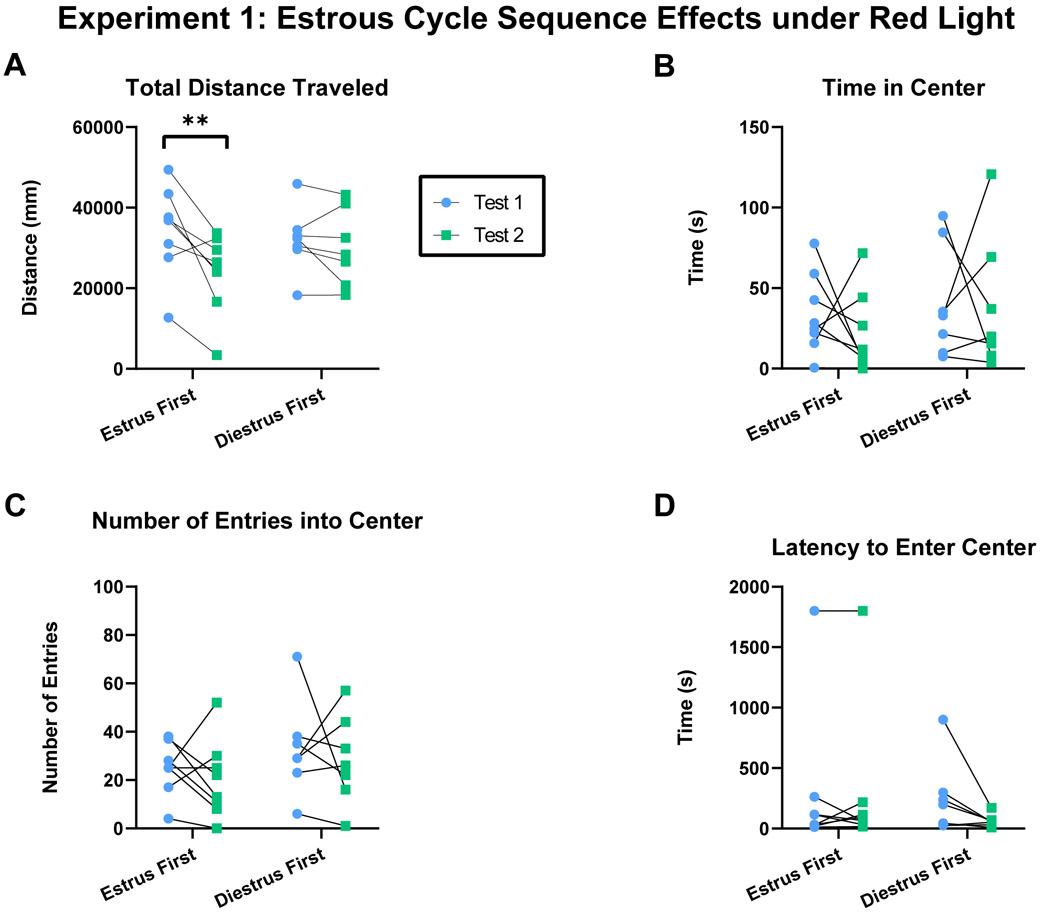
Figure 1.
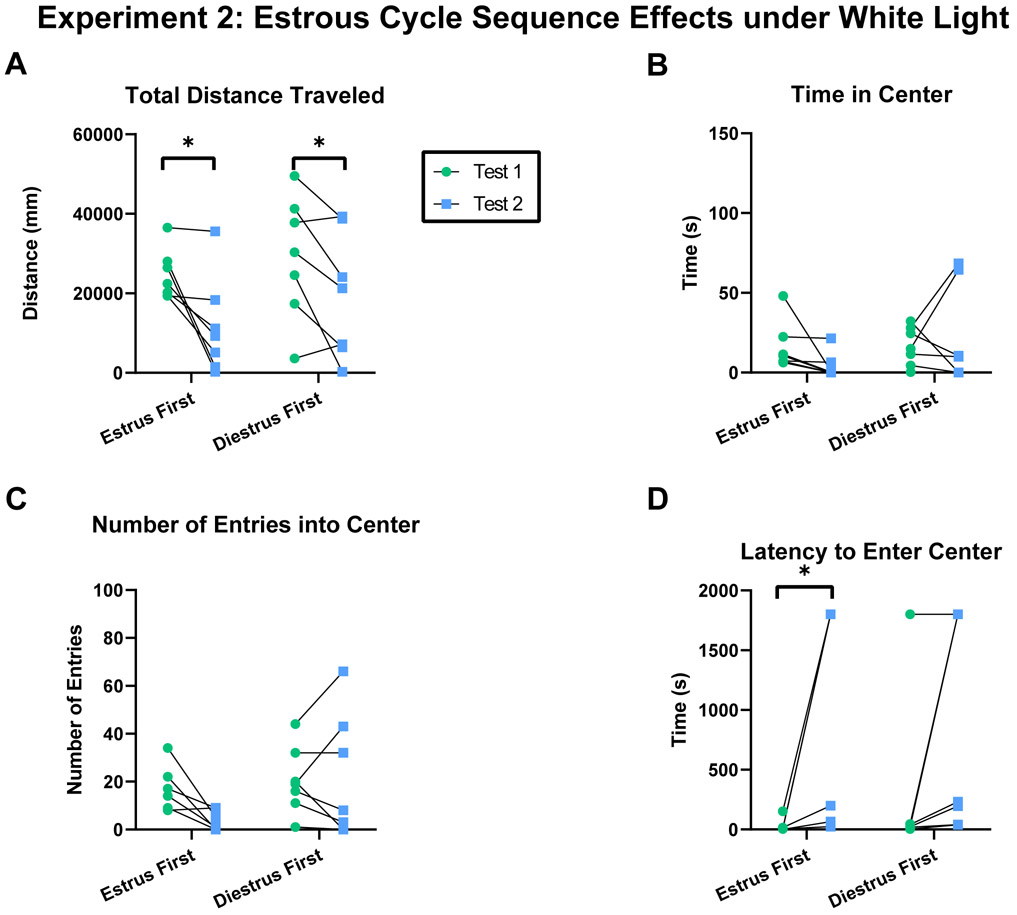
Schematic depicting experimental design. A) Experiment 1: Female rats were tested twice under red light in both estrus and diestrus phases, separated into two cycle sequence groups: estrus first or diestrus first. B) Experiment 2: Female rats were tested twice under white light in both estrus and diestrus phases, separated into two cycle sequence groups: estrus first or diestrus first. C) Female rats were tested twice under red light in either estrus or diestrus phases. D) Male rats were tested twice under either red or white light.
Experiment 2:
Behavioral testing was conducted under white light (250±10 lux) with verified equal illumination across the open field arena. Female rat open field behavior was conducted in the same manner as in experiment one, with two cycle sequence groups of either diestrus first (n=7) or estrus first (n=7) (Figure 1B). Rats experienced at least one full estrous cycle between the first and second behavioral test (10±1 days).
Experiment 3:
Behavioral testing was conducted under red light (0.5±0.5 lux). Female rats were tested twice in either diestrus (n=7) or estrus (n=8) (Figure 1C). Rats experienced at least one full estrous cycle between the first and second behavioral test (13±1 days).
Experiment 4:
Male rats were tested twice under either red light (n=8, 0.5±0.5 lux) or white light (n=6, 250±10 lux) (Figure 1D). Males were given 8±1 days in between the first and second behavioral test.
