Abstract
Transient potential receptor vanilloid 4 (TRPV4) is an ion channel responsible for sensing osmotic and mechanical signals, which in turn regulates calcium signaling across cell membranes. TRPV4 is widely expressed throughout the body, and plays an important role in normal physiological function, as well as different pathologies, however, its role in the eye is not well known. In the eye, TRPV4 is expressed in various tissues, such as the retina, corneal epithelium, ciliary body, and the lens. In this review, we provide an overview on TRPV4 structure, activation, mutations, and summarize the current knowledge of TRPV4 function and signaling mechanisms in various locations throughout the eye, as well as its role in ocular diseases, such as glaucoma and diabetic retinopathy. Based on the available data, we highlight the therapeutic potential of TRPV4 as well as the shortcomings of current research. Finally, we provide future perspectives on the implications of targeting TRPV4 to treat various ocular pathologies.
Keywords: angiogenesis, calcium channel, cornea, diabetic retinopathy, glaucoma, lens, osmolarity, retina, TRPV4
1. Introduction
Transient receptor potential (TRP) channels are a superfamily of non-selective cation channels, first identified as a mutant photoreceptor that responds transiently to the light in Drosophila eyes(Hardie and Juusola, 2015). To date, more than 30 subtypes of TRP channels have been identified, which are divided into 6 major families such as canonical (TRPC), vanilloid (TRPV), melastatin (TRPM), ankyrin (TRPA), mucolipin (TRPML), and polycystic (TRPPP)(Himmel and Cox, 2020). TRP channels are expressed in all eye tissues including the cornea, lens, ciliary body, trabecular mesh work, and retina(Reinach et al., 2015a; Reinach et al., 2015b) (Table.1). While TRPC4 and TRPV4 are expressed ubiquitously in all tissues, TRPV1 was shown to be present in corneal epithelium, lens, and retina (Delamere et al., 2016; Gilliam and Wensel, 2011; Mergler et al., 2012a; Mergler et al., 2012b; Mergler et al., 2011; Nakazawa et al., 2019; Okada et al., 2016; Ryskamp et al., 2016; Ryskamp et al., 2011; Takai et al., 2004; Yang et al., 2005; Zhao et al., 2015). TRPM family members (TRPM1, TRPM3 and TRPML1) were shown to be expressed in the retina and lens(Bennett et al., 2014; Gilliam and Wensel, 2011; Shiels, 2020). Although different TRP channels participate in distinct ocular functions, the role of TRPV4 in the eye is largely unknown and is the focus of this review. TRPV4 is a mechanosensitive ion channel belonging to the vanilloid subfamily, which consists of 7 subtypes ranging from TRPV1-TRPV7. All members of the TRPV family are known to be activated by vanilloid compounds.
Table 1.
TRP subfamily distribution throughout eye tissue.
| Tissue | TRP channel expression | Reference |
|---|---|---|
| Corneal epithelium |
|
|
| Lens |
|
|
| Ciliary body |
|
|
| Trabecular meshwork |
|
|
| Retina |
|
1.1. Structure of TRPV4
In humans, the TRPV4 gene is located on position 24.11 of the long (q) arm of chromosome 12 (Genetics Home Reference – NIH). Its protein is 871 amino acids in length, and structurally, the channel contains six transmembrane domains (S1-S6) with a pore forming between S5 and S6 (Plant and Strotmann, 2007). Recently, cryo-EM revealed that S3 and S4 interact with S5 and S6 of the pore domain, with S3 firmly against S6 (Deng et al., 2018). The TRPV4 protein contains both N and C terminal domains. The N terminal domain, which contains 6 ankyrin (ANK) repeats, is presumptively involved in protein-protein interactions(Chun et al., 2012; Everaerts et al., 2010). Moreover, five variants of TRPV4 have been identified as TRPV4-A-E, where TRPV4-A and D, are localized to the plasma membrane, and TRPV4-B, C, and E, are contained inside the endoplasmic reticulum due to the lack of certain ANK components(Arniges et al., 2006). Additionally, cryo-EM identified only one ion-binding site that and did not discriminate against charges, which is believed to be a reason for TRPV4’s non-selective nature (Deng et al., 2018).
1.2. Activation
TRPV4 is a polymodal channel known to be activated by cell swelling, heat, mechanical stretch, shear stress, arachidonic acid metabolites; EETs (Epoxyeicosatrienoic acids), endocannabinoids and phorbol esters (White et al., 2016). However, TRPV4 is characterized as a mechanosensitive ion channel activated by osmotic and mechanical stimuli(Liedtke, 2005). In TRPV4−/− mice, there is an impaired response to pressure sensing and osmolarity(Liedtke and Friedman, 2003; Suzuki et al., 2003). Under highly viscous conditions, TRPV4 activation is dependent on PLA2, an enzyme involved in the release of arachidonic acids, which then activate the channel(Andrade et al., 2005). In ciliated cells, arachidonic acid activates TRPV4 via IP3 signaling(Fernandes et al., 2008), and in chondrocytes, the inhibition of TRPV4 blocks the Ca2+ signaling response to changes in osmolarity(Phan et al., 2009), providing further evidence of the channel’s osmotic-dependent gating.
In the vasculature, TRPV4 is shown to be an important regulator of vascular tone via Ca2+ signaling(Kohler et al., 2006; Mendoza et al., 2010), thus indicating its importance in vascular pathologies. In response to shear stress, the force generated by blood flow, vessels either dilate or constrict. In endothelial cells, TRPV4 is the mechanosensor responsible for mediating this reaction. Kohler et al provided the first evidence for the requirement of endothelial TRPV4 in shear induced NO/EDHF-mediated vasodilation(Kohler et al., 2006). Later, they demonstrated that shear stress-induced vasodilation is completely attenuated in In TRPV4−/− mice (Hartmannsgruber et al., 2007), where the relaxation effects of both nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) are reduced in small mesenteric arteries (Mendoza et al., 2010). Additionally, shear stress-induced vasodilation is attenuated by ruthenium red, a TRPV4 antagonist (Kohler and Hoyer, 2007). Mechanistically, in the presence of shear stress, intracellular clusters of TRPV4 dislodge into lone channels, and in endothelial cells, break previous interactions with β catenin(Baratchi et al., 2016; Baratchi et al., 2019; Baratchi et al., 2017), which is indicative of TRPV4’s role in maintaining endothelial permeability. Another type of hemodynamic force within the endothelium is cyclic strain, and along with shear stress, is involved in vascular maintenance and remodeling. We, for the first time, have shown that TRPV4 is required for cyclic strain-induced reorientation of endothelial cells. Mechanistically, we demonstrated that TRPV4 channels mediate cyclic strain-induced EC reorientation through intergrin to intergrin signaling via activation of PI3 Kinase and modulation of the cytoskeleton (Thodeti et al., 2009). Further, we demonstrated ultra-rapid activation of TRPV4 channels in response to mechanical forces applied to β1 integrins (Matthews et al., 2010). Furthermore, we found that an adapter protein CD98 which binds to the β1 integrin cytoplasmic tail is required for the mechanical force transfer between β1 integrins and TRPV4 (Matthews et al., 2010). In addition to direct mechanical forces, we demonstrated an ECM stiffness-dependent role for TRPV4-dependent Ca2+ influx in the differentiation of cardiac fibroblasts into myofibroblasts (Adapala et al., 2013). In fact, we showed that TRPV4 integrates mechanical (matrix stiffness) and soluble (TGF-β1) signaling in fibroblast differentiation to myofibroblasts(Adapala et al., 2020; Adapala et al., 2013). We further demonstrated that TRPV4 is required for sensing matrix stiffness by endothelial cells in vitro and in vivo, and deletion or reduction of TRPV4 in endothelial cells results in aberrant mechanosensitivity and abnormal angiogenesis via activation of Rho/Rho kinase pathway. Interestingly, activation of TRPV4 or inhibition of Rho/Rho kinase restores mechanosensitivity of EC to matrix stiffness and induced vascular normalization(Adapala et al., 2016; Thoppil et al., 2016).
Heat and temperature changes have also been shown to activate TRPV4 channels. Interestingly, TRPV4 is activated at a range of temperatures depending on cell type(Gao et al., 2003; Guler et al., 2002). TRPV4’s response to heat is due in part to its N-terminal ankyrin binding domains; when there is a mutation in the ankyrin binding domains, heat is unable to activate TRPV4, however, mutations in this region do not prevent activation due to cell swelling (Watanabe et al., 2002b), indicating that specific stimuli activate TRPV4 differently. Further, PACSIN 3, a substrate that plays a role in vesicle formation, has been shown to inhibit the activation of TRPV4 by heat, whereas activation by other stimuli, such as phorbol esters are unaltered (D’Hoedt et al., 2008).
The focus of pharmacological activation of TRPV4 has been mostly limited to two small molecules: 4α-phorbol-12,13-didecanoate (4α-PDD), a phorbol ester, and GSK1016790A (GSK1), a synthetic agonist(Vincent and Duncton, 2011). Mechanistically, 4α-PDD activates TRPV4 channels by binding to transmembrane domain 3 and 4 (TM3-TM4), which is due to the characteristics of the A, B ring junction on 4α-PDD (Klausen et al., 2009). GSK1 differs in structure from 4α-PDD, and its mechanism of TRPV4 activation is less understood. In endothelial cells, stimulation with GSK1 leads to the increased expression of TRPV4 channels or recruitment of inactive TRPV4 channels (Adapala et al., 2016; Baratchi et al., 2019; Sullivan et al., 2012). Recently, it was shown that this recruitment is accomplished via exocytosis of TRPV4 channels to the plasma membrane, which then cause an influx of Ca2+ into the cytoplasm. Further, endocytosis of TRPV4 into the cytoplasm assembles into recycling endosomes(Baratchi et al., 2019), which causes a desensitization effect (Dai et al., 2010).
