Abstract
Anti-müllerian hormone (AMH) is an established marker of ovarian reserve that decreases with age. Though the pool of ovarian follicles is established during fetal development, impacts of in utero exposures on AMH are uncertain. Thus, we sought to evaluate associations of in utero exposures with AMH of adult daughters with a prospective cohort study of adult daughters at university medical centers. Women noted their mother’s reported use of diethylstilbestrol (DES), vitamins, tobacco, alcohol, and caffeine during pregnancy, and their mother’s occupation during pregnancy. All participants were reproductive age women (18–40 years) enrolled in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial. Serum AMH concentrations were measured at baseline prior to conception and categorized using clinical guidelines. Multinomial regression models estimated associations between each exposure and high (>3.5 ng/mL) and low (<1.0 ng/mL) versus normal AMH (1.0–3.5 ng/mL), adjusting for participant’s age, mother’s age, mother’s history of fertility treatment, and mother’s use of vitamins. In 1202 women with available data, maternal caffeine use was associated with an increased risk of low AMH, compared to normal (relative risk [RR] 1.90, 95% confidence interval [CI] 1.09, 3.30). Vitamins were associated with an increased risk of high AMH compared to normal (RR 1.93, 95% CI 1.24, 3.00). Other exposures were not associated with AMH concentrations in offspring. Maternal caffeine and vitamin use during pregnancy may be associated with ovarian reserve in adult offspring, highlighting the potential importance of pregnancy lifestyle on the reproductive health of daughters.
Keywords: anti-müllerian hormone, caffeine use, vitamins, intergenerational effects
Capsule:
Examining reported in utero exposures and the AMH of adult offspring suggests that maternal caffeine and vitamin use during pregnancy may be associated with AMH in offspring.
Introduction:
All ovarian follicles are developed during fetal development and therefore, the maternal environment plays a critical role in the health of these developing follicles. A woman’s reproductive lifespan is related to the number of ovarian follicles, each containing a single oocyte, that are established in utero and how long it takes this number to undergo atresia1. The majority of follicles will never complete maturation. Given that the primordial follicle pool is established during fetal development, understanding the role of the maternal environment during pregnancy is critical for understanding factors associated with downstream fertility and reproductive health outcomes of daughters.
AMH is a widely used marker related to ovarian reserve, and more specifically the current pool of antral follicles, that is secreted by the granulosa cells of the ovarian follicles2,3. It may be a particularly relevant endpoint for examining impacts of in utero exposures on reproductive health since it is a simple circulating marker. AMH does not measure egg quality or failure to conceive, but is more specific for ovarian reserve, which can be evaluated independently, but also with other hormonal markers like testosterone1,2.
It is hypothesized that as ovarian follicular reserve is affected by certain lifestyle risk factors, AMH may aid in understanding mechanisms for infertility. Several studies have evaluated the impact of maternal tobacco and caffeine use during pregnancy on age of menarche, fecundability, and live birth rates of the offspring with conflicting results1,4–20. In utero exposure to diethylstilbestrol (DES), which was historically used to reduce the risk of pregnancy complications and losses, has also been extensively studied on the outcomes of exposed offspring noting increased risk for gynecological cancers, malformation of the genitourinary tract, and infertility related to these poor developmental outcomes. However, in women with normal anatomy and a DES exposure, specific relation to fertility hormones have not been evaluated. Despite that ovarian follicles are established during the fetal development, a potential role of maternal lifestyle risk factors during pregnancy for daughter’s reproductive lifespan is less understood. To date, only a single study evaluated maternal risk factors that were not external exposures in relation to AMH, but among daughters in 14–16 years of age1. As such it is unknown whether these lifestyle factors may influence ovarian reserve among reproductive age women, particularly among those without known infertility where effects may be more subtle1.
Therefore, the objective of this study is to evaluate the associations of reported in utero lifestyle exposures, including prenatal smoking, alcohol use, vitamin intake, caffeine use, DES use, and maternal employment status as a proxy for stress and socioeconomic status, on the serum AMH, a widely used marker of ovarian reserve, of adult daughters.
Materials and Methods:
Study Population
This report is a secondary analysis of women participating in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial21,22.This trial was a multi-center, double-blind, block-randomized, placebo-controlled trial evaluating the effects of low dose aspirin (LDA) on live birth. The detailed study design and methods have been previously published22. In brief, 1228 women trying to conceive were enrolled from 2007–2011 at four U.S. clinical centers. Inclusion criteria were a history of one or two documented pregnancy losses and age 18–40 years. Exclusion criteria included a history of major medical disorders, infertility, pelvic inflammatory disease, tubal occlusion, endometriosis, anovulation, uterine abnormalities, or polycystic ovarian syndrome. Institutional Review Board authorization was obtained for the data coordinating center and at all clinical centers and all participants provided written informed consent. Patient safety was monitored by the Data Safety and Monitoring Board (DSMB) and the trial was registered with ClinicalTrials.gov, number NCT00467363.
Exposure Assessment:
Exposure information was collected via questionnaire at baseline. Women participating in the EAGeR trial were asked to answer based on their knowledge of their mother’s exposures during the participant’s in utero development. For example, women were asked: “To the best of your knowledge, did your mother take vitamins while pregnant with you?”, with possible responses of yes, no, or don’t know. In utero exposures that were investigated included DES exposure, vitamin use, smoking, alcohol intake, caffeine use, and working either full or part time during pregnancy.
