Abstract
Background
The Wisconsin Cystic Fibrosis Neonatal Screening Project was a randomized clinical trial (RCT) revealing that children receiving an early diagnosis of CF via newborn screening (NBS) had improved nutritional outcomes but similar lung disease severity compared to those who presented clinically. Because the evaluations of these subjects by protocol ended in 2012, our objective was to assess long-term pulmonary and mortality outcomes.
Methods
Retrospective analysis of the RCT cohort utilized longitudinal outcome measures obtained from the Cystic Fibrosis Foundation Patient Registry (CFFPR). Data included screening assignment, clinical characteristics, percent predicted forced expiratory volume in 1 second (ppFEV1) and mortality. A random intercept model was used to compare the ppFEV1 decline of subjects between the two groups up to age 26 years. Mortality was analyzed using the Kaplan-Meier method.
Results
Of the 145 subjects who consented to the original study, 104 subjects met inclusion criteria and had adequate data in the CFFPR. Of 57 subjects in the screened group and 47 in the control group, the rates of ppFEV1 decline were 1.76%/year (95% CI 1.62 to 1.91%) and 1.43%/year (95% CI 1.26 to 1.60%), respectively (p<0.0002). Pseudomonas aeruginosa acquired before 2 years was partially responsible. There was no difference in mortality between the two groups.
Conclusions
NBS alone does not improve pulmonary outcomes in CF, particularly when other risk factors supervene. In an era prior to strict infection control and current therapies, NBS for CF may be associated with worse pulmonary outcomes.
Keywords: cystic fibrosis, newborn screening, forced expiratory volume, mortality, phenotype, Pseudomonas aeruginosa
1. Introduction
Although convincing evidence exists that newborn screening (NBS) for cystic fibrosis (CF) leads to better nutritional outcomes [1–4], there has been a paucity of studies evaluating long-term pulmonary and mortality outcomes. To our knowledge, only one previously published study compared cohort mortality with or without screening, and this evaluation suggested that better survival occurred after early diagnosis in New South Wales, Australia [5]. Our previous comparative assessment of lung function parameters in children up to the age of 16 years old enrolled and followed in the Wisconsin CF Neonatal Screening Project (hereafter, the Project) found no difference between the group identified early by NBS and the concurrent (“control”) group which generally presented with signs/symptoms [6]. However, the lack of difference was due in part to the young ages of the subjects at the time of analysis since most had typically normal spirometry data [6]. Subsequently, Tluczek et al reported an assessment at ages 8 to 18 years using the Wisconsin chest radiograph scoring system that showed more severe lung disease in the early diagnosis group [7]. Now, as the surviving subjects from the Project have reached adulthood, we hypothesized that re-evaluation of pulmonary and mortality outcomes could provide valuable information. The purpose of this study is to extend the analysis of outcomes by using data from the Cystic Fibrosis Foundation Patient Registry (CFFPR) [8].
When CF NBS was initiated with immunoreactive trypsinogen (IRT) in the 1980s [9], it was unknown if the benefits of NBS for CF would outweigh the risks [10]. In order to address this issue, the Project was designed as a prospective, comprehensive randomized clinical trial (RCT) involving statewide collaboration and longitudinal, systematic evaluations at the University of Wisconsin and the Children’s Hospital of Wisconsin [1,11]. The randomization method was valid [1] and designed to avoid selection bias. However, after 9 years of patient accrual, group variation was seen to occur [1,12,13]. A striking finding of the Project was that subjects in the control group had significantly more evidence of malnutrition throughout childhood compared to the screened group, despite having a lower prevalence of pancreatic insufficiency (PI) and receiving the same protocol-guided therapy [1,14]. In addition, patients with greater degrees of malnutrition were shown at 6 and 12 years of age to have more severe lung disease [15,16]. On the other hand, while the Project clearly demonstrated nutritional benefits of NBS for CF and suggested a favorable pulmonary association, the lung disease severity outcomes were unclear. This study addresses the long-term pulmonary and mortality outcomes of this cohort and provides long-term follow-up data regarding newborn screening as recommended by the Centers for Disease Control and Prevention (CDC) [17].
