Abstract
Extracellular electron transfer via filamentous protein appendages called ‘microbial nanowires’ has long been studied in Geobacter and other bacteria because of their crucial role in globally-important environmental processes and their applications for bioenergy, biofuels, and bioelectronics. Thousands of papers thought these nanowires as pili without direct evidence. Here, we summarize recent discoveries that could help resolve two decades of confounding observations. Using cryo-electron microscopy with multimodal functional imaging and a suite of electrical, biochemical, and physiological studies, we find that rather than pili, nanowires are composed of cytochromes OmcS and OmcZ that transport electrons via seamless stacking of hemes over micrometers. We discuss the physiological need for two different nanowires and their potential applications for sensing, synthesis, and energy production.
Keywords: Electrochemically-active bacteria, Geobacter, Microbial fuel cells, Biofilms, Interspecies electron transfer, Microbial nanowires, Pili, Cytochromes, Electron transport, Multimodal imaging, Atomic force microscopy, cryo-electron microscopy, Protein structure, Conformational change
Introduction—blind men and an elephant: critical need for multidisciplinary approaches
In the ancient Indian tale of blind men encountering an elephant, each man approaches the creature from a different direction [1]. One finds the trunk, another a leg, the third the tail, and so on, whereupon they naturally disagree with each other as to the true appearance of the elephant. Each person experiences something different depending on their angle of approach.
And so it is with microbial nanowires, genetic and electrical properties of nanowires seem to depend on the discipline from which you view them. In 2002, Childers et al. [2] pioneered the field by finding that Geobacter produce filaments during extracellular electron transfer. Since then, microbiologists thought that these ‘microbial nanowires’ are pili filaments made up of PilA protein [3] and that monomeric cytochromes bind to pili [4] (Fig. 1a). Electrochemists found a cytochrome signal in biofilms and suggested that pili are a scaffold for these monomeric cytochromes [5] (Fig. 1a). Biophysicists, like us, found that the filaments are intrinsically conductive, and not a scaffold [6,7], but did not know the mechanism. Lack of nanowire structure was everybody’s elephant in the room. It had become clear that understanding the nanowire structure and function would require an unusually wide range of interdisciplinary knowledge.
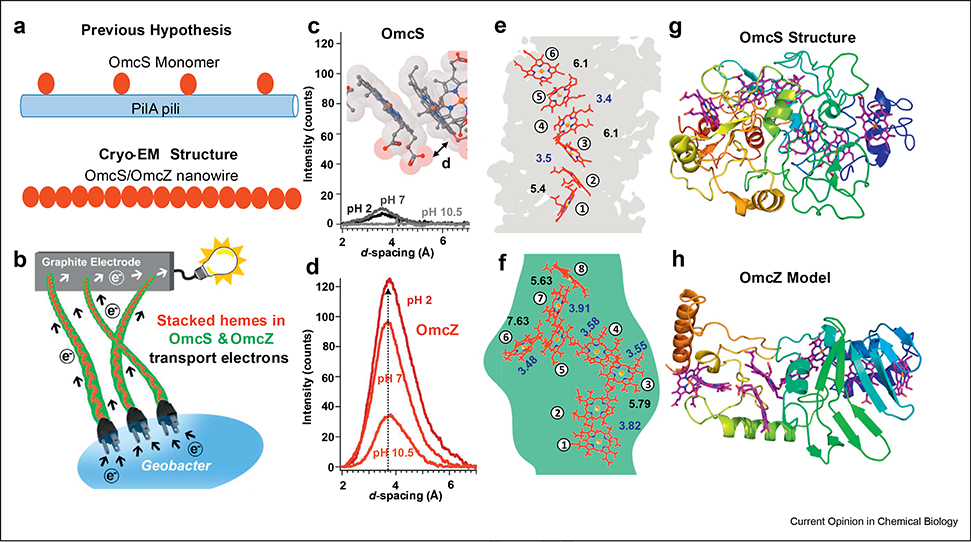
Figure 1. Microbial OmcZ nanowires show improved π stacking between hemes than OmcS, and protonation enhances π stacking.
(a) Hypothesis of pili-based nanowires versus structural evidence for cytochrome nanowires. (b) Schematic of nanowires helping to export electrons outside the cell (Modified from the study by Malvankar et al. [22] with permission.). X-ray diffraction of (c) OmcS and (d) OmcZ nanowires revealed increased intensity for a peak at d-spacing 3.6 Å (pH 10.5 → pH 2), suggesting improved π stacking between hemes that correlated with enhanced conductivity. Inset for (c): Cryo-EM structure of OmcS nanowires with hemes as stick models colored by chemical elements. (gray carbon, blue nitrogen, red oxygen, orange iron), with van der Waals radii translucent. Arrangement of hemes (red) in (e) OmcS and (f) 8-heme proteins. Cryo-EM density is in gray and green for OmcS and OmcZ, respectively. Edge-to-edge distances within π-stacking distance (3.5–4 Å) between heme pairs in OmcS and 8-heme proteins are shown in blue. (g) Cryo-EM structure of OmcS protomer and (h) model of OmcZ, colored by residue number, N-terminus (blue) and fading to red at the C-terminus. ((c)–(h) Adapted from the study by Yalcin et al. [9] with permission.). Cryo-EM; cryo-electron microscopy.
