Abstract
Background:
Inflammatory bowel disease (IBD) refers to a group of incurable, gastrointestinal diseases, common among young adults. The purpose of this study was to describe dietary intake, self-modifications, and beliefs among adults ages 18–35 with IBD and to compare those with active versus inactive disease. National guidelines for daily intake include: 34g of fiber for males and 28g of fiber for females, 3oz of whole grains, 1,000mg of calcium, <10% of added sugars, 3 cups of dairy, 2.5 cups of vegetables, and 2 cups of fruit.
Methods:
Individuals with a diagnosis of IBD were recruited online using convenience sampling (N=147). Data included a dietary screening questionnaire, self-directed diet modifications, dietary beliefs questionnaire, and demographics. Chi-square and t-tests were used to compare those with active versus inactive disease.
Results:
The sample was predominantly female (90%) and diagnosed with Crohn’s disease (64%). Daily intake for females was 9.7 gm fiber, 0.3 oz whole grains, 683.8 gm calcium, 1.1 cups vegetables, and 0.5 cups fruit. Daily intake for males was 14.2 gm fiber, 0.4 oz whole grains, 882.9 gm calcium, 1.4 cups vegetables, and 0.5 cups fruit. Participants most often modified fiber (73%), fruits and vegetables (71%), grains (67%), and dairy (66%) due to their IBD. 83% believed that modifying their diet could reduce IBD symptoms.
Conclusions:
Both men and women with IBD struggle to meet the national guidelines for intake of fiber, whole grains, fruits, and vegetables. The majority reported modifying their dietary intake due to IBD and expressed belief that diet could reduce symptoms.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, diet
Graphical Abstract
The purpose of this study was describe dietary intake, self-modifications, and beliefs among adults ages 18–35 with inflammatory bowel disease and to compare those with active versus inactive disease. Individuals with inflammatory bowel disease commonly modified their diet, especially individuals who had active disease. The majority are modifying their diet without the guidance of a dietitian or nutritionist.
Introduction
Inflammatory Bowel Disease (IBD) refers to a group of chronic inflammatory diseases of the gastrointestinal system. IBD is a heterogeneous disease which is typically classified into ulcerative colitis and Crohn’s disease. The disease course varies with individuals experiencing periods of active disease and remission. IBD is a growing public health concern as the incidence has been increasing worldwide (1). IBD is associated with gastrointestinal symptoms (e.g., abdominal cramps, abdominal pain, and diarrhea) and inflammation of the gastrointestinal tract (2,3). Since IBD affects the GI system, patients often question how modifying their diet may influence IBD symptoms (4–6). Some studies have shown the benefits of diet modification, through altering food intake, on symptom reduction and quality of life in IBD patients (5,7–9). In fact, children with Crohn’s disease on 8-weeks of exclusive enteral nutrition, a nutritionally complete liquid diet, demonstrated greater likelihood of remission compared to children who do not use exclusive enteral nutrition (10). While studies demonstrate the effectiveness of dietary interventions (i.e., Specific Carbohydrate Diet or low Fermentable Oligo-, Di-, Mono-saccharides And Polyols [FODMAP] diet) in reducing symptoms, few studies have demonstrated changes in markers of inflammation (11–16). Although dietary recommendations have been published for adults with IBD, recommendations primarily report insufficient evidence to recommend dietary changes and are based on expert opinions (17, 18). Therefore, providers have been hesitant to provide specific recommendations due to the complex role of diet in IBD and the lack of a dietary gold standard (5,19).
Even without clear dietary recommendations, individuals with IBD anecdotally report diet modifications. Adults with IBD are interested in diet since patients believe modifying their diet could decrease IBD symptoms and improve health (20–23). Yet, dietary information is reported as the least adequately addressed topic among individuals recently diagnosed and with longstanding disease (>10 years) (24,25). Furthermore, even though diet modification is commonly reported in clinical settings, few studies have characterized dietary intake (i.e., grams of fiber, cups of fruits and vegetables) among adults with IBD (26). Characterization of dietary intake is the first step towards better understanding nutrient deficiencies and developing personalized dietary recommendations for patients.
