Abstract
Background
Accumulation of lipoprotein X (LpX) in blood can cause severe hypercholesterolemia and cutaneous xanthomas. Monocytes sensitively sense lipid changes in circulation and contribute to inflammation. However, how monocytes respond to LpX is undefined.
Objective
We examined the phenotype of monocytes from a subject, who had LpX, severe hypercholesterolemia, and extensive cutaneous xanthomas, and effects of semiselective plasmapheresis therapy (SPPT).
Method
Fluorescence-activated cell sorting and adhesion assays were used to examine monocyte phenotype and ex vivo oxLDL uptake and adhesion in the patient before and after treatment with SPPT. Effects of plasma from the patient on the phenotype and adhesion of monocytes from a healthy subject were determined.
Results
SPPT improved hypercholesterolemia and cutaneous xanthomas. Before treatment, the patient had lower frequency of non-classical monocytes but higher frequency of intermediate monocytes than control subject. Before treatment, monocytes from the LpX patient showed more intracellular lipid accumulation, alterations in several cell surface markers and intracellular cytokines as well as enhanced oxLDL uptake and reduced adhesion compared to control. After SPPT, the phenotypes of monocytes from the LpX patient were similar to control monocytes. Incubation with plasma from the patient before treatment as compared to plasma from the control subject or the patient after treatment increased CD11c expression and adhesion of monocytes from a healthy subject.
Conclusion
LpX-induced hypercholesterolemia increased lipid accumulation and altered the phenotype of monocytes, which may contribute to cutaneous xanthoma development. Removal of LpX by SPPT reduced lipid accumulation and improved monocyte phenotype, likely contributing to xanthoma resolution.
Keywords: Lipoprotein X, hypercholesterolemia, foamy monocyte, primary biliary cirrhosis
Introduction
Lipoprotein X (LpX) is an abnormal lipoprotein that commonly emerges and accumulates in circulation in patients with primary biliary cirrhosis, obstructive liver disease, and lecithin–cholesterol acyltransferase deficiency.1–3 Severe LpX elevation in circulation can cause hypercholesterolemia that is associated with generalized skin xanthomas in patients.4, 5 The predominant composition in xanthoma of LpX patients is foamy cells derived from lipid-laden macrophages, suggesting an interaction between circulating LpX and immune cells.6, 7
Circulating monocytes are a major source of macrophages in tissues. During inflammation, activated monocytes infiltrate into tissue and differentiate to macrophages. Elevated plasma lipid is an inflammatory factor that activates circulating monocytes and changes monocyte phenotype and function. Hypercholesterolemia and/or hypertriglyceridemia induce intracellular lipid accumulation in monocytes and foamy monocyte formation in the circulation of mice and human.8, 9 Foamy monocytes express higher levels of adhesion molecules, which enhances monocyte adhesion and infiltration and contributes to foam macrophage accumulation in arterial walls and possibly other tissues. However, how monocytes respond to LpX in the circulation is ill defined.
Here, we report the clinical management and monocyte phenotype and functional changes in a case of LpX syndrome before and after semiselective apheresis treatment.
Case
A 36-year-old woman with a medical history of prehypertension, primary biliary cirrhosis, and autoimmune hepatitis was referred to the lipidology clinic due to severe elevation in total cholesterol in May 2015. At the time, she was noted to have abnormal lipid profile (Table 1) with total cholesterol of 2324 mg/dL and abnormal liver function test (total bilirubin 9.2 mg/dL, alkaline phosphatase 1124 U/L, aspartate aminotransferase [AST] 116 U/L, alanine aminotransferase [ALT] 111 U/L) consistent with obstructive liver disease. She also had widespread skin lesion that were consistent with eruptive and planar xanthomas on biopsy. Prior to the onset of obstructive liver disease, she had a normal lipid profile with total cholesterol of 156 mg/dL, triglycerides of 77 mg/dL, high-density lipoprotein cholesterol (HDL-C) of 52 mg/dL, calculated low-density lipoprotein cholesterol (LDL-C) of 89 mg/dL and non–high-density lipoprotein cholesterol of 104 mg/dL. All other secondary causes of hypercholesterolemia besides obstructive liver disease were ruled out. Although baseline apolipoprotein B (apoB) levels (226 mg/dL in May 2015 and 114 mg/dL in February 2016) were slightly discrepant, given the onset of elevated cholesterol levels and eruptive xanthomas in the setting of primary biliary cirrhosis in an adult with prior low levels of total cholesterol and LDL-C, the patient was diagnosed to have LpX syndrome.
Table 1.