Geobacter sulfurreducens produces current densities in microbial fuel cells that are among the highest known for pure cultures [8]. As discovered recently [9], such high current density is possible because of the unique ability of Geobacter to produce nanowires in biofilms with conductivity rivaling synthetic polymers [6,10,11]. Nanowires enable bacteria to transport electrons over hundreds of cell lengths [6,12]. However, the nanowire composition, structure, and conduction mechanism had remained unknown.
The recent discoveries of microbial nanowire structures have forced us to rethink the aforementioned decade-old beliefs [9,13]. Rather than pili, we found nanowires made up of cytochromes with seamlessly stacked hemes over the entire nanowire length, providing a continuous path for electrons [9,13] (Figs. 1 and 2).
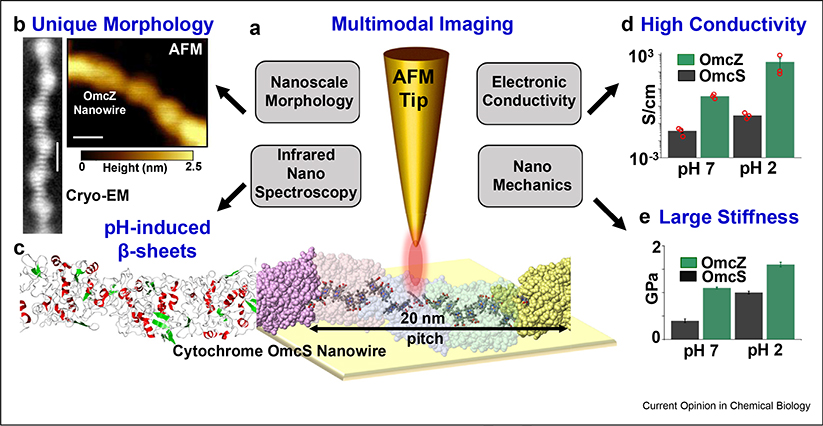
Figure 2. Correlating nanowire structure with electrical and mechanical properties.
(a) Multimodal imaging platform, with OmcS nanowire structure and stacked hemes providing electron transport. (b) Unique globular morphology of OmcZ nanowires revealed by cryo-EM and AFM. Scale bars, cryo-EM, 5 nm; AFM, 20 nm c, Secondary structure of the OmcS nanowire with loops in gray, α-helices in red, and beta strands in green. Low pH converts loop and helices into beta sheets. Low pH enhances (d) conductivity and (e) stiffness of OmcS and OmcZ nanowires (Adapted from the study by Yalcin et al. [9] with permission.). AFM, atomic force microscopy; cryo-electron microscopy.
We have combined cryo-electron microscopy (cryo-EM) with multimodal atomic force microscopy (AFM), to determine composition and structure of nanowires in biofilms to correlate with their conductivity and stiffness [9,13] (Figs. 2 and 3). Using mass spectrometry and near-atomic resolution, cryo-EM has allowed ‘sequencing’ the protein forming the nanowires without knowing its identity a priori [13,14]. Many other studies are now using this approach [15,16]. Multimodal imaging enabled the discovery of OmcZ nanowires by identifying their composition using infrared nanospectroscopy–based chemical imaging [17] and prediction of their structure with computational modeling [9] (Figs. 1–2). As proteins are widely considered as nonconductors [18], these studies will help understand the electron transport mechanism and why these two different nanowires are essential for various physiological roles such as iron reduction [19], current production [20], and direct interspecies electron transfer [21] (Fig. 4).
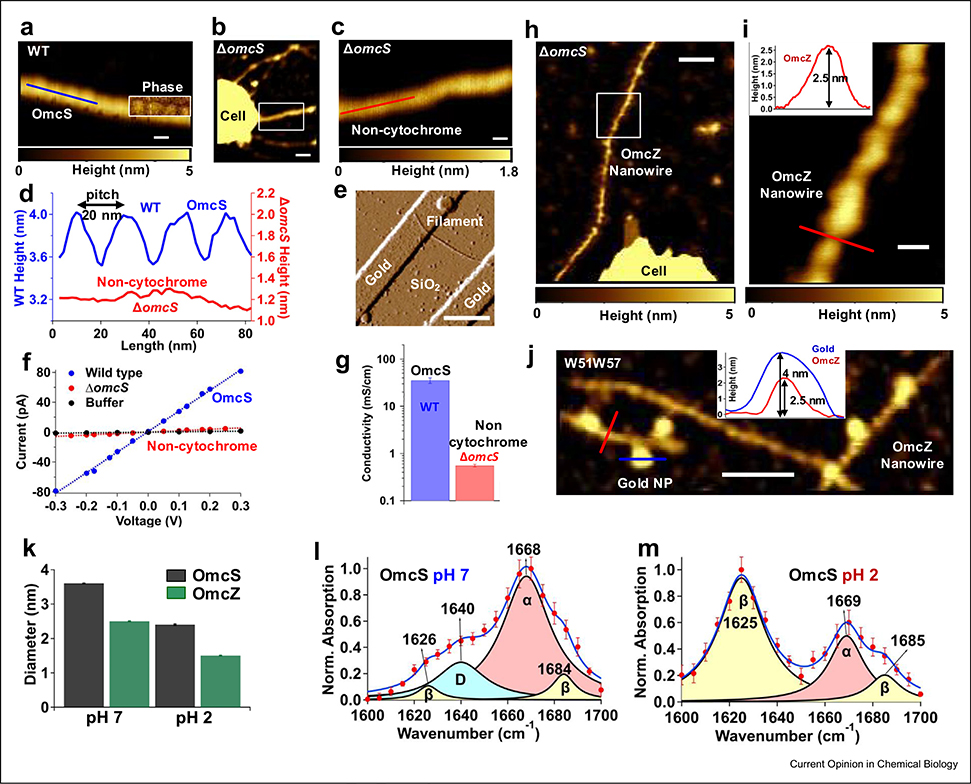
Figure 3. Correlating filament structure with conductivity reveals pH-induced conformational changes in nanowires.
