Abstract
Background:
Structural abnormalities in the orbitofrontal cortex (OFC) of incarcerated psychopaths have been well documented. However, the neural correlates of psychopathic traits in younger and nonclinical samples remain poorly understood.
Aim:
The present study aimed to examine the structural brain asymmetry in the OFC in relation to dimensions of psychopathic traits in adolescents from the community.
Method:
In 29 youths from the community, childhood psychopathic traits including narcissism, impulsivity, and callous- unemotional traits were assessed when they were 7- to 10 years old (Time 1), and their gray matter (GM) volumes were measured using structural Magnetic Resonance Imaging when they were 10- to 14 years old (Time 2).
Results:
After controlling for age, sex, IQ, pubertal stage, and whole-brain volumes, callous-unemotional traits were associated with right-left asymmetry in the medial OFC (mOFC), that is, smaller right mOFC GM as compared to the left. Impulsivity was associated with left-right asymmetry in the mOFC, that is, smaller left mOFC than the right. Narcissism was not associated with any GM asymmetry measure. No significant association was found for the lateral OFC, amygdala, caudate and putamen.
Conclusion:
The present findings provide further support that dimensions of psychopathic traits may have distinct neurobiological correlates.
Keywords: orbitofrontal cortex, psychopathy, adolescents, brain imaging, gray matter asymmetry
Introduction
Prior literature has shown that psychopathic traits are among the most significant risk factors for antisocial behaviors (Hoppenbrouwers et al., 2016). Specifically, half of the crimes in the U.S. were committed by adults who had shown psychopathic traits during adolescence (Loeber & Farrington, 2000). These criminal behaviors incur enormous psychological and financial burden for individuals’ families and society (e.g., Brodaty & Low, 2003). In addition, these psychopathic traits and antisocial behaviors are associated with a wide range of functioning deficits during adolescence and adulthood, including poor academic performance, reckless behaviors, neurocognitive impairments, and dysfunctional interpersonal relationship, as well as mental health problems in adulthood (Asscher et al., 2011; Benbenishty et al., 2016; Blair et al., 2006; Salekin, 2006; Viding et al., 2005; Wertz et al., 2018). Hence, it is essential to understand the etiology of these problematic traits and behaviors at early ages.
Psychopathy is a multi-faceted personality construct that is comprised of callous, antisocial, narcissistic, and impulsive features (Frick et al., 2003; Hare, 1991). In particular, callous-unemotional traits have been incorporated into the Diagnostic and Statistical Manual of Mental Disorders, the fifth edition (DSM–5; APA, 2013) and are being considered for the International Classification of Diseases, the 11th Revision (ICD-11; WHO, 2016). A number of assessment tools have been developed to assess psychopathic traits in adolescents and adults (e.g., Frick et al., 2014). Among them, the Inventory of Callous-Unemotional Traits (ICU) and the Antisocial Process Screening Device (APSD) are two of the most widely used ones in adolescent samples (Frick & Hare, 2001; Frick et al., 2014). The factor structure of the ICU has been validated in adolescents and juvenile offenders in different countries including Germany and the United States (Essau et al., 2006; Kimonis et al., 2008), and recently two meta-analyses have demonstrated the reliabilities of this tool (Deng et al., 2019; Ray & Frick, 2018). Similarly, the psychometric properties of the APSD (Frick & Hare, 2001) that assesses narcissism, impulsivity, and callous-unemotional traits have been well established in young populations (Munoz & Frick, 2007). Collectively, these results indicate that the ICU and APSD yield good reliability and validity in youth samples. In terms of the relationship between clinical measures of psychopathy and narcissism, narcissism and impulsivity are suggested to bear some resemblance to the interpersonal component of clinical psychopathy. However, the relationship for narcissism in a large sample of youth was not significant (Fink et al., 2012). Most of the prior studies focusing on the neural correlates in youth (Blair, 2003; White et al., 2012) have treated psychopathy as a unidimensional construct. Therefore, investigation of the neural correlates for the sub-dimensions of psychopathy is warranted. The present study aimed to investigate the multifaceted construct of psychopathy in adolescents: callous, narcissistic and impulsive features (Frick et al., 2003; Hare, 1991). Although the validated components of clinical measures of psychopathy in adults and in adolescents are more generally known as the interpersonal, affective, lifestyle, and antisocial components or facets, the present study aimed to examine three psychopathic traits including callous-unemotional traits, impulsivity and narcissism that are the three components that emerged in factor analyses of APSD present in non-clinical individuals.
Neuroimaging literature in recent years has implicated a set of brain regions in psychopathy (e.g., Anderson & Kiehl, 2012), broadly supporting the development of two neurobiological theories of psychopathy proposed by Blair (2006) and Kiehl (2006). Specifically, Blair’s model (2006, 2007) has mainly emphasized the role of the amygdala and the ventromedial prefrontal cortex (vmPFC)/OFC in psychopathy, while Kiehl’s model (2006) has primarily focused on the paralimbic system suggesting that all the paralimbic regions (e.g., insula) are dysfunctional in psychopathic individuals. There is one limitation of Kiehl’s hypothesis because of its lack of specificity in the paralimbic regions in relation to psychopathy. Because of this limitation in the paralimbic hypothesis and the fact that the anomalous activations in these regions vary from study to study, it is important to study which specific regions in the paralimbic system are implicated in psychopathy. Supporting Blair’s model, structural and functional abnormalities in the orbitofrontal cortex (OFC), a part of the prefrontal cortex that is responsible for generating flexible and adaptive behavior such as planning and executive control, have been documented in incarcerated psychopaths (e.g., Bertsch et al., 2013; Espinoza et al., 2018; Gregory et al., 2012; Koenigs, 2012; Korponay et al., 2017; Yang & Raine, 2009), although a few studies have failed to identify the OFC abnormalities in psychopathy (e.g., Poeppl et al., 2017). In contrast, the neuropsychological literature fails to support the idea of dysfunction in a number of regions implicated by the paralimibic hypothesis (e.g., Christianson et al., 1996). In a meta-analysis, Yang and Raine (2009) found reduced prefrontal structure and function (specifically in the right OFC, dorsolateral frontal, and anterior cingulate cortex) in antisocial, violent, and psychopathic individuals. Although there were no differences between the effects for antisocial samples described as psychopathy samples than for samples described as non-psychopathy samples, the OFC appears to be implicated in those with antisocial and psychopathic traits. Similarly, another review study also supported the association between psychopathy and structural and functional abnormalities in vmPFC/OFC and the anterior cingulate cortex (Koenigs, 2012). In addition, Knutson et al. (2001, 2003) found that the brain activities in the striatum and the vmPFC/OFC are implicated in reward anticipation and outcomes, respectively. Accordingly, structural and/or functional abnormalities in the OFC and striatum may contribute to poor decision-making and atypical reward processing seen in psychopathic individuals. Although prior studies found abnormalities in a number of brain regions other than the OFC, the findings were not consistent while the OFC has been consistently found to be associated with psychopathy across studies (e.g., Anderson & Kiehl, 2012; Bertsch et al., 2013; Ermer et al., 2012; Ermer et al., 2013; Yang & Raine, 2009). Since there have been more consistent findings regarding this brain region when compared to the others (e.g., caudate and putamen) across studies (e.g., Anderson & Kiehl, 2012; Bertsch et al., 2013; Ermer et al., 2012; Ermer et al., 2013; Yang & Raine, 2009), the present study took an exploratory approach and the OFC was chosen as the specific region of interest (ROI) among all other brain regions.
More importantly, although the OFC abnormality has been found to be associated with psychopathy, limited studies have examined the abnormalities in the OFC subregions (e.g., Raine et al., 2004). In terms of the anatomical structure of the OFC, the lateral part is more closely related to multiple sensory modalities while the medial part has a close relationship with the limbic structures (Carmichael & Price, 1995a,b). In addition, the damage to the medial OFC (mOFC) has been associated with increased risk-taking behavior that is highly correlated with the impulsive traits of psychopathy (Clark et al., 2008). Similarly, Veit et al. (2010) observed increased brain activation in the dorsal and vmPFC that was associated with impulsivity and antisocial behavior in psychopaths when watching their opponents being punished. Along the same line, functional Magnetic Resonance Imaging (fMRI) studies (McClure et al., 2004; 2007) have shown that the lOFC is involved in the choice of delay rewards while the mOFC is responsible for the choice of immediate rewards. This suggests that the mOFC is more related to the impulsive traits of psychopathy while the lOFC is not. Additionally, the mOFC is involved in instrumental learning and moral reasoning, concepts that are highly related to social conduct and callous emotionality and narcissism, whereas the lOFC is involved in suppression and inhibition of a response previously associated with rewards which is not related to psychopathy conceptually (Elliot et al., 2000). Taken together, the medial but not the lateral part of the OFC may be associated with psychopathic traits.
Some studies have also suggested asymmetry of the OFC in relation to psychopathy, although the findings are mixed. For example, significant GM loss in the PFC which includes the OFC, particularly in the left hemisphere, has been linked to psychopathy (Müller et al., 2008). In contrast, Yang and Raine’s (2009) meta-analysis which reviewed 43 structural and functional imaging studies has suggested reduced volume in the overall and right OFC, right anterior cingulate cortex, and left dorsolateral PFC in antisocial, violent, and psychopathic individuals. In addition, Tranel et al. (2002) found that individuals with lesions to the right OFC had deficits in social conduct, decision-making and emotional processing, all having been associated with callous-unemotional traits. Potenza et al. (2003) reported decreased left vmPFC/OFC activity in pathological gamblers with impulsivity problems and Jollant et al. (2010) found that suicide attempters who often had high impulsivity had reduced activation during risky decision-making in the left OFC. Furthermore, Nenadic et al. (2015) found reduced GM in the right prefrontal and bilateral medial prefrontal/ anterior cingulate cortices in patients with narcissistic personality disorder. Taken together, evidence has suggested that abnormalities in the right OFC are related to higher levels of callous-unemotional traits and narcissism (Nenadic et al., 2015; Tranel et al., 2002), whereas deficits in the left OFC are related to more impulsivity problems (Potenza et al., 2003).
Nonetheless, the neural correlates of antisocial behavior and psychopathy in younger and nonclinical samples remain poorly understood. A few studies in adolescents have suggested that psychopathic traits in youth are associated with dysfunction in the vmPFC/OFC and other brain regions including the amygdala and striatum, generally consistent with findings in adults (Blair, 2003; Herpers et al., 2014). Along the same line, White et al. (2012) found that youth with higher psychopathic traits exhibit reduced activation in the right amygdala in response to fearful faces when compared to those with lower levels of psychopathic traits. Moreover, it has been found that adolescents with either Oppositional Defiant Disorder (ODD) or Conduct Disorder (CD) and high psychopathic traits have reduced amygdala-prefrontal functional connectivity (Finger et al., 2008, 2012; Marsh et al., 2011), broadly consistent with prior findings that linked psychopathic traits to the PFC and limbic structures (Carmichael & Price, 1995a). Taken together, similar neurobiological abnormalities in the OFC and the amygdala have been found in youth and adults with psychopathic traits. Yet, the possibility of some discrepant findings in youth versus adults signifies the importance to examine structural brain volumes in youth. Differences between youth and adults might have implications for the development of psychopathy.