Changes in Lipid Profile with Plasmapheresis in Patient with Lipoprotein X Syndrome
| Baseline | First Abnormal Lipid Profile | First Presentation Lipid Clinic | Follow-up Lipid Clinic | Re-established Lipid Clinic Care | Post First 2 Cycles of Plasmapheresis | Established Biweekly Plasmapheresis | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Date of presentation(mm/yy) | 1/08 | 3/14 | 5/15 | 2/16 | 10/17 | 1/18 (before treatment) | 1/18 | 3/18 | 4/18 | 5/18 |
| Triglycerides(mg/dL) | 77 | 171 | 408 | 184 | 163 | 194 | 177 | 165 | 173 | 210 |
| Total cholesterol (mg/dL) | 156 | 528 | 2324 | 1449 | 2033 | 1821 | 1096 | 822 | 472 | 341 |
| High-density lipoprotein cholesterol(mg/dL)* | 52 | 41 | 23 | 22 | >180 | >180 | 31 | 24 | 17 | 12 |
| Non-high-density lipoprotein cholesterol(mg/dL)# | 104 | 487 | 2301 | 1427 | <1853 | <1641 | 1065 | 858 | 455 | 329 |
| Apolipoprotein B (mg/dL) | N/A | N/A | 226 | 114 | N/A | N/A | N/A | N/A | N/A | N/A |
Because of assay limitation, the values may not represent the accurate high-density lipoprotein cholesterol levels at times of severe hypercholesterolemia.
Calculated values, which, depending on the accuracy of high-density lipoprotein cholesterol levels, may not represent accurate non-high-density lipoprotein cholesterol levels.
She was started by hepatology on a dual regimen of cholestyramine and ursodeoxycholic acid for her primary biliary cirrhosis and symptoms of pruritus; however, after this she was not seen for follow-up in our clinic for ~2 years.
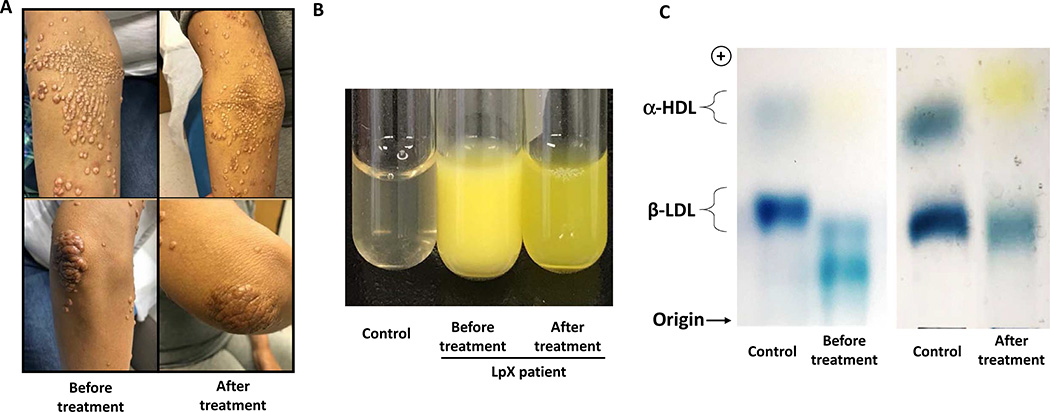
On follow-up to the lipid clinic in October 2017, she had total cholesterol of 2033 mg/dL, total bilirubin of 4.1 mg/dL, AST of 80 U/L, and ALT of 76 U/L. She also reported worsening in her eruptive xanthomas which were a source of severe discomfort due to pruritus and resultant bleeding. She also disliked the cosmetic appearance of her xanthomas (Figure 1A).
Figure 1.
Skin xanthomas and confirmation of LpX in the patient. A. The regression of skin xanthomas after treatment (20 weeks of regular biweekly plasmapheresis therapy), compared with the patient’s first presentation in Oct 2017. B. Plasma samples from a control subject and the LpX patient before (Jan 2018) and after treatment (May 2018). C. Plasma lipoprotein electrophoresis showing some typical features of LpX from the patient’s plasma before treatment and recovery of the β-lipoprotein band after treatment.
For symptomatic relief, she was scheduled for semi--selective plasmapheresis trial therapy every 2–4 weeks from January 2018 which she pursued over next 6 months till May 2018. Successive plasmapheresis treatments were associated with a pronounced reduction in lipid levels (Table 1) as well as xanthomas (Figure 1A) and associated itching/discomfort.
Laboratory studies
We performed laboratory tests in January 2018 (before treatment) and in May 2018 (after 20 weeks on treatment), for the following studies. Two sex-, age-, and race-matched healthy volunteers with normal plasma levels of cholesterol and triglyceride were recruited as control subjects, one for each timepoint, and blood was collected and processed simultaneously from the LpX patient and the controls after an overnight fast. The studies were approved by the Institutional Review Board of Baylor College of Medicine, and all participants provided written informed consent.
Electrophoresis
Plasma was collected from K2EDTA-anticoagulated blood by 800 g centrifugation and then was diluted 1:1.5 with medical-grade saline. Two-hundred microliters diluted plasma was stained with 20 μL 0.5% Sudan Black B solution at 37°C for 30 minutes and run on 0.5% agarose gel under 15 V/cm in barbital buffer for 1 hour. Lipoprotein bands were captured by a Nikon digital camera.
