Abstract
Background:
Homeobox B13 (HOXB13) expression regulates normal prostate development and mutations are associated with prostate cancer (PCa) formation.
Objective:
To assess the role of HOXB13 mRNA expression in PCa progression following radical prostatectomy.
Design, setting, and participants:
Genome-wide expression profiles were queried from two retrospective prostatectomy cohorts with follow-up data (Mayo Clinic, n = 780; Johns Hopkins Medical Institute [JHMI], n = 355), and a prospective genomic registry (n = 5239).
Outcome measurements and statistical analysis:
Multivariable Cox regressions were used to analyze metastasis-free survival (MFS).
Results and limitations:
HOXB13 expression in primary PCa increased with increasing tumor grade and with high metastatic potential based on a genomic signature. The highest quartile of HOXB13 expression was associated with worse MFS compared with the lowest quartile (Mayo Clinic: adjusted hazard ratio [AHR] 1.46, 95% confidence interval [CI] 1.03–2.06, and JHMI: AHR 1.80, 95% CI 1.02–3.19). The combinations of high HOXB13 expression and low expression of its binding partner, MEIS1 (AHR 2.03, 95% CI 1.54–2.66) or MEIS2 (AHR 1.73, 95% CI 1.33–2.26), portended worse MFS. Additionally, high HOXB13 expression in combination with low MEIS1/2 expression correlated with high expression of androgen receptor–mediated genes. The retrospective nature of this study subjects the findings to a bias due to unmeasured variables.
Conclusions:
Primary PCa tumors with increased HOXB13 expression have an increased propensity for metastases following prostatectomy, particularly in the setting of low MEIS1/2 expression. High androgen receptor output may account for worse outcomes for these tumors and suggests heightened sensitivity to androgen suppression.
Patient summary:
Using genomic data from a large number of prostate cancer (PCa) tumors, we found that increased expression of homeobox B13 (HOXB13), a gene related to normal prostate development, was associated with worse outcomes following surgery for PCa. A biomarker signature suggests that these tumors would be more susceptible to androgen suppression, a common treatment for PCa.
Keywords: Prostatic neoplasms, Homeobox B13, Homeodomain proteins, Disease progression
1. Introduction
The homeobox B13 (HOXB13) protein interacts with the androgen receptor (AR) and contributes to the regulation of AR-mediated transcriptomes critical for prostate growth [1]. Contributions of HOXB13 in prostate cancer (PCa) began with the discovery of the G84E mutation at the HOXB13 MEIS–binding site, which confers an increased risk of PCa [2]. However, the G84E mutation is rare in the population (1–2% of all PCa), and there are limited data supporting an association between G84E and tumor aggressiveness [2–4]. Conversely, decreased expression of the HOXB13 cofactors MEIS1 and MEIS2 has been linked to PCa metastatic progression [5]. Thus, despite links between MEIS1/2 and HOXB13, a more generalized association between somatic changes in tumor expression of HOXB13 and outcomes following a diagnosis of PCa has not been explored fully.
Associations between deregulated HOXB13 expression and cancer outcomes are not without precedent. For example, in breast, ovarian, and endometrial cancers, overexpression of HOXB13 correlates with aggressive tumor phenotypes and a poor response to hormonal therapies [6–8]. Despite the similarities between these hormonally regulated cancers, correlations with clinical outcomes for PCa have yet to be demonstrated.
We postulated that somatic changes in tumors that result in increased HOXB13 expression correlate with aggressive PCa based on pathologic and genomic features, as well as metastasis-free survival (MFS) following radical prostatectomy. We used a cohort of over 6000 radical prostatectomy specimens with genome-wide mRNA expression profiles. Additionally, we aimed to characterize the relationship between HOXB13 and MEIS1 and MEIS2 expression, and oncologic outcomes. Finally, we sought to assess HOXB13 expression as it relates to genes mediated by AR.
2. Patients and methods
2.1. Data and patient cohorts
Pan-Cancer samples across 32 primary organ sites were used to compare the expression of HOX genes across cancers and benign tissue types [9]. To further characterize HOXB13 expression in primary PCa, we used radical prostatectomy tumor samples from a prospectively maintained cohort retrieved from a prospective registry of genomic data (NCT02609269; n = 5239) [10] and two retrospective cohorts with follow-up for the endpoint of MFS (Mayo Clinic cohort, n = 780, and Johns Hopkins Medical Institute [JHMI] cohort, n = 355) [11,12]. The characteristics of our prospective and retrospective cohorts are shown in Supplementary Table 1, and more detailed descriptions including gene expression profiling can be found in our Supplementary material.