This study describes the current dietary intake, modifications, and beliefs among adults ages 18–35 with IBD and compares dietary modifications between individuals with active and inactive disease. Dietary research among individuals with IBD is limited and primarily focuses on patients beliefs about specific types of food (20,22,23,). Since dietary modification is common, there is a need to describe the current dietary intake and modifications among individuals with IBD in the United States. Dietary intake will be compared to the United States Department of Agriculture (USDA) recommendations. These recommendations indicate 34g of fiber for males and 28g of fiber for females, 3oz of whole grains, 1,000mg of calcium, <10% of added sugars, 3 cups of dairy, 2.5 cups of vegetables, and 2 cups of fruit (27).
This study addresses three research questions:
Among individuals with IBD, does dietary intake (fiber, whole grains, calcium, sugar, dairy, vegetables, and fruit) differ between those with active and inactive disease?
Are individuals with active IBD more likely to modify their diet than individuals with inactive IBD?
Do dietary beliefs differ between individuals with active and inactive IBD?
Methods
Study Design
A cross-sectional study design was conducted to describe the current dietary intake, modifications, and beliefs among adults ages 18–35 with IBD. This study was approved by the Michigan State University Institutional Review Board.
Participants
Individuals were recruited through ResearchMatch, Facebook, or referral from a friend between January and February 2018. Individuals were screened for participation if they had a diagnosis of ulcerative colitis or Crohn’s disease and were between the ages of 18–35. Inclusion criteria were: currently prescribed medication to manage their IBD, living in the United States, understand written English, and have access to the internet. Those hospitalized within the past month or currently pregnant were excluded.
Procedures
All data was collected using Qualtrics. Those interested in participating reviewed an online consent form describing the purpose of the study, study procedures, and voluntary participation. Participants first completed required screening questions to assess for eligibility. These questions were automatically scored, and those meeting inclusion/exclusion criteria continued to the survey.
Measures
Participant Characteristics.
Participants were asked about their age, gender, and marital status. Participants reported type of IBD (ulcerative colitis or Crohn’s disease), time since diagnosis, and current medications type (aminosalicylates, biologics, corticosteroids, and/or immunomodulators). Disease activity was assessed using the Manitoba Inflammatory Bowel Disease Index (MIBDI), where participants report their disease activity on a six-point scale ranging from “constantly active, giving me symptoms every day” or “I was well in the past 6 months, what I consider a remission or absence of disease.” A cut-off of 1–4 for active disease and 5–6 for inactive disease was used based on existing literature (28).
Dietary Intake.
Dietary Intake was assessed by the 26-item National Health and Nutrition Examination Survey (NHANES) and National Cancer Institute dietary screener questionnaire. Participants reported the frequency of food and drink consumption over the past month as the number of times per day, week, or month. Estimates for intake of food and nutrient groups were calculated using publicly available scoring algorithms (29). The algorithms were developed based on 24-hour dietary recalls (30) and account for participant age and sex. Food and nutrient groups include per day measures of: fiber (gm), calcium (mg), whole grains (ounce equivalents), total added sugars (tsp equivalents), dairy (cup equivalents), fruits and vegetables (cup equivalents), and added sugars from sugar-sweetened beverages (tsp equivalents).
Dietary Modification.
After each item on the Dietary Screener Questionnaire, (7,30) participants responded to the following statement: “I alter my intake of [name of food] due to my IBD” on a Likert scale (0=never to 5=always). Responses were then categorized into subscales (fruit and vegetables, dairy, whole grains, added sugars, sugar-sweetened beverages, meat, and dietary fiber) based on the scoring algorithms. In addition, the most frequently modified individual foods were reported. Participants who reported never or rarely were categorized as no diet modification; participants responding sometimes, often, or always were categorized as modifying their diet.
Dietary Beliefs.
Dietary beliefs were assessed using investigator-developed questions, which were pre-tested prior to survey use. Participants were asked questions such as “Do you think that diet modification can reduce IBD symptoms?” and “Does your healthcare provider think that diet modification can reduce IBD symptoms?” Participants could respond “yes”, “no”, or “don’t know”. In addition, participants were asked if they could identify foods that made their symptoms better or worse and if they had ever seen or were currently seeing a dietitian/nutritionist for their IBD.
Statistical Analysis
Data were analyzed using STATA 15.1 and SAS 14.1. Demographic statistics were calculated using means and standard deviations for continuous variables, while counts and percentages for categorical variables. Dietary intake was calculated in SAS using scoring procedures published by the National Cancer Institute (29). Data were reported as means and standard deviations. Comparisons were made between individuals with active and inactive disease using t-tests.