Fluorescence-activated cell sorting (FACS)
For surface marker staining, 100 μL blood was incubated with antibody mixtures (Table 2) at 4°C for 30 minutes and then was fixed with BD lysing solution, which also lysed red blood cells. Leukocytes were pelleted and washed with phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin and stored in 2% paraformaldehyde in PBS for detection by a Gallios cytometer (Beckman Coulter).
Table 2.
List of antibodies used in the flow cytometry experiments
| Marker | Clone | Company |
|---|---|---|
| CD14 | RMO52 | Beckman Coulter |
| CD16 | 3G8 | Beckman Coulter |
| CD11c | 3.9 | BioLegend |
| CD36 | FA6.152 | Beckman Coulter |
| SRA | 351615 | R&D systems |
| CCR2 | K036C2 | BioLegend |
| CX3CR1 | 2A9–1 | BioLegend |
| CD62L | DREG56 | Beckman Coulter |
| HLA-DR | Immu-357 | Beckman Coulter |
| CD18 | m24 | BioLegend |
| VLA4 | 9F10 | BioLegend |
| IL1β | CRM56 | eBioscience |
| TNFα | MAb11 | BioLegend |
| LRP1 | A2MR-a2 | BD Biosciences |
| CCR5 | J418F1 | BioLegend |
| IL6 | MQ2–13A5 | BioLegend |
For intracellular cytokine expression assay, 100 μL blood was mixed with equal volume of complete RPMI-1640 medium and incubated with 5 mg/mL of LPS (Sigma-Aldrich) in the presence of Golgiplug (BD) for 4 hours at 37°C. After incubation, red blood cells were lysed by red blood cell lysis buffer (Biolegend). Then, the surface markers of monocytes were stained. For intercellular staining, leukocytes were permeabilized and then incubated with antibodies for intercellular markers following the instruction of Fixation and Permeabilization Solution kit (BD).
For Nile red staining of lipids, blood was subjected to red blood cell lysis and then stained for monocyte surface markers. After fixation, cells were stained with Nile red at 0.1 μM in PBS for 20 minutes. Then, cytospin was performed, and cells were mounted in permanent DAPI medium and imaged by Nikon LSM 780 confocal microscope.
For oxidized low-density lipoprotein (oxLDL) uptake assay, 100 μL blood was mixed with equal volume of complete RPMI-1640 medium and incubated with 120 μg/mL DiI-labeled human oxLDL (Kalen Biomedical) and incubated for 1 hour. After incubation, samples were subjected to surface antibody staining as described above.
On-chip adhesion assay
Design and assembly of the microfluidic device and the whole blood adhesion assay were performed as previously reported.9 Briefly, coverslips that were coated with recombinant hVCAM-1/Fc chimera (R&D Systems) were assembled with a 4-channel polydimethylsiloxane device and kept at 37°C. Heparin-anticoagulated blood (100 μL) was stained with PE–anti-CD16 and AF488–anti-CD14 antibodies at room temperature for 20 minutes. Then, after 1:3 dilution in PBS with calcium and magnesium, 60 μL diluted blood was introduced into the polydimethylsiloxane device at a flow rate that produced a shear stress of 2 dynes/cm2 at fluid–glass interface and was perfused for 5 minutes followed by fixation and mounting in permanent DAPI medium. The number of adherent monocytes (subsets) was counted and normalized by total infused number of each monocyte subset.
In vitro plasma treatment of monocytes
Blood (100 μL) from the healthy control subject was washed by PBS to remove endogenous lipoproteins and resuspended in 100 μL RPMI-1640 medium (without fetal bovine serum). Then, the blood samples were incubated in triplicate with 200 μL plasma from the control subject or from the LpX patient before and after treatment. At 0.5, 1, 3, and 6 hours of incubation, the cells were washed and subjected to surface marker staining for FACS assay or perfused in polydimethylsiloxane device for adhesion assay.
Results
Confirmation of LpX
Compared with that from the control subject, plasma from the patient before treatment was cloudy and yellowish (Figure 1B). The yellow color was consistent with the elevated blood level of bilirubin (see above) and also possibly extremely high blood level of cholesterol in the patient. The cloudy appearance of plasma, which does not usually occur in severe hypercholesterolemia caused by LDL-C, such as familial hypercholesterolemia, because LDL particles are not large enough to scatter visible light, supported the presence of LpX, which can form disk-like lipoprotein particles of varying size that can scatter visible light and, at room temperature (at which the picture was taken), can form rouleaux to amplify light scattering, causing cloudiness.10 After treatment, plasma from the patient was more transparent than before treatment (Figure 1B). The yellow color of plasma after treatment was likely because of persistent hyperbilirubinemia in the patient. Agarose electrophoresis also showed that, compared to the control, the plasma sample from the patient before treatment showed some typical features of LpX: 1) absence of α−lipoprotein, indicating low HDL contents; 2) low concentration of β−lipoprotein, indicating low abundance of the LDL fraction; 3) presence of a band below β−lipoprotein, a pattern similar to that as previously reported for LpX cases.11, 12 After 20 weeks of treatment, the plasma of the patient recovered the β-lipoprotein band, but not α-lipoprotein (Figure 1C).