Since psychopathy is a multifaceted construct, are there specific neural correlates associated with different psychopathic dimensions in youth? Fan et al. (2011) investigated the psychological and neural correlates of narcissism in adults, and found that individuals with high narcissistic traits had lower deactivation in the insula during an empathy task. As for the impulsivity dimension of psychopathy, Brown et al. (2006) found that impulsivity was negatively associated with activity of the dorsal amygdala and ventral PFC in adults. To the best of the authors’ knowledge, very few brain imaging studies have investigated differential neural correlates of the dimensions of psychopathic traits in youth. Herpers and colleagues (2014) examined 75 peer-reviewed papers and reported a reduced response of the amygdala and a weaker functional connectivity between the amygdala and the vmPFC/OFC in adolescents with callous-unemotional traits. In addition, De Brito et al. (2009) found increased GM concentration in the OFC and anterior cingulate cortex in boys with callous-unemotional conduct problems when compared with healthy boys. Taken together, previous studies examining the neural correlates of psychopathic traits in youths are scarce and most of them suffer from two limitations: 1) psychopathic traits were treated as a unidimensional construct; and 2) the specific subregion and the right-left asymmetries of the OFC were not examined.
Taking all these findings together, the dimensions of psychopathic traits may be potentially associated with structural abnormalities in different parts of the OFC. More importantly, prior studies have largely focused on adults and whether the same neural correlates apply to adolescents whose neuropsychological functioning is drastically changing over time warrants investigation. To address the issues above, the present study examined structural brain abnormalities in relation to the dimensions of psychopathic traits in a group of adolescents from the community. Based on prior literature, this study took an exploratory approach to investigate the OFC as the region of interest (ROI). A ROI analysis is often performed when there is a priori hypothesis and it has a number of advantages over whole- brain analysis including the increase of statistical power (Brette et al., 2002; Saxe et al., 2006). Also, ROI analysis has been used in numerous neuroimaging studies previously (e.g., Lam et al., 2015; Wolf et al., 2015). Therefore, the ROI analysis would be performed to test the aims and hypotheses of the present study. It was hypothesized that the dimensions of psychopathic traits would be differentially associated with structural abnormalities in specific parts of the OFC (lateral vs. medial parts). Based on prior findings (e.g., Nenadic et al., 2015; Potenza et al., 2003; Tranel et al., 2002), it was hypothesized that callous-unemotional and narcissistic traits would be associated with right-left asymmetries in the mOFC (smaller GM volumes in the right as compared to the left mOFC), and that impulsivity would be associated with left-right asymmetries in the mOFC (smaller GM volumes in the left as compared to the right mOFC). In contrast, it was hypothesized that the GM volumes asymmetries in the lOFC would not be associated with any psychopathic traits. Finally, the structural abnormalities in other brain regions that were previously found to be implicated in psychopathy, including the amygdala and striatum (caudate and putamen), were also examined. Also, a number of brain regions including lingual and postcentral region that are not related to psychopathy were chosen as the comparison regions in the present study. However, no specific hypothesis was formed due to lack of consistent and less well-established evidence when compared to the OFC. Although brain changes over time and there might be variances that affect the brain- psychopathic traits associations during adolescence, the present study attempted to adjust different variances that might affect these associations by controlling for age, IQ, pubertal stage, sex and whole-brain volumes which are some common covariates included in adolescent brain studies (e.g., Huang et al., 2019). Moreover, instead of the brain changes over time during adolescence, the present study aimed to investigate the relationship between the psychopathic traits at the baseline and subsequent structural abnormalities in specific parts of the OFC and other brain regions of interest.
Material and methods
Participants
Twenty-nine 10-to 14-year-old participants living in Brooklyn, New York, were recruited as part of a larger ongoing longitudinal investigation on the development of behavioral problems in children and adolescents. The original cohort (T1) consisted of 340 children (49.1% male; mean age = 9.05, SD = 0.60, range = 7.51 - 10.75 years) living in the study area. The study was promoted publicly to all children in the community and the participants who met the inclusion criteria joined the study on a voluntary basis. Recruitment for the follow-up assessment (T2) was open to the entire sample, but only 32 participants were available to participate in this study and 3 of them were excluded after a quality check for motion and dropout artifacts. This subsample (n= 29) did not significantly differ from the larger cohort in terms of sex, age, IQ, or the psychopathic traits of interest (e.g., callous-unemotionality, narcissism, and impulsivity) (ps > 0.05). Children diagnosed with psychiatric disorder, intellectual disability, or a pervasive developmental disorder as well as those with orthodontia, head gear or foreign objects that could potentially cause distortion or artifacts on the brain imaging data were excluded. Demographic information, behavioral problems, and psychopathic traits were assessed when the families visited the laboratory at Brooklyn College. There was variability in the sample with respect to psychopathy for original cohort and follow-up subsample (Table 1) and the percentage who can be categorized as high level of psychopathy was about 3% in the present study. The prevalence was similar to previous study with 9415 students (Perenc & Radochonski, 2014). More details of the full cohort can be found in Zhang and Gao (2015) and Zhang et al. (2017). Regarding the full cohort, there were three time points (T1= baseline, T2= 2- year follow-up, and T3= 4-year follow-up). The 4-year follow-up was relabelled as T2 for this study to avoid confusion because brain scans were only collected at the 4-year follow-up. Two-year follow-up data (labelled as T2 in Zhang et al. (2017)) was not the focus of this manuscript and hence it was not included in the present study. That is, T2 (4-year follow-up) in the present study is equivalent to T3 in the full cohort (Zhang et al., 2017).
Table 1.
Participant demographic information, psychopathic traits and neural correlates.
| Demographics | Baseline T1 Full Cohort |
|---|---|
| Mean (Range; SD) | |
| Males (%) | 163 (47.8%) |
| IQa | 104.76 (70–155; 18.01) |
| Agea | 9.05 (7.51–10.75; 0.60) |
| Psychopathic traitsa | |
| Callous-unemotional traits | 4.29 (0–24; 3.46) |
| Narcissism trait | 1.87 (0–12; 2.01) |
| Impulsivity trait | 2.60 (0–8; 1.76) |
| Follow-up T2 Cohort | |
| Demographics | Mean (SD) |
| Males (%) | 16 (55.2%) |
| IQa | 99.52 (22.68) |
| Age (years)b | 12.28 (0.84) |
| Whole brain volumes (×1000mm3)b | 1565.13 (139.75) |
| Puberty scoreb | 2.63 (0.72) |
| Psychopathic traitsa | |
| Callous- unemotional traits | 3.98 (2.74) |
| Narcissism trait | 1.76 (1.35) |
| Impulsivity trait | 2.66 (1.78) |
| Neural correlatesb | |
| mOFC GM (combined) | 10.84 (1.32) |
| Left | 5.30 (0.73) |
| Right | 5.54 (0.72) |
| lOFC GM (combined) | 17.11 (2.13) |
| Left | 8.60 (1.15) |
| Right | 8.50 (1.04) |
| Amygdala (combined) | 3.02 (0.39) |
| Left | 1.50 (0.20) |
| Right | 1.52 (0.21) |
| Caudate (combined) | 8.16 (1.02) |
| Left | 3.92 (0.50) |
| Right | 4.23 (0.54) |
| Putamen (combined) | 12.41 (1.38) |
| Left | 6.25 (0.78) |
| Right | 6.15 (0.64) |
Note: Numbers in brackets represent the standard deviation (SD); lateral orbitofrontal cortex-lOFC; medial orbitofrontal cortex-mOFC; gray matter volumes-GM.
P ≤ 0.05
P ≤ 0.01
P ≤ 0.001.
×1000 mm3
Baseline assessment (Time 1).
Follow-up assessment (Time 2).
At T2, the participants were 10 to 14 years old (55% male; mean age = 12.8, SD = 0.84). The ethnic breakdown of these 29 participants was as follows: 58.6% Black, 24.1% Hispanic, 10.3% Caucasian, and 6.9% mixed-race/other. Youth participants with any history of drug use, psychiatric disorders, intellectual disabilities, or developmental disorders, and metal/foreign objects in the body were excluded from the follow-up. More details of the participant profile for the baseline cohort (T1) and follow-up cohort (T2) are listed in Table 1. These participants were invited to the Mount Sinai Icahn School of Medicine Translational and Molecular Imaging Institute in Upper Manhattan for a 90-minute assessment that included a 60-minute brain scan and questionnaires.
At both T1 and T2, families were financially compensated with monetary reward for their participation. All procedures were approved by the university Institutional Review Board, and both parental informed written consent and child/adolescent informed written consent were obtained. The details of the assessment tools used in the present study are described below.
Measures
Psychopathic Traits (Time 1)
Inventory of Callous-Unemotional Traits (ICU).
The parent filled out the 24-item Inventory of Callous Unemotional traits (ICU; Frick, 2004), a questionnaire developed to provide a more comprehensive assessment of CU traits, composited of the callous, uncaring, and unemotional subscales. It consists of a four-point rating scale ranging from 0 (not at all true) to 3 (definitely true). A total CU score for each participant was computed by the summation of the scores from the items in all three subscales. The reliability and validity information in the original cohort can be found in Gao and Zhang (2016). Internal consistency of the caregiver-report CU scores for our sample was high (α = 0.89).
Antisocial Personality Screening Device (APSD).
The parent also filled out the APSD (Frick & Hare, 2001; Vitacco et al., 2003) to assess the narcissism (7 items) and impulsivity (5 items) dimensions of the psychopathic traits. It is a three-point rating scale ranging from 0 (not at all true) to 2 (definitely true). Impulsivity (e.g., “acts without thinking”) and narcissism (e.g., “thinks he or she is more important than others”) subscales of the APSD were used in the current study. The reliability of the subscales was acceptable (Gao & Zhang, 2016). The CU subscale of the APSD was not administered to the participants in the current study because it showed poor reliability in adolescent samples due to the limited number of items used to assess this dimension of psychopathy (Pardini et al. 2003; Poythress et al. 2006). Instead, we used the ICU (see above) to measure the affective features of psychopathy in the present study.
Intelligence (IQ; Time 1)
The intelligence quotient (IQ) of the participants was measured using four subtests of the fourth edition of the Wechsler Intelligence Scale for Children (WISC-IV; Wechsler, 2003) at T1. Estimates of IQ scores were created by prorating four subscales of the WISC-IV (Vocabulary, Digit Span, Coding, and Matrix Reasoning) and a full-scale IQ score was calculated for each participant.
