Abstract
Introduction
There is little consensus of quality measurements for restorative proctocolectomy with ileal pouch-anal anastomosis(RPC-IPAA) performed for ulcerative colitis(UC). The National Surgical Quality Improvement Program(NSQIP) cannot accurately classify RPC-IPAA staged approaches. We formed an IBD-surgery registry that added IBD-specific variables to NSQIP to study these staged approaches in greater detail.
Methods
We queried our validated database of IBD surgeries across 11 sites in the US from March 2017 to March 2019, containing general NSQIP and IBD-specific perioperative variables. We classified cases into delayed versus immediate pouch construction and looked for independent predictors of pouch delay and postoperative Clavien-Dindo complication severity.
Results
430 patients received index surgery or completed pouches. Among completed pouches, 46(28%) and 118(72%) were immediate and delayed pouches, respectively. Significant predictors for delayed pouch surgery included higher UC surgery volume(p=0.01) and absence of colonic dysplasia(p=0.04). Delayed pouch formation did not significantly predict complication severity.
Conclusions
Our data allows improved classification of complex operations. Curating disease-specific variables allows for better analysis of predictors of delayed versus immediate pouch construction and postoperative complication severity.
Keywords: Inflammatory bowel disease, ulcerative colitis, NSQIP, outcomes, restorative proctocolectomy
Short Summary
We applied our previously validated novel NSIP-IBD database for classifying complex, multi-stage surgical approaches for UC to a degree that was not possible prior to our collaborative effort. From this, we describe predictive factors for delayed pouch formation in UC RPC-IPAA with the largest multicenter effort to date.
Precis
Using two years of data from our novel multicenter inflammatory bowel disease database, we demonstrate the utility of collecting disease-specific data for perioperative and postoperative data not currently available in mainstream registries. We also describe current trends in delaying pouch formation in restorative proctocolectomy for ulcerative colitis.
Introduction
Ulcerative colitis(UC)is a chronic inflammatory disease that has seen increasing prevalence and incidence in North America, with current prevalence estimated at 286 cases per 100,000 person-years.1,2 North American UC incidence ranges between two and 19 cases per 100,000 person-years.1,3 Despite advances in medical management, operation remains the only curative modality for the colorectal manifestations of UC including medically refractory colitis and colorectal neoplasia.4 Restorative proctocolectomy with ileal pouch anal anastomosis(RPC-IPAA)is the procedure of choice for UC patients who wish to maintain transanal defecation. This procedure is often performed in a staged manner as the operation itself is complex and patients often require the initial surgical intervention when they are medically-refractory, receiving multiple immunosuppressive medications, and experiencing other sequelae of UC including anemia and malnutrition.5 Currently, much of the North American literature comparing RPC-IPAA staged approaches has been limited to single-center retrospective cohorts.
The American College of Surgeons National Surgical Quality Improvement Program(ACS-NSQIP)is a well-established retrospective database of over 200 preoperative, intraoperative, and postoperative variables collected by over 700 hospitals in the US. While ACS-NSQIP and its procedure-targeted datasets remain invaluable resources for surgical outcomes research, there is limited data specific to inflammatory bowel disease(IBD)operation.6,7 Important granular data that was previously unavailable include preoperative biologic use, presence of colonic dysplasia, anastomotic technique where applicable, and stoma formation and type. Because of this, our understanding of the key factors contributing to outcomes in pouch operation is incomplete at best.
To mitigate this, the NSQIP-IBD Collaborative was formed as a working group of clinicians and surgical quality experts from 11 sites in the US. The Collaborative’s mission is to build upon the strong foundation provided by ACS-NSQIP and collect more granular data specific to IBD surgical patients. This effort is the first to use ACS-NSQIP data to generate a disease-specific surgical outcomes database with statistical validity.8 In this descriptive report, we further demonstrate the utility of these previously validated data points, which may be used to accurately classify RPC-IPAA staged approaches and provide insight into the current patterns of RPC-IPAA decision making. We then explored potential preoperative predictors of whether patients received immediate or delayed pouch construction operation.
Materials and methods
Data collection
A multicenter retrospective deidentified NSQIP-based IBD database based on the colectomy and proctectomy modules from March 2017 to March 2019 was queried. The five new variables which were designed, collected and validated has been previously published.8 Since that publication we have also added a sixth variable which attempts to overcome deficiencies in Current Procedural Terminology(CPT)coding for IPAA to assess whether or not, during a proctectomy case, an IPAA was constructed. We included cases with a postoperative diagnosis of UC and who received either 1)index total proctocolectomy with IPAA(TPC-IPAA)or index subtotal colectomy(STC)or 2)completion proctectomy with IPAA(CP-IPAA).
Documented diagnosis of UC was determined using ICD-9 and ICD-10 codes. NSQIP-collected characteristics were gathered: age, sex, race, smoking status, body mass index(BMI), American Society of Anaesthesiologists(ASA)class, preoperative steroid use, albumin, hematocrit, Current Procedural Terminology(CPT)codes,9 and wound class. Variables unique to the IBD Collaborative database were also collected: immune modulator (e.g. 6MP, azathioprine, cyclosporine, and methotrexate) use, biologic use, 10% weight loss prior to operation, IBD type, anastomotic technique, and ileostomy/IPAA formation.