Monocyte counts and phenotypes
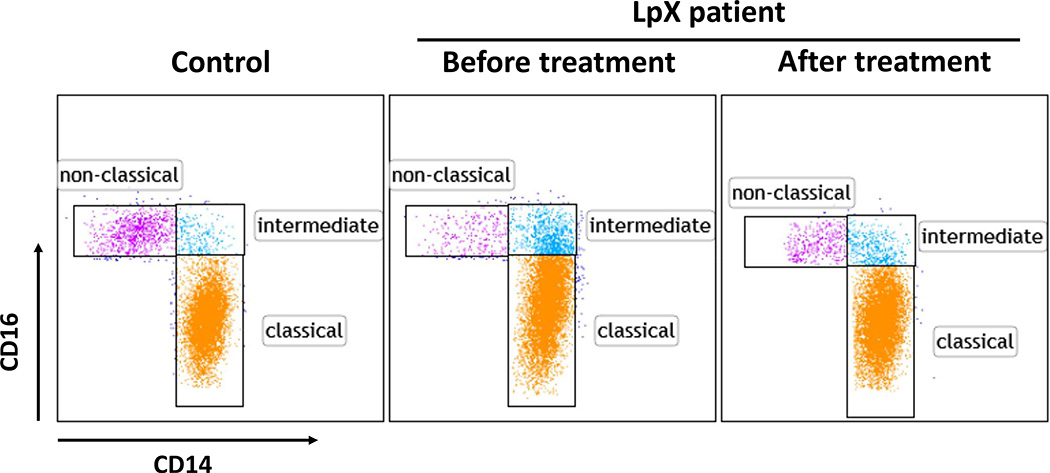
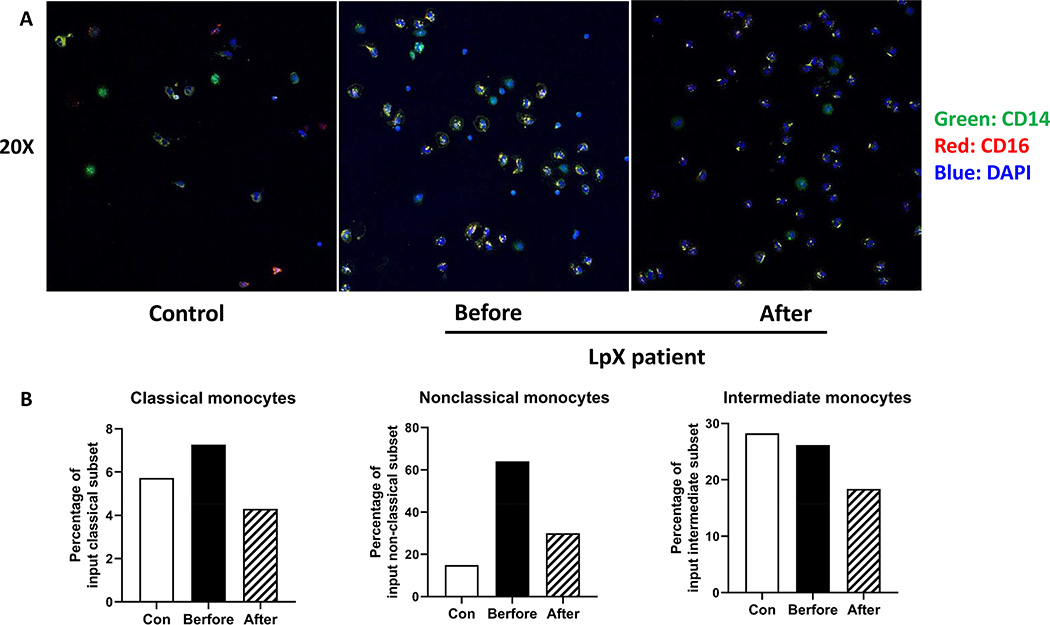
Based on CD14 and CD16, human monocytes can be categorized as classical (CD14+CD16-), intermediate (CD14+CD16low), and nonclassical (CD14lowCD16+) subsets (Figure 2). Total monocytes/mL in blood in the LpX patient were higher before and after treatment than the average in control subjects. Compared with control subjects, the patient before treatment had a higher proportion of the intermediate subset, a lower proportion of the nonclassical subset, and a slight increase in the classical subset. After treatment, the monocyte subset proportions in the patient trended toward normal status (Table 3).
Figure 2.
Monocyte subsets of the patient and the control subject were examined by fluorescence-activated cell sorting (FACS) showing CD14+CD16− classical subset, CD14+CD16low intermediate subset, and CD14lowCD16+ nonclassical subset.
Table 3.