Pubertal Status (Time 2)
The Self-Rating Scale for Pubertal Development (Carskadon & Acebo, 1993) was administered to measure the pubertal status of the participants at T2. Its rating is based on a four-point scale: 1 (has not yet begun), 2 (has barely begun), 3 (definitely underway), 4 (seems complete), or unknown (I don’t know). It contains three identical questions for both boys and girls regarding growth in height, body hair growth, and skin changes. Girls were asked to answer two additional questions about breast growth and menstruation (and a third question about age of menstruation, if applicable), while boys were asked to answer two additional questions regarding deepening of the voice and facial hair growth. Based on guidelines from Crockett (1988, unpublished), a score of pubertal development status (1= pre-pubertal, 2 = early pubertal, 3 = mid pubertal, 4 = late pubertal, and 5 = post pubertal) was computed for each participant by summating all sex-relevant items and obtaining an average. Then, the score was recoded into puberty category scores based on the following guidelines: for males, it included body hair growth, voice change, and facial hair growth (prepubertal = 3, early pubertal = 4 or 5, midpubertal = 6, 7, or 8, late pubertal = 9-11, and postpubertal = 12). For females, the computation included body hair growth, breast development, and menarche (prepubertal = 2 and no menarche, early pubertal = 3 and no menarche, midpubertal = >3 and no menarche, postpubertal = 8 and menarche). The reliability (student version= 0.67 to 0.70) and validity of this scale has been established (Carskadon & Acebo, 1993).
Magnetic Resonance Imaging Acquisition (Time 2)
A 3T Siemens Magnetom Skyra Neuro Suite MRI scanner with a 32-channel phase array coil was used for brain image acquisition. The participant was guided by the technician into the scanner and instructed to remain still throughout data collection. Foam padding was used to reduce head motion. A high-resolution T1-weighted anatomical volume of the whole brain was acquired with a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with the following parameters: 176 axial slices of 1.0 mm thick, skip = 0 mm, TR = 2400 ms, TE = 1.94 ms, flip angle = 8°, FOV = 256 mm, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3.
Brain Preprocessing and Statistical Analysis
A quality check for motion and dropout artifacts was conducted before the structural MRI data were preprocessed. The original sample size for this study (T2) was 32 and we excluded 3 participants due to their excessive head motion during the MRI scanning based on this metric (> 8 mm in translation or > 5° in rotation). Structural MRI data including volumetric segmentation were preprocessed using FreeSurfer software (FreeSurfer 4.0.5, http://surfer.nmr.mgh.harvard.edu). The following preprocessing steps were conducted to estimate the GM in cortical and subcortical areas: motion correction, non-parametric non-uniform intensity correction, transformation of the original volume to the MNI305 atlas using the MINC program mritotal, intensity normalization, skull stripping, automatic subcortical segmentation, GCA atlas registration, removal of neck region, EM registration with the skull, CA labeling (labeling of subcortical structures using the GCA model), white matter segmentation, and cutting of the mid- brain from the cerebrum, and the hemispheres from each other. No manual corrections of the automaged outputs from FreeSurfer were performed.
Upon completion of all preprocessing, analyses of the primary ROI (lOFC and mOFC) were conducted using SPSS 24 (Chicago, IL, USA). In order to examine their relationships with different dimensions of psychopathic traits, Pearson correlation and hierarchical linear regression analyses were performed. Significance was set based on a two-tailed alpha level of .05 with Bonferroni correction for the regression analyses. Specifically, the Bonferroni correction rejects the null hypothesis for each p<= α/number of multiple comparisons, thereby controlling the family-wise error rate (FWER) at α<= 0.05. For example, the null hypothesis would be rejected for each p<= 0.05/2= 0.025 for each primary ROI while it would be rejected for each p<= 0.05/10= 0.005 for each comparison brain region (non- ROI). In the linear regressions, age, sex, IQ, puberty score, and whole- brain volumes were entered in the first step with three dimensions of psychopathic traits (callous- unemotional, narcissism, and impulsivity) entered simultaneously in the second step. The lOFC gray matter (GM) asymmetry (right - left lOFC GM) and the mOFC GM asymmetry (right - left mOFC GM) were treated as the dependent variables in each set of regression models. Similar regression models were also performed with amygdala GM asymmetry (right - left amygdala GM), caudate GM asymmetry (right - left caudate GM), putamen (right - left putamen GM) and the GM asymmetry in the comparison brain regions as the dependent variables. Since psychopathic traits were assessed at Time 1 and structural MRI data were collected at Time 2, psychopathic traits were entered as independent variables and GM asymmetry measures were entered as dependent variables in the regression analyses to test the association between the two in the present study. In addition, more rationales are described below (see “Hierarchical Linear Regression”). To examine the “pure” relationship between each dimension of psychopathic traits and dependent variables (Figures 1-3), residualized measures of these major variables were computed using regression analysis in order to assess the correlates of “pure” callous-unemotional independent of the other two psychopathic traits (narcissism and impulsivity); of “pure” narcissism independent of the other two psychopathic traits; and of “pure” impulsivity independent of the other two psychopathic traits. For instance, in order to compute “pure” callous-unemotional traits and residualized mOFC GM asymmetry, the covariates (e.g., IQ and whole-brain volumes), raw narcissism and raw impulsivity scores were treated as the independent variables to predict raw callous-unemotional traits (dependent variable) in one linear regression model; while the same set of independent variables would be used to predict raw mOFC GM asymmetry in a separate regression model. The residualized estimates would be computed and recorded accordingly. The correlates of the rest of the “pure” variables were computed using the same method. This method was adopted from a previous study by Raine et al. (2011).
Figure 1. Scatterplot for Residualized Callous- unemotional Traits and Residualized Medial OFC (mOFC) GM Asymmetry.
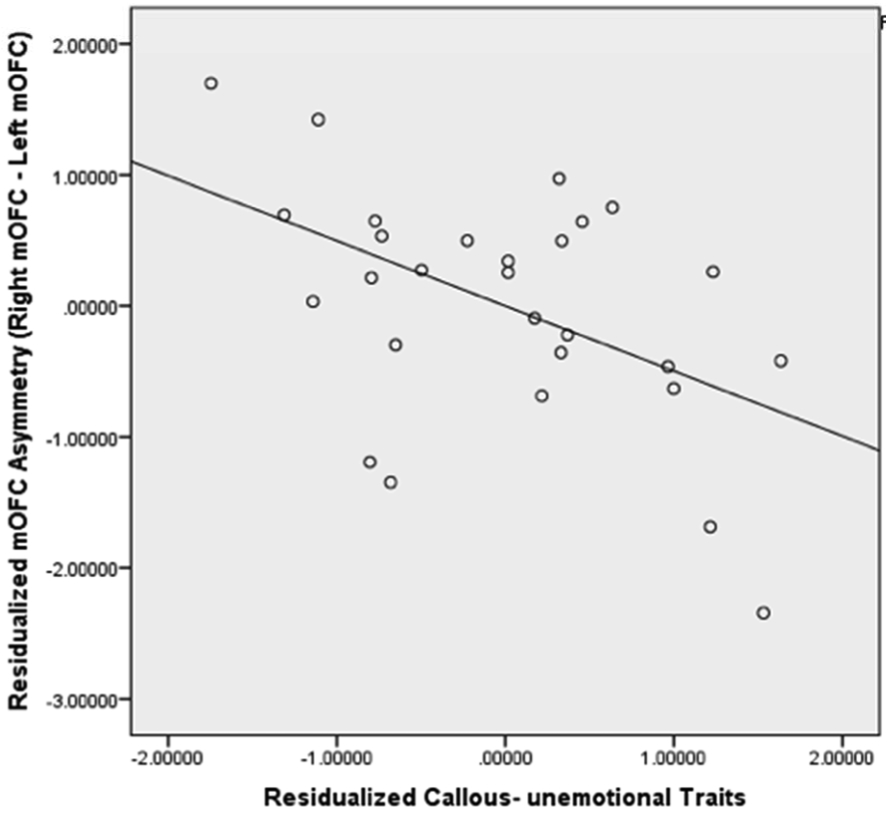
The scatterplot showed that residualized callous-unemotional traits were negatively correlated with residualized mOFC GM asymmetry (p< 0.05) and 24.8% of variances of pure mOFC GM asymmetry were explained by pure callous-unemotional traits.
Figure 3. Scatterplot for Residualized Narcissism Trait and Residualized Medial OFC (mOFC) GM Asymmetry.
The scatterplot showed that residualized narcissism trait was not significantly correlated with residualized mOFC GM asymmetry (p> 0.05) and 0.06% of variances of pure mOFC GM asymmetry were explained by pure narcissism trait.
Results
Pearson Correlations
Pearson correlations among all variables are listed in Table 2. IQ and pubertal status were not significantly correlated with any of the major variables (raw or pure psychopathic traits and the GM volumes in the OFC) (ps> 0.05), suggesting that these covariates did not contribute to psychopathy and the OFC GM asymmetry in this study. However, age measured at T2 was correlated positively with the raw impulsivity score (r = 0.42, p = 0.02). The correlations among three psychopathic dimensions were moderate (r = 0.36- 0.45, ps < 0.05). In terms of the pure psychopathic dimensions, significant correlations were found for the mOFC GM asymmetry but not for the lOFC GM asymmetry. Specifically, pure impulsivity (r = 0.51, p = 0.01) and pure callous-unemotional traits (r = −0.41, p = 0.03) were significantly correlated with the mOFC GM asymmetry, respectively. Furthermore, correlational analyses regarding the overall subregional volumes in the right, left and overall mOFC and lOFC were also performed. Only left mOFC was found to have marginal significant association with pure callous-unemotional traits (r= 0.36, p= 0.06) and pure impulsivity (r=−0.33, p=0.08). The correlations between right and overall mOFC, lOFC (left, right and overall) and all three pure psychopathic traits were not significant (ps> 0.05).
Table 2.