2.2. Statistical analysis and outcomes
Our primary outcome was MFS following radical prostatectomy, as defined by radiographic evidence of metastatic disease by imaging with computed tomography or technetium-99 bone scans following biochemical recurrence or new symptoms. To assess this outcome, the Mayo Clinic and JHMI cohorts served as our discovery and validation cohorts, respectively. For the prospective cohorts, a high genomic risk score (Decipher score ≥0.6) was used as a surrogate for high metastatic potential [10]. To test our primary outcome of MFS, we categorized our primary exposure (HOXB13 expression levels) by quartile of expression within our two retrospective cohorts. This was done (1) as there is no set cut-point for “high” HOXB13 expression and (2) to show a continuous relationship between MFS and HOXB13 expression over the range of expression levels within our cohorts. All other outcome analyses should be considered exploratory and hypothesis generating. Overall survival was assessed as a secondary outcome. Time to distant metastasis following radical prostatectomy was modeled using multivariable Cox proportional hazards adjusting for tumor characteristics. Spearman’s correlation was used for correlation analyses. Wilcoxon rank sum test was used for continuous statistical analyses, and chi-square test was used for categorical associations. All tests were performed in R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria), and all tests used a 5% significance level.
3. Results
3.1. HOXB13 is overexpressed in PCa
We first characterized the expression of 39 HOX genes in 32 different cancer primaries in Pan-Cancer, including PCa. Only HOXB13 was significantly overexpressed in PCa relative to the average of other cancers (Supplementary Fig. 1A). HOXB13 was also expressed more in PCa than in benign prostate, but both were notable for greater expression relative to other individual benign and cancerous tissues (Supplementary Fig. 1B).
3.2. Greater HOXB13 expression predicts worse outcomes for localized PCa
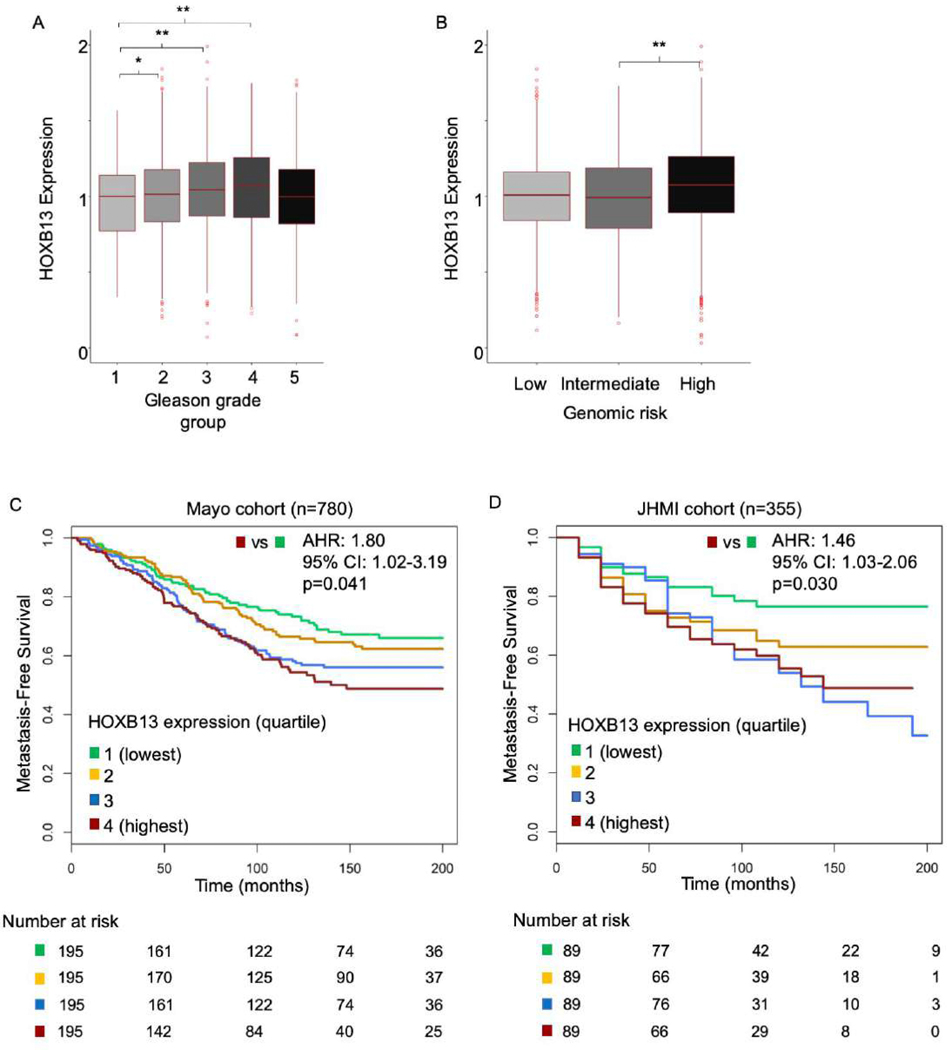
To assess the prognostic impact of HOXB13 in primary tumors, we investigated its expression by pathologic Gleason grade group and metastatic potential in a prospective cohort of over 5000 men with PCa [10]. HOXB13 expression increased with increasing Gleason grade groups 1–4 (Fig. 1A) and was also upregulated in tumors with high metastatic potential (Fig. 1B). Within the Mayo Clinic cohort, median follow-up was 156 mo and 288 men (37%) developed metastatic disease, while in the JHMI cohort, median follow-up was 108 mo and 127 men (36%) developed metastatic disease (Supplementary Table 1). High HOXB13 expression was also associated with worse MFS in both the Mayo Clinic (top quartile vs lowest quartile: adjusted hazard ratio [AHR] 1.46, 95% confidence interval [CI] 1.03–2.06, p = 0.030) and the JHMI (AHR 1.80, 95% CI 1.02–3.19, p = 0.041) cohort (Fig. 1C and 1D, and Table 1). When looking at metastatic outcomes across expression levels, HOXB13 showed a significant trend between increasing expression and incidence of metastasis at 10-yr follow-up (Mann-Kendall trend test p < 0.05 for both; Supplementary Fig. 2). Tumors in the top 10% of HOXB13 expression had about twice the incidence of metastasis compared with those in the lowest 10% in both the Mayo Clinic (42% vs 22%) and the JHMI (46% vs 22%) cohort.