AMH Assessment:
AMH concentrations in women in the EAGeR trial (the daughters of the women in whom exposures were assessed) were measured in serum samples collected during preconception at baseline, which occurred between days 2–4 of the menstrual cycle. Serum samples were stored at −80°C until analyses. Concentrations of AMH were determined in serum samples using the GEN II ELISA assay protocol with correction for complement interference (Beckman Coulter)23. AMH data were available for 1202 of the 1228 women in the cohort. All machine observed concentrations were used without substitution of concentration below the limits of detection (0.006 ng/mL) to avoid bias24. The inter-assay laboratory coefficients of variation (CVs) were 6.2% and 6.6% at mean concentrations of 8.9 and 3.1 ng/mL, respectively, for lyophilized manufacturer’s controls and 6.3% for an in-house pooled serum control. To maximize the measurement precision of AMH, we evaluated for any improper calibration curves or out-of-range values for manufacturer-provided control samples using a pooled standard curve and confirmed that sample recalibration was not required25. AMH concentrations for this analysis were categorized using clinical thresholds based on data from infertile women with low AMH as under 1.00 ng/mL, normal AMH as 1.00–3.5 ng/mL, and high AMH as over 3.5 ng/mL1,3,23. In prior studies, AMH is not a valuable tool for fecund women and therefore, the thresholds are importantly based on infertile women3. Categorizing, allows for generalization of high, normal, and low, in a variable that specific values are less meaningful than the category women may fall in after a diagnosis of infertility.
Testosterone Measurement:
Liquid chromatography and tandem mass spectrometry using a Shimadzu Prominence Liquid Chromatogram (Shimadzu Scientific Instruments, Inc., Columbia, MD) with an ABSceix 5500 tandem mass spectrometer (AB SCIEX, Framingham, MA) was utilized to measure the patient’s total testosterone for evaluations. Sex hormone binding globulin (SHBG) concentration was determined by SHBG reagent/sandwich immunoassay method/electrochemiluminescence (Roche Diagnostics, Indianapolis, IN) utilizing a Roche COBAS 6000 chemistry analyzer (Roche Diagnostics). Interassay CVs were 3.0% at 55.64 nmol/L and 3.8% at 19.74 nmol/L and limit of detection was 0.800 nmol/L. From this value, along with the patient’s total testosterone, the free testosterone was able to be calculated as 24.00314 × T/log10S – 0.0499 × T2 and free androgen index (FAI) as 100*(T/S), where T = total testosterone in nmol/L and S = SHBG in nmol/L26.
Statistical Analysis:
Demographic characteristics of the daughters (i.e. EAGeR participants) were compared by AMH category. ANOVA or Fisher’s exact test were used, as appropriate, to determine differences in characteristics across low, normal, and high AMH categories. Geometric mean AMH concentrations and 95% confidence intervals (CIs) were also compared between women with versus without the specific in utero exposures of interest. Multinomial regression was used to evaluate the associations between reported in utero exposures and the risk of low or high AMH compared to normal levels. Models were adjusted for relevant confounding factors including, EAGeR participant age, mother’s age at birth of daughter, mother’s history of fertility treatment, and mother’s history of vitamin use (except where history of vitamin use was the main exposure of interest), as these maternal factors may be related to offspring reproductive health as well as the given exposures of interest.
As there was missing information on maternal exposures (ranging from 8% for smoking status to 40% for DES exposure; most factors were missing approximately 12%) we completed a sensitivity analysis for missing data to evaluate the degree to which this missing data may have influenced the findings. We imputed, from the digital data set of completed surveys, exposure information under every scenario in the low, normal, and high AMH groups, as well as using multiple imputation with 1000 imputed datasets. P-values for each scenario were plotted, along with the level of missing data imputed for each multiple imputation for the association between each exposure and AMH. SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.2 (R Foundation, Vienna, Austria) were used for the statistical analyses.
Results:
Among 1228 women enrolled in the trial, 1202 completed the questionnaire regarding in utero exposures and had measured AMH concentrations available. Participants were primarily white (94.7%) with a mean body mass index (BMI) of 26.3 (standard deviation [SD] 6.5) kg/m2 and mean age of 28.7 (SD 4.8) years (Table 1). Participant’s mother’s age at time of questionnaire was 46 to 83 and their ages when they gave birth to participants in EAGeR ranged from 15 to 44. Women in the low AMH group tended to be older, had lower testosterone, and were more likely to be parous. There were no differences in BMI, race, treatment assignment, and number of prior pregnancy losses by AMH category.
Table 1:
Demographics of Participants of the EAGeR Cohort with Recorded AMH
| Characteristics | Total | Low AMH <1.0 ng/ml | Normal AMH 1.0–3.5 ng/ml | High AMH >3.5 ng/ml | P-Value |
|---|---|---|---|---|---|
| N | 1202 | 121 | 596 | 485 | |
| Age, y; mean ± SD | 28.7 ± 4.8 | 32.8 ± 5.1 | 29.1 ± 4.6 | 27.3 ± 4.2 | <0.0001 |
| BMI, kg/m2; mean ± SD | 26.3 ± 6.5 | 27 ± 7.1 | 26.4 ± 6.5 | 25.8 ± 6.2 | 0.12 |
| Race | |||||
| White | 1138 (94.7) | 117 (96.7) | 563 (94.5) | 458 (94.4) | 0.65 |
| Non-white | 64 (5.3) | 4 (3.3) | 33 (5.5) | 27 (5.6) | |
| Treatment Assignment; n (%) | |||||
| Placebo | 600 (49.9) | 56 (46.3) | 310 (52) | 234 (48.2) | 0.33 |
| Low Dose Aspirin | 602 (50.1) | 65 (53.7) | 286 (48) | 251 (51.8) | |
| Parity; n (%) | |||||
| Nulliparous | 512 (42.6) | 45 (37.2) | 228 (38.3) | 239 (49.3) | 0.0006 |
| Parous (1 or 2 prior births) | 690 (57.4) | 76 (62.8) | 368 (61.7) | 246 (50.7) | |
| Testosterone Level; n (%) | |||||
| Low <15ng/dl | 284 (23.7) | 52 (43) | 186 (31.3) | 46 (9.5) | <0.0001 |
| Normal 15.0– 75.0 ng/dl | 914 (76.2) | 69 (57) | 408 (68.6) | 437 (90.3) | |
| Elevated >75 ng/dl | 2 (0.2) | 0 (0) | 1 (0.2) | 1 (0.2) | |
| Number of previous pregnancy losses; n (%) | |||||
| 1 | 804 (66.9) | 80 (66.1) | 392 (65.8) | 332 (68.5) | 0.64 |
| 2 | 398 (33.1) | 41 (33.9) | 204 (34.2) | 153 (31.5) | |
AMH values of the participants were then compared between those with and without the specific exposures (Table 2). Participants who reported that their mothers were taking vitamin supplements while they were pregnant with them also had higher AMH than those who did not report exposure to vitamins (3.0 ng/mL versus 2.3 ng/mL, p=0.002). Self-reported in utero caffeine exposure was associated with lower AMH (2.7 ng/mL versus 3.0 ng/mL, p-value=0.04). Exposure to DES was very limited with only seven participants reporting this exposure and differences in AMH were similar (2.4 ng/mL versus 2.8 ng/mL, p-value=0.59). Geometric mean AMH concentrations were similar between those reported to be exposed and not exposed to tobacco, alcohol, and both maternal full and part time work.