Dietary modification was scored as a dichotomous variable and presented as the percentage of individuals modifying or not modifying their diet. χ2 was used to assess the relationship between diet modification and disease activity. Dietary beliefs were presented as counts and percentages.
Results
Participant Characteristics
One-hundred and forty-seven individuals met inclusion criteria. The sample was predominantly female (90%), with a mean age of 28.8 (SD = 4.6), and diagnosed with Crohn’s disease (64%). The majority of participants were on a biologic medication (64.6%), and 38.1% took more than one medication type. Based on the MIBDI scale, 75% of the sample reported active disease (Table 1).
Table 1:
Demographics of Individuals with Inflammatory Bowel Diseases, ages 18–35
| M | SD | |
|---|---|---|
| Age | 28.8 | 4.6 |
| Time since diagnosis (in years) | 7.6 | 5.6 |
| N | % | |
| Female sex | 132 | 89.8 |
| Single | 79 | 53.7 |
| Type of IBD | ||
| Ulcerative Colitis | 53 | 36 |
| Crohn’s Disease | 94 | 64 |
| Medication Type | ||
| Aminosalicylates | 51 | 34.5 |
| Biologics | 95 | 64.6 |
| Corticosteroids | 22 | 15.0 |
| Immunomodulators | 33 | 22.5 |
| Manitoba IBD Index | ||
| Active disease | 110 | 74.8 |
| Inactive disease | 37 | 25.2 |
Note: some individuals were using multiple medications
Dietary Intake
Dietary intake was reported for individuals who did not have missing data on the individual scale items. The estimated dietary intake for males (n=12) each day was 14.2 gm of fiber, 0.4 oz of whole grains, 882.9 gm of calcium, 1.4 cups of vegetables, and 0.5 cups of fruit. Estimated dietary intake for females (n=113) each day was 9.7 gm of fiber, 0.3 oz of whole grains, 683.8 gm of calcium, 1.1 cups of vegetables, and 0.5 cups of fruit. Females with active compared to inactive disease differed on predicted intake of fiber (p=0.006), calcium (p=0.013), and fruit and vegetable intake (all <0.01). See Table 2 for additional dietary intake information.
Table 2:
Predicted dietary intake for males and females with inflammatory bowel disease
| Females | ||||||
|---|---|---|---|---|---|---|
| USDA Recommendations | Males (N=12) |
Females (N=113) |
Active disease (N=82) |
Inactive disease (N=30) |
Active vs. Inactive disease | |
| M (SD) | M (SD) | M (SD) | M (SD) | p-value | ||
| Fiber (gm) | 34 M / 28 F | 18.2 (3.3) | 14.3 (2.8) | 13.9 (2.4) | 15.5 (3.3) | 0.006 |
| Predicted intake of calcium (mg) | 1,000 | 1129.3 (257.9) | 853.3 (104.6) | 838.8 (88.1) | 894.5 (134.8) | 0.013 |
| Whole grains (ounce equivalents) | 3 | 0.92 (0.5) | 0.6 (0.3) | 0.6 (0.3) | 0.7 (0.4) | 0.16 |
| Total added sugars (tsp equivalents) | <10% | 15.8 (2.5) | 15.9 (5.3) | 16.2 (5.7) | 15.0 (4.1) | 0.29 |
| Dairy (cup equivalents) | 3 | 1.8 (0.7) | 1.4 (0.4) | 1.4 (0.3) | 1.5 (0.5) | 0.20 |
| Fruits and vegetables including legumes and French fries (cup equivalents) | 4.5 | 2.7 (0.7) | 2.2 (0.5) | 2.1 (0.4) | 2.4 (0.6) | 0.003 |