Fig. 1 –
HOXB13 expression in nonmetastatic prostate adenocarcinoma and oncologic outcomes. (A) HOXB13 expression increased with increasing Gleason grade group, but did not differ between grade groups 1 (lowest) and 5 (highest) in the prospective cohort (n = 5239). (B) HOXB13 is overexpressed in patients at a high risk of developing metastatic disease following radical prostatectomy in the prospective cohort (n = 5239). Kaplan-Meier analysis of HOXB13 expression quartiles in (C) Mayo and (D) JHMI cohorts showing that higher expression is associated with poor metastasis-free survival.
AHR = adjusted hazard ratio; CI = confidence interval; HOXB3 = homeobox B13; JHMI = Johns Hopkins Medical Institute.
* p < 0.05.
** p < 0.001.
Table 1 –
Multivariable Cox regression analysis of HOXB13 expression for metastasis following radical prostatectomy
| Covariate | JHMI | Mayo Clinic | ||
|---|---|---|---|---|
|
| ||||
| AHR (95% CI) | p value | AHR (95% CI) | p value | |
|
| ||||
| HOXB13 expression (quartile) | ||||
| First (lowest) | Reference | Reference | ||
| Second | 1.78 (1.00–3.17) | 0.050 | 1.06 (0.74–1.51) | 0.7 |
| Third | 1.71 (0.98–2.98) | 0.060 | 1.22 (0.86–1.73) | 0.3 |
| Fourth (highest) | 1.80 (1.02–3.19) | 0.041 | 1.46 (1.03–2.06) | 0.030 |
| Grade group (per increase 1 group) | 2.06 (1.68–2.51) | <0.001 | 1.69 (1.49–1.91) | <0.001 |
| Log of PSA (ng/ml) | 0.79 (0.6–1.04) | 0.095 | 0.93 (0.80–1.08) | 0.3 |
| SVI | 2.01 (1.32–3.06) | 0.001 | 1.44 (1.10–1.89) | 0.008 |
| LNI | 2.15 (1.42–3.25) | <0.001 | 1.17 (0.85–1.60) | 0.3 |
| EPE | 1.28 (0.76–2.14) | 0.3 | 1.22(0.94–1.60) | 0.120 |
| PSM | 1.00 (0.68–1.45) | 0.9 | 1.06 (0.82–1.35) | 0.6 |
AHR = adjusted hazard ratio; CI = confidence interval; EPE = extraprostatic extension; HOXB3 = homeobox B13; JHMI = Johns Hopkins Medical Institute; LNI = lymph node invasion; PSA = prostate-specific antigen; PSM = positive surgical margin; SVI = seminal vesicle invasion.
In exploratory analyses, in both the Mayo Clinic and the JHMI cohort, HOXB13 expression was not associated with overall survival (Supplementary Table 2). We also constructed a separate Cox regression with the Mayo Clinic cohort adjusting for the 202 (26%) patients who received adjuvant androgen deprivation following surgery. In a multivariable regression adjusting for all the variables listed in Table 1 and adjuvant androgen deprivation (yes vs no), we found that both quartile 3 and quartile 4 were associated with shorter time to development of metastatic disease (AHR 1.50, 95% CI 1.06–2.10, p = 0.020, and AHR 1.66, 95% CI 1.18–2.24, p = 0.003, respectively).
