Abstract
Introduction
Radiolabeling of stem cells with a positron emitting radioisotope represents a major advancement in regenerative biotherapy enabling non-invasive imaging. To assess the value of such an approach in a clinically relevant scenario, the tolerability and therapeutic aptitude of [89Zr]zirconium-p-isothiocyanatobenzyl-desferrioxamine ([89Zr]Zr-DBN) labeled human cardiopoietic stem cells (CPs) were evaluated in a model of ischemic heart failure.
Methods and Results
[89Zr]Zr-DBN based radiolabeling of human CPs yielded [89Zr]Zr-DBN-CPs with radioactivity yield of 0.70 ± 0.20 MBq/106 cells and excellent label stability. Compared to unlabeled cell counterparts, [89Zr]Zr-DBN-CPs maintained morphology, viability, and proliferation capacity with characteristic expression of mesodermal and pro-cardiogenic transcription factors defining the cardiopoietic phenotype. Administered in chronically infarcted murine hearts, [89Zr]Zr-DBN-CPs salvaged cardiac pump failure, documented by improved left ventricular ejection fraction not inferior to unlabeled CPs and notably superior to infarcted hearts without cell treatment.
Conclusion
The present study establishes that [89Zr]Zr-DBN labeling does not compromise stem cell identity or efficacy in the setting of heart failure, offering a non-invasive molecular imaging platform to monitor regenerative biotherapeutics post-transplantation.
Keywords: Cardiopoietic stem cells, Imaging, Radiolabeling, Positron-Emission Tomography, Myocardial infarction, Regenerative medicine
1. Introduction
Stem cell-based regenerative therapies have advanced into human testing for the management of heart failure [1–4]. Refined assessment requires stem cell fate monitoring following delivery [5–8]. In this regard, nuclear medicine compatible tools would offer opportunities for non-invasive imaging expanding the knowledge base pertinent to the behavior of transplanted stem cells.
To this end, there is increased focus on using the positron emitting radionuclide zirconium-89 (T1/2 = 78.4 h) for direct radiolabeling of cells [9–12]. This approach allows for an expanded follow-up compared to shorter lived positron emitter alternatives such as [18F]-FDG [13–15]. There are currently two major strategies to radiolabel cells with Zr-89 using either: (i) cell-permeable [89Zr]Zr-oxine or (ii) [89Zr]zirconium-p-isothiocyanatobenzyl-desferrioxamine ([89Zr]Zr-DBN). [89Zr]Zr-DBN covalently binds with the primary amine groups of membrane proteins based on subcellular protein fractionation study [9]. Further assessment of the utility of [89Zr]Zr-DBN based radiolabeling would require testing in the setting of disease.
Lineage guidance, known as cardiopoiesis, promotes cardioreparative potential in otherwise refractory heart failure following myocardial infarction [16–18]. Cardiopoietic stem cells (CPs) have reached advanced clinical development, and have been tested in the largest ischemic heart failure cohort to date [19–22]. The present study evaluates the effect of [89Zr]Zr-DBN radiolabeling on the cardiopoietic phenotype, biodistribution, and therapeutic aptitude.
2. Materials and Methods
2.1. Cardiopoietic stem cell labeling with synthesized [89Zr]Zr-DBN
89Zr4+ was produced via the 89Y(p,n)89Zr nuclear reaction using a solution or solid target on a cyclotron, and isolated in K2HPO4/ KH2PO4 buffer [23, 24]. Radiosynthesis of [89Zr]Zr-DBN followed established protocols [9]. Human mesenchymal stem cells were isolated from bone marrow aspirates and cultured at 37°C in Advanced Minimum Essential Medium (AMEM) supplemented with 5% human platelet lysate. Cardiopoietic stem cells (CPs) were generated by exposing mesenchymal stem cells to a cardiogenic cocktail of growth factors - Transforming Growth Factor beta 1 (TGF-β1, 2.5 ng/ml), Bone Morphogenetic Protein 4 (BMP-4, 5 ng/ml), Activin-A (5 ng/ml), Fibroblast Growth Factor 2 (FGF-2, 10 ng/ml), InterLeukin 6 (IL-6, 100 ng/ml), Human Thrombin Factor IIa (h-α-thrombin, 1 U/ml), Insulin-like Growth Factor 1 (IGF-1, 50 ng/ml) and/or retinoic acid (1 μM) [17]. The cell labeling reaction was performed using 6 × 106 CPs in 500 μL Hanks balanced salt solution buffered with 10 mM HEPES (H-HBSS) at pH 7.5. To this, ~100 μL [89Zr]Zr-DBN (~6 MBq) was added [9]. The final pH of the reaction mixture was adjusted to pH 7.5 and the mixture was incubated at 37°C for 30 min using a thermomixer at 550 rpm. Free 89Zr or unreacted [89Zr]Zr-DBN/DBN were separated from radiolabeled cells by triple washing/centrifugation with H-HBSS. The final labeling efficiency was calculated in triplicates from the radioactivity bound to cells after triple cell washing with H-HBSS.
