Abstract
BACKGROUND:
Twin studies show that age-related change in symptoms of attention-deficit/hyperactivity disorder (ADHD) is heritable. However, we do not know the heritability of the development of the neural substrates underlying the disorder. Here, we estimated the heritability of developmental change in white matter tracts and the brain’s intrinsic functional connectivity using longitudinal data. We further determined associations with change in ADHD symptoms.
METHODS:
The study reports on 288 children, which included 127 siblings, 19 cousins, and 142 singletons; 150 (52%) had a diagnosis of ADHD (determined by clinician interview with parent); 188 were male. All had 2 clinical assessments (overall baseline mean age: 9.4 ± 2.4 years; follow-up: 12.5 ± 2.6 years). Diffusion tensor imaging estimated microstructural properties of white matter tracts on 252 participants. Resting-state functional magnetic resonance imaging estimated intrinsic connectivity within and between major brain networks on 226 participants. Total additive genetic heritability (h2) of the annual rate of change in these neural phenotypes was calculated using SOLAR (Sequential Oligogenic Linkage Analysis Routines).
RESULTS:
Significant heritability was found for the rates of change of 6 white matter tract microstructural properties and for change in the connectivity between the ventral attention network and both the cognitive control and dorsal attention networks. Change in hyperactivity-impulsivity was associated with heritable change in white matter tracts metrics and change in the connectivity between the ventral attention and cognitive networks.
CONCLUSIONS:
The relatively small number of heritable, ADHD-associated developmental neural phenotypes can serve as phenotypes for future gene discovery and understanding.
Keywords: ADHD, Connectome, Developmental, DTI, Heritability, Longitudinal, Resting-state fMRI
Longitudinal twin studies have shown that age-related change in symptoms of attention-deficit/hyperactivity disorder (ADHD) is heritable (1). Specifically, over half of the variance in the course of symptoms of hyperactivity-impulsivity and inattention is attributable to genetic factors. However, we do not know the heritability of the developing neural substrates that underlie this change in symptoms. Against this background, we aimed to identify neurodevelopmental endophenotypes pertinent to ADHD, i.e., phenotypes of brain development that are both heritable and associated with developmental change in symptoms. Such neural features could serve as phenotypes for the discovery of genes underpinning brain growth and plasticity in ADHD (2).
Here, we focused on the development of the brain’s structural and functional connectivity. We did so as ADHD is increasingly viewed as a “developmental dysconnectome,” in which anomalous connectivity underpins developmental disruptions to brain systems that are tied to the symptom trajectories of ADHD (3-6). We first estimated the heritability of change in the microstructural properties of white matter tracts, as defined by prospectively acquired diffusion tensor imaging (DTI). We asked which facets of developmental change in white matter microstructure are both heritable and associated with ADHD symptom change. We also estimated the heritability of developmental change in the brain’s intrinsic functional connectivity, as defined by serial resting-state functional magnetic resonance imaging (rsfMRI). Intrinsic connectivity refers to the coordinated patterns of neural activity or intrinsic networks that emerge when an individual is not engaged in task-oriented behavior (7,8). We acquired data prospectively to delineate developmental change in the activity within and between intrinsic networks, particularly the default mode network, which is prominent during internally directed thought, and the networks supporting cognitive control and attention. An imbalance in activity within and between these networks is thought to underpin many of the core symptoms of ADHD (5,9,10). This study complements mostly cross-sectional studies that show both structural and functional connectivity in typically developing children to have strong genetic influences (11-17). We extended this work by studying a longitudinal cohort enriched for ADHD, allowing us to delineate how change in connectivity tracks with changing symptoms.
In summary, by using longitudinal data acquired on biologically related children, we aimed to estimate the heritability of developmental change in structural and functional connectivity. We further aimed to determine associations with change in ADHD symptoms, allowing us to identify developmental connectivity phenotypes, pertinent to ADHD, for future gene discovery.
METHODS AND MATERIALS
We included 288 youths, comprising 127 (61 sets of) siblings, 19 first-degree cousins (5 sets), and 142 singletons. Given our interest in ADHD, the cohort was enriched for ADHD, with 150 (52%) of all participants meeting DSM-5 diagnostic criteria during the study. This was attained partly by including children from our study of multigenerational extended families that have a high prevalence of ADHD. We earlier reported on the heritability of “baseline” connectivity phenotypes (18) and now report on developing connectivity on children from these families. We also include additional longitudinal data from 208 new subjects, not previously reported upon. In total, 496 of the 576 (86%) observations have not been previously reported. All children had at least 2 clinical assessments, conducted using the parental Diagnostic Interview for Children and Adolescents-IV (19), which establishes the number of symptoms of inattention and hyperactivity-impulsivity (with a range from 0 to a maximum of 9 symptoms in each category). Interviews were conducted by 2 experienced clinicians (W.S. and P.S.) with interrater reliabilities of κ > 0.9. Exclusion criteria were an IQ <80 [determined with Wechsler Intelligence Scales (20)], neurologic disorders affecting brain structure, current substance dependence, or psychotic disorders. Medication histories were obtained from the parents. The institutional review board of the National Human Genome Research Institute approved the research protocol, and written informed consent was obtained from adult participants and parents; children gave written assent. The study and data analysis were conducted from April 1, 2010, to June 1, 2019.