Monocyte Count in Blood and Proportions of Monocyte Subsets
| Subject | Treatment (mm/yy) | Monocyte Count (105/mL blood) | Monocyte Subsets (% of Total Monocytes) |
||
|---|---|---|---|---|---|
| Classical | Intermediate | Nonclassical | |||
| Control | 3.40 | 85.8 | 3.1 | 11.1 | |
| Before treatment (1/18) | 4.49 | 86.4 | 10.7 | 2.9 | |
| Patient | |||||
| After treatment (5/18) | 4.92 | 90.6 | 5.3 | 4.1 | |
Lipid accumulation in monocytes
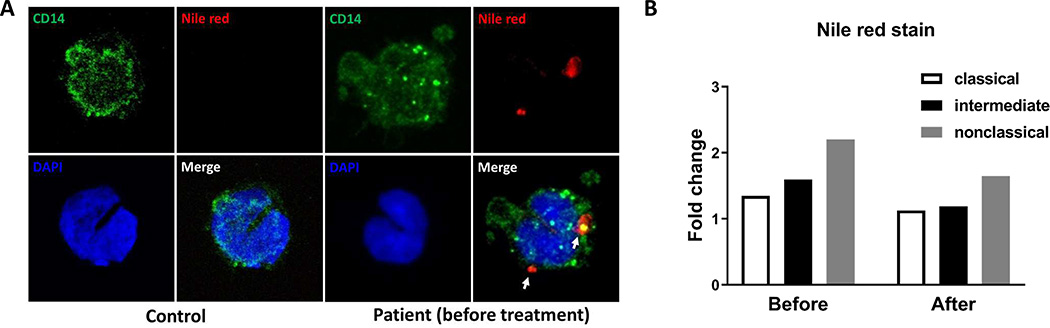
By using Nile red staining, we found that monocytes from the LpX patient before treatment had positive lipid staining in cytoplasm, which was not observed in monocytes from control subjects, indicating intracellular lipid accumulation in monocytes from the patient (Figure 3A). Quantitation by FACS showed that nonclassical monocytes had higher mean fluorescence intensity (MFI) levels of Nile red, indicating more lipid, than classical and intermediate monocytes in the patient before treatment and that Nile red levels were reduced in all monocyte subsets after treatment (Figure 3B).
Figure 3.
Lipid accumulation in monocytes from the patient. A. Confocal images of circulating monocytes from a control subject and the patient stained with AF488–anti-CD14 and Nile red. White arrows indicate positive Nile red staining. B. The fold changes of Nile red mean fluorescence intensity (MFI) of monocytes from the LpX patient relative to corresponding control subjects.
Changes in monocyte phenotypes
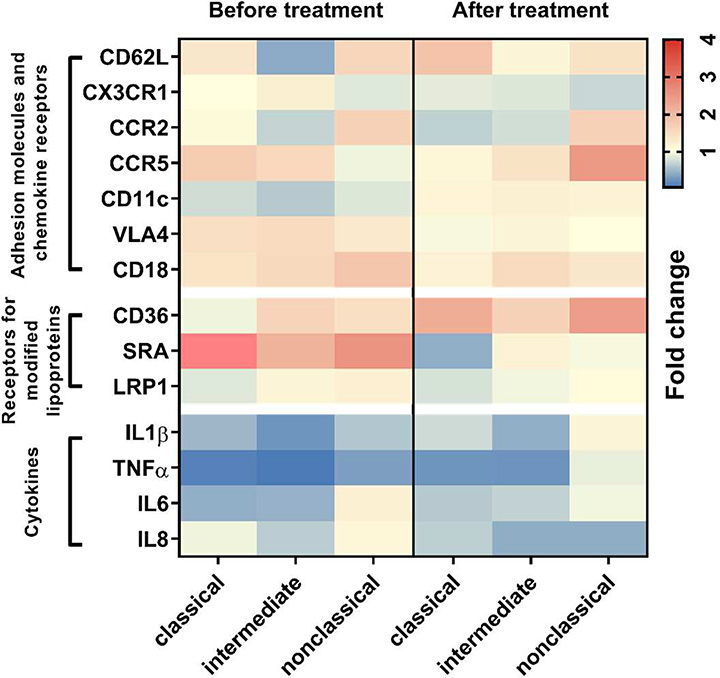
Monocyte expression of several surface markers that are involved in monocyte migration and modified lipoprotein uptake was examined by FACS. The heatmap plot in Figure 4 shows the fold changes in MFI level of detected markers on monocytes from the LpX patient relative to those from the control subject. The most striking differences were found in CD11c and class A scavenger receptor (SRA) expression between the patient before treatment and the control subject. CD11c, an adhesion molecule, was unexpectedly decreased in all subsets of monocytes in the LpX patient before treatment and returned to normal level after treatment. SRA, a scavenger receptor mediating pattern recognition and oxLDL uptake, was increased in all monocyte subsets in the patient before treatment and returned to a normal or lower expression level after treatment.
Figure 4.
Heatmap of phenotypic profiles of monocyte subsets. The expression of adhesion molecules, chemokine receptors, modified lipoprotein receptors, and cytokines on monocytes was detected by FACS. Fold changes in MFI of each marker from the patient before and after treatment versus corresponding control subjects are shown.