2.2. Cell proliferation and viability
With unlabeled CPs serving as control, the effect of radiolabeling on cellular proliferation and viability of CPs was assessed by the CyQUANT DNA content assay (Thermo Fisher, Waltham, MA, USA) and trypan blue exclusion assay at 48 h. For the CyQUANT DNA content assay, 30,000 of either unlabeled CPs cells or [89Zr]Zr-DBN-CPs counterparts were plated on two 96-well plates (n=3 for each group). Plates were allowed to incubate at 37 °C for ~16 h to allow cells to attach. Medium from one of the two 96 well plate was removed and cells in the microplate frozen and stored at −70 °C (designated T = 0). Cells in the second 96-well plate were allowed to grow at 37 °C for additional 48 h. At the end of 48 h, the medium was removed and the cells frozen and stored at −70 °C (designated T = 48 h). After the plates were allowed to decay for 10 half-lives (~30 days), the plates were thawed and processed as per instructions for CyQUANT assay. Fluorescence from each well was measured using a fluorescent microplate reader (480 nm excitation and 520 nm emission). The fluorescence values for T = 0 and T = 48 h was used to calculate the doubling time based using the following formula: DT (h) = T × ln(2) / ln (Nf / Ni), where DT is time needed to double a cell population, T is the incubation time in hours, i.e. 48 h, Ni is the fluorescence value at T = 0 and Nf is the fluorescence value at T = 48 h. The viability assay, based on trypan blue exclusion, was performed in accordance with current European Union guidelines and International Atomic Energy Agency recommendations [25]. In the assay, 10 μL of trypan blue dye 0.4% solution (Biorad, Hercules, CA, USA) was added to 10 μL of the labeled [89Zr]Zr-DBN CP cell suspension and gently mixed. Ten μL of the resultant cell suspension was pipetted onto counting slides and the percentage of live cells was determined using a TC20 automated cell counter. Unlabeled CPs served as control.
The effect of radiolabeling on apoptosis, viability and cytotoxicity of CPs was assessed using ApoTox-Glo Triplex Assay Kit, Promega Corporation, Madison, WI, USA. Unlabeled CPs served as control. A known number of unlabeled and [89Zr]Zr-DBN CP cells (approximately 104 /well) were plated in a 96-well culture plate. The culture medium was replaced daily and maintained at 37°C in a CO2 incubator. As positive controls for viability, cytotoxicity and apoptosis assays, unlabeled CPs were incubated with 30 μg/mL digitonin for 30 min for the viability and cytotoxicity assays, while unlabeled CPs were incubated with 2 μM staurosporine for 16 h for the caspase 3/7 dependent apoptosis assay.
2.3. Cellular efflux of radioactivity
[89Zr]Zr-DBN-CPs (0.3 × 106 cells) were plated into wells of a six-well culture plate. Medium was replaced daily for 2 days, and radioactivity in the replaced medium counted.
2.4. Cardiopoietic markers
[89Zr]Zr-DBN-CPs and unlabeled CPs were seeded on 6-well plates and cultured in AMEM with 5% PLT Max, 1% Glutamax, 1 unit/mL heparin, and 1% penicillin-streptomycin. After two days of culture, cells were counter-stained with 4’,6-diamidino-2-phenylindole (DAPI) and immunostained with primary antibodies against myocyte enhancer factor 2C (MEF2C, 1:400, Cell Signaling Technologies, TX, USA), T-box transcription factor 5 (TBX5, 1:5000, Abcam, MA, USA) and mesoderm posterior bHLH transcription factor 1 (MESP1,1:250, Novus Bio, CO, USA) for cardiopoietic phenotyping [26]. The secondary antibody against MEF2C and TBX5 was conjugated with Alexa Fluor 555 (Excitation (Ex): 555 nm, Emission (Em): 590 nm), and a secondary antibody against MESP1 was conjugated with Alexa Fluor 488 (Ex: 496 nm, Em: 519 nm). Fluorescent images were acquired using either a Zeiss Axioplan epifluorescence wide field microscope (Carl Zeiss AG, Oberkochen, Germany) using a 10X non-immersion objective (numerical aperture 0.3) with an HBO mercury lamp for DAPI, Alexa Fluor 488 and Alexa Fluor 555 channels or with LSM780 Confocal microscope (40X Water; numerical aperture 1.2). Expression of MEF2C, TBX5 and MESP1 in [89Zr]Zr-DBN-CPs was quantified using Zen software (Carl Zeiss) and compared with unlabeled CPs (control) (n > 200 cells from independent experiments) [17, 26].
2.5. Cell delivery in infarcted heart
Animal study protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee. All procedures on live animals were performed under isoflurane (2–3%) general anesthesia. Myocardial infarction was surgically imposed in 8–10 week old, male immunodeficient mice (Hsd: Athymic Nude_Foxn1nu, Envigo, Indianapolis, IN, USA) [27]. The left anterior descending coronary artery was ligated with a 9–0 suture for 70 min followed by reperfusion. One-month post-surgery, infarcted mice were randomized into three groups, using a 1:1:1 design, received saline (n = 6), or CPs with (n = 6) or without (n = 6) [89Zr]Zr-DBN labeling, and were prospectively followed for 1 month. In cell-treated cohorts, a total of 600,000 CPs per heart were epicardially delivered (in 15 μL of phosphate buffered saline) into 6 sites of the left ventricular (LV) anterior wall (100,000 cells/site/2.5 μL) using a 31-gauge needle and 1 mL micro-syringe connected to a programmable syringe pump (Pump Pico Plus Elite; Harvard Apparatus, Holliston, MA, USA) [28]. In infarcted animals assigned to saline treatment alone, the same procedure was applied to inject saline without cells. In addition to 18 animals with 1-month follow-up, 6 infarcted animals were enrolled for ex vivo validation at 2 days after intramyocardial delivery of [89Zr]Zr-DBN-CPs. Prophylactically, all animals undergoing thoracotomy were administered perioperative analgesics through a combination of buprenorphine SR-LAB (0.5–1 mg/kg, sc, once intra-operatively and every 72 h as needed; ZooPharm, Windsor, CO, USA) and ibuprofen (40 mg/kg in drinking water, 2 days before and 5 days after surgery).