Neuroimaging
DTI data were collected were acquired (3T HDx MRI system; GE Healthcare, Milwaukee, WI) with a single-shot dual spin echo echo-planar imaging sequence with the following pa-rameters: echo time = 85 ms, repetition time = 18.5 seconds, field of view = 240 mm, 96 × 96 matrix, slice thickness = 2.5 mm, gap = 0 mm, and acceleration factor = 2. A custom set of diffusion directions and weightings were acquired for a total of 60 volumes: 6 volumes at b value = 0 s/mm2, 12 volumes with evenly distributed directions at b value = 300 s/mm2, and 42 volumes with evenly distributed directions at b value = 1100 s/mm2. Preprocessing, and tensor fitting are described in the Supplemental Methods.
Quality-control measures included the reacquisition of corrupted data in real time, visual inspection, and removal of corrupted data. Participants were excluded if they had more than 10% of corrupted volumes. Next, 2 machine learning algorithms (local outlier factor and isolation forest) were applied to detect outliers, using as input 6 motion parameters (translation and rotation in X, Y, Z) and total number of retained volumes after visual inspection. A second round of outlier detection applied the same algorithm to the mean axial and radial diffusivity values of the white matter tracts. Finally, the best 2 scans for each participant were selected based on output from the outlier detection algorithms. Of 954 DTI datasets initially considered, 504 (53%) were retained in the final analyses following these steps. Motion parameters were entered as possible covariates in all heritability analyses, retaining any that were associated with the outcome at p < .1, thus ensuring that change in motion with age did not confound heritability estimates. Software (DTI-TK, version 2.3.1 for Linux; http://dti-tk.sourceforge.net/) registered the diffusion tensors into a common template space. We then extracted the 20 tracts defined in the Johns Hopkins University tract atlas (21). For each tract, axial diffusivity and radial diffusivity were measured, which are proxies for the flow of water along the axis and the radius of the axon, respectively.
Intrinsic functional connectivity was defined using a gradient echo echo-planar series with whole-brain coverage, acquired on the same scanner as DTI. Parameters were the following: repetition time = 2500 ms, echo time = 27 ms, flip angle = 90° 44 axial contiguous interleaved slices per volume, slice thickness = 2.8 mm, field of view = 22 cm, 64 × 64 acquisition matrix. Participants were instructed to remain still for 5.25 minutes and gaze at a fixation point. Preprocessing used the 36-parameter despike functional pipeline implemented through fmriprep and xcpengine (see Supplemental Methods). We examined connectivity within and between 4 intrinsic connectivity networks of interest (default mode network, cognitive control network, and dorsal and ventral attention networks). Each network has been implicated in the pathogenesis of ADHD. The 4 networks comprised a total of 64 brain regions, as defined by the Schaefer atlas (22). Connectivity was calculated as the Pearson correlation between residualized time series in the 64 brain regions. The number of significant connections (at p < .05) within and between each network was then estimated, and the annual rate of change in this connectivity “count” was used as the phenotype in heritability estimates.
Quality control of these data involved 3 steps, detailed in the Supplement. First, any scans with poor alignment (as judged by visual inspection) between anatomical to Montreal Neurological Institute template, or functional data to anatomical alignment, were removed. Next, outlier detection was conducted (local outlier factor and isolation forest) first using 7 variables from fmriprep (reflecting brain coverage, motion, voxel variance, and data spikes). Finally, the connectivity count from each scan was used as input for another round of outlier detection. These steps resulted in the retention of 452 scans from an initial 726 (62%) considered rsfMRI datasets (details in Figure S1). Measures of the age-related change in motion were considered as covariates.
Statistical Analysis
We calculated the rate of change for all connectivity phenotypes as (metrictime2 – metrictime1)/(time2 – time1). The heritability of change in these connectivity phenotypes was estimated using SOLAR (Sequential Oligogenic Linkage Analysis Routine) (23). This routine uses a variance component method to estimate the proportion of phenotypic variance due to additive genetic factors (i.e., narrow sense heritability [h2]) (Supplemental Methods). In line with recent recommendations, inverse normalization was applied to phenotypes, and all covariates were treated in a uniform manner (24).
In addition to the heritability analysis, we tested for associations between rates of change of each heritable connectivity trait and rates of symptom change [symptomstime2 – symptomstime1]/[time2 – time1] using a mixed-effects model (implemented in the R package nlme v.3.1-140) (R Foundation for Statistical Computing, Vienna, Austria). The full model is
where di is a random effect accounting for within-family dependence and eij represents residual random error. Fixed effects include b0 (the intercept) and b1, which models the relationship between change in the brain metric and change in symptoms. We used a t test to test the significance of b1.