3.3. Prognostic value of HOXB13 is dependent on MEIS1 and MEIS2 expression
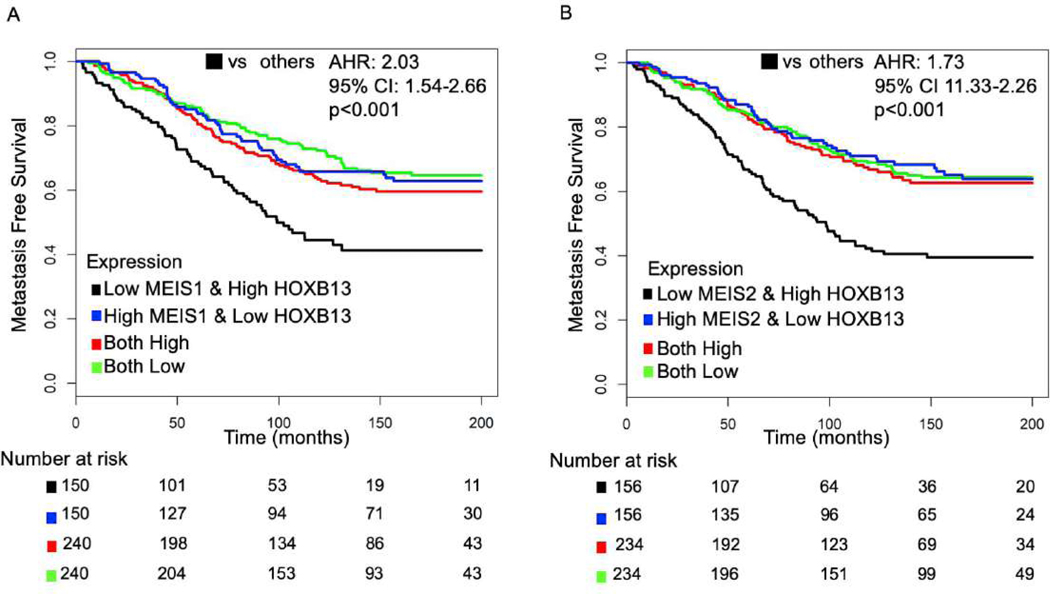
Previous work from our group has suggested that variable expression of the key binding partners for HOXB13, MEIS1/2, is associated with divergent oncologic outcomes following radical prostatectomy [5]. Thus, we explored interactions between these proteins and HOXB13. We first validated our prior work by assessing MFS following radical prostatectomy based on expression levels of MEIS1 and MESI2 in the Mayo Clinic cohort (Supplementary Fig. 3A and 3B). Compared with tumors with low MEIS1 or MEIS2 expression, tumors with high expression were associated with improved MFS (highest versus lowest quartile of expression: AHR 0.78, 95% CI 0.62–0.99, p = 0.046, and AHR 0.80, 95% CI 0.63–1.02, p = 0.080, respectively; Supplementary Table 3). To explore the interplay between MEIS1/2 and HOXB13 expression, we then tested the association of the expression of both HOXB13 and MEIS1/2 with MFS, and found that above median expression levels of both HOXB13 and MEIS1/2 were independently associated with MFS (Supplementary Table 4). We thus stratified the Mayo Clinic and JHMI cohorts by combined expression of these genes. Tumors with high (above median) HOXB13 expression and low (below median) MEIS1 (Mayo Clinic: AHR 2.03, 95% CI 1.54–2.66, p < 0.001) or MEIS2 (AHR 1.73, 95% CI 1.33–2.26, p < 0.001) expression were at a higher risk of developing metastases (Fig. 2A and 2B, and Table 2). The results were similar in the JHMI cohort (Supplementary Table 5) and in an assessment of overall survival (Supplementary Fig. 4A and 4B, and Supplementary Table 6), suggesting a converging pathway for HOXB13 and MEIS1/2 expression and aggressive PCa phenotype.
Fig. 2 –
Metastases-free survival based on HOXB13 and MEIS expression. Kaplan-Meier analysis of metastasis-free survival in patients from the Mayo retrospective cohort stratified by above versus below median expression of HOXB13 and (A) MEIS1 or (B) MEIS2 expression following radical prostatectomy.
AHR = adjusted hazard ratio; CI = confidence interval; HOXB3 = homeobox B13.