2.6. PET imaging, biodistribution and quantification of radiolabeled cells post-delivery
Under anesthesia, [89Zr]Zr-DBN-CPs (~0.4 MBq; 600,000 CPs per heart) were injected intramyocardially and PET images were obtained as 10 min static scans at 24 h and 48 h post-administration using a small animal PET/X-ray system (Sofie BioSystems Genesys4, Culver City, CA, USA). The anatomic reference skeleton images were formed by using the mouse atlas registration system algorithm with information obtained from the stationary top-view planar x-ray projector and side-view optical camera. Co-registration was performed using the image analysis software AMIDE [29]. PET images were normalized to units of standardized uptake value (SUV = activity concentration in tissue / injected dose / g whole body weight). Mice were either sacrificed for ex vivo studies or recruited for prospective 1-month follow-up. The region of highest Zr-89 SUV signal within each tissue/organ was used to quantify PET images [29].
At 48 h post-administration following the final PET scan, the concentration of 89Zr activity in tissues via ex vivo biodistribution was determined. After radioactivity counting, CP cell retention in the recipient heart was quantified by qPCR. To validate long-term safety, qPCR and histopathological assessment were performed on tissues sampled at 2 months following CP cell intramyocardial delivery. The radioactivity (% injected dose) in each organ was calculated as: (decay corrected radioactivity in organ/decay corrected radioactivity injected in the animal) × 100. For qPCR estimation, after radioactivity counting, total genomic deoxyribonucleic acid (DNA) was extracted from each heart using DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany). The uptake of labeled human CPs within the mouse was quantified by qPCR using human DNA specific primers by iQ SYBR Green Supermix Assay (Biorad) in CFX-Connect thermocycler (Biorad). For qPCR quantification [30], a standard curve was made using human DNA extracted from 195, 390, 781, 1562, 3125, 6250, 12500, 25000, 50000, and 100000 human cells plotted against their respective threshold cycle (Cq) value in a qPCR reaction. A pair of primers (forward: 5’-ATGCTGATGTCTGGGTAGGGTG-3’ reverse: 5’-TGAGTCAGGAGCCAGCGTATG-3’) that generate a 141-bp fragment of human Down syndrome region at chromosome 21 was used for quantification of human DNA. For unknown samples, the amount of human cells in each qPCR assay was estimated using the Cq value of unknowns plotted against above-mentioned standard curve (Fig. S1). Each ~20 μL qPCR assay contained a fraction (1/360) of total genomic DNA extracted from whole heart or brain with human cells. Appropriate dilution factor was used to finally estimate the total number of human cells in whole heart or brain for calculating uptake of human cells in mouse heart or brain.
2.7. Therapeutic outcome
As a therapeutic functional outcome in heart failure [31, 32], improvement of left ventricular (LV) ejection fraction was assessed at 1 month following intervention [33]. Head-to-head comparison of saline (n = 6), CPs with (n = 6) or without (n = 6) [89Zr]Zr-DBN labeling was implemented (2 months post-infarction) with reference to pre-intervention (1 month post-infarction). LV ejection fraction was documented by echocardiography (Vevo2100 with MS-400 transducer; FUJIFILM VisualSonics, Toronto, ON, Canada). LV ejection fraction (%) was defined as [(EDV - ESV) / EDV] × 100, EDV is end-diastolic volume, and ESV is end-systolic volume [34].
2.8. Statistical analysis
Transcription factors (TF) were tested for equivalence between labeled and unlabeled CPs using two one-sided Mann-Whitney-Wilcoxon tests (one-sided alpha of 0.025). Data were log-transformed prior to comparison and the equivalence bound was set at +/− 10% of the unlabeled CP mean expression for each TF. Wilcoxon signed-rank tests were used to compare between standardized uptake values (SUVs) at 24 and 48 h and between LV ejection fraction at pre- and post-intervention. Correlation between biodistribution and qPCR estimation methods was assessed using Spearman’s rho. Kruskal-Wallis non-parametric ANOVA was used to evaluate change in LV ejection fraction during follow-up among the three groups, with subsequent pairwise comparisons using Dunn’s test if the omnibus test was found to be significant. Except as specified for transcription factors’ TOST, tests were two-sided. P-values less than 0.05 were considered to be significant. P-values were not adjusted for multiple testing. Student t-test analysis for viability and CyQUANT assay data was performed using GraphPad QuickCalcs (GraphPad Software, La Jolla, CA, USA). All other statistical analyses were performed in R (R Foundation, Vienna, Austria; version 3.4.2).