Correlations between covariates, psychopathic traits and neural correlates.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. IQa | – | |||||||||||||||
| 2. Puberty scoreb | −0.19 | – | ||||||||||||||
| 3. Ageb | 0.04 | 0.15 | – | |||||||||||||
| 4. Raw callous-unemotional traits (Pure callous-unemotional traits)a,c | 0.08 (−) | –0.05 (−) | 0.29 (−) | – | ||||||||||||
| 5. Raw Narcissism (Pure narcissism)a,d | −0.03 (−)< | 0.25 (−) | 0.28 (−) | 0.36 (0.23)f | – | |||||||||||
| 6. Raw Impulsivity (Pure impulsivity)a,d | 0.18 (−) | 0.00 (−) | 0.42* (−) | 0.45* (−0.30)f | 0.45* (09.37)f | – | ||||||||||
| 7. Lateral OFC asymmetryb,e | 0.03 | −0.03 | 0.03 | 0.03 (0.04) | 0.13 (0.04) | −020 (−0.11) | – | |||||||||
| 8. Medial OFC asymmetryb,e | 0.24 | −022 | −0.25 | −0.25 (−0.41*) | 0.02 (−0.02) | 0.18 (0.51*) | 0.20 | – | ||||||||
| 9. Medial OFC GM-combined | 0.06 | −0.10 | −0.33 | −0.11 (0.21) | −0.31 (−0.26) | −0.18 (−0.14) | −0.35 | −0.03 | – | |||||||
| 10. Lateral OFC GM-combined | 0.04 | −0.15 | −0.36 | −0.06 (0.23) | −0.31 (−0.30) | −0.13 (−0.04) | −0.23 | 0.08 | 0.79*** | – | ||||||
| 11. Lateral OFC- Left [Right] | 0.03 [0.05] | −013 [−0.16] | −0.34 [−0.37] | −0.06 [−0.05]; (0.20 [0.24]) | −0.32 [−0.29]; (−0.29 [−0.30]) | −0.08 [−0.18]; (−0.02 [−0.07]) | −0.43* [0.01] | 0.03 [0.13] | 0.81*** [0.73***] | – | ||||||
| 12. Medial OFC-Left [Right] | −0.05 [0.16] | −0.001 [−0.19] | −0.19 [−0.41*] | 0.01 [−0.21]; (0.36 [0.02]) | −0.28 [−0.28]; (−0.23 −0.25]) | −0.24 [−0.9]; (−0.33 [0.08]) | −0.39 [−0.23] | −0.44* [0.40*] | 0.91*** [0.91***] | 0.97*** [0.97] | 0.72** [0.74***]g | – | ||||
| 13. Amygdala asymmetryb,e | 0.13 | 0.03 | 0.50** | 0.24 (0.21) | 0.01 (−0.16) | 0.17 (−010) | 0.13 | −0.27 | −0.02 | −0.05 | −0.07 [−0.02] | 0.09 [−0.13] | – | |||
| 14. Putamen asymmetryb,e | −0.17 | 0.01 | 0.35 | 0.05 (0.09) | −0.19 (−0.09) | −0.12 (−0.28) | −0.05 | −0.29 | −0.08 | −0.16 | −0.14 [−0.17] | −0.20 [0.04] | 0.28 | – | ||
| 15. Caudate asymmetryb,e | 0.22 | −0.05 | 0.09 | −0.09 (0.08) | −0.33 (−0.24) | −0.16 (−0.22) | 0.07 | −0.20 | 0.18 | 0.06 | 0.04 [0.07] | 0.24 [0.08] | 0.27 | −0.04 | – | |
| 16. Whole brain volumesb | 0.05 | −0.20 | −0.01 | −0.09 (−) | 0.09 (−) | 0.11 (−) | −0.22 | 0.04 | 0.68** | 0.62** | 0.62** [0.58**] | 0.64** [0.59**] | 0.20 | −0.16 | 0.17 | – |
P < = 0.05;
P < = 0.01;
P < = 0.001.
Parenthetical terms reflect partial correlation estimates after controlling for the covariates (IQ, age, sex, puberty score, and whole-brain volumes).
Baseline assessment.
Follow-up assessment.
Measured by Inventory of Callous Unemotional Traits (ICU).
Measured by Antisocial Process Screening Device (APSD).
Lateral OFC asymmetry = right lOFC - left lOFC gray matter volumes; medial OFC asymmetry = right mOFC – left mOFC gray matter volumes.
Correlation coefficients between three raw psychopathic traits (correlation coefficients between three pure psychopathic traits).
Correlation coefficients between left lateral OFC and left medial OFC [correlation coefficients between right lateral OFC and right medial OFC].
Hierarchical Linear Regression
Hierarchical linear regression analyses were performed to test the independent contributions of covariates (Age, sex, IQ, puberty score, and whole-brain volumes; Step 1) and three dimensions of psychopathic traits (callous-unemotional, narcissism, impulsivity; Step 2) to the primary ROI neural correlates (lOFC and mOFC asymmetry). The covariates were entered in Step 1 so that the unique contributions and variances of the three dimensions of psychopathic traits after controlling for the covariates could be examined (Step 2). All three dimensions of psychopathic traits were entered in the same level of the regression model (Step 2) in order to examine the relationship between each pure psychopathic trait (e.g., pure impulsivity after controlling the variance associated with the other two psychopathic traits) and the ROI neural correlates. Stepwise regression analyses were not performed because this study intended to examine the predictive value of each dimension of psychopathic traits on the ROI neural correlates. Most importantly, all three dimensions of psychopathic traits were entered simultaneously in the same level of the regression model to address the suppressor effects and increase the predictive power. This method was adopted from previous studies to test the association between psychopathic traits and brain correlates (Caldwell et al., 2019; Cohn et al., 2016; DeBellis et al., 2001; Gao et al., 2018; Hicks & Patrick, 2006; Leutgeb et al., 2015; Korponay et al., 2017; Korponay et al., 2017; Wolf et al., 2015). For instance, DeBellis et al. (2001) and Gao et al. (2018) treated the demographic and psychopathic traits as the independent variables (IV) and the brain correlates as the dependent variables (DV) in the regression models respectively. In addition, Korponay et al. (2017) investigated the functional connectivity and volumes in psychopathy in a sample of adult male prison inmates. By treating psychopathy as the IV and prefrontal volumes and functional connectivity as the DV, it was found that impulsivity trait was associated with more functional connectivity and larger prefrontal subregion, particularly in the medial orbitofrontal cortex and dorsolateral prefrontal cortex. It was suggested that the positive association could potentially be due to the aberrant neurodevelopment. In addition, similar statistical approach to investigate the relationship between brain correlates (DV) and psychopathy (IV) in incarcerated criminal offenders was adopted in the diffusion tensor imaging study by Wolf et al. (2015). It was found that psychopathy was associated with reduced rational anisotropy in the right uncinate fasiculus which indicated a neural marker for psychopathic symptomatology. In summary, although it might be a natural option to treat brain correlates as IV and psychopathic traits as DV for studies that test the causal relationship between the two, the present study aimed to test the association instead of a causal relationship. In addition, this analytical method was adopted from previous studies that shared similar aims of the present study and there was a temporal difference between the assessments of psychopathic traits (Time 1) and brain correlates (Time 2) in the present study. Therefore, psychopathic traits and brain correlates were treated as IV and DV respectively in the present study. The hierarchical linear regression results for the OFC, amygdala, putamen and caudate are shown in Table 3 and the results for those randomly selected regions for which no prior results indicate an association with psychopathy are reported in Table 4.
Table 3.
Hierarchical linear regression models predicting mOFC/lOFC, amygdala, caudate and putamen GM asymmetry.
| Dependent variable |
mOPC GM asymmetry | IOFC GM asymmetry | Amygdala GM asymmetry | Caudate GM asymmetry | Putamen GM asymmetry | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictor variables |
Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 |
| Age | β = −0.23, t = −1.03, p = 0.31 | β = −0.33, t = −0.16, p = 0.13 | β = 0.18, t = 0.80, p = 0.43 | β = 20, t = 0.76, p = 0.46 | β = 0.46, t = 2.30, p = 0.03* | β = 0.52, t = 2.30, p = 0.03* | β = 0.06, t = 0.27, p = 0.79 | β = 0.26, t = 1.08, p = 0.30 | β = 0.37, t = 1.67, p = 0.11 | β = 0.52, t = 2.18, p = 0.04* |
| IQ | β = 0.23 t = 1.15, p = 0.26 | β = 0.13, t = 0.77, p = 0.45 | β = 0.08, t = 0.41, p = 0.69 | β = 0.11, t = 0.48, p = 0.64 | β = 0.10, t = 0.54, p = 0.60 | β = 0.12, t = 0.61, p = 0.55 | β = 0.21, t = 1.00, p = 0.33 | β = 0.27, t = 1.31, p = 0.21 | β = −0.22, t = −1.08, p = 0.29 | β = −0.15, t = −0.72, p = 0.48 |
| Puberty score | β = −0.11, t = −0.43, p = 0.67 | β = −0.32, t = −1.42, p = 0.17 | β = −0.17, t = −0.66, p = 0.52 | β = −0.14, t = −0.47, p = 0.64 | β = 0.06, t = 0.26, p = 0.80 | β = 0.14, t = 0.57, p = 0.58 | β = −0.01, t = −0.03, p = 0.98 | β = 0.10, t = 0.35, p = 0.73 | β = −0.15, t = −0.56, p = 0.58 | β = −0.04, t = −0.15, p = 0.88 |
| Sex | β = −0.08, t = −0.25, p = 0.80 | β = −0.07, t = −0.25, p = 0.80 | β = 0.11, t = 0.38, p = 0.71 | β = 0.10, t = 0.31, p = 0.76 | β = −0.07, t = −0.26, p = 0.80 | β = −0.03, t = −0.10, p = 0.92 | β = 0.06, t = 0.19, p = 0.85 | β = 0.14, t = 0.45, p = 0.66 | β = 0.08, t = 0.27, p = 0.79 | β = 0.11, t = 0.38, p = 0.71 |
| Whole-brain volumes | β = −0.07, t = −0.31, p = 0.76 | β = −0.25, t = −1.25, p = 0.23 | β = −0.34, t = −1.54, p = 0.14 | β = −0.32, t = −1.28, p = 0.22 | β = 0.18, t = 0.89, p = 0.38 | β = 0.27, t = 1.23, p = 0.24 | β = 0.22, t = 0.94, p = 0.36 | β = 0.33, t = 1.43, p = 0.17 | β = −0.09, t = −0.42, p = 0.68 | β = 0.01, t = 0.04, p = 0.97 |
| Psychopathic traits | ||||||||||
| Callout-unemotional | β = −0.50, t = −2.51, p = 0.02* | β = 0.05, t = 0.21, p = 0.84 | β = 0.25, t = 1.16, p = 0.26 | β = 0.09, t = 0.38, p = 0.71 | β = 0.10, t = 0.45, p = 0.66 | |||||
| Narcissism | β = −0.02 t = −0.11, p = 0.91 | β = 0.05, t = 0.19, p = 0.85 | β = −0.21, t = −0.88, p = 0.39 | β = −0.31, t = −1.21, p = 0.24 | β = −0.12, t = −0.46, p = 0.65 | |||||
| Impulsivity | β = 0.70, t = 3.08, p = 0.01** | β = −0.15, t = −0.53, p = 0.61 | β = −0.14, t = −0.57, p = 0.58 | β = −0.30, t = −1.10, p = 0.28 | β = −0.38, t = −1.44, p = 0.17 | |||||
| F statistics | F5,22 = 0.75, p = 0.59 | F8,19 = 2.23, p = 0.07 | F5,22 = 0.85, p = 0.53 | F8,19 = 0.50, p = 0.84 | F5,22 = 1.92, p = 0.13 | F8,19 = 1.41, p = 0.26 | F5,22 = 0.42, p = 0.33 | F5,22 = 0.87, p = 0.56 | F5,22 = 0.89, p = 0.51 | F8,19 = 0.99, p = 0.48 |
| R2 | 0.146 | 0.484 | 0.162 | 0.174 | 0.304 | 0.373 | 0.088 | 0.268 | 0.168 | 0.294 |
| R2 change | – | 0.338 | – | 0.012 | – | 0.069 | – | 0.180 | – | 0.126 |
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
Note: β represents standardized coefficient.
Table 4.