Statistical Analysis:
All experimental data was analyzed via Graphpad Prism version 8.3 (La Jolla, CA). Behavioral data was first analyzed using a two-way repeated measures ANOVA with Holm-Sidak’s multiple comparisons tests and unpaired t-tests. Further analysis was taken to decompose the effects of light, sex, and estrous cycle phase using unpaired t-tests and a one-way ANOVA with Holm-Sidak’s multiple comparisons test to maximize statistical power. Significance was given a priori to P values of less than 0.05. Effect sizes were calculated as partial eta-squared (η2p) for F-tests and Cohen’s d (d) for t-tests. Data is reported as mean ± SEM.
Results
Experiment 1: Estrous cycle phase and novelty interact to influence locomotion but not anxiety-related behaviors under red light.
Females first tested in estrus showed increased total distance traveled in the open field compared to a second test in diestrus (Figure 2A; Two-way RM ANOVA: Cycle Sequence x Test: F(1,13)=4.858, p=0.046, η2p=0.272; Cycle Sequence: F(1,13)=0.171, p=0.686; Test: F(1,13)=10.05, p=0.007; Subject: F(13,13)=5.532, p=0.002; Estrus first test 1- test 2: t(13)=3.934, p=0.003; Diestrus first test 1-test 2: t(13)=0.662, p=0.769). This is in contrast to females first tested in diestrus and then in estrus, which showed no difference in total distance traveled between tests. No significant differences in the time spent in the center of the open field were detected in either estrous cycle group (Figure 2B; Two-way RM ANOVA: Cycle Sequence x Test: F(1,13)=0. 178, p=0.680, η2p=0.014; Cycle Sequence: F(1,13)=1.252, p=0.283; Test: F(1,13)=0.316, p=0.584; Subject: F(13,13)=0.786, p=0.665). No significant differences in the number of entries into the center of the open field were detected in either estrous cycle group (Figure 2C; Two-way RM ANOVA: Cycle Sequence x Test: F(1,13)<0.001, p=0.988, η2p<0.001; Cycle Sequence: F(1,13)=1.687, p=0.217; Test: F(1,13)=0.672, p=0.427; Subject: F(13,13)=1.237, p=0.354). No significant differences in latency to enter the center of the open field were detected in either estrous cycle group (Figure 2D; Two-way RM ANOVA: Cycle Sequence x Test: F(1,13)=3.31, p=0.092, η2p=0.203; Cycle Sequence: F(1,13)=0.362, p=0.558; Test: F(1,13)=3.161, p=0.099; Subject: F(13,13)=22.05, p<0.001). Overall this data indicates that females exhibited increased locomotor behavior in estrus under red light, but this effect is masked by an effect of novelty occurring during the first test. Estrous cycle phase- or novelty- induced differences in anxiety-related behaviors were not observed when females were tested in both estrous cycle phases under red light.
Figure 2.
Estrous cycle phase sequence and novelty influenced locomotor but not anxiety-related behavior in females tested in both estrus and diestrus phase under red light. A) Total distance traveled in the open field within subject during test 1 and test 2. B) Time spent in the center of the open field within subject during test 1 and test 2. C) Number of entries into the center of the open field within subject during test 1 and test 2. D) Latency to enter the center of the open field within subject during test 1 and test 2. Acronyms: **=P<0.01
Experiment 2: Novelty independent of estrous cycle phase influences locomotor but not anxiety-related behaviors under white light.
Females showed increased total distance traveled in the open field during the first compared to the second test regardless of estrous cycle phase sequence (Figure 3A; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=0.401, p=0.538, η2p=0.032; Cycle Sequence: F(1,12)=0.968, p=0.345; Test: F(1,12)=17.36, p=0.001; Subject: F(12,12)=5.565, p=0.002; Estrus first test 1- test 2: t(12)=3.395, p=0.011; Diestrus first test 1-test 2: t(12)=2.499, p=0.028). No significant differences in time spent in the center of the open field were detected in either estrous cycle group (Figure 3B; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=1.902, p=0.193, η2p=0.137; Cycle Sequence: F(1,12)=1.396, p=0.260; Test: F(1,12)=0.282, p=0.605; Subject: F(12,12)=1.488, p=0.251). No significant differences in the number of entries into the center of the open field were detected in either estrous cycle group (Figure 3C; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=3.799, p=0.075, η2p=0.240; Cycle Sequence: F(1,12)=2.263, p=0.158; Test: F(1,12)=2.532, p=0.138; Subject: F(12,12)=4.364, p=0.008). Females first tested in estrus showed decreased latency to enter the center of the open field compared to a second test in diestrus (Figure 3D; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=1.073, p=0.321, η2p=0.082; Cycle Sequence: F(1,12)=0.002, p=0.968; Test: F(1,12)=12.22, p=0.004; Subject: F(12,12)=1.824, p=0.156; Estrus first test 1- test 2: t(12)=3.204, p=0.015; Diestrus first test 1-test 2: t(12)=1.739, p=0.108). Overall this data indicates that under white light, females exhibited increased locomotor behavior during the first compared to the second behavioral test due to novelty, and that the estrous cycle phase did not robustly influence locomotor or anxiety-related behaviors.
Figure 3.
Novelty independent of estrous cycle phase influenced locomotor but not anxiety-related behavior in females tested in both estrus and diestrus phase under white light. A) Total distance traveled in the open field within subject during test 1 and test 2. B) Time spent in the center of the open field within subject during test 1 and test 2. C) Number of entries into the center of the open field within subject during test 1 and test 2. D) Latency to enter the center of the open field within subject during test 1 and 2. Acronyms: *=P<0.05
Experiment 3: Novelty does not robustly influence locomotor or anxiety-related behaviors under red light in females repeatedly tested in estrus or diestrus.
Experiments 1 and 2 establish that novelty can potentially modulate female behavior in the open field under specific variables, such as light type and estrous cycle phase sequence. To further decompose the specific effects of novelty, we repeatedly tested females in either estrus or diestrus under red light. We chose red light given that novelty and the estrous phase showed a significant interaction in experiment 1. We reasoned that if the effects of novelty were more influential than the effects of the estrous cycle phase, novelty would exert a robust and similar effect across experimental groups. Furthermore, it is possible that novelty would exert differential effects between females in estrus or diestrus.
No significant differences in total distance traveled in the open field were detected in females repeatedly tested in estrus compared to those tested in diestrus (Figure 4A; Two-way RM ANOVA: Cycle Phase x Test: F(1,13)=0.066, p=0.801, η2p=0.005; Cycle Phase: F(1,13)=1.887, p=0.193; Test: F(1,13)=1.553, p=0.235; Subject: F(13,13)=4.230, p=0.007). No significant differences in time spent in the center of the open field were detected in females repeatedly tested in estrus compared to those tested in diestrus (Figure 4B; Two-way RM ANOVA: Cycle Phase x Test: F(1,13)=0.363, p=0.557, η2p=0.027; Cycle Phase: F(1,13)=0.893, p=0.362; Test: F(1,13)=0.114, p=0.742; Subject: F(13,13)=3.363, p=0.019). No significant differences in the number of entries into the center of the open field were detected in females repeatedly tested in estrus compared to those tested in diestrus (Figure 4C; Two-way RM ANOVA: Cycle Phase x Test: F(1,13)=0.837, p=0.377, η2p=0.060; Cycle Phase: F(1,13)=3.049, p=0.104; Test: F(1,13)=1.008, p=0.334; Subject: F(13,13)=2.651, p=0.045). No significant differences in latency to enter the center of the open field were detected in females repeatedly tested in estrus compared to those tested in diestrus (Figure 4D; Two-way RM ANOVA: Cycle Phase x Test: F(1,13)=0.323, p=0.580, η2p=0.024; Cycle Phase: F(1,13)=1.095, p=0.314; Test: F(1,13)=1.533, p=0.238; Subject: F(13,13)=131.4, p<0.001). Overall these findings indicate that when tested twice within the same estrous cycle phase under red light, the potential effects of novelty and estrous cycle phase on female open field behavior can be mitigated, allowing for the accurate analysis of other variables.