2. Materials and methods
2.1. Study design, population, and setting
This retrospective analysis of the Project’s RCT population included patients who were enrolled in the original trial and had longitudinal data captured in the CFFPR but excluded those patients who did not consent to participate in the original study, those without data in the CFFPR, those who presented with meconium ileus, and patients with intermediate (40–59 mmol/L) sweat chloride tests. All patients were derived from a complex design intended to avoid selection bias that may be summarized as follows [1,11,18]. Between April 15, 1985 and June 30, 1994, all newborns in the state of Wisconsin had an IRT screening test performed after randomization based on the laboratory-assigned number’s terminal digit — odd numbers being the “early diagnosis” or screened group — but the data were blinded and computer-stored in the other half (“standard diagnosis,” control, or comparison group) unless the parents (0.03%) requested the result. Families of neonates in the screened, early diagnosis group were notified via their primary care providers of positive results during the first postnatal month and seen for a sweat chloride test (pilocarpine iontophoresis) at approximately 6 weeks of age. Those infants diagnosed with CF (sweat chloride was ≥60 mmol/L) were invited to enroll in the longitudinal evaluation phase of the Project. If the family consented, the child was started on a standardized Evaluation and Treatment Protocol. Infants were followed every 6 weeks for the first year of life, and then every 3 months until age 21 years. In contrast, the IRT (and later IRT/DNA) results for neonates in the comparison group were not unveiled until the children in this group presented with symptoms of CF or reached 4 years of age when the screening results were “unblinded” [11,18]. Those who met the diagnostic threshold for a CF diagnosis were enrolled in the study and followed/treated just like the screened group [1]. With parental consultations and legal analyses, the Project was approved by the Institutional Review Board (IRB) of the University of Wisconsin-Madison in 1983 when all ethical dilemmas were resolved [18]. This long-term follow-up study was approved by the IRB of the University of Wisconsin-Madison in 2017.
2.2. Outcome measures
The primary outcomes assessed were percent predicted forced expiratory volume in 1 second (ppFEV1) decline over time and mortality. Percent predicted FEV1 was selected as a primary outcome due to its record of reproducibility and availability as a longitudinal measure of lung function over time [19]. The secondary outcome was the annual decline in percent predicted forced vital capacity (ppFVC). We also assessed data on acquisition of Pseudomonas aeruginosa (PA) and on survival.
2.3. Data collection
The names and study group assignment of subjects enrolled in the original Project were submitted to the 2016 CFFPR, and key variables were requested including those listed in Table 1, serial lung function data, indices of nutritional status, and other clinical information such as signs/symptoms at diagnosis and whether or not lung transplantation occurred. Annual data available in the CFFPR differed from the more frequent PA results in the RCT database because the original Project included respiratory cultures at least every 6 months (13). The CFFPR provided the research team with data sheets with the requested subjects’ names and their associated relevant data. The data was stored on a secure computer in the University of Wisconsin School of Medicine and Public Health Department of Pediatrics per IRB and CFFPR policies.
Table 1:
Demographic and Clinical Characteristics
| Screened N=57 |
Control N=47 |
||||
|---|---|---|---|---|---|
| Median | Rang‡ | Median | Range‡ | p-value | |
| Age at diagnosis (years) | 0.1 | 0–5.4 | 0.6 | 0–7.1 | <0.001 |
| Age of first positive Pseudomonas aeruginosa culture | 5.1 | 0.7–15.9 | 6.6 | 0.4–21.2 | 0.0460 |
| Baseline ppFEV1at age 7 years old | 96% | 43–120% | 98% | 30–122% | 0.1979 |
| N | % | N | % | ||
| Gender Female | 21 | 37% | 17 | 36% | 0.9999 |
| F508del homozygous | 32 | 57% | 21 | 45% | 0.3339 |
| Pancreatic Insufficient † | 53 | 93% | 38 | 81% | 0.1178 |
| Ever had a lung transplant | 4 | 7% | 4 | 9% | 0.9999 |
| Positive Pseudomonas aeruginosa culture by age 18 years | 51 | 89% | 37 | 80% | 0.2153 |
Pancreatic enzyme use detected during the follow-up period between age 7 and age 26
Range (smallest to ’arĝst value)
2.4. Statistical analysis
Demographics variables and baseline clinical characteristics were summarized in terms of medians and ranges or frequencies and percentages. A linear mixed model with subject specific random effects was used to analyze the decline of ppFEV1 between age 7 to 26 years. In the initial analysis, rounded age values were included as categorical variables. Predicted ppFEV1 values, along with the corresponding 95% confidence intervals, were calculated and shown in graphical format using smoothing splines. In order to quantify and compare the annual decline in lung function, we performed a regression analysis of lung function parameters on age as continuous values using a random intercept regression model. Both univariate and multivariate analyses were conducted to account for imbalance in clinical characteristics and prognostic factors between study arms. Specifically, in the multivariate analyses, age at diagnosis, infection with PA and pancreatic insufficient status were included as (time-varying) covariates. Furthermore, based on our a priori hypothesis, stratified analyses of lung function decline were conducted using random intercept regression models. Specifically, in these analyses, we performed a regression analysis of ppFEV1 values on age and stratified by age of first positive PA culture (≤2 years vs. > 2 years, and ≤5 years vs. > 5 years). Overall survival was defined from date of birth to date of death (any cause) or date of last follow-up if alive. The survival times of subjects alive at the last date of follow-up were censored. Overall survival was shown in graphical format using the Kaplan-Meier method and compared between groups using univariate Cox proportional hazard regression analysis. All reported p-values are two-sided and P<0.05 was used to define statistical significance. Statistical analyses were conducted using SAS software (SAS Institute Inc., Cary NC), version 9.4.