We also observed differences among the three monocyte subsets in the change of expression levels of certain surface markers in the patient versus control. CD36, another scavenger receptor for oxLDL uptake, was decreased on the classical subset, but was increased on intermediate and nonclassical subsets from the patient before treatment compared to control subjects. After treatment, CD36 expression was increased on all monocyte subsets of the patient.
We then examined intracellular cytokine expression of circulating monocytes and found that monocytes from the LpX patient before and after treatment mostly expressed lower levels of proinflammatory cytokines, including interleukin-1β, tumor necrosis factor α, interleukin-6, and interleukin-8, than those from control subjects (Figure 4).
OxLDL uptake by monocytes
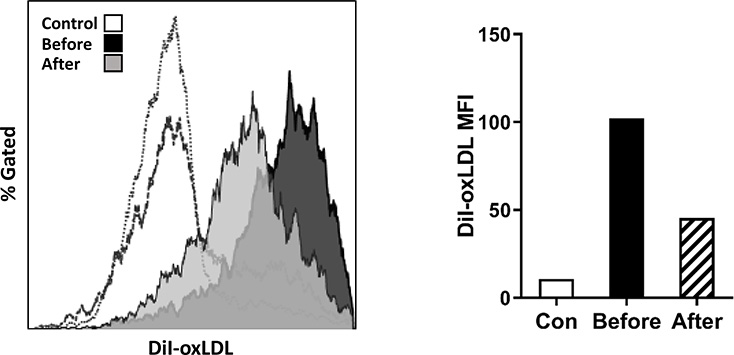
To test whether the altered expression of scavenger receptors in the patient impact monocyte uptake of modified lipoprotein, a process for foam cell formation, we examined monocyte uptake of DiI-oxLDL ex vivo and found that after incubation with DiI-oxLDL, monocytes from LpX patient before treatment vs the control subjects exhibited higher MFI levels of DiI, indicating increased uptake capacity of oxLDL. After treatment, monocyte uptake of oxLDL was reduced in the patient but was still higher than that in control subjects (Figure 5).
Figure 5.
Uptake of oxidized low-density lipoprotein (oxLDL) by monocytes from the patient. Monocytes from control subjects and the patient before and after treatment were incubated with DiI-oxLDL for 1 hour; the Dil MFI was examined by FACS.
On-chip adhesion assay of monocytes
Using a laboratory-on-a-chip analysis, we tested the adhesion capacity of monocytes from the LpX patient and control subjects. The percentages of arrested classical and nonclassical subsets were higher and percentage of the intermediate subset was slightly lower in the patient before treatment than in the control subject. After treatment, adhesion capacity of all monocyte subsets was reduced than before treatment in the patient (Figure 6).
Figure 6.
On-chip adhesion assay of monocyte subsets. A. Representative images showing monocytes arrested on VCAM-1–coated slices; 20x magnification. B. Percentages of arrested monocytes.
Effect of LpX plasma on normal monocytes
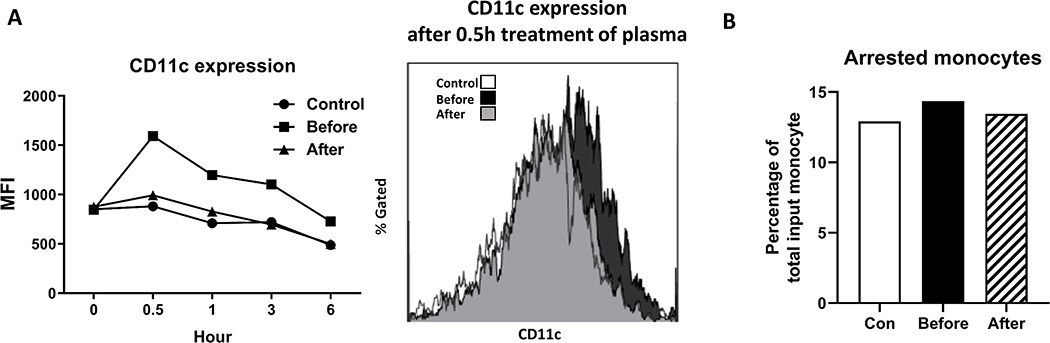
Finally, we examined the effects of plasma from the LpX patient on monocytes from a healthy subject. Ex vivo incubation with plasma from the patient before treatment increased CD11c levels on monocytes from the healthy subject. In contrast, incubation with plasma from the patient after treatment or the control subject had minor effect on CD11c expression on monocytes of the healthy subject (Figure 7A).
Figure 7.
Incubation with LpX plasma changed CD11c expression and adhesion capacity of monocytes from the control subject. A. Monocytes from a healthy control were incubated ex vivo with plasma from control subjects or the LpX patient before and after treatment, and the CD11c expression on monocytes was detected by FACS. B. After incubation for 0.5 hours, adhesion capacity of monocytes was examined and percentages of arrested monocytes to total monocytes were presented.
