Abstract
Background:
(1–3)-b-D-glucan (BDG) is a fungal cell wall component and, in the absence of invasive fungal infection, a novel biomarker for microbial translocation of endogenous fungal products from the gastrointestinal tract into systemic circulation. However, its value as a marker of fungal translocation is limited by a concern that plant BDG-rich food influences blood BDG levels.
Methods:
We conducted a pilot clinical trial to evaluate the impact of a standardised oral BDG challenge on blood BDG levels in participants with and without elevated microbial translocation. We enrolled 14 participants including 8 with HIV infection, 2 with advanced liver cirrhosis, and 4 healthy controls. After obtaining a baseline blood sample, participants received a standardised milkshake containing high levels of BDG followed by serial blood samples up to 8 hours after intake.
Results:
The standardised oral BDG challenge approach did not change the blood BDG levels over time in all participants. We found consistently elevated blood BDG levels in one participant with advanced liver cirrhosis and a single person with HIV with a low CD4 count of 201 cells/mm3.
Conclusion:
Our findings indicate that BDG blood levels were not influenced by plant origin BDG-rich nutrition in PWH, people with advanced liver cirrhosis, or healthy controls.
Future studies are needed to analyse gut mycobiota populations in individuals with elevated blood BDG levels.
Keywords: 1-3-beta-D-glucan, fungal translocation, healthy controls, liver cirrhosis, microbial translocation, non-AIDS events, plasma
1 |. INTRODUCTION
(1–3)-beta-D-glucan (BDG) is a fungal cell wall component, currently used as a marker for the presumptive diagnosis and treatment monitoring of invasive fungal infections (IFI).1–4 Recently, in the absence of IFI, plasma levels of BDG also emerged as candidate biomarker of gut fungal translocation.5,6 Fungal translocation is the passage of fungal components through a compromised intestinal epithelial barrier due to immune dysfunction, gut damage or altered gut microbiota composition. However, its clinical relevance is less studied compared with gut bacterial translocation.5,6 The merit of plasma BDG as an indicator of gut mucosal barrier permeability leading to fungal translocation is supported by studies reporting increased BDG levels during haemodialysis,7,8 most likely due to transiently reduced blood flow within the splanchnic region,9 leading to ischaemia and, consequently, gut barrier damage. Fungal translocation with elevated BDG levels also occurs in various diseases associated with ‘leaky gut’ such as sepsis, kidney injury and liver cirrhosis,10–12 and in animal models has been shown to contribute to alcoholic liver disease progression.13 In people with HIV (PWH), BDG has been used as a fungal translocation marker associated with increased gut permeability, microbial translocation and consequently with inflammation contributing to risk of developing non-AIDS comorbidities.14–17 Specifically, BDG blood levels in blood correlated with the gram-negative bacterial endotoxin lipopolysaccharide (LPS), soluble CD14, the inflammatory cytokines interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha (TNF-alpha) as well as with non-AIDS comorbidities such as cardiopulmonary dysfunction and HIV-associated neurocognitive disorders (HAND).14,16,18–20 These studies suggest that excessive fungal or fungal cell wall component translocation is associated with greater risk of comorbidities and negative health outcomes in at-risk groups such as those with liver cirrhosis, PWH, and also acute respiratory distress syndrome.21
BDG is also a component of foods such as cereals, mushrooms, seaweeds and yeast, and often utilised as nutritional supplements.22 One potential limitation of its use as a marker of microbial translocation is the high inter-individual variability of mycobiome burden in the intestinal luminal contents and the unknown impact of daily and postprandial variation.4,23–25 In this pilot trial, we utilised a controlled and standardised BDG-rich food regimen, to determine whether food intake affects BDG levels in blood and alters its validity as a fungal translocation marker.