3. Results
3.1. Characteristics of the study population
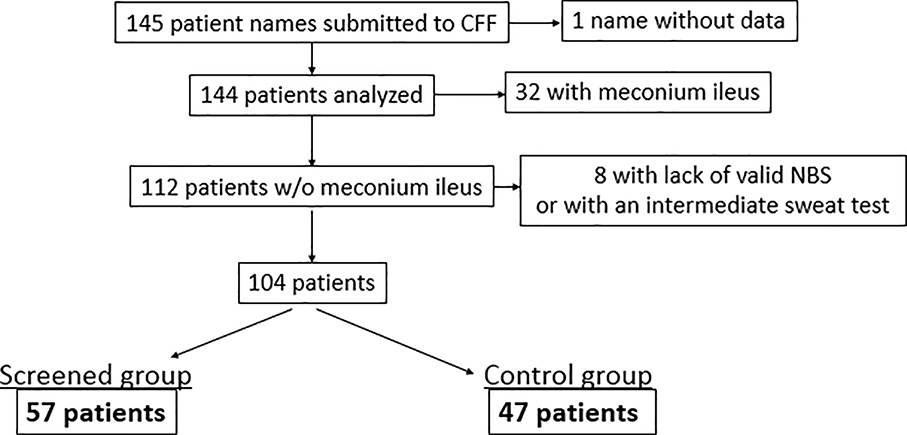
Of the 145 subjects who were enrolled in the Project, 144 had associated data in the 2016 CFFPR; 32 of these subjects were excluded because they presented with meconium ileus, and an additional 8 were excluded from analysis due to an invalid NBS test or an intermediate sweat chloride test result. This resulted in 104 subjects for analysis — 57 in the screened, early diagnosis group and 47 in the standard diagnosis (comparison) group (Figure 1).
Figure 1:
Consort diagram
Table 1 provides data on the key demographic and clinical characteristics of the two groups. As expected, the screened group had a significantly earlier age of diagnosis, with a median age of diagnosis of 0.1 years, compared to the median age of diagnosis of 0.6 years in the control group. As described previously [1], the screened group contains five patients with false negative NBS test results that delayed their diagnoses to 6.9, 19, 21, 124, and 281 weeks. It is notable that at baseline, the screened group had a greater percent PI compared to the control group, which previous studies have demonstrated to be due to a chance occurrence rather than a failure of randomization [1]. This feature and other characteristics listed in Table 1 were similar to those reported for the RCT cohorts, which confirms the representative nature of our sample. Notably, the age of first PA positive culture, defined as age of first positive culture recorded in the CFFPR, was younger in the screened group, with a median age of 5.1 years compared to 6.6 years in the comparison group. In addition, the percentage of patients with a positive PA culture prior to 18 years of age was greater in the screened group in keeping with previous observations [12,13].