Table 2 –
Multivariable Cox regression analysis of HOXB13 and MEIS expression for metastasis following radical prostatectomy in the Mayo cohort
| Covariate | MEIS1 | MEIS2 | ||
|---|---|---|---|---|
|
| ||||
| AHR (95% CI) | p value | AHR (95% CI) | p value | |
|
| ||||
| High HOXB13 and low MEIS expression (vs others) | 2.03 (1.54–2.66) | <0.001 | 1.73 (1.33–2.26) | <0.001 |
| Grade group (per increase 1 group) | 1.72 (1.52–1.94) | <0.001 | 1.67 (1.48–1.9) | <0.001 |
| Log of PSA (ng/ml) | 0.87 (0.76–1.01) | 0.080 | 0.89 (0.77–1.03) | 0.140 |
| SVI | 1.47 (1.13–1.93) | 0.004 | 1.51 (1.15–1.97) | 0.002 |
| LNI | 1.19 (0.87–1.62) | 0.3 | 1.08 (0.78–1.49) | 0.6 |
| EPE | 1.24 (0.95–1.61) | 0.100 | 1.19 (0.91–1.55) | 0.180 |
| PSM | 1.03 ( 0.81–1.32) | 0.8 | 1.05 (0.82–1.34) | 0.7 |
AHR = adjusted hazard ratio; EPE = extraprostatic extension; HOXB3 = homeobox B13; LNI = lymph node invasion; PSA = prostate-specific antigen; PSM = positive surgical margin; SVI = seminal vesicle invasion.
3.4. AR activity correlates with HOXB13 and MEIS1/2 expression
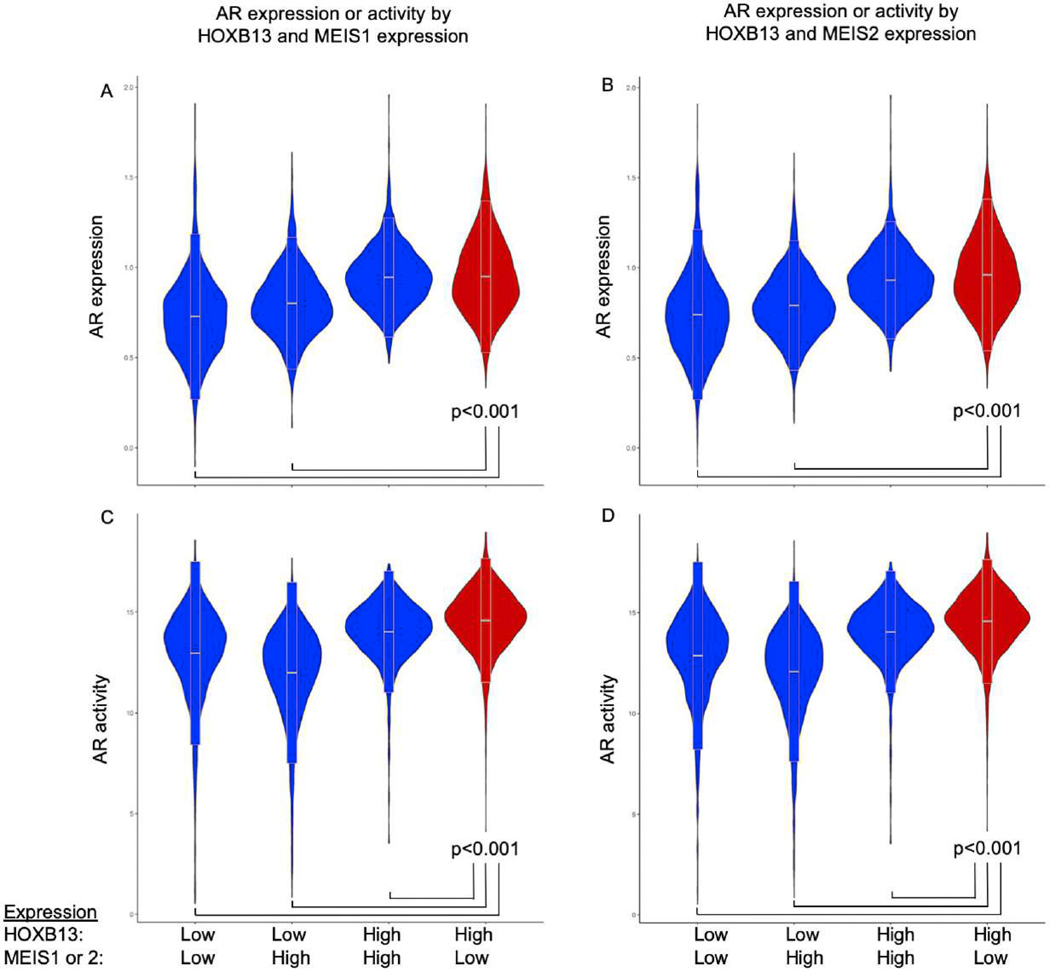
Based on the positive correlation between HOXB13 and AR activity in primary PCa, and the prognostic value of combined high HOXB13 and low MEIS1/2 expression following prostatectomy (Fig. 2A and 2B), we theorized that the combination of high HOXB13 and low MEIS1/2 expression occurs in tumors that also have increased expression of AR and AR-mediated genes. To test this, we assessed the expression of the AR gene as well as AR activity based on the stratifications used in Figures 2A and 2B (low/high HOXB13 and low/high MEIS1/2 expression). Tumors with the combination of high HOXB13 and low MEIS1/2 expression also expressed the highest levels of AR (Fig. 3A and 3B) and AR activity (Fig. 3C and 3D).