2 |. METHODS
2.1 |. Participant recruitment
A total of 14 adult participants were enrolled in this study between January 2018 and May 2019. Exclusions were invasive fungal infection, haemodialysis with cellulose-containing membranes, diabetes mellitus or low blood sugar, history of high/low blood pressure, intake of nutritional supplements containing high doses of BDG within 24 hours of the study visit and use of antibiotics or anticholinergic drugs within the last 14 days. Of these, (a) four were PWH with CD4 T-cell count < 350 cells/mm3 and/or on antiretroviral therapy (ART) for less than eight weeks with viral load (VL) > 1000 copies/mL, (b) four were PWH on ART with suppressed VL and with CD4+ T-cell count > 350 cells/mm3, (c) two were participants without HIV but with hepatitis-C (HCV)-associated liver cirrhosis Child-Pugh class B or C,26 and (d) four were controls without HIV and HCV.
2.2 |. Preparation of standardised BDG-rich food shake
Twenty aliquots of a standardised food shake were prepared by the University of California San Diego (UCSD) nutritional service in late 2017 using ingredients high in BDG, including (ie per aliquot) 150 mL of almond milk, 50 g of oat bran, 25 g of bananas and 25 g of dates, generating a Fungitell signal equivalent to 234 mg of Pachyman signal per gram of foodstuff. Aliquots were frozen at −20°C and thawed the day before a planned study visit. On the day of study visit, the milkshake was fed to participants and a 0.5 mL aliquot was obtained and stored for retrospective BDG testing.
The Fungitell-based estimation of BDG content of poorly soluble BDG containing plant material is difficult due to the complex and particulate nature of the material. The estimate of beta-glucan content is further complicated by the alkaline pretreatment of the material prior to reaction with the Fungitell reagent. This has the effect of converting triple helical (1→3)-β-glucan to single helical (1→3)-β-glucan with a concomitant multifold increase in reactivity with the Fungitell reagent. This is the same procedure used for the estimation of BDG titre in biological fluids such as serum. Using this approach, it was estimated the signal level of BDG signal in the foodstuff challenge represented approximately 234 mg/g, as Pachyman (the assay’s (1→3)-β-glucan standard) equivalents. This exceeds the likely unprocessed BDG mass available in the ingredients. Oat bran, the ingredient with the highest level of (1→3)-β-glucan, is typically estimated to contain 4%−22% beta-glucan.27,28 However, the Fungitell estimate is based upon reactivity which can be highly variable for beta-glucans based upon their tertiary structure, branching, molecular weight, and processing history with heat and/or alkali.29 The data do, however, demonstrate the overwhelmingly large level of signal introduced with the BDG-rich food challenge in this study. A large level of challenge signal was utilised to ensure that the quantity of Fungitell signal-capable BDG available for translocation was not a limiting factor.
2.3 |. Study visit and laboratory testing
Participants completed a prestudy visit where they signed the informed consent and were instructed to fast or consume only non-BDG-containing food during the 12 hours before the study visit and enter all nutritional intake during these 12 hours into a food log. On the morning of the study visit, a baseline plasma sample was taken, and participants were instructed to drink the milkshake aliquot within 5 minutes. Blood was collected from participants at 0.5, 1, 1.5, 2, 3, 4, 6 and 8 hours after consumption of the BDG-rich food shake (Figure 1). During the study visit, participants were served only non-BDG containing food and drinks. Plasma samples were stored at −80°C within 90 minutes of collection and retrospectively evaluated for BDG levels using the Fungitell® assay at the facilities at Associates of Cape Cod, Inc, (Associates of Cape Cod, Inc, East Falmouth, USA). LPS levels were measured in all baseline samples and a subset of follow-up samples (ie to assess daily variation) at the Research Institute of the McGill University Health Centre, Montreal, QC, Canada, using a standardised ELISA assay (Cusabio, China).
FIGURE 1.

Study design
2.4 |. Statistical analysis
For statistical analysis, SPSS 25 (SPSS Inc, Chicago, IL, USA) was used. BDG and LPS levels over time were compared using paired t test. The study protocol and all study-related procedures were approved by the Human Research Protections Program at the University of California, San Diego, United States.
3 |. RESULTS
In total, 14 participants (13 men, 1 woman; 7 Hispanic, 5 Caucasian, 1 African-American, 1 Asian) were enrolled in this study. Participants were 19–56 years of age, and among the two participants with liver cirrhosis, each one had Child-Pugh B or Child-Pugh C liver cirrhosis. The study design is depicted in Figure 1.