3. Results
3.1. [89Zr]Zr-DBN labeling of lineage-specified CPs
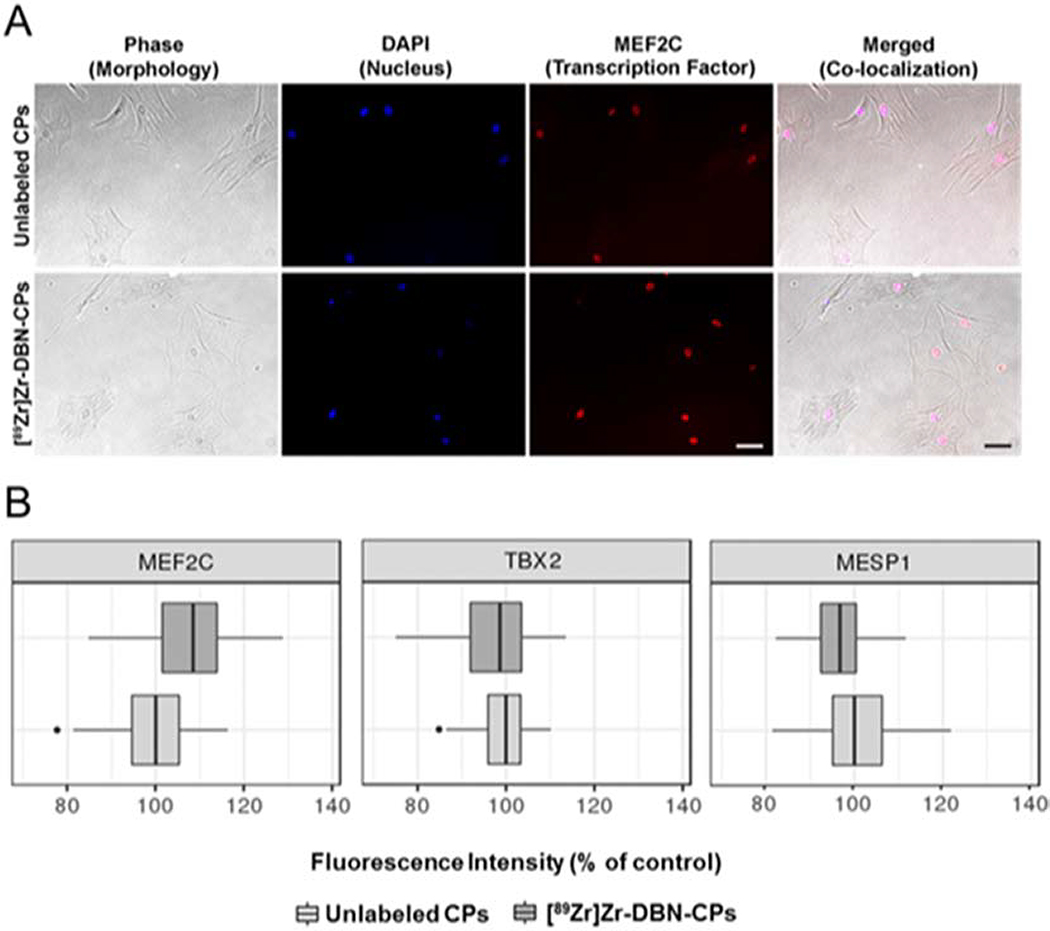
89Zr was readily chelated by its functionalized chelator DFO-NCS to form the protein reactive [89Zr]Zr-DBN complex. After 60 min of incubation, 90 ± 5.0% of 89Zr was converted to the [89Zr]Zr-DBN complex. CPs were incubated with [89Zr]Zr-DBN for 30 min at 37°C (pH 7.5) with a cell labeling efficiency of 30–40 % that yielded 0.70 ± 0.20 MBq radioactivity per 106 cells. Based on the labeling efficiency, it is estimated that 1.26–1.68 × 1014 DBN molecules (both labeled and unlabeled) will covalently bind to 6 × 105 cells. With the assumption of homogenous radiolabeling, each cell was conjugated to at least 2.1–2.8 × 108 DBN molecules (0.35–0.70 femtomoles of DBN). The DBN molecules consists of both [89Zr]Zr-DBN and free DBN. Both [89Zr]Zr-DBN and free DBN with their reactive isothiocyante group were conjugated to the primary amines on the cell. Based on the scale of labeling, it is unlikely that there were unlabeled cells in the population of labeled cells. Cellular efflux of Zr-89, determined by radioactivity in the medium in which cells were rinsed, was below the detection threshold of < 2%. Radiolabeled CPs were morphologically indistinguishable from unlabeled counterparts in phase contrast images (Fig. 1A). Moreover, the cardiopoietic-defining phenotype quantified by expression of cardiac transcription factors was documented following 89Zr-DBN radiolabeling (Fig. 1A and Fig. S2). All radiolabeled CPs expression levels were within the equivalence limits of +/−10% of unlabeled CPs fluorescence (MEF2C p=0.027, MESP1 p<0.001, TBX5 p<0.001) (Fig. 1B).
Fig. 1.
Cardiopoietic phenotype maintained in radiolabeled 89Zr CPs. (A) Unlabeled and [89Zr]Zr-DBN-labeled CPs, 2 days in culture, displayed an indistinguishable morphology and nuclear localization of MEF2C. (B) Box and whisker plots of expression of cardiogenic transcription factors, MEF2C, TBX5 and MESP1, in unlabeled and [89Zr]Zr-DBN-labeled CPs (relative to unlabeled CPs (control) after 2 days in culture). Thicker center lines represent group medians, box edges represent 25th and 75th percentiles, and the lines/whiskers extend to +/− 1.5*IQR (interquartile range, distance between 25th and 75th percentile). Bar, 100 μm.