To date, NSQIP does not classify a unique ileostomy, IPAA formation, or anastomotic technique variable, nor does it specify the type of ileostomy received. Due to the limitations of CPT coding which current NSQIP relies on, we classified RPC-IPAA procedures staged approaches by supplementing preexisting NSQIP CPT codes with our novel ileostomy, IPAA and anastomotic technique variables. Additionally, our ileostomy data was compared to NSQIP’s data collected on concurrent procedures to assess how robust our ileostomy data was.
RPC-IPAA staged approach classification
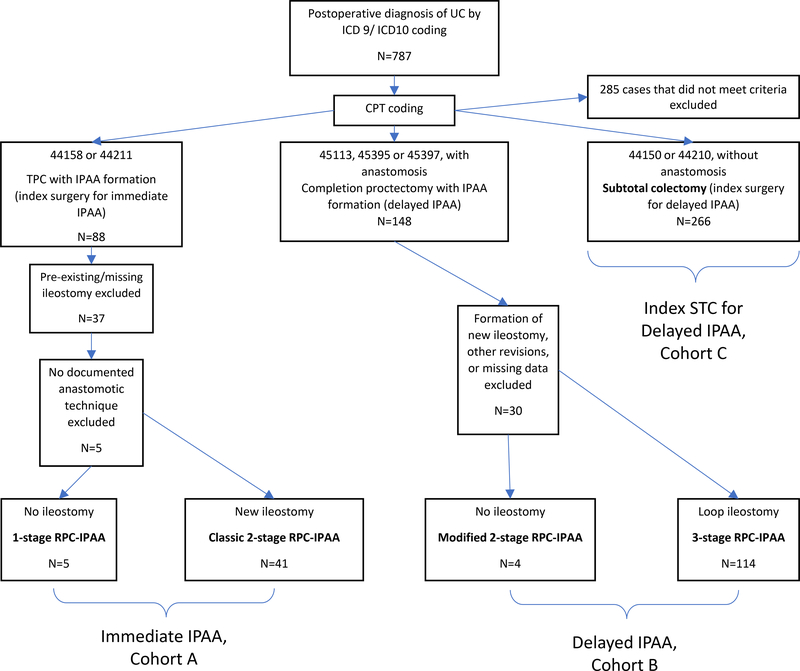
Principal CPT codes and data on ileostomy/IPAA formation were used to stratify cases into one of two RPC-IPAA staging approaches: 1-stage and classic 2-stage(immediate pouch construction) versus modified 2-stage and 3-stage(delayed pouch construction). We classified total proctocolectomies with IPAA(TPC)and subtotal colectomy(STC)as index surgeries for immediate and delayed pouch construction, respectively. For delayed pouch construction, we classified IPAA formation after delayed pouch construction as completion proctectomy with IPAA(CP-IPAA). Immediate and delayed pouches were defined as TPC-IPAA(Cohort A) and CP-IPAA(Cohort B), respectively; STCs for delayed IPAA during the study period are reported separately(Cohort C). Appendix B describes the CPT coding and ileostomy status used to classify these staged approaches.9 Figure 1 summarizes our classification schema and cohort allocation included operations. While CPT codes 45397 and 45395 are not standard codes for RPC-IPAA we have previously demonstrated that they are regularly used as a surrogate for laparoscopic completion proctectomy with IPAA.8 For all cases determined to involve pouch formation, we confirmed that they had an answer entered for RPC-IPAA anastomotic technique, a previously validated NSQIP-IBD targeted variable.8 This classification was then combined with IBD-specific and NSQIP baseline characteristics to determine the staged approach in each patient.
Figure 1:
Classification schema for RPC-IPAA staged approaches based on ICD coding, CPT coding, and ileostomy status.
Outcomes
We first analyzed preoperative characteristics to look for predictors of delayed versus immediate IPAA construction. Our postoperative outcome of interest was composite postoperative complication severity between immediate versus delayed pouch construction at IPAA formation(i.e. TPC vs. completion proctectomy w/ IPAA)as measured by the Clavien-Dindo scale derived from a previously published composite of 23 postoperative complications(30 days post-primary procedure)included in NSQIP.10
Statistical analysis
We presented preoperative characteristics of patients receiving each staged RPC-IPAA approach while comparing differences in delayed versus IPAA formation at both index operation and IPAA formation. (Tables 1(Cohort A vs. C) and Table 2(Cohort A vs. B)) Analysis was performed using SPSS(version 26,IBM 2019). Non-parametric tests(Mann-Whitney U, Kruskal-Wallis, and Fisher’s exact)were used to compare preoperative and intraoperative characteristics between the cohorts. We performed logistic binary regressions for predictors of IPAA delay. Significant predictors with p<0.05 in the final model predicting IPAA delay were reported with odds ratios(OR), 95% confidence interval(CI), and p-value by likelihood ratios.(Table 3) For Clavien-Dindo grade, we used IPAA delay as our predictor of interest, and assessed preoperative and intraoperative confounders using single-predictor linear regression. Again, predictors in the final model were presented with our predictor of interest as the model parameter B, 95% CI, and p-value.(Table 4) For both models, we entered predictors/confounders with p≤0.2 into a backwards stepwise selection process with a threshold of p=0.2. Finally, we compared 30-day anastomotic leak rates following immediate versus delayed pouch construction at the IPAA creation step.