| Vegetables including legumes and French fries (cup equivalents) | 2.5 | 1.7 (0.5) | 1.4 (0.2) | 1.2 (0.2) | 1.5 (0.3) | 0.0001 |
| Fruits and vegetables including legumes and excluding French fries (cup equivalents) | 4.5 | 2.5 (0.7) | 2.1 (0.5) | 2.0 (0.5) | 2.3 (0.6) | 0.01 |
| Vegetables including legumes and excluding French fries (cup equivalents) | 2.5 | 1.6 (0.5) | 1.2 (0.3) | 1.2 (0.2) | 1.4 (0.3) | 0.0001 |
| Fruits (cup equivalents) | 2 | 0.9 (0.4) | 0.8 (0.3) | 0.8 (0.3) | 0.9 (0.3) | 0.21 |
| Added sugars from sugar-sweetened beverages (tsp equivalents) | <10% | 0.2 (0.1) | 7.1 (4.7) | 7.5 (4.9) | 6.2 (3.8) | 0.19 |
Note: All are presented as predicted intake, per day; dietary analysis was performed on individuals with complete dietary intake responses
Dietary Modifications
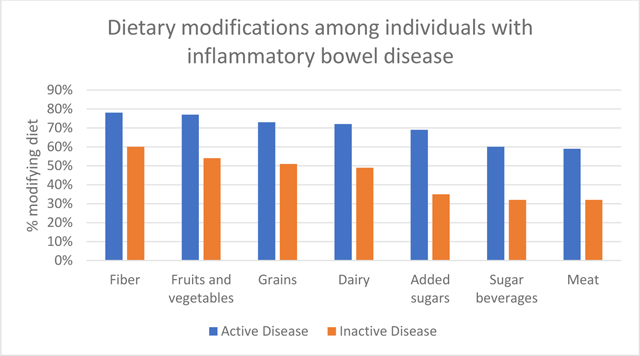
Dietary modification was common within the sample with the majority of individuals (83%) modifying their diet due to IBD (Table 3). Participants most commonly reported modifying intake of fiber (73%), fruits and vegetables (71%), grains (67%), and dairy (66%). Dietary modification was greater among individuals with active disease compared to inactive disease (all p<0.05). The individual food items most frequently modified included: salad (72%), popcorn (72%), other vegetables (67%), fruit (66%), and pizza (62%).
Table 3:
Dietary modifications among individuals with inflammatory bowel disease based on food subgroups
| Total Sample | Active Disease | Inactive Disease | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| Modify Fiber | 0.026 | ||||||
| Yes | 108 | 73.5 | 86 | 78.2 | 22 | 59.5 | |
| No | 39 | 26.5 | 24 | 21.8 | 15 | 40.5 | |
| Modify Fruits and Vegetables | 0.007 | ||||||
| Yes | 105 | 71.4 | 85 | 77.3 | 20 | 54.1 | |
| No | 42 | 28.6 | 25 | 22.7 | 17 | 46.0 | |
| Modify Grains | 0.016 | ||||||
| Yes | 99 | 67.4 | 80 | 72.7 | 19 | 51.4 | |
| No | 48 | 32.7 | 30 | 27.3 | 18 | 48.7 | |
| Modify Dairy | 0.01 | ||||||
| Yes | 97 | 66.0 | 79 | 71.8 | 18 | 48.7 | |
| No | 50 | 34.0 | 31 | 28.2 | 19 | 51.4 | |
| Modify Added Sugar | 0.001 | ||||||
| Yes | 89 | 60.5 | 76 | 69.1 | 13 | 35.1 | |
| No | 58 | 39.5 | 34 | 31.0 | 24 | 64.9 | |
| Modify Sugar Beverages | 0.004 | ||||||
| Yes | 78 | 53.1 | 66 | 60.0 | 12 | 32.4 | |
| No | 69 | 46.9 | 44 | 40.0 | 25 | 67.6 | |
| Modify Meat | 0.005 | ||||||
| Yes | 77 | 52.4 | 65 | 59.1 | 12 | 32.4 | |
| No | 70 | 47.6 | 45 | 40.9 | 25 | 67.6 | |
Note: p-value comparing active vs. inactive disease.
Dietary Beliefs
Sixty-nine percent of participants reported that diet modification could reduce IBD symptoms (Table 4). Only 47.6% of participants reported their healthcare provider thought diet modification could reduce IBD symptoms. Twenty-five percent reported ever visiting a dietitian and/or nutritionist for their IBD and only 5% were currently seeing a dietitian and/or nutritionist. There were no differences in dietary beliefs based on disease activity.