3.2. Proliferation and viability of [89Zr]Zr-DBN-CPs
Cell population doubling time, assessed by the CyQUANT DNA content assay, was 86.1 ± 0.5 h in unlabeled versus [89Zr]Zr-DBN-CPs 88.3 ± 2.0 h in labeled CPs; mean difference 2.2 (95% CI −1.1–5.5); p value = 0.1383, indicating that [89Zr]Zr-DBN labeling did not impair cellular proliferative capacity. Trypan blue viability test showed no difference in the percentage of live cells in the absence or presence of labeling, i.e., 90 ± 5% without label versus 90 ± 5% with label. Furthermore, the ApoTox-Glo assay showed absence of induction of apoptosis or cytotoxicity or negative impact on viability in [89Zr]Zr-DBN-CPs (Fig. 2).
Fig. 2.
Assessment of viability, cytotoxicity and apoptosis in [89Zr]Zr-DBN-CPs and unlabeled CPs. No statistically significant differences were observed between [89Zr]Zr-DBN-CPs and unlabeled CPs after 2 days of culture with regard to (A) apoptosis, (C) viability and (E). For positive controls, 30 μg/mL digitonin was used for viability test (D) and cytotoxicity test (F), and 2 μΜ staurosporine was used for apoptosis test (B). *p < 0.05 versus assessments in unlabeled cells and staurosporine treated unlabeled cells using unpaired t-test and #p < 0.05 versus assessments in unlabeled cells and digitonin treated unlabeled cells using unpaired t-test.
3.3. Distribution following intra-cardiac delivery
PET based tracking of [89Zr]Zr-DBN-CPs, following intramyocardial administration, showed a predominant heart distribution, followed by lung, liver and brain as captured in maximum intensity projection scan image (Fig. 3A). Comparing PET images acquired at 24 h versus 48 h post-injection showed a 33% decrease in signal, expressed as standardized uptake value (SUV; from 98.87 ± 23.00 in heart at 24 h to 65.87 ± 19.55 in hearts at 48 h, p = 0.031; Fig. 3B). Transverse, coronal and sagittal sections of PET image of heart overlaid on software generated atlas shows injection site to be left ventricle region of the heart (Figure 3C). Ex vivo biodistribution of 89Zr signal at 48 h post-injection (Fig. 4) shows 40.26 ± 13.6% of the injected [89Zr]Zr-DBN-CPs in heart followed by lung (20.42 ± 14.18%), liver (15.56 ± 8.72%), and brain (4.56 ± 3.50%). Organ retention was independently tracked using qPCR based demonstration of human specific gene markers, with a correlation of ρ = 0.143 (p = 0.80) for the biodistribution and qPCR methods (Fig. 5). At 2 months following intramyocardial delivery of human stem cells, mouse brains demonstrated absence of human DNA signal documented in qPCR (0.01 ± 0.01% with [89Zr]Zr-DBN-CPs treatment, n = 3, 0.02 ± 0.01% with saline injection and no human cell delivery, n = 3, NS) and maintained brain structure with no uncontrolled cell growth on histology examination (Fig. S3), ruling out long-term pathological engraftment.
Fig. 3.
PET based monitoring of [89Zr]Zr-DBN-CPs following intramyocardial delivery. (A) Prospective maximum intensity projection scan showing distribution of 89Zr activity primarily in the heart at 24 h and 48 h post-administration. (B) Relative to 24 h post-injection, radioactive signal declined at 48 h (dashed lines: change of SUV in individual mouse; solid line: change in average SUV). For each animal, ~ 0.4 MBq radioactivity was injected and images were obtained by 10 min long static scan using a small animal PET/X-ray system. (C) Representative PET image of cardiac retention in transverse (left), coronal (middle) and sagittal (right) sections at 24h post-delivery. R, right; L, left.
Fig. 4.
Biodistribution of [89Zr]Zr-DBN-CPs at 48 h post-myocardial injection in heart, lung, liver, brain, kidney, intestine (large and small) and spleen of six tested animals. Cell retention is predominant in the cardiopulmonary system.
Fig. 5.
Detection by qPCR of human specific gene markers in murine hearts. PET- versus. qPCR based signal independently validate successful intramyocardial delivery of [89Zr]Zr-DBN-CPs.
3.4. Disease rescue
Cardiac performance following myocardial infarction was assessed by high-resolution ultrasound in saline, [89Zr]Zr-DBN-CPs or unlabeled CP treated cohorts. With equivalent disease severity (i.e., equivalent LV ejection fraction on echocardiography) at the time of randomization, saline treatment was ineffective whereas cardiopoietic stem cell (CP) injection improved cardiac function quantified by LV ejection fraction regardless of whether CPs were unlabeled or labeled with 89Zr-DBN (Figs. 6A and 6B). Median change in LV ejection fraction between pre- and post-intervention was −0.4% (IQR −4.9, 0.2) with saline treatment, 9.6% (IQR 0.1, 14.8) with unlabeled CPs (p = 0.008 versus saline), and 12.7% (IQR 3.6, 12.8) with [89Zr]Zr-DBN-CPs (p = 0.02 versus saline, p = 0.39 versus unlabeled CPs; Fig. 6C). Of note, no animals treated with [89Zr]Zr-DBN-CPs demonstrated signs of systemic toxicity, adverse effects, or mortality.
Fig. 6.