Figure 4.
Novelty does not robustly influence locomotor or anxiety-related behaviors under red light in females tested twice in either estrus or diestrus phase. A) Total distance traveled in the open field within subject during test 1 and test 2. B) Time spent in the center of the open field within subject during test 1 and 2. C) Number of entries into the center of the open field within subject during test 1 and 2. D) Latency to enter the center of the open field within subject during test 1 and 2.
Animals in estrus show decreased anxiety-related behaviors under red light during the second behavioral test.
The results of experiments 1 and 3 predict that mean differences in open field behaviors between females in estrus and diestrus phases may manifest in large populations, but only when the influences of novelty are controlled, such as in the second test. To test this prediction, we analyzed data from experiments 1 and 3. Females were sorted by estrous cycle phase and test number, independent of estrous cycle phase sequence. For example, data from all females who were in the estrus phase during test 1 in experiment 1 and 3 was combined and analyzed with the combination of data from all females who were in the diestrus phase during test 1 in experiment 1 and 3.
No significant differences in total distance traveled in the open field were detected between estrous cycle phases in the first (Figure 5A: t(28)=1.169, p=0.252, d=0.424) or second (Figure 5B: t(28)=1.971, p=0.059, d=0.720) behavioral test. No significant differences in time spent in the center of the open field were detected between estrous cycle phases in the first (Figure 5C: t(28)=0.119, p=0.907, d=0.043) or second (Figure 5D: t(28)=1.544, p=0.134, d=0.564) behavioral test. No significant differences in the number of entries into the center of the open field were detected between estrous cycle phases in the first behavioral test (Figure 5E: t(28)=0.179, p=0.859, d=0.065). However, females in estrus showed an increased number of entries compared to diestrus during the second test (Figure 5F: t(28)=2.281, p=0.030, d=0.833). No significant differences in latency to enter the center of the open field were detected between estrous cycle phases in the first (Figure 5G: t(28)=0.596, p=0.556, d=0.217) or second (Figure 5H: t(28)=1.476, p=0.151, d=0.539) behavioral test. This data indicates that under red light females exhibited decreased anxiety-related behavior in estrus compared to diestrus and that these effects were seen only during the second behavioral test.
Figure 5.
Females in estrus exhibit lower anxiety-related behavior compared to females in diestrus during test 2 but not test 1 under red light. Locomotor behavior did not significantly differ between phases during either test. A) Total distance traveled in the open field during test 1. B) Total distance traveled in the open field during test 2. C) Time spent in the center of the open field during test 1. D) Time spent in the center of the open field during test 2. E) Number of entries into the center of the open field during test 1. F) Number of entries into the center of the open field during test 2. G) Latency to enter the center of the open field during test 1. H) Latency to enter the center of the open field during test 2. Acronyms: *=P<0.05
Females exhibit increased locomotor and decreased anxiety-related behaviors under red light compared to white light.
The differing results of experiments 1 and 2 suggest that light exposure considerably influences open field behaviors. To test this conjecture, data from experiments 1 and 2 was sorted by estrous cycle phase and light type to analyze the effect of light on open field behavior independent of test number. Females showed increased total distance traveled in the open field under red light compared to white light in both estrous cycle phases (Figure 6A; Two-way ANOVA: Interaction: F(1,82)=0.415, p=0.521, η2p=0.005; Cycle Phase: F(1,82)=2.730, p=0.102; Light: F(1,82)=14.71, p<0.001; Diestrus white light – red light: t(82)=2.245, p=0.028; Estrus white light – red light: t(82)=3.184, p =0.004). Females showed increased time spent in the center of the open field under red light compared to white light in both estrous cycle phases (Figure 6B; Two-way ANOVA: Interaction: F(1,82)=0.110, p=0.741, η2p=0.001; Cycle Phase: F(1,82)=1.561, p=0.215; Light: F(1,82)=17.08, p<0.001; Diestrus white light – red light: t(82)=2.675, p=0.009; Estrus white light – red light: t(82)=3.173, p =0.004). Females showed increased number of entries into the center of the open field under red light compared to white light in both estrous cycle phases (Figure 6C; Two-way ANOVA: Interaction: F(1,82)=0.031, p=0.860, η2p<0.001; Cycle Phase: F(1,82)=3.331, p=0.072; Light: F(1,82)=15.25, p<0.001; Diestrus white light – red light: t(82)=2.622, p=0.010; Estrus white light – red light: t(82)=2.901, p =0.009). Females showed a decrease in overall latency to enter the center of the open field under red light compared to white light, although this effect was not so robust as to be seen in individual estrous cycle phases (Figure 6D; Two-way ANOVA: Interaction: F(1,82)=0.071, p=0.790, η2p=0.001; Cycle Phase: F(1,82)=2.304, p=0.133; Light: F(1,82)=6.915, p=0.010; Diestrus white light – red light: t(82)=2.037, p=0.088; Estrus white light – red light: t(82)=1.679, p =0.097). Overall, this data indicates that females traveled more and exhibited decreased anxiety-related behaviors under red light than white light regardless of estrous cycle phase.
Figure 6.
Females in both estrus and diestrus phases exhibited reduced locomotor behavior and increased anxiety-related behavior when tested under white compared to red light. A) Total distance traveled in the open field. B) Time spent in the center of the open field. C) Number of entries into the center of the open field. D) Latency to enter the center of the open field. Acronyms: *=P<0.05, **=P<0.01
Experiment 4: Novelty influences male locomotor behaviors independent of light type, but influences anxiety-related behaviors only under red light.
Males were sequentially tested in the open field under both red and white light. Males showed a decrease in total distance traveled in the open field from the first test to the second test regardless of light type (Figure 7A; Two-way RM ANOVA: Light type x Test: F(1,12)=0.206, p=0.658, η2p=0.017; Light type: F(1,12)=0.189, p=0.672; Test: F(1,12)=18.68, p =0.001; Subject: F(12,12)=7.084, p =0.001; Red light test 1-test 2: t(12)=3.648, p=0.007; White light test 1-test 2: t(12)=2.559, p=0.025). Males tested under red light showed a decrease in time spent in the center of the open field from the first to the second test (Figure 7B; Two-way RM ANOVA: Light type x Test: F(1,12)=7.723, p=0.017, η2p=0.392; Light type: F(1,12)=5.897, p=0.032; Test: F(1,12)=10.67, p =0.007; Subject: F(12,12)=1.174, p =0.393; Red light test 1-test 2: t(12)=4.617, p=0.001; White light test 1-test 2: t(12)=0.322, p=0.753). This difference between tests was not seen when males were tested under white light. Males tested under red light showed a decrease in number of entries into the center of the open field from the first to the second test (Figure 7C; Two-way RM ANOVA: Light type x Test: F(1,12)=5.317, p=0.040, η2p=0.307; Light type: F(1,12)=8.20, p=0.014; Test: F(1,12)=7.427, p =0.018; Subject: F(12,12)=1.428, p =0.274; Red light test 1-test 2: t(12)=3.843, p=0.005; White light test 1-test 2: t(12)=0.277, p=0.786). This difference between tests was not seen when males were tested under white light. No significant differences in latency to enter the center of the open field were detected between tests, but latency was significantly decreased in both tests under red light (Figure 7D; Two-way RM ANOVA: Light type x Test: F(1,12)=0.086, p=0.774, η2p=0.007; Light type: F(1,12)=20.820, p=0.001; Test: F(1,12)=0.676, p =0.427; Subject: F(12,12)=0.650, p =0.767). Overall, this data indicates that males exhibited decreased locomotor behavior from the first to the second test independent of light type. Males exhibited decreased anxiety-related behaviors during the first compared to the second test only under red light.
Figure 7.