The first known antagonist of TRPV4 is ruthenium red(Watanabe et al., 2002a). However, ruthenium red is nonspecific and can inhibit activities of other calcium channels including TRP channels independent of TRPV4. Ruthenium red was shown to block calcium uptake and release from mitochondria, as well as calcium release from ryanodine-sensitive intracellular stores(Bernardi et al., 1984; Xu et al., 1999). Importantly, ruthenium red was also shown to inhibit cell membrane-located capsaicin-activated cation and voltage-sensitive Ca2+ channels (Amann and Maggi, 1991; Szallasi and Blumberg, 1999). To circumvent the non-specificity of ruthenium red, new lines of TRPV4 specific antagonists were developed. The first developed specific inhibitor of TRPV4 was AB-159908, also known as RN-1734 (Vincent et al., 2009), followed by RN-9893, Capsazepine, Citral, and HC-067047 (Vincent and Duncton, 2011). GSK2193874 (GSK2) is the most widely used selective synthetic small molecule inhibitor of TRPV4, which inhibits Ca2+ influx into cells (Cheung et al., 2017; Thorneloe et al., 2017). Since its discovery, GSK2 has been used in a wide array of studies used to investigate the effects of TRPV4 antagonism. In a mouse model of sepsis, a condition caused by an overreaction of the immune system in response to foreign bodies, blockade of TRPV4 channels caused less fatality by reducing cytokine production and restoring the function of endothelial cells (Dalsgaard et al., 2016). Further, GSK2 has been shown to resolve pulmonary edema in heart failure models (Thorneloe et al., 2012). Because of its promise in animal models, clinical trials for GSK2 are underway for patients with pulmonary edema, and safety profiles have shown promise (Goyal et al., 2019).
1.3. Mutations
It is well established that mutations in the TRPV4 gene cause various neuropathologies (Zimon et al., 2010). An autosomal dominant peripheral nervous system disorder, Charcot-Marie-Tooth disease type 2C (CMT 2C), is caused by mutations in the TRPV4 gene. Specifically, the R269 residue of the ANK domain has been linked to CMT 2C (Landoure et al., 2010). Vocal cord paresis, a characteristic of CMT 2C, has also been tied to specific mutations within the TRPV4 gene (Chen et al., 2010). Additionally, gain-of-function mutations in the TRPV4 gene are shown to cause brachyolmia, a type of skeletal dysplasia. In both disorders, increased TRPV4 activation causes Ca2+ neurotoxicity (Rock et al., 2008). Recently a missense variant p.S94L, found on the N-terminal of the TRPV4 protein was linked to the recessive form of congenital distal spinal muscular atrophy and arthrogryposis (Velilla et al., 2019). Although most diseases related to mutations of TRPV4 are neurologic in nature, another condition, hyponatremia is associated with a nonsynonymous polymorphism which causes a Pro to Ser substitution at reside 19 of the TRPV4 gene (Tian et al., 2009). Furthermore, polymorphisms in the TRPV4 gene have been associated with chronic obstructive pulmonary disease (COPD) (Zhu et al., 2009). Based on TRPV4’s involvement in various pathologies, further research is needed to target TRPV4 therapeutically. Because of its role in various physiologic functions, TRPV4 has become an attractive target for many disease states. Here, we review the literature on TRPV4 in the context of ocular function and disease (Table.2), highlighting the importance of this ion channel in the discovery of ocular therapeutics.
Table. 2.
TRPV4 expression and function in various tissues of the eye.
| Tissue | Cell type | Function | Pathology | Reference |
|---|---|---|---|---|
| Corneal epithelium | Outermost layer of epithelial sheets |
|
Corneal injury |
|
| Lens | Epithelium |
|
Lens swelling | |
| Fiber cells of outer cortex |
|
|||
| Ciliary body | Nonpigmented epithelial cells |
|
- |
|
| Trabecular meshwork | - |
|
Glaucoma | |
| Retina | Müller cells |
|
Retinal damage from trauma |
|
| Retinal endothelial cells |
|
Retinopathy Glaucoma |
||
| Bipolar cells & Retinal ganglion cells |
|
Glaucoma | ||
| Photoreceptor cells (PRC) |
|
Retinal detachment | Matsumoto et al., 2018 |
2. TRPV4 expression and function in ocular tissue
2.1. Corneal epithelium
The corneal epithelium is a layer of epithelial cells covering the cornea and is responsible for its protection from environmental hazards. TRPV4 expression is confined to the atypical domain of the outermost layer of epithelial sheets, and its function is crucial to the formation of tight junctions within the corneal epithelium(Martinez-Rendon et al., 2017) (Fig.1). Osmolarity changes in the tear film can compromise the integrity of the tissue, and eventually lead to vision problems. Corneal epithelial cells have been shown to regulate volume after insult by activation of various ion channels (Capo-Aponte et al., 2005). Because TRPV4 is an important ion channel involved in osmolarity, its function in corneal epithelial cells in the context of volume control has been studied. When TRPV4 is knocked down using siRNA, regulatory volume decrease (RVD) activity is inhibited (Pan et al., 2008), indicating that TRPV4 plays an important role in regulating osmolarity within the corneal epithelium. Further, damage to the cornea causes a release of inflammatory cytokines, which lead to fibrosis. Okada et al (2016) used a mouse model of burned corneas to study the effects of TRPV4 antagonism and preservation of the cornea after injury (Okada et al., 2016). It was found that inhibition of TRPV4 leads to less fibrosis and subsequent preservation of corneas (Okada et al., 2019). In contrast, insertion of the TRPV4 gene into impaired trigeminal nerves restores healing of the corneal epithelium via the upregulation of nerve growth factor (Okada et al., 2019), indicating the complex function TRPV4 plays in the maintenance and repair of the corneal epithelium (Fig.1).
Figure 1. TRPV4 channels in the maintenance and repair of the corneal epithelium.
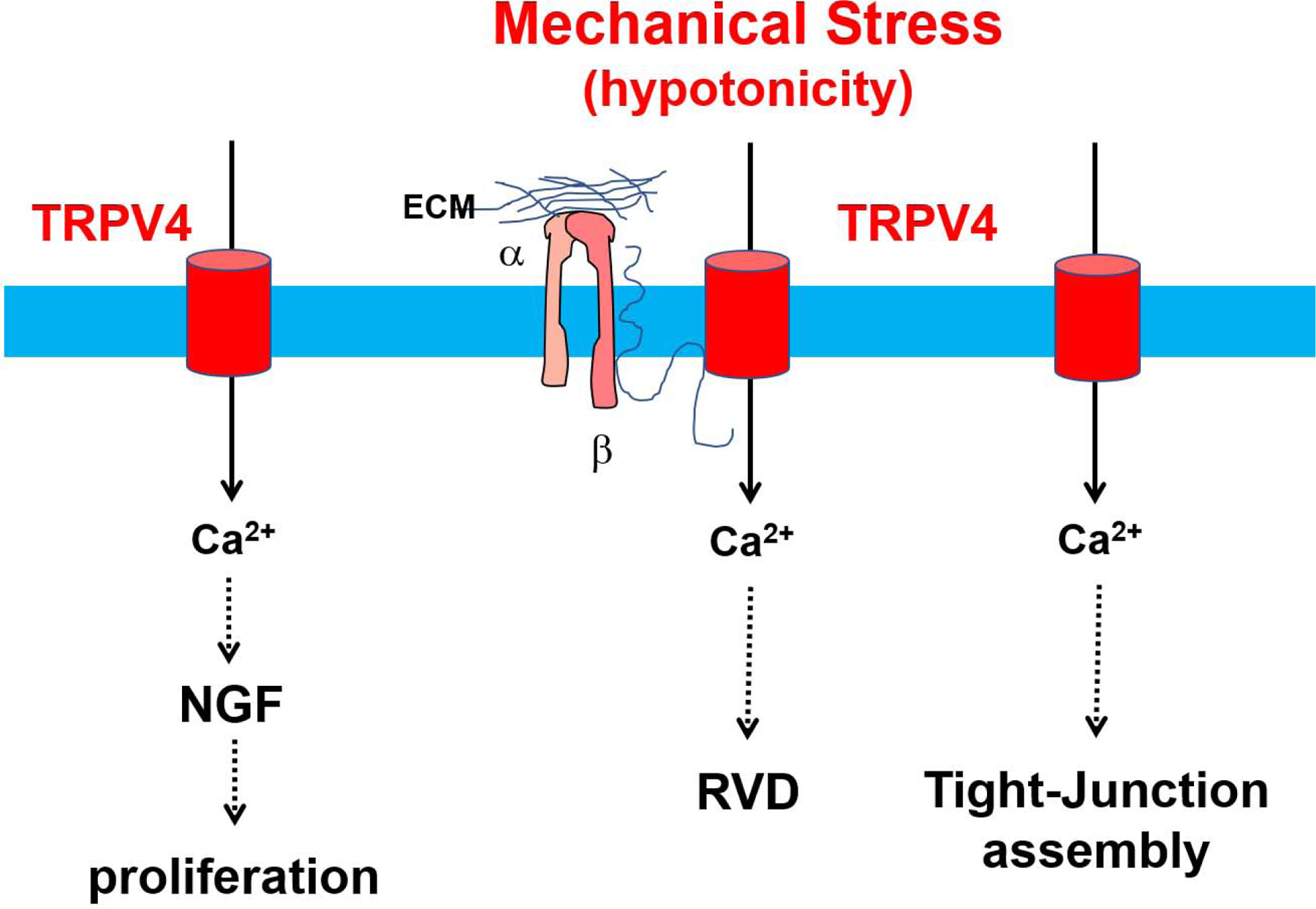
Hypotonicity induced membrane stretch activates TRPV4 channels which normalizes cell volume by mediating regulatory volume decrease (RVD). Membrane stretch may activate TRPV4 channels through phospholipase A2 (PLA2) dependent production of arachidonic acid (AA) and its metabolite EET (not shown). Alternatively, membrane stretch may activate TRPV4 through β1 integrin/CD98 pathway. TRPV4 activation in the corneal epithelium causes Ca2+ influx, leading to increased transepithelial resistance (TER), which is needed for the assembly of tight junctions and, has also been implicated in the release of nerve growth factor and proliferation of epithelial cells in the cornea.