The AFM height image of (a) OmcS nanowires from WT strain, (b) noncytochrome filaments from a ΔomcS strain, and (c) a zoomed image of the region shown in (b). Inset in (a): The AFM phase image overlaid on the height image showing the periodicity. Scale bars: (a), (c), 20 nm; (b), 100 nm (d) The longitudinal height profile for filaments at locations shown in (a) and (c). (e) The AFM image of noncytochrome filament by ΔomcS strain across gold electrodes. Scale bar, 500 nm. (f) The current–voltage profile for individual OmcS nanowires and noncytochrome filaments compared with buffer alone. (g) Comparison of their conductivity. (h) OmcZ nanowires produced by ΔomcS strain grown under conditions that overexpress OmcZ. (i) The zoomed image of the OmcZ nanowire shown in the box in (h). Inset: height of the OmcZ nanowire taken at the red line in (i). (j) The AFM image of nanowires by W51W57 strain labeled with anti-OmcZ immunogold. Inset: heights of the nanowire (red) and gold nanoparticle (blue) at locations shown in (j). Scale bars: (h), 100 nm, (i), 20 nm, (j), 100 nm, (k) Relative heights and pH-induced reduction in the nanowire diameter. Infrared nanospectroscopy of OmcS nanowires at (l) pH 7 and (m) pH 2 showing low pH–induced beta sheets ((a)–(g) adapted with permission from the study by Wang et al. [13] and (h)–(m) from the study by Yalcin et al. [9]). AFM, atomic force microscopy.
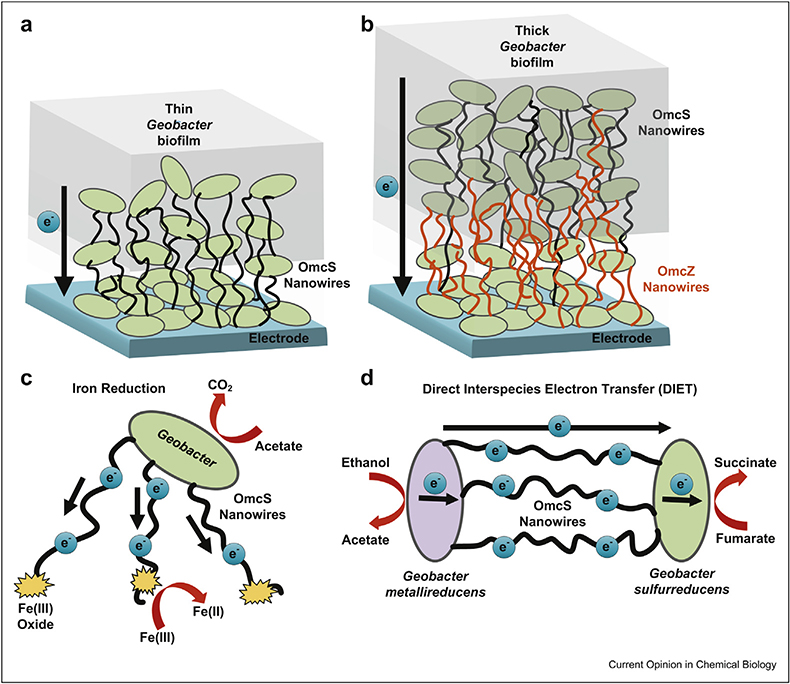
Figure 4. model for physiologically distinct roles of OmcS and OmcZ nanowires.
(a) OmcS nanowires are essential for electricity-producing biofilms during initial stages of growth. (b) OmcZ nanowires are essential for the formation of thick, mature biofilms capable of high current density. OmcS nanowires are also essential for (c) iron reduction and (d) direct interspecies electron transfer.