Table 2:
Geometric Mean (95% confidence interval [CI]) AMH Levels (ng/mL) by In Utero Exposure Category
| Exposures | Total Positive Exposures N (%) | AMH in Positive Exposure Mean (95% CI) | AMH in Negative Exposure Mean (95% CI) | p-value |
|---|---|---|---|---|
| DES | 7 (1.0) | 2.4 (1.0, 5.8) | 2.8 (2.6, 2.9) | 0.59 |
| Vitamins | 666 (88.3) | 3.0 (2.8, 3.2) | 2.3 (1.9, 2.7) | 0.002 |
| Cigarettes | 103 (9.3) | 2.5 (2.1, 2.9) | 2.8 (2.7, 2.9) | 0.14 |
| Alcohol Use | 50 (4.6) | 2.7 (2.1, 3.5) | 2.8 (2.6, 2.9) | 0.85 |
| Caffeine Use | 469 (51.2) | 2.7 (2.5, 2.9) | 3.0 (2.8, 3.2) | 0.04 |
| Working | ||||
| Any work (part time + full time) | 348 (32.6) | 2.7 (2.5, 2.9) | 2.7 (2.6, 2.9) | 0.69 |
| Part Time | 103 (9.7) | 2.4 (2.1, 2.9) | 2.7 (2.6, 2.9) | 0.18 |
| Full Time | 250 (23.4) | 2.8 (2.6, 3.1) | 2.7 (2.5, 2.9) | 0.48 |
When evaluating the relationship of reported in utero exposures to low and high versus normal AMH categories, exposure to caffeine and DES were associated with an increased risk of low AMH in the offspring in unadjusted models (Table 3), though after adjustment only caffeine was significantly associated with risk of low AMH. Conversely, report of mothers’ vitamin use during pregnancy was associated with high AMH in adjusted models. Further, while maternal employment was associated with a lower risk of high AMH before adjustment, results were attenuated after adjusting for potential confounders. No associations were noted for both in utero exposure to cigarette and alcohol use. Similar conclusions were observed across all exposures in sensitivity analyses to address missing exposure information (Figures 1 and 2 for vitamin and caffeine exposure respectively; figures for other exposures not shown). Specifically, results indicated that under most scenarios the association between vitamin use and AMH would remain statistically significant, indicated by results from all multiple imputation scenarios lying in the white region of Figure 1 (as represented by the blue triangles). Further, under reasonable scenarios the association between caffeine and AMH would remain statistically significant, indicated by the majority of the blue triangles from multiple imputation scenarios lying in the white region of Figure 2, though for some imputations the association could be attenuated.
Table 3:
Associations between In Utero Exposures and Risk of High and Low AMH Compared to Normal AMH
| Exposures | Low AMH <1.0ng/ml RR (95% CI) | Normal AMH 1.0 – 3.5ng/ml | High AMH >3.5 ng/ml RR (95% CI) |
|---|---|---|---|
| DES | |||
| Unadjusted | 2.94 (0.89, 9.70) | Ref | 1.44 (0.75, 2.75) |
| Adjusted | 4.54 (0.61, 33.65) | Ref | 0.98 (0.26, 3.73) |
| Vitamins | |||
| Unadjusted | 0.53 (0.30, 0.93) | Ref | 2.05 (1.36, 3.08) |
| Adjusted | 0.67 (0.38, 1.19) | Ref | 1.93 (1.24, 3.00) |
| Cigarettes | |||
| Unadjusted | 1.46 (0.86, 2.47) | Ref | 0.85 (0.65, 1.12) |
| Adjusted | 1.14 (0.61, 2.11) | Ref | 0.99 (0.72, 1.36) |
| Alcohol Use | |||
| Unadjusted | 1.03 (0.44, 2.41) | Ref | 0.99 (0.70, 1.4) |
| Adjusted | 0.90 (0.29, 2.80) | Ref | 1.06 (0.73, 1.56) |
| Caffeine Use | |||
| Unadjusted | 1.80 (1.16, 2.80) | Ref | 0.91 (0.78, 1.07) |
| Adjusted | 1.90 (1.09, 3.30) | Ref | 0.93 (0.77, 1.11) |
| Any work (full or part time) | |||
| Unadjusted | 0.99 (0.68, 1.45) | Ref | 0.83 (0.71, 0.99) |
| Adjusted | 0.96 (0.59, 1.55) | Ref | 0.86 (0.71, 1.05) |
| Working Part Time | |||
| Unadjusted | 1.39 (0.83, 2.35) | Ref | 0.79 (0.59, 1.06) |
| Adjusted | 1.03 (0.55, 1.92) | Ref | 0.93 (0.68, 1.28) |
| Working Full Time | |||
| Unadjusted | 0.79 (0.50, 1.24) | Ref | 0.91 (0.75, 1.09) |
| Adjusted | 0.84 (0.47, 1.51) | Ref | 0.88 (0.71, 1.09) |
Bold indicates statistical significance at the α=0.05 level.