Table 1:
Baseline characteristics and operative approach of cases with index surgeries, stratified by staged approach and IPAA delay. P-values represent comparisons between Cohorts A and C.
| Index Operation, UC patients
RPC-IPAA n=312 |
||||
|---|---|---|---|---|
| Cohort A, Immediate
IPAA N=46 |
Cohort C, Delayed
IPAA N=266 |
p-value | ||
| Parameter | One Stage RPC IPAA n=5 |
Proctocolectomy with IPAA and loop
ileostomy for classic 2 stage RPC-IPAA n=41 |
Subtotal Colectomy for modified 2 stage
or 3 stage RPC IPAA n=266 |
|
| Age at time of
Operation years median (IQR) |
24.3 (8.3) | 39.3 (25.4) | 38.4 (28.4) | 0.538 |
| Females n (%) |
2 (40) | 21 (51) | 115 (43) | 0.424 |
| White n (%) |
4 (80) | 34 (83) | 211 (79) | 0.870 |
| BMI kg/m^2 median (IQR) |
23.8 (8.5) | 24.5 (8.6) | 24.5 (7.6) | 0.704 |
| ASA Classification n (%) |
||||
| ASA 2 | 5 (100) | 26 (63.4) | 128 (48.1) | 0.076 |
| ASA 3 | 0 (0) | 15 (36.6) | 127 (47.7) | |
| ASA 4 | 0 (0) | 0 (0) | 10 (3.8) | |
| ASA 5 | 0 (0) | 0 (0) | 1 (0.4) | |
| Smoker within 1 year of
operation n (%) |
0 (0) | 0 (0) | 17 (6) | 0.087 |
| 10% weight loss in 6 mo. prior to
operation n (%) |
1 (20) | 2 (5) | 74 (28) | 0.001 |
| Steroid use for chronic
condition n (%) |
4 (80) | 21 (51) | 216 (81) | <0.001 |
| Biologic agent within 60 days prior to
operation n (%) |
3 (60) | 18 (44) | 168 (63) | 0.035 |
| Immune modulator use within 60 days prior
to operation n (%) |
0 (0) | 6 (15) | 49 (18) | 0.550 |
| Serum albumin (g/dL) mean (SD) |
4.5 (0.2) | 4.0 (0.6) | 3.2 (0.9) | <0.001 |
| HCT % median (IQR) |
43.1 (7.0) | 38.6 (6.3) | 35.1 (10.1) | 0.001 |
| WBC 103 per μL median (IQR) |
7.3 (4.1) | 7.4 (4.4) | 9.1 (5.4) | 0.002 |
| UC operation volume n (%) |
0.001 | |||
| <89 | 3 (60) | 31 (75.6) | 123 (46.2) | |
| 89+ | 2 (40) | 10 (24.4) | 143 (53.8) | |
| Colonic Dysplasia n (%) |
||||
| None | 4 (80.0) | 23 (56.1) | 234 (88.0) | |
| Low grade dysplasia | 1 (20.0) | 7 (17.1) | 9 (3.4) | <0.001 |
| High grade dysplasia | 0 (0) | 5 (12.2) | 2 (0.8) | |
| Colorectal cancer | 0 (0) | 1 (2.4) | 7 (2.6) | |
| Unable to determine/Not applicable | 0 (0) | 5 (12.2) | 14 (5.3) | |
| Approach n (%) |
||||
| MIS | 2 (40) | 30 (73.2) | 225 (84.6) | <0.001 |
| Open | 1 (20) | 9 (22.0) | 41 (15.4) | |
| Unknown | 2 (40) | 2 (4.9) | 0 (0) | |
BMI=body mass index, ASA=American Society of Anesthesiologists, IPAA=Ileal pouch anal anastomosis, IQR=interquartile range, RPC=restorative proctocolectomy, SD=standard deviation, HCT=hematocrit, WBC=white blood cells, UC=ulcerative colitis
Table 2:
Baseline characteristics and operative approach of cases with completed IPAA procedures, stratified by staged approach. P-values represent comparisons between Cohorts A and B.
