Abstract
Phthalates are known endocrine-disrupting chemicals that are found in many consumer products. Our laboratory previously developed a relevant phthalate mixture consisting of six phthalates and found that it disrupted female fertility in mice. However, it is unknown if prenatal exposure to phthalate mixtures can accelerate reproductive aging and if this occurs in multiple generations. Thus, we tested the hypothesis that prenatal exposure to a mixture of phthalates accelerates biomarkers of reproductive aging in multiple generations of female mice. Pregnant CD-1 mice were orally dosed with vehicle control or a phthalate mixture (20μg/kg/day-500mg/kg/day) daily from gestational day 10 to birth. Adult F1 females born to these dams were used to create the F2 and F3 generations by mating them with unexposed males. At 13 months, estrous cyclicity was monitored and ovaries and sera were collected for analysis. In the F1 generation, the mixture decreased testosterone and inhibin B levels, but increased follicle-stimulating hormone and luteinizing hormone levels compared to control. In the F2 generation, the phthalate mixture decreased the percent of antral follicles and testosterone hormone levels compared to control. In the F3 generation, prenatal exposure to the phthalate mixture increased ovarian weight, increased the time in metestrus/diestrus, altered follicle numbers, and decreased the levels of luteinizing hormone compared to control. Collectively, these data suggest that prenatal exposure to a phthalate mixture may accelerate several biomarkers of reproductive aging in a multi- and transgenerational manner in female mice.
Keywords: phthalates, mixture, reproductive aging, ovary, hormone, cyclicity, transgenerational
Introduction
Phthalates are a group of chemicals found in many different consumer products including building materials, personal care products, children’s toys, and food storage containers [1]. Phthalates are found ubiquitously in the environment, and more than 18 billion pounds of phthalates are produced annually [1]. Low molecular weight phthalates and high molecular weight phthalates are present in different types of products to provide plasticity or act as solvents. Low molecular weight phthalates include diethyl phthalate (DEP), dibutyl phthalate (DBP), and diisobutyl phthalate (DiBP) and are found in colognes, perfumes, nail polish, pharmaceuticals, and some adhesives [2, 3]. High molecular weight phthalates include di(2-ethylhexyl) phthalate (DEHP), benzylbutyl phthalate (BzBP), and di-isononyl phthalate (DiNP) and are heavily used as plasticizers in polyvinyl chloride plastics, flooring, food packaging, medical tubing, plastic toys, and paints [2]. In addition to being present in consumer products, phthalates are detected in indoor air and household dust [4]. Due to the widespread use of phthalates, humans are exposed daily to these chemicals. Exposure occurs via inhalation, ingestion, and dermal contact, but the most common route of exposure is from oral ingestion [5]. Due to widespread exposure, phthalates and/or their metabolites have been measured in urine, blood, semen, breast milk, amniotic fluid, umbilical cord blood, and follicular fluid [6, 7].
Phthalates are known endocrine disrupting chemicals that have been shown to have negative effects on reproduction [8]. Exposure to single phthalates has been shown to accelerate folliculogenesis, decrease fertility, and alter steroidogenesis in female rodents [8–14]. Specifically, prenatal exposure to DEHP accelerated the transition of germ cells to primordial follicles in the neonatal ovary in the F3 generation of female mice [12]. In addition, adult exposure to DEHP accelerated folliculogenesis after 10 days of exposure [9]. Moreover, prenatal exposure and adult exposure to DEHP decreased the primordial follicle pool, leading to early reproductive senescence in aging female mice [10, 15].
Although these previous studies on single phthalates provide important information, humans are exposed to mixtures of phthalates on a daily basis. Due to limited studies on the effects of phthalate mixtures on reproductive outcomes, our laboratory developed a phthalate mixture consisting of DEHP, DEP, DBP, BzBP, DiNP, and DiBP [16–18] based on the phthalate metabolite levels found in the urine of pregnant women in central Illinois [19] and examined the effects of prenatal exposure to this mixture on female reproductive outcomes [16, 17]. We previously found that prenatal exposure to the mixture increased uterine weights, altered anogenital distance, and caused fertility complications in the F1, F2, and F3 generations of female mice [16, 17]. However, we did not examine the effects of the mixture on reproductive aging in the female mice.
In addition to limited information on the effects of a relevant phthalate mixture on reproductive aging in females, very few studies have focused on the transgenerational effects of mixtures of chemicals like phthalates on female reproduction. Zhou et al. [16, 17] found that the phthalate mixture described above disrupted female reproduction in the F1, F2, and F3 generations of female mice. Specifically, prenatal exposure to the mixture increased the occurrence of grossly visible cystic ovaries in the F1 and F2 generations, which is a sign of aging of the ovary in the female rodent [16, 17]. In addition, the estrous cycles of the female mice were monitored for 14 days prior to tissue collections at 13 months and it was found that this mixture did not alter cycles in the F1 and F2 generations [16, 17]. Moreover, collaborative studies found that prenatal exposure to this mixture decreased levels of progesterone in the F2 generation, indicating a decrease in sex steroid hormone levels that occurs with normal reproductive aging [20].
Although these studies have examined how prenatal exposure to phthalates affects female reproduction in multiple generations of female mice, they did not examine all endpoints to determine if prenatal exposure to a relevant phthalate mixture accelerates reproductive aging in a transgenerational manner. Thus, the current study tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture accelerates reproductive aging in multiple generations of female mice. To test this hypothesis, we determined whether prenatal exposure to the mixture causes acyclicity in the F3 generation at time-points sooner than controls. In addition, we examined whether the phthalate mixture decreased the ovarian follicle pool and increased the occurrence of cystic ovaries in the F1, F2, and F3 generations because a reduced ovarian follicle pool and increased occurrence of cysts are key characteristics of reproductive aging. Lastly, we examined whether the mixture alters hormones that fluctuate with reproductive aging.
Methods
Chemicals
The chemicals used in the mixture include DEP, DEHP, DBP, DiBP, DiNP, and BzBP and were purchased from Sigma-Aldrich (St. Louis, MO). The mixture consisted of 35% DEP, 21% DEHP, 15% DBP, 15% DiNP, 8% DiBP, and 5% BzBP and the percentages were calculated from levels of phthalate metabolites measured in urine from pregnant women in central Illinois [16, 17, 19]. After creating the mixture, it was thoroughly mixed and diluted in tocopherol-stripped corn oil (vehicle control). The doses used for this study included 20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, and 500 mg/kg/day. We selected these doses because the two lower doses used in this study fall within the range of human exposure to most of the phthalates in the mixture or are very close to those levels [21, 22]. The estimated range of daily human exposure to DEHP is about 3–30 μg/kg/day and the 20 and 200 μg/kg/day doses of the mixture contain approximately 4.2 and 42 μg of DEHP, which are close to human exposure levels. For the phthalate DEP, human exposure ranges from 2.32–12 μg/kg/day and the 20 μg/kg/day dose of the mixture contains approximately 7 μg of DEP. Next, the daily human exposure to phthalate BzBP is 0.26–0.88 μg/kg/day and the 20 μg/kg/day dose of the mixture contains approximately 1 μg of BzBP, which is close to the high range of human exposure. Human exposure to DBP falls within 0.84–5.22 μg/kg/day and the 20 μg/kg/day dose of the mixture contains around 3 μg of DBP, making it environmentally relevant. The phthalate DiBP has daily exposure levels ranging from 0.12–1.4 μg/kg/day and the 20 μg/kg/day dose of mixture contains approximately 1.6 μg of DiBP. Last, daily human exposure for DiNP is not known, but occupational exposure levels reach up to 26 μg/kg/day, whereas exposure in infants can reach levels of up to 120 μg/kg/day [23, 24]. The 200 μg/kg/day dose of the mixture is calculated to contain around 30 μg of DiNP, which is in the range of occupational and infant exposure. Furthermore, the two higher doses in this study (200 and 500 mg/kg/day) were chosen so we can compare our results to other studies examining single phthalate exposure [9, 10, 12, 13, 15, 25–27].