Hierarchical linear regression models predicting the asymmetry in other brain regions.
| Dependent variable |
Postcentral GM asymmetry | Lingual GM asymmetry | Middle Temporal GM asymmetry |
Posterior Cingulate GM asymmetry |
Temporal Pole GM asymmetry |
Fusiform GM asymmetry | Hippocampus GM asymmetry | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor variables |
Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 | Step 1 | Step 2 |
| Age | β = −0.26, t = −1.15, p = 0.26 | β = −0.31, t = −1.17, p = 0.26 | β = −0.02, t = −0.09, p = 0.93 | β = −0.06, t = −0.24, p = 0.81 | β = −0.09, t = −0.40, p = 0.70 | β = −0.06, t = −0.22, p = 0.83 | β = −0.23, t = −0.99, p = 0.33 | β = −0.07, t = −0.27, p = 0.79 | β = 0.23, t = 1.09, p = 0.29 | β = 0.25, t = 0.99, p = 0.33 | β = 0.31, t = 1.40, p = 0.18 | β = 0.21, t = 0.91, p = 0.38 | β = 0.13, t = 0.54, p = 0.59 | β = 0.02, t = 0.10, p = 0.92 |
| IQ | β = −0.03, t = −0.14, p = 0.89 | β = −0.06, t = −0.28, p = 0.78 | β = 0.12, t = 0.62, p = 0.54 | β = 0.04, t = 0.23, p = 0.82 | β = 0.02, t = 0.07, p = 0.94 | β = 0.02, t = 0.09, p = 0.93 | β = −0.08, t = −0.36, p = 0.72 | β = −0.03, t = −0.15, p = 0.88 | β = −0.05, t = −0.23, p = −0.82 | β = −0.05, t = −0.23, p = 0.82 | β = −0.05, t = −0.23, p = 0.82 | β = −0.02, t = −0.09, p = 0.93 | β = −0.07, t = −0.32, p = 0.75 | β = −0.14, t = −0.76, p = 0.46 |
| Puberty score | β = 0.03, t = 0.12, p = 0.91 | β = −0.004, t = −0.02, p = 0.99 | β = 0.08, t = 0.32, p = 0.75 | β = −0.03, t = −0.11, p = 0.92 | β = −0.30, t = −1.11, p = 0.28 | β = −0.28, t = −0.93, p = 0.36 | β = 0.16, t = 0.59, p = 0.56 | β = 0.18, t = 0.62, p = 0.54 | β = 0.29, t = 1.17, p = 0.26 | β = 0.26, t = 0.92, p = 0.37 | β = −0.16, t = −0.61, p = 0.55 | β = −0.14, t = −0.51, p = 0.61 | β = −0.37, t = −1.38, p = 0.18 | β = −0.32, t = −1.35, p = 0.19 |
| Sex | β = 0.11, t = 0.34, p = 0.74 | β = 0.12, t = 0.36, p = 0.73 | β = −0.54, t = −1.89, p = 0.07 | β = −0.48, t = −1.71, p = 0.10 | β = 0.34, t = 1.09, p = 0.29 | β = 0.36, t = 1.08, p = 0.30 | β = −0.34, t = −1.10, p = 0.29 | β = −0.30, t = −0.94, p = 0.36 | β = −0.35, t = −1.22, p = 0.24 | β = −0.35, t = −1.13, p = 0.27 | β = −0.20, t = −0.68, p = 0.50 | β = −0.30, t = −1.02, p = 0.32 | β = 0.34, t = 1.10, p = 0.28 | β = 0.42, t = 1.59, p = 0.13 |
| Whole-brain volumes | β = 0.13, t = 0.57, p = 0.58 | β = 0.10, t = 0.40, p = 0.69 | β = −0.27, t = −1.27, p = 0.22 | β = −0.34, t = −1.60, p = 0.13 | β = 0.03, t = 0.12, p = 0.91 | β = 0.05, t = 0.21, p = 0.84 | β = −0.29, t = −1.26, p = 0.22 | β = −0.26, t = −1.09, p = 0.29 | β = −0.26, t = −1.23, p = 0.23 | β = −0.30, t = −1.25, p = −0.23 | β = −0.10, t = −0.47, p = 0.65 | β = −0.12, t = −0.52, p = 0.61 | β = 0.15, t = 0.66, p = 0.52 | β = 0.23, t = 1.13, p = 0.27 |
| Psychopathic traits: | ||||||||||||||
| Callous-unemotional | β = ,0.002 t = 0.007, p = 0.96 | β = −0.17, t = −0.78, p = 0.45 | β = 0.05, t = 0.21, p = 0.84 | β = −0.21, t = −0.85, p = 0.40 | β = −0.16, t = −0.68, p = 0.51 | β = 0.06, t = 0.27, p = 0.79 | β = 0.59, t = 2.90, p = 0.01 | |||||||
| Narcissism | β = −0.07, t = −0.26, p = 0.80 | β = −0.26, t = −1.12, p = 0.28 | β = −0.11, t = −0.38, p = 0.71 | β = −0.12, t = −0.45, p = 0.66 | β = 0.03, t = 0.10, p = 0.92 | β = 0.42, t = 1.73, p = 0.10 | β = −0.43, t = −1.99, p = 0.06 | |||||||
| Impulsivity | β = 0.20, t = 0.69, p = 0.50 | β = 0.51, t = 2.07, p = 0.05* | β = −0.02, t = −0.07, p = 0.94 | β = −0.14, t = −0.49, p = 0.63 | β = 0.07, t = 0.26, p = 0.80 | β = −0.25, t = −0.94, p = 0.36 | β = 0.25, t = 1.08, p = 0.29 | |||||||
| F statistics | F5,22 = 0.50, p = 0.78 | F8,19 = 0.34, p = 0.94 | F5,22 = 1.30, p = 0.30 | F8,19 = 1.44, p = 0.25 | F5,22 = 0.56, p = 0.73 | F8,19 = 0.33, p = 0.94 | F5,22 = 0.48, p = 0.79 | F8,19 = 0.64, p = 0.74 | F5,22 = 1.26, p = 0.32 | F8,19 = 0.76, p = 0.65 | F5,22 = 1.04, p = 0.42 | F8,19 = 1.10, p = 0.41 | F5,22 = 0.45, p = 0.81 | F8,19 = 2.01, p = 0.10 |
| R2 | 0.101 | 0.126 | 0.228 | 0.377 | 0.113 | 0.122 | 0.098 | 0.211 | 0.223 | 0.241 | 0.191 | 0.316 | 0.092 | 0.458 |
| R2 change | – | 0.025 | – | 0.049 | – | 0.009 | – | 0.113 | – | 0.018 | – | 0.125 | – | 0.366 |
P ≤ 0.05;
P ≤ 0.01;
P≤ 0.001.
Note: β represents standardized coefficient.
After controlling for age, sex, IQ, pubertal stages, and whole brain volumes, the model with three dimensions of psychopathic traits measured at T1 showed marginally significant association with mOFC GM asymmetry at T2 (F8,19= 2.23, p= 0.07). After controlling for covariates (accounted for 14.6% of the variance), the three dimensions of psychopathic traits still accounted for 33.8% of the variance in the mOFC GM asymmetry. Callous-unemotional traits (β = −0.50, t = −2.51, p= 0.02; see Figure 1) and impulsivity (β = 0.70, t = 3.08 , p= 0.01; see Figure 2) were marginally significantly and significantly associated with mOFC GM asymmetry, while the effect of narcissism was not significant (β = −0.02, t = −0.11, p= 0.91; see Figure 3). Age, sex, IQ, puberty score, and whole-brain volumes were not associated with mOFC GM asymmetry (ps> 0.05) (Table 3).
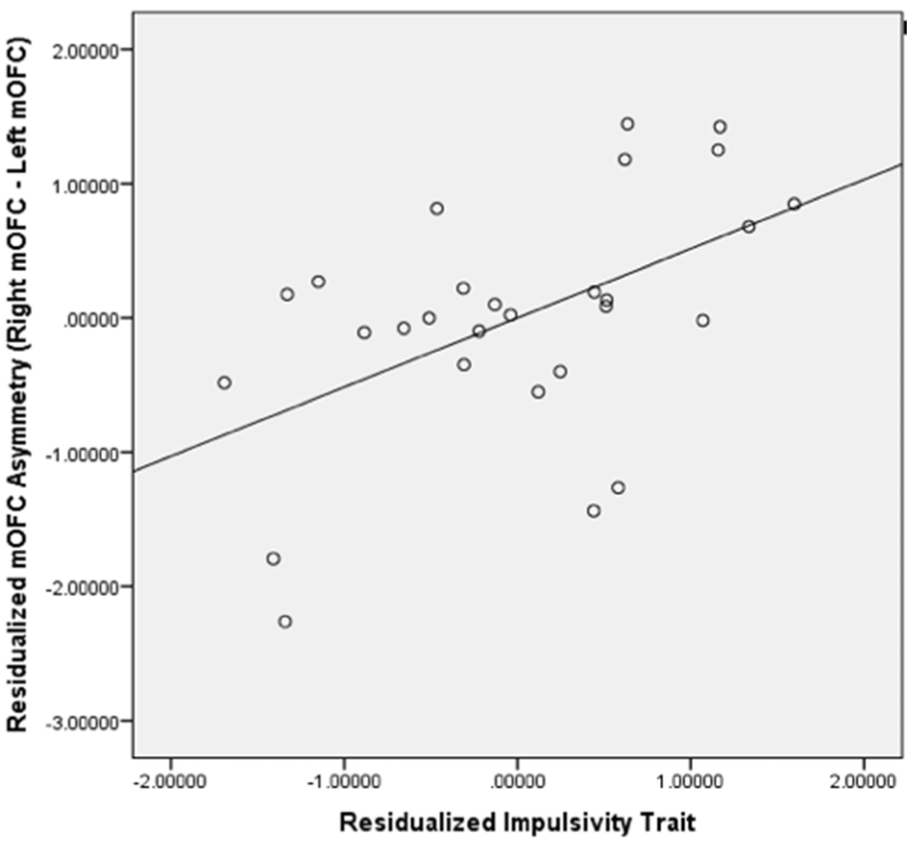
Figure 2. Scatterplot for Residualized Impulsivity Trait and Residualized Medial OFC (mOFC) GM Asymmetry.