We confined these association analyses to children who had clinically meaningful number of symptoms (4 or more) at one or both assessments. We considered rates of change of hyperactivity-impulsivity and inattention separately, given evidence of their distinct developmental trajectories and partly distinct genetic determinants. We applied a false discovery rate (FDR) procedure to correct for multiple comparisons across all 50 phenotypes tested (the 40 white matter tract microstructural and 10 intrinsic connectivity phenotypes). We report heritability estimates that are significant at FDRs of .1 and .05, to highlight results that hold at different levels of adjustment for false discoveries (10% and 5%, respectively).
RESULTS
The age distribution of the entire cohort is shown in Figure 1. For the DTI analyses, the mean age of the first observation was 9.3 ± 2.2 years (range, 4.1–16.4 years). For the functional connectivity analyses, the mean age of first observation was 10.4 ± 2.5 years (range, 4.5–16.7 years). Age distribution by diagnosis are given in Table S1. Overall, 150 of the 288 (52%) participants had a diagnosis of ADHD at some point in the study, and 188 (65%) were male. Details of medication and comorbidity are given in Table S1.
Figure 1.
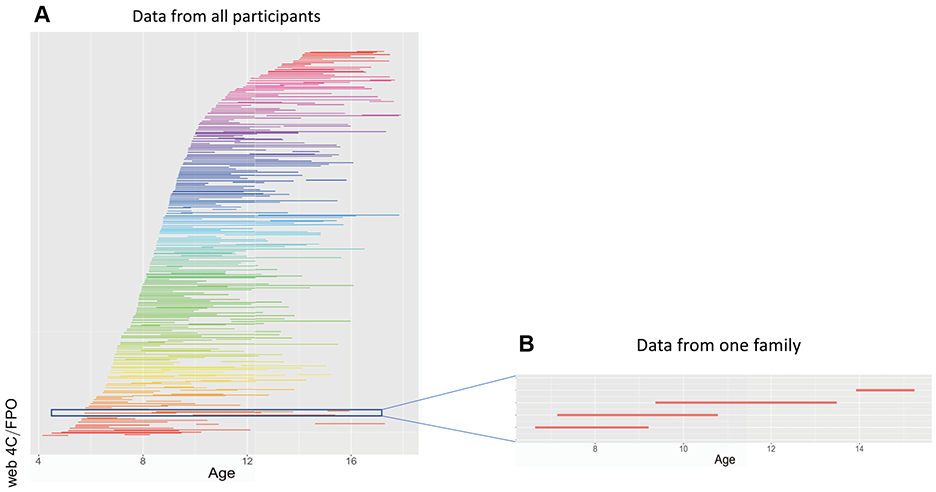
Participants. (A) Each child is shown as a line, connecting the two ages of their assessment. Each family is shown in the same color. (B) The data from one family with 4 siblings is shown.
Developing Structural Connectivity
We found that both radial and axial diffusivity showed significant decreases with age (Table S2). These age-related changes were heritable in 6 tracts at an FDR of < .05 and a further 2 tracts at an FDR < .1. Specifically, heritable change that was significant at an FDR of .05 was found for 6 tracts and ranged from h2 = 0.78 ± 0.2 (p = .0002) for radial diffusivity of the right uncinate fasciculus to h2 = 0.57 ± 0.21 (p = .004) for axial diffusivity of the forceps minor (Figure 2 and Table S2 give the full results). Heritable change at an FDR of .1 was found for a further 2 tract properties.
Figure 2.
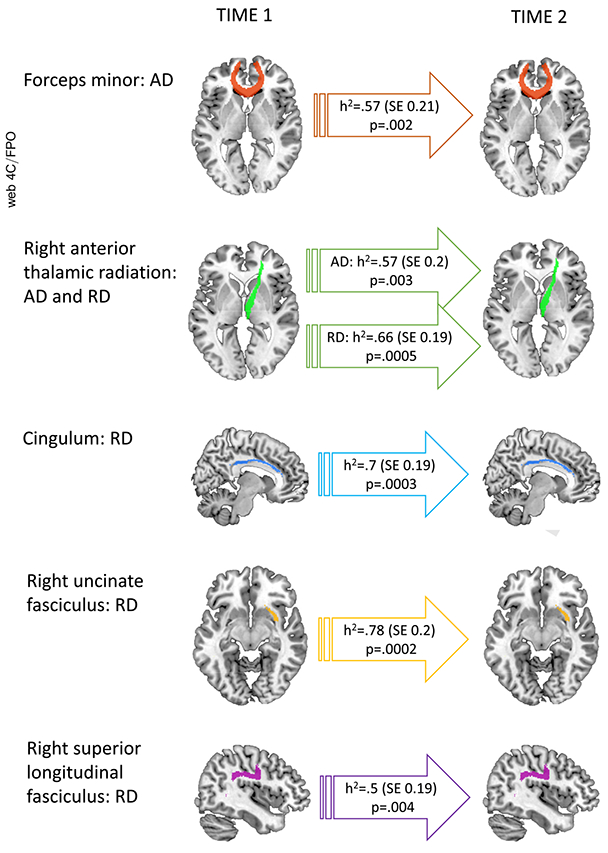
Heritability of white matter tracts. Heritability of age-related change in axial diffusivity (AD) and radial diffusivity (RD) of white matter tracts is shown (all significant at false discovery rate q = .05).