Novelty influenced locomotor behavior in males under both red and white light, and anxiety-related behavior in males under red light only. A) Total distance traveled in the open field within subject during test 1 and test 2. B) Time spent in the center of the open field within subject during test 1 and test 2. C) Number of entries into the center of the open field within subject during test 1 and test 2. D) Latency to enter the center of the open field within subject during test 1 and test 2. Acronyms: *=P<0.05, **=P<0.01
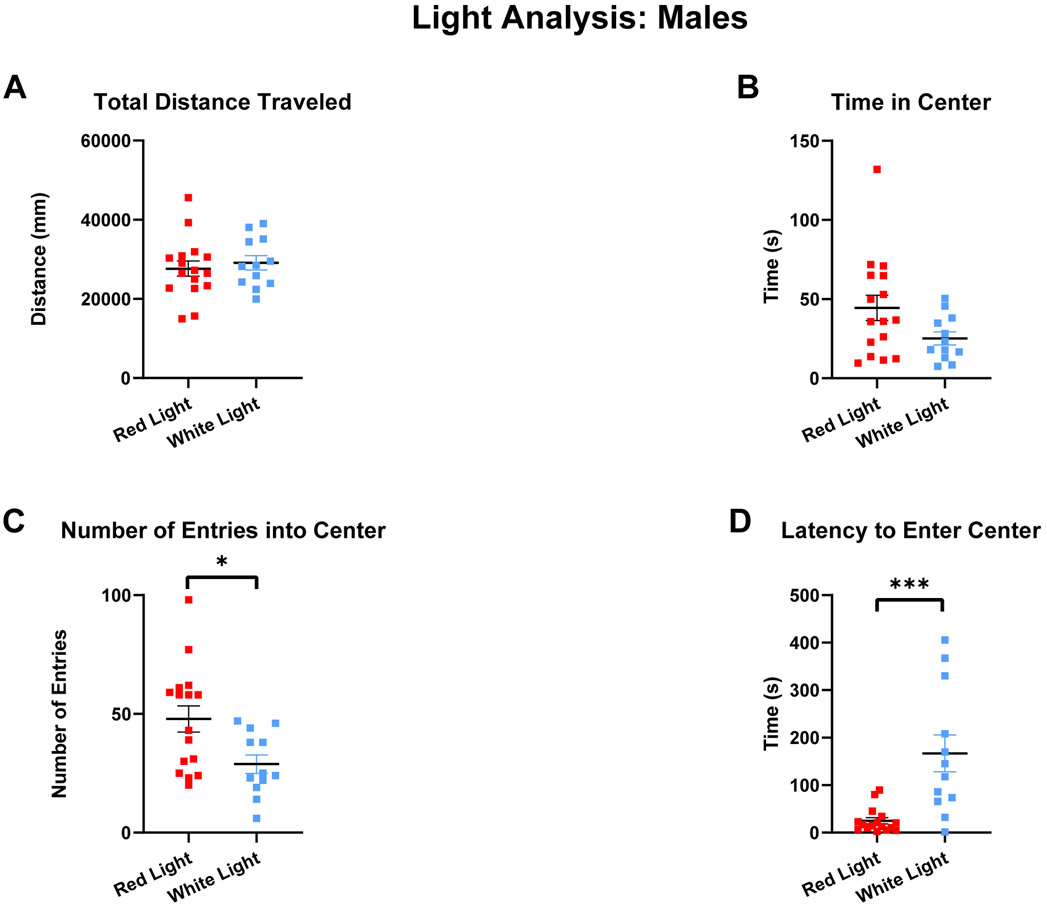
Males exhibit decreased anxiety-related behaviors but no differences in locomotor behaviors under red light compared to white light.
Results from experiment 4 establish that light type influences male open field behavior. To further test this conclusion, data from experiment 4 was analyzed independent of test number. No significant differences were found in the total distance traveled in the open field in males between light types (Figure 8A: t(26)=0.545, p=0.590, d=0.211). No significant differences were found in the time spent in the center of the open field in males between light types (Figure 8B: t(26)=1.946, p=0.063, d=0.780). Males showed increased number of entries into the center of the open field under red light compared to white light (Figure 8C: t(26)=2.631, p=0.014, d=1.040). Males showed decreased latency to enter the center of the open field under red light compared to white light (Figure 8D: t(26)=4.142, p<0.001, d=1.464). Overall, this data indicates that males exhibited decreased anxiety-related behaviors but no differences in locomotor behaviors under red light compared to white light.
Figure 8.
Males exhibited increased anxiety-related behavior under white compared to red light. Male locomotor behavior was not influenced by light. A) Total distance traveled in the open field. B) Time spent in the center of the open field. C) Number of entries into the center of the open field. D) Latency to enter the center of the open field. Acronyms: *=P<0.05, ***=P<0.001
Sex differences: Females in estrus exhibit decreased anxiety-related but not locomotor behavior compared to males under red light.
The investigation for females and males alone suggest the hypothesis that estrous cycle-induced sex differences exist in open field behavior between tests 1 and 2. To test this hypothesis, data from males tested under red light from experiment 4 was compared with data from females under red light from experiment 3. No significant differences were detected in total distance traveled in the open field when data was analyzed as the change between the two tests and compared across groups (Figure 9A; One-way ANOVA: F(2,20)=0.320, p=0.730, η2p=0.031). Females in estrus significantly differed from males when the time spent in the center of the open field was analyzed as the change between the two tests and compared across groups (Figure 9B; One-way ANOVA: F(2,20)=4.362, p=0.027, η2p=0.304; Males vs Females Estrus: t(20)=2.824, p=0.031; Males vs. Females Diestrus: t(20)=2.121, p=0.091; Females Estrus vs Females Diestrus: t(20)=0.608, p=0.550). No significant differences were detected in the number of entries into the center of the open field when the data was analyzed as the change between the two tests and compared across groups (Figure 9C; One-way ANOVA: F(2,20)=3.158, p=0.064, η2p=0.240). No significant differences were detected in latency to enter the center of the open field when the data was analyzed as the change between the two tests and compared across groups (Figure 9D; One-way ANOVA: F(2,20)=0.248, p=0.783, η2p=0.024). Overall, this data indicates that under red light females in estrus exhibited decreased anxiety-related behaviors than males but no differences in locomotor behaviors.
Figure 9.