2.2. Lens
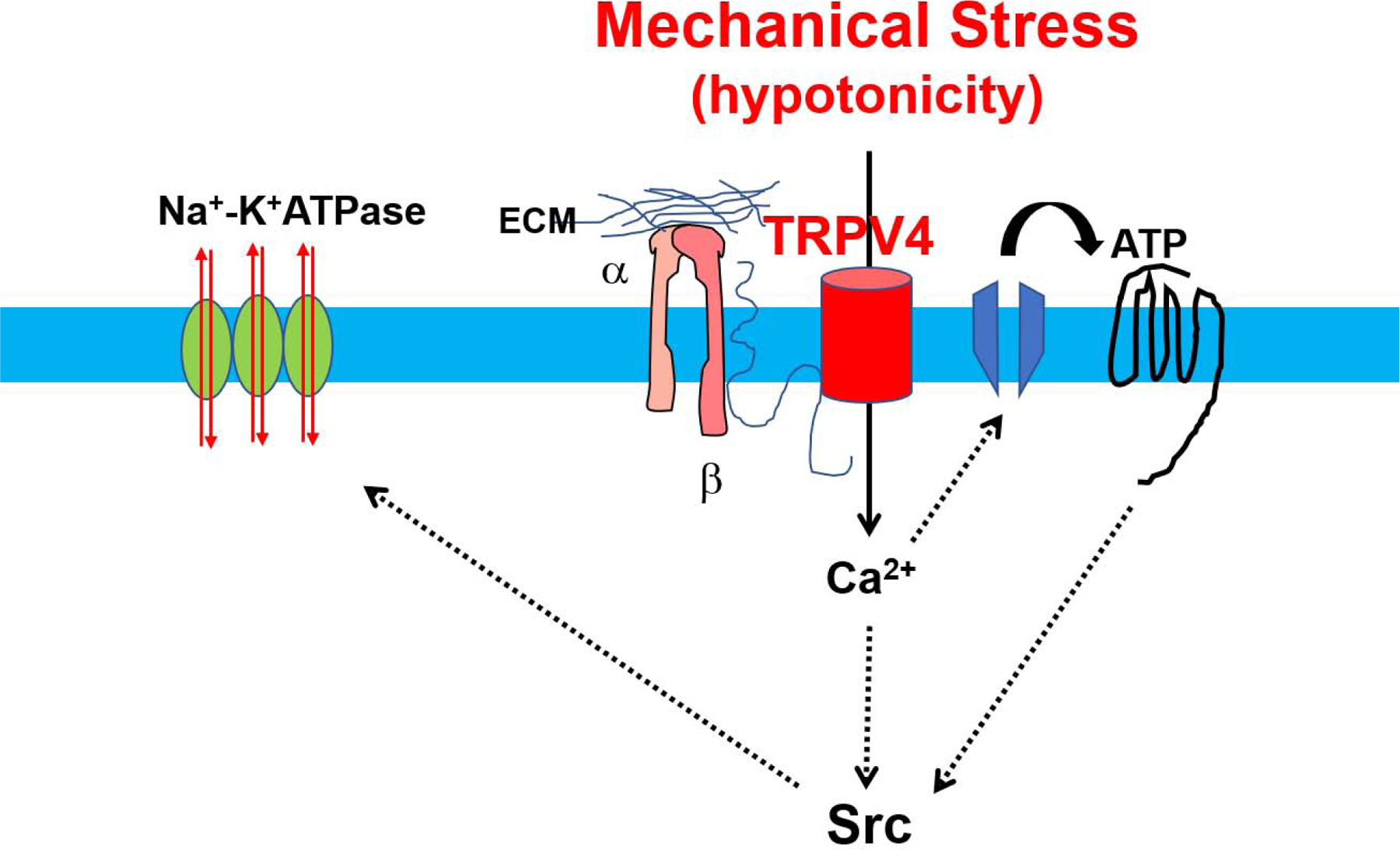
The lens is located behind the cornea and in front of the retina, where its main function is focusing light rays that are then passed and focused onto the retina. The majority of the lens is made up of fiber cells that communicate with the anterior epithelium (Bassnett et al., 1994). TRPV4 expression was initially thought to be localized to the epithelium of the lens (Shahidullah et al., 2015), however, a recent study has shown that TRPV4 is indeed present within fiber cells as well (Nakazawa et al., 2019). Within the lens, the function of TRPV4 is to sense mechanical and osmotic changes. In response to hyposmotic shock, ATP production increases, which has implications in RVD (Andersson et al., 2004). The inhibition of TRPV4 channels blocks the stimulation of Na-K-ATPase by Src family tyrosine kinase which in turn is activated in response to ATP release via connexin and pannexin hemichannels (Shahidullah et al., 2012). Similarly, damage to the fiber mass causes the activation of Src family tyrosine kinase activity within the epithelium, and can be inhibited by TRPV4 antagonism(Shahidullah et al., 2015, 2017) (Fig.2). Taken together, it is evident that TRPV4 is necessary for maintaining homeostasis within the lens, however, the number of studies are limited.
Figure 2. Regulation of ion and water homeostasis by TRPV4 channels in the lens.
TRPV4 is activated in response to osmotic stress or damage to the lens epithelium, TRPV4-mediated calcium influx induces releases of ATP through connexin and pannexin hemichannels. ATP then binds to purinergic receptor and induces Src-dependent Na-KATPase activation restoring volume/ion homeostasis in the lens. Note that TRPV4 activation could be mediated through either integrin/CD98 or AA/EET pathways.
2.3. Ciliary body
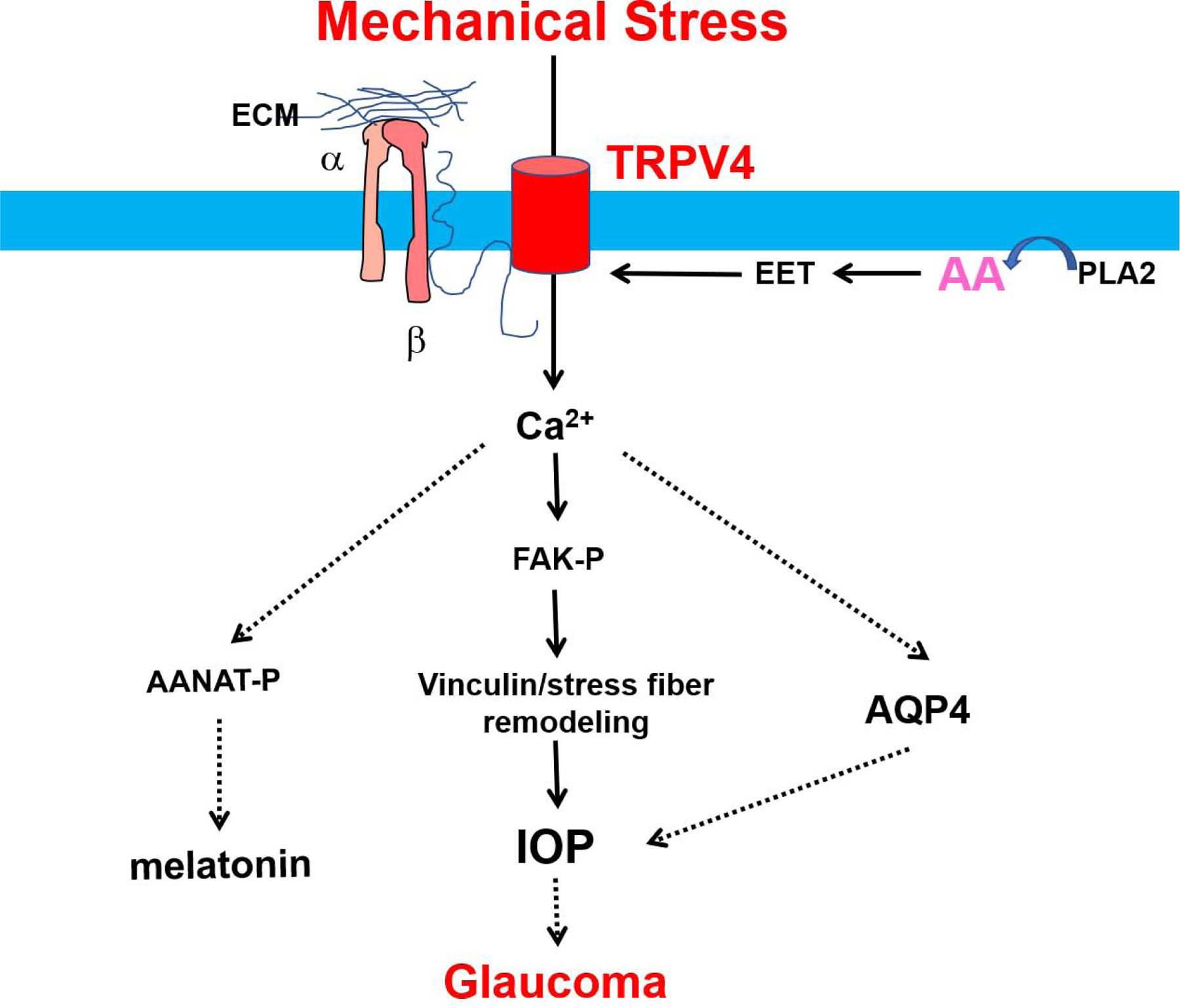
The ciliary body connects the iris to the choroid and has two main functions: the production/drainage of aqueous humor, which is a fluid that supplies nutrients to structures lacking a vasculature, and securing the lens in place(Delamere et al., 2016). The ciliary body contains nonpigmented epithelial cells (NPE), pigmented epithelial cells (PE), and the ciliary muscle(Coca-Prados and Escribano, 2007). Functional expression of TRPV4 has been found within the NPE, where it senses osmotic changes, and regulates swelling-induced Ca2+ signaling within the ciliary body via possible activation by the PLA2 pathway’s release of arachidonic acid and its derivatives(Jo et al., 2016). Responsible for the drainage of aqueous humor, the trabecular meshwork (TM) is located near the ciliary body. TRPV4 channels have been implicated in mechanotransduction within the TM, where stretching of TM cells or activation via a TRPV4 agonist causes Ca2+ influx, phosphorylation of focal adhesion kinases (FAK), restructuring of vinculin, and thickening of stress fibers (Ryskamp et al., 2016), which are all crucial to the maintenance of intraocular pressure (IOP). A lesser-known function of the ciliary body is the production of melatonin, which is involved in the light/dark cycle (Aimoto et al., 1985; Rohde et al., 1985). The synthesis of melatonin is dependent on the conversion of tryptophan to 4-hydroxytyrptophan by tryptophan hydroxylase (TPH), the conversion of serotonin to N-acetylserotonin via N-acetyltransferase (AANAT), and the conversion of N-acetylserotonin to melatonin via O-methyltransferase (HIOMT)(Bernard et al., 1999). Activation of TRPV4 by GSK1 is shown to increase phosphorylation of the AANAT enzyme in NPE cells of the ciliary body after 5 minutes, and may be the result of calmodulin binding to activate protein kinase II (Alkozi et al., 2017a; Alkozi et al., 2017b), which is implicated in the maintenance of IOP (Fig.3).