Animals
Adult cycling female and adult male CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign (Champaign, IL). The mice were acclimated to the facility for at least 1 week prior to beginning the experiment. The mice were housed individually at 25°C in polysulfone cages with a light/dark cycle of 12 hours light and 12 hours dark. Mice were given the Teklad Rodent Diet 8604 (Envigo, Huntingdon, United Kingdom) and reverse-osmosis filtered high-purity water ad libitum. Animal procedures including euthanasia and tissue collections were approved the University of Illinois Institutional Animal Care and Use Committee.
Study design and dosing
Female mice (F0) were mated with non-treated males, both 8 weeks of age, to create the F1 generation of mice. A female was considered pregnant when a vaginal sperm plug was present. After a vaginal sperm plug was observed, females were separated from males and individually housed. To control for litter effects, the females were randomly divided into the 5 different treatment groups. Once the pregnant females reached gestational day 10, dosing began at the same time every day and continued until the delivery of pups. Pregnant females were dosed orally by inserting a pipette tip into the cheek of the mouse containing either the vehicle control (tocopherol-stripped corn oil) or one of the doses of the mixture (20 μg/kg/day-500 mg/kg/day). Females were weighed daily to determine the correct dosing volume. Oral dosing by pipette was chosen to mimic daily human exposure and the dosing window was chosen because this is a critical time period for ovarian development in the mouse.
The pups born from these dosed females were considered the F1 generation. Each dam was considered the experimental unit and at least 1 female per litter from at least 3 different exposed dams in each treatment group was used in the analyses. At 3 months of age, the F1 generation was mated with non-treated males to create the F2 generation, and then the F2 females were mated with non-treated males to create the F3 generation. The F1 generation was exposed directly as the developing pup within the F0 pregnant dam, the F2 generation was exposed as the gametes within the developing pups of the F1 generation, and the F3 generation was the first generation that was not directly exposed to the phthalate mixture.
Estrous cyclicity
Previous studies showed that prenatal exposure to the mixture did not affect estrous cyclicity in the F1 or F2 generations of 13 month old mice [16, 17]. Thus, we expanded these results by monitoring the estrous cycles of 13 month old mice in the F3 generation. The cycles were monitored for 14 days before tissue collections by performing daily vaginal lavage. Stages of estrus were determined by previously defined criteria [28]. The percentage of days spent in each cycle was calculated by the number of days in each cycle divided by 14 and multiplying that value by 100. For statistical analysis, our laboratory combined metestrus and diestrus because they are similar in both hormone profile and cytology.
Tissue collection
Tissues were collected in the F1 and F2 generations and data on theses tissues were published [16, 17]. However data were not previously published for the F3 generation. Therefore, when females reached 13 months of age in the F3 generation, mice were euthanized and tissues were collected. The morning of collections, the cyclicity of the mice was observed approximately the same time every day, and tissues were collected if mice were in the estrous stage of diestrus. Body weight in grams and anogenital distance (AGD) in mm were recorded before tissues were collected. The AGD was normalized to body weight by taking the AGD measurement and dividing it by the cubic root of the body weight [29]. Sera were collected to use for hormone assays described below. Ovaries, uteri, and livers were collected and weights were recorded in grams. Many mice had enlarged cystic ovaries at 13 months, so ovarian weights were reported for one healthy ovary per mouse. If both ovaries were cystic, no ovarian weights were recorded for that mouse. The ovaries were considered cystic if they were very enlarged in size and/or had visible fluid-filled cysts. If one or both ovaries were grossly cystic during tissue collections, percentages of cystic ovaries were calculated by taking the number of ovaries with cysts divided by the total number of ovaries in that treatment group multiplied by 100. One ovary was fixed in Dietrich’s fixative and used for histological evaluation as described below and the other ovary was snap-frozen and stored for future studies.
Histological examination of ovaries
Ovaries in each generation were collected at 13 months of age. After being fixed in Dietrich’s fixative, ovaries were embedded in paraffin, and sectioned. Ovaries in the F1 generation were sectioned at 8 μm and ovaries in the F2 and F3 generations were sectioned at 5 μm. After sectioning, slides were stained with hematoxylin and eosin to prepare for counting follicle types and determining if cysts were present. The follicle types counted included primordial, primary, preantral, and antral follicles. Further, atretic follicles, abnormal follicles, and number of corpora lutea were counted. Criteria to determine each follicle type were described previously by our laboratory [9, 15]. Primordial and primary follicles were counted whether or not nuclear material was present, but preantral, antral, and atretic follicles were required to have nuclear material present to avoid double counting of follicles that span multiple sections. Total number of follicles, number and percent of each type of follicle, percent of atretic follicles, number of abnormal follicles, corpora lutea per number of total sections, and presence of cysts were recorded without knowledge of the treatment group. In addition, the percent of each follicle type was examined to observe the effects of the phthalate mixture on shifts in the follicle pool because an accelerated shift in the follicle pool could be contributing to overall reproductive aging.
Analysis of sex steroid, gonadotropin, and peptide hormone levels
During tissues collections at 13 months of age, sera were collected from each generation of female mice. The sera were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for measurement of testosterone, inhibin B, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) by enzyme-linked immunosorbent assays (progesterone, estradiol, testosterone, and inhibin B) or radioimmunoassays (RIAs) (FSH and LH). Progesterone and estradiol data were not included in this study because Li et al. [20] published the results using sera from the same mice in this current study. Li et al. determined that prenatal exposure to the mixture caused a borderline increase in estradiol levels and significantly decreased progesterone levels compared to control mice in the F2 generation. The lowest limits of detection were 10 ng/dL for testosterone, 2 ng/mL for FSH, 0.04 ng/mL for LH, and 10.4 pg/mL for inhibin B. If the measurement was lower than the lowest limit of detection, the value was substituted with the lowest limit of detection/√2. The intra- and inter-assay coefficients of variability were less than 10%.
Statistical analysis
The software SPSS (SPSS Inc., Chicago, IL) was used for data analysis. If data were normally distributed, a one-way analysis of variance (ANOVA) was performed. The post hoc test of Dunnett (2-sided) was used if equal variances were assumed and the post hoc test of Games-Howell was used if equal variances were not assumed. If data were not equally distributed or were presented as percentages, the independent sample Kruskal-Wallis H tests were used to compare each treatment group, followed by the Mann-Whitney U test. To evaluate cystic ovaries, mice were assigned a 0 (indicates no cysts) or 1 (cysts observed), and Fisher’s exact tests were used to compare treatment groups to the control group. Statistical significance was assigned p ≤ 0.05, but when p-values were greater than 0.05, but less than 0.1, data were considered to exhibit a trend towards significance.
Results
Effect of the phthalate mixture on estrous cyclicity of F3 female mice
We previously determined that in the F1 and F2 generations, the phthalate mixture did not alter estrous cyclicity in 13 month old female mice compared to control [16, 17]. Interestingly, in the current study in the F3 generation, the mixture did not affect time spent in proestrus, however, it (20 and 200 μg/kg/day, 500 mg/kg/day) caused a borderline decrease in the time spent in estrus, and it (20 and 200 μg/kg/day, 500 mg/kg/day) increased time spent in metestrus/diestrus compared to control (Figure 1; n = 3 to 9 females per treatment, * p ≤ 0.05, ^ 0.05 < p < 0.1, borderline significance).
Figure 1.