Females in estrus exhibit decreased anxiety-related behavior compared to males but no differences in locomotor behavior. A) Difference in the total distance traveled in the open field from test 2 to test 1. B) Difference in the time spent in the center of the open field from test 2 to test 1. C) Difference in the number of entries into the center of the open field from test 2 to test 1. D) Difference in the latency to enter the center of the open field from test 2 to test 1. Acronyms: *=P<0.05
Minor differences emerge when assessing behavior over a 10 versus 30 minute period
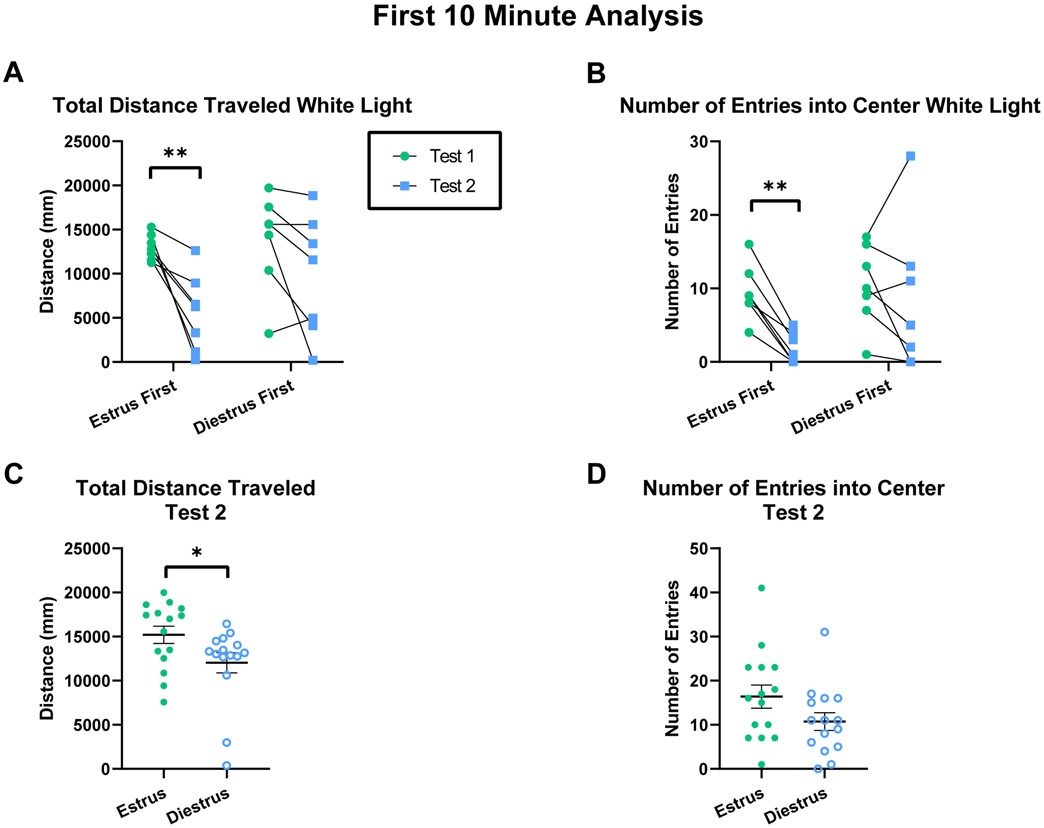
Given that open field activity is typically assessed within ten minutes, it is possible that this study’s test length of thirty minutes reveals different results than a shorter behavioral assessment. To test this, data from each analysis was reanalyzed for the first ten minutes of open field exposure and the analyses that differ are depicted here.
When analyzing the first 10 minutes of behavior from experiment 2 under white light, females first tested in estrus showed increased total distance traveled in the open field compared to a second test in diestrus, but no differences between tests were observed when females were first tested in diestrus (Figure 10A; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=1.728, p=0.213, η2p=0.126; Cycle Sequence: F(1,12)=1.187, p=0.297; Test: F(1,12)=18.81, p=0.001; Subject: F(12,12)=3.050, p=0.032; Estrus first test 1- test 2: t(12)=3.997, p=0.004; Diestrus first test 1-test 2: t(12)=2.138, p=0.054). When analyzing the first 10 minutes of behavior from experiment 2 under white light, females first tested in estrus showed an increased number of entries into the center of the open field compared to a second test in diestrus, but no differences between tests were observed when females were first tested in diestrus (Figure 10B; Two-way RM ANOVA: Cycle Sequence x Test: F(1,12)=3.545, p=0.084, η2p=0.228; Cycle Sequence: F(1,12)=1.681, p=0.219; Test: F(1,12)=10.46, p=0.007; Subject: F(12,12)=3.895, p=0.013; Estrus first test 1- test 2: t(12)=3.619, p=0.007; Diestrus first test 1-test 2: t(12)=0.956, p=0.358). When analyzing the first 10 minutes of the second test from experiments 1 and 3 under red light, estrus females traveled significantly more than diestrus females (Figure 10C; t(28)=2.107, p=0.044, d=0.769). When analyzing the first 10 minutes of the second test from experiments 1 and 3 under red light, no significant differences were found in the number of entries into the center of the open field between estrus and diestrus females (Figure 10D; t(28)=1.711, p=0.098, d=0.625). Overall, this data indicates that differences may be either more or less robust when analyzing 10 compared to 30 minutes of open field behavior.
Figure 10.
Differing results after analyzing the first 10 minutes of behavior. A) Total distance traveled in the open field within the first 10 minutes under white light. B) Number of entries into the center of the open field within the first 10 minutes under white light. C) Total distance traveled in the open field within the first 10 minutes of test 2 under red light. D) Number of entries into the center of the open field within the first 10 minutes of test 2 under red light. Acronyms: *=P<0.05, **=P<0.01
Discussion
Our findings highlight the importance of multiple interacting factors that can influence a specific animal behavior, in this case rat open field behavior in the context of the female estrous cycle, exploration of a novel environment, and light. To summarize, these findings indicate that under red light, both novelty and the estrous cycle influence female rat locomotor and anxiety-related behaviors. However, under red light, novelty abrogates the impact of the estrous cycle on locomotor and anxiety-related behavior. Under white light, novelty but not the estrous cycle impacts locomotor behaviors. Anxiety-related behaviors are elevated in white compared to red light, and neither novelty nor estrous cycle exert as robust an impact. White light appears to be a more robust modulator of locomotor behavior in females than males.
This summary is premised upon seven key findings generated by this series of experiments. First, in females tested under red light, an interaction between novelty and estrous cycle phase influenced locomotor behaviors. Second, anxiety-related behaviors were decreased in females in estrus compared to diestrus on the second but not first day of behavioral testing under red light. Third, in females tested under white light, novelty influenced locomotor but not anxiety-related behaviors and no estrous cycle effects were observed. Fourth, novelty influenced male locomotor behaviors independent of light exposure, but influenced anxiety-related behaviors only under red light. Fifth, females exhibited increased locomotor behavior under red light. Sixth, no differences were observed in male locomotor behavior under red or white light exposure. Seventh, females and males exhibited increased anxiety-related behaviors under white compared to red light.
Throughout the literature studies have observed that female rats during the estrus phase are more active than females in the diestrus phase [4, 7, 8, 29-31]. This observation has been further explored with exogenous estradiol treatment, where increased levels of estradiol increase locomotor behavior [8, 9, 23, 32-35]. However, occasional studies targeting hormone-induced locomotor behavior have detected no differences in behavior across the estrous cycle or with estradiol replacement [2, 11, 32, 36-38]. Similarly, anxiety-related behaviors in the context of the estrous cycle and ovarian hormone levels have conflicting results across experiments and behavioral tests. Generally, decreased anxiety-related behaviors are seen in estrus and proestrus phases compared to diestrus, as well as with the treatment of exogenous estradiol in ovariectomized females [2, 5, 6, 10, 11, 39]. However, some studies have not detected any changes in anxiety-related behavior across the estrous cycle or in the presence of exogenous estradiol [11, 37, 38, 40]. Likely, difference in test length, lighting, habituation times, estradiol dosage, light cycle, and other factors can explain these inconsistent findings [41]. Our current study observed changes in locomotor behavior between estrous cycle phases only when analyzing the differences between estrus and diestrus as a within-subject comparison, and observed anxiety-related estrous cycle changes only during the second test. Additionally, both of these observations occurred only under red light. Test length also played a role in identifying these estrous cycle phase differences, as no significant differences were found in anxiety-related behavior between estrus and diestrus phases during the second test when only the first ten minutes were analyzed. Conversely, female locomotor behavior during the second test was significantly increased during estrus compared to diestrus when only the first ten minutes were analyzed. This may explain some of the previous inconsistencies, as many behavioral tests do not control for the novelty of the behavioral test, use varying levels of light in the testing arena, and differ in test length [27, 41]. These interacting and concurrent factors during behavioral testing may lead to undetectable effects of the estrous cycle. Thus, to maximally detect estrous cycle effects in the open field, testing should occur under red light and novelty must be controlled. We recommend that researchers interested in estrous cycle effects should expose rats more than once to the open field.