Figure 3. Mechanisms of activation and role of TRPV4 channels in the ciliary body/trabecular mesh work.
Osmotic cell swelling stretches the plasma membrane and activate the PLA2 enzyme, which breaks down phospholipids into arachidonic acid and its metabolite EET. EET activates TRPV4 channels, leading to Ca2+ influx and subsequent signaling cascades. TRPV4 mediated calcium influx-dependent phosphorylation of FAK induces restructuring of vinculin and thickening of stress fibers, which are implicatied in enhancing IOP. The activation of AQP4 by TRPV4-mediated Ca2+ influx also contributes to the maintenance of IOP. Thus, TRPV4 mechanotransduction has been implicated in glaucoma, as TRPV4 antagonists lowered IOP in glaucomatous mouse eyes and protected retinal neurons from IOP-induced death. Additonally, TRPV4 activation implicated in the phosphorylation of AANAT leading to the production of melatonin (Alkozi, Perez de Lara, & Pintor, 2017).
2.4. Retina
Among the various eye tissues discussed, the functional expression of retinal TRPV4 is the most studied. The retina lies in the back of the eye where its function is to convert incoming light into neuronal signals, which are then processed by the brain. Photoreceptor cells such as rods and cones, ganglion cells, bipolar cells, horizontal cells, and amacrine cells are the 5 main cell types comprising the retina(Mahabadi and Al Khalili, 2020). Additionally, Müller cells are the main type of retinal glial cell, which are non-neuronal and both support and regulate extracellular fluid within the retina(Reichenbach and Bringmann, 2013). TRPV4 has been identified as the sensor responsible for Müller cell response to changes in osmolarity. Mechanistically, cell swelling activates the PLA2 pathway, where CYP450 converts arachidonic acid into 5,6-EET, which goes on to activate TRPV4 channels and subsequent Ca2+ release and reactive gliosis(Ryskamp et al., 2014; Toft-Bertelsen et al., 2017). Furthermore, the TRPV4-dependent release of Ca2+ activates gene expression of AQP4, which mediates fluid exchange in the blood retinal and blood brain barriers(Jo et al., 2015). A common cause of vision loss, retinal detachment, causes cell death of photoreceptors (Wubben et al., 2016). In mice, retinal detachment causes Müller cell swelling, and downstream activation of TRPV4. Further, TRPV4-dependent Ca2+ release provokes the release of cytokine MCP-1 from Müller cells, leading to apoptosis of photoreceptor cells (Matsumoto et al., 2018), indicating that inhibition of TRPV4 could preserve vision loss in patients with retinal detachment. Interestingly, the loss of TRPV4 does not appear to alter outer retinal light signaling(Yarishkin et al., 2018b), demonstrating the complexity of retinal TRPV4 function.
Retinal endothelial cells (RECs) line the vessels within the retina, where they supply nutrients, are involved in maintenance of the blood-retinal barrier (BRB), and their dysfunction lays the foundation for various ocular pathologies(Bharadwaj et al., 2013). Functional expression of TRPV4 has been identified in RECs, where its job is to regulate Ca2+ entry into the cells. Moreover, inhibition of TRPV4 either pharmacologically or using shRNA hinders the migration of RECs, which causes a subsequent decrease of tube formation(Wen et al., 2018); both are critical steps in angiogenesis. Additionally, TRPV1, another member of the transient receptor potential vanilloid family, forms heteromeric channels with TRPV4 in retinal microvascular endothelial cells (RMECs) at the plasma membrane level, indicating the strong need for intracellular Ca2+ in retinal angiogenesis (O’Leary et al., 2019).
Retinal bipolar cells, a type of neuron that joins the inner and outer layers of the retina, feed signals to the inner plexiform layer where their axon terminals synapse onto retinal ganglion cells (RGCs) and amacrine cells, are further encoded, and eventually reach the optic nerve(Euler et al., 2014). TRPV4 expression has been found in RGC somas, inner and outer plexiform layers, and bipolar cells (Gao et al., 2019). Additionally, TRPV4 expression is noted in αRGCs, which are especially receptive to changes in IOP(Lakk et al., 2018). Functionally, TRPV4 channels regulate RGC firing rate via regulation of Ca2+ influx. Further, continuous activation of TRPV4 causes apoptosis in RGC (Ryskamp et al., 2011), where inhibition of TRPV4 increases RGC survival(Taylor et al., 2017). Other mechanistic studies show that the addition of a TRPV4 agonist shortens the delay time of Na+ currents evoked by depolarizing pulses, demonstrating that TRPV4 activation increases membrane excitability in RGCs (Gao et al., 2019).
3. TRPV4 and ocular pathologies
3.1. Glaucoma
Intraocular pressure (IOP) is controlled by the drainage of aqueous humor by the trabecular meshwork and the uveoscleral outflow pathway. Primary open-angle glaucoma (POAG) occurs when there is a slow block at the entrance to the drainage canals, whereas primary closed-angle glaucoma (PCAG) occurs when there is an acute blockage(Weinreb et al., 2014). In both types of glaucoma, increased IOP leads to the loss of RGCs, and a subsequent loss of vision. Other types of glaucoma include but are not limited to normal tension glaucoma (NTG) and congenital glaucoma. In NTG, there is damage to the optic nerve head; however, IOP is not severely elevated (Mallick et al., 2016). Congenital glaucoma is seen in patients less than three years of age and occurs when development of the trabecular meshwork and anterior chamber angle is abnormal(Badawi et al., 2019). Though there are subtypes of the differing glaucomas, we will focus on those listed above, as they are the most common.
Though the cause of glaucoma is not completely understood, there are certain risk factors. For example, in POAG, genetic evidence has revealed mutations of Myocilin(McMonnies, 2017), a protein found in the trabecular meshwork. Moreover, in NTG, a study found the presence of TANK binding kinase1 (TBK1) copy number variants, something that is not seen in glaucomas with elevated IOP (Awadalla et al., 2015). Studies have been limited in the genetic profile of patients with PCAG; however, the gene nanophthalmos 1 (NNO1) has been identified as a cause of PCAG (Othman et al., 1998). Additionally, some susceptible genetic loci, such as PLEKHA7, COL11A1, and PCMTD1 have been shown to play a role in PCAG (Vithana et al., 2012), as well as variants in the matrix metalloproteinase 9 (MMP9) gene (Ahram et al., 2015). Concerning congenital glaucoma, variants in the CYP1B1 and LTBP2 are inherited in an autosomal recessive manner, whereas a variant in TEK is autosomal dominant (Abu-Amero and Edward, 1993). In addition to genetic abnormalities, it appears that race, age, and gender also play a role in the development of glaucomas, but they will not be discussed in this review.
Because TRPV4 is both an osmotic and mechanosensor, it has been studied in the context of glaucoma. As explained above, a hallmark of glaucoma is the death of RGCs. Immunochemistry has shown that a substantial amount of TRPV4 is localized to the optic nerve head and the nerve fiber layer of axons in RGCs. The same study found that TRPV4 agonists increase the firing rate of RGCs, cause an influx in Ca2+, and sustained activation of these channels lead to RGC death (Ryskamp et al., 2011). Additionally, inhibition of TRPV4 channels within the retina improves the survival of RGCs(Taylor et al., 2017), indicating that inhibition of TRPV4 could be used as a potential treatment for glaucoma.
Lowe syndrome is an X-linked disease causing both cataracts and glaucoma. The OCRL1 gene, which codes for the OCRL1 protein, a type of inositol polyphosphate 5-phosphatase, is mutated in patients with Lowe syndrome (Loi, 2006). This mutation leads to abnormal cilia development and abnormal trafficking of endosomes (Coon et al., 2012; Luo et al., 2014). Coimmunoprecipitation assays have shown that TRPV4 interacts with OCRL, where they both localize in the primary cilia. Further, OCRL siRNA-treated trabecular meshwork cells show a decrease in Ca2+ influx in the presence of a TRPV4 agonist, and TRPV4 channels in keratinocytes isolated from a Lowe syndrome patient are unable to react to TRPV4 agonists (Luo et al., 2014). Therefore, it appears that TRPV4 trafficking to primary cilia is important for Ca2+ signaling within the trabecular meshwork. A follow-up study revealed that TRPV4 channels are activated via the PLA2 pathway within the trabecular meshwork. Additionally, this study found that TRPV4 activation causes stress fiber and focal adhesion remodeling in trabecular meshwork cells when Ca2+ is elevated, where 3D trabecular meshwork models treated with GSK1 increase formation of F-actin stress fibers and fibronectin expression, TRPV4 antagonists can attenuate these effects. Importantly, it was revealed that intraocular injections of a TRPV4 antagonist are able to lower IOP in a mouse model of glaucoma (Ryskamp et al., 2016).