Fig. 3 –
Correlation of HOXB13 and MEIS expression with AR expression and activity. The combination of high HOXB13 expression and low MEIS1 and MEIS2 expression was associated the greatest expression of (A and B) AR and (C and D) AR activity.
AR = androgen receptor; HOXB3 = homeobox B13.
** p < 0.001.
4. Discussion
Genetic alterations and increased expression of HOXB13 have previously been linked to PCa development [2,13]. However, there are very limited data correlating this key hereditary PCa gene with oncologic outcomes. Using multiple cohorts across the spectrum of localized and advanced disease, we provide unique insights into HOXB13 expression and PCa progression. In primary PCa, increased HOXB13 expression was associated with an increased risk of developing metastatic disease following local treatment in two large academic cohorts. Additionally, this association was confirmed in a large, prospectively assembled cohort using a validated biomarker of metastatic potential.
In this analysis, we characterize the relationship between HOXB13 expression and PCa progression. We then confirm the previous work that showed that increased expression of MEIS1 and MEIS2, binding partners of HOXB13, portended a decreased risk of developing metastatic disease [5]. Additionally, we demonstrate interplay between HOXB13 and MEIS1/2. More specifically, our results indicate that tumors with the combination of high HOXB13 and low MEIS1/2 expression had an increased propensity for developing metastatic disease by two-fold in a multivariable model.
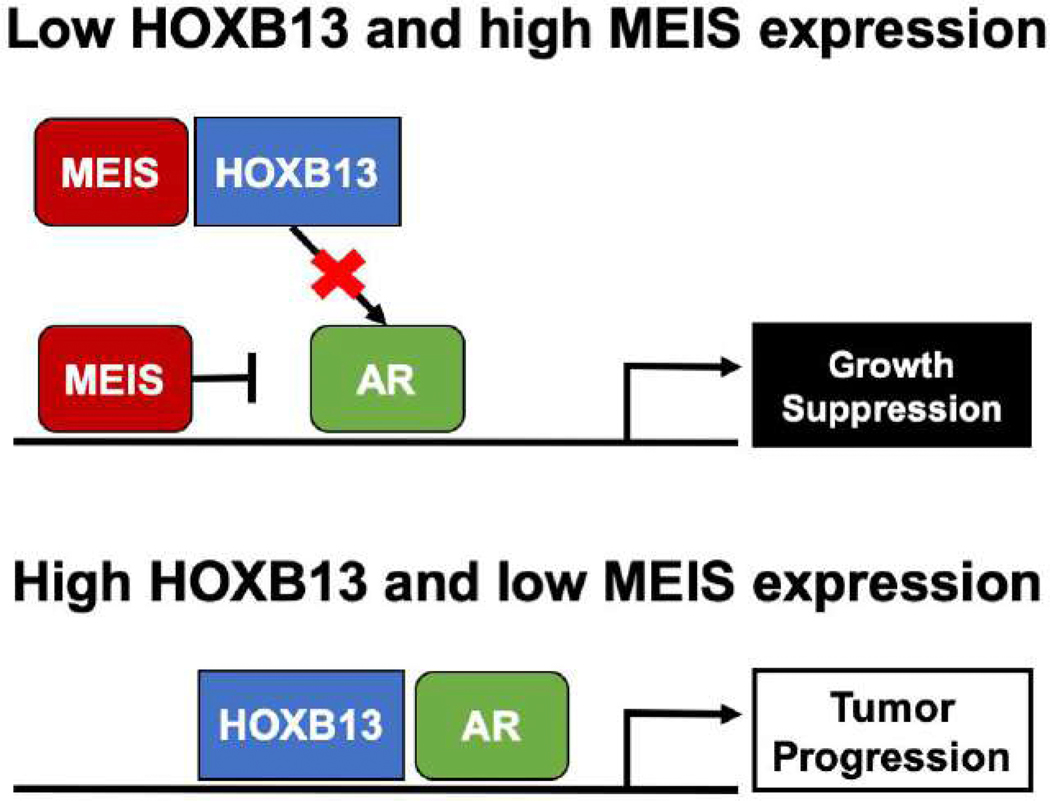
To better understand the relationship between HOXB13 and MEIS1/2 expression with MFS and overall survival, we noted that tumors with high HOXB13 and low MEIS1/2 expression also showed high expression levels of AR activity. We also noted that HOXB13 expression is higher in tumors with higher-grade disease except for Gleason grade group 5 disease, which is consistent with previous data showing that localized tumors with low AR activity are enriched in high-grade tumors [14]. Although our observational data cannot ascribe a causal relationship to explain these finding, one theoretical hypothesis to explain the relationship between HOXB13 and MEIS1/2 expression and AR activity is considering HOXB13 binding as a potential “sink” for the AR-suppressing qualities of MEIS [5,15]. Thus, when HOXB13 levels are high, MEIS is no longer available to suppress AR activity. Alternatively, binding of MEIS to HOXB13 may prevent HOXB13 from driving AR activation and subsequent tumor progression (Fig. 4). However, in light of the limitation of the current study, the precise biochemical interactions that mediate any relationship between HOXB13 and MEIS1/2 expression and AR activity are an active component of ongoing research.