The introduction of a novel environment both increases the motivation to explore and induces a stress response, whereas additional exposure to the same environment results in a decrease of behavioral responses, or habituation [13, 42]. This effect can be seen after repeated exposure to the open field, where locomotor activity generally decreases after the first test [32, 43]. In some studies, the effect of novelty is considered to occur within the first five minutes of the open field test, however others use multiple exposures of the open field to assess learning and memory by observing the habituation response between tests [43]. Testing rats twice in the open field for 30 minutes each is useful in the current study to both assess the role of novelty on locomotor and anxiety-related behavior and to better illustrate the effects of the estrous cycle by assessing general activity over a longer testing period and using the change in behavior between tests to account for individual variation. Variation of response to novelty in the open field may lead to an oversight of behavioral patterns that can only be seen with repeated testing, especially in the context of the estrous cycle. It is common to observe high individual variation in the responses of rats to a novel environment, and by separating rats into groups of high and low responders to novelty one study revealed that females in estrus and proestrus may be more influenced by novelty than those in diestrus [44]. As our results have demonstrated, under red light a response to novelty results in decreased locomotor behaviors between tests in males and in females tested in estrus first. Interestingly, this novelty effect is not seen under red light in females tested in diestrus first or in the same estrous cycle phase, demonstrating that females may respond differently to novelty across the estrous cycle. In other studies using males and gonadectomized females in the open field, activity levels decrease across tests, but in some cases estradiol treatment in females can increase or eliminate this change [32, 35]. This suggests that similar or increased levels of ovarian hormones from the first to second test preclude the effect of habituation on activity levels. Similarly, under red light anxiety-related behaviors decreased across tests in males but remained the same across tests in females. Because introduction of a novel environment leads to an approach-avoidance conflict, male rats upon first exposure to the open field may highly explore the perimeters but avoid the center of the open field, where females under the influence of ovarian hormones may additionally display reduced anxiety-related behaviors in response to the novel environment [45, 46]. A second exposure to the open field may result in lower activity in males due to habituation and thus lower entries into the center, but females under the simultaneous influence of ovarian hormones may still exhibit increased exploratory behavior and decreased anxiety-related behavior [2, 9, 42]. This could explain the similarities in female anxiety-related behavior seen between tests. Together, these findings reveal a strong interaction between novelty and the estrous cycle on locomotor and anxiety-related behaviors, where not only novelty may conceal estrous cycle effects, but the estrous cycle can in turn conceal the effect of novelty. To summarize, when attempting to analyze the influence of novelty on female locomotor and anxiety-related behavior under red light, testing should account for individual variation and estrous cycle effects.
As it is important to consider that novelty interacts with estrous cycle phase, it is also important to consider that the effect of novelty is not separate from the effect of light on locomotor and anxiety-related behaviors. The introduction of a novel environment may increase exploratory behaviors, but the presence of light often has the opposite effect [13, 16, 40, 47]. In experiments that assess novelty-induced exploratory behavior in male rats, the presence of light decreases overall locomotion, which may overshadow the novelty response [48, 49]. Although our results revealed no differences in male locomotor behavior between white and red light, we see a more robust decrease in locomotion between tests under red light than under white light which may be due to the interacting effect of light on novelty. In contrast, females show reduced locomotor behaviors between tests under white light regardless of estrous cycle phase, unlike red light where reduced locomotion between tests is estrous cycle sequence dependent. This suggests that novelty or the effects of habituation may be highlighted by the presence of white light, however this conclusion does not take into account the effects of the estrous cycle which may differentially influence rodent locomotor behavior under white compared to red light [50]. In both males and females, no effects of novelty or habituation on anxiety-related behavior are observed under white light. Both of these factors usually exert an increase in anxiety-related behavior [5, 13, 47, 51, 52]. Indeed, the combined effect of light and novelty during test one and the continued effect of light during test two likely explains the minimal differences in anxiety-related behavior between tests in both males and females. In females, light sensitivity in respect to anxiety-related behaviors may even vary across the estrous cycle, providing an even more complex interaction of factors on behavior [5, 50]. Curiously, an interaction between novelty and the estrous cycle is detected in the presence of light when assessing only the first ten minutes of behavior. Within the first ten minutes, females tested in estrus first exhibit a decrease between tests in locomotion and entries into the center where females in tested in diestrus first do not, an effect not observed when assessing the full thirty minutes of behavior. This detection suggests that a novelty-estrous cycle interaction may occur under white light similar to that under red light, but can only be seen with a shorter test duration. Due to the anxiogenic effect of light and the decreased effect of novelty-induced exploration over time, limited activity during the later period of the test may explain why this detection occurs only during a shorter testing period. Consequently, to maximally detect the influence of novelty on locomotor and anxiety-related behavior, we recommend that testing should be conducted under red light. Additionally, although testing the influence of light on locomotor and anxiety-related behaviors will likely be robust in any experimental paradigm, light effects may best be observed when controlling for novelty, using a shorter test duration, and considering potential estrous cycle interactions.
Interactions of the estrous cycle, novelty, and light may be further evident when comparing male and female behavior in the open field and placing our findings into the context of previously identified sex differences. For example, the current experiment observed a consistent influence of novelty and habituation under red light on locomotor and anxiety-related behaviors in males, but these effects on female behaviors were less robust and were estrous cycle dependent. Females are historically indicated to be more exploratory and active than males, and may even show reduced habituation responses [8, 35, 53]. One explanation for the reduced habituation response observed in our study and the commonly observed increased exploration in females compared to males is evolutionary, as it may be necessary for females to gain more familiarity with the environment to ensure safety when mothering [54]. Increased activity in females, particularly occurring during estrus and proestrus, could also arise from a motivation to travel greater distances in search of a mate and to signal receptivity for copulation [55]. Furthermore, an increase of locomotion in females during times of sexual receptivity may represent antecedent solicitation behaviors induced by ovarian hormones such as pacing or darting, although these behaviors typically occur in the presence of a male [55-57]. Our study did not detect a significant difference between males and females in the change in locomotor behaviors across test days, but as some measurements are better than others in detecting sex differences [24], it may be that the open field is not as robust an indicator of sex-differences in locomotion than a test such as the voluntary running wheel task. In fact, other studies have also been unable to detect sex differences in locomotor activity in the open field task, even though they find these differences using other behavioral tests [11, 25]. Our study observed decreased anxiety-related behavior in females in estrus compared to males when analyzing the differences between tests, which is consistent with previous studies that show a lower anxiety phenotype in female rodents compared to males [11, 58, 59]. Interestingly, exposure to light exerted similar effects to increase anxiety in both males and females, but light exposure reduced locomotor activity only in females. Light can be seen to reduce male locomotor activity, but also in other situations induces no effect on locomotion which may be due to other environmental variables such as light intensity or testing method [47, 52, 60, 61]. An increased sensitivity to light and greater increase of locomotion in response to darkness seen in females compared to males could explain the more robust effect of light on female locomotion seen in our study [5, 62]. These sex differences further present the estrous cycle in females as an important modulator of behavior, exhibiting interactions with both novelty and light.
Our experiment focused on females in the diestrus and estrus phases, but it is important to mention that in addition to estrus, females in proestrus also exhibit increased locomotion and decreased anxiety-related behavior compared to diestrus [2, 5, 6, 29]. The present study chose to test during the first day of estrus, or behavioral estrus, due to the timing of behavioral testing to ensure the continued effect of ovarian hormones. During proestrus, estradiol levels typically peak in the middle of the animals’ light cycle, followed by a surge of progesterone, before returning to baseline and inducing behavioral effects [24]. As our behavioral testing occurred during the first hours of the rats’ dark cycle to achieve a natural level of activity, it was important to measure behavior in behavioral estrus following these hormonal surges to maximize the impact of ovarian hormones. Testing females in the early proestrus phase during their dark cycle could have been premature and resulted in behavioral measurements occurring before hormonal surges, explaining why experiments that include the proestrus phase effects often take place during the animals’ light cycle [2, 5].