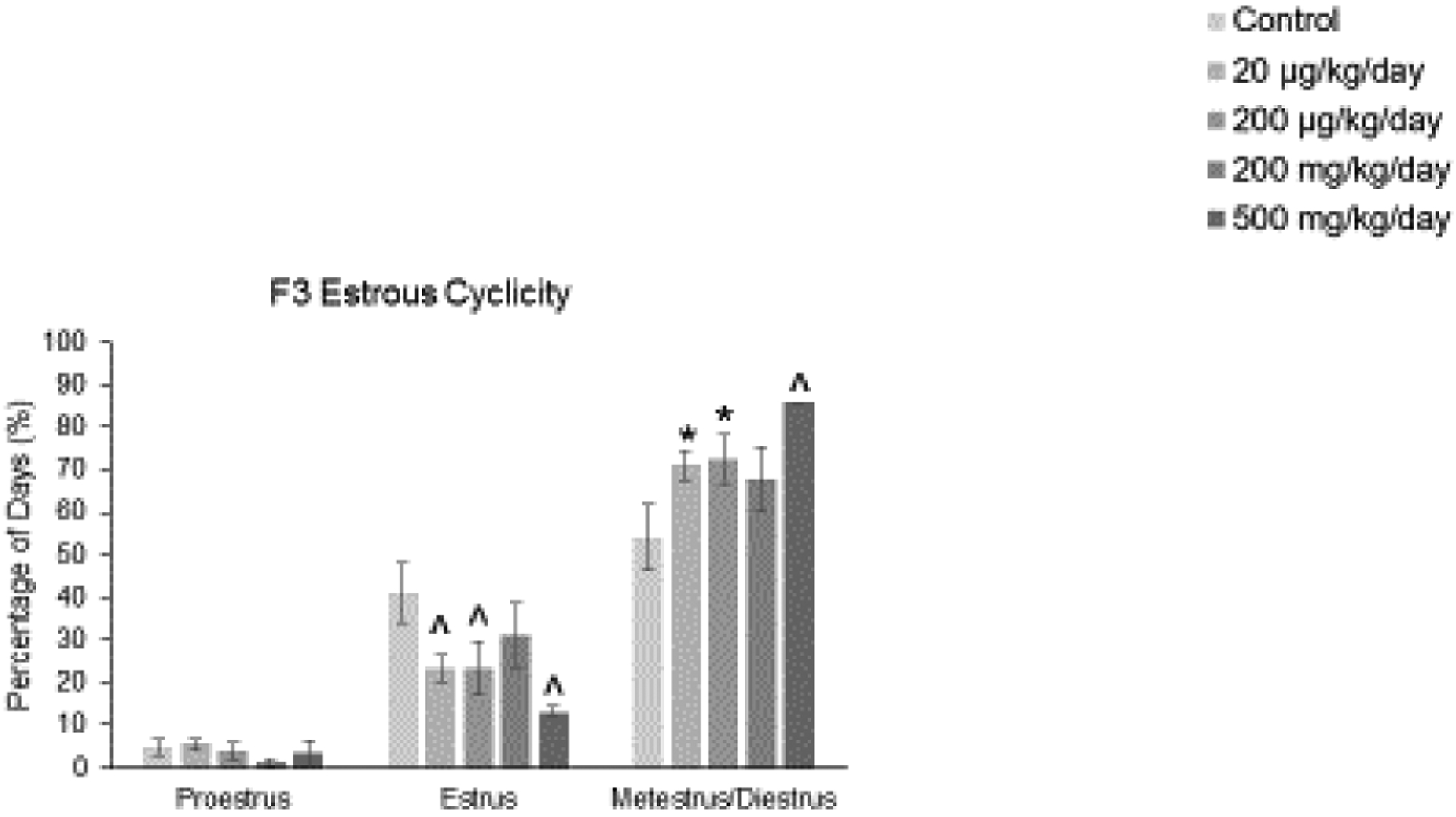
Effect of prenatal exposure to the phthalate mixture on estrous cyclicity at 13 months of age in the F3 generation of mice. Percentage of days in proestrus, estrus, and metestrus/diestrus were calculated and compared to controls in each treatment group (control = 6 females/treatment group, 20 μg/kg/day = 9 females/treatment group, 200 μg/kg/day = 7 females/treatment group, 200 mg/kg/day = 6 females/treatment group, 500 mg/kg/day = 3–4 females/treatment group). Graphs represent means ± SEM. Asterisks (*) indicate significant differences compared to the control (p < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < p < 0.1).
Effect of the phthalate mixture on body weight, tissue weights, and grossly visible cystic ovaries of F3 female mice
A previous study showed that in the F1 generation, the phthalate mixture increased the occurrence of grossly visible cystic ovaries in all treatment groups except for the control, but it did not affect body weight, ovary weight, uterine weight, liver weight, or AGD normalized to body weight in 13 month old female mice [16]. However, in the F2 generation, prenatal exposure to the phthalate mixture (200 μg/kg/day) significantly increased body weight and it (20 μg/kg/day) caused a borderline increase in the occurrence of grossly visible cystic ovaries, but did not affect other tissue weights [17]. In this current study on the F3 generation, prenatal exposure to the environmentally relevant phthalate mixture did not significantly affect body weight, uterine weight, liver weight, AGD normalized to body weight, or the number of grossly visible cystic ovaries compared to control (Table 1; n = 4 to 9 females per treatment group). However, the mixture (20 μg/kg/day) significantly increased ovarian weight compared to control in the F3 generation (Table 1; n = 4 to 9 females per treatment group, * p ≤ 0.05).
Table 1.
Effects of prenatal exposure to phthalate mixture on body weight, tissue weight, and occurrence of grossly visible cystic ovaries in the F3 generation of 13 month old female mice
| F3 Generation | ||
|---|---|---|
| Treatment | ||
| Body Weight (g) | Control | 46.01 ± 3.32 |
| 20 μg/kg/day | 46.73 ± 3.65 | |
| 200 μg/kg/day | 51.28 ± 3.58 | |
| 200 mg/kg/day | 49.19 ± 2.72 | |
| 500 mg/kg/day | 40.47 ± 2.26 | |
| Single Ovary Weight (g) | Control | 0.0080 ± 0.0010 |
| 20 μg/kg/day | 0.0148 ± 0.0006* | |
| 200 μg/kg/day | 0.0092 ± 0.0015 | |
| 200 mg/kg/day | 0.0118 ± 0.0019 | |
| 500 mg/kg/day | 0.0072 ± 0.0010 | |
| Uterine Weight (g) | Control | 0.2041 ± 0.0244 |
| 20 μg/kg/day | 0.2337 ± 0.0228 | |
| 200 μg/kg/day | 0.2192 ± 0.0239 | |
| 200 mg/kg/day | 0.2278 ± 0.0402 | |
| 500 mg/kg/day | 0.2786 ± 0.0131 | |
| Liver Weight (g) | Control | 2.5658 ± 0.1604 |
| 20 μg/kg/day | 2.7343 ± 0.1709 | |
| 200 μg/kg/day | 2.9306 ± 0.2055 | |
| 200 mg/kg/day | 2.7714 ± 0.1444 | |
| 500 mg/kg/day | 2.4965 ± 0.0760 | |
| AGD (mm)/∛Body Weight (g) | Control | 1.7313 ± 0.0665 |
| 20 μg/kg/day | 1.7435 ± 0.0506 | |
| 200 μg/kg/day | 1.5799 ± 0.0972 | |
| 200 mg/kg/day | 1.6454 ± 0.0721 | |
| 500 mg/kg/day | 1.6727 ± 0.1193 | |
| Percent Grossly Visible Cystic Ovaries (%) | Control | 33.3 |
| 20 μg/kg/day | 44.4 | |
| 200 μg/kg/day | 33.3 | |
| 200 mg/kg/day | 33.3 | |
| 500 mg/kg/day | 50.0 | |
Asterisks (*) indicate significant differences compared to the control (p < 0.05).