Developing Functional Connectivity
Next, we examined the heritability of age-related change in connectivity within and between functional networks (given in Table S3). Change in connectivity between the ventral attention and cognitive control networks emerged as heritable (h2 = 0.52 ± 0.23; p = .01) (Figure 3 and Table S3). Similarly, change in connectivity between the ventral and dorsal attention networks also emerged as significant (h2 = 0.55 ± 0.24; p = .02). Both heritability estimates were significant at an FDR q < .1. All other within- and between-network connectivity measures were not significantly heritable.
Figure 3.
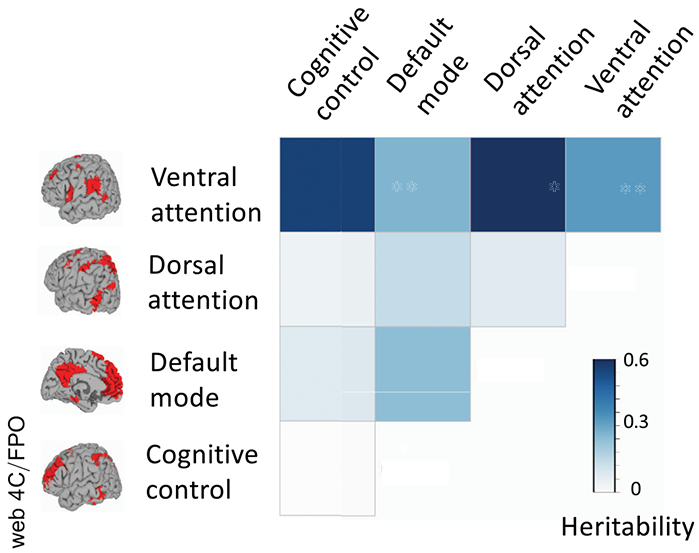
Heritability of functional connectivity. Heritability of rate of change of connectivity within and between all networks is shown.
Change in ADHD Symptoms and Association With Heritable Connectivity
There was a significant improvement with age in symptoms of hyperactivity-impulsivity both for the cohort used in DTI analyses (mean rate of change was a decrease of 0.25 symptoms/year, SE = 0.07 [t = −3.4 p = .0007]) and for the cohort used in rsfMRI analyses (decrease of 0.3 symptoms/year, SE = 0.07 [t = −4.38, p < .0001]). Symptoms of inattention showed less pronounced age-related change. For the cohort used in DTI, there was a mean decrease of 0.07 symptoms per year (SE = 0.08 [t = −0.89, p =.37]), and for the rsfMRI cohort, there was a 0.18-symptom/year decrease (SE = 0.08 [t = −2.24, p =.03]). Rates of symptom change did not vary by average age of assessment (all p > .05). DSM-5 categorical outcomes are detailed in Table S1.
We tested for associations between the rate of change of symptoms and the heritable changes in structural and functional connectivity reported above. Associations with change in hyperactivity-impulsivity emerged for all 8 heritable tracts (Table S4). Worsening symptoms of hyperactivity-impulsivity were associated with greater age-related decreases in both white matter tract microstructural properties. The strongest association, shown in Figure 4A, was between change in hyperactivity-impulsivity and change in axial diffusivity of the forceps minor (β = −4.62 × 10−3, SE = 1.14 × 10−3 [t = 4.1, p = .0007]). For intrinsic connectivity, there was an association between worsening symptoms of hyperactivity-impulsivity and decreased connectivity between the ventral attention and cognitive control networks (Figure 4B) (β = −1.83, SE = 0.68 [t = −2.69, p = .017]). There were no significant associations between baseline symptoms of hyperactivity-impulsivity and change in either the white matter or functional connectivity metrics (all nominal p > .05) (full results on baseline symptoms and change in connectivity in the Supplement).
Figure 4.
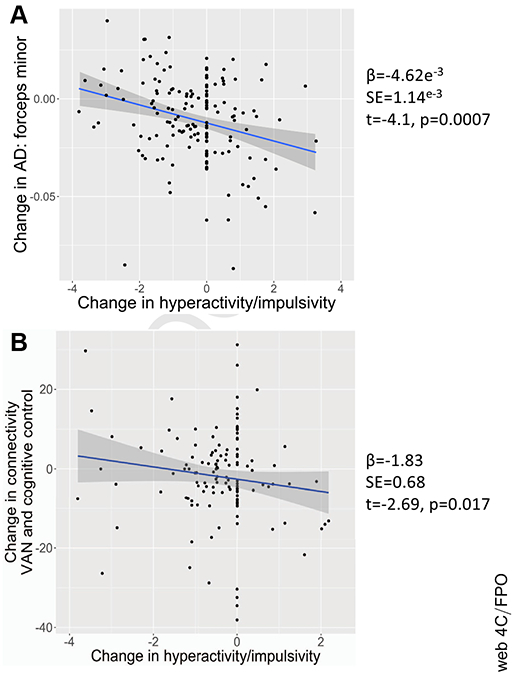
Associations between change in hyperactive-impulsive symptoms with age. (A) Association with change in axial diffusivity (AD) of the forceps minor; (B) association with change in connectivity between the cognitive control network and ventral attention network (VAN).