Fig. 4 –
Theoretical mechanism of HOXB13 and MEIS1/2 interaction with the androgen receptor.
AR = androgen receptor; HOXB3 = homeobox B13.
Several biomarkers have been investigated as predictors of adverse pathology at the time of radical prostatectomy, or biochemical or metastatic recurrence following surgery [16–18]. In a recent consensus statement, standards for new biomarkers include (1) assessment in multivariable models that incorporate multiple known clinical risk factors and (2) intervention that can lead to improved quantity or quality of life, as assessed by relevant outcome measures [19]. Our study demonstrates an association between high HOXB13 and low MEIS1/2 RNA expression and MFS, as well as overall survival when adjusting for relevant clinical covariates. These findings in large cohorts with relevant oncologic outcomes are pertinent in the era of precision medicine and oncology care. For instance, men with disease recurrence following local treatment can benefit from radiotherapy; however, the timing, duration, and benefit of additional hormone therapy are not fully understood [20]. Our data, which suggest that localized tumors with high HOXB13 and low MEIS1/2 expression identify patients with high AR output, may act as a framework to develop molecular signatures that predict response to androgen deprivation in a cohort at a high risk of developing recurrent metastatic disease. Thus, our work nominates these markers for future studies investigating mechanistic underpinnings and prospective validation.
4.1. Limitations
Our study is not without limitations. These data suggest a correlation between HOXB13 and MEIS expression and oncologic outcomes for localized PCa. While our study shows an association between high HOXB13 and low MEIS1/2 expression with increased AR activity, the actual mechanistic underpinning for HOXB13 and MEIS expression, cancer progression, and AR activity remains a worthy subject of future work. Future work on this topic with proteomic and immunohistochemistry data would further validate our findings. Importantly, while the G84E mutation is at the binding site for MEIS, the mutation does not seem to alter HOXB13 expression in primary PCa or the interaction between HOXB13 and MEIS [21,22]; we were unable to assess the connection between HOXB13 and germline mutations in this study’s cohort. Additionally, assessments of HOXB13 expression and prognosis in the setting of metastatic PCa, castration-resistant PCa, and neuroendocrine PCa were not completed in this study, but could further our understanding of the role of HOXB13 and PCa progression. Our analysis of the retrospective cohorts with clinical outcomes is also subject to a bias related to unmeasured covariates, and thus our findings require future validating study. Although the patients in the JHMI cohort did not receive any adjuvant treatments, a more complete analysis of the possible effect of adjuvant treatment in the Mayo Clinic cohort would include radiation therapy and a landmark analysis starting after adjuvant treatments were given. Finally, follow-up was modest at about 9 yr in the JHMI cohort, which was shorter than that for the Mayo Clinic cohort (about 13 yr), particularly for our analysis of overall survival.
5. Conclusions
Our study provides novel insight into the correlation between HOXB13 and localized PCa progression. High HOXB13 expression portends a greater propensity for metastases following local treatment. This effect was noted only in the setting of low MEIS1/2 expression, suggesting a coregulatory role between the two genes and PCa progression. Tumors with high HOXB13 and low MEIS1/2 expression were enriched with high AR activity, suggesting a mechanistic connection to cancer outcomes and sensitivity to androgen suppression.
Supplementary Material
In multiple large cohorts, prostate cancer tumors with high homeobox B13 (HOXB13) expression and low expression of its binding partner MEIS1/2 were enriched with high androgen receptor output and had an increased propensity for metastases following surgery.
Acknowledgments
A poster version of these data was presented at the annual meeting of the American Urological Association (Chicago, IL, USA) in May 2019 and at the annual meet of the Society of Urology Oncology (Washington, DC, USA) in December 2019.