Table 4:
Beliefs about diet among individuals with inflammatory bowel disease
| Total Sample | Active Disease | Inactive Disease | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| Have you seen a dietitian and/or nutritionist for your IBD? | 0.72 | ||||||
| Yes | 36 | 24.5 | 27 | 24.6 | 9 | 24.3 | |
| No | 109 | 74.2 | 82 | 74.2 | 27 | 73.0 | |
| Are you currently seeing a dietitian and/or nutritionist for your IBD? | 0.98 | ||||||
| Yes | 9 | 6.2 | 7 | 6.4 | 2 | 5.4 | |
| No | 136 | 93.8 | 103 | 93.6 | 35 | 94.6 | |
| Do YOU think that diet modification can reduce IBD symptoms? | 0.6 | ||||||
| Yes | 102 | 69.4 | 78 | 70.9 | 24 | 64.9 | |
| No | 21 | 14.3 | 16 | 14.6 | 5 | 13.5 | |
| Did not respond | 24 | 16.3 | 16 | 14.6 | 8 | 21.6 | |
| Does your healthcare provider think that diet modification can reduce IBD symptoms? | 0.6 | ||||||
| Yes | 70 | 47.6 | 53 | 48.2 | 17 | 46.0 | |
| No | 39 | 26.5 | 27 | 24.6 | 12 | 32.4 | |
| Did not respond | 38 | 25.9 | 30 | 27.3 | 8 | 21.6 | |
| Are you able to identify foods that make your IBD worse? | 0.18 | ||||||
| Yes | 124 | 87.3 | 94 | 89.5 | 30 | 81.1 | |
| No | 18 | 12.7 | 11 | 10.5 | 7 | 18.9 | |
| Are you able to identify foods that make your IBD better? | 0.16 | ||||||
| Yes | 55 | 41.0 | 38 | 37.6 | 17 | 51.5 | |
| No | 79 | 59.0 | 63 | 62.4 | 16 | 48.5 | |
Note: p-value comparing active vs. inactive disease.
Discussion
Among an online sample of primarily females ages 18–35 with IBD, food intakes based on a dietary screener questionnaire did not meet USDA recommendations (27). Participants reported commonly modifying foods due to their IBD, with the most commonly modified foods being fiber, fruits and vegetables, and grains. Individuals were more likely to modify their diet during active disease.
Individuals with IBD often do not obtain the necessary nutrients. For instance, the USDA recommended an intake of 34g of fiber for males and 28g of fiber for females per day (27). Participants in the current study had a predicted fiber intake of 18g for males and 14g for females, which is similar to actual fiber intake in the general U.S. population (17g) (31,32). A Canadian study found significant micronutrients deficits (Vitamins C, D, thiamin, and niacin) among individuals with Crohn’s disease compared to a representative sample of Canadians (26). Increased dietary fiber intake was found in patients with Crohn’s disease compared to the representative sample of Canadians (26) whereas an Italian cohort found decreased dietary fiber, increased lipids, and increased calories among IBD patients compared to controls (33). Differences in fiber intake may be due to the percentage of individuals following a low-residue or low-fiber diet. Such variations demonstrate a need to characterize the current dietary intake of individuals with IBD prior to providing dietary recommendations.
Dietary modification was common among the sample, with individuals in active disease more likely to modify their diet than individuals in inactive disease. Yet, during inactive disease, one-third to two-thirds of participants still engaged in some type of dietary modification. A Dutch study reported 76.5% of their sample of individuals with IBD omitted foods to reduce disease symptoms (34). Specifically, participants felt that omitting foods reduced abdominal pain/cramps and diarrhea. Another study reported that 66.8% of IBD patients avoid certain food to prevent a relapse (23). Individuals with IBD focus on eliminating foods that worsen symptoms and few individuals focus on incorporating foods that improve symptoms (35). In the current sample, 41% of participants were able to identify foods that made their IBD better. This points to a possible need to reframe nutritional instructions.
Within this sample, 24.8% of individuals visited a dietitian or nutritionist and only 6% were currently receiving services from a dietitian or nutritionist. This is similar to findings within a Dutch cohort in which 25.3% of individuals obtained nutrition information from a dietitian (34). Although few individuals have obtained guidance from a dietitian or nutritionist, the majority of participants in the current study reported modifying their diet. This is especially concerning, as recent research in an Italian cohort demonstrated an association between self-prescribed dietary restrictions and abnormal bone density (DXA) scans (36). Furthermore, individuals with IBD are at risk for nutrient deficiencies due to bowel malabsorption (37). Future research could target those with self-prescribed dietary modifications in the absence of a dietitian/nutritionist. Such modifications could have a social or emotional component, as well as physical discomfort. With advanced knowledge, more tailored interventions could be designed to address the rationale for self-prescribed changes. In the meantime, providers should encourage patients to enlist the help of a Registered dietitian/nutritionist that specialists in nutritional therapy for IBD in order to prevent nutritional deficits. A registered dietitian can help patients identify foods that affect their symptoms, prevent and help with recovery from malnutrition, nutrient deficiencies and the fear of eating that often accompanies IBD.