Adjusted models are adjusted for EAGeR participant age, mother’s age at birth of daughter, mother’s history of fertility treatment, and mother’s history of vitamin use (except where history of vitamin use was the main exposure of interest).
CI, confidence interval; RR, relative risk.
Figure 1.
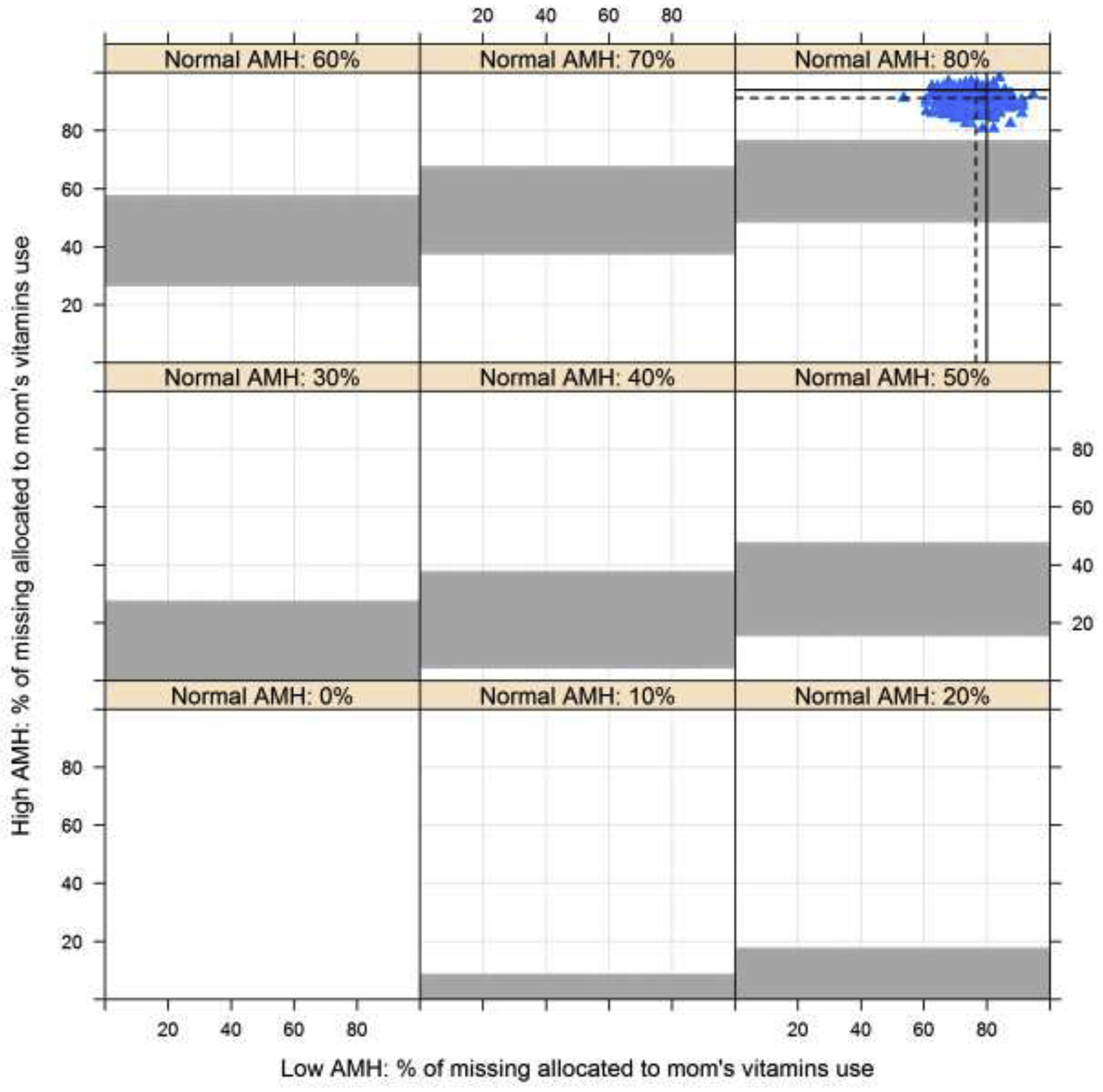
Association between in utero exposures to vitamin use and risk of high AMH compared to normal AMH with missing exposure information imputed across the range of possible scenarios (0–100% exposed in the low and high AMH categories by varying exposure in the normal AMH category). White and grey regions indicate p≤0.05 and p>0.05, respectively. Values from multiple imputation with 1000 datasets are indicated using the blue triangles. Dashed lines represent the mean of the imputed values and solid lines indicated the observed proportions.
Figure 2.