Critical need to correlate nanowire structure with function
Previous studies presumed that nanowires are pili based on indirect genetic evidence that pilA mutant did not produce filaments and because of a lack of high-resolution structural methods [3,4]. Therefore, it is critical to correlate nanowire structure with functional studies. We have correlated atomic structure with AFM imaging to confirm that the same OmcS and OmcZ nanowires were studied for both conductivity measurements and atomic structure determination [9,13]. For example, AFM revealed an axial height periodicity with a 20-nm pitch for OmcS nanowires, consistent with the helical pitch determined by cryo-EM, whereas no such pitch was observed for noncytochrome filaments (Fig. 3a,c) [13]. Furthermore, at pH 7, the nanowire heights for OmcS and OmcZ are 3.6 and 2.5 nm, respectively, and the nanowires undergo very large conformational changes to beta sheets at pH 2 that reduce their diameter to 2.4 and 1.5 nm, respectively [9] (Fig. 3k). This distinct axial periodicity and the substantial thickness difference observed for OmcS and OmcZ nanowires versus other filaments were used to confirm that the same nanowires were studied for both structural as well as conductivity and stiffness studies. These studies revealed that in comparison with OmcS nanowires, noncytochrome filaments show 100-fold lower conductivity [13] (Fig. 3e–g), whereas OmcZ nanowires show 1000-fold higher conductivity [9] (Fig. 2d). Such correlative imaging studies will ensure that identical filaments are examined via multiple methods by first mapping their structural features and then linking them with different functional properties.
Chemically tuning the nanowire structure and conductivity
Our previous studies had shown that lowering the pH enhances nanowire conductivity [6] and alters their conformation [22]. However, the identity of nanowires and the underlying mechanism for low pH–enhanced conductivity were unknown. We have found that lowering the pH enhances conductivity of OmcS and OmcZ nanowires by 100-fold because of protein conformational changes to a β sheet–rich structure [9] (Fig. 3l and m). X-ray diffraction studies showed that this structural change improves the stacking of hemes in nanowires [9] (Fig. 1c–d). This enhanced π stacking between hemes can increase the effective conjugation length, yielding a longer mean free path for electrons that enhances conductivity [24].
Why two nanowires?—physiologically distinct roles of OmcS and OmcZ
The role of OmcS as nanowires was overlooked because ΔomcS biofilms were conductive and produced high current densities in microbial fuel cells when grown over prolonged growth conditions. Therefore, we evaluated the possibility of proteins other than OmcS capable of forming nanowires in biofilms [9]. Using our AFM-based multimodal imaging platform, we have found that growing G. sulfurreducens biofilms, under current-producing conditions that require an electric field, stimulates production of OmcZ nanowires that exhibit 1000-fold higher conductivity than OmcS nanowires (Fig. 2d) [9]. The electric field is maximum near the biofilm–electrode interface and decreases away from the electrode. Therefore, OmcZ expression will be maximum at the interface. This could explain the maximum accumulation of OmcZ [9] and the highest metabolic activity [9] observed near the biofilm–electrode interface (Fig. 4b).
Both OmcS and OmcZ are important for electricity production: deletion of omcS inhibits electricity production during the early stages of biofilm growth [6,26], whereas deletion of omcZ precludes formation of thick, high–current density biofilms [20]. In wild-type biofilms, OmcZ accumulates near the electrode, whereas OmcS is distributed throughout the biofilm [25]. Based on all these findings, we propose a new model that OmcS nanowires are involved in the biofilm growth during early stages, whereas OmcZ nanowires help bacteria form 100 μm–thick biofilms because of their high conductivity (Fig. 4a and b).
The OmcS is also essential for Fe (III) oxide reduction [19] and direct interspecies electron transfer between Geobacter co-cultures [21,27], with cells connecting each other via anti-OmcS–labeled filaments [21]. Analysis of such anti-OmcS–labeled filaments revealed structure similar to OmcS nanowires [13]. We therefore propose that G. sulfurreducens use OmcS nanowires to transfer electrons to Fe(III) oxide (Fig. 4c) and to accept electrons from Geobacter metallireducens (Fig. 4d).
The regulatory role of pili in secretion of cytochrome nanowires
The nanowires were thought to be type IV pili composed of PilA protein [3] because ΔpilA cells did not produce conductive filaments [6] and could not transfer electrons to extracellular acceptors such as iron [3] or electrodes in microbial fuel cells [28]. However, the presence of PilA in a filament of wild-type cells has not been established, only inferred from indirect evidence such as the presence of PilA monomer in filament preparations that also contain OmcS nanowires [13]. It is important to note that the conduction along the length of a single bona fide PilA filament has not been demonstrated and theoretical studies did not find conductivity in modeled PilA filaments [29,30], except when hypothesized that aromatic residues are within 3–4 Å of each other [31]. It is possible that synthetic pilA could assemble into a filament under artificial conditions [32–34]. However, these individual synthetic filaments’ conductivity has not been shown along their length, only across their diameter, and their exact composition and structure is unknown.
We propose that, rather than serving as nanowires, pili are involved in the translocation of OmcS and OmcZ nanowires to the outer surface. The deletion of pilA inhibits the extracellular translocation of OmcS [23,27] and OmcZ [23] nanowires, which are essential for extracellular electron transfer to iron [19] and high–current density biofilms [20], respectively. Furthermore, overexpression of PilA is accompanied by over-production of OmcS [25], OmcZ [20], and extracellular filaments that result in the formation of highly conductive biofilms with enhanced current density [25]. Cryo-EM studies did not find any filaments with structure consistent with type IV pili either in filaments from current-producing wild-type biofilms or in previously published images of intact, cell-attached filaments [13]. Analysis of previously published filament images, that were thought to be pili, showed structure similar to OmcS nanowires [13]. Furthermore, conductivity measurements along the length of individual OmcS and OmcZ nanowires showed values similar to conductivity values [35,36] for filaments of wild-type [13] and the W51W57 strain [9], respectively. All these results suggest that these previous studies, including some of our own [7], interpreted OmcS and OmcZ nanowires as pili. It is, therefore, important to identify the conditions under which G. sulfurreducens can naturally show pili and determine their composition, structure, and conductivity to evaluate their exact function.