Effect of the phthalate mixture on folliculogenesis of F1, F2, and F3 female mice
In the F1 generation, prenatal exposure to the phthalate mixture (200 μg/kg/day) caused a borderline decrease in primordial follicles compared to control (Figure 2-A; n = 6 to 9 females per treatment group, ^ 0.05 < p < 0.1, borderline significance). In the F2 generation, the mixture did not affect the number of follicles compared to control (Figure 2-B; n = 6 to 11 females per treatment group). However, in the F3 generation, the phthalate mixture (20 μg/kg/day and 500 mg/kg/day) increased the number of primary follicles compared to control (Figure 2-C; n = 4 to 9 females per treatment group, * p ≤ 0.05, ^ 0.05 < p < 0.1, borderline significance).
Figure 2.
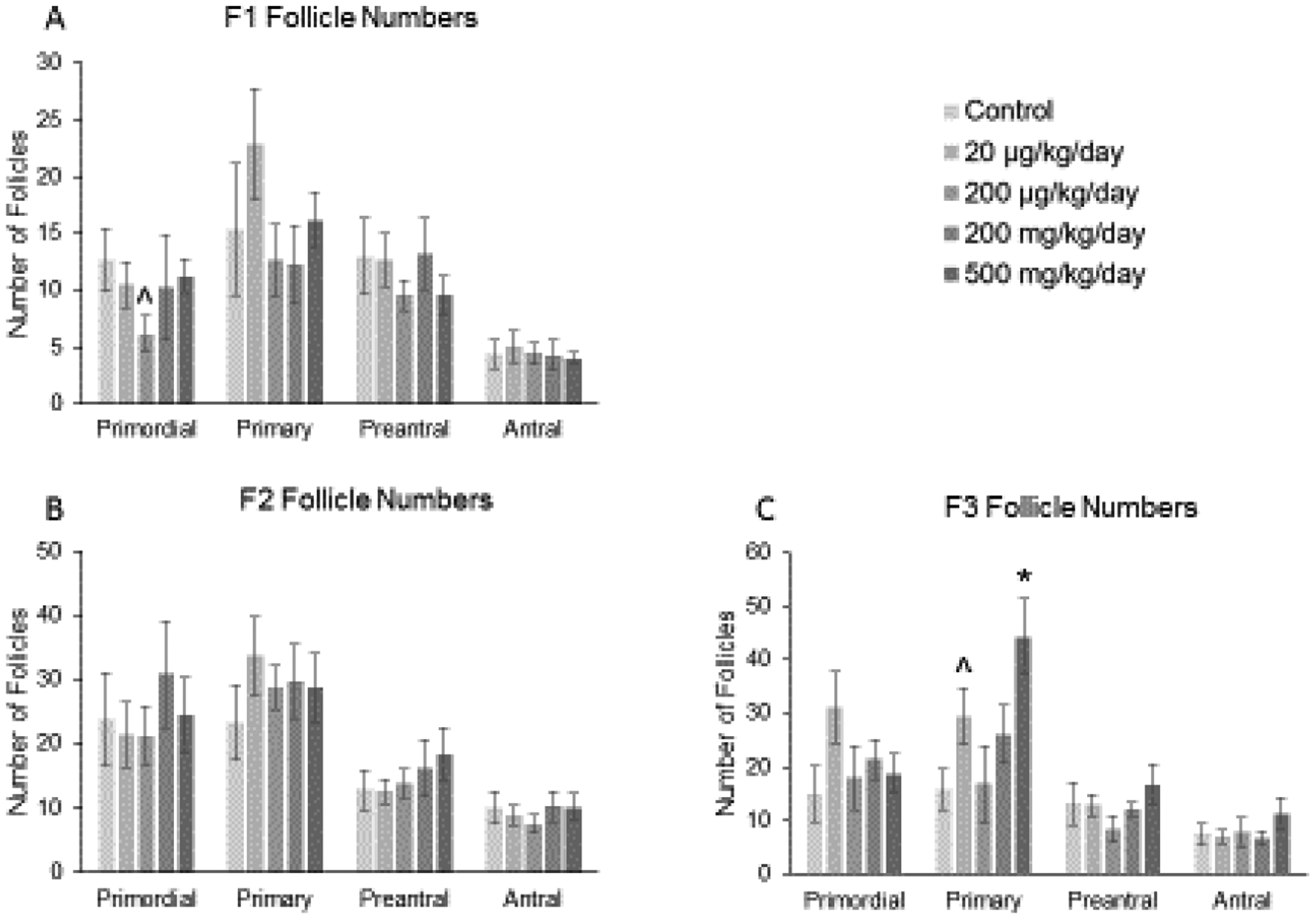
Effect of prenatal exposure to the phthalate mixture on follicle numbers at 13 months of age in the F1, F2, and F3 generations of mice. Ovaries from the F1, F2, and F3 generations were subjected to histological evaluation of follicle numbers. Follicles in the F1 generation (panel A; control = 6 females/treatment group, 20 μg/kg/day = 8–9 females/treatment group, 200 μg/kg/day = 9 females/treatment group, 200 mg/kg/day = 7–8 females/treatment group, 500 mg/kg/day = 9 females/treatment group), F2 generation (panel B; control = 7 females/treatment group, 20 μg/kg/day = 10–11 females/treatment group, 200 μg/kg/day = 7 females/treatment group, 200 mg/kg/day = 9 females/treatment group, 500 mg/kg/day = 6 females/treatment group), and the F3 generation (panel C; control = 6 females/treatment group, 20 μg/kg/day = 8–9 females/treatment group, 200 μg/kg/day = 6 females/treatment group, 200 mg/kg/day = 6–7 females/treatment group, 500 mg/kg/day = 4 females/treatment group) were counted and separated by stage of development in each treatment group. Graphs represent means ± SEM in the F1 generation, F2 generation, and the F3 generation. Asterisks (*) indicate significant differences compared to the control (p < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < p < 0.1).
In addition, the percent of each follicle type was examined to observe the effects of the phthalate mixture on shifts in folliculogenesis, or the ability of the follicles to progress from one follicle type to another. In the F1 generation, the phthalate mixture did not affect folliculogenesis (Figure 3-A; n = 5 to 9 females per treatment group), but in the F2 generation, the mixture (500 mg/kg/day) significantly decreased the percent of antral follicles compared to control (Figure 3-B; n = 5 to 11 females per treatment group, * p ≤ 0.05). In the F3 generation, the phthalate mixture (500 mg/kg/day) caused a borderline increase in the percent of primary follicles, it (20 and 200 μg/kg/day, 200 mg/kg/day) decreased the percent of preantral follicles, and it (20 μg/kg/day) caused a borderline decrease in the percent of antral follicles compared to the control group (Figure 3-C; n = 3 to 9 females per treatment group, * p ≤ 0.05, ^ 0.05 < p < 0.1, borderline significance).
Figure 3.