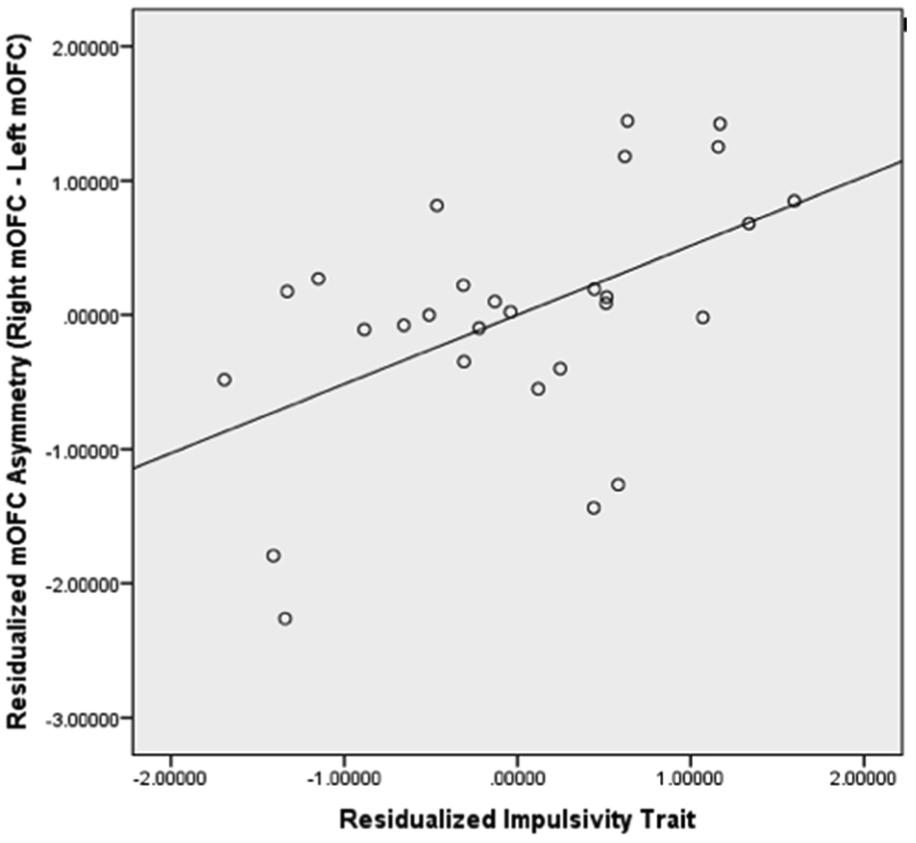
The scatterplot showed that residualized impulsivity trait was positively correlated with residualized mOFC GM asymmetry (p< 0.05) and 33.3% of variances of pure mOFC GM asymmetry were explained by pure impulsivity trait.
It is noted that there were moderate correlations between age and the callousness-unemotional, narcissism, and impulsivity scores (rs = .28 - .45) (Table 2). Although the other correlations were not significant in this sample, these moderate correlations suggested that some variance in psychopathic traits was removed in assessing the relationships being studied. Therefore, age was removed from the regression analyses. In the regression model with other covariates (except age), callous-unemotional traits (β = −0.52, t = −2.54, p= 0.02) and impulsivity (β = 0.64, t = 2.75, p= 0.01) were still significantly and significantly associated with mOFC GM asymmetry, while the effect of narcissism was not significant (β = −0.09, t = −0.40, p= 0.70). Similar findings are found with or without age being included as one of the covariates.
The regression model with the covariates and three dimensions of psychopathic traits measured at T1 were not significantly associated with lOFC GM asymmetry (F8,19= 0.50, p= 0.84). After controlling for the variance accounted for by the covariates (16.2 %), three dimensions of psychopathic traits only accounted for 1.2% of the variance in the lOFC GM asymmetry. None of the variables were associated with the lOFC GM asymmetry (ps > 0.05) (see Table 3). Because of the aforementioned reason, age was removed from the regression analyses. In the regression model with other covariates (except age), callous-unemotional traits (β = 0.07, t = 0.27, p= 0.79), narcissism (β = 0.09, t = 0.34, p= 0.74) and impulsivity (β = −0.11, t = −0.41, p= 0.69) were not significantly associated with mOFC GM asymmetry. Similar findings are found with or without age being included as one of the covariates. Finally, none of the psychopathic traits significantly were associated with asymmetry measures in the amygdala, caudate, and putamen as well as in the comparison brain regions (ps > .05) except for lingual. Specifically, lingual GM asymmetry was significantly related to impulsivity (β = 0.51, t = 2.07, p= 0.05) but not the other two psychopathic traits (Table 4). However, after Bonferroni correction, this association was also not significant (p> 0.005).
Discussion
Although abnormalities in the PFC, in particular the OFC, have been consistently implicated in psychopathy (e.g., Koenigs, 2012; Yang & Raine, 2009), previous studies have largely focused on adults, and research on the specificity and asymmetry is lacking. The present study examined differential relationships between structural brain correlates in the subregions of the OFC and the three dimensions of psychopathic traits (narcissism, impulsivity and callous- unemotional traits) during adolescence. Consistent with the hypotheses, psychopathic traits at T1 were significantly associated with T2 GM asymmetry in the mOFC. Specifically, it was found that callous-unemotional traits were associated with right- left asymmetry in the mOFC (smaller right mOFC as compared to the left), while the impulsivity was linked to left- right asymmetry in the mOFC (smaller left mOFC when compared to the right). Broadly consistent with prior findings in adults, these results support the notion of the lateralization of the OFC functions (Tranel et al., 2002) and that various dimensions of psychopathic traits are differentially associated with specific parts of the OFC. The present findings help us better delineate the relationship between the brain correlates (particularly in the OFC) and different aspects of psychopathy in adolescence. In addition, the present findings can set up the foundation to understand the relationship between the two.
Broadly, our findings are consistent with prior findings that suggest that different structural correlates in the brain are associated with different psychopathic and antisocial traits (Bertsch et al., 2013; Gregory et al., 2012; Lam et al., 2017). For instance, reduced GM in the PFC and temporal poles were found in individuals with antisocial personality disorder and psychopathy when compared to controls (Gregory et al., 2012) and significant reduction in the midline cortical areas (e.g., dorsomedial prefrontal cortex, posterior cingulate/precuneus) were found in antisocial offenders with high psychopathic traits (Bertsch et al., 2013). In addition to the findings pertaining to the left-right asymmetry in the mOFC, it was also found that left mOFC had marginal significant association with pure callous-unemotional traits (r= 0.36) and pure impulsivity (r=−0.33) while the associations between right mOFC and all three pure psychopathic traits were not significant. These findings suggest that the left and right mOFC are differentially associated with callous-unemotional traits and impulsivity respectively. Although the association between reduced/ increased gray matter volume in the left mOFC and psychopathic traits is not equivalent to the relationship between left-right asymmetry in the mOFC and psychopathic traits, the significant correlational findings pertaining to the left mOFC are consistent with the asymmetry findings yielded from the regression analyses in the present study. Moreover, the overall mOFC or lOFC effect was not significant on all three psychopathic traits in the present study which is not consistent with previous finding in adults (Yang & Raine, 2009). The possible reason for the discrepant findings might be because different age groups were studied. Specifically, structural brain findings in youth in the present study might be different from the adult findings in previous studies (e.g., Yang & Raine, 2009) which signifies the importance of studying the structural brain volumes in youth.
Furthermore, the significant findings in the OFC but not other brain regions of interest (e.g., caudate and putamen) support Blair’s model (2006, 2007) of psychopathy which has focused on the role of the OFC in psychopathy. Although impulsivity trait was found to be positively related to lingual GM asymmetry unexpectedly (it became not significant after Bonferroni correction), there is a lack of previous findings regarding lingual and psychopathy that can provide sufficient theoretical basis for this significant relationship. For instance, Deeley et al. (2006) found greater increased activation in right lingual and fusiform cortices when processing happy faces versus neutral faces in the control group than the psychopathy group. However, this finding about lingual was task-specific (functional brain correlates) and the structural volumes were not compared between the two groups. In terms of the neuroanatomical functions, lingual gyrus is involved in ideational fluency (Jauk et al., 2015) and visual imagery (Zhang et al., 2014). In summary, there is a lack of theoretical basis from previous literature to understand how lingual GM is related to psychopathy. A more recent study found that lower inhibition was associated with larger GM in the lingual gyrus, which in turn was associated with higher divergent thinking (Zhang et al., 2016). It was suggested that the significant relationship between inhibition and lingual GM might be because inhibition function would activate a greater number of concepts in working memory and concept activation (Dietrich, 2004; Radel et al., 2015). This previous finding (Zhang et al., 2016) was consistent with the positive relationship between impulsivity and lingual GM asymmetry found in the present study. However, this exploratory finding in a homogeneous group (healthy male adults) in Zhang et al.’s study (2016) has to be further investigated in future studies.
Consistent with our hypothesis, psychopathic traits were found to be associated with structural correlates in the medial but not the lateral parts of the OFC. Specifically, the mOFC GM volumes were associated with the callous-unemotional traits and impulsivity in adolescents. This structural finding in the mOFC is in line with the functional brain imaging studies showing that the mOFC is more involved in instrumental learning and moral reasoning as well as the choice for immediate rewards (Elliot et al., 2000; Knutson et al., 2001; 2003; McClure et al., 2004;2007). The deficits in these functions are often found in psychopathic individuals who have impulsive and callous- unemotional characteristics (Blair, 1995). In contrast, the lOFC is more involved in reward processing (Elliot et al., 2000), and psychopathic individuals do not consistently show salient reward processing deficits (Buckholtz et al., 2010). Although a recent finding shows that both subregions are associated with instrumental learning and reward processing, they are associated with different aspects of learning and reward processing (Noonan et al., 2017).
The three dimensions of psychopathic traits altogether explained a significant amount of variances (33.8 %) in the mOFC asymmetry even after controlling for the covariates including age, sex, IQ, puberty status, and whole-brain volumes. More specifically, we found that higher levels of callous-unemotional traits were associated with smaller right as compared to left mOFC. This might be due to the right-sided prefrontal pathology, as suggested by Tranel et al. (2002), who found impairments in social conduct, decision-making, and emotional processing in individuals with lesions to their right but not the left OFC. In contrast, higher levels of impulsivity were found to be associated with smaller left as compared to right mOFC. This finding is in line with prior studies that showed decreased activation in the left vmPFC in pathological gamblers with impulsivity problems (Potenza et al., 2003).
Inconsistent with prior findings (e.g., Nenadic et al., 2015), narcissism was not significantly related to the mOFC asymmetry or any other ROIs. This inconsistency may be partly due to the differences in sample characteristics. Specifically, Nenadic et al. (2015) investigated clinical adult patients with narcissistic personality disorder while the present study examined non-clinical adolescents with high or low psychopathy-related narcissistic traits. The variability in the degree and nature of narcissism between the two samples might have affected the results in relation to the mOFC GM volumes. In addition, the non- significant findings for narcissism in the present study are consistent with previous findings (Fink et al., 2012). Specifically, it has been argued that the narcissism factor bears some resemblance to the interpersonal component of clinical psychopathy, however, this relationship in a large sample of youth was not significant (Fink et al., 2012). Since three psychopathic traits that were examined in the present study are the three components emerged in factor analyses of the Antisocial Processes Screening Device (APSD) while the clinical measures of psychopathy validated in adults and in adolescents are more generally known as the interpersonal, affective, lifestyle, and antisocial components or facets, the non-significant findings in narcissism in non-clinical individuals are inconsistent with previous findings with clinical psychopathic measures. Furthermore, the discrepant findings in youth versus adults also highlight the importance of studying the relationship between psychopathic traits and structural brain volumes in youth. Besides, there is a paucity of research into narcissistic traits or narcissistic personality disorder. In addition to the prefrontal cortex, the insula and premotor cortex were also found to be associated with narcissism (Fan et al., 2011; Schulze et al., 2013), although these findings could not be replicated in the study by Nenadic et al. (2015).