Resolving the controversy about nanowires by reconciling conflicting results
The discovery of OmcZ nanowires helps address the following concerns about the physiological role of OmcS nanowires by Lovley and Walker [37], and numbered the same as recently summarized [38]:
-
1
Long-range electron transport requires the formation of thick (>50 μm) electrically-conductive biofilms. The omcS gene deletion has no obvious impact on current production in biofilms.
The omcS deletion inhibits current production during early stages of biofilm growth [26], suggesting that OmcS nanowires are involved in the current production. Our discovery of OmcZ nanowires helps to explain how cells could compensate for the loss of OmcS nanowires during later stages of thick biofilm growth.
-
2
OmcS filaments do not participate in long-range electron transport as the expression of a pilin gene in which 5 aromatics were mutated (“Aro5’) generates a less conductive biofilm; heterologous expression of pilin genes from other bacteria in G. sulfurreducens yielded strains expressing pili with low conductivity, but that expressed even more outer-surface OmcS.
Pili are required for the secretion of both OmcS and OmcZ cytochromes and not just OmcS alone [23]. Therefore, it is necessary to evaluate the expression and localization of both OmcS and OmcZ nanowires, as well as other outer-surface cytochromes, before attributing the aforementioned phenotypes solely to pili.
G. sulfurreducens strain KN400, which expresses much less OmcS and much more PilA than wild-type G. sulfurreducens, generates higher current and much more conductive biofilms.
We found that this strain produces OmcZ nanowires [9] with 1000-fold higher conductivity than OmcS nanowires. This could also explain the ability of KN400 strain to generate higher current and more conductive biofilms. Therefore, it is not surprising that OmcS is not essential for the KN400 strain.
-
3
E-pili expression is required for Fe(III) oxide reduction, but there are substitutes for the deletion of OmcS, such as magnetite.
Without an atomic resolution structure of a Geobacter filament composed of PilA, there seems to be no conclusive proof that Geobacter can make such a filament. As the deletion of pilA inhibits the extracellular translocation of OmcS [23,27] nanowires, it is not possible to attribute lack of Fe(III) oxide reduction in a pilA mutant to pili alone. Furthermore, magnetite is electrically conductive and is shown to facilitate extracellular electron transfer [39]. We have previously shown that in a sediment environment conductive minerals can transport electrons over centimeters, 10,000 times the size of a cell [40]. Therefore, introducing such conductive minerals can compensate for the loss of OmcS nanowires.
-
4
Iron corrosion, current production, and syntrophic growth do not require OmcS filaments emanating at distances from the cell.
If gene deletion studies show that a cytochrome is not essential, it does not mean that it has no function under wild-type growth conditions. Bacteria use multiple approaches and redundant pathways for growth. The discovery of OmcZ nanowires shows that many cytochromes could form nanowires, not just OmcS.
-
5
There is no correlation between the expression level of PilA and the secretion of OmcS.
The lack of correlation does not necessarily mean a lack of causation. Deletion of pilA inhibits the secretion of OmcS and OmcZ, establishing that PilA is required for nanowire secretion [23,27]. No prior studies have ever quantified the density of either OmcS or OmcZ nanowires in current-producing biofilms or visualized their network to determine the percolation threshold that determines biofilm conductivity. The lack of correlation between the total amount of OmcS/OmcZ in biofilms and measured biofilm conductivity does not mean that cytochrome nanowires do not confer conductivity to biofilms [41].
-
6
The culture conditions of Wang and co-workers’ research [13] are not good for the expression of e-pili [37]. That is the reason that PilA is barely detectable in their filament preparation.
The culture conditions for current-producing biofilms [42], that led to discovery of OmcS and OmcZ nanowires [9,13], are identical to those previously used by Lovley and colleagues to evaluate long-range electron transport in biofilms where they found overexpression of PilA and OmcZ [20]. Both SDS-PAGE gel and western immunoblotting confirm the presence of abundant PilA monomer in our filament preparations from these biofilms [13]. However, cryo-EM revealed that all the filaments in current-producing highly-conductive biofilms are OmcS and OmcZ nanowires and not pili [9,13].
Outlook
When asked about the importance of discovering conducting polymers that started the field of plastic electronics, Nobel laureate Alan Heeger offered two basic answers: (i) they did not previously exist and (ii) they offer a unique combination of properties not available from any other known materials [43]. The first expressed an intellectual challenge; the second expressed a promise for a wide range of applications.
The discovery and many properties of microbial nanowires are just like conducting polymers. Without interdisciplinary approaches, none of these discoveries would have been possible. Moreover, the ability to modulate their conductivity by targeted changes in the sequence or environment is particularly exciting because it can provide a foundation for a new field of research on the boundaries between molecular biology, microbiology, biophysical chemistry, and physics.