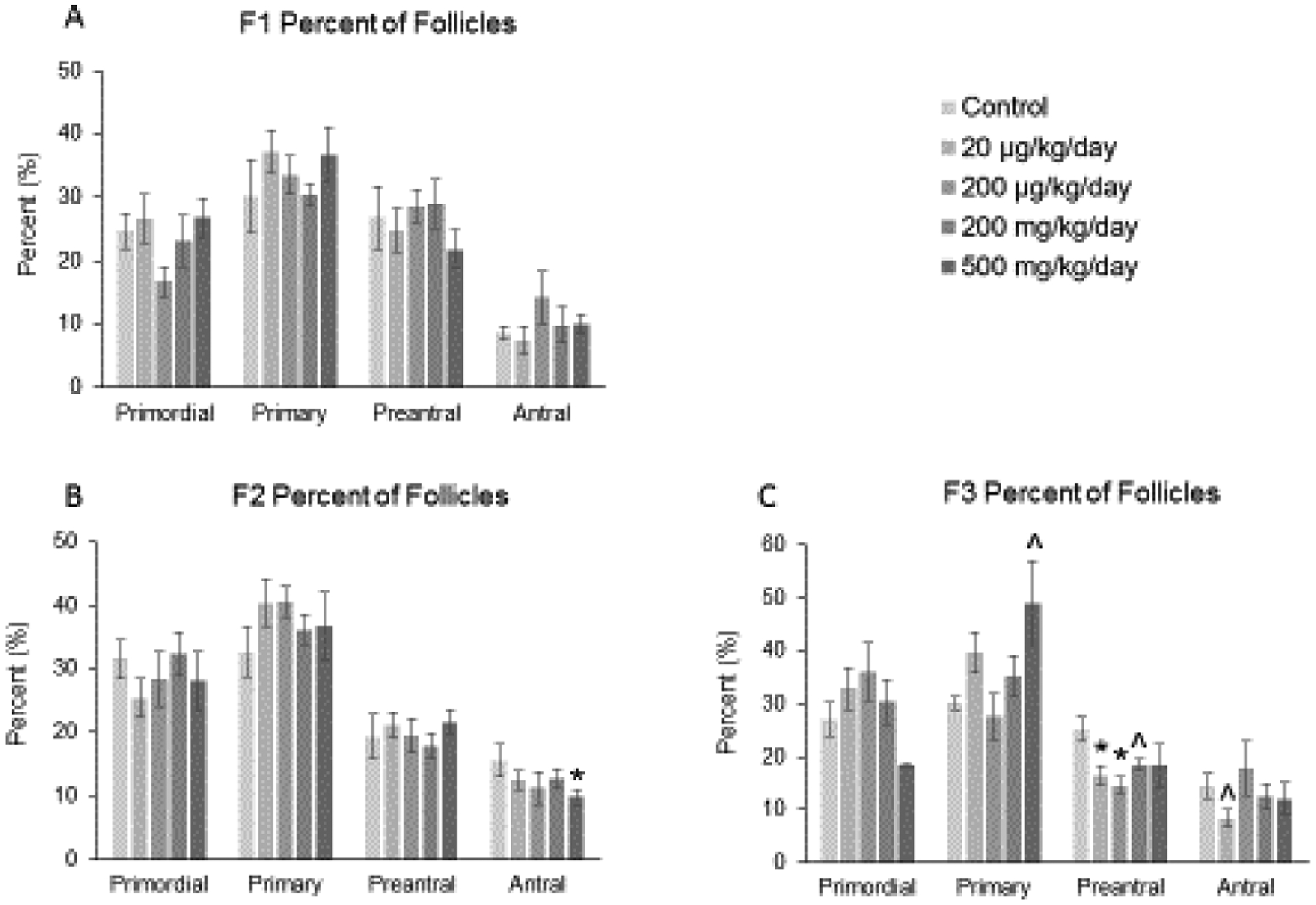
Effect of prenatal exposure to the phthalate mixture on the percentage of follicle types per ovary at 13 months of age in the F1, F2, and F3 generations of mice. Ovaries from the F1, F2, and F3 generations were subjected to histological evaluation for the percentage of each follicle type. Follicles in the F1 generation (panel A; control = 5–6 females/treatment group, 20 μg/kg/day = 9 females/treatment group, 200 μg/kg/day = 9 females/treatment group, 200 mg/kg/day = 7–8 females/treatment group, 500 mg/kg/day = 9 females/treatment group), F2 generation (panel B; control = 7 females/treatment group, 20 μg/kg/day = 11 females/treatment group, 200 μg/kg/day = 7 females/treatment group, 200 mg/kg/day = 9 females/treatment group, 500 mg/kg/day = 5–6 females/treatment group), and the F3 generation (panel C; control = 5–6 females/treatment group, 20 μg/kg/day = 8–9 females/treatment group, 200 μg/kg/day = 5–6 females/treatment group, 200 mg/kg/day = 6–7 females/treatment group, 500 mg/kg/day = 3–4 females/treatment group) were counted and separated by stage of development, and percentages of each follicle type were calculated for each treatment group. Graphs represent means ± SEM in the F1 generation, F2 generation, and the F3 generation. Asterisks (*) indicate significant differences compared to the control (p < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < p < 0.1).
Prenatal exposure to the phthalate mixture did not affect total follicle numbers, the number of corpora lutea, or the number of abnormal follicles in the F1, F2, or F3 generations of female mice (data not shown). However, the mixture (200 μg/kg/day) caused a borderline decrease in the percent of atretic follicles in the F2 generation compared to the control group (n = 6 to 11 females per treatment group, ^ 0.05 < p < 0.1, borderline significance; data not shown).
No statistically significant changes were observed in the number of ovaries with cysts compared to the control treatment group (Table 2). Although it should be mentioned that in the F1 generation, the 200 μg/kg/day group had 77.8% of ovaries with cysts and the 200 mg/kg/day group had 62.5% of ovaries with cysts compared to the control group with 33.3% of ovaries with cysts (Table 2; n = 6 to 9 females per treatment group). Moreover, in the F3 generation, the 200 μg/kg/day group had 85.7% of ovaries with cysts and the 500 mg/kg/day group had 100% of ovaries with cysts compared to the control group with 66.7% of cysts (Table 2; n = 4 to 9 females per treatment group).
Table 2.
Effects of prenatal exposure to phthalate mixture on the percent of histologically visible cystic ovaries in 13 month old mice
| Percent (%) Females with Cystic Ovaries | |||
|---|---|---|---|
| Generation | |||
| Treatment | F1 | F2 | F3 |
| Control | 33.3 | 71.4 | 66.7 |
| 20 μg/kg/day | 55.6 | 81.8 | 55.6 |
| 200 μg/kg/day | 77.8 | 85.7 | 85.7 |
| 200 mg/kg/day | 62.5 | 55.6 | 57.1 |
| 500 mg/kg/day | 55.6 | 50.0 | 100.0 |
Effect of the phthalate mixture on serum sex steroid, gonadotropin, and peptide hormone levels of F1, F2, and F3 female mice
In collaboration with Li et al. [20], we previously determined that in the F2 generation, prenatal exposure to the mixture (500 mg/kg/day) caused a borderline increase in estradiol levels and it (200 μg/kg/day and 200 mg/kg/day) significantly decreased progesterone levels compared to control mice. However, the mixture did not affect levels of estradiol or progesterone in the F1 or F3 generations of female mice [20]. In the current study, in the F1 generation, the phthalate mixture (200 and 500 mg/kg/day) significantly decreased the levels of testosterone compared to control (Figure 4-A; n = 7 to 9 females per treatment group, * p ≤ 0.05). In the F2 generation, it (20 and 200 μg/kg/day, 200 and 500 mg/kg/day) decreased levels of testosterone compared to control (Figure 4-A; n = 5 to 11 females per treatment group, * p ≤ 0.05, ^ 0.05 < p < 0.1, borderline significance). However, in the F3 generation, the mixture did not affect the levels of testosterone compared to the control group (Figure 4-A; n = 4 to 9 females per treatment group).
Figure 4.