Limitations
The limitations of the study include the small sample size may give rise to issues related to the statistical power and representativeness of the samples. For instance, the probability value for functional effects of lateral OFC gray matter asymmetry on pure callous- unemotional traits might become significant with larger samples. However, the current findings were essential to set up the foundation for future studies with a larger sample size. Nevertheless, it should be noted that non- significant findings in the present study does not inspire absolute confidence that there would be no important differences with larger sample sizes. For instance, it is plausible that the nonsignificant effects for middle temporal lobe would have proven significant if these effect sizes proved are stable in a larger sample. In addition, potential differences between the original and follow-up samples may also raise questions about the representativeness of the Time 2 sample as a possible additional limitation on the generalizability of findings. Moreover, the current sample only included adolescents without a history of substance abuse. This might limit the generalizability of the findings to the community where the psychopathic adolescents might have the substance use problems (Smith & Newman, 1990). In addition, although psychopathic traits were assessed by ages 7-10, the structural MRI brain scan was administered about four years later. Future longitudinal studies may acquire both psychopathic traits and brain imaging data at two time points to examine if the asymmetry findings can be found in young individuals with a higher level of psychopathic traits over the time. Also, only whole-brain volumes instead of left and right brain volumes were controlled because it is commonly used as the covariate instead of splitting them up into two in previous asymmetry brain studies (e.g., Liu et al., 2016). Another reason is to avoid including too many covariates in the regression model that would result in overfitting problems in the present study. The left and right brain volumes in the regression analyses in future studies in order to reduce the potential confounding effect of left and right brain asymmetry. It should also be cautious that the current findings only support the association between psychopathic traits and laterality in the mOFC, and the nature of the laterality as well as whether similar result can be found if the position of the IV and DV is swapped warrants further investigation in future studies. Last but not least, future studies should investigate an adolescent group with conduct disorder and compare them to a neurotypical sample.
Conclusion
Despite these limitations, the present findings provide further support to the notion that the three dimensions of psychopathic traits are associated with differential structural correlates in the brain (particularly in the OFC). In terms of clinical implication, although the present study did not confirm the causal relationship between psychopathic traits and the mOFC, the present findings can set up the foundation to test the relationship between the two in future studies. Ultimately, the current research study furthers our understanding of the early neural correlates of psychopathic traits and may potentially aid in the prevention of crime in adulthood.
Supplementary Material
Highlights.
Callous-unemotional traits were associated with right-left asymmetry in the mOFC
Impulsivity was associated with left-right asymmetry in the mOFC
Dimensions of psychopathic traits may have distinct neurobiological correlates
Acknowledgments
Funding
This work was supported by the National Institute of General Medical Science of the National Institutes of Health [SC3GM118233 to Y.G.].
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andershed H, Gustafson SB, Kerr M, Stattin H, 2002. The usefulness of self-reported psychopathy-like traits in the study of antisocial behaviour among non-Preferred adolescents. Eur. J. Psychol. Assess 16(5), 383–402. 10.1002/per.455 [DOI] [Google Scholar]
- Anderson NE, Kiehl KA 2012. The psychopath magnetized: insights from brain imaging. Trends Cogn. Sci 16(1), 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth ed. Arlington, VA: Author. doi: 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Asscher JJ, van Vugt ES, Stams GJJ, Deković M, Eichelsheim VI, Yousfi S 2011. The relationship between juvenile psychopathic traits, delinquency and (violent) recidivism: A meta-analysis. J. Child Psychol. Psychiatry 52(11), 1134–1143. 10.1111/j.1469-7610.2011.02412.x [DOI] [PubMed] [Google Scholar]
- Blair RJR 1995. A cognitive developmental approach to morality: Investigating the psychopath. Cognition 57(1), 1–29. 10.1016/0010-0277(95)00676-P [DOI] [PubMed] [Google Scholar]
- Blair RJR 2006. The emergence of psychopathy: Implications for the neuropsychological approach to developmental disorders. Cognition 101(2), 414–442. [DOI] [PubMed] [Google Scholar]
- Blair RJR 2007. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn. Sci 11(9), 387–392. [DOI] [PubMed] [Google Scholar]
- Blair RJR 2010. Neuroimaging of psychopathy and antisocial behavior: a targeted review. Curr. Psychiatr. Rep 12(1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR 2013. The neurobiology of psychopathic traits in youths. Nature Rev. Neurosci 14(11), 786 10.1038/nrn3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbenishty R, Astor RA, Roziner I, Wrabel SL 2016. Testing the causal links between school climate, school violence, and school academic performance: A cross-lagged panel autoregressive model. Educ. Res 45(3), 197–206. 10.3102/0013189X16644603 [DOI] [Google Scholar]
- Bertsch K, Grothe M, Prehn K, Vohs K, Berger C, Hauenstein K, Keiper P, Domes G, Teipel S, Herpertz SC 2013. Brain volumes differ between diagnostic groups of violent criminal offenders. Eur. Arch. Psychiatry Clin. Neurosci 263(7), 593–606. 10.1007/s00406-013-0391-6 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB 2002, June Region of interest analysis using an SPM toolbox. In 8th International Conference on Functional Mapping of the Human Brain (Vol. 16, No. 2, p. 497). [Google Scholar]
- Brodaty H, Low LF 2003. Aggression in the elderly. J. Clin. Psychiatry 64, 36–43. [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR 2006. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion, 6(2), 239 10.1037/1528-3542.6.2.239 [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH 2010. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neurosci. 13(4), 419 10.1038/nn.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Anderson NE, Harenski KA, Sitney MH, Caldwell MF, Van Rybroek GJ, Kiehl KA 2019. The structural brain correlates of callous-unemotional traits in incarcerated male adolescents. NeuroImage: Clinical, 22, 101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL 1995a. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363(4), 615–641. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL 1995b. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol 363(4), 642–664. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C 1993. A self-administered rating scale for pubertal development. J. Adolesc. Health 14(3), 190–195. 10.1016/1054-139X(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Christianson SÅ, Forth AE, Hare RD, Strachan C, Lidberg L, Thorell LH 1996. Remembering details of emotional events: A comparison between psychopathic and nonpsychopathic offenders. Pers. Individ. Diff 20(4), 437–443. [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW 2008. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131(5), 1311–1322. 10.1093/brain/awn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MD, Viding E, McCrory E, Pape L, van den Brink W, Doreleijers TA, Veltman DJ, Popma A (2016). Regional grey matter volume and concentration in at-risk adoles cents: Untangling associations with callous-unemotional traits and conduct disorder symptoms. Psychiatry Research: Neuroimaging, 254, 180–187. [DOI] [PubMed] [Google Scholar]
- Crockett L 1988. Pubertal development scale: Pubertal categories. Unpublished manuscript.
- DeBellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM 2001. Sex differences in brain maturation during childhood and adoles cence. Cereb. Cortex 11(6), 552–557. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E 2009. Size matters: Increased grey matter in boys with conduct problems and callous–unemotional traits. Brain 132(4), 843–852. 10.1093/brain/awp011 [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, Ambikapathy A, Robert son D, Giampietro V, Brammer MJ, Clarke A, Dowsett J, Fahy T, Philips ML, Murphy DG 2006. Facial emotion processing in criminal psychopathy: Preliminary functional magnetic resonance imaging study. B. J. Psychiatry, 189(6), 533–539. [DOI] [PubMed] [Google Scholar]
- Deng J, Wang MC, Zhang X, Shou Y, Gao Y, and Luo J 2019. The Inventory of Callous Unemotional Traits: A reliability generalization meta-analysis. Psychol. Assess 31(6), 765–780. 10.1037/pas0000698 [DOI] [PubMed] [Google Scholar]
- Dietrich A (2004). The cognitive neuroscience of creativity. Psychonomic bulletin & review, 11(6), 1011–1026. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD 2000. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb. Cortex 10(3), 308–317. 10.1093/cercor/10.3.308 [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA 2012. Aberrant paralimbic gray matter in criminal psychopathy. J. Abnorm. Psychol 121(3), 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA (2013). Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. J. Am. Acad. Child Adolesc. Psychiatry, 52(1), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza FA, Vergara VM, Reyes D, Anderson NE, Harenski CL, Decety J, Rachakonda S, Damaraju E, Rashid B, Miller RL, Koenigs M, Kosson DS, Harenski K, Kiehl KA, Calhoun VD 2018. Aberrant functional network connectivity in psychopathy from a large (N= 985) forensic sample. Hum. Brain Mapp. 39(6), 2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S, Frick PJ 2006. Callous-unemotional traits in a community sample of adolescents. Assessment 13(4), 454–469. 10.1177/1073191106287354 [DOI] [PubMed] [Google Scholar]
- Fink BC, Tant AS, Tremba K, Kiehl KA 2012. Assessment of psychopathic traits in an incarcerated adolescent sample: a methodological comparison. J. Abnorm. Child Psych 40(6), 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, Kiehl KA, Koenigs M 2017. Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Soc. Cogn. Affect. Neurosci 12(7), 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Wonneberger C, Enzi B, De Greck M, Ulrich C, Tempelmann C, Bogerts B, Doering S, Northoff G 2011. The narcissistic self and its psychological and neural correlates: an exploratory fMRI study. Psychol. Med 41(8), 1641–1650. 10.1017/S003329171000228X [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR 2008. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. Gen. Psychiatry 65(5), 586–594. 10.1001/archpsyc.65.5.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, Schneider MR, Sims C, Pope K, Fowler K, Sinclair S, Tovar-Moll F, Pine D, Blair RJ 2012. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res Neuroimaging 202(3), 239–244. 10.1016/j.pscychresns.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ 2004. The inventory of callous-unemotional traits. Unpublished rating scale.