3.2. Lung function
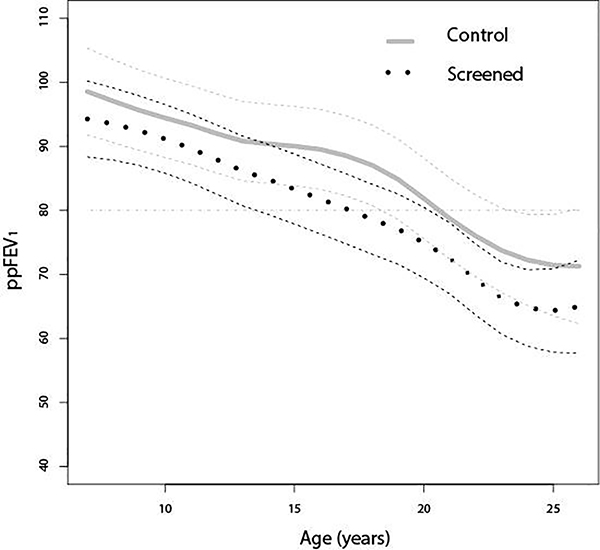
The ppFEV1 declined over time in both groups. However, the rate of decline was greater in the screened group compared with the control group (Figure 2). There was a mean ppFEV1 loss of 1.76% per year (95% CI: 1.62 to 1.91%) in the screened group compared to 1.43% per year (95% CI: 1.26 to 1.60%) in the control group between the ages of 7 years old and 26 years old (p<0.0002) (Table 2). Likewise, the screened group’s ppFVC decline at 1.11% per year (95% CI: 0.99 to 1.24%) was greater (p<0.0001) over time than the comparison, control group at 0.83% per year (95% CI: 0.68 to 0.97%).
Figure 2.
Smoothing Splines of Predicted Values and Corresponding 95% Confidence Interval Limits of Percent Predicted Forced Expiratory Volume in 1 Second (ppFEV1) Over Time in Screened Group and Control Group between age 7 and 26 years old
Table 2:
Unadjusted and adjusted comparisons of ppFEV1 and ppFVC decreases for screened vs. control arm from age 7 to age 26
| Screened N=57 |
Control N=47 |
|||||
|---|---|---|---|---|---|---|
| Lung Function Parameter | Slope1 (%/year) |
95% CI2 | Slope1 (%/year) |
95% CI2 | p-value3 | |
| Unadjusted Analysis | pp FEV1 | 1.76 | 1.62–1.91 | 1.43 | 1.21–1.60 | <0.0001 |
| ppFVC | 1.11 | 0.99–1.24 | 0.83 | 0.68–0.97 | <0.0001 | |
| Adjusted Analysis† | pp FEV1 | 1.79 | 1.64–1.94 | 1.52 | 1.34–1.70 | 0.0130 |
| ppFVC | 1.15 | 1.02–1.28 | 0.86 | 0.70–1.01 | 0.001 | |
ppFEV1 = percent predicted forced expiratory volume in 1 second
ppFVC = percent predicted forced vital capacity
: Adjusted by age at diagnosis, infection with Pseudomonas aeruginosa and pancreatic insufficient status
: Regression slope for decrease in ppFEV1 (%/year)
: 95% CI of regression slope
: Comparison of regression slope between Screened vs. Control
Given the greater rates of pancreatic insufficiency (PI), PA, and younger diagnosis age n the screened group, a repeat analysis was performed adjusting for PI and PA status as well as age at diagnosis. This analysis confirmed the greater decline in ppFEV1 (p=0.013) and ppFVC (p=0.001) in the screened group compared with the control group (Table 3).