Another channel responsible for mechanotransduction within the trabecular meshwork is TREK-1, which belongs to the two-pore domain potassium channel family (K2P)(Meadows et al., 2000). It is believed that both TRPV4 and TREK-1 channels work together to control IOP (Fig.3), which is evidenced by residual outward conductance when TRPV4 is antagonized in the trabecular meshwork, indicating that another channel is at work. Additionally, this study demonstrates that treatment with a TREK-1 activator causes elevated Ca2+ in the trabecular meshwork where subsequent ruthenium red (a non-specific TRPV4 antagonist) treatment abrogates these effects(Yarishkin et al., 2018a). l
Endothelial dysfunction, such as vascular abnormality is another risk factor for glaucoma (Resch et al., 2009) and can contribute to the breakdown of the BRB. Retinal microvascular monolayers exposed to GSK1, the TRPV4 agonist, show increased permeability, where GSK2 can attenuate these effects. Further, the zigzag-like overlap of VE-cadherin and β-catenin that is usually present, is decreased after treatment with GSK1, where quantification revealed a ~32.1% reduction in β-catenin, ~25.6% reduction in VE-cadherin, and a ~40% decrease in occludin; important proteins involved in maintaining endothelial integrity. GSK1 also causes a loss of cell cortex actin, and a subsequent accumulation of actin within the perinuclear region of retinal microvascular endothelial cells, causing a spike in Ca2+ (Phuong et al., 2017), which taken together, show that TRPV4 is indeed a crucial regulator of endothelial stability.
3.2. Retinopathy
Endothelial dysfunction is a hallmark of retinopathy, a microvascular condition in which there is significant damage to the retina. Many studies have investigated the role of TRPV4 on endothelial dysfunction in the context of other microvascular pathologies. As briefly mentioned above, TRPV4 has been implicated in the regulation of vascular tone in response to vasodilators, such as acetylcholine or shear stress. First, using TRPV4−/− mice, Kohler et al demonstrated that arterial response to shear stress is dependent on TRPV4(Hartmannsgruber et al., 2007; Kohler et al., 2006). The TRPV4 agonist, GSK1, was shown to increase endothelial Ca2+ and relaxation in small mesenteric arteries from wild-type (WT) but not TRPV4−/− mice(Mendoza et al., 2010). Mechanistically, this flow-induced dilation is mediated through the release of NO and other endothelial relaxing factors. Deletion of TRPV4 also attenuates acetylcholine-induced vasodilation in mesenteric arteries(Zhang et al., 2009). Acetylcholine appears to mediate these effects through the activation of TRPV4 via PKCα(Adapala et al., 2011). Recently, it was shown that activation of TRPV4 leads to Ca2+ influx, which triggers Ca2+-induced-Ca2+ release from internal stores via activation of IP3 receptors causing a further increase in Ca2+ leading to vasodilation (Heathcote et al., 2019). One of the key factors in determining CVD prognosis is vascular stiffness leading to endothelial dysfunction. A possible mechanism involving TRPV4 and substrate stiffness has been outlined, where increasing stiffness leads to reduced TRPV4 expression and increased endothelin-1 expression in endothelial cells (Song et al., 2019). Additionally, post spinal cord injury in rats, TRPV4 expression is significantly increased at endothelial-microglial junctions and leads to endothelial damage and poor prognosis (Kumar et al., 2020). In retinas, inhibition of TRPV4 (or TRPV1) arrests angiogenesis both in vitro and in vivo via modulation of tubulogenesis (O’Leary et al., 2019). The evidence is abundantly clear that TRPV4 is involved in endothelial dysfunction, and therefore should be studied in the context of retinopathies.
Diabetes is a widespread debilitating disease characterized by uncontrolled glucose levels due to either insulin resistance by cells or destruction of pancreatic β cells, which produce insulin. The effects of hyperglycemia cause significant changes in signaling pathways of various tissues, including the retina. Diabetic retinopathy (DR) is classified according to two different types: non-proliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR). Features of NPDR include microaneurysms, hemorrhages, and changes to the retinal microvasculature, whereas PDR is seen at later stages and involves neovascularization(Duh et al., 2017). Further, DR can affect the macula, an area that contains a high number of photoreceptor cells, when this occurs, it is termed diabetic maculopathy, which causes vascular leakage, swelling, and eventual vision loss(Klein et al., 1989; Stitt et al., 2016). Though there are many signaling mechanisms leading to DR, this review focuses mainly on TRPV4 in relation to DR.
When retinal endothelial cells are subjected to high levels of glucose, functional expression of TRPV4 decreases in vitro. Further, diabetic rats show a downregulation in retinal TRPV4 expression (Monaghan et al., 2015). One of the key features of DR is the breakdown of the blood retinal barrier (BRB). This occurs when there are elevated levels of certain growth factors, cytokines, and loss of pericyte coverage leading to an increase in endothelial cell permeability (Klausen et al., 2014). A recent study investigated the role TRPV4 plays in the breakdown of the BRB. Here, it was found that TRPV4 activation causes an increase in BRB permeability, and that vasoinhibins can inhibit the activation of TRPV4, leading to a decrease in permeability, and further inhibition by a TPRV4 antagonist arrests the breakdown of the BRB under diabetic conditions(Arredondo Zamarripa et al., 2017). Additionally, diabetic TRPV4−/− mice have shown significantly less BRB breakdown than diabetic wild type mice, and structurally there is a thicker inner nuclear layer in nondiabetic TRPV4−/− mice. This same study found that deletion of TRPV4 is able to abrogate retinal edema, a crucial step in the breakdown of the BRB, however, a single intravitreal injection of the TRPV4 antagonist, GSK2, is not enough to mimic these effects, although reduced retinal thinning is seen (Orduna Rios et al., 2019). Taken together, these studies highlight TRPV4 as a potential target in treating diabetic retinopathy.
When premature babies are placed inside an incubator, they are exposed to high levels of oxygen. Because the main function of blood vessels is to deliver oxygen to tissues, when there is an overabundance of oxygen (hyperoxia), less vessels form. Once the babies are removed from the high oxygen environment of the incubator, new abnormal vessels form rapidly, causing leakage, bleeding, and eventual blindness. This condition is known as retinopathy of prematurity, a type of ischemic retinopathy. A common laboratory technique used to mimic this is the oxygen induced retinopathy model (OIR)(Connor et al., 2009). Here, mouse pups are placed in a hyperoxia chamber for a period, and then moved to conventional housing. Recently, TRPV4 has been studied in the context of this type of retinopathy. TRPV4 (or TRPV1) antagonists, HC067047 (20 μM) or RN1734 (15 μM) were injected on day P15 of hypoxic phase P13-P17(though the pups were kept in normal air) and neovascularization was measured on the final day of P17. Findings from this study revealed that injection of TRPV4 (or TRPV1) antagonists into the vitreous space inhibited retinal neovascularization in OIR mice (O’Leary et al., 2019) (Fig.4), indicating that TRPV4 antagonists have potential in treating retinopathy of prematurity. Although the concentration of HC06 is many folds higher than reported IC50, both HC06 and RN1734 have been demonstrated to be specific antagonist for TRPV4 (Vincent et al., 2009; Vincent and Duncton, 2011). In fact, TRPV4 presence but not absence appear to be critical for diabetes-induced retinopathy, disruption of BBB, and OIR, however, findings from our laboratory on tumor vasculature tells a different story. We have demonstrated that TRPV4 is functionally downregulated in tumor endothelial cells that exhibit aberrant mechanosensitivity and tube formation in vitro and abnormal angiogenesis characterized by leaky vessels in vivo (Adapala et al., 2016) which is mediated through activation of Rho/Rho kinase pathway(Thoppil et al., 2016) (Fig.4). Further, the activation of TRPV4 with GSK1 or inhibition of Rho/Rho kinase pathway was able to normalize the vasculature and improve the delivery of anti-cancer drugs (Adapala et al., 2016; Thoppil et al., 2016). Our latest findings demonstrate that tumor-derived extracellular vesicles downregulate TRPV4 channels and transform normal endothelial cells into a tumor endothelial cell-like phenotype (Guarino et al., 2019). These findings suggest that TRPV4 is required for vascular stability and pathological stimuli may disrupt vascular integrity via downregulation of TRPV4 in endothelial cells (Cappelli et al., 2019), which may be applicable to OIR models. Since the one OIR study is based on pharmacological agents, we have performed OIR studies using TRPV4−/− mice and observed increased angiogenesis in retinas subjected to OIR, which exhibit reduced vascular integrity (Manuscript in revision).
Figure 4. TRPV4 channels regulate retinal angiogenesis.