| IPAA operations for UC
patients n=164 |
|||||
|---|---|---|---|---|---|
| Cohort A, Immediate
IPAA N=46 |
Cohort B, Delayed
IPAA N=118 |
p-value | |||
| Parameter | One Stage RPC IPAA n=5 |
Classic Two Stage
RPC-IPAA n=41 |
Modified Two Stage
RPC-IPAA n=4 |
Three Stage RPC-IPAA n=114 |
|
| Age at time of
Operation years median (IQR) |
24.3 (8.3) | 39.3 (25.4) | 40.6 (6.8) | 33.9 (20.4) | 0.493 |
| Females n (%) |
2 (40) | 21 (51) | 4 (100) | 45 (40) | 0.382 |
| White n (%) |
4 (80) | 34 (83) | 3 (75) | 96 (84) | 0.390 |
| BMI kg/m^2 mean (SD) |
23.9 (4.4) | 25.9 (5.3) | 28.0 (5.7) | 25.9 (5.1) | 0.673 |
| ASA Classification n (%) |
|||||
| ASA 1 | 0 (0) | 0 (0) | 0 (0) | 2 (1.8) | 0.684 |
| ASA 2 | 5 (100) | 26 (63.4) | 4 (100) | 65 (57) | |
| ASA 3 | 0 (0) | 15 (36.6) | 0 (0) | 46 (40.4) | |
| None assigned | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | |
| Smoker within 1 year of
operation n (%) |
0 (0) | 0 (0) | 0 (0) | 7 (6) | 0.192 |
| 10% weight loss in 6 mo. prior to
operation n (%) |
1 (20) | 2 (5) | 0 (0) | 6 (5) | 0.712 |
| Steroid use for chronic
condition n (%) |
4 (40) | 21 (51) | 1 (25) | 14 (12) | <0.001 |
| Biologic agent within 60 days prior to
operation n (%) |
3 (60) | 18 (44) | 1 (25) | 9 (8) | <0.001 |
| Immune modulator use within 60 days prior
to operation n (%) |
0 (0) | 6 (15) | 0 (0) | 3 (2.6) | 0.015 |
| Colonic Dysplasia n (%) |
|||||
| None | 4 (80) | 23 (56.1) | 4 (100) | 95 (83.3) | |
| Low grade dysplasia | 1 (20) | 7 (17.1) | 0 (0) | 2 (1.8) | <0.001 |
| High grade dysplasia | 0 (0) | 5 (12.2) | 0 (0) | 0 (0) | |
| Colorectal cancer | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) | |
| Unable to determine/Not applicable | 0 (0) | 5 (12.2) | 0 (0) | 17 (14.9) | |
| Serum albumin (g/dL) mean (SD) |
4.5 (0.2) | 4.0 (0.6) | 3.9 (0.3) | 4.2 (0.4) | 0.210 |
| HCT % median (IQR) |
43.1 (7.0) | 38.6 (6.4) | 38.8 (0.9) | 40.1 (7.1) | 0.371 |
| WBC 103 per μL median (IQR) |
7.3 (4.1) | 7.4 (4.4) | 6.7 (2.4) | 7.0 (2.5) | 0.530 |
| UC operation volume, n
cases n (%) |
0.014 | ||||
| <89 | 3 (60) | 31 (75.6) | 0 (0) | 62 (54.4) | |
| 89+ | 2 (40) | 10 (24.4) | 4 (100) | 52 (45.6) | |
| Approach n (%) |
|||||
| MIS | 2 (40) | 30 (73.2) | 2 (50) | 50 (43.9) | 0.011 |
| Open | 1 (20) | 9 (22.0) | 0 (0) | 53 (46.5) | |
| Unknown | 2 (40) | 2 (4.9) | 2 (50) | 11 (9.6) | |
BMI=body mass index, ASA=American Society of Anesthesiologists, IPAA=Ileal pouch anal anastomosis, IQR=interquartile range, RPC=restorative proctocolectomy, SD=standard deviation, HCT=hematocrit, WBC=white blood cells, UC=ulcerative colitis
Table 3:
Single-predictor and multi-predictor models for independent predictors of delayed versus immediate pouch operation at the index operation (i.e. Cohort C vs. Cohort A). Predictors were selected if there were significant at p<0.2 on single-predictor analysis. We then performed backward stepwise selection of predictors significant at p<0.2 with significant predictors in the final model shown. P-vales presented are based on likelihood ratios.