Funding/Support and role of the sponsor: This work was supported in part by the National Institutes of Health grant 5U01CA196390 and the Prostate Cancer Foundation (Edward M. Schaeffer), as well as the 2019 Urology Care Foundation Residency Research Award Program and the Russell Scott, Jr., MD Urology Research Fund (Adam B. Weiner). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Financial disclosures: Edward M. Schaeffer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Elai Davicioni reports personal fees from GenomeDx (Decipher Biosciences) outside the submitted work. R. Jeffrey Karnes reports royalties from GenomeDx (Decipher Biosciences) outside the submitted work. Tamara L. Lotan reports grants from the Prostate Cancer Foundation outside the submitted work. The other authors made no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Norris JD, Chang CY, Wittmann BM, et al. The homeodomain protein HOXB13 regulates the cellular response to androgens. Mol Cell 2009;36:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nyberg T, Govindasami K, Leslie G, et al. Homeobox B13 G84E mutation and prostate cancer risk. Eur Urol 2019;75:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Storebjerg TM, Hoyer S, Kirkegaard P, et al. Prevalence of the HOXB13 G84E mutation in Danish men undergoing radical prostatectomy and its correlations with prostate cancer risk and aggressiveness. BJU Int 2016;118:646–53. [DOI] [PubMed] [Google Scholar]
- [4].Kote-Jarai Z, Mikropoulos C, Leongamornlert DA, et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann Oncol 2015;26:756–61. [DOI] [PubMed] [Google Scholar]
- [5].Bhanvadia RR, VanOpstall C, Brechka H, et al. MEIS1 and MEIS2 expression and prostate cancer progression: a role for HOXB13 binding partners in metastatic disease. Clin Cancer Res 2018;24:3668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao Y, Yamashita T, Ishikawa M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol Rep 2005;13:721–6. [PubMed] [Google Scholar]
- [7].Miao J, Wang Z, Provencher H, et al. HOXB13 promotes ovarian cancer progression. Proc Natl Acad Sci U S A 2007;104:17093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jansen MP, Sieuwerts AM, Look MP, et al. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: a retrospective study. J Clin Oncol 2007;25:662–8. [DOI] [PubMed] [Google Scholar]
- [9].Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013;45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spratt DE, Yousefi K, Deheshi S, et al. Individual patient-level meta-analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J Clin Oncol 2017;35:1991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Erho N, Crisan A, Vergara IA, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 2013;8:e66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ross AE, Johnson MH, Yousefi K, et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur Urol 2016;69:157–65. [DOI] [PubMed] [Google Scholar]
- [13].Pomerantz MM, Li F, Takeda DY, et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat Genet 2015;47:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spratt DE, Alshalalfa M, Fishbane N, et al. Transcriptomic heterogeneity of androgen receptor activity defines a de novo low AR-active subclass in treatment naive primary prostate cancer. Clin Cancer Res 2019;25:6721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cui L, Li M, Feng F, et al. MEIS1 functions as a potential AR negative regulator. Exp Cell Res 2014;328:58–68. [DOI] [PubMed] [Google Scholar]
- [16].Hamid AA, Gray KP, Huang Y, et al. Loss of PTEN expression detected by fluorescence immunohistochemistry predicts lethal prostate cancer in men treated with prostatectomy. Eur Urol Oncol 2019;2:475–82. [DOI] [PubMed] [Google Scholar]
- [17].Jeyapala R, Savio AJ, Olkhov-Mitsel E, et al. GBX2 methylation is a novel prognostic biomarker and improves prediction of biochemical recurrence among patients with prostate cancer negative for intraductal carcinoma and cribriform architecture. Eur Urol Oncol 2019;2:231–8. [DOI] [PubMed] [Google Scholar]
- [18].Wu Y, Yu H, Li S, et al. Rare germline pathogenic mutations of DNA repair genes are most strongly associated with grade group 5 prostate cancer. Eur Urol Oncol 2020;3:224–30. [DOI] [PubMed] [Google Scholar]
- [19].Cooperberg MR, Carroll PR, Dall’Era MA, et al. The state of the science on prostate cancer biomarkers: the San Francisco Consensus statement. Eur Urol 2019;76:268–72. [DOI] [PubMed] [Google Scholar]
- [20].Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017;376:417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johng D, Torga G, Ewing CM, et al. HOXB13 interaction with MEIS1 modifies proliferation and gene expression in prostate cancer. Prostate 2019;79:414–24. [DOI] [PubMed] [Google Scholar]
- [22].Lotan TL, Torres A, Zhang M, et al. Somatic molecular subtyping of prostate tumors from HOXB13 G84E carriers. Oncotarget 2017;8:22772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.