Although the open field test is widely used for the measurement of locomotor and anxiety-related behavior in various contexts [17, 27, 63], inconsistent results have led to a discussion of its ethological relevance and validity [15, 64]. In particular, it is suggested that natural activity may be more accurately assessed in a familiar environment, that the test may measure risk aversion or lack of exploratory interest rather than anxiety, and that overgeneralization may occur due to the variation of animal personalities [64-66]. These concerns originate from discrepancies across studies, where although the open field test was validated by detecting rodent sensitivity to anxiolytic benzodiazapenes and activity-inducing psychostimulants, using this test to measure sensitivity to other pharmaceuticals intended to treat anxiety and depression do not generate the same results [17, 65]. It is possible that these treatments as well as other behavioral constructs such as the estrous cycle are not as robust as the drugs used for validation during the first exposure to the open field. As noted in the current study, several factors may contribute to this including the influence of novelty on open field behavior which obscures estrous cycle effects on locomotor and anxiety-related behaviors during the first open field exposure and anxiety-related behavior during the initial ten minutes of testing. Other testing parameters that are not always held consistent such as light level, apparatus set up, sex, animal handling, etc. may further account for the discrepancies of the open field and should be carefully considered prior to experimental design [15, 41]. Acknowledging these cautions, the open field remains a highly useful test to include among other behavioral assessments that assess similar traits because of its ease of use, abundance of data, and versatility [27, 66]. Thus, the goal of the present study to identify and dismantle the factors influencing behavior within the open field will be useful for many laboratories that incorporate this test into their batteries of behavioral assessment.
In conclusion, our findings indicate that interpretation of female open field locomotor and anxiety-related behavior may be most accurate with the consideration of interacting factors such as the estrous cycle, novelty, and light exposure. Our results observed independent interactions between the estrous cycle and novelty, the estrous cycle and light, and novelty and light wherein each factor is concurrently influencing behavior. To minimize the interactions of these factors when analyzing locomotor and anxiety-related behavior in the open field, we have the following recommendations. First, if considering the role of the estrous cycle on behavior, we recommend testing under red light and controlling for novelty. Second, if analyzing the role of novelty on behavior, we recommend testing under red light and to account for influences of the estrous cycle. Third, if analyzing the role of light on behavior, we recommend controlling for novelty and accounting for potential estrous cycle interactions. Collectively, this research illustrates how multiple variables can acutely modulate each other in different contexts and demonstrates the importance of considering each of these factors when assessing behavior.
Highlights.
Estrous cycle phase interacts with novelty to modulate the total distance traveled in the open field.
Anxiety-related behaviors on the second test day are decreased in females during the estrus compared to diestrus phase.
Novelty and light exposure differentially influence female and male open-field behavior.
Acknowledgements
We thank Dr. Amanda Krentzel and David Dorris for technical assistance, and Stephanie Proaño for sharing equipment.
Funding Sources
Funding: This work was supported by the NIH R01 MH109471 (JM) and NIH P30ES025128 (Center for Human Health and the Environment).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Gonda X, Telek T, Juhasz G, Lazary J, Vargha A, Bagdy G Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Progress in neuro-psychopharmacology & biological psychiatry. 2008,32:1782–8. [DOI] [PubMed] [Google Scholar]
- [2].Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & behavior. 2001,74:435–40. [DOI] [PubMed] [Google Scholar]
- [3].Becker JB Behavioral endocrinology. 2nd ed. Cambridge, Mass: MIT Press; 2002. [Google Scholar]
- [4].Slonaker JR The effect of pubescence, oestruation, and menopause on the voluntary activity in the albino rat. Am J Physiol. 1924,68:294–345. [Google Scholar]
- [5].Mora S, Dussaubat N, Diaz-Veliz G Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996,21:609–20. [DOI] [PubMed] [Google Scholar]
- [6].Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacology, biochemistry, and behavior. 1997,58:637–42. [DOI] [PubMed] [Google Scholar]
- [7].Burke AW, Broadhurst PL Behavioural correlates of the oestrous cycle in the rat. Nature. 1966,209:223–4. [DOI] [PubMed] [Google Scholar]
- [8].Beatty WW Gonadal hormones and sex differences in nonreproductive behaviors in rodents: organizational and activational influences. Hormones and behavior. 1979,12:112–63. [DOI] [PubMed] [Google Scholar]
- [9].Stewart J, Cygan D Ovarian hormones act early in development to feminize adult open-field behavior in the rat. Hormones and behavior. 1980,14:20–32. [DOI] [PubMed] [Google Scholar]
- [10].Frye CA, Petralia SM, Rhodes ME Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacology, biochemistry, and behavior. 2000,67:587–96. [DOI] [PubMed] [Google Scholar]
- [11].Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL Sex differences in anxiety-like behaviors in rats. Physiology & behavior. 2019,211:112670. [DOI] [PubMed] [Google Scholar]
- [12].Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes, brain, and behavior. 2007,6:192–200. [DOI] [PubMed] [Google Scholar]
- [13].van den Buuse M, van Acker SA, Fluttert MF, de Kloet ER Involvement of corticosterone in cardiovascular responses to an open-field novelty stressor in freely moving rats. Physiology & behavior. 2002,75:207–15. [DOI] [PubMed] [Google Scholar]
- [14].Claustrat B, Valatx JL, Harthe C, Brun J Effect of constant light on prolactin and corticosterone rhythms evaluated using a noninvasive urine sampling protocol in the rat. Hormone and metabolic research. 2008,40:398–403. [DOI] [PubMed] [Google Scholar]
- [15].Walsh RN, Cummins RA The Open-Field Test: a critical review. Psychological bulletin. 1976,83:482–504. [PubMed] [Google Scholar]
- [16].Miller CK, Krentzel AA, Patisaul HB, Meitzen J Metabotropic glutamate receptor subtype 5 (mGlu5) is necessary for estradiol mitigation of light-induced anxiety behavior in female rats. Physiology & behavior. 2020,214:112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Prut L, Belzung C The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003,463:3–33. [DOI] [PubMed] [Google Scholar]
- [18].Mani SK, Reyna AM, Alejandro MA, Crowley J, Markaverich BM Disruption of male sexual behavior in rats by tetrahydrofurandiols (THF-diols). Steroids. 2005,70:750–4. [DOI] [PubMed] [Google Scholar]
- [19].Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, et al. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environmental health perspectives. 2002,110:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Villalon Landeros R, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-alpha expression in the brain. Endocrinology. 2012,153:949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hubscher CH, Brooks DL, Johnson JR A quantitative method for assessing stages of the rat estrous cycle. Biotechnic & histochemistry. 2005,80:79–87. [DOI] [PubMed] [Google Scholar]
- [22].Proano SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. Journal of neurophysiology. 2018,120:1356–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Krentzel AA, Proano S, Patisaul HB, Meitzen J Temporal and bidirectional influences of estradiol on voluntary wheel running in adult female and male rats. Hormones and behavior. 2020,120:104694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005,146:1650–73. [DOI] [PubMed] [Google Scholar]