Table 3:
Comparisons of ppFEV1 (decreases for screened vs. control arm from age 7 to age 26, stratified by age of first positive Pseudomonas aeruginosa culture
| Screened N=57 |
Control N=47 |
||||
|---|---|---|---|---|---|
| Age of first positive Pseudomonas aeruginosa culture |
Slope1 (%/year) |
95% CI2 | Slope1 (%/year) |
95% CI2 | p-value3 |
| ≤2 years (N=12) | 1.87 | 1.38–2.37 | 0.33 | −008–0.73 | <0.0001 |
| >2 years (N=7 5) | 1.74 | 1.54–1.94 | 2.22 | 1.94–2.51 | <0.0001 |
| ≤5 years (N=39) | 1.69 | 1.44–1.93 | 2.28 | 1.92–2.64 | <0.0001 |
| >5 years (N=48) | 1.83 | 1.56–2.11 | 1.68 | 1.27–2.10 | 0.3890 |
ppFEV1 = percent predicted forced expiratory volume in 1 seconc
: Regression slope for decrease in ppFEV1 (%/year)
: 95% CI of regression slope
: Comparison of regression slope between Screened vs. Control
3.3. Mortality
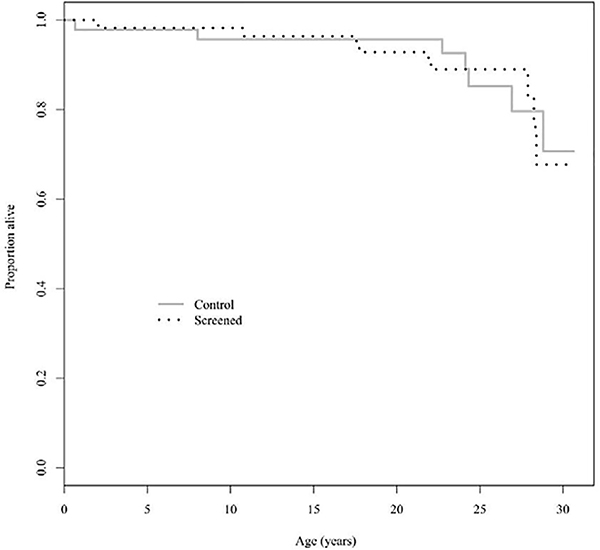
Figure 3 depicts the Kaplan-Meier curves for overall survival of each group in the study. With a hazard ratio of 1.07 (95% CI: 0.40–2.88) in the screened versus control group, there was no significant difference detected in overall survival between groups. At 25 years of age, 89% of screened group enrollees in the CFFPR and 85% of the control patients were alive.
Figure 3:
Kaplan-Meier Curve for Overall Survival
3.4. Stratified analyses ppFEV1 decline
Based on our a priori hypothesis, and previous suggestive data that earlier acquisition and infection with PA may have contributed to the worse pulmonary outcomes identified in the screened group, we also conducted an exploratory stratified analyses of ppFEV1 decline for the screened and control group. Specifically, by comparing ppFEV1 decline was compared between the screened group and control group after stratifying by age of first positive PA culture (Table 3). These results revealed that colonization with PA at ≤2 years of age was associated with a greater adjusted decline in ppFEV1 in the screened group compared to the control group. Conversely, infection with PA ≤5 years of age was associated with a greater adjusted decline in the ppFEV1 in the control group compared with the screened group.
4. Discussion
4.1. Longitudinal lung function decline
Based largely on CFFPR-accumulated data reflecting CF care practices in the final two decades of the 20th century, we found that lung function declined after age 10 years in both the screened and comparison group of the Wisconsin RCT, but was significantly greater in the screened group. This suggests that while NBS is effective at identifying cases of CF and early diagnosis of CF by NBS with subsequent management of nutrition can lead to improved nutritional and growth outcomes [1,14], NBS may be insufficient to improve pulmonary and mortality outcomes for patients with CF. Although the Wisconsin Project was designed to evaluate both nutritional and pulmonary outcomes with the best methods then available, it was much easier in retrospect to evaluate longitudinal nutritional status than the course of lung disease. Many intrinsic and extrinsic variables (risk factors) influence the clinical course of CF. It often takes a longer time period to appreciate the impact of variables affecting lung disease compared to the impact of variables affecting nutritional status. In particular, numerous environmental factors influence lung disease, including exposures to smoke, virulent respiratory bacterial pathogens such as mucoid PA, respiratory virus epidemics, etc. Results reported recently by Schlüter et al [20] from a 15-year longitudinal study revealed “no significant association between diagnosis by NBS and the rate of change in lung function, i.e., ppFEV1 decline.
Our research confirms that ppFEV1 decline over time is a valuable objective outcome measure to follow longitudinal lung function and compare populations This index of obstructive dysfunction was initially established by the Toronto team [19] and has more recently been useful to quantitate the impact of CFTR modulators over time [21]. In contrast, our attempt to compare the occurrence of pulmonary exacerbations in the two groups was not revealing [22].