Therapeutic effectiveness in myocardial infarction of CPs post-labeling with 89Zr. (A) In failing hearts, 1 month post-myocardial infarction, saline (left), CPs without (89Zr-unlabeled, middle) or with [89Zr]Zr-DBN labeling (89Zr-labeled, right) were delivered by intramyocardial route. One month post-intervention, echocardiography demonstrated extensive wall motion abnormalities (arrows) in the saline-treated heart (left). In contrast, left ventricular segments with contractile defect (arrows) were limited in infarcted hearts following CPs without (89Zr-unlabeled, middle) or with [89Zr]Zr-DBN labeling (right) transplantation. Ao, aorta. (B and C) Left ventricular ejection fraction was compromised throughout follow-up in the saline group, whereas cardiac contractility significantly recovered following CPs administration in both 89Zr-unlabeled (p = 0.008 versus saline) and 89Zr-labeled groups (p = 0.02 versus saline). Unlabeled CPs and 89Zr-labeled CPs were equally effective (p = 0.39) in the setting of chronic ischemic heart failure.
4. Discussion
There is a growing need to develop imaging modalities compatible for non-invasive monitoring of regenerative biotherapy in a disease heart. This proof-of-concept study establishes the suitability of a [89Zr]Zr-DBN-empowered radiolabeling strategy for cardiopoietic stem cell tracking by PET without altering cellular identity and therapeutic efficacy in ischemic heart failure.
Currently in advanced translational and clinical development [18, 20, 35, 36], human cardiopoietic stem cells (CPs), derived through growth factor-based guidance, were here employed as a cell prototype with the capacity to transition a heart failure trajectory towards pre-disease [37]. The labeling efficiency of CPs with the amine-reactive synthon [89Zr]Zr-DBN was comparable with that achieved with the naïve stem cell source [9]. Maintenance of cell morphology, proliferative capacity, viability, and cardiopoietic indices, exemplified by expression of MEF2C, TBX5 and MESP1 transcription factors, qualifiers of the cardiopoietic phenotype [17, 26], indicated CPs tolerability towards [89Zr]Zr-DBN. Similar to other cell types, such as melanoma, mesenchymal and dendritic cell types [9], there was minimal efflux of radioactivity in [89Zr]Zr-DBN-CPs.
Whole body PET imaging of infarcted animals showed preferential uptake in the heart, with kinetics consistent with reported rates of cell clearance after myocardial transplantation [38]. Biodistribution of 89Zr-radioacitivity in the heart, and to lesser degrees in non-cardiac tissues, was independently validated by human DNA detection in the xenogeneic host in line with the documented fate of other labeled stem cells [9, 39]. Radioactive signal beyond the originally targeted organ could be the consequence of multiple mechanisms, including circulation via blood flow, cell entrapment in the lungs, macrophage phagocytosis followed by clearance of cell membrane fragments in the liver and spleen [40, 41]. Indeed, accumulating data demonstrate short-term distribution of intramyocardially-delivered stem cells to extra-cardiac organs mainly through circulating blood flow [40–42], which is consistent with our observation at 2 days. At 2 days post-injection, bone uptake of free Zr-89 is less than detection threshold in PET images which is consistent with our observation in our previous study [9]. While cell fate is yet to be fully identified, the majority of circulating exogenous stem cells will be cleared due to mechanical stress and interaction with the host immune system [17]. In this study, signals from the head region may be emitted from live [89Zr]Zr-DBN-CP cells, or cell membranes, following circulation via blood flow and/or recruitment into organs, e.g., central nerve system, eye, skull. Entry of injected stem cells into the brain is unlikely due to an intact blood brain barrier or in the absence of whole-body irradiation [43]. While engraftment of a pluripotent cell type may engender uncontrolled growth within the host environment, CP cells are pharmacologically pre-guided into cardiac lineage achieving a lower proliferation rate with a documented safety profile achieved both clinically and pre-clinically up to 2-year of follow-up [17, 44, 45].
Benefit in the infarcted heart model was here shown to be bioequivalent between unlabeled and [89Zr]Zr-DBN-CPs. Following 89Zr-DBN labeled CP therapy, significant improvement of cardiac pump function, an established readout of successful heart failure treatment [46, 47], provided evidence of non-inferiority indicating thereby that the [89Zr]Zr-DBN synthon does not negatively impact the regenerative response of the diseased failing heart.
While the developed 89Zr-based PET methodology is applicable in a clinically relevant scenario, PET imaging of the 89Zr signal does not provide information on the cellular status, including cell viability or cell number. As 89Zr-labeled cells are broken down over time, the retention of 89Zr-radioactivity in membrane fragments rather than intact cells would compromise interpretation in a long-term chronic follow-up. Moreover, should the labeled cells undergo cell division, the 89Zr-activity will be split between dividing cells. A recognized challenge for cardiac cell therapy is early cell loss, especially within the first 2 days, under currently available procedures [2]. This study employed [89Zr]Zr-DBN labelling and PET to validate cell retention within infarcted hearts in the setting of CP cell therapy. Over half (~60%) of delivered CP cells were lost from the heart within 2 days following intramyocardial cell injection. Independent qPCR assessment showed that CP cell retention in the heart slowly declined from ~35% at 2 day (this study) to ~14% at 3 months and ~10% at 2 years [17]. These results highlight the need for further optimization of early cell retention. Accordingly, reliable interpretation would be expected to be obtained from the 89Zr-labeled stem cell tracking within the acute phase post-administration reflective of early trafficking patterns.