Aliquots from milkshakes yielded high BDG content on the order of 234 mg/g, by Fungitell estimate, with measured concentrations between 3 × 106 and 75 × 108 pg/mL.
Baseline plasma BDG levels ranged between 5 pg/mL and 95 pg/mL, with the highest level observed in the participant with Child-Pugh C liver cirrhosis. No significant change in plasma BDG levels between baseline and any of the later timepoints was observed (all P > .149). Plasma BDG levels following shake intake remained low and stable for the entire duration of the study for most participants, while small peaks (ie single higher levels) were observed in 4 of the participants between one and two hours after the oral challenge. Two participants, one with Child-Pugh C liver cirrhosis and one PWH not taking ART, both conditions that are associated with leaky gut and microbial translocation, demonstrated relatively high levels of plasma BDG for the duration of the study visit (Figure 2).
FIGURE 2.

BDG levels at baseline and over 8 h after milkshake consumption in participants with and without fungal translocation; namely HCV associated advanced liver cirrhosis (n = 2; red), HIV infection with detectable viral load (n = 4; purple), HIV infection with suppressed viral load (n = 4; pink), and HIV and HCV negative controls (n = 4; blue)
LPS levels in baseline samples varied between 0 and 111 pg/mL, with the two highest levels observed in the two individuals with advanced liver cirrhosis. Furthermore, in a subset of samples from three PWH taking ART and two liver cirrhosis participants, LPS levels seemed to show wider ranges of undirected intra-timepoint variation (n.s.) (Figure 3).
FIGURE 3.
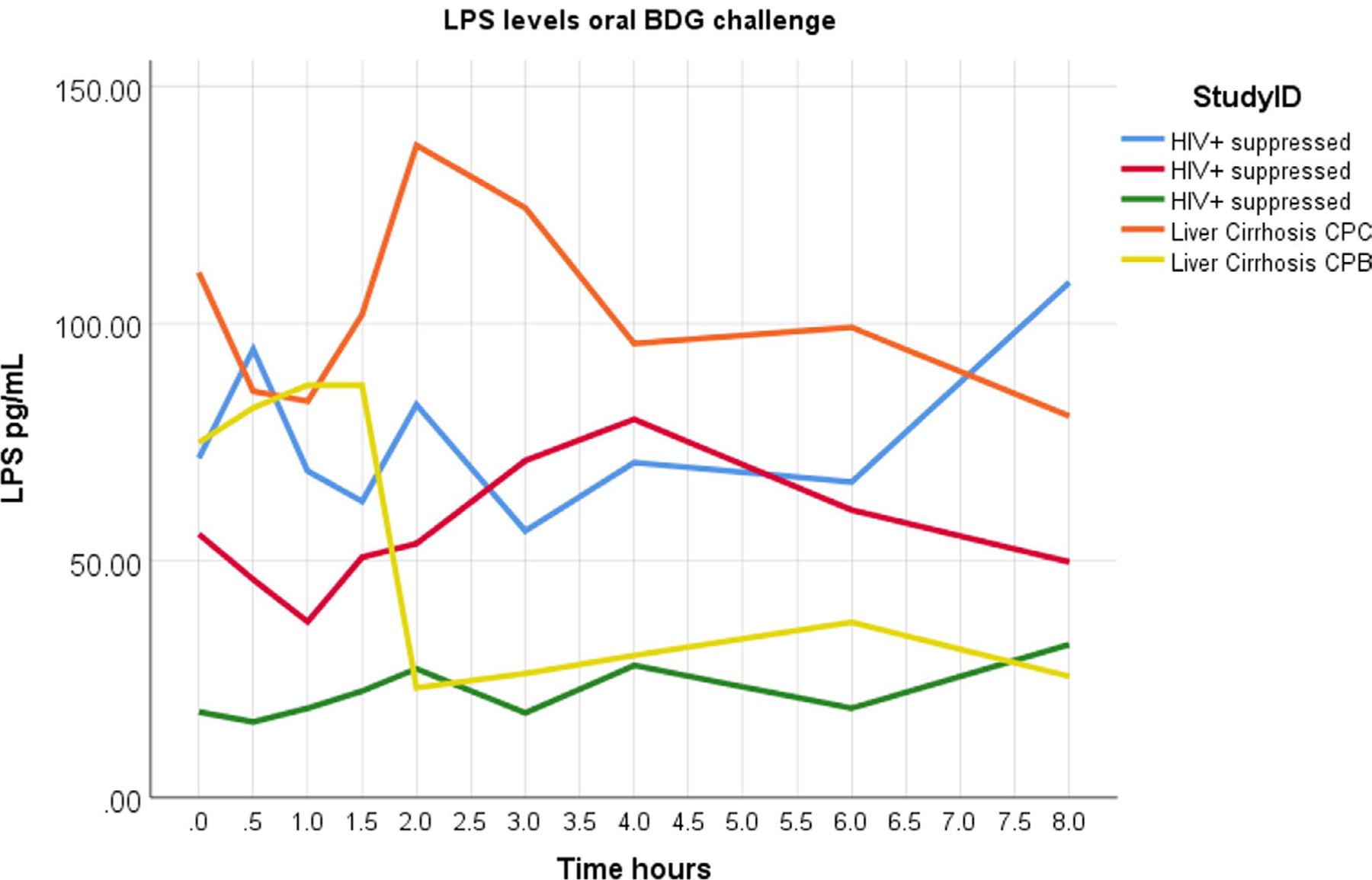
LPS levels at baseline and over 8 h after milkshake consumption in a subset of participants with advanced liver cirrhosis or HIV infection suppressed on antiretroviral therapy
4 |. DISCUSSION
The composition of the human gut microbiome is influenced by multiple intrinsic and extrinsic factors, including host genetics, age, diet, lifestyle and antibiotic use. Likewise, the gut microbiome and mycobiome influence the propensity to develop various diseases, such as alcoholic liver disease as described by Yang et al (2017).13,30 Furthermore, damage to the physiological barrier of the gut allows translocation of fungal products from the intestinal lumen into the blood.31 Certain contributors that give rise to fungal translocation include medical procedures