The findings of our study are in contrast to some other short term studies that have reported better pulmonary function in children with CF identified by NBS compared to those who presented clinically, although none of those studies were RCTs and some assessed small, non-concurrent groups [23–27]. One possible explanation for this difference is that our Project was impaired by cumulative confounders such as genotype distribution, pancreatic functional status, and differential exposures to respiratory pathogens [1,12,13]. In addition, the Wisconsin RCT cohort that we followed experienced the health care setting in a different era. The enrolled pediatric CF patients grew up during 1980–2000 when the standard of care was very different from current practice, including 1) limited choices of oral antibiotics; 2) lack of PA eradication protocols; 3) absence of CFTR modulator therapies; 4) unavailability of dornase alfa until December 1993 when most of the subjects had been enrolled; 5) no use of inhaled hypertonic saline; and 6) no infection control protocols.
One hypothesis for our findings of a greater lung function decline over time in the screened group is an earlier acquisition of PA in the screened group [12,13]. In a previous publication [13], we reported data showing that the higher percentage of PA-positive cultures in patients who had been randomized to the screened group was not attributable to a higher number of overall cultures. Since this cohort entered the health care setting at a time prior to strict infection control, the screened group’s earlier diagnosis led them to become infected with pathogenic bacteria earlier than the comparison group as reported previously [12,13]. It is important to note that many of the patients with CF in the study were not initially segregated from each other and used common waiting rooms [12] which is no longer a practice in today’s health care setting. This confirms the report of Sanders et al [28] and suggests that very early PA colonization (at age 2 years or less) plays a significant role in lung function decline later in life, and therefore strict infection control policies, starting at the time of diagnosis, are critical.
4.2. Survival studies
As a comprehensive investigation of childhood CF epidemiology [11], the Wisconsin Neonatal Screening Project was planned to evaluate mortality at an appropriate time. Additionally, in an era of improved therapeutics, including the recent advent of CFTR modulator therapy [20], it is not surprising that we did not detect a survival difference. However, our survival findings are different from other studies. Although Dijk et. al [5] reported “a nonsignificant 43% mortality risk reduction in screened patients as compared to non-screened patients,” the Kaplan-Meier graphs they published reveal improved survival at age 25 years with 75% surviving in 60 screened patients compared to approximately 50% in 57 non-screened patients identified the previous 3 years (1978–81). Yet, their retrospective observational study excluded approximately one-third of the patients in each group with many lost to follow-up. Grosse et al. [2] also suggested better survival with screening from an analysis of 2001 CFFPR data assessing children without meconium ileus who were born between 1987 and 1991; specifically, the authors found that CF-related deaths in this cohort up to the age of 10 were lower by 1.5–2 per 100 children with CF born in states that performed NBS for CF compared to those born in states that did not screen [2].
4.3. Implications
A previous analysis of younger subjects in the Wisconsin Project found that significant determinants of pulmonary disease included poor growth reflecting malnutrition, infection with mucoid PA, hospitalizations, genotype, and meconium ileus [28]. This led the authors to conclude that “Early diagnosis of CF after NBS provides an opportunity for better care but does not ensure better outcomes, making NBS necessary but not sufficient to optimize outcomes for the majority of patients with CF” [28]. Fortunately, there are many modifiable extrinsic risk factors that also contribute to lung function decline, and the medical community has improved treatment of these since the cohort in this study entered the health care system.
4.4. Limitations
There are several limitations in this study, which is in essence a retrospective analysis of subjects enrolled in a previous prospective study. The original study was designed and powered primarily to assess nutritional status early in life. By examining outcomes such as mortality that the RCT was not designed to evaluate, there is the risk of identifying differences where none truly exist. Pulmonary function test values were also limited to patients older than 6 years who were capable of performing spirometry as more recent and sensitive methods were not available. In addition, the results in this study were limited to data available in the CFFPR. This is particularly significant when it comes to the PA observations reported herein.
It is important that these results be interpreted within context of current treatment standards. While the screened group in our cohort acquired PA at an earlier age than the control group [12,13], more recent studies have not shown an association between NBS and earlier acquisition of PA [29], and the article by Schlüter et al [20] reports that NBS can delay chronic PA infection. Now that strict infection control measures are in place, transmission between patients is less likely. We attempted to account for the intrinsic difference between the groups by adjusting for PI status, but we were unable to adjust for all of the possible confounding intrinsic differences between the two groups.