In summary, change in hyperactivity-impulsivity emerged as significantly associated with heritable changes in the microstructural properties of major white matter tracts and with heritable patterns of functional connectivity. Worsening symptoms of hyperactivity-impulsivity were associated with age-related decreases in white matter microstructural measures and decreased internetwork connectivity. Note that in all analyses, measures of the age-related change in motion were considered as covariates.
Robustness Analyses
We considered the impact of medication on heritability estimates by entering as a covariate the proportion of time each subject was on psychostimulant medication (Table 1). This did not change the pattern of findings. We also entered as a covariate the use of nonstimulant psychotropics (mainly selective serotonin reuptake inhibitors) as a covariate: this had little impact on findings. We removed those who developed comorbid psychiatric disorders during the study (n = 41), which also did not change heritability estimates meaningfully. We entered the average age of assessment as a covariate, and the heritability estimates did not change substantially. Finally, heritability estimates did not change on entering socioeconomic status and IQ as covariates (Table S5).
Table 1.
Impact of Medication on Heritability Estimates
| Modality | Phenotype | Original |
Psychostimulant Medication (as Proportion) |
Other Psychotropics |
Excluding Comorbidities |
||||
|---|---|---|---|---|---|---|---|---|---|
| h2 | p | h2 | p | h2 | p | h2 | p | ||
| White Matter Tract Microstructure | R uncinate fasciculus: RD | 0.78 | .0002 | 0.76 | .0003 | 0.77 | .0003 | 0.73 | .001 |
| L cingulum (cingulate gyrus): RD | 0.7 | .0003 | 0.72 | .0003 | 0.71 | .0003 | 0.71 | .0006 | |
| R anterior thalamic radiation: RD | 0.66 | .0005 | 0.62 | .001 | 0.62 | .001 | 0.64 | .001 | |
| Forceps minor: AD | 0.57 | .004 | 0.59 | .004 | 0.6 | .003 | 0.66 | .003 | |
| R anterior thalamic radiation: AD | 0.57 | .003 | 0.57 | .003 | 0.57 | .003 | 0.57 | .003 | |
| R superior longitudinal fasciculus: RD | 0.5 | .004 | 0.49 | .006 | 0.51 | .004 | 0.48 | .010 | |
| L superior longitudinal fasciculus (temporal part): RD | 0.44 | .02 | 0.50 | .008 | 0.52 | .006 | 0.47 | .021 | |
| L corticospinal tract: RD | 0.41 | .02 | 0.41 | .018 | 0.42 | .015 | 0.46 | .014 | |
| Intrinsic Functional Connectivity | Dorsal attention to ventral attention network | 0.55 | .02 | 0.54 | .014 | 0.54 | .016 | 0.54 | .019 |
| Ventral attention to cognitive control network | 0.52 | .01 | 0.54 | .009 | 0.52 | .014 | 0.39 | .046 | |
Analyses repeated entering the proportion of time on psychostimulant medication or use of other psychotropics as covariates, and after excluding the 41 participants who developed comorbidities during the follow-up.
AD, axial diffusivity; L, left; R, right; RD, radial diffusivity.
DISCUSSION
We identified heritable facets of childhood change in struc-tural and functional connectivity; most were associated with age-related improvement in hyperactivity-impulsivity. Specif-ically, significant heritability was found for developmental change in the microstructure of association and commissural tracts and for change in the connectivity between the ventral attention and both the cognitive control and dorsal attention networks. We also found that change in hyperactivity-impulsivity was associated with a subset of these heritable properties: worsening hyperactivity-impulsivity was associated with decreases in white matter microstructural measures and decreased internetwork functional connectivity. The study thus provides a relatively small number of developing connectivity features that are both heritable and associated with symptom change.
As reported elsewhere, we found age-related decreases in both axial and radial diffusivity for the major white matter tracts during late childhood into adolescence (25). This developmental change was heritable for the tracts that are most consistently implicated in pathogenesis of ADHD. For example, the uncinate fasciculus is pivotal in impulse control and reward processing (26,27), anomalies of the cingulum bundle are associated with behavioral dysregulation and impaired motor inhibition (28-30), and the anterior corpus callosum is integral to higher-order cognitive and motor control (31,32). Meta-analyses of DTI studies converge to implicate atypical microstructure in these same tracts in ADHD (33-35), and cross-sectional twin and family studies find these tracts to be under genetic control (18,36,37). Four of the 5 most heritable tracts were in the right hemisphere, consistent with lateralized models of attention and reports of associations between the microstructural properties of the right superior longitudinal fasciculus, working memory, and sustained attention (38-40).