such as surgery and haemodialysis, as well as conditions like liver cirrhosis, immune dysfunction, sepsis, intestinal hypoperfusion and persistent inflammation.11,26,32–36 In the setting of HIV, higher blood BDG levels are associated with increased fat gain in ART-naïve PWH initiating treatment and with immune activation, increased systemic inflammation in ART-treated PWH and non-AIDS clinical events.5,16–20,37,38 Thus, there is a need to determine when best to measure markers of microbial translocation and gut damage in order to deliver accurate diagnostics and to assess treatment responses.
Interestingly, microbial composition changes throughout the day and are time-dependent due to food intake and sleep cycle.39 Of note, in a mouse model, plasma LPS levels directly increased following food intake, especially when mice were fed a high-fat diet.40 Similarly, in participants with metabolic syndrome, a high saturated-fat diet was associated with increased LPS levels in the blood.41 Robust markers of microbial translocation that do not require timing and are not influenced by diet are needed to gauge levels of microbial translocation in certain patients. In a recent study measuring daily variations of microbial translocation and gut damage markers in 11 PWH receiving ART, we determined that BDG remained stable across 24 hours, including after food intake as well as during sleep, while LPS showed variations.42
Herein, we showed that BDG plasma levels remained stable over 8 hours after a high-dose oral BDG challenge even in those with significant fungal translocation. In other words, higher blood BDG levels do not appear to be impacted by time of day and are not enhanced by high intestinal luminal contents of plant-based beta-glucan.
The use of plant-based BDG instead of fungal-based BDG in our study is a potential limitation. Plant-based BDG (callose), in addition to the beta-1–3-linked backbone, has β-(1→4)-linked side chains while fungal-based BDG has β-(1→6)-linked side chains, which may result in differing absorption through the intestinal epithelium.43 Additionally, our study had a small sample size and similar future studies will require a larger sample size to increase statistical power. Despite these limitations, our study confirmed that BDG is a biomarker that is not affected by food intake or variation over time, which, in-line with previous studies, may be considered as a reliable indicator of gut fungal translocation.