These discoveries are creating many opportunities:
First, microbial nanowires are opening the way for understanding the fundamental chemistry and physics of electron transport in proteins over micrometer distances at rates not previously known in biomolecules. Recent theoretical studies have suggested quantum-coherence effects in the conductivity of OmcS nanowires [44] that need to be examined using the atomic structure of nanowires.
Second, microbial nanowires are providing an opportunity to address questions that had been of interest, such as polymerization of cytochrome c in apoptosis [45] and design of synthetic metalloprotein nanowires [46–48].
Third, the ability to function at a low pH is a unique strength of these materials [9]. There is no other protein-based electronic material that shows such high electronic conductivity at low pH, to our knowledge. Improved conductivity at low pH in polyaniline, discovered by Nobel laureate Alan MacDiarmid, was critical for the development of conducting polymer–based sensors [49]. Therefore, we anticipate that the discovery of protein-based electronic materials that can withstand and function in extreme environments will serve as a foundation for future developments of biosensor and pH sensors. Improved conductivity will enhance the performance of protein nanowire–based devices used for energy harvesting [50,51], sensors [52], ultra-low power computing [53], and bioelectronics such as living transistors [6] and supercapacitors [54].
Finally, microbial nanowires offer promise for achieving a new class of electronic materials that could exhibit the electrical and optical properties of metals and semiconductors but retain mechanical properties and demonstrate versatile functionalities of proteins to bring together synthetic biology with semiconducting technology [55].
Acknowledgments
The authors thank all lab members and Eric Martz for helpful discussions. This research was supported by the Career Award at the Scientific Interfaces from Burroughs Wellcome Fund, the National Institutes of Health Director’s New Innovator award no. 1DP2AI138259-01, the National Science Foundation (NSF) CAREER award no. 1749662 and NSF Early-Concept Grant for Exploratory Research (EAGER) award no. 2038000. Research was sponsored by the Defence Advanced Research Project Agency Army Research Office and was accomplished under Cooperative Agreement Number W911NF-18-2-0100. Research in the laboratory is also supported by the Charles H. Hood Foundation Child Health Research Award, and The Hartwell Foundation Individual Biomedical Research Award.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.Stolow A: Molecular conformations fielded. Nature 2009, 461: 1063–1064. [DOI] [PubMed] [Google Scholar]
- 2.Childers SE, Ciufo S, Lovley DR: Geobacter metallireducens accesses insoluble Fe(iii) oxide by chemotaxis. Nature 2002, 416:767–769. [DOI] [PubMed] [Google Scholar]
- 3.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR: Extracellular electron transfer via microbial nanowires. Nature 2005, 435:1098–1101. [DOI] [PubMed] [Google Scholar]
- 4.Leang C, Qian XL, Mester T, Lovley DR: Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl Environ Microbiol 2010, 76:4080–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, Tender LM: Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci U S A 2012, 109:15467–15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, et al. : Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 2011, 6:573–579. [DOI] [PubMed] [Google Scholar]
- 7.Malvankar NS, Yalcin SE, Tuominen MT, Lovley DR: Visualization of charge propagation along individual pili proteins using ambient electrostatic force microscopy. Nat Nanotechnol 2014, 9:1012–1017. [DOI] [PubMed] [Google Scholar]
- 8.Yi H, Nevin KP, Kim BC, Franks AE, Klimes A, Tender LM, Lovley DR: Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens Bioelectron 2009, 24:3498–3503. [DOI] [PubMed] [Google Scholar]
- 9.Yalcin SE, O’Brien JP, Gu Y, Reiss K, Yi SM, Jain R, Srikanth V,Dahl PJ, Huynh W, Vu D, et al. : Electric field stimulates production of highly conductive microbial OmcZ nanowires. Nat Chem Biol 2020, 16:1136–1142. 10.1038/s41589-020-0623-9.●● This study uses multiomodal imaging to reveal that the electric field, required for current-production by G. sulfurreducens biofilms, causes overexpression of OmcZ nanowires tha are 1000-fold more conductive than OmcS nanowires.
- 10.Malvankar NS, Tuominen MT, Lovley DR: Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energy Environ Sci 2012, 5:5790–5797. [Google Scholar]
- 11.Malvankar NS, Lou J, Nevin K, Franks AE, Tuominen MT, Lovley DR: Electrical conductivity in a mixed-species biofilm. Appl Environ Microbiol 2012, 78:5967–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malvankar NS, Lovley DR: Electronic conductivity in living biofilms: physical meaning, mechanisms, and measurement methods In Biofilms in bioelectrochemical systems. John Wiley & Sons, Inc; Beyenal H and Babauta J (Eds.), 2015:211–248. [Google Scholar]
- 13.Wang F, Gu Y, O’Brien J, Patrick Yi SM, Yalcin SE, Vishok Srikanth, Shen C, Vu D, Ing NL, Hochbaum AI, et al. : Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell 2019, 177: 361–369.●● Both these studies use cryo-electron microscopy to reveal that microbial nanowires in current-producing G. sulfurreducens biofilms are made up of OmcS and not pili.