| Single-predictor Analysis | Multi-predictor Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | ||
| Lower | Upper | Lower | Upper | |||||
| Age, per year | 0.99 | 0.97 | 1.01 | 0.359 | ||||
| Female sex | 0.76 | 0.41 | 1.43 | 0.395 | ||||
| White race | 0.58 | 0.13 | 2.61 | 0.752 | ||||
| BMI, per kg/m2 | 1.00 | 0.95 | 1.05 | 0.966 | ||||
| ASA Classification, per class | 2.26 | 1.21 | 4.23 | 0.007 | ||||
| Current smoker within 1 year of operation | 2.98e8 | 0.00 | - | 0.018 | ||||
| 10% weight loss in 6 mo. prior to operation | 5.52 | 1.66 | 18.36 | 0.001 | ||||
| Steroid use for chronic condition | 3.63 | 1.88 | 7.00 | <0.001 | ||||
| Biologic use within 60 days of operation | 2.17 | 1.15 | 4.10 | 0.021 | ||||
| Immune modulator use within 60 days of operation | 1.54 | 0.62 | 3.84 | 0.28 | ||||
| Albumin, per g/dL | 0.27 | 0.15 | 0.49 | <0.001 | 0.27 | 0.13 | 0.56 | <0.001 |
| Hematocrit, per % | 0.91 | 0.85 | 0.96 | <0.001 | ||||
| WBC, per 10^3/μL | 1.18 | 1.05 | 1.32 | <0.001 | 1.27 | 1.07 | 1.50 | 0.002 |
| Upper quantile (89 or more) UC cases | 3.29 | 1.63 | 6.64 | <0.001 | 3.36 | 1.25 | 9.05 | 0.012 |
| Colonic Dysplasia (including colorectal cancer) | 0.15 | 0.07 | 0.33 | <0.001 | 0.26 | 0.08 | 0.9 | 0.036 |
WBC=White blood count
Table 4:
Multi-predictor model for significant predictors of greater Clavien-Dindo postoperative complication severity following IPAA formation for immediate vs. delayed pouch operation(i.e. Cohort A vs. Cohort B), respectively. Predictors, including pouch delay, in the final multi-predictor model are shown on the right.
| Single-predictor analysis | Multi-predictor analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Parameter Estimate | 95% CI |
p-value, Between-subjects effects | Parameter Estimate | 95% CI |
p-value | ||
| Lower | Upper | Lower | Upper | |||||
| Immediate versus delayed pouch formation | 0.34 | −0.264 | 0.945 | 0.268 | 0.38 | −0.22 | 0.98 | 0.21 |
| Age at time of Operation, per year | 0.01 | −0.01 | 0.029 | 0.331 | ||||
| Female sex | −0.013 | −0.563 | 0.536 | 0.962 | ||||
| White | 0.08 | −0.965 | 1.126 | 0.25 | ||||
| BMI, per kg/m^2 | 0.009 | −0.045 | 0.062 | 0.746 | ||||
| ASA Classification, per class | 0.489 | 0.023 | 0.954 | 0.04 | 0.51 | 0.042 | 0.97 | 0.033 |
| Smoker within 1 year of operation | −0.451 | −1.798 | 0.896 | 0.509 | ||||
| 10% weight loss in 6 mo. prior to operation | 0.668 | −0.525 | 1.861 | 0.27 | ||||
| Steroid use for chronic condition | 0.335 | −0.297 | 0.968 | 0.297 | ||||
| Biologic agent within 60 days prior to operation | 0.461 | −0.277 | 1.11 | 0.238 | ||||
| Immunomodulation within 60 days prior to operation | −0.136 | −1.328 | 1.055 | 0.262 | ||||
| Serum albumin, per g/dL | 0.034 | −0.642 | 0.709 | 0.921 | ||||
| HCT, per % | 0.004 | −0.049 | 0.057 | 0.88 | ||||
| WBC, per 10^3 per μL | 0.012 | −0.101 | 0.125 | 0.837 | ||||
| UC operative volume | 0.658 | |||||||
| <89 | 0.124 | −0.429 | 0.677 | |||||
| 89+ | Reference | |||||||
| Colonic Dysplasia (including CRC) | 0.71 | −0.203 | 1.623 | 0.203 | ||||
| Operative approach | ||||||||
| MIS | Reference | 0.625 | ||||||
| Open | −0.067 | −0.65 | 0.515 | |||||
| Unknown | 0.398 | −0.53 | 1.327 | |||||
| Anastomotic Technique | ||||||||
| Double staple | Reference | |||||||
| Hand sewn w/out mucosectomy | −1.179 | −4.685 | 2.326 | 0.624 | ||||
| Mucosectomy w/ hand sew | −0.179 | −1.949 | 1.59 | |||||
| Other | −1.179 | −3.216 | 0.857 | |||||
| Diverting ileostomy formation | −0.08 | −1.277 | 1.117 | 0.895 | ||||
BMI=body mass index, ASA=American Society of Anesthesiologists, IPAA=Ileal pouch anal anastomosis, IQR=interquartile range, RPC=restorative proctocolectomy, SD=standard deviation, HCT=hematocrit, WBC=white blood cells, UC=ulcerative colitis
The ACS-NSQIP and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Results
Descriptive Statistics
Of a total of 1809 operations for IBD over a two-year period, 787(44%) unique UC operations are reported. Appendix C summarizes the 10 most common CPT codes used for UC cases and their definitions. Age, gender, BMI, and description of other important baseline characteristics are shown in Tables 1 and 2. Our NSQIP-IBD database captured 629 ileostomies created in 787 UC cases(80%), compared to seven(0.9%)captured by NSQIP’s concurrent procedures variable via CPT code 44310: ileostomy or jejunostomy, non-tube. Four of these ileostomies were also captured by our database. Overall standard NSQIP did not properly capture 99% of all ileostomies using a concurrent procedures CPT code in these patients. Of the 787 cases, 266(34%)were subtotal colectomy (Cohort C) and 164(21%)were for IPAA. The overall median age was 37.3 years(IQR 26.1 years). 187(43.5%)of the patients were female. 46 patients received immediate IPAA (Cohort A) and 118 received delayed IPAA (Cohort B). Among immediate pouches, five(11%)were 1-stage and 41(89%)were classic 2-stage procedures. We also found 118 completed delayed pouches; four(3%)were modified 2-stage, and 114(97%)were 3-stage. Among 88 modified 2-stage and 3-stage IPAA cases coded using CPT 45113, 25(28%)were found to be minimally invasive(MIS). In contrast, 26/29(90%) of IPAA cases coded using CPT 45397 were MIS when using the operative approach variable in the targeted colectomy and proctectomy data sets.