Corresponding to the increase in CD11c expression, incubation with plasma from the LpX patient before treatment compared with that from the control subject or the patient after treatment increased monocyte firm arrest on chips (Figure 7B).
Discussion
Elevated levels of circulating lipids, including LDL and triglyceride-rich lipoprotein, promote intracellular lipid accumulation in monocytes, leading to foamy monocyte formation.8, 9, 13–15 In the current case, we report that LpX accumulation in an individual’s circulation induced foamy monocyte formation, changed monocyte phenotypes, and enhanced monocyte adhesion and uptake of oxLDL. Our findings indicate that monocytes may be a responsive immune component in LpX patients, which may contribute to tissue inflammation and damage.
Intracellular lipid accumulation can drive monocyte phenotypic changes. One change is the upregulation of scavenger receptors, such as SRA and CD36, with enhanced uptake of modified LDL, the major lipoprotein that causes foam cell formation in atherosclerotic lesions.16, 17 In line with this, in the current case of LpX, the increased foamy monocyte formation was accompanied by increased SRA expression, which correlated with increased oxLDL uptake.
Other phenotypic changes in monocytes associated with intracellular lipid accumulation include regulation of cytokine expression and adhesion molecules such as CD11c, a β2 integrin that plays an important role in monocyte adhesion to endothelial cells and atherosclerosis.9, 13, 18 However, opposite to our expectation, we observed downregulation of several proinflammatory cytokines and CD11c in/on monocytes in the LpX patient before treatment compared to the control subject. The mechanisms for these unexpected changes are unknown. We speculate that monocytes with large amounts of lipid accumulation and possibly increased inflammation and CD11c may have migrated into tissues quickly, whereas the monocytes that we examined in the study represent those that stayed in the circulation and may have included relatively lesser lipids with lower levels of inflammation and CD11c expression. In support of this speculation are our observations that in vitro treatment with plasma from the LpX patient increased CD11c expression and adhesion of monocytes from a healthy subject and a previous study showing that hyperlipidemia in mice induced by an inhibitor of lipoprotein lipase resulted in monocyte, particularly nonclassical monocyte, extravasation into tissues, leading to decreases in circulating nonclassical monocytes.19 Consistent with these findings, the individual with LpX before treatment compared to control subjects had reduced nonclassical monocytes and a drastic increase in adhesion capacity of nonclassical monocytes, which may play crucial roles in development of skin xanthoma. In contrast, other changes in monocyte phenotypes (Figs. 3 and 4) were moderate, possibly for the reason discussed above.
Removal of LpX particles through therapeutic plasma exchange is effective to reduce the total cholesterol level.5 In the current case, therapeutic plasma exchange treatment gradually decreased total plasma cholesterol and reduced intercellular lipid accumulation in circulating monocytes. Furthermore, expression of most surface markers on monocytes returned to normal levels after treatment.
Our study had several limitations. First, this is a single case report of LpX, which may not be generalizable to all LpX cases. Second, a limitation to extrapolating our findings to other patients with LpX is that this individual had higher levels of baseline apoB than most patients with LpX. Thus, she may have had higher circulating levels of modified LDL or more oxidized phospholipids associated with apoB particles that also contributed to the altered phenotype of the monocyte subset. Lipoprotein(a) is one of the modified lipoproteins containing oxidized phospholipids and apoB. However, we did not observe an obvious lipoprotein(a) fraction in the plasma of the LpX patient in electrophoretic results (Figure 1C), indicating that it is less likely that lipoprotein(a) contributed to the apoB elevation and changes in monocyte phenotypes in the current case. Another limitation is that in our ex vivo examination of the effect of LpX plasma on monocyte phenotypes, we cannot exclude potential effects of other components in plasma of the patient. However, given the enrichment of LpX in the patient’s plasma, we speculate that the effects were mainly derived from LpX in the plasma. Furthermore, because of our limited access to plasma samples of the patient with SPPT treatment, we could not continuously monitor monocyte phenotypes for a long term, during which monocyte phenotypes may change differently.
In conclusion, our study shows that LpX-induced hypercholesterolemia resulted in lipid accumulation in circulating monocytes with alterations in monocyte phenotypes in an individual with LpX syndrome. These LpX-associated alterations in monocytes may have contributed to tissue inflammation such as planar and eruptive xanthomas observed in this case. Treatment with selective lipoprotein apheresis effectively reduced the LpX concentration with decreases in monocyte lipid accumulation and changes of monocyte phenotypes, which may also have contributed to the improvement of planar xanthomas in the current case.
Highlights.
Cutaneous xanthomas presented in a woman with lipoprotein X induced hypercholesterolemia
Lipoprotein X-induced hypercholesterolemia increased lipid accumulation in monocytes
Lipoprotein X-induced hypercholesterolemia altered phenotypes of circulating monocytes
Removal of lipoprotein X by plasma exchange reduced size of the cutaneous xanthomas
Removal of lipoprotein X by plasma exchange improved monocyte phenotypes
Acknowledgement
We thank Kerrie Jara for editorial assistance.