Limitations of this study include that the sample was recruited online using convenience sampling and may not be representative of individuals in clinic settings. Specifically, the small sample size of males limited understanding of the dietary intake specific to males. Future studies with a larger sample size can better characterize differences based on sex, disease activity, and other factors which may influence diet. While a validated dietary screener was used to predict food intake, the use of a three-day dietary intake would provide more detailed information on both micro and macronutrients.
Conclusions
This study addressed the pressing clinical challenge of patient dietary modification by providing new information on current dietary intake, modifications, and beliefs among individuals with IBD living in the United States. Findings indicated the majority of individuals are modifying their diet without the guidance of a dietitian or nutritionist. Participants had lower dietary intake of fiber, whole grains, fruits and vegetables than recommendations. Larger studies would be beneficial in assessing dietary intake for both men and women. If sex differences were found, tailored interventions could be designed and tested for their effectiveness in curbing symptoms and nutritional concerns. Longitudinal studies examining dietary intake over time may also provide additional insights into factors that influence dietary intake. This work provides the foundation for future work in the area of dietary intervention trials to determine the best diet composition for individuals with IBD, and perhaps unique components for men versus women.
Acknowledgements:
This work was supported by a grant from Sigma Theta Tau International. Kendra J. Kamp was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases, T32DK007742. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
Institution where the work was performed:
Michigan State University, College of Nursing, 1355 Bogue St, East Lansing, MI 48824
Transparency Declaration
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported. The reporting of this work is compliant with STROBE guidelines. The lead author affirms that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
Contributor Information
Kendra Kamp, University of Washington, Seattle, WA.
Breanna Pennings, Eagle Global Scientific, Atlanta, GA.
Diane Javelli, University of Washington, Seattle, WA.
Gwen Wyatt, Michigan State University, East Lansing, MI.
Barbara Given, Michigan State University, East Lansing, MI.
References
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Kamp K, Dudley-Brown S, Heitkemper M, Wyatt G, Given B. Symptoms among emerging adults with inflammatory bowel disease: a descriptive study. Res Nurs Health. 2020;43:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crohn’s and Colitis Foundation of America. The facts about inflammatory bowel diseases. 2015.
- 4.Knight-Sepulveda K, Kais S, Santaolalla R, Abreu MT. Diet and inflammatory bowel disease. Gastroenterol Hepatol. 2015;11:511–520. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D, Albenberg L, Compher C, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterol. 2015;148:1087–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong C, Harris PJ, Ferguson LR. Potential benefits of dietary fibre intervention in Inflammatory Bowel Disease. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlebois A, Rosenfeld G, Bressler B. The impact of dietary interventions on the symptoms of Inflammatory Bowel Disease: A systematic review. Crit Rev Food Sci Nutr. 2016;56:1370–1378. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekaran A, Groven S, Lewis JD, et al. An Autoimmune Protocol Diet Improves Patient-Reported Quality of Life in Inflammatory Bowel Disease. Crohn’s & Colitis 360. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McVeigh L, Payne A. Inducing remission in paediatric Crohn’s disease using nutritional therapies – A systematic review. J Hum Nurt Diet. 2020;33(2):170–186. [DOI] [PubMed] [Google Scholar]
- 10.Adamji M, Day AS. An overview of the role of exclusive enteral nutrition for complicated Crohn’s disease. Intest Res. 2019;17:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohn Colitis. 2009;3:8–14. [DOI] [PubMed] [Google Scholar]
- 12.Joyce T, Staudacher H, Whelan K, Irving P, Lomer M. PWE-092 Symptom response following advice on a diet low in short-chain fermentable carbohydrates (fodmaps) for functional bowel symptoms in patients with IBD. Gut. 2014;63:A164. [Google Scholar]