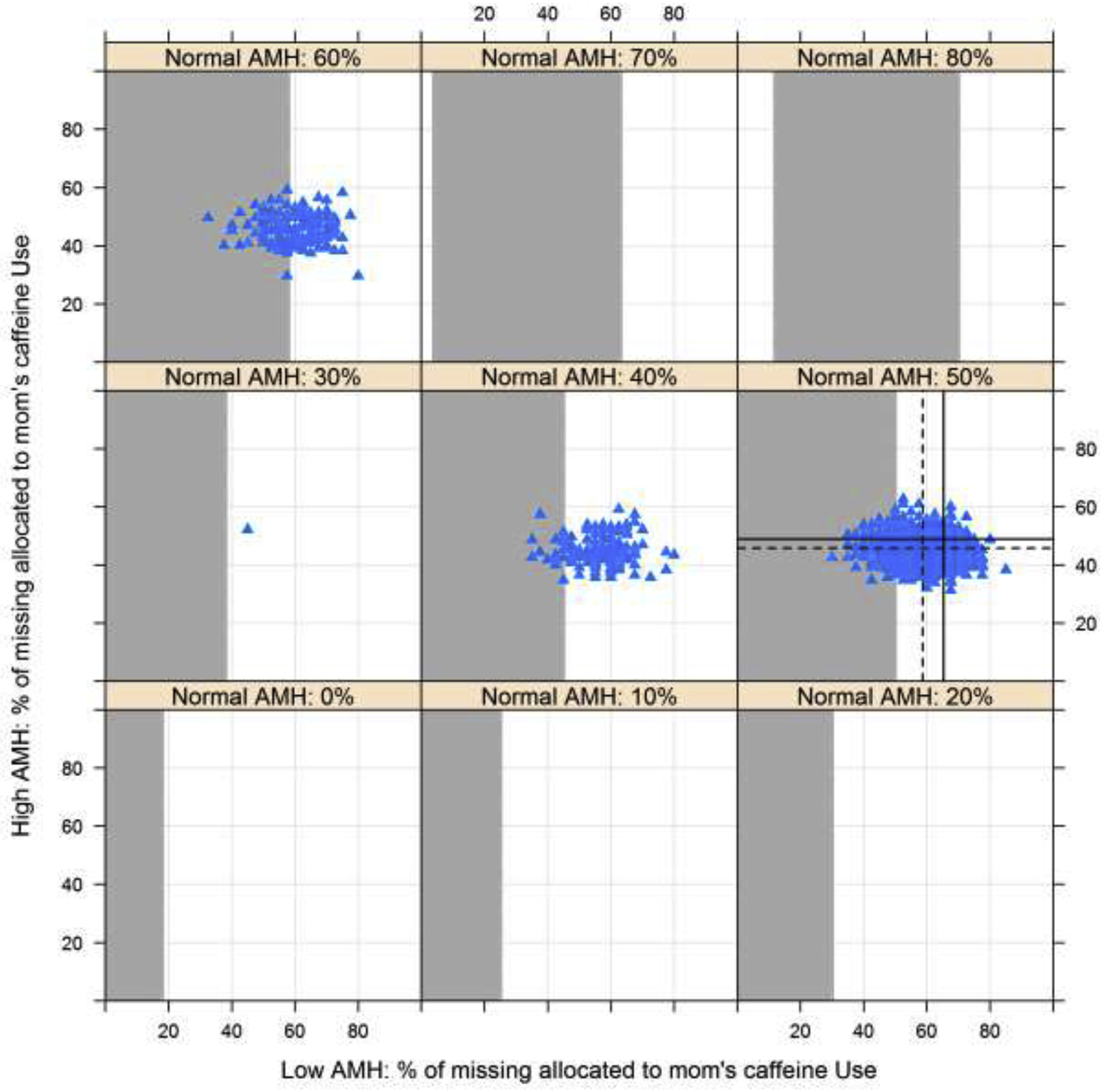
Association between in utero exposure to caffeine use and risk of low AMH compared to normal AMH with missing exposure information imputed across the range of possible scenarios (0–100% exposed in the low and high AMH categories by varying exposure in the normal AMH category). White and grey regions indicate p≤0.05 and p>0.05, respectively. Values from multiple imputation with 1000 datasets are indicated using the blue triangles. Dashed lines represent the mean of the imputed values and solid lines indicated the observed proportions.
Discussion:
Overall, this study demonstrates potential intergenerational effects between reported in utero exposures during pregnancy and AMH in female offspring. Specifically, women who reported that their mothers were taking vitamin supplements while they were pregnant with them may have higher AMH in adulthood, whereas women whose mothers used caffeine during pregnancy may have lower AMH. This is one of two studies to evaluate the relationship between multiple in utero exposures and AMH in female offspring, and the results highlight the potential importance of lifestyle factors during pregnancy on reproductive health of the offspring1. Given that our findings were limited by reliance on daughters report of the maternal exposures, further studies with more detailed exposure information are needed to corroborate these results.
Our findings are largely consistent with available evidence and proposed biological rationale, though direct comparison is difficult given the paucity of data for specific exposures. We observed that report of maternal caffeine use during pregnancy was associated with an increased risk of low AMH in the daughters. While caffeine use during pregnancy has been widely studied with respect to pregnancy outcomes, often with conflicting results, evaluation in relation to fertility of offspring is rare27,28. A study of rats exposed to caffeine showed no changes in fertility of offspring27, though a study in humans found that daughters of tea drinkers had delayed puberty, whereas daughters of caffeine drinkers did not28. To our knowledge, no other studies have evaluated intergenerational effects of caffeine use during pregnancy on fecundability or AMH in female offspring. The American College of Obstetricians and Gynecologists recommends limiting the amount of caffeine exposure in pregnancy, as it is known to limit blood flow to the uterus and placenta. This inhibition in blood flow may divert blood supply away from developing the reproductive organs. It is important to note that our data on caffeine exposure were limited by self-report of the daughters of any versus no exposure. Future studies should evaluate the dose of caffeine consumed and the timing during pregnancy.
Though our findings with respect to DES exposure were not statistically significant and based on few events, our data indicate a potential increased risk of low AMH. This is consistent with the large body of evidence regarding the deleterious effects of DES on offspring fertility. Women exposed to DES in utero are also known to have menstrual irregularities and early menopause which may be due to the lack of complete development of the menstrual tract, and further support the negative, though insignificant, association we found in this study29.