Funding: This work was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK121348 (H.W.), National Heart, Lung, and Blood Institute grant R01HL098839 (H.W.), An American Diabetes Association award 1-17-IBS-082 (H.W.), and an American Heart Association award 16GRNT30410012 (H.W.).
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felker TE, Hamilton RL and Havel RJ. Secretion of lipoprotein-X by perfused livers of rats with cholestasis. Proc Natl Acad Sci U S A. 1978;75:3459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel D, Alaupovic P, Furman RH and McConathy WJ. A lipoprotein characterizing obstructive jaundice. II. Isolation and partial characterization of the protein moieties of low density lipoproteins. J Clin Invest. 1970;49:2396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utermann G, Schoenborn W, Langer KH and Dieker P. Lipoproteins in LCAT-deficiency. Humangenetik. 1972;16:295–306. [DOI] [PubMed] [Google Scholar]
- 4.Rosenson RS, Baker AL, Chow MJ and Hay RV. Hyperviscosity Syndrome in a Hypercholesterolemic Patient With Primary Biliary Cirrhosis. Gastroenterology. 1990;98:1351–1357. [DOI] [PubMed] [Google Scholar]
- 5.Brandt EJ, Regnier SM, Leung EK, Chou SH, Baron BW, Te HS, Davidson MH and Sargis RM. Management of lipoprotein X and its complications in a patient with primary sclerosing cholangitis. Clin Lipidol. 2015;10:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata Y, Okawa K, Ikeda M, Seike M, Matsumoto M and Kodama H. Low density lipoprotein oxidized in xanthoma tissue induces the formation and infiltration of foam cells. Journal of Dermatological Science. 2002;30:248–255. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki L, Hirayama S, Fukui M, Sasaki M, Hiroi S, Ayaori M, Terai S, Tozuka M, Watada H and Miida T. Lipoprotein-X in cholestatic patients causes xanthomas and promotes foam cell formation in human macrophages. J Clin Lipidol. 2017;11:110–118. [DOI] [PubMed] [Google Scholar]
- 8.Lian Z, Perrard XD, Peng X, Raya JL, Hernandez AA, Johnson CG, Lagor WR, Pownall HJ, Hoogeveen RC, Simon SI, Sacks FM, Ballantyne CM and Wu H. Replacing Saturated Fat With Unsaturated Fat in Western Diet Reduces Foamy Monocytes and Atherosclerosis in Male Ldlr(−/−) Mice. Arterioscler Thromb Vasc Biol. 2020;40:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA and Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellin R and Manzato E. Lipoprotein-X fifty years after its original discovery. Nutr Metab Cardiovasc Dis. 2019;29:4–8. [DOI] [PubMed] [Google Scholar]
- 11.Ćwiklińska A, Mickiewicz A, Kowalski R, Kortas-Stempak B, Kuchta A, Mucha K, Makowiecki M, Gliwińska A, Lewandowski K, Pączek L, Fijałkowski M, Gruchała M and Jankowski M. Detection of lipoprotein X (LPX) – a challenge in patients with severe hypercholesterolaemia. Journal of Medical Biochemistry. 2019;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kattah L, Gomez A, Gutierrez S, Puerto K, Moreno-Pallares ED, Jaramillo A and Mendivil CO. Hypercholesterolemia Due to Lipoprotein X: Case Report and Thematic Review. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551419878687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI and Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernelot Moens SJ, Neele AE, Kroon J, van der Valk FM, Van den Bossche J, Hoeksema MA, Hoogeveen RM, Schnitzler JG, Baccara-Dinet MT, Manvelian G, de Winther MPJ and Stroes ESG. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J. 2017;38:1584–1593. [DOI] [PubMed] [Google Scholar]
- 15.Khan IM, Pokharel Y, Dadu RT, Lewis DE, Hoogeveen RC, Wu H and Ballantyne CM. Postprandial Monocyte Activation in Individuals With Metabolic Syndrome. J Clin Endocrinol Metab. 2016;101:4195–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap SR, Ioachimescu AG, Gornik HL, Gopan T, Davidson MB, Makdissi A, Major J, Febbraio M and Silverstein RL. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity (Silver Spring). 2009;17:2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL and Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Dai Perrard X, Perrard JL, Yang D, Xiao X, Teng BB, Simon SI, Ballantyne CM and Wu H. Foamy Monocytes Form Early and Contribute to Nascent Atherosclerosis in Mice With Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saja MF, Baudino L, Jackson WD, Cook HT, Malik TH, Fossati-Jimack L, Ruseva M, Pickering MC, Woollard KJ and Botto M. Triglyceride-Rich Lipoproteins Modulate the Distribution and Extravasation of Ly6C/Gr1(low) Monocytes. Cell Rep. 2015;12:1802–15. [DOI] [PMC free article] [PubMed] [Google Scholar]