- 14.Filman DJ, Marino SF, Ward JE, Yang L, Mester Z, Bullitt E, Lovley DR, Strauss M: Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire. Commun Biol 2019, 2:219.●● Both these studies use cryo-electron microscopy to reveal that microbial nanowires in current-producing G. sulfurreducens biofilms are made up of OmcS and not pili.
- 15.Ho C-M, Li X, Lai M, Terwilliger TC, Beck JR, Wohlschlegel J, Goldberg DE, Fitzpatrick AWP, Zhou ZH: Bottom-up structural proteomics: cryoEM of protein complexes enriched from the cellular milieu. Nat Methods 2020, 17:79–85.● This study presents a protocol for using mass spectrometry and cryo-electron microscopy to determine the composition and structure of proteins without knowing their identity a priori.
- 16.Wang F, Cvirkaite-Krupovic V, Kreutzberger MA, Su Z, de Oliveira GA, Osinski T, Sherman N, DiMaio F, Wall JS, Prangishvili D, Krupovic M, Egelman EH: An extensively glycosylated archaeal pilus survives extreme conditions. Nature Microbiol 2019, 4:1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yalcin SE, Legg BA, Yesilbas M, Malvankar NS, Boily J-F: Direct observation of anisotropic growth of water films on minerals driven by defects and surface tension. Sci Adv 2020, 6, eaaz9708.● This study uses infrared nanospectroscopybased chemical imaging to directly visualize water molecules as they bind to individual minerals that could serve as extracellular electron acceptors.
- 18.Zhang B, Song W, Pang P, Lai H, Chen Q, Zhang P, Lindsay S: Role of contacts in long-range protein conductance. Proc Natl Acad Sci USA 2019, 116:5886.● This study shows that non-redox proteins are conductive in single-molecule measurements, with small electron decay with distance, provided charge is injected into the protein interior via good contact.
- 19.Mehta T, Coppi MV, Childers SE, Lovley DR: Outer membrane c-type cytochromes required for Fe (III) and Mn (IV) oxide reduction in Geobacter sulfurreducens. Applied and Environmental Microbiology 2005, 71:8634–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevin KP, Kim BC, Glaven RH, Johnson JP, Woodard TL, Methé BA, DiDonato RJ Jr, Covalla SF, Franks AE, Liu A, et al. : Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PloS One 2009, 4:e5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR: Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330:1413–1415. [DOI] [PubMed] [Google Scholar]
- 22.Malvankar NS, Vargas M, Nevin K, Tremblay PL, Evans-Lutterodt K, Nykypanchuk D, Martz E, Tuominen MT, Lovley DR: Structural basis for metallic-like conductivity in microbial nanowires. mBio 2015, 6 e00084–00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter LV, Sandler SJ, Weis RM: Two isoforms of Geobacter sulfurreducens PilA have distinct roles in pilus biogenesis, cytochrome localization, extracellular electron transfer, and biofilm formation. J Bacteriol 2012, 194:2551–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K, Cho S, Park S, Heeger A, Lee C, Lee S: Metallic transport in polyaniline. Nature 2006, 441:65–68. [DOI] [PubMed] [Google Scholar]
- 25.Leang C, Malvankar NS, Franks AE, Nevin KP, Lovley DR: Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ Sci 2013, 6:1901–1908. [Google Scholar]
- 26.Holmes DE, Chaudhuri SK, Nevin KP, Mehta T, Methe BA, Liu A, Ward JE, Woodard TL, Webster J, Lovley DR: Microarray and genetic analysis of electron transfer to electrodes in Geobacter sulfurreducens. Environ Microbiol 2006, 8: 1805–1815. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Zhuo S, Rensing C, Zhou S: Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species. ISME J 2018, 12:2142–2151.● This study shows that Geobacter co-cultures require OmcS but not pili for DIET
- 28.Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR: Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 2006, 72:7345–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebedev N, Mahmud S, Griva I, Blom A, Tender LM: On the electron transfer through Geobacter sulfurreducens PilA protein. J Polym Sci B Polym Phys 2015, 53:1706–1717. [Google Scholar]
- 30.Yan H, Chuang C, Zhugayevych A, Tretiak S, Dahlquist FW, Bazan GC: Inter-aromatic distances in Geobacter sulfurreducens pili relevant to biofilm charge transport. Adv Mater 2015, 27:1908–1911. [DOI] [PubMed] [Google Scholar]
- 31.Ru X, Zhang P, Beratan DN: Assessing possible mechanisms of micrometer-scale electron transfer in heme-free Geobacter sulfurreducens pili. J Phys Chem B 2019, 123:5035–5047.● This theoretical study suggests that to account for experimentally-measured conductivity, aromatic residues in pili need to be within 3–4 Å and oxidized using a strong oxidizing agent.