Maternal vitamin use during pregnancy was positively associated with an elevated AMH. This positive association is consistent with data showing that adequate maternal nutrition status in pregnancy is protective for healthy endocrine pathways that influence fertility30, though AMH in the daughters has not been previously evaluated. Our findings are in line with studies that have shown that maternal vitamin use is associated with beneficial health outcomes in the offspring, including healthy body weight31, reduced risk of asthma32, and leukemia33, among others. It has been suggested that maternal nutrition plays a critical role on these later offspring health outcomes through changes in DNA methylation34, and due to nutrient status, endocrine health of the mother, and oxidative stress on the developing fetus35. Though our exposure information was limited in that we do not know the formulation, dose, or frequency of vitamin use during pregnancy, these results add to our knowledge regarding the use of vitamins on offspring health and suggest that further research on the reproductive health outcomes of the offspring is needed. Caution should be noted as elevated AMH can also be a marker of other reproductive issues in a young female such as polycystic ovarian syndrome (PCOS). While there are women in the EAGeR trial who may have a subclinical phenotype with mild elevations in both AMH and testosterone (Table 1), none of the women in this trial had known PCOS, so no comment can be made on associations with high AMH in that setting36,37.
Further, we found that employment status of the mothers was not related to the AMH of the female offspring. There are no studies that directly examine the impact of maternal employment on the AMH or fertility of the offspring. Given the lack of detailed data regarding work during pregnancy, it is difficult to make strong conclusions and more research is needed in this area.
Our finding that cigarette use during pregnancy was not associated with the AMH of the offspring is somewhat inconsistent with prior data that found decreased fertility and fecundability in the offspring of women who smoked while pregnant7, 8,12,17,19, as well as both earlier and later menarche4–6,14–16, 18, 20,38, and earlier menopause9. Though it is important to note that not all prior studies have shown associations with these outcomes16, an association may be expected based on this collective prior evidence and biological rationale. However, AMH in the offspring has only been evaluated in girls 14–16 years old1. Animal studies show maternal tobacco exposure inhibits granulosa cell proliferation and promotes apoptosis39,40. The authors of the referenced study hypothesized that this effect on the ovaries may not affect AMH because the effects are not long term, and therefore adult AMH values are normal1. Interestingly, there was an association between paternal smoking during pregnancy and lower AMH of the offspring1. Cigarette smoking is known to be associated with prolonged time to pregnancy, adverse pregnancy outcomes, spontaneous abortion, preterm delivery, and low birth weight12,17,41. However, there are also several other studies that do not demonstrate these effects1,42,43. One possibility is that external smoke exposure damages the DNA of cord blood of infants44, but the authors of the referenced study believe that the change in AMH was also potentially from the effect of post-natal smoking and second-hand smoking during infancy and childhood with paternal smoking1. Our study found no relationship between smoking or alcohol use in pregnancy on the AMH of the adult daughters. While these data are self-reported by the adult daughter, this finding is surprising based on the well-known effects of smoking on personal fertility. However, this observation is consistent with the other two published studies on the intergenerational effects of smoking1,38.
Alcohol abuse and fetal alcohol syndrome can have devastating effects on the child including severe developmental delays. Different mechanisms have been offered to explain the teratogenic effects of alcohol on the developing embryo including oxidative stress, disturbed metabolism of vital nutrients and proteins, impaired neurogenesis, increased cellular apoptosis, and effects on gene expression45. While there is evidence that spermatogenesis, and therefore male infertility in offspring, is adversely affected by prenatal alcohol exposure, there are a handful of reports of female infertility with alcohol exposure, however, they does not reference prenatal exposure, but rather exposure while trying to concieve45,46. This is consistent with the lack of association elucidated from this study.
The strengths of this study include measurement of AMH in a large cohort of 1202 healthy women with an assurance of fecundity. Our study design allowed the evaluation to be adjusted for known confounders, BMI, and age, as well as maternal age at conception and maternal use of infertility treatments to conceive. This is consistent with the adjustments made by the other large study evaluating the effects of AMH1. Importantly, this study is limited in that it relies on the participants’ recall and knowledge of their mother’s health during their in utero development, which raises concerns regarding potential recall bias. Evaluations on maternal recall of medication use in pregnancy have varied in prior reports, but generally are universally present throughout similar studies47,48. Finally, as previously discussed, AMH values were selected based on studies of infertile women and therefore, the cut-offs used in this investigation are not applicable to fertile women3. In light of this limitation, our findings should be considered as hypothesis generating and as a proof of concept for potential in utero effects on ovarian reserve of the offspring and additional studies are needed to further refine the exposure assessment. Moreover, each question was listed only as a binary response of “yes” or “no”. This limits the ability to evaluate whether a dose-response or threshold effects are present. We also chose to include women with a history of one to two prior spontaneous abortions which limits this utility of this study to all healthy women and increases the chance for a selection bias. Finally, the utility of AMH as a marker for fertility is still under debate as it does not correlate directly with ability to conceive or quality of eggs, but at this time remains an important marker for ovarian reserve2.
Conclusion:
As ovarian follicles develop in utero, there is concern that prenatal exposures during this follicular formation can have an impact on the future fertility of the offspring. Though our results are based on daughter’s report of the maternal exposures, our results are suggestive of the potential for in utero exposure to vitamins and caffeine to influence AMH concentrations in female offspring. Given the importance of prenatal health to optimize the health of the offspring, additional studies are needed to confirm these findings.
Highlights.
Maternal caffeine use during pregnancy may be associated with ovarian reserve in adult female offspring.
Vitamin use in pregnancy may be associated with ovarian reserve in adult female offspring.
Intergenerational effects of medications or toxins in pregnancy may have consequences through multiple generations.