Funding information
This work was supported by a CFAR Developmental Grant from National Institutes of Health AI036214, and further supported by funds from MH113477. Supported in part the Fonds de la Recherche Québec-Santé (FRQS): Réseau SIDA/Maladies infectieuses and Thérapie cellulaire; the Canadian Institutes of Health Research (CIHR; Grants HOP 103230 and PTJ 166049). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST
MH received grant funding from Gilead and Pfizer. YZ and MF are employees of Associates of Cape Cod. All other authors declare no conflicts of interest.
OFF-LABEL USE
Fungitell®, the FDA-cleared IVD kit used for the measurement of (1→3)-β-glucan in serum, does not have an indication for the diagnostic use of BG titres in the assessment of intestinal translocation. The data presented here represents research use only.
REFERENCES
- 1.Prattes J, Hoenigl M, Rabensteiner J, et al. Serum 1,3-beta-D-glucan for antifungal treatment stratification at the intensive care unit and the influence of surgery. Mycoses. 2014;57(11):679–686. [DOI] [PubMed] [Google Scholar]
- 2.Reischies FMJ, Prattes J, Woelfler A, Eigl S, Hoenigl M. Diagnostic performance of 1,3-beta-D-glucan serum screening in patients receiving hematopoietic stem cell transplantation. Transpl Infect Dis. 2016;18(3):466–470. [DOI] [PubMed] [Google Scholar]
- 3.Heldt S, Prattes J, Eigl S, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Infect. 2018;77(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro E, Calder P, Roche H. β−1,3/1,6-glucans and Immunity: state of the art and future directions. Mol Nutr Food Res. 2020. 10.1002/mnfr.201901071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner LD, Retuerto M, Hager CL, et al. Fungal translocation is associated with immune activation and systemic inflammation in treated HIV. AIDS Res Hum Retroviruses. 2019;35(5):461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoenigl M Fungal translocation: a driving force behind the occurrence of non-AIDS events? Clin Infect Dis. 2020;70(2):242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 → 3)-β-d-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2012;18(5):E122–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theel ES, Jespersen DJ, Iqbal S, et al. Detection of (1, 3)-β-d-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175(1):33–41. [DOI] [PubMed] [Google Scholar]
- 9.Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J. Splanchnic perfusion during hemodialysis: evidence for marginal tissue perfusion. Crit Care Med. 2001;29(7):1393–1398. [DOI] [PubMed] [Google Scholar]
- 10.Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, et al. Gastrointestinal leakage detected by serum (1→3)-β-D-glucan in mouse models and a pilot study in patients with sepsis. Shock. 2016;46(5):506–518. [DOI] [PubMed] [Google Scholar]
- 11.Simões-Silva L, Araujo R, Pestana M, Soares-Silva I, Sampaio-Maia B. The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis. Pharmacol Res. 2018;130:143–151. [DOI] [PubMed] [Google Scholar]
- 12.Issara-Amphorn J, Surawut S, Worasilchai N, et al. The synergy of endotoxin and (1→3)-β-D-glucan, from gut translocation, worsens sepsis severity in a lupus model of fc gamma receptor IIb-deficient mice. J Innate Immun. 2018;10(3):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang A-M, Inamine T, Hochrath K, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoenigl M, Pérez-Santiago J, Nakazawa M, et al. (1→3)-β-d-glucan: a biomarker for microbial translocation in individuals with acute or early HIV infection? Front Immunol. 2016;7:1–7. 10.3389/fimmu.2016.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhour Z, Mehraj V, Chen J, Ramendra R, Lu H, Routy J-P. Use of (1→3)-β-d-glucan for diagnosis and management of invasive mycoses in HIV-infected patients. Mycoses. 2018;61(10):718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramendra R, Isnard S, Mehraj V, et al. Circulating LPS and (1→3)-β-D-Glucan: a folie à deux contributing to HIV-associated immune activation. Front Immunol. 2019;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehraj V, Ramendra R, Isnard S, et al. Circulating (1→3)-β-D-glucan is associated with immune activation during human immunodeficiency virus infection. Clin Infect Dis. 2020;70(2):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris A, Hillenbrand M, Finkelman M, et al. Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immuno-suppression, inflammation, and cardiopulmonary function. JAIDS J Acquir Immune Defic Syndr. 