Single-predictor Analysis
Compared to immediate IPAA, patients receiving index STCs for delayed IPAA were more likely to be taking steroids(p<0.001)and have 10% or greater weight loss in the 6 months prior to operation(p=0.002), lower serum albumin and hematocrit (p<0.001), and higher white blood cell count (p=0.025). Moreover, index STCs were more likely to be completed at higher volume centers(p=0.001).(Table 1) In contrast, patients receiving delayed IPAAs were less likely to be on biologics or steroids than their immediate IPAA counterparts. In this series the traditional 2-stage approach is currently the most commonly performed procedure for dysplasia or cancer, in 13/16 (81%) of cases.(Tables 1 and 2) Among index surgeries, STCs were most likely to be performed via MIS(p<0.001), while delayed IPAA cases evenly split between open and MIS approaches(p=0.001, Tables 1 and 2).
Multi-predictor Analysis
We performed multi-predictor analysis of index surgeries for immediate versus delayed pouch formation using predictors significant at p=0.2. Higher WBC(OR=1.27;95%CI(1.07,1.50))and upper quantile of operation volume(89 or more UC cases of any kind)(OR=3.36;95%CI(1.25,9.05))were independent predictors for receiving delayed pouch operation. Higher serum albumin(OR=0.27;95%CI(0.13,0.56))and colonic dysplasia(OR=0.26;95%CI(0.08,0.90))were independent predictors against receiving delayed pouch operation.(Table 3)
In our multi-predictor analysis of postoperative complication severity, we found that ASA classification(p=0.033) was an independent predictor of complication severity following pouch construction regardless of timing. Biologics, immunomodulator use, weight loss, diverting ileostomy formation, and delayed versus immediate pouch formation were not significant predictors for complication severity following pouch creation.(Table 4)
Finally, we found that 6 of 147 reporting cases(4.1%, 95% CI(1.3,7.7)%)experienced anastomotic leak within 30 days of their pouch operation. Three(7%) immediate pouches and three(3%) delayed pouches experienced anastomotic leak.
Discussion
Our description of preoperative characteristics for IPAA formation suggests that delaying pouch operation appears to be fruitful; while they tend to be less healthy at the index operation, these patients much more closely resemble their immediate pouch construction counterparts when they are ready for IPAA.(Tables 1 and 2) We found that delayed pouches were more likely to be performed at higher volume centers, for sicker patients, and for patients without colonic dysplasia.(Table 3)
Delaying pouch construction was not an independent predictor of worse or better postoperative complication severity after index operation or pouch construction, thus suggesting that surgeons are delaying pouch construction only when necessary based on preoperative presentation.(Table 4) While UC case volume was an independent predictor of delaying pouch construction, it did not predict complication severity following index operation or pouch creation. Unfortunately, this statement is limited by lack of longitudinal data currently available in NSQIP and our database. Immediate pouch operation appeared to be more likely than delayed pouch cases to result in anastomotic leak. This is likely due to the relatively low number of events in the entire sample of completed pouch surgeries with anastomotic leak data(6/142,4.2%)which limits our ability to perform an adjusted analysis. This question is still a matter of debate and driven by surgeon opinion, but we anticipate more clarity as our database matures and collects more longitudinal data.