5. Conclusions
Early diagnosis of CF by NBS alone is not sufficient to improve longitudinal lung function decline. While there are risks to CF NBS, most notably psychosocial harms and early exposure to the healthcare [11], the benefits of early identification and initiation of treatment are sufficient that nearly worldwide implementation has occurred wherever CF is prevalent [30]. Dramatic advancements of the past decade in particular make it essential to interpret the results of this study in the context of current therapies. Thus, despite the risk we identified of worse pulmonary dysfunction, we conclude that NBS for CF is “necessary but not sufficient” [27] by itself to optimize outcomes.
Highlights.
The decline of ppFEV1 /year is a valuable index in longitudinal studies of lung disease
Survival data are no longer useful in cystic fibrosis cohort comparison studies
Newborn screening leads to better nutrition but may not lessen lung disease severity
Newborn screening is necessary but not sufficient to decease lung disease severity
Acknowledgements
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry.
In addition, we would like to thank funding from the NIH grant R01DK034108.
We also thank all the members of the Wisconsin Cystic Fibrosis Neonatal Screening Project team, including: Jeff Douglas, PhD, Norman Fost, MD, MPH, Christopher Green, MD, Ronald Gregg, PhD, Michael Kosorok, PhD, Ronald Laessig, PhD, Zhanhai Li, PhD, Mari Palta, PhD, Michael Rock, MD, Margie Rosenberg, PhD, Audrey Tluzcek, PhD, L. J. Wei, PhD, SusanWest, PhD, and Benjamin Wilfond, MD (University of Wisconsin Medical School, Madison); and W. Theodore Bruns, MD, William Gershan, MD, Elaine Mischler, MD, Mark Splaingard, MD, and Lee Rusakow (Medical College of Wisconsin, Milwaukee). The study was coordinated and managed superbly on a day-to-day basis at both sites by Anita Laxova. In addition, the group includes outstanding teams of biostatisticians (Rebecca Koscik, Sharon Shen, and Lan Zeng), nurses (Karen Moucha, Miriam Block, Holly Colby, Lynn Feenan, Mary Ellen Freeman, Catherine McCarthy, and Darci Pfeil), nutritionists (Lisa Davis, Mary Marcus, and Tami Miller), and the outstanding laboratory leaders of the Wisconsin State Laboratory of Hygiene’s newborn screening program (David Hassamer and Gary Hoffman).
Funding source: This study was supported by NIH grant R01DK034108 and funding from the Cystic Fibrosis Foundation.
Abbreviations
- CF
cystic fibrosis
- NBS
newborn screening
- ppFEV1
percent predicted forced expiratory volume in 1 second
Footnotes
Financial Disclosure Statement: Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai H, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics 2001;107(1):1–13. [DOI] [PubMed] [Google Scholar]
- 2.Grosse SD, Rosenfeld M, Devine OJ, Lai H, Farrell PM. Potential impact of newborn screening for cystic fibrosis on child survival: A systematic review and analysis. J Pediatr. 2006;149(3):362–6. [DOI] [PubMed] [Google Scholar]
- 3.Castellani C Evidence for newborn screening for cystic fibrosis. Paediatr Respir Rev. 2003;4(4):278–84. [DOI] [PubMed] [Google Scholar]
- 4.Waters DL, Wilcken B, Irwig L, Van Asperen P, Mellis C, Simpson JM, et al. Clinical outcomes of newborn screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed. 1999;80(1):F1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dijk FN, McKay K, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Arch Dis Child. 2011;96(12):1118–23. [DOI] [PubMed] [Google Scholar]
- 6.Farrell PM, Li Z, Kosorok MR, Laxova A, Green CC, Collins J, et al. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med. 2003;168(9):1100–8. [DOI] [PubMed] [Google Scholar]
- 7.Tluczek A, Becker T, Laxova A, Grieve A, Racine Gilles CN, Rock MJ, et al. Relationships among health-related quality of life, pulmonary health, and newborn screenin g for cystic fibrosis. Chest. 2011;140(1):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The cystic fibrosis foundation patient registry: Design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13(7):1173–9. [DOI] [PubMed] [Google Scholar]
- 9.Crossley JR, Elliott RBSP. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet. 1979;1(8114):472–4. [DOI] [PubMed] [Google Scholar]