5. Conclusion
In summary, we here document that the [89Zr]Zr-DBN synthon is well tolerated by human cardiopoietic stem cells which retain their identity and therapeutic potential following radioactive labeling. Accordingly, non-invasive molecular imaging of cardiopoietic stem cells is thus feasible, providing a PET compatible tool for the further development of regenerative therapies.
Supplementary Material
Fig. S1. Assessment of cell retention. Mouse hearts, treated with multiple doses of human CPs, underwent qPCR analysis using human DNA specific primers. A reference standard curve of Cq values (diamond) was used to estimate cell number retained in each heart following delivery (square).
Fig. S2. Representative fluorescence images showing phase image of cells, DAPI stained nucleus, cytosolic expression of MESP1 and nuclear localization of TBX5 in unlabeled-CPs and [89Zr]Zr-DBN-CPs. Bar, 20 μm.
Fig. S3. Absence of uncontrolled growth in mouse brain sections at 2 months following intramyocardial delivery of human cardiopoietic stem cells.
Acknowledgments
The authors thank Lois Rowe and Ryan Mahlberg for histology, and Paul G. Stalboerger and Jilian L. Foxen for project management. Authors were supported by NIH 5R21HL127389-02, NIH 4T32HL007111-39, NIH R01HL134664, Mayo Clinic Department of Radiology, Accelerated Regenerative Medicine Award, Mayo Clinic Center for Regenerative Medicine, Van Cleve Cardiac Regenerative Medicine Program, Marriott Foundation, and Michael S. and Mary Sue Shannon Family.
Disclosures
S.Y., A. Behfar, and A.T. are co-inventors on regenerative sciences related intellectual property disclosed to Mayo Clinic. A. Bansal., M.P., and T.D. are co-inventors on 89Zr related intellectual property disclosed to Mayo Clinic. Previously, Mayo Clinic has administered research grants from Celyad. Mayo Clinic, A. Behfar, and A.T. have interests in Rion LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Menasche P Cell therapy trials for heart regeneration - lessons learned and future directions. Nat Rev Cardiol 2018;15:659–71. [DOI] [PubMed] [Google Scholar]
- [3].Peng H and Abdel-Latif A. Cellular Therapy for Ischemic Heart Disease: An Update. Adv Exp Med Biol 2019;1201:195–213. [DOI] [PubMed] [Google Scholar]
- [4].Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, and Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lezaic L, Socan A, Peitl PK, Poglajen G, Sever M, Cukjati M, et al. Imaging and 1-day kinetics of intracoronary stem cell transplantation in patients with idiopathic dilated cardiomyopathy. Nucl Med Biol 2016;43:410–4. [DOI] [PubMed] [Google Scholar]
- [6].Li X and Hacker M. Molecular imaging in stem cell-based therapies of cardiac diseases. Adv Drug Deliv Rev 2017;120:71–88. [DOI] [PubMed] [Google Scholar]
- [7].Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging 2012;5:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nguyen PK, Riegler J, and Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell 2014;14:431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bansal A, Pandey MK, Demirhan YE, Nesbitt JJ, Crespo-Diaz RJ, Terzic A, et al. Novel (89)Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asiedu KO, Koyasu S, Szajek LP, Choyke PL, and Sato N. Bone Marrow Cell Trafficking Analyzed by (89)Zr-oxine Positron Emission Tomography in a Murine Transplantation Model. Clin Cancer Res 2017;23:2759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Charoenphun P, Meszaros LK, Chuamsaamarkkee K, Sharif-Paghaleh E, Ballinger JR, Ferris TJ, et al. [(89)Zr]oxinate4 for long-term in vivo cell tracking by positron emission tomography. Eur J Nucl Med Mol Imaging 2015;42:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sato N, Wu H, Asiedu KO, Szajek LP, Griffiths GL, and Choyke PL. (89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies. Radiology 2015;275:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elhami E, Dietz B, Xiang B, Deng J, Wang F, Chi C, et al. Assessment of three techniques for delivering stem cells to the heart using PET and MR imaging. EJNMMI Res 2013;3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lang C, Lehner S, Todica A, Boening G, Franz WM, Bartenstein P, et al. Positron emission tomography based in-vivo imaging of early phase stem cell retention after intramyocardial delivery in the mouse model. Eur J Nucl Med Mol Imaging 2013;40:1730–8. [DOI] [PubMed] [Google Scholar]
- [15].Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 2009;54:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Behfar A, Faustino RS, Arrell DK, Dzeja PP, Perez-Terzic C, and Terzic A. Guided stem cell cardiopoiesis: discovery and translation. J Mol Cell Cardiol 2008;45:523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol 2010;56:721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Terzic A and Behfar A. Stem cell therapy for heart failure: Ensuring regenerative proficiency. Trends Cardiovasc Med 2016;26:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 2013;61:2329–38. [DOI] [PubMed] [Google Scholar]
- [20].Bartunek J, Terzic A, Behfar A, and Wijns W. Clinical Experience With Regenerative Therapy in Heart Failure: Advancing Care With Cardiopoietic Stem Cell Interventions. Circ Res 2018;122:1344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 2017;38:648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, et al. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail 2017;19:1520–9. [DOI] [PubMed] [Google Scholar]