- [25].Baldwin KR, Phillips AL, Horman B, Arambula SE, Rebuli ME, Stapleton HM, et al. Sex Specific Placental Accumulation and Behavioral Effects of Developmental Firemaster 550 Exposure in Wistar Rats. Sci Rep. 2017,7:7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor DL, Patisaul HB Impact of Low-Dose Oral Exposure to Bisphenol A (BPA) on Juvenile and Adult Rat Exploratory and Anxiety Behavior: A CLARITY-BPA Consortium Study. Toxicological sciences. 2015,148:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gould TD Mood and anxiety related phenotypes in mice : characterization using behavioral tests. New York, NY: Humana Press; 2009. [Google Scholar]
- [28].Blizard DA, Takahashi A, Galsworthy MJ, Martin B, Koide T Test standardization in behavioural neuroscience: a response to Stanford. Journal of psychopharmacology. 2007,21:136–9. [DOI] [PubMed] [Google Scholar]
- [29].Scimonelli T, Marucco M, Celis ME Age-related changes in grooming behavior and motor activity in female rats. Physiology & behavior. 1999,66:481–4. [DOI] [PubMed] [Google Scholar]
- [30].Petersson M, Ahlenius S, Wiberg U, Alster P, Uvnas-Moberg K Steroid dependent effects of oxytocin on spontaneous motor activity in female rats. Brain research bulletin. 1998,45:301–5. [DOI] [PubMed] [Google Scholar]
- [31].Martin JR, Battig K Exploratory behaviour of rats at oestrus. Animal behaviour. 1980,28 Pt 3:900–5. [DOI] [PubMed] [Google Scholar]
- [32].Fahrbach SE, Meisel RL, Pfaff DW Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiology & behavior. 1985,35:985–92. [DOI] [PubMed] [Google Scholar]
- [33].Espinosa E, Curtis KS Increased locomotor activity in estrogen-treated ovariectomized rats is associated with nucleus accumbens dopamine and is not reduced by dietary sodium deprivation. Integrative zoology. 2018,13:783–94. [DOI] [PubMed] [Google Scholar]
- [34].Slater J, Blizard DA A reevaluation of the relation between estrogen and emotionality in female rats. Journal of comparative and physiological psychology. 1976,90:755–64. [DOI] [PubMed] [Google Scholar]
- [35].Blizard DA, Lippman HR, Chen JJ Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiology & behavior. 1975,14:601–8. [DOI] [PubMed] [Google Scholar]
- [36].Palermo-Neto J, Dorce VA Influences of estrogen and/or progesterone on some dopamine related behavior in rats. General pharmacology. 1990,21:83–7. [DOI] [PubMed] [Google Scholar]
- [37].Gogos A, McCarthy M, Walker AJ, Udawela M, Gibbons A, Dean B, et al. Differential effects of chronic 17beta-oestradiol treatment on rat behaviours relevant to depression. Journal of neuroendocrinology. 2018,30:e12652. [DOI] [PubMed] [Google Scholar]
- [38].Byrnes EM, Bridges RS Reproductive experience alters anxiety-like behavior in the female rat. Hormones and behavior. 2006,50:70–6. [DOI] [PubMed] [Google Scholar]
- [39].Bowman RE, Ferguson D, Luine VN Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002,113:401–10. [DOI] [PubMed] [Google Scholar]
- [40].Sakae DY, Sakae TM, Paschoalini MA, Faria MS Relative luminosity in the plus maze upon the exploratory behaviour of female Wistar rats. Arquivos de neuro-psiquiatria. 2015,73:601–6. [DOI] [PubMed] [Google Scholar]
- [41].Sestakova N, Puzserova A, Kluknavsky M, Bernatova I Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdisciplinary toxicology. 2013,6:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thompson RF, Spencer WA Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological review. 1966,73:16–43. [DOI] [PubMed] [Google Scholar]
- [43].Leussis MP, Bolivar VJ Habituation in rodents: a review of behavior, neurobiology, and genetics. Neuroscience and biobehavioral reviews. 2006,30:1045–64. [DOI] [PubMed] [Google Scholar]
- [44].Sell SL, Dillon AM, Cunningham KA, Thomas ML Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behavioural brain research. 2005,161:69–74. [DOI] [PubMed] [Google Scholar]
- [45].Montgomery KC The relation between fear induced by novel stimulation and exploratory behavior. Journal of comparative and physiological psychology. 1955,48:254–60. [DOI] [PubMed] [Google Scholar]
- [46].Leret ML, Molina-Holgado F, Gonzalez MI The effect of perinatal exposure to estrogens on the sexually dimorphic response to novelty. Physiology & behavior. 1994,55:371–3. [DOI] [PubMed] [Google Scholar]
- [47].Valle FP Effects of strain, sex, and illumination on open-field behavior of rats. The American journal of psychology. 1970,83:103–11. [PubMed] [Google Scholar]
- [48].Roth KA, Katz RJ Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neuroscience and biobehavioral reviews. 1979,3:247–63. [DOI] [PubMed] [Google Scholar]
- [49].Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain research bulletin. 2007,72:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Datta S, Samanta D, Tiwary B, Chaudhuri AG, Chakrabarti N Sex and estrous cycle dependent changes in locomotor activity, anxiety and memory performance in aged mice after exposure of light at night. Behavioural brain research. 2019,365:198–209. [DOI] [PubMed] [Google Scholar]
- [51].Crawley J, Goodwin FK Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980,13:167–70. [DOI] [PubMed] [Google Scholar]
- [52].Morato S, Castrechini P Effects of floor surface and environmental illumination on exploratory activity in the elevated plus-maze. Brazilian journal of medical and biological research. 1989,22:707–10. [PubMed] [Google Scholar]
- [53].de Cabo de la Vega C, Pujol A, Paz Viveros M Neonatally administered naltrexone affects several behavioral responses in adult rats of both genders. Pharmacology, biochemistry, and behavior. 1995,50:277–86. [DOI] [PubMed] [Google Scholar]
- [54].Dubovicky M, Skultetyova I, Jezova D Neonatal stress alters habituation of exploratory behavior in adult male but not female rats. Pharmacology, biochemistry, and behavior. 1999,64:681–6. [DOI] [PubMed] [Google Scholar]
- [55].Calhoun JB The ecology and sociology of the Norway rat. Bethesda, Md,: U.S. Dept. of Health, Education, and Welfare for sale by the Superintendent of Documents, U.S. Govt. Print. Off; 1963. [Google Scholar]
- [56].Erskine MS Solicitation behavior in the estrous female rat: a review. Hormones and behavior. 1989,23:473–502. [DOI] [PubMed] [Google Scholar]
- [57].Mermelstein PG, Becker JB Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behavioral neuroscience. 1995,109:354–65. [DOI] [PubMed] [Google Scholar]
- [58].Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacology, biochemistry, and behavior. 2004,78:445–58. [DOI] [PubMed] [Google Scholar]
- [59].Johnston AL, File SE Sex differences in animal tests of anxiety. Physiology & behavior. 1991,49:245–50. [DOI] [PubMed] [Google Scholar]
- [60].Garcia AM, Cardenas FP, Morato S Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiology & behavior. 2005,85:265–70. [DOI] [PubMed] [Google Scholar]
- [61].Becker A, Grecksch G Illumination has no effect on rats' behavior in the elevated plus-maze. Physiology & behavior. 1996,59:1175–7. [DOI] [PubMed] [Google Scholar]
- [62].Nasello AG, Machado C, Bastos JF, Felicio LF Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiology & behavior. 1998,63:451–4. [DOI] [PubMed] [Google Scholar]
- [63].Hall C, Ballachey EL A study of the rat's behavior in a field: a contribution to method in comparative psychology. University of California Publications in Psychology; 1932,6:1–12. [Google Scholar]
- [64].Stanford SC The Open Field Test: reinventing the wheel. Journal of psychopharmacology. 2007,21:134–5. [DOI] [PubMed] [Google Scholar]
- [65].Ennaceur A Tests of unconditioned anxiety - pitfalls and disappointments. Physiology & behavior. 2014,135:55–71. [DOI] [PubMed] [Google Scholar]
- [66].Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R Animal personality: what are behavioural ecologists measuring? Biological reviews of the Cambridge Philosophical Society. 2013,88:465–75. [DOI] [PubMed] [Google Scholar]