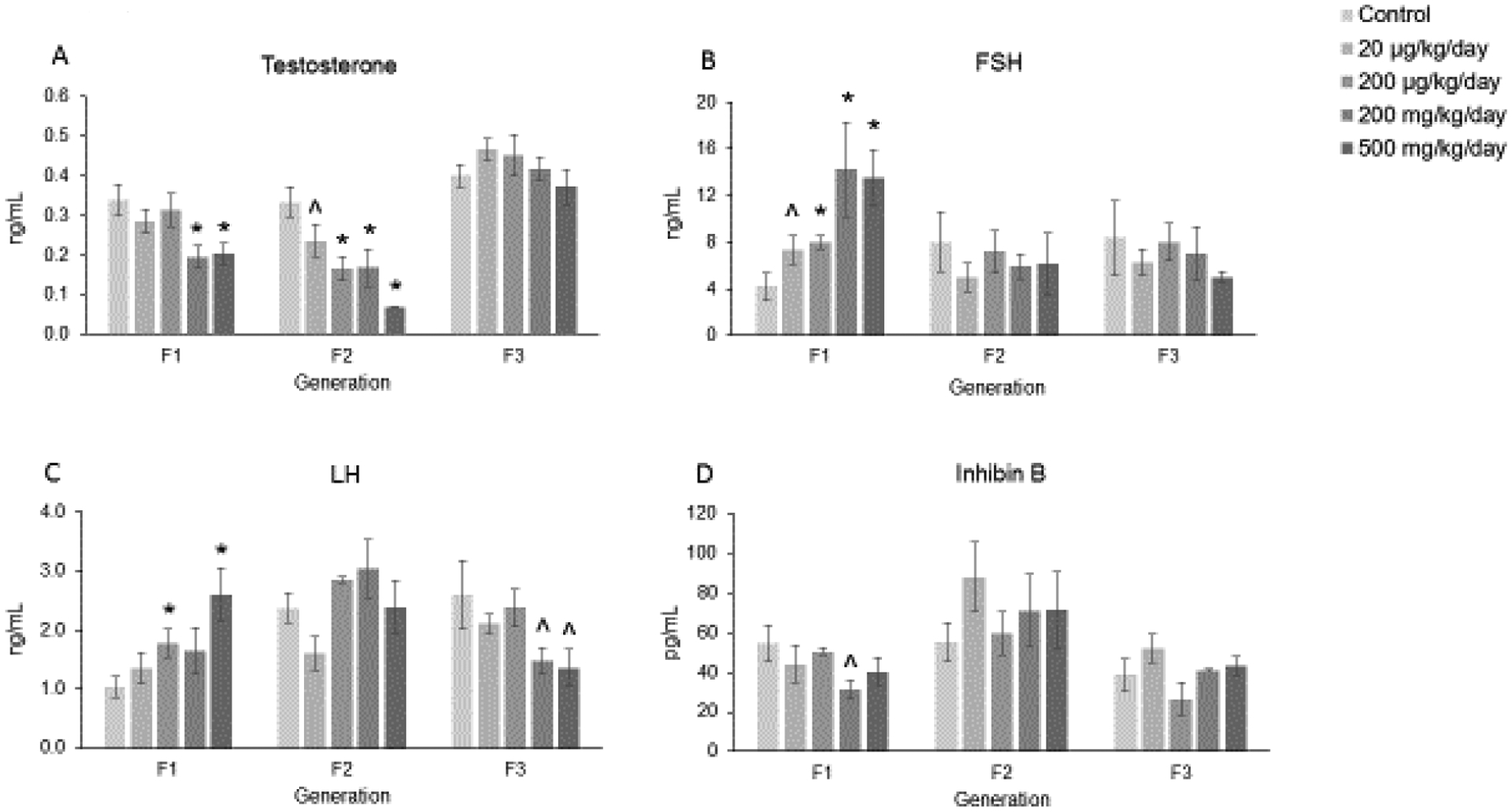
Effect of prenatal exposure to the phthalate mixture on serum sex steroid, gonadotropin, and peptide hormone levels at 13 months of age in the F1, F2, and F3 generations of mice. Sera were subjected to ELISAs or RIAs for the measurements of testosterone (panel A; F1 = 7–9 females/treatment group, F2 = 5–11 females/treatment group, F3 = 4–9 females/treatment group), FSH (panel B; F1 = 5–9 females/treatment group, F2 = 6–10 females/treatment group, F3 = 4–9 females/treatment group), LH (panel C; F1 = 6–9 females/treatment group, F2 = 6–11 females/treatment group, F3 = 4–9 females/treatment group), and inhibin B (panel D; F1 = 7–9 females/treatment group, F2 = 6–10 females/treatment group, F3 = 4–9 females/treatment group). Graphs represent means ± SEM in the F1 generation, F2 generation, and the F3 generation. Asterisks (*) indicate significant differences compared to the control (p < 0.05) and carets (^) indicate borderline significance compared to the control (0.05 < p < 0.1).
The mixture affected levels of FSH and LH in the F1 and F3 generations of female mice. In the F1 generation, prenatal exposure to the mixture (20 and 200 μg/kg/day, 200 and 500 mg/kg/day) significantly increased levels of FSH compared to the control treated mice (Figure 4-B; n = 5 to 9 females per treatment group, * p ≤ 0.05, ^ 0.05 < p < 0.1, borderline significance). In contrast, the mixture did not affect levels of FSH in the F2 or F3 generations compared to control (Figure 4-B; n = 4 to 10 females per treatment group). When examining levels of LH in the F1 generation, the mixture (200 μg/kg/day and 500 mg/kg/day) significantly increased levels of LH compared to control (Figure 4-C; n = 6 to 9 females per treatment group, * p ≤ 0.05). In the F2 generation, the mixture did not affect levels of LH compared to control (Figure 4-C; n = 6 to 11 females per treatment group). However, in the F3 generation, prenatal exposure to the phthalate mixture (200 and 500 mg/kg/day) caused borderline decreases in LH levels compared to the control group (Figure 4-C; n = 4 to 9 females per treatment group, ^ 0.05 < p < 0.1, borderline significance).
In the F1 generation, the mixture (200 mg/kg/day) caused a borderline decrease in the level of inhibin B compared to the control group (Figure 4-D; n = 7 to 9 females per treatment group, ^ 0.05 < p < 0.1, borderline significance). In the F2 and F3 generations, the mixture did not affect levels of inhibin B mice compared to control (Figure 4-D; n = 4 to 10 females per treatment group).
Discussion
In this study, we tested the hypothesis that prenatal exposure to an environmentally relevant phthalate mixture accelerates biomarkers of reproductive aging in multiple generations of female mice. Biomarkers of reproductive aging include acyclicity, a decrease in the ovarian follicle pool, an increase in the occurrence of cystic ovaries (rodents), and a dysregulation of the hormones involved in the hypothalamic-pituitary-ovarian (HPO) axis [30–32]. Previously, we found that prenatal exposure to the single phthalate DEHP accelerates reproductive aging in multiple generations of female mice [15]. The current study expands our previous work by using a relevant phthalate mixture consisting of six phthalates instead of a single phthalate.
In the F1 generation of female mice, we found that prenatal exposure to the phthalate mixture had minimal effects on the follicle pool, but it did alter hormones involved in the HPO axis, including testosterone, FSH, LH, and inhibin B. In the F2 generation, the mixture decreased the percent of antral follicles and affected estradiol, progesterone, and testosterone hormone levels in aging females. Finally, in the F3 generation, prenatal exposure to the phthalate mixture increased ovarian weight, caused irregular cyclicity, altered follicle numbers, and decreased the levels of LH in female mice. It is interesting that the effects of the phthalate mixture were not the same in each generation of mice. This is most likely due to the window of exposure being different for each generation. The F1 generation was directly exposed as the developing pup within the pregnant dam, the F2 generation was directly exposed via the developing ovaries within the developing pup, and the F3 generation is the first generation that does not have direct exposure to the mixture. Effects observed in the F1 and F2 generations are considered multigenerational, but effects observed in the F3 generation are considered transgenerational. In addition, the results did not follow a linear dose-response curve. Non-monotonic dose responses are typical of endocrine disrupting chemicals, are characterized by a U-shaped or bell-shaped curve, and have been observed in other studies examining phthalate exposure [8–18, 20, 25].