- [23].Holland JP, Sheh Y, and Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol 2009;36:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pandey MK, Engelbrecht HP, Byrne JP, Packard AB, and DeGrado TR. Production of 89Zr via the 89Y(p,n)89Zr reaction in aqueous solution: effect of solution composition on in-target chemistry. Nucl Med Biol 2014;41:309–16. [DOI] [PubMed] [Google Scholar]
- [25].Roca M, de Vries EF, Jamar F, Israel O, and Signore A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur J Nucl Med Mol Imaging 2010;37:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Crespo-Diaz R, Yamada S, Bartunek J, Perez-Terzic C, de Waele P, Mauen S, et al. Cardiopoietic index predicts heart repair fitness of patient-derived stem cells. Biomark Med 2015;9:639–49. [DOI] [PubMed] [Google Scholar]
- [27].Yamada S, Arrell DK, Rosenow CS, Bartunek J, Behfar A, and Terzic A. Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy. Stem Cells Transl Med 2020;9:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nelson TJ, Martinez-Fernandez A, Yamada S, Mael AA, Terzic A, and Ikeda Y. Induced pluripotent reprogramming from promiscuous human stemness related factors. Clin Transl Sci 2009;2:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loening AM and Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2003;2:131–7. [DOI] [PubMed] [Google Scholar]
- [30].Zhou R, Li Z, He C, Li R, Xia H, Li C, et al. Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model. PLoS One 2014;9:e104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Banovic M, Loncar Z, Behfar A, Vanderheyden M, Beleslin B, Zeiher A, et al. Endpoints in stem cell trials in ischemic heart failure. Stem Cell Res Ther 2015;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yamada S, Arrell DK, Martinez-Fernandez A, Behfar A, Kane GC, Perez-Terzic CM, et al. Regenerative Therapy Prevents Heart Failure Progression in Dyssynchronous Nonischemic Narrow QRS Cardiomyopathy. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamada S, Nelson TJ, Kane GC, Martinez-Fernandez A, Crespo-Diaz RJ, Ikeda Y, et al. Induced pluripotent stem cell intervention rescues ventricular wall motion disparity, achieving biological cardiac resynchronization post-infarction. J Physiol 2013;591:4335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yamada S, Arrell DK, Kane GC, Nelson TJ, Perez-Terzic CM, Behfar A, et al. Mechanical dyssynchrony precedes QRS widening in ATP-sensitive K(+) channel-deficient dilated cardiomyopathy. J Am Heart Assoc 2013;2:e000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Behfar A, Crespo-Diaz R, Terzic A, and Gersh BJ. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol 2014;11:232–46. [DOI] [PubMed] [Google Scholar]
- [36].Emmert MY, Wolint P, Jakab A, Sheehy SP, Pasqualini FS, Nguyen TDL, et al. Safety and efficacy of cardiopoietic stem cells in the treatment of post-infarction left-ventricular dysfunction - From cardioprotection to functional repair in a translational pig infarction model. Biomaterials 2017;122:48–62. [DOI] [PubMed] [Google Scholar]
- [37].Arrell DK, Rosenow CS, Yamada S, Behfar A, and Terzic A. Cardiopoietic stem cell therapy restores infarction-altered cardiac proteome. NPJ Regen Med 2020;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, et al. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med 2009;50:1088–94. [DOI] [PubMed] [Google Scholar]
- [39].Fabian C, Naaldijk Y, Leovsky C, Johnson AA, Rudolph L, Jaeger C, et al. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res Ther 2017;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dow J, Simkhovich BZ, Kedes L, and Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res 2005;67:301–7. [DOI] [PubMed] [Google Scholar]
- [41].Kierdorf K, Katzmarski N, Haas CA, and Prinz M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS One 2013;8:e58544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].van den Akker F, Feyen DA, van den Hoogen P, van Laake LW, van Eeuwijk EC, Hoefer I, et al. Intramyocardial stem cell injection: go(ne) with the flow. Eur Heart J 2017;38:184–6. [DOI] [PubMed] [Google Scholar]
- [43].Lampron A, Lessard M, and Rivest S. Effects of myeloablation, peripheral chimerism, and whole-body irradiation on the entry of bone marrow-derived cells into the brain. Cell Transplant 2012;21:1149–59. [DOI] [PubMed] [Google Scholar]
- [44].Karantalis V and Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mathur A, Fernandez-Aviles F, Dimmeler S, Hauskeller C, Janssens S, Menasche P, et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur Heart J 2017;38:2930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Normand C, Kaye DM, Povsic TJ, and Dickstein K. Beyond pharmacological treatment: an insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 2019;393:1045–55. [DOI] [PubMed] [Google Scholar]
- [47].Rossignol P, Hernandez AF, Solomon SD, and Zannad F. Heart failure drug treatment. Lancet 2019;393:1034–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Assessment of cell retention. Mouse hearts, treated with multiple doses of human CPs, underwent qPCR analysis using human DNA specific primers. A reference standard curve of Cq values (diamond) was used to estimate cell number retained in each heart following delivery (square).
Fig. S2. Representative fluorescence images showing phase image of cells, DAPI stained nucleus, cytosolic expression of MESP1 and nuclear localization of TBX5 in unlabeled-CPs and [89Zr]Zr-DBN-CPs. Bar, 20 μm.
Fig. S3. Absence of uncontrolled growth in mouse brain sections at 2 months following intramyocardial delivery of human cardiopoietic stem cells.