Longitudinal studies of heritability are sparse: one study of adolescent twins found that white matter microstructure and network characteristics of structural connectivity were heritable throughout adolescence, with largely stable genetic influences (17,41). Similarly, an infant study found stable heritability for white matter tracts measured at 1 and 2 years of age (15). We built on these studies reporting heritable change in white matter microstructure in a clinical cohort. We found more heritable change than was noted in the earlier studies, which may reflect our use of an extended family rather than a twin design, our examination of change (rather than shifts in heritability measured at different ages), and differing lengths of follow-up across studies. Our study benefited from the use of one scanner throughout, a high number of noncollinear diffusion gradients, and the 3T field strength, all of which may decrease measurement error, thus aiding the detection of underlying heritability.
We found an association between change in white matter tracts and change in hyperactivity-impulsivity but not change in inattention. This might reflect the more robust improvement during adolescence of symptoms of hyperactivity-impulsivity compared with inattention, which has long been noted (42,43). Recent studies using data-driven methods such as growth mixture modeling to map adolescent symptom trajectories also found that the subgroup with clear age-related improvement (ranging from between 33% to 66% of affected children) consistently shows more improvement in hyperactivity-impulsivity than in inattention (44-46). Additionally, change in hyperactive-impulsive symptoms during adolescence is more heritable than change in inattention (1); thus, it is perhaps unsurprising that changing hyperactivity-impulsivity is associated with heritable neural features.
There are mixed findings on the nature of developmental change in connectivity within and between functional networks (11,47-52). The disparate findings likely reflect differences in connectivity metrics employed and study design, particularly the use of cross-sectional versus longitudinal data. We found a significance increase in connectivity within the default mode network, as has been reported by some other longitudinal studies (11,49,50,52,53).
Our finding of heritable change in the intrinsic connectivity between the ventral attention network and both the dorsal attention and cognitive control networks can be compared with the only two other longitudinal studies of heritability, both on typically developing cohorts (11,14). A study of adolescent twins found that incorporating dynamic genetic factors provided a better fit compared with models with purely static genetic factors for connectivity measures within and between the default mode and attention networks (11). This study also reported dynamic additive genetic effects in the cognitive control network. A longitudinal study that included infant twins found complex, heterogeneous patterns of network heritability, with a predominant increase in genetic influences during the first year of life, followed by a decrease in the second year of life (14). As this infant study examined heritability at 3 time points (shortly after birth, 1 year of age, 2 years of age), as opposed to our study of the heritability of change metrics, direct comparisons are limited. Nonetheless, the initial studies converge to find heritability in some but not all facets of the developing functional connectome. Like prior longitudinal studies, we found more modest heritability in age-related change in intrinsic functional connectivity compared with change in white matter microstructure.
Our report of heritable development in internetwork connectivity is of particular interest. As noted earlier, ADHD is often viewed as the result of dysregulated connectivity between multiple brain networks, which leads to core features such as impulsivity, and impaired motor planning (5,9,10). By extension, it might be expected that developmental change in this internetwork connectivity would be associated with change in symptoms with age. Indeed, we found such an association: worsening of symptoms of hyperactivity-impulsivity is tied to a decrease in functional connectivity between the ventral attention and cognitive control networks.
Finally, we consider the roles that data quality, medication, and comorbidity may play. Neuroimaging quality control included real-time reacquisition of corrupted data, visual inspection, and machine learning–based outlier detection. These steps resulted in the exclusion of around half of the initially considered DTI and rsfMRI datasets. Additionally, motion parameters were considered as covariates in the analysis band; thus, changes in motion were unlikely to drive the findings. Turning to medication, around two-thirds of our subjects with ADHD were treated with psychostimulant medication. However, our findings were robust in analyses that entered the proportion of time on medication as a covariate, and also in analyses that considered other psychotropic medications. During the study, some participants developed comorbid disorders; excluding these participants did not meaningfully alter findings. As only two scans were included for each individual, we could only consider linear change at the individual level. The acquisition of more observations would allow the delineation of individual nonlinear trajectories, which may provide further insight into genetic influences on developing connectivity. While we did not find that the average age of assessment contributed significantly to heritability estimates, our study may have been underpowered to detect developmental shifts in the heritability of connectivity (36).
This study delineates heritable, ADHD-associated developmental phenotypes that are well suited to act as phenotypes for future gene discovery. It is feasible that the genes underlying these developmental phenotypes may point to neuroplastic processes that are particularly amenable to therapeutic interventions.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type |
Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. |
Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. |
Include any additional information or notes if necessary. |
| Antibody | NA | |||
| Bacterial or Viral Strain | NA | |||
| Biological Sample | NA | |||
| Cell Line | NA | |||
| Chemical Compound or Drug | NA | |||
| Commercial Assay Or Kit | NA | |||
| Deposited Data; Public Database | Whole genome SNP array data | Illumina HumanOmniExpressExome-8v1-2 array | bGaP_phs000962.v1.p1_NHGRI/CIDR_BrainGrowth_Children | Further genotypes are being posted |
| Genetic Reagent | NA | |||
| Organism/Strain | NA | |||
| Peptide, Recombinant Protein | NA | |||
| Recombinant DNA | NA | |||
| Sequence-Based Reagent | NA | |||
| Software; Algorithm | NA | |||
| Transfected Construct | NA | |||
| Other | NA |
ACKNOWLEDGMENTS AND DISCLOSURES
This work utilized the computational resources of the National Institutes of Health High-Performance Computation Biowulf cluster.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2020.06.007.