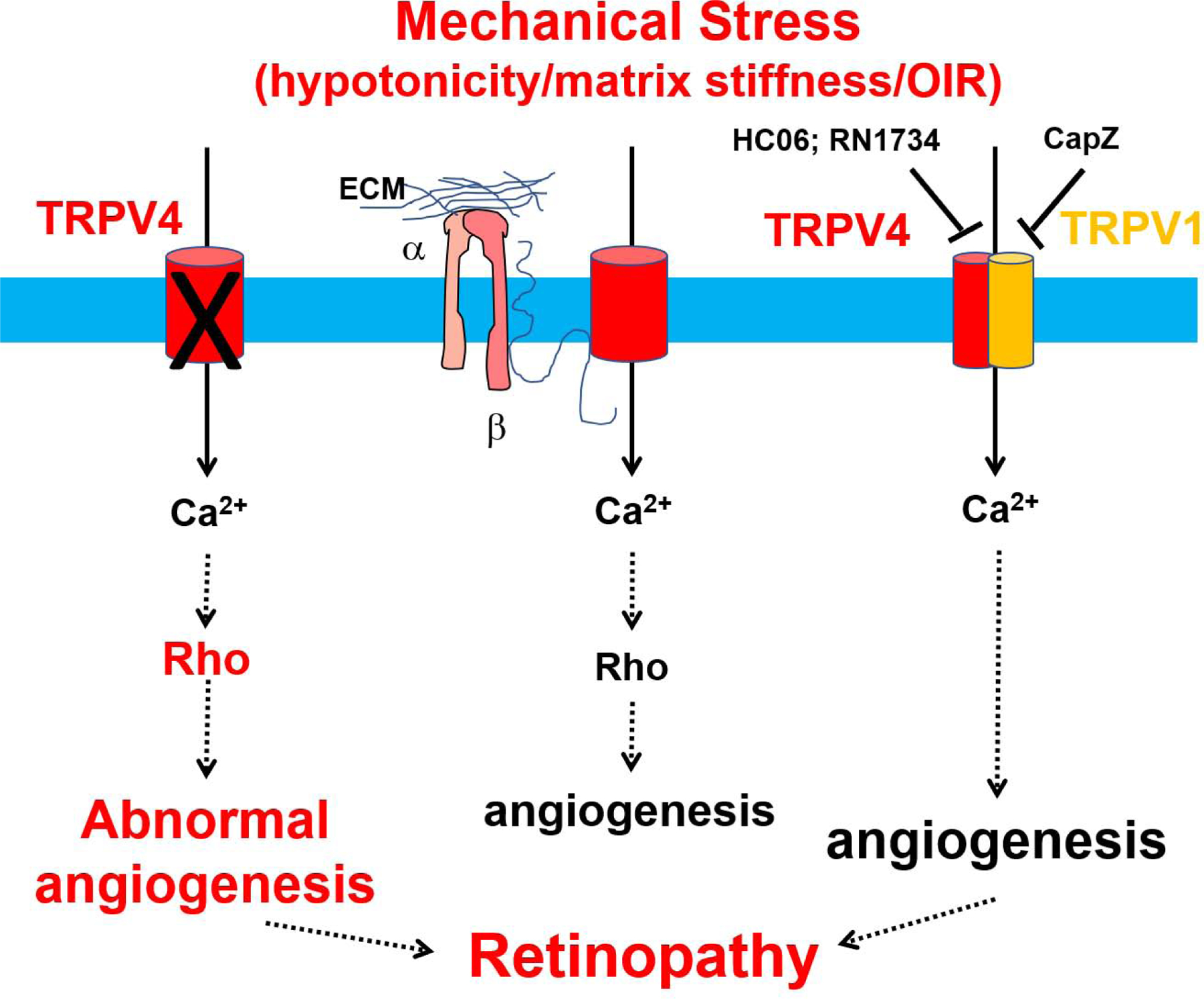
TRPV4 acts as a mechanosensor of cyclic strain, shear stress, matrix stiffness, ischemia, and keeps Rho/Rho kinase activity at optimal level in endothelial cells leading to which results in physiological (optimal) angiogenesis. However, reduction or deletion of TRPV4 results in aberrant mechanosensing, enhanced Rho/Rho kinase activation, leading to abnormal angiogenesis which may cause retinopathy. Recently, TRPV4 was shown to form heteromeric channels with TRPV1 channels in retinal endothelial cells. Inhibition of either of these channels appear to inhibit abnormal angiogenesis induced by OIR. In contrast, our unpublished results show that genetic deletion of TRPV4 does not impact developmental angiogenesis but enhances pathological angiogenesis in response to OIR in mouse retinas. Though contrasting, these findings suggest TRPV4 plays an important role in neovascularization seen in proliferative diabetic retinopathy.
4. Conclusion
TRPV4 channels are essential for maintaining normal ocular physiology. These mechanosensitive channels detect changes in IOP, shear stress, and maintain osmolarity within the eye. Based on its role in ocular pathologies, more research needs to be done regarding TRPV4 in both retinopathies and glaucoma. TRPV4 dysfunction is, in part responsible for the phenotypic changes associated with these disease states, however, studies are limited. Further investigation of TRPV4 in the context of retinal angiogenesis is needed. For example, how pericyte coverage is affected in the retinal vasculature of TRPV4−/− mice. It is possible that the deletion of TRPV4 reduces pericyte coverage leading to compromised vascular integrity. Another example of a less understood concept is how TRPV4 channels affect the vasculature in glaucoma. A possible approach would include enucleating a mouse model of glaucoma and using immunohistochemistry to stain for TRPV4 expression colocalized with the well-known endothelial marker, CD31. Additionally, opposing observations of the possible role for TRPV4 in regulating neovascularization in OIR and tumor could be addressed using endothelial specific TRPV4−/− mice to unequivocally confirm the role of endothelial TRPV4 channel. Despite limitations, TRPV4 should be considered as a potential target in treating ocular pathologies.
Highlights.
TRPV4 is expressed in various cells and tissues of the eye.
TRPV4 is mechanosensor and regulates ion and water homeostasis in the eye.
TRPV4 modulates intraocular pressure and involved in glaucoma.
TRPV4 is implicated in ischemia-induced neovascularization and diabetic retinopathy.
Acknowledgements
This work was supported by National Institutes of Health (R15CA202847, R01HL119705 and R01HL148585; CKT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Amero KK, Edward DP, 1993. Primary Congenital Glaucoma, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), Seattle (WA). [PubMed] [Google Scholar]
- Adapala RK, Kanugula AK, Paruchuri S, Chilian WM, Thodeti CK, 2020. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic research in cardiology 115, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapala RK, Talasila PK, Bratz IN, Zhang DX, Suzuki M, Meszaros JG, Thodeti CK, 2011. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 301, H757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapala RK, Thoppil RJ, Ghosh K, Cappelli HC, Dudley AC, Paruchuri S, Keshamouni V, Klagsbrun M, Meszaros JG, Chilian WM, Ingber DE, Thodeti CK, 2016. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 35, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK, 2013. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. Journal of molecular and cellular cardiology 54, 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahram DF, Alward WL, Kuehn MH, 2015. The genetic mechanisms of primary angle closure glaucoma. Eye 29, 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimoto T, Rohde BH, Chiou GC, Lauber JK, 1985. N-acetyltransferase activity and melatonin level in the eyes of glaucomatous chickens. Journal of ocular pharmacology 1, 149–160. [DOI] [PubMed] [Google Scholar]
- Alkozi HA, Perez de Lara MJ, Pintor J, 2017a. Melatonin synthesis in the human ciliary body triggered by TRPV4 activation: Involvement of AANAT phosphorylation. Experimental eye research 162, 1–8. [DOI] [PubMed] [Google Scholar]
- Alkozi HA, Perez de Lara MJ, Sanchez-Naves J, Pintor J, 2017b. TRPV4 Stimulation Induced Melatonin Secretion by Increasing Arylalkymine N-acetyltransferase (AANAT) Protein Level. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R, Maggi CA, 1991. Ruthenium red as a capsaicin antagonist. Life sciences 49, 849–856. [DOI] [PubMed] [Google Scholar]
- Andersson RM, Aizman O, Aperia A, Brismar H, 2004. Modulation of Na+,K+-ATPase activity is of importance for RVD. Acta physiologica Scandinavica 180, 329–334. [DOI] [PubMed] [Google Scholar]
- Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA, 2005. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 168, 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA, 2006. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem 281, 1580–1586. [DOI] [PubMed] [Google Scholar]
- Arredondo Zamarripa D, Noguez Imm R, Bautista Cortes AM, Vazquez Ruiz O, Bernardini M, Fiorio Pla A, Gkika D, Prevarskaya N, Lopez-Casillas F, Liedtke W, Clapp C, Thebault S, 2017. Dual contribution of TRPV4 antagonism in the regulatory effect of vasoinhibins on blood-retinal barrier permeability: diabetic milieu makes a difference. Scientific reports 7, 13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadalla MS, Fingert JH, Roos BE, Chen S, Holmes R, Graham SL, Chehade M, Galanopolous A, Ridge B, Souzeau E, Zhou T, Siggs OM, Hewitt AW, Mackey DA, Burdon KP, Craig JE, 2015. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. American journal of ophthalmology 159, 124–130 e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA, 2019. Primary congenital glaucoma: An updated review. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society 33, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Almazi JG, Darby W, Tovar-Lopez FJ, Mitchell A, McIntyre P, 2016. Shear stress mediates exocytosis of functional TRPV4 channels in endothelial cells. Cellular and molecular life sciences : CMLS 73, 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Keov P, Darby WG, Lai A, Khoshmanesh K, Thurgood P, Vahidi P, Ejendal K, McIntyre P, 2019. The TRPV4 Agonist GSK1016790A Regulates the Membrane Expression of TRPV4 Channels. Frontiers in pharmacology 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Knoerzer M, Khoshmanesh K, Mitchell A, McIntyre P, 2017. Shear Stress Regulates TRPV4 Channel Clustering and Translocation from Adherens Junctions to the Basal Membrane. Scientific reports 7, 15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC, 1994. Intercellular communication between epithelial and fiber cells of the eye lens. J Cell Sci 107 ( Pt 4), 799–811. [DOI] [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Siegfried CJ, Shiels A, 2014. Mutation of the melastatin-related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One 9, e104000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M, Guerlotte J, Greve P, Grechez-Cassiau A, Iuvone MP, Zatz M, Chong NW, Klein DC, Voisin P, 1999. Melatonin synthesis pathway: circadian regulation of the genes encoding the key enzymes in the chicken pineal gland and retina. Reproduction, nutrition, development 39, 325–334. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Paradisi V, Pozzan T, Azzone GF, 1984. Pathway for uncoupler-induced calcium efflux in rat liver mitochondria: inhibition by ruthenium red. Biochemistry 23, 1645–1651. [DOI] [PubMed] [Google Scholar]
- Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, Benedetti EE, Zamora DO, Choi D, David LL, Smith JR, 2013. Role of the retinal vascular endothelial cell in ocular disease. Progress in retinal and eye research 32, 102–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Aponte JE, Iserovich P, Reinach PS, 2005. Characterization of regulatory volume behavior by fluorescence quenching in human corneal epithelial cells. The Journal of membrane biology 207, 11–22. [DOI] [PubMed] [Google Scholar]
- Cappelli HC, Kanugula AK, Adapala RK, Amin V, Sharma P, Midha P, Paruchuri S, Thodeti CK, 2019. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer letters 442, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH, Sul Y, Weiss M, Hillel A, Lipe H, Wolff J, Matsushita M, Raskind W, Bird T, 2010. CMT2C with vocal cord paresis associated with short stature and mutations in the TRPV4 gene. Neurology 75, 1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Bao W, Behm DJ, Brooks CA, Bury MJ, Dowdell SE, Eidam HS, Fox RM, Goodman KB, Holt DA, Lee D, Roethke TJ, Willette RN, Xu X, Ye G, Thorneloe KS, 2017. Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS medicinal chemistry letters 8, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Shin SH, Kang SS, 2012. The negative feedback regulation of TRPV4 Ca2+ ion channel function by its C-terminal cytoplasmic domain. Cellular signalling 24, 1918–1922. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Escribano J, 2007. New perspectives in aqueous humor secretion and in glaucoma: the ciliary body as a multifunctional neuroendocrine gland. Progress in retinal and eye research 26, 239–262. [DOI] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE, 2009. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nature protocols 4, 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, Lowe M, Beales PL, Aguilar RC, 2012. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Human molecular genetics 21, 1835–1847. [DOI] [PubMed] [Google Scholar]
- D’Hoedt D, Owsianik G, Prenen J, Cuajungco MP, Grimm C, Heller S, Voets T, Nilius B, 2008. Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem 283, 6272–6280. [DOI] [PubMed] [Google Scholar]