- Frick PJ, Hare RD 2001. Antisocial process screening device. Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE 2014. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol. Bull 140(1), 1 10.1037/a0033076 [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE 2003. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. J. Abnorm. Child Psych 31(4), 457–470. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Chan F, Venables P, Mednick S 2010. Early maternal and paternal bonding, childhood physical abuse and adult psychopathic personality. Psychol. Med 40(6), 1007–1016. 10.1017/S0033291709991279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang W 2016. Confirmatory factor analyses of self-and parent-report inventory of callous-unemotional traits in 8-to 10-year-olds. J. Psychopathol. Behav. Assess. 38(3), 331–340. 10.1007/s10862-015-9527-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang W, Eisenbarth H, Fung ALC, Lu M, Raine A, Lee TMC, Li X 2018. P3 amplitude and psychopathic traits in youths: Distinct contributions of the grandiose-manipulative and daring-impulsivity traits. Pers. Individ. Diff 120, 87–94. [Google Scholar]
- Gregory S, Simmons A, Kumari V, Howard M, Hodgins S, Blackwood N 2012. The antisocial brain: psychopathy matters: a structural MRI investigation of antisocial male violent offenders. Arch. Gen. Psychiatry 69(9), 962–972. 10.1001/archgenpsychiatry.2012.222 [DOI] [PubMed] [Google Scholar]
- Hare RD (1991). The Psychopathy Checklist-Revised (PCL-R). USA: Multi Health Systems. [Google Scholar]
- Herpers PC, Scheepers FE, Bons DM, Buitelaar JK, Rommelse NN 2014. The cognitive and neural correlates of psychopathy and especially callous–unemotional traits in youths: A systematic review of the evidence. Dev. Psychopathol 26(1), 245–273. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ 2006. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. J. Abnorm. Psychol 115(2), 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Bulten BH, Brazil IA 2016. Parsing fear: A reassessment of the evidence for fear deficits in psychopathy. Psychol. Bull 142(6), 573 10.1037/bul0000040 [DOI] [PubMed] [Google Scholar]
- Huang Y, Wu T, Luo Y, Wu Z, Fagan S, Leung S, … & Gao Y 2019. The impact of callous-unemotional traits and externalizing tendencies on neural responsivity to reward and punishment in healthy adolescents. Front. Neurosci 13, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isen J, Raine A, Baker L, Dawson M, Bezdjian S, Lozano DI 2010. Sex-specific association between psychopathic traits and electrodermal reactivity in children. J. Abnorm. Psychol 119(1), 216 10.1037/a0017777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauk E, Neubauer AC, Dunst B, Fink A, Benedek M 2015. Gray matter corre lates of creative potential: A latent variable voxel-based morphometry study. Neu roImage, 111, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Olie E, O'daly O, Malafosse A, Courtet P, Philips ML 2010. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. Neuroimage 51(3), 1275–1281. 10.1016/j.neuroimage.2010.03.027 [DOI] [PubMed] [Google Scholar]
- Kiehl KA 2006. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiat. Res 142(2-3), 107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, Aucoin KJ, Morris AS 2008. Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional Traits. Int. J. Law Psychiat 31(3), 241–252. 10.1016/j.ijlp.2008.04.002 [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Skeem JL, Cauffman E, and Dmitrieva J 2011. Are secondary variants of juvenile psychopathy more reactively violent and less psychosocially mature than primary variants? Law. Hum. Behav 35(5), 381–391. 10.1007/s10979-010-9243-3 [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12(17), 3683–3687. 10.1097/00001756-200112040-00016 [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18(2), 263–272. 10.1016/S1053-8119(02)00057-5 [DOI] [PubMed] [Google Scholar]
- Koenigs M 2012. The role of prefrontal cortex in psychopathy. Rev. Neurosci 23(3), 253–262. 10.1515/revneuro-2012-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, Kiehl KA, Koenigs M 2017. Impulsive-antisocial dimension of psychopathy linked to enlargement and abnormal functional connectivity of the striatum. Biol. Psychiat. Cogn. Neurosci. Neuroimaging, 2(2), 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C, Pujara M, Deming P, Philippi C, Decety J, Kosson DS, Kiehl KA, Koenigs M 2017. Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Soc. Cogn. Affect. Neurosci 12(7), 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BYH, Yang Y, Raine A, Lee TMC 2015. Neural mediator of the schizotypy– antisocial behavior relationship. Translational Psychiatry, 5(11), e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BY, Yang Y, Schug RA, Han C, Liu J, Lee TMC 2017. Psychopathy moderates the relationship between orbitofrontal and striatal alterations and violence: the investigation of individuals accused of homicide. Front. Hum. Neurosci 11, 579 10.3389/fnhum.2017.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb V, Leitner M, Wabnegger A, Klug D, Scharmuller W, Zussner T, Schienle A 2015. Brain abnormalities in high-risk violent offenders and their association with psy chopathic traits and criminal recidivism. Neuroscience, 308, 194–201. [DOI] [PubMed] [Google Scholar]
- Liu W, Mao Y, Wei D, Yang J, Du X, Xie P, Qiu J 2016. Structural asymmetry of dorsolateral prefrontal cortex correlates with depressive symptoms: evidence from healthy individuals and patients with major depressive disorder. Neurosci. Bull 32(3), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Farrington DP 2000. Young children who commit crime: Epidemiology, developmental origins, risk factors, early interventions, and policy implications. Dev. Psychopathol 12(4), 737–762. 10.1017/S0954579400004107 [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW 2011. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci 31(17), 6398–6404. 10.1523/JNEUROSCI.6620-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Henry HY, et al. (2011). Reduced amygdala–orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res. Neuroimaging 194(3), 279–286. 10.1016/j.pscychresns.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Gänßbauer S, Sommer M, Döhnel K, Weber T, Schmidt-Wilcke T, Hajak G 2008. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Res. Neuroimaging 163(3), 213–222. 10.1016/j.pscychresns.2007.08.010 [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD 2004. Separate neural systems value immediate and delayed monetary rewards. Science 306(5695), 503–507. 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD 2007. Time discounting for primary rewards. J. Neurosci 27(21), 5796–5804. 10.1523/JNEUROSCI.4246-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz LC, Frick PJ 2007. The reliability, stability, and predictive utility of the self-report version of the Antisocial Process Screening Device. Scand. J. Psychol 48(4), 299–312. 10.1111/j.1467-9450.2007.00560.x [DOI] [PubMed] [Google Scholar]
- Nenadic I, Güllmar D, Dietzek M, Langbein K, Steinke J, Gaser C 2015. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. Neu roimaging 231(2), 184–186. 10.1016/j.pscychresns.2014.11.001 [DOI] [PubMed] [Google Scholar]
- Noonan MP, Chau B, Rushworth M, Fellows LK 2017. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Hu mans. J. Neurosci 37(29), 7023–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenc L, Radochonski M 2014. Psychopathic traits and reactive-proactive aggression in a large community sample of polish adolescents. Child Psychiatry Hum. Dev 45(4), 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Santtila P, Schecklmann M, Langguth B, Greenlee MW Oster heider M, Mokros A 2013. Association between brain structure and phenotypic charac teristics in pedophilia. J. Psychiat. Res 47(5), 678–685. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC 2003. An FMRI Stroop task study of ventrome dial prefrontal cortical function in pathological gamblers. Am. J. Psychiatry 160(11), 1990–1994. 10.1176/appi.ajp.160.11.1990 [DOI] [PubMed] [Google Scholar]
- Radel R, Davranche K, Fournier M, Dietrich A 2015. The role of (dis) inhibition in cre ativity: Decreased inhibition improves idea generation. Cognition, 134, 110–120. [DOI] [PubMed] [Google Scholar]
- Raine A 2002. Biosocial studies of antisocial and violent behavior in children and adults: A review. J. Abnorm. Child Psych 30(4), 311–326. [DOI] [PubMed] [Google Scholar]
- Raine A, Fung ALC, Lam BYH 2011. Peer victimization partially mediates the schizotypy-aggression relationship in children and adolescents. Schizophr. Bull 37(5), 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, LaCasse L, Colletti P 2004. Hippocampal structural asymmetry in unsuccessful psychopaths. Biol. Psychiatry 55(2), 185–191. 10.1016/S0006-3223(03)00727-3 [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y 2006. Neural foundations to moral reasoning and antisocial behavior. Soc. Cognit. Affect. Neurosci 1, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JV, Frick PJ 2018. Assessing Callous-Unemotional Traits Using the Total Score from the Inventory of Callous-Unemotional Traits: A Meta-Analysis. J. Clin. Child Adolesc. Psychol 1–10. 10.1080/15374416.2018.1504297 [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N 2006. Divide and conquer: a defense of functional localizers. Neuroimage, 30(4), 1088–1096. [DOI] [PubMed] [Google Scholar]
- Salekin RT 2006. Psychopathy in children and adolescents: Key issues in conceptualization and assessment, in: Patrick CJ (Ed.), Handbook of Psychopathy. Guilford Press, New York, pp. 389–414. [Google Scholar]
- Schulze L, Dziobek I, Vater A, Heekeren HR, Bajbouj M, Renneberg B, Heuser I, Roepke S 2013. Gray matter abnormalities in patients with narcissistic personality disorder. J. Psychiatr. Res 47(10), 1363–1369. [DOI] [PubMed] [Google Scholar]
- Smith SS, Newman JP 1990. Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. J.Abnorm. Psychol 99(4), 430. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL 2002. Asymmetric functional d of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex 38(4), 589–612. 10.1016/S0010-9452(08)70024-8 [DOI] [PubMed] [Google Scholar]
- Veit R, Lotze M, Sewing S, Missenhardt H, Gaber T, Birbaumer N 2010. Aberrant social and cerebral responding in a competitive reaction time paradigm in criminal psychopaths. Neuroimage, 49(4), 3365–3372. [DOI] [PubMed] [Google Scholar]
- Viding E, Blair RJR, Moffitt TE, Plomin R 2005. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J. Child Psychol. Psychiatry 46(6), 592–597. 10.1111/j.1469-7610.2004.00393.x [DOI] [PubMed] [Google Scholar]
- Vitacco MJ, Rogers R, Neumann CS 2003. The Antisocial Process Screening Device: An examination of its construct and criterion-related validity. Assessment, 10(2), 143–150. [DOI] [PubMed] [Google Scholar]
- Wang MC, Gao Y, Deng J, Lai H, Deng Q, Armour C 2017. The factor structure and construct validity of the inventory of callous-unemotional traits in Chinese undergraduate students. PloS One 12(12), e0189003 10.1371/journal.pone.0189003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 2003. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wertz J, Caspi A, Belsky DW, Beckley AL, Arseneault L, Barnes JC, Corcoran DL, Hogan S, Houts RM, Morgan N, Odgers CL, Prinz JA, Sugden K, Williams BS, Poulton R, Moffitt TE 2018. Genetics and crime: Integrating new genomic discoveries into psychological research about antisocial behavior. Psychol. Sci 29(5), 791–803. 10.1177/0956797617744542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, Sinclair S, Pine DS, Blair RJR 2012. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am. J. Psychiatry 169(7), 750–758. 10.1176/appi.ajp.2012.11081270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Pujara MS, Motzkin JC, Newman JP, Kiehl KA, Decety J, Kosson DS, Koenigs M 2015. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum. Brain Mapp 36(10), 4202–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2016. International classification of diseases. http://www.who.int/classifications/icd/en/ (accessed 20 Oct 2016). [Google Scholar]
- Yang Y, Raine A 2009. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res Neuroimaging 174(2), 81–88. 10.1016/j.pscychresns.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fagan SE, Gao Y 2017. Respiratory Sinus Arrhythmia Activity Predicts Internalizing and Externalizing Behaviors in Non-referred Boys. Front. Psychol 8, 1496 10.3389/fpsyg.2017.01496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao Y 2015. Interactive effects of social adversity and respiratory sinus arrhythmia activity on reactive and proactive aggression. Psychophysiology 52(10), 1343–1350. 10.1111/psyp.12473 [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Zhang Q 2014. Neural representations for the generation of inventive conceptions inspired by adaptive feature optimization of biological species. Cortex, 50, 162–173. [DOI] [PubMed] [Google Scholar]
- Zhang L, Qiao L, Chen Q, Yang W, Xu M, Yao X, Qiu J, Yang D 2016. Gray matter volume of the lingual gyrus mediates the relationship between inhibition function and divergent thinking. Front. Psychol 7, 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