Funding:
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The opinions expressed in this manuscript are those of the authors and do not represent the Department of Health and Human Services or the Department of Defense.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Clinical Trials Number: NCT00467363
https://clinicaltrials.gov/ct2/show/NCT00467363?term=EAGeR&draw=2&rank=1
References
- 1.Fraser A, McNally W, Sattar N, Anderson EL, Lashen H, Fleming R, Lawlor DA and Nelson SM, 2013. Prenatal exposures and anti-müllerian hormone in female adolescents: the Avon Longitudinal Study of Parents and Children. American journal of epidemiology, 178(9), pp.1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL, Silver RM, Stanford JB, Galai N, White MV, Schliep KC, DeCherney AH and Schisterman EF, 2015. Is anti-Müllerian hormone associated with fecundability? Findings from the EAGeR trial. The Journal of Clinical Endocrinology & Metabolism, 100(11), pp.4215–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL, Silver RM, Stanford JB, Galai N, Schliep KC, Radin RG, Plowden TC and DeCherney AH, 2016. Antimüllerian hormone and pregnancy loss from the Effects of Aspirin in Gestation and Reproduction trial. Fertility and sterility, 105(4), pp.946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behie AM and O’Donnell MH, 2015. Prenatal smoking and age at menarche: influence of the prenatal environment on the timing of puberty. Human Reproduction, 30(4), pp.957–962. [DOI] [PubMed] [Google Scholar]
- 5.Ernst A, Kristensen SL, Toft G, Thulstrup AM, Håkonsen LB, Olsen SF and Ramlau-Hansen CH, 2012. Maternal smoking during pregnancy and reproductive health of daughters: a follow-up study spanning two decades. Human reproduction, 27(12), pp.3593–3600. [DOI] [PubMed] [Google Scholar]
- 6.Ferris JS, Flom JD, Tehranifar P, Mayne ST and Terry MB, 2010. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatric and perinatal epidemiology, 24(6), pp.515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler PA, Childs AJ, Courant F, MacKenzie A, Rhind SM, Antignac JP, Le Bizec B, Filis P, Evans F, Flannigan S and Maheshwari A, 2014. In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. Human reproduction, 29(7), pp.1471–1489. [DOI] [PubMed] [Google Scholar]
- 8.Håkonsen LB, Ernst A and Ramlau-Hansen CH, 2014. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian journal of andrology, 16(1), p.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honorato TC, Haadsma ML, Land JA, Boezen MH, Hoek A and Groen H, 2018. In-utero cigarette smoke exposure and the risk of earlier menopause. Menopause (New York, NY), 25(1), pp.54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton LC, Goldberg M, Wei Y, Cirillo PM, Cohn BA, Michels KB and Terry MB, 2018. Why do studies show different associations between intrauterine exposure to maternal smoking and age at menarche?. Annals of epidemiology, 28(3), pp.197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloway AC, Kellenberger LD and Petrik JJ, 2006. Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine, 30(2), pp.213–216. [DOI] [PubMed] [Google Scholar]
- 12.Jensen TK, Henriksen TB, Hjollund NHI, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE and Olsen J, 1998. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. American journal of epidemiology, 148(10), pp.992–997. [DOI] [PubMed] [Google Scholar]
- 13.Lutterodt MC, Sørensen KP, Larsen KB, Skouby SO, Andersen CY and Byskov AG, 2009. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Human reproduction, 24(10), pp.2558–2566. [DOI] [PubMed] [Google Scholar]
- 14.Morris DH, Jones ME, Schoemaker MJ, Ashworth A and Swerdlow AJ, 2010. Determinants of age at menarche in the UK: analyses from the Breakthrough Generations Study. British journal of cancer, 103(11), p.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrestha A, Nohr EA, Bech BH, Ramlau-Hansen CH and Olsen J, 2010. Smoking and alcohol use during pregnancy and age of menarche in daughters. Human reproduction, 26(1), pp.259–265. [DOI] [PubMed] [Google Scholar]
- 16.Tweed S, Bhattacharya S and Fowler PA, 2017. Effects of maternal smoking on offspring reproductive outcomes: an intergenerational study in the North East of Scotland. Human Reproduction Open, 2017(2), p.hox006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg CR, Wilcox AJ and Baird DD, 1989. Reduced fecundability in women with prenatal exposure to cigarette smoking. American journal of epidemiology, 129(5), pp.1072–1078. [DOI] [PubMed] [Google Scholar]
- 18.Windham GC, Zhang L, Longnecker MP and Klebanoff M, 2008. Maternal smoking, demographic and lifestyle factors in relation to daughter’s age at menarche. Paediatric and perinatal epidemiology, 22(6), pp.551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Skjaerven R, Basso O, Baird DD, Eggesbo M, Uicab LAC, Haug K and Longnecker MP, 2010. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Human reproduction, 25(11), pp.2901–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yermachenko A and Dvornyk V, 2015. A meta-analysis provides evidence that prenatal smoking exposure decreases age at menarche. Reproductive Toxicology, 58, pp.222–228. [DOI] [PubMed] [Google Scholar]
- 21.Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, Lynch AM, Perkins NJ, Mumford SL and Galai N, 2014. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. The Lancet, 384(9937), pp.29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, Lesher LL, Faraggi D, Wactawski-Wende J, Browne RW and Townsend JM, 2013. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatric and perinatal epidemiology, 27(6), pp.598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson SM, Iliodromiti S, Fleming R, Anderson R, McConnachie A and Messow CM, 2014. Reference range for the antimüllerian hormone Generation II assay: a population study of 10,984 women, with comparison to the established Diagnostics Systems Laboratory nomogram. Fertility and sterility, 101(2), pp.523–529. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Harel O and Little RJ, 2010. How well quantified is the limit of quantification?. Epidemiology, pp.S10–S16. [DOI] [PubMed] [Google Scholar]