- 10.Rock MJ, Mischler EH, Farrell PM, Wei; Lee-Jen Bruns; Theodore W, Hassemer DJ, et al. Newborn Screening for Cystic Fibrosis Is Complicated by Age-Related Decline in Immunoreactive Trypsinogen Levels. Pediatrics. 1990;85(6):1001–7. [PubMed] [Google Scholar]
- 11.Farrell PM. Advances in Pediatrics. Volume 47. St. Louis: Mosby, Inc, 2000. Chapter 3: Improving the health of patients with cystic fibrosis through newborn screening. p.79–115. [PubMed] [Google Scholar]
- 12.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, et al. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26(2):81–8. [DOI] [PubMed] [Google Scholar]
- 13.Farrell PM, Shen G, Splaingard M, Colby CE, Laxova A, Kosorok MR, et al. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics. 1997;100(5):E2. [DOI] [PubMed] [Google Scholar]
- 14.Farrell PM, Lai HJ, Li Z, Kosorok MR, Laxova A, Green CG, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J Pediatr. 2005;147(3 Suppl):S30–6. [DOI] [PubMed] [Google Scholar]
- 15.Lai HJ, Shoff SM, Farrell PM. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders DB, Zhang Z, Farrell PM, Lai H. Early life growth patterns persist for 12 years and impact outcomes in cystic fibrosis. J Cyst Fibros. 2018;17(4):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell RR. CDC Grand Rounds: Newborn Screening and Improved Outcomes [Internet]. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report (MMWR). 2012. [cited 2019 Sep 16]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6121a2.htm [PubMed]
- 18.Fost N, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: a unique ethical dilemma. Clin Res. 1989;37(3):495–500. [PubMed] [Google Scholar]
- 19.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131(6):809–14. [DOI] [PubMed] [Google Scholar]
- 20.Schlüter DK, Southern KW, Dryden C, Diggle P, Taylor-Robinson D. Impact of newborn screening on outcomes and social inequalities in cystic fibrosis: A UK CF registry-based study. Thorax. 2020;75(2):123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192(7):836–42. [DOI] [PubMed] [Google Scholar]
- 22.Sanders DB, Li Z, Parker-McGill K, Farrell P, Brody AS. Quantitative chest computerized tomography and FEV1 equally identify pulmonary exacerbation risk in children with cystic fibrosis. Pediatr Pulmonol. 2018;53(10):1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Accurso FJ, Sontag MK, Wagener JS. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J Pediatr. 2005;147(3 Suppl):S37–41. [DOI] [PubMed] [Google Scholar]
- 24.Mérelle ME, Schouten JP, Gerritsen J, Dankert-Roelse JE, Mérelle JMEP, Schouten J, et al. Influence of neonatal screening and centralized treatment on long-term clinical outcome and survival of CF patients. Eur Respir J. 2001;18(2):306–15. [DOI] [PubMed] [Google Scholar]
- 25.McKay KO, Waters DL, Gaskin KJ. The influence of newborn screening for cystic fibrosis on pulmonary outcomes in New South Wales. J Pediatr. 2005;147(3 Suppl):S47–50. [DOI] [PubMed] [Google Scholar]
- 26.Coffey MJ, Whitaker V, Gentin N, Junek R, Shalhoub C, Nightingale S, et al. Differences in outcomes between early and late diagnosis of cystic fibrosis in the newborn screening era. J Pediatr. 2017;181:137–45. [DOI] [PubMed] [Google Scholar]
- 27.Collins MS, Abbott MA, Wakefield DB, Lapin CD, Drapeau G, Hopfer SM, et al. Improved pulmonary and growth outcomes in cystic fibrosis by newborn screening. Pediatr Pulmonol. 2008;43(7):648–55. [DOI] [PubMed] [Google Scholar]
- 28.Sanders DB, Li Z, Laxova A, Rock MJ, Levy H, Collins J, et al. Risk factors for the progression of cystic fibrosis lung disease throughout childhood. Ann Am Thorac Soc. 2014;11(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SS, FitzSimmons SC, O’Leary LA, Rock MJ, Gwinn ML, Khoury MJ. Early diagnosis of cystic fibrosis in the newborn period and risk of Pseudomonas aeruginosa acquisition in the first 10 years of life: A registry-based longitudinal study. Pediatrics. 2001;107(2):274–9. [DOI] [PubMed] [Google Scholar]
- 30.Scotet V, Gutierrez H, Farrell P. Newborn screening for CF across the globe - where is it worthwhile? Intern J Neonatal Screen. 2020;6(18). [DOI] [PMC free article] [PubMed] [Google Scholar]