These results show the utility of our database’s granularity in categorizing complex surgical procedures essential to UC management. Indeed, the preoperative characteristics displayed among our RPC-IPAA and STC cohorts are like those previously reported. For example, the literature has reported an upward trend of 3 stage and modified 2 stage RPC-IPAA, due in part to the advent of potent biologic immunosuppressants in UC management leading to patients delaying their surgeries to a time at which they have experienced more sequelae of living with this chronic disease than in the past. This trend is reflected in our population of patients receiving index STCs for RPC-IPAA. Most patients will no longer be on these medications after their STC and prior to their IPAA and most will have had the opportunity to recover their nutritional status and overall health and wellbeing. This is then reflected in the relative lack of immunosuppressant use in our modified 2-stage and 3-stage IPAA group. Together, these findings show that our data collection and classification are clinically accurate using variables not currently available through traditional NSQIP colectomy and proctectomy modules, which are primarily focused on cancer-specific variables.6,7
Our data also represent the collaborative’s ability to overcome important obstacles currently faced by retrospective IBD surgical research. First, large retrospective surgical outcomes research is reliant on CPT coding to determine whether a procedure of interest was performed. No single CPT code can capture the complexity of staged approaches performed for RPC-IPAA as they all include phrases such as “with or without ileostomy.” Additionally, secondary CPT capture in NSQIP is inadequate. Since 44310 could be either ileostomy or jejunostomy, the remaining 3 cases not captured by our ileostomy variable were assigned indeterminate ileostomy status and may have either been incorrectly classified or represent jejunostomies. Thus, there is significant heterogeneity in how different studies classify RPC-IPAAs using NSQIP data. Our study is unique in its inclusion of CPT codes 45397 and 45395 for modified 2- or 3-stage IPAA surgeries; previous studies often omitted this code when using NSQIP to classify RPC-IPAAs.11–17 Clearly both codes do not describe an IPAA procedure, however, with the advent of minimally invasive operation, there is no clear alternative to describe a minimally invasive completion proctectomy with IPAA, thus coders opted to select the procedure which sounded like a close match even if it was an inaccurate description of the procedure.9 In the past, uncertainty surrounding the accuracy of using these codes has forced investigators to exclude codes such as 45397 and 45395 when categorizing modified 2-stage versus 3-stage RPC-IPAAs due to general ambiguity. Exclusion of these codes also means previous studies potentially ignored IPAA cases performed using MIS, or that MIS cases may be inaccurately classified under other codes. In our study we found that 30/118(25%)of all completion proctectomies with IPAA were coded as 45397 or 45395 and it can be therefore understood that at least a quarter of all procedures are being missed when these codes are excluded. Moreover, all 30 cases coded by these codes were performed MIS and accounted for 58% of completion proctectomies with IPAA performed via MIS, highlighting their use as surrogates for laparoscopic completion proctectomies with IPAA. By adding a simple yes/no IPAA variable and coupling this with ileostomy status and operative approach we can almost completely circumvent the weaknesses which CPT codes present when used in isolation.
These issues shed light on a larger problem: because CPT codes were originally designed for standardized billing purposes, they are inherently unreliable data sources for research. CPT coding may depend on how an institution’s billing practices document procedures, and study investigators may differ in which CPT codes they choose for retrospective studies. For example, almost 30% of IPAA cases coded using CPT 45113 were minimally invasive and could have just as easily been coded as 45397 instead. Often which is selected has more to do with the level at which they reimburse than their actual accuracy. For example, 45113 only reimburses 33.22 work relative value units(wRVUs)while 45397 offers 36.50 wRVUs.18 This makes standardizing definitions for complex surgeries such as RPC-IPAA a challenge in the current literature and limits an individual retrospective study’s generalizability. Yet, established multicenter databases, including ours, still must rely on CPT coding as other procedural coding systems are either less accurate or too difficult to extract in large scale.
Large, retrospective database studies can be quite difficult when the database was not specifically designed to answer the question that is being asked. Clearly NSQIP was not specifically designed to describe outcomes for just UC operation. Here we demonstrate that by simply adding a few key targeted variables we can open NSQIP up for use in outcomes research in UC. This effort has optimized NSQIP for IBD analysis and quality improvement and assessment initiatives.
We demonstrated that most pouches being done in these high volume IBD centers are being performed in a delayed pouch construction approach. This is likely due to the relative infirmity of patients undergoing index operation for UC as significantly more of these patients are malnourished, anemic, and on steroids and biologics. Indeed, our multi-predictor analysis found that that elevated WBC and lower albumin were predictors of STC for delayed pouches instead of total proctocolectomy for immediate pouches. Higher institutional volume was also an independent predictor of delayed pouch operation; this may be due to more complex UC presentation at higher volume centers, though institutional preference or experience for one approach versus another should also be considered. Whether one explanation is dominant over the other is difficult to determine.(Table 3)
Interestingly, the traditional 2-stage procedure appears to be the procedure of choice for patients with dysplasia, who tend to have a more indolent disease course and are generally healthier at the time of operation. Colonic dysplasia was also an independent predictor of receiving immediate versus delayed pouch operation. Additionally, there are high rates of MIS within these cohorts, demonstrating the greater adoption of these approaches in the treatment of complex UC.
Like any large retrospective database study, ours has weaknesses. As mentioned, our early series is small, especially among 1- and modified 2-stage procedures; this limits the power of some of our analyses, such as for anastomotic leak. This will allow us to set baselines, however, and as the collaborative expands, we expect our yearly numbers to grow exponentially. Data entry can be difficult for these complex procedures and we have spent much time ensuring the NSQIP surgical clinical reviewers have the appropriate training for this project. But more so than any previous study using NSQIP to look at outcomes in RPC-IPAA, studies employing this data set will be able to demonstrate a level of validity which was previously not possible.