- Dai J, Kim OH, Cho TJ, Schmidt-Rimpler M, Tonoki H, Takikawa K, Haga N, Miyoshi K, Kitoh H, Yoo WJ, Choi IH, Song HR, Jin DK, Kim HT, Kamasaki H, Bianchi P, Grigelioniene G, Nampoothiri S, Minagawa M, Miyagawa SI, Fukao T, Marcelis C, Jansweijer MC, Hennekam RC, Bedeschi F, Mustonen A, Jiang Q, Ohashi H, Furuichi T, Unger S, Zabel B, Lausch E, Superti-Furga A, Nishimura G, Ikegawa S, 2010. Novel and recurrent TRPV4 mutations and their association with distinct phenotypes within the TRPV4 dysplasia family. Journal of medical genetics 47, 704–709. [DOI] [PubMed] [Google Scholar]
- Dalsgaard T, Sonkusare SK, Teuscher C, Poynter ME, Nelson MT, 2016. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Scientific reports 6, 33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere NA, Mandal A, Shahidullah M, 2016. The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 32, 504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, Hite RK, Yuan P, 2018. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nature structural & molecular biology 25, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW, 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Haverkamp S, Schubert T, Baden T, 2014. Retinal bipolar cells: elementary building blocks of vision. Nature reviews. Neuroscience 15, 507–519. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Nilius B, Owsianik G, 2010. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol 103, 2–17. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA, 2008. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5’−6’-epoxyeicosatrienoic acid. The Journal of general physiology 131, i2. [DOI] [PubMed] [Google Scholar]
- Gao F, Yang Z, Jacoby RA, Wu SM, Pang JJ, 2019. The expression and function of TRPV4 channels in primate retinal ganglion cells and bipolar cells. Cell death & disease 10, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wu L, O’Neil RG, 2003. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278, 27129–27137. [DOI] [PubMed] [Google Scholar]
- Gilliam JC, Wensel TG, 2011. TRP channel gene expression in the mouse retina. Vision research 51, 2440–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A, Norton N, Sprecher DL, Cheriyan J, 2019. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. American journal of cardiovascular drugs : drugs, devices, and other interventions 19, 335–342. [DOI] [PubMed] [Google Scholar]
- Guarino BD, Adapala RK, Kanugula AK, Lenkey NM, Dougherty JA, Paruchuri S, Khan M, Thodeti CK, 2019. Extracellular Vesicles From Pathological Microenvironment Induce Endothelial Cell Transformation and Abnormal Angiogenesis via Modulation of TRPV4 Channels. Frontiers in cell and developmental biology 7, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M, 2002. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22, 6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Juusola M, 2015. Phototransduction in Drosophila. Current opinion in neurobiology 34, 37–45. [DOI] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R, 2007. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2, e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote HR, Lee MD, Zhang X, Saunter CD, Wilson C, McCarron JG, 2019. Endothelial TRPV4 channels modulate vascular tone by Ca(2+) -induced Ca(2+) release at inositol 1,4,5-trisphosphate receptors. British journal of pharmacology 176, 3297–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel NJ, Cox DN, 2020. Transient receptor potential channels: current perspectives on evolution, structure, function and nomenclature. Proceedings. Biological sciences 287, 20201309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Frye AM, Phuong TT, Redmon SN, Roberts R, Berkowitz BA, Yarishkin O, Krizaj D, 2016. Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci U S A 113, 3885–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N, Krizaj D, 2015. TRPV4 and AQP4 Channels Synergistically Regulate Cell Volume and Calcium Homeostasis in Retinal Muller Glia. J Neurosci 35, 13525–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen TK, Janssens A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Nilius B, 2014. Single point mutations of aromatic residues in transmembrane helices 5 and −6 differentially affect TRPV4 activation by 4alpha-PDD and hypotonicity: implications for the role of the pore region in regulating TRPV4 activity. Cell Calcium 55, 38–47. [DOI] [PubMed] [Google Scholar]
- Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B, 2009. Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. Journal of medicinal chemistry 52, 2933–2939. [DOI] [PubMed] [Google Scholar]
- Klein R, Moss SE, Klein BE, Davis MD, DeMets DL, 1989. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology 96, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J, 2006. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26, 1495–1502. [DOI] [PubMed] [Google Scholar]
- Kohler R, Hoyer J, 2007. Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells. [PubMed]
- Kumar H, Lim CS, Choi H, Joshi HP, Kim KT, Kim YH, Park CK, Kim HM, Han IB, 2020. Elevated TRPV4 Levels Contribute to Endothelial Damage and Scarring in Experimental Spinal Cord Injury. J Neurosci 40, 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakk M, Young D, Baumann JM, Jo AO, Hu H, Krizaj D, 2018. Polymodal TRPV1 and TRPV4 Sensors Colocalize but Do Not Functionally Interact in a Subpopulation of Mouse Retinal Ganglion Cells. Frontiers in cellular neuroscience 12, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, Gaudet R, Kleta R, Fischbeck KH, Sumner CJ, 2010. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2 C. Nature genetics 42, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, 2005. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol 567, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM, 2003. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A 100, 13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, 2006. Lowe syndrome. Orphanet journal of rare diseases 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF, Joos KM, Iomini C, Obukhov AG, Sun Y, 2014. Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci U S A 111, 12871–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi N, Al Khalili Y, 2020. Neuroanatomy, Retina, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Mallick J, Devi L, Malik PK, Mallick J, 2016. Update on Normal Tension Glaucoma. Journal of ophthalmic & vision research 11, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rendon J, Sanchez-Guzman E, Rueda A, Gonzalez J, Gulias-Canizo R, Aquino-Jarquin G, Castro-Munozledo F, Garcia-Villegas R, 2017. TRPV4 Regulates Tight Junctions and Affects Differentiation in a Cell Culture Model of the Corneal Epithelium. J Cell Physiol 232, 1794–1807. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Sugio S, Seghers F, Krizaj D, Akiyama H, Ishizaki Y, Gailly P, Shibasaki K, 2018. Retinal Detachment-Induced Muller Glial Cell Swelling Activates TRPV4 Ion Channels and Triggers Photoreceptor Death at Body Temperature. J Neurosci 38, 8745–8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE, 2010. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMonnies CW, 2017. Glaucoma history and risk factors. Journal of optometry 10, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows HJ, Benham CD, Cairns W, Gloger I, Jennings C, Medhurst AD, Murdock P, Chapman CG, 2000. Cloning, localisation and functional expression of the human orthologue of the TREK-1 potassium channel. Pflugers Archiv : European journal of physiology 439, 714–722. [DOI] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX, 2010. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298, H466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler S, Cheng Y, Skosyrski S, Garreis F, Pietrzak P, Kociok N, Dwarakanath A, Reinach PS, Kakkassery V, 2012a. Altered calcium regulation by thermosensitive transient receptor potential channels in etoposide-resistant WERI-Rb1 retinoblastoma cells. Experimental eye research 94, 157–173. [DOI] [PubMed] [Google Scholar]
- Mergler S, Garreis F, Sahlmuller M, Lyras EM, Reinach PS, Dwarakanath A, Paulsen F, Pleyer U, 2012b. Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochemistry and cell biology 137, 743–761. [DOI] [PubMed] [Google Scholar]
- Mergler S, Garreis F, Sahlmuller M, Reinach PS, Paulsen F, Pleyer U, 2011. Thermosensitive transient receptor potential channels in human corneal epithelial cells. J Cell Physiol 226, 1828–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, McGeown JG, Curtis TM, 2015. Hyperglycemia and Diabetes Downregulate the Functional Expression of TRPV4 Channels in Retinal Microvascular Endothelium. PLoS One 10, e0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Donaldson PJ, Petrova RS, 2019. Verification and spatial mapping of TRPV1 and TRPV4 expression in the embryonic and adult mouse lens. Experimental eye research 186, 107707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary C, McGahon MK, Ashraf S, McNaughten J, Friedel T, Cincola P, Barabas P, Fernandez JA, Stitt AW, McGeown JG, Curtis TM, 2019. Involvement of TRPV1 and TRPV4 Channels in Retinal Angiogenesis. Investigative ophthalmology & visual science 60, 3297–3309. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shirai K, Miyajima M, Reinach PS, Yamanaka O, Sumioka T, Kokado M, Tomoyose K, Saika S, 2016. Loss of TRPV4 Function Suppresses Inflammatory Fibrosis Induced by Alkali-Burning Mouse Corneas. PLoS One 11, e0167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sumioka T, Ichikawa K, Sano H, Nambu A, Kobayashi K, Uchida K, Suzuki Y, Tominaga M, Reinach PS, Hirai SI, Jester JV, Miyajima M, Shirai K, Iwanishi H, Kao WW, Liu CY, Saika S, 2019. Sensory nerve supports epithelial stem cell function in healing of corneal epithelium in mice: the role of trigeminal nerve transient receptor potential vanilloid 4. Lab Invest 99, 210–230. [DOI] [PubMed] [Google Scholar]