- 13.de Silva PS, Ahrens S, Cole W, Korzenik JR. Response to the Specific Carbohydrate Diet amongst individuals with Inflammatory Bowel Disease - a survey of 122 patients Digestive Disease Week; 2015; Washington DC. [Google Scholar]
- 14.Cox SR, Lindsay JO, Fromentin S, et al. Effects of Low-FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterol. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Keshteli AH, Madsen KL, Dieleman LA. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients. 2019;11:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comeche JM, Caballero P, Gutierrez-Hervas A, et al. Enteral Nutrition in Patients with Inflammatory Bowel Disease. Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients. 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine A, Rhodes JM, Lindsay JO, et al. Dietary Guidance for Patients with Inflammatory Bowel Disease from the International Organization for the Study of Inflammatory Bowel Disease. Clinical Gastroenterology & Hepatology. 2020;18(6):1381–1392. [DOI] [PubMed] [Google Scholar]
- 18.Limketkai BN, Gordon M, Mutlu EA, De Silva PS, Lewis JD. Diet Therapy for Inflammatory Bowel Diseases: A Call to the Dining Table. Inflammatory Bowel Diseases. 2020;26(4):510–514. [DOI] [PubMed] [Google Scholar]
- 19.Holt DQ, Strauss BJ, Moore GT. Patients with inflammatory bowel disease and their treating clinicians have different views regarding diet. J Hum Nurt Diet. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Jowett SL, Seal CJ, Phillips E, Gregory W, Barton JR, Welfare MR. Dietary beliefs of people with ulcerative colitis and their effect on relapse and nutrient intake. Clin Nutr. 2004;23:161–170. [DOI] [PubMed] [Google Scholar]
- 21.Kinsey L, Burden S. A survey of people with inflammatory bowel disease to investigate their views of food and nutritional issues. Eur J Clin Nutr. 2016;70:852–854. [DOI] [PubMed] [Google Scholar]
- 22.Limdi JK, Aggarwal D, McLaughlin JT. Dietary practices and beliefs in patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Zallot C, Quilliot D, Chevaux JB, et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:66–72. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein KI, Promislow S, Carr R, Rawsthorne P, Walker JR, Bernstein CN. Information needs and preferences of recently diagnosed patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:590–598. [DOI] [PubMed] [Google Scholar]
- 25.Wong S, Walker JR, Carr R, et al. The information needs and preferences of persons with longstanding inflammatory bowel disease. Can J Gastroenterol. 2012;26:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor L, Almutairdi A, Shommu N, et al. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USDA. Dietary Guidelines for Americans 2015–2020. 8 ed2015. [Google Scholar]
- 28.Clara I, Lix LM, Walker JR, et al. The Manitoba IBD Index: Evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009;104:1754–1763. [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. Dietary Screener Questionnaires (DSQ) in the NHANES 2009–10: SAS Pograms. https://epi.grants.cancer.gov/nhanes/dietscreen/programs.html. Published 2018. Accessed.
- 30.Thompson FE, Midthune D, Kahle L, Dodd KW. Development and Evaluation of the National Cancer Institute’s Dietary Screener Questionnaire Scoring Algorithms. J Nutr. 2017;147:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S, Lanlan W, Wang W, Li N, Wiaoyan W. Trends in Dietary Nutrients by Demographic Characteristics and BMI among US Adults, 2003–2016. Nutrients. 2019;11(11):2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD). What We Eat in America, NHANES 2015–2016, individuals 2 years and over (excluding breast-fed children), day 1. Available: www.ars.usda.gov/nea/bhnrc/fsrg
- 33.Principi M, Losurdo G, Iannone A, et al. Differences in dietary habits between patients with inflammatory bowel disease in clinical remission and a healthy population. Ann Gastroenterol. 2018;31:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries JHM, Dijkhuizen M, Tap P, Witteman BJM. Patient’s Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig Dis Sci. 2019;37:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58:1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larussa T, Suraci E, Marasco R, Imeneo M, Abenavoli L, Luzza F. Self-Prescribed Dietary Restrictions are Common in Inflammatory Bowel Disease Patients and Are Associated with Low Bone Mineralization. Medicina. 2019;55:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilby K, Mathias H, Boisvenue L, Heisler C, Jones JL. Micronutrient Absorption and Related Outcomes in People with Inflammatory Bowel Disease: A Review. Nutrients. 2019;11:1388. [DOI] [PMC free article] [PubMed] [Google Scholar]