Conclusions
We demonstrate that the NSQIP-IBD Collaborative database’s data curation allows for more granular classification of complex IBD surgeries than what was previously possible with standard NSQIP. We also show that collection of disease-specific variables give insight to the disease-specific profiles of patients receiving each type of staged operation. Moreover, we show that delaying pouch operation for patients with worse disease presentation allows for comparable outcome severity following index operation; patients requiring delayed pouches are optimized for eventual IPAA formation. While NSQIP has proven to be a strong foundation for outcomes-based operation research nationwide and was the foundation for our own database, large-scale studies of IBD surgeries such as RPC-IPAA cannot be reliably conducted without the disease specific variables that our Collaborative has been able to collect. This study can serve as a model for not just IBD operation, but also for continued improvement of NSQIP and other large databases.
Supplementary Material
Disclosures
WYL was supported by the National Institutes of Health TL1 Training Grant #1TL1TR001443. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV Jr., Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin Gastroenterol Hepatol. 2017;15(6):857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 3.Loftus CG, Loftus EV Jr., Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13(3):254–261. [DOI] [PubMed] [Google Scholar]
- 4.Cima RR, Pemberton JH. Medical and surgical management of chronic ulcerative colitis. Arch Surg. 2005;140(3):300–310. [DOI] [PubMed] [Google Scholar]
- 5.Swenson BR, Hollenbeak CS, Koltun WA. Factors affecting cost and length of stay associated with the ileal pouch-anal anastomosis. Dis Colon Rectum. 2003;46(6):754–761. [DOI] [PubMed] [Google Scholar]
- 6.NSQIP PUF User Guide 2018. American College of Surgeons National Surgical Quality Improvement Program 2018; https://www.facs.org/-/media/files/quality-programs/nsqip/nsqip_puf_userguide_2018.ashx.
- 7.Procedure Targeted NSQIP PUF User Guide 2018. American College of Surgeons National Surgical Quality Improvement Program 2018; https://www.facs.org/-/media/files/quality-programs/nsqip/pt_nsqip_puf_userguide_2018.ashx.
- 8.Eisenstein S, Holubar SD, Hilbert N, et al. The ACS National Surgical Quality Improvement Program-Inflammatory Bowel Disease Collaborative: Design, Implementation, and Validation of a Disease-specific Module. Inflamm Bowel Dis. 2019;25(11):1731–1739. [DOI] [PubMed] [Google Scholar]
- 9.CPT 2019 professional edition. 4th ed., revised. ed. [Chicago, Ill: ]: American Medical Association; 2019. [Google Scholar]
- 10.Gurien LA, Ra JH, Crandall M, Kerwin AJ, Tepas JJ 3rd. Clavien-Dindo Analysis of NSQIP Data Objectively Measures Patient-Focused Quality. Am Surg. 2019;85(8):789–793. [PubMed] [Google Scholar]
- 11.McKenna NP, Habermann EB, Glasgow AE, Mathis KL, Lightner AL. Risk factors for readmission following ileal pouch-Anal anastomosis: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Surg Res. 2018;229:324–331. [DOI] [PubMed] [Google Scholar]
- 12.de Campos-Lobato LF, Wells B, Wick E, et al. Predicting organ space surgical site infection with a nomogram. J Gastrointest Surg. 2009;13(11):1986–1992. [DOI] [PubMed] [Google Scholar]
- 13.Kauffman JD, Snyder CW, Danielson PD, Chandler NM. 30-Day Outcomes of Laparoscopic Versus Open Total Proctocolectomy with Ileoanal Anastomosis in Children and Young Adults: A Combined Analysis of the National Surgical Quality Improvement Project Pediatric and Adult Databases. J Laparoendosc Adv Surg Tech A. 2019;29(3):402–408. [DOI] [PubMed] [Google Scholar]
- 14.McKenna NP, Behm KT, Ubl DS, et al. Analysis of Postoperative Venous Thromboembolism in Patients With Chronic Ulcerative Colitis: Is It the Disease or the Operation? Dis Colon Rectum. 2017;60(7):714–722. [DOI] [PubMed] [Google Scholar]
- 15.McKenna NP, Glasgow AE, Cima RR, Habermann EB. Risk factors for organ space infection after ileal pouch anal anastomosis for chronic ulcerative colitis: An ACS NSQIP analysis. Am J Surg. 2018;216(5):900–905. [DOI] [PubMed] [Google Scholar]
- 16.McKenna NP, Potter DD, Bews KA, Glasgow AE, Mathis KL, Habermann EB. Ileal-pouch anal anastomosis in pediatric NSQIP: Does a laparoscopic approach reduce complications and length of stay? J Pediatr Surg. 2019;54(1):112–117. [DOI] [PubMed] [Google Scholar]
- 17.Wertzberger BE, Sherman SK, Byrn JC. Differences in short-term outcomes among patients undergoing IPAA with or without preoperative radiation: a National Surgical Quality Improvement Program analysis. Dis Colon Rectum. 2014;57(10):1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Work RVU Calculator (Relative Value Units). https://www.aapc.com/practice-management/rvu-calculator.aspx. Accessed February 8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.