Previous studies in this laboratory found that prenatal exposure to the phthalate mixture did not alter estrous cyclicity at 13 months of age in female mice in the F1 or F2 generations [16, 17]. In the current study, in the F3 generation, female mice spent less time in estrus and more time in diestrus/metestrus compared to control. Increased time spent in diestrus, and eventually acyclicity in rodents, is consistent with reproductive aging [33]. Moreover, the estrous cycle was only affected in the F3 generation, but not in the F1 or F2 generations. This could be due to the window of exposure being different in each generation. In a similar study from our laboratory, we found that prenatal exposure to DEHP increased time spent in metestrus/diestrus in the F1 and F3 generations, but not the F2 generation in one year old female mice [15]. It is interesting that at approximately one year of age, prenatal exposure to the single phthalate DEHP and the mixture of phthalates did not affect estrous cyclicity in the F2 generation of female mice. In addition, time spent in estrus and metestrus/diestrus was altered in every treatment group except for the 200 mg/kg/day group. In collaboration with Li et al. we found that in the F3 generation, the mice in the 200 mg/kg/day group had increased levels of estradiol, however this was not statistically significant compared to control [20]. Further, it was found that prenatal exposure to the mixture caused a borderline significant decrease in the levels of LH in the 200 mg/kg/group compared to control. Together, it is possible that because of the trend towards increased estradiol levels and decreased levels of LH, mice may have been spending more time in estrus compared to the other treatment groups in the F3 generation. However, future studies should examine this in greater detail.
Prenatal exposure to the phthalate mixture increased body weight of female mice in the F2 generation [17], but it did not affect body weight in the F1 or F3 generations of mice [16, 17]. In addition, the mixture increased ovarian weight of 13 month old mice in the F3 generation, but it did not affect other tissue weights in the F1, F2, or F3 generations of female mice [16, 17]. It is possible that the mixture increased ovarian weight by increasing the number of total follicles or corpora lutea compared to control treated animals. However, when we examined the effects of the mixture on total follicle numbers and corpora lutea, we did not observe statistically significant differences compared to the control groups (data not shown). Although the mixture did not significantly affect the number of follicles or corpora lutea, the size of the structures may have been larger in diameter, leading to increased ovarian weight. For example, a study examining the ovary at advanced reproductive age found that corpora lutea from reproductively old mice, with increased ovarian weight, had a larger average cross-sectional area than corpora lutea from young controls [34]. Additionally, in previous studies, the mixture significantly increased the occurrence of grossly visible cystic ovaries compared to control [16, 17]. Although, in the current study in the F3 generation, the mixture did not significantly increase the occurrence of grossly visible ovarian cysts compared to controls. Future studies should examine the diameter of corpora lutea and antral follicles in aging female mice.
Due to some cysts not being grossly visible during tissue collection, ovaries with cysts observed during histological examination were noted. Despite not observing increases in gross appearances, when histologically examining the cysts in the F3 generation, we found that the mixture (200 μg/kg/day and 500 mg/kg/day) increased the occurrence of histologically visible cysts compared to the control group. These increases were not statistically significant, most likely due to our small sample size in the F3 generation. Nilsson et al. found that prenatal exposure to endocrine disrupting chemicals including pesticides and a plastic mixture from embryonic day 8 to 14 increased the number of ovarian cysts in the F1 and F3 generations of one year old female mice [35]. The formation of cystic ovaries is common as rodents age [36]. Therefore, the increase in cystic ovaries in our treated groups could be occurring due to accelerated reproductive aging in these female mice.
At 13 months of age, we found that prenatal exposure to the mixture caused a borderline decrease in the absolute number of primordial follicles in the F1 generation, but it did not affect the overall percentage of different follicle types compared to control. A decreased primordial follicle pool is indicative of reproductive aging in females [32], so the mixture may be accelerating the natural decline in the number of primordial follicles. In the F2 generation, the mixture did not affect the absolute number of follicles, but it did decrease the percentage of antral follicles compared to control. Lastly, in the F3 generation, the phthalate mixture increased the number and percent of primary follicles and it decreased the percent of preantral and antral follicles compared to control. Although we did not observe major effects of the mixture on follicle numbers, it is possible that the health of the oocyte may be compromised with mixture exposure. As females age, reactive oxygen species accumulate in the ovary, causing oxidative stress [37–40]. Oxidative stress can lead to mitochondrial dysfunction, accumulated DNA damage, and telomere shortening in aging oocytes [39]. In addition, the DNA damage caused by oxidative stress can disrupt methylation processes, modify chromatin organization, and lead to hypermethylation of gene promoter regions, which could lead to epigenetic alterations [41–43]. Phthalates have been shown to produce oxidative stress and induce epigenetic changes in female mice [44, 45]. Specifically, adult exposure to DEHP increased levels of reactive oxygen species, caused DNA and mitochondrial damage, and affected epigenetic alterations in mouse oocytes [44]. Furthermore, prenatal exposure to DEHP altered oxidative stress factors and DNA methylation processes in multiple generations of female mice, suggesting that DNA methylation may serve as an epigenetic mechanism underlying the observed transgenerational effects [45]. Future studies should examine if prenatal exposure to the phthalate mixture accelerates an increase in oxidative stress in aging females earlier than in control treated females and determine if this occurs in multiple generations of mice.
Our data showed that prenatal exposure to the phthalate mixture dysregulated some of the hormones involved in the HPO axis in the F1, F2, and F3 generations of aging female mice. In the F1 generation, the mixture decreased testosterone and inhibin B levels, but increased FSH and LH levels compared to control. Decreases in sex steroids like testosterone and the peptide hormone inhibin B and increases in gonadotropin hormones are normal with reproductive aging [46]. Therefore, the phthalate mixture may be accelerating reproductive aging by dysregulating the HPO axis sooner than the control treated animals. In the F2 generation, the mixture decreased testosterone and in collaboration with Li et al. [20], we found that the mixture decreased progesterone hormone levels compared to control. Another study from our laboratory found that prenatal exposure to DEHP decreased levels of testosterone and progesterone in the F2 generation in aging female mice compared to controls [15]. Last, in the F3 generation, the mixture only caused a borderline decrease in the levels of LH compared to control.
The results in the current study examining a mixture of six phthalates varies from the previous study from our laboratory examining the same endpoints, but with the single phthalate DEHP. Many of the effects observed in the DEHP study occurred in the higher treatment groups of 500 and 750 mg/kg/day [15], however in the mixture study, the levels of DEHP in each treatment group were much lower than in previous studies with DEHP alone. The amount of DEHP in the phthalate mixture falls between 4.2 μg/kg/day (20 μg/kg/day mixture dose) to 105 mg/kg/day (500 mg/kg/day mixture dose), clearly displaying a large difference in the amount of DEHP the mice were exposed to prenatally. It is possible that the mixture of phthalates may be causing synergistic or additive effects compared to the exposure to the single phthalate. Future studies should examine how prenatal exposure to the individual phthalates in the mixture affect reproductive aging and if these effects occur at relevant exposure levels or much higher levels used in toxicology studies. In addition, it would be interesting to determine if mixtures of high molecular weight phthalates versus low molecular weight phthalates display different effects on female reproductive aging.
Collectively, this study found that prenatal exposure to an environmentally relevant phthalate mixture may accelerate some biomarkers of reproductive aging in multiple generations of aging female mice. In the F1 generation, the mixture altered hormone levels of testosterone, FSH, LH, and inhibin B. In addition, it increased the occurrence of cystic ovaries in the F1 generation, but this was not statistically significant, most likely due to our small sample size. In the F2 generation, the mixture decreased testosterone levels. Last, in the F3 generation, the mixture increased time spent in metestrus/diestrus, a marker of reproductive aging, and it altered follicle numbers and the shift in the follicle pool, another marker of reproductive aging. However, further work is needed to elucidate how the mixture is affecting the ovary before 13 months of age, if the mixture is affecting the health of the oocyte in aging females, and the mechanisms of reproductive aging across generations. In addition, the ovary can show direct signs of aging by increasing fibrosis, inflammation, and oxidative stress [37, 38, 40, 47]. Further, some studies suggest that low levels of anti-Müllerian hormone are a good measure of ovarian aging [31]. Therefore, future studies should examine if prenatal exposure to the phthalate mixture increases signs of direct ovarian aging such as fibrosis, inflammation, oxidative stress, and low anti-Müllerian hormone levels.