- 25.Whitcomb BW, Perkins NJ, Albert PS and Schisterman EF, 2010. Treatment of batch in the detection, calibration, and quantification of immunoassays in large-scale epidemiologic studies. Epidemiology (Cambridge, Mass.), 21(Suppl 4), p.S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sartorius G, Ly LP, Sikaris K, McLachlan R and Handelsman DJ, 2009. Predictive accuracy and sources of variability in calculated free testosterone estimates. Annals of clinical biochemistry, 46(2), pp.137–143. [DOI] [PubMed] [Google Scholar]
- 27.Pollard I and Claassens R, 1992. Caffeine-mediated effects on reproductive health over two generations in rats. Reproductive Toxicology, 6(6), pp.541–545. [DOI] [PubMed] [Google Scholar]
- 28.Windham GC, Bottomley C, Birner C and Fenster L, 2004. Age at menarche in relation to maternal use of tobacco, alcohol, coffee, and tea during pregnancy. American journal of epidemiology, 159(9), pp.862–871. [DOI] [PubMed] [Google Scholar]
- 29.Reed CE and Fenton SE, 2013. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Research Part C: Embryo Today: Reviews, 99(2), pp.134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhind SM, Rae MT and Brooks AN, 2001. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction, 122(2), pp.205–214. [DOI] [PubMed] [Google Scholar]
- 31.Dougan MM, Willett WC and Michels KB, 2015. Prenatal vitamin intake during pregnancy and offspring obesity. International Journal of Obesity, 39(1), p.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parr CL, Magnus MC, Karlstad Ø, Holvik K, Lund-Blix NA, Haugen M, Page CM, Nafstad P, Ueland PM, London SJ and Håberg SE, 2018. Vitamin A and D intake in pregnancy, infant supplementation, and asthma development: the Norwegian Mother and Child Cohort. The American journal of clinical nutrition, 107(5), pp.789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metayer C, Milne E, Dockerty JD, Clavel J, Pombo-de-Oliveira MS, Wesseling C, Spector LG, Schüz J, Eleni P, Sameera E and Armstrong BK, 2014. Maternal supplementation with folic acid and other vitamins and risk of leukemia in the offspring: A childhood leukemia international consortium study. Epidemiology (Cambridge, Mass.), 25(6), p.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGee M, Bainbridge S and Fontaine-Bisson B, 2018. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutrition reviews, 76(6), pp.469–478. [DOI] [PubMed] [Google Scholar]
- 35.Chavatte-Palmer P, Al KG, Picone O and Heyman Y, 2008. Maternal nutrition: effects on offspring fertility and importance of the periconceptional period on long-term development. Gynecologie, obstetrique & fertilite, 36(9), pp.920–929. [DOI] [PubMed] [Google Scholar]
- 36.Sjaarda LA, Mumford SL, Kissell K, Schliep KC, Hammoud AO, Perkins NJ, Weck J, Wactawski-Wende J and Schisterman EF, 2014. Increased androgen, anti-Müllerian hormone, and sporadic anovulation in healthy, eumenorrheic women: a mild PCOS-like phenotype?. The Journal of Clinical Endocrinology & Metabolism, 99(6), pp.2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjaarda LA, Mumford SL, Kuhr DL, Holland TL, Silver RM, Plowden TC, Perkins NJ and Schisterman EF, 2018. Association of testosterone and antimüllerian hormone with time to pregnancy and pregnancy loss in fecund women attempting pregnancy. Fertility and sterility, 109(3), pp.540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst A, Kristensen SL, Toft G, Thulstrup AM, Håkonsen LB, Olsen SF, & Ramlau-Hansen CH (2012). Maternal smoking during pregnancy and reproductive health of daughters: a follow-up study spanning two decades. Human reproduction, 27(12), 3593–3600. [DOI] [PubMed] [Google Scholar]
- 39.Bordel R, Laschke MW, Menger MD and Vollmar B, 2005. Nicotine does not affect vascularization but inhibits growth of freely transplanted ovarian follicles by inducing granulosa cell apoptosis. Human Reproduction, 21(3), pp.610–617. [DOI] [PubMed] [Google Scholar]
- 40.Paixão LL, Gaspar-Reis RP, Gonzalez GP, Santos AS, Santana AC, Santos RM, Spritzer PM and Nascimento-Saba CCA, 2012. Cigarette smoke impairs granulosa cell proliferation and oocyte growth after exposure cessation in young Swiss mice: an experimental study. Journal of ovarian research, 5(1), p.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Petersen I and Christensen K, 2006. Early exposure to smoking and future fecundity among Danish twins. International journal of andrology, 29(6), pp.603–613. [DOI] [PubMed] [Google Scholar]
- 42.Baird DD and Wilcox AJ, 1986. Future fertility after prenatal exposure to cigarette smoke. Fertility and sterility, 46(3), pp.368–372. [PubMed] [Google Scholar]
- 43.Joffe M and Barnes I, 2000. Do parental factors affect male and female fertility?. Epidemiology, 11(6), pp.700–705. [DOI] [PubMed] [Google Scholar]
- 44.Laubenthal J, Zlobinskaya O, Poterlowicz K, Baumgartner A, Gdula MR, Fthenou E, Keramarou M, Hepworth SJ, Kleinjans JC, van Schooten FJ and Brunborg G, 2012. Cigarette smoke-induced transgenerational alterations in genome stability in cord blood of human F1 offspring. The FASEB Journal, 26(10), pp.3946–3956. [DOI] [PubMed] [Google Scholar]
- 45.Ornoy A and Ergaz Z, 2010. Alcohol abuse in pregnant women: effects on the fetus and newborn, mode of action and maternal treatment. International journal of environmental research and public health, 7(2), pp.364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Heertum K, & Rossi B (2017). Alcohol and fertility: how much is too much?. Fertility research and practice, 3(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockenbauer M, Olsen J, Czeizel AE, Pedersen L, Sørensen HT and The EuroMAP Group, 2001. Recall bias in a case-control surveillance system on the use of medicine during pregnancy. Epidemiology, pp.461–466. [DOI] [PubMed] [Google Scholar]
- 48.Sundermann AC, Hartmann KE, Jones SH, Torstenson ES and Edwards DRV, 2017. Validation of maternal recall of early pregnancy medication exposure using prospective diary data. Annals of epidemiology, 27(2), pp.135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]