2012;61(4):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianella S, Letendre SL, Iudicello J, et al. Plasma (1 → 3)-β-d-glucan and suPAR levels correlate with neurocognitive performance in people living with HIV on antiretroviral therapy: a CHARTER analysis. J NeuroVirol. 2019;25(6):837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoenigl M, Moser CB, Funderburg N, et al. Soluble urokinase plasminogen activator receptor is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression. Clin Infect Dis. 2019;69(4):676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyland D, Jiang X, Day AG, Laverdiere M. Serum β-d-glucan of critically ill patients with suspected ventilator-associated pneumonia: Preliminary observations. J Crit Care. 2011;26(5):536.e1–536. e9. [DOI] [PubMed] [Google Scholar]
- 22.El Khoury D, Cuda C, Luhovyy BL, Anderson GH. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metab. 2012;2012:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. (1,3)-β-D-glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis. 2012;55(4):521–526. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima A, Yamada K, Iwata O, et al. β-glucan in foods and its physiological functions. J Nutr Sci Vitaminol. 2018;64(1):8–17. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman M, Lempitski SJ. (1→3)-β-D-Glucan (BG) testing in invasive ungal infection (IFI): Potential sources of contamination. Presented at the: ISHAM 2006; 2006. [Google Scholar]
- 26.Moon MS, Quinn G, Townsend EC, et al. Bacterial translocation and host immune activation in chronic hepatitis C infection. Open Forum Infect Dis. 2019;6:ofz255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan MA, Nadeem M, Rakha A, Shakoor S, Shehzad A, Khan MR. Structural characterization of Oat Bran (1→3), (1→4)-β-D-glucans by lichenase hydrolysis through high-performance anion exchange chromatography with pulsed amperometric detection. Int J Food Prop. 2016;19(4):929–935. [Google Scholar]
- 28.Zielke C, Kosik O, Ainalem M-L, Lovegrove A, Stradner A, Nilsson L. Characterization of cereal β-glucan extracts from oat and barley and quantification of proteinaceous matter. PLoS One. 2017;12(2):e0172034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelman MA, Tamura H. Detection and measurement of (1→3)-β-D-glucan In: Young S, Castranova V, eds. Toxicology of (1→3)-Beta-Glucans. New York, NY: CRC Press; 2005. [Google Scholar]
- 30.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690–699. [DOI] [PubMed] [Google Scholar]
- 31.Wiest R, Rath HC. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17(3):397–425. [DOI] [PubMed] [Google Scholar]
- 32.Szyszkowitz A, Zurl C, Herzeg A, et al. Serum 1,3-beta-D-glucan values during and after laparoscopic and open intestinal surgery. Open Forum Infect Dis. 2018;5(12):ofy296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med. 2016;375(8):767–777. [DOI] [PubMed] [Google Scholar]
- 34.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60(1):197–209. [DOI] [PubMed] [Google Scholar]
- 35.Haussner F, Chakraborty S, Halbgebauer R, Huber-Lang M. Challenge to the intestinal mucosa during sepsis. Front Immunol. 2019;10:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirajlal-Fargo S, Moser C, Rodriguez K, et al. Changes in the fungal marker β-D-glucan after antiretroviral therapy and association with adiposity. Open Forum Infect Dis. 2019;6(11):ofz434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoenigl M, de Oliveira MF, Pérez-Santiago J, et al. (1→3)-β-D-glucan levels correlate with neurocognitive functioning in HIV-infected persons on suppressive antiretroviral therapy: a cohort study. Medicine (Baltimore). 2016;95(11):e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deaver JA, Eum SY, Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front Microbiol. 2018;9:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 41.López-Moreno J, García-Carpintero S, Jimenez-Lucena R, et al. Effect of dietary lipids on endotoxemia influences postprandial inflammatory response. J Agric Food Chem. 2017;65(35):7756–7763. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang J, Isnard S, Lin J, et al. Daily variations of gut microbial translocation markers in ART-treated HIV-infected people. AIDS Res Ther. 2020;17(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz-Herrera J, Ortiz-Castellanos L. Cell wall glucans of fungi. A review. Cell Surf. 2019;5:100022. [DOI] [PMC free article] [PubMed] [Google Scholar]