- 32.Cosert K, Castro-Forero A, Steidl RJ, Worden RM, Reguera G: Bottom-up fabrication of protein nanowires via controlled self-assembly of recombinant Geobacter pilins. mBio 2019:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueki T, Walker DJ, Woodard TL, Nevin KP, Nonnenmann SS, Lovley DR: An Escherichia coli chassis for production of electrically conductive protein nanowires. ACS Synth Biol 2020, 9:647–654. [DOI] [PubMed] [Google Scholar]
- 34.Ueki T, Walker DJ, Tremblay P-L, Nevin KP, Ward JE, Woodard TL, Nonnenmann Ss, Lovley DR: Decorating the outer surface of microbially produced protein nanowires with peptides. ACS Synth Biol 2019, 8:1809–1817. [DOI] [PubMed] [Google Scholar]
- 35.Adhikari R, Malvankar N, Tuominen M, Lovley D: Conductivity of individual Geobacter pili. RSC Adv 2016, 6:8354–8357. [Google Scholar]
- 36.Tan Y, Adhikari RY, Malvankar NS, Pi S, Ward JE, Woodard TL, Nevin KP, Xia Q, Tuominen MT, Lovley DR: Synthetic biological protein nanowires with high conductivity. Small 2016, 12: 4481–4485. [DOI] [PubMed] [Google Scholar]
- 37.Lovley DR, Walker DJF: Geobacter protein nanowires. Front Microbiol 2019, 10:2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Dong F, Minteer SD: The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nature Catal 2020, 3:225–244. [Google Scholar]
- 39.Kato S, Hashimoto K, Watanabe K: Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci Unit States Am 2012, 109: 10042–10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malvankar NS, King GM, Lovley DR: Centimeter-long electron transport in marine sediments via conductive minerals. ISME J 2014, 9:527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malvankar NS, Tuominen MT, Lovley DR: Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energy Environ Sci 2012, 5:8651–8659. [Google Scholar]
- 42.O’Brien JP, Malvankar NS: A simple and low-cost procedure for growing Geobacter sulfurreducens cell cultures and biofilms in bioelectrochemical systems. Curr Protoc Microbiol 2017, 43:A.4K.1–A.4K.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeger AJ: Semiconducting polymers: the third generation. Chem Soc Rev 2010, 39:2354–2371. [DOI] [PubMed] [Google Scholar]
- 44.Eshel Y, Peskin U, Amdursky N: Coherence-assisted electron diffusion across the multi-heme protein-based bacterial nanowire. Nanotechnology 2020, 31:314002.● This theoretical study on model heme systems suggests that to account for expreimentally-measued condcutivity in OmcS nanowires, quantum-coherence effects need to be invoked.
- 45.Hirota S, Hattori Y, Nagao S, Taketa M, Komori H, Kamikubo H, Wang Z, Takahashi I, Negi S, Sugiura Y: Cytochrome c polymerization by successive domain swapping at the C-terminal helix. Proc Natl Acad Sci Unit States Am 2010, 107: 12854–12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altamura L, Horvath C, Rengaraj S, Rongier A, Elouarzaki K, Gondran C, Maçon AL, Vendrely C, Bouchiat V, Fontecave M: A synthetic redox biofilm made from metalloprotein–prion domain chimera nanowires. Nat Chem 2017, 9:157–163. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, Zarzycki J, Gunner MR, Parson WW, Kern JF, Yano J, Ducat DC, Kramer DM: Mesoscopic to macroscopic electron transfer by hopping in a crystal network of cytochromes. J Am Chem Soc 2020, 142:10459–10467. [DOI] [PubMed] [Google Scholar]
- 48.Chen YX, Ing NL, Wang F, Xu D, Sloan NB, Lam NT, Winter DL, Egelman EH, Hochbaum AI, Clark DS: Structural determination of a filamentous chaperone to fabricate electronically conductive metalloprotein nanowires. ACS Nano 2020, 14:6559–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDiarmid AG: Nobel Lecture: synthetic metals:A novel role for organic polymers. Rev Mod Phys 2001, 73:701–712. [DOI] [PubMed] [Google Scholar]
- 50.Malvankar NS, Tuominen MT, Lovley DR: Biofilm conductivity is a decisive variable for high-current-density microbial fuel cells. Energy Environ Sci 2012, 5:5790–5797. [Google Scholar]
- 51.Liu X, Gao H, Ward JE, Liu X, Yin B, Fu T, Chen J, Lovley DR, Yao J: Power generation from ambient humidity using protein nanowires. Nature 2020, 578:550–554.●● This study shows a unique application to generate electricity out of thin air using microbial nanowires.
- 52.Smith AF, Liu X, Woodard TL, Fu T, Emrick T, Jiménez JM, Lovley DR, Yao J: Bioelectronic protein nanowire sensors for ammonia detection. Nano Res 2020:1–6. [Google Scholar]
- 53.Fu T, Liu X, Gao H, Ward JE, Liu X, Yin B, Wang Z, Zhuo Y, Walker DJ, Yang jJ: Bioinspired bio-voltage memristors. Nat Commun 2020, 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malvankar NS, Mester T, Tuominen MT, Lovley DR: From the Cover: supercapacitors based on c-type cytochromes using conductive nanostructured networks of living bacteria. ChemPhysChem 2012, 13:463–468. [DOI] [PubMed] [Google Scholar]
- 55.Bathe M, Chrisey LA, Herr DJC, Lin Q, Rasic D, Woolley AT, Zadegan RM, Zhirnov VV: Roadmap on biological pathways for electronic nanofabrication and materials. Nano Futures 2019, 3, 012001.● This review summarizes key emerging technologies for biologically-produced electronic materials.