Highlights.
Prenatal phthalate exposure decreased sex steroid hormones in the F1 generation.
Prenatal phthalate exposure increased gonadotropin hormones in the F1 generation.
Phthalate exposure decreased percentage of antral follicles in the F2 generation.
Prenatal phthalate exposure caused irregular cyclicity in the F3 generation.
Prenatal phthalate exposure altered follicle numbers in the F3 generation.
Acknowledgments
The authors thank the member of the Flaws laboratory group for their assistance. We would also like to thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core that is supported by the supported by the Eunice Kennedy Shriver NICHD Grant R24 HD102061 for assistance with measuring serum hormone levels. This work was supported by R01 ES032163 (JF), the NIH Office for Research on Women’s Health, a Toxicology Scholar Award (CZ), and a Billie A. Field Fellowship (EB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest Statement
The authors do not have any conflicts of interest or potential conflicts of interest to disclose.
References
- 1.Schettler T, Human exposure to phthalates via consumer products. Int J Androl, 2006. 29(1): p. 134–9; discussion 181–5. [DOI] [PubMed] [Google Scholar]
- 2.Braun JM, Sathyanarayana S, and Hauser R, Phthalate exposure and children’s health. Curr Opin Pediatr, 2013. 25(2): p. 247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Council NR, Phthalates and Cumulative Risk Assessment: The Tasks Ahead. 2008, Washington, DC: The National Academies Press; 208. [PubMed] [Google Scholar]
- 4.Rudel RA, et al. , Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environmental Science & Technology, 2003. 37(20): p. 4543–4553. [DOI] [PubMed] [Google Scholar]
- 5.Wittassek M, et al. , Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res, 2011. 55(1): p. 7–31. [DOI] [PubMed] [Google Scholar]
- 6.Johns LE, et al. , Exposure assessment issues in epidemiology studies of phthalates. Environ Int, 2015. 85: p. 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, et al. , Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertil Steril, 2019. 111(5): p. 953–961. [DOI] [PubMed] [Google Scholar]
- 8.Hannon PR and Flaws JA, The effects of phthalates on the ovary. Front Endocrinol (Lausanne), 2015. 6: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannon PR, Peretz J, and Flaws JA, Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod, 2014. 90(6): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon PR, Niermann S, and Flaws JA, Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol Sci, 2016. 150(1): p. 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niermann S, et al. , Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol, 2015. 53: p. 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rattan S, et al. , Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod, 2018. 98(1): p. 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rattan S, et al. , Di(2-Ethylhexyl) Phthalate Exposure During Prenatal Development Causes Adverse Transgenerational Effects on Female Fertility in Mice. Toxicol Sci, 2018. 163(2): p. 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang C and Flaws JA, Subchronic Exposure to Di(2-ethylhexyl) Phthalate and Diisononyl Phthalate During Adulthood Has Immediate and Long-Term Reproductive Consequences in Female Mice. Toxicol Sci, 2019. 168(2): p. 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brehm E, et al. , Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 2018. 159(2): p. 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C, Gao L, and Flaws JA, Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol, 2017. 318: p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou C, Gao L, and Flaws JA, Exposure to an Environmentally Relevant Phthalate Mixture Causes Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 2017. 158(6): p. 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C and Flaws JA, Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol Sci, 2017. 156(1): p. 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdy MM, et al. , A possible approach to improving the reproducibility of urinary concentrations of phthalate metabolites and phenols during pregnancy. J Expo Sci Environ Epidemiol, 2018. 28(5): p. 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li K, et al. , Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol, 2020. 93: p. 178–190. [DOI] [PubMed] [Google Scholar]
- 21.Kavlock R, et al. , NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reproductive Toxicology, 2002. 16(5): p. 529–653. [DOI] [PubMed] [Google Scholar]
- 22.Koch HM and Calafat AM, Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci, 2009. 364(1526): p. 2063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hines CJ, et al. , Occupational exposure to diisononyl phthalate (DiNP) in polyvinyl chloride processing operations. Int Arch Occup Environ Health, 2012. 85(3): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 24.Babich MA, et al. , Risk assessment of oral exposure to diisononyl phthalate from children’s products. Regul Toxicol Pharmacol, 2004. 40(2): p. 151–67. [DOI] [PubMed] [Google Scholar]
- 25.Pocar P, et al. , Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology, 2012. 153(2): p. 937–48. [DOI] [PubMed] [Google Scholar]
- 26.Neier K, et al. , Longitudinal Metabolic Impacts of Perinatal Exposure to Phthalates and Phthalate Mixtures in Mice. Endocrinology, 2019. 160(7): p. 1613–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X and Craig ZR, Environmentally relevant exposure to dibutyl phthalate disrupts DNA damage repair gene expression in the mouse ovarydagger. Biol Reprod, 2019. 101(4): p. 854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman CG, Some New Observations on the Vaginal Smear of the Rat. Yale J Biol Med, 1944. 17(1): p. 99–112. [PMC free article] [PubMed] [Google Scholar]
- 29.Gallavan RH Jr., et al. , Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol, 1999. 13(5): p. 383–90. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Endocrinology of the Menopause. Endocrinol Metab Clin North Am, 2015. 44(3): p. 485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meczekalski B, et al. , Fertility in women of late reproductive age: the role of serum anti-Mullerian hormone (AMH) levels in its assessment. J Endocrinol Invest, 2016. 39(11): p. 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.te Velde ER and Pearson PL, The variability of female reproductive ageing. Hum Reprod Update, 2002. 8(2): p. 141–54. [DOI] [PubMed] [Google Scholar]
- 33.Scarbrough K and Wise PM, Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology, 1990. 126(2): p. 884–90. [DOI] [PubMed] [Google Scholar]
- 34.Mara JN, et al. , Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging, 2020. 12(10): p. 9686–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson E, et al. , Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One, 2012. 7(5): p. e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal J, et al. , Reproductive System and Mammary Gland, in Toxicologic Pathology. 2013, CRC Press. p. 717–830. [Google Scholar]
- 37.Agarwal A, Gupta S, and Sharma RK, Role of oxidative stress in female reproduction. Reprod Biol Endocrinol, 2005. 3: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim J and Luderer U, Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod, 2011. 84(4): p. 775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki H, et al. , Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front Endocrinol (Lausanne), 2019. 10: p. 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatone C, et al. , Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update, 2008. 14(2): p. 131–42. [DOI] [PubMed] [Google Scholar]
- 41.Menezo YJ, et al. , Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online, 2016. 33(6): p. 668–683. [DOI] [PubMed] [Google Scholar]
- 42.Menezo Y, Dale B, and Elder K, The negative impact of the environment on methylation/epigenetic marking in gametes and embryos: A plea for action to protect the fertility of future generations. Mol Reprod Dev, 2019. 86(10): p. 1273–1282. [DOI] [PubMed] [Google Scholar]
- 43.Guillaumet-Adkins A, et al. , Epigenetics and Oxidative Stress in Aging. Oxidative Medicine and Cellular Longevity, 2017. 2017: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Z, et al. , Plasticizer Bis(2-ethylhexyl) Phthalate Causes Meiosis Defects and Decreases Fertilization Ability of Mouse Oocytes in Vivo. J Agric Food Chem, 2019. 67(12): p. 3459–3468. [DOI] [PubMed] [Google Scholar]
- 45.Rattan S, et al. , Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicol Appl Pharmacol, 2019. 379: p. 114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santoro N, The menopause transition: an update. Hum Reprod Update, 2002. 8(2): p. 155–60. [DOI] [PubMed] [Google Scholar]
- 47.Briley SM, et al. , Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction, 2016. 152(3): p. 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]