- Orduna Rios M, Noguez Imm R, Hernandez Godinez NM, Bautista Cortes AM, Lopez Escalante DD, Liedtke W, Martinez Torres A, Concha L, Thebault S, 2019. TRPV4 inhibition prevents increased water diffusion and blood-retina barrier breakdown in the retina of streptozotocin-induced diabetic mice. PLoS One 14, e0212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman MI, Sullivan SA, Skuta GL, Cockrell DA, Stringham HM, Downs CA, Fornes A, Mick A, Boehnke M, Vollrath D, Richards JE, 1998. Autosomal dominant nanophthalmos (NNO1) with high hyperopia and angle-closure glaucoma maps to chromosome 11. American journal of human genetics 63, 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, Kao WW, Koziel H, Pleyer U, Reinach PS, 2008. Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium 44, 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F, 2009. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis and rheumatism 60, 3028–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TTT, Redmon SN, Yarishkin O, Winter JM, Li DY, Krizaj D, 2017. Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. J Physiol 595, 6869–6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TD, Strotmann R, 2007. TRPV4: A Multifunctional Nonselective Cation Channel with Complex Regulation, in: Liedtke WB, Heller S (Eds.), TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Boca Raton (FL). [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A, 2013. New functions of Muller cells. Glia 61, 651–678. [DOI] [PubMed] [Google Scholar]
- Reinach PS, Chen W, Mergler S, 2015a. Polymodal roles of transient receptor potential channels in the control of ocular function. Eye and vision 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinach PS, Mergler S, Okada Y, Saika S, 2015b. Ocular transient receptor potential channel function in health and disease. BMC ophthalmology 15 Suppl 1, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L, 2009. Endothelial dysfunction in glaucoma. Acta ophthalmologica 87, 4–12. [DOI] [PubMed] [Google Scholar]
- Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, Lachman RS, Wilcox WR, Reyno S, Quadrelli R, Vaglio A, Owsianik G, Janssens A, Voets T, Ikegawa S, Nagai T, Rimoin DL, Nilius B, Cohn DH, 2008. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nature genetics 40, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde BH, McLaughlin MA, Chiou LY, 1985. Existence and role of endogenous ocular melatonin. Journal of ocular pharmacology 1, 235–243. [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Frye AM, Phuong TT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY, Krizaj D, 2016. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Scientific reports 6, 30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, Krizaj D, 2014. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci 34, 15689–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Renteria RC, Liedtke W, Krizaj D, 2011. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci 31, 7089–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA, 2012. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol 302, C1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA, 2015. Damage to lens fiber cells causes TRPV4-dependent Src family kinase activation in the epithelium. Experimental eye research 140, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA, 2017. A Role for Calcium-Activated Adenylate Cyclase and Protein Kinase A in the Lens Src Family Kinase and Na,K-ATPase Response to Hyposmotic Stress. Investigative ophthalmology & visual science 58, 4447–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, 2020. TRPM3_miR-204: a complex locus for eye development and disease. Human genomics 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Sun Z, Chen G, Shang P, You G, Zhao J, Liu S, Han D, Zhou H, 2019. Matrix stiffening induces endothelial dysfunction via the TRPV4/microRNA-6740/endothelin-1 mechanotransduction pathway. Acta biomaterialia 100, 52–60. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simo R, Lois N, 2016. The progress in understanding and treatment of diabetic retinopathy. Progress in retinal and eye research 51, 156–186. [DOI] [PubMed] [Google Scholar]
- Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S, 2012. Optical Recording Reveals Novel Properties of GSK1016790A-Induced TRPV4 Channel Activity in Primary Human Endothelial Cells. Mol Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M, 2003. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278, 22664–22668. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM, 1999. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacological reviews 51, 159–212. [PubMed] [Google Scholar]
- Takai Y, Sugawara R, Ohinata H, Takai A, 2004. Two types of non-selective cation channel opened by muscarinic stimulation with carbachol in bovine ciliary muscle cells. J Physiol 559, 899–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Arner K, Ghosh F, 2017. Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Experimental eye research 154, 10–21. [DOI] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE, 2009. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoppil RJ, Cappelli HC, Adapala RK, Kanugula AK, Paruchuri S, Thodeti CK, 2016. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget 7, 25849–25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN, 2012. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Science translational medicine 4, 159ra148. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Holt DA, Willette RN, 2017. PROPERTIES OF THE TRPV4 AGONIST GSK1016790A AND the TRPV4 ANTAGONIST GSK2193874. Physiol Rev 97, 1231–1232. [DOI] [PubMed] [Google Scholar]
- Tian W, Fu Y, Garcia-Elias A, Fernandez-Fernandez JM, Vicente R, Kramer PL, Klein RF, Hitzemann R, Orwoll ES, Wilmot B, McWeeney S, Valverde MA, Cohen DM, 2009. A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci U S A 106, 14034–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen TL, Krizaj D, MacAulay N, 2017. When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor. J Physiol 595, 3287–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velilla J, Marchetti MM, Toth-Petroczy A, Grosgogeat C, Bennett AH, Carmichael N, Estrella E, Darras BT, Frank NY, Krier J, Gaudet R, Gupta VA, 2019. Homozygous TRPV4 mutation causes congenital distal spinal muscular atrophy and arthrogryposis. Neurology. Genetics 5, e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA, 2009. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389, 490–494. [DOI] [PubMed] [Google Scholar]
- Vincent F, Duncton MA, 2011. TRPV4 agonists and antagonists. Curr Top Med Chem 11, 2216–2226. [DOI] [PubMed] [Google Scholar]
- Vithana EN, Khor CC, Qiao C, Nongpiur ME, George R, Chen LJ, Do T, Abu-Amero K, Huang CK, Low S, Tajudin LA, Perera SA, Cheng CY, Xu L, Jia H, Ho CL, Sim KS, Wu RY, Tham CCY, Chew PTK, Su DH, Oen FT, Sarangapani S, Soumittra N, Osman EA, Wong HT, Tang G, Fan S, Meng H, Huong DTL, Wang H, Feng B, Baskaran M, Shantha B, Ramprasad VL, Kumaramanickavel G, Iyengar SK, How AC, Lee KY, Sivakumaran TA, Yong VHK, Ting SML, Li Y, Wang YX, Tay WT, Sim X, Lavanya R, Cornes BK, Zheng YF, Wong TT, Loon SC, Yong VKY, Waseem N, Yaakub A, Chia KS, Allingham RR, Hauser MA, Lam DSC, Hibberd ML, Bhattacharya SS, Zhang M, Teo YY, Tan DT, Jonas JB, Tai ES, Saw SM, Hon DN, Al-Obeidan SA, Liu J, Chau TNB, Simmons CP, Bei JX, Zeng YX, Foster PJ, Vijaya L, Wong TY, Pang CP, Wang N, Aung T, 2012. Genome-wide association analyses identify three new susceptibility loci for primary angle closure glaucoma. Nature genetics 44, 1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B, 2002a. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277, 13569–13577. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B, 2002b. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277, 47044–47051. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. Jama 311, 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Wen YC, Ke GJ, Sun SQ, Dong K, Wang L, Liao RF, 2018. TRPV4 regulates migration and tube formation of human retinal capillary endothelial cells. BMC ophthalmology 18, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I, 2016. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev 96, 911–973. [DOI] [PubMed] [Google Scholar]
- Wubben TJ, Besirli CG, Zacks DN, 2016. Pharmacotherapies for Retinal Detachment. Ophthalmology 123, 1553–1562. [DOI] [PubMed] [Google Scholar]
- Xu L, Tripathy A, Pasek DA, Meissner G, 1999. Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 274, 32680–32691. [DOI] [PubMed] [Google Scholar]
- Yang H, Mergler S, Sun X, Wang Z, Lu L, Bonanno JA, Pleyer U, Reinach PS, 2005. TRPC4 knockdown suppresses epidermal growth factor-induced store-operated channel activation and growth in human corneal epithelial cells. J Biol Chem 280, 32230–32237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M, Crandall A, Heurteaux C, Hartnett ME, Krizaj D, 2018a. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. The Journal of general physiology 150, 1660–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O, Phuong TTT, Lakk M, Krizaj D, 2018b. TRPV4 Does Not Regulate the Distal Retinal Light Response. Adv Exp Med Biol 1074, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD, 2009. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53, 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao PY, Gan G, Peng S, Wang SB, Chen B, Adelman RA, Rizzolo LJ, 2015. TRP Channels Localize to Subdomains of the Apical Plasma Membrane in Human Fetal Retinal Pigment Epithelium. Investigative ophthalmology & visual science 56, 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Investigators I, Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, Silverman EK, Pillai SG, 2009. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Human molecular genetics 18, 2053–2062. [DOI] [PubMed] [Google Scholar]
- Zimon M, Baets J, Auer-Grumbach M, Berciano J, Garcia A, Lopez-Laso E, Merlini L, Hilton-Jones D, McEntagart M, Crosby AH, Barisic N, Boltshauser E, Shaw CE, Landoure G, Ludlow CL, Gaudet R, Houlden H, Reilly MM, Fischbeck KH, Sumner CJ, Timmerman V, Jordanova A, Jonghe PD, 2010. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain : a journal of neurology 133, 1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]