Contributor Information
Gustavo Sudre, Neurobehavioral Clinical Research Section, Social and Behavioral Research Branch, National Human Genome Research Institute, National Institutes of Health.
Marine Bouyssi-Kobar, Neurobehavioral Clinical Research Section, Social and Behavioral Research Branch, National Human Genome Research Institute, National Institutes of Health.
Luke Norman, Neurobehavioral Clinical Research Section, Social and Behavioral Research Branch, National Human Genome Research Institute, National Institutes of Health.
Wendy Sharp, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland.
Saadia Choudhury, Neurobehavioral Clinical Research Section, Social and Behavioral Research Branch, National Human Genome Research Institute, National Institutes of Health.
Philip Shaw, Neurobehavioral Clinical Research Section, Social and Behavioral Research Branch, National Human Genome Research Institute, National Institutes of Health.
REFERENCES
- 1.Pingault JB, Viding E, Galéra C, Greven C, Zheng Y, Plomin R, Rijsdik F (2015): Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry 72:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glahn DC, Thompson PM, Blangero J (2007): Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Hum Brain Mapp 28:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, et al. (2014): Unraveling the miswired connectome: A developmental perspective. Neuron 83:1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konrad K, Eickhoff SB (2010): Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 31:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre G, Szekely E, Sharp W, Kasparek S, Shaw P (2017): Multimodal mapping of the brain’s functional connectivity and the adult outcome of attention deficit hyperactivity disorder. Proc Natl Acad Sci U S A 114:11787–11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudre G, Shaw P, Wharton A, Weingart D, Sharp W, Sarlls J (2015): White matter microstructure and the variable adult outcome of childhood attention deficit hyperactivity disorder. Neuropsychopharmacology 40:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damoiseaux J, Rombouts S, Barkhof F, Scheltens P, Stam C, Smith SM, et al. (2006): Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sripada CS, Kessler D, Angstadt M (2014): Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc Natl Acad Sci U S A 111:14259–14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonuga-Barke EJS, Castellanos FX (2007): Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev 31:977–986. [DOI] [PubMed] [Google Scholar]
- 11.Teeuw J, Brouwer RM, Guimarães JP, Brandner P, Koenis MM, Swagerman SC, et al. (2019): Genetic and environmental influences on functional connectivity within and between canonical cortical resting-state networks throughout adolescent development in boys and girls. Neuroimage 202:116073. [DOI] [PubMed] [Google Scholar]
- 12.Teeuw J, Brouwer RM, Koenis MM, Swagerman SC, Boomsma DI, Hulshoff Pol HE (2018): Genetic influences on the development of cerebral cortical thickness during childhood and adolescence in a Dutch longitudinal twin sample: The brainscale study. Cereb Cortex 29:978–993. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore JH, Knickmeyer RC, Gao W (2018): Imaging structural and functional brain development in early childhood. Nat Rev Neurosci 19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao W, Elton A, Zhu H, Alcauter S, Smith JK, Gilmore JH, et al. (2014): Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J Neurosci 34:11288–11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Zhang J, Neale MC, Styner M, Zhu H, Gilmore JH (2019): Quantitative tract-based white matter heritability in 1-and 2-year-old twins. Hum Brain Mapp 40:1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richmond S, Johnson KA, Seal ML, Allen NB, Whittle S (2016): Development of brain networks and relevance of environmental and genetic factors: A systematic review. Neurosci Biobehav Rev 71:215–239. [DOI] [PubMed] [Google Scholar]
- 17.Koenis MM, Brouwer RM, van den Heuvel MP, Mandl RC, van Soelen IL, Kahn RS, et al. (2015): Development of the brain’s structural network efficiency in early adolescence: A longitudinal DTI twin study. Hum Brain Mapp 36:4938–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudre G, Choudhuri S, Szekely E, Bonner T, Goduni E, Sharp W, et al. (2017): Estimating the heritability of structural and functional brain connectivity in families affected by attention-deficit/hyperactivity disorder. JAMA Psychiatry 74:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich W (2000): Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry 39:59–66. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D (2011): Wechsler Abbreivated Scale of Intelligence, 2nd ed. San Antonio, TX: Psychologial Corporation. [Google Scholar]
- 21.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. (2008): Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. (2017): Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex 28:3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almasy L, Blangero J (1998): Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochunov P, Patel B, Ganjgahi H, Donohue B, Ryan M, Hong E, et al. (2019): Homogenizing estimates of heritability among SOLAR-Eclipse, OpenMx, APACE, and FPHI software packages in neuroimaging data. Front Neuroinform 13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebel C, Deoni S (2018): The development of brain white matter microstructure. Neuroimage 182:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson IR, Heide RJVD, Alm KH, Vyas G (2015): Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci 14:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw P, Stringaris A, Nigg J, Leibenluft E (2014): Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 171:276–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikuta T, Shafritz KM, Bregman J, Peters BD, Gruner P, Malhotra AK, et al. (2014): Abnormal cingulum bundle development in autism: A probabilistic tractography study. Psychiatry Res 221:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. (2008): Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex 18:1210–1220. [DOI] [PubMed] [Google Scholar]
- 30.Cooper M, Thapar A, Jones DK (2015): ADHD severity is associated with white matter microstructure in the subgenual cingulum. Neuroimage Clin 7:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. (2004): Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology 30:610–617. [DOI] [PubMed] [Google Scholar]
- 32.Gilliam M, Stockman M, Malek M, Sharp W, Greenstein D, Lalonde F, et al. (2011): Developmental trajectories of the corpus callosum in attention-deficit/hyperactivity disorder. Biol Psychiatry 168:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki Y, Cortese S, Castellanos FX (2018): Research Review: Diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: Meta-analyses and reflections on head motion. J Child Psychol Psychiatry 59:193–202. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, et al. (2016): A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 68:838–847. [DOI] [PubMed] [Google Scholar]
- 35.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J (2012): Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- 36.Kochunov P, Jahanshad N, Marcus D, Winkler A, Sprooten E, Nichols TE, et al. (2015): Heritability of fractional anisotropy in human white matter: A comparison of Human Connectome Project and ENIGMA-DTI data. Neuroimage 111:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang M-C, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, et al. (2011): Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage 54:2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Schotten MT, Dell’Acqua F, Forkel S, Simmons A, Vergani F, Murphy DG, et al. (2011): A lateralized brain network for visuo-spatial attention. Nat Neurosci 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- 39.Klarborg B, Skak Madsen K, Vestergaard M, Skimminge A, Jernigan TL, Baaré WF (2013): Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum Brain Mapp 34:3216–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vestergaard M, Madsen KS, Baaré WF, Skimminge A, Ejersbo LR, Ramsøy TZ, et al. (2011): White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci 23:2135–2146. [DOI] [PubMed] [Google Scholar]
- 41.Brouwer RM, Mandl RC, Schnack HG, van Soelen IL, van Baal GC, Peper JS, et al. (2012): White matter development in early puberty: A longitudinal volumetric and diffusion tensor imaging twin study. PLoS One 7:e32316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ (1995): Developmental change in attention-deficit hyperactivity disorder in boys: A four-year longitudinal study. J Abnorm Child Psychol 23:729–749. [DOI] [PubMed] [Google Scholar]
- 43.Larsson H, Dilshad R, Lichtenstein P, Barker ED (2011): Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: Genetic effects, family risk and associated psychopathology. J Child Psychol Psychiatry 52:954–963. [DOI] [PubMed] [Google Scholar]
- 44.Riglin L, Collishaw S, Thapar AK, Dalsgaard S, Langley K, Smith GD, et al. (2016): Association of genetic risk variants with attention-deficit/ hyperactivity disorder trajectories in the general population. JAMA Psychiatry 73:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tandon M, Tillman R, Agrawal A, Luby J (2016): Trajectories of ADHD severity over 10 years from childhood into adulthood. Atten Defic Hyperact Disord 8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malone PS, Van Eck K, Flory K, Lamis DA (2010): A mixture-model approach to linking ADHD to adolescent onset of illicit drug use. Dev Psychol 46:1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long X, Benischek A, Dewey D, Lebel C (2017): Age-related functional brain changes in young children. Neuroimage 155: 322–330. [DOI] [PubMed] [Google Scholar]
- 48.Xiao Y, Friederici AD, Margulies DS, Brauer J (2016): Longitudinal changes in resting-state fMRI from age 5 to age 6 years covary with language development. Neuroimage 128:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wendelken C, Ferrer E, Ghetti S, Bailey SK, Cutting L, Bunge SA (2017): Frontoparietal structural connectivity in childhood predicts development of functional connectivity and reasoning ability: A large-scale longitudinal investigation. J Neurosci 37:8549–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sylvester CM, Whalen DJ, Belden AC, Sanchez SL, Luby JL, Barch DM (2018): Shyness and trajectories of functional network connectivity over early adolescence. Child Dev 89:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strikwerda-Brown C, Davey CG, Whittle S, Allen NB, Byrne ML, Schwartz OS, et al. (2014): Mapping the relationship between subgenual cingulate cortex functional connectivity and depressive symptoms across adolescence. Soc Cogn Affect Neurosci 10:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard JA, Orr JM, Mittal VA (2016): Differential motor and prefrontal cerebello-cortical network development: Evidence from multimodal neuroimaging. Neuroimage 124:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M (2014): Development of the default mode and central executive networks across early adolescence: A longitudinal study. Dev Cogn Neurosci 10:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


