Abstract
The Hsp90 molecular chaperone is required for the function of hundreds of different cellular proteins. Hsp90 and a cohort of interacting proteins called cochaperones interact with clients in an ATP-dependent cycle. Cochaperone functions include targeting clients to Hsp90, regulating Hsp90 ATPase activity, and/or promoting Hsp90 conformational changes as it progresses through the cycle. Over the last 20 years, the list of cochaperones identified in human cells has grown from the initial six identified in complex with steroid hormone receptors and protein kinases to about fifty different cochaperones found in Hsp90-client complexes. These cochaperones may be placed into three groups based on shared Hsp90 interaction domains. Available evidence indicates that cochaperones vary in client specificity, abundance, and tissue distribution. Many of the cochaperones have critical roles in regulation of cancer and neurodegeneration. A more limited set of cochaperones have cellular functions that may be limited to tissues such as muscle and testis. It is likely that a small set of cochaperones are part of the core Hsp90 machinery required for the folding of a wide range of clients. The presence of more selective cochaperones may allow greater control of Hsp90 activities across different tissues or during development.
Electronic supplementary material
The online version of this article (10.1007/s12192-020-01167-0) contains supplementary material, which is available to authorized users.
Keywords: Molecular chaperone, Hsp90, Cochaperone, Tetratricopeptide repeat, Aha1, Cdc37
The Hsp90 folding cycle
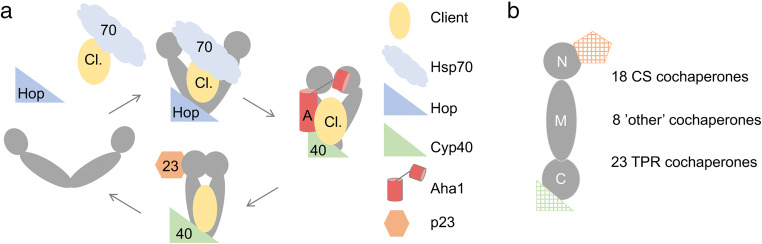
The predominant model of Hsp90 and cochaperone interaction with client proteins is based on analysis of proteins that associate with steroid hormone receptors, such as the glucocorticoid receptor (GR). During GR folding and activation, Hsp90 progresses through a series of distinct complexes characterized by the presence of different cochaperones. This cycle was first established using cellular extracts and has since been confirmed using purified components. In a simplified model (Fig. 1a), the chaperones Hsp70 and Hsp40 interact with the client first. Hop (human gene name STIP1) is able to bind simultaneously to both Hsp70 and Hsp90, facilitating transfer of client to Hsp90. Coordinated action of Aha1 (human gene name AHSA1) and Cyp40 (human gene name PPID) displace Hop, allowing formation of the closed nucleotide-bound state characterized by interaction with p23 (human gene name PTGES3). As the cycle progresses, Hsp90 undergoes dramatic structural changes, cycling from the open form dimerized at only the carboxyl-terminus to the closed form that additionally dimerizes the amino-terminal domains. Hsp90 ATPase activity is inhibited while bound to Hop and activated by Aha1. Details about cochaperone function and structure and functional interaction between Hsp90 and the GR are available (Kirschke et al. 2014; Lorenz et al. 2014; Prodromou 2012; Schopf et al. 2017).
Fig. 1.
a Simplified model of the Hsp90 folding pathway. The client protein (yellow, Cl.) bound to Hsp70 (light blue) is targeted to the open conformation of Hsp90 (dark gray) in a process facilitated by Hop (dark blue). Nucleotide binding promotes dimerization of the amino-termini of Hsp90 and displacement of Hop by Aha1 (red, A) and Cyp40 (green, 40). The closed conformation is further stabilized by p23 (orange, 23). b Additional cochaperones that share a CS domain with p23, or a TPR domain with Hop or Cyp40 have also been found in Hsp90 complexes. Other cochaperones do not contain either of those domains
The above model does not account for increasing evidence that different clients interact with different cochaperone proteins. The extent of overlap of the folding pathways for diverse clients is not known. As discussed below, almost 50 proteins have been identified in complex with Hsp90 clients. These proteins have been established as cochaperones based on shared characteristics with known cochaperones. These include the ability to directly interact with Hsp90, the ability to modulate Hsp90 ATPase activity, the ability to prevent aggregation of other proteins, and/or the presence of conserved domains known to promote Hsp90 interaction. Another property is that overexpression or knockdown of cochaperones alters resistance to Hsp90 inhibitors (Forafonov et al. 2008; Smith et al. 2009). It is important to note that some cochaperones also have Hsp90-independent functions (Echtenkamp et al. 2011; Freeman and Yamamoto 2002; Sager et al. 2018).
The large number of cochaperones is likely due to the diverse roles of Hsp90, which is both abundant and essential in eukaryotes (Borkovich et al. 1989). Hsp90 inhibition directly or indirectly impacts the function of 10–15% of all human proteins (Wu et al. 2012). Hsp90 interaction promotes client stability, provides a means of regulating client activity, and/or promotes assembly into multiprotein complexes. Some cochaperones exhibit preferential binding to proteins with specific folds or domains. For example, Cdc37 binds directly to protein kinases, while Sgt1 binds clients with leucine-rich repeats (Taipale et al. 2014; Verba et al. 2016). A likely source of variation in Hsp90-client complexes is that many cochaperones share a common Hsp90-interacting domain (Fig. 1b), allowing swapping of one cochaperone for another. For instance, there are 18 proteins that share homology with p23, in what is now referred to as a cysteine and histidine-rich domain (CHORD) domain, or CS domain (Garcia-Ranea et al. 2002). An additional 23 cochaperones, including Hop and Cyp40, are characterized by the presence of a tetratricopeptide repeat (TPR) domain (Schopf et al. 2017). Considering that an Hsp90 dimer has binding sites for two CS cochaperones and two TPR cochaperones, there is potential for vast variability in Hsp90 complexes. A further set of eight cochaperones do not contain either of those domains. We summarize what is known about this collection of human cochaperones, most of which have been diligently tracked and curated by Didier Picard over more than 20 years (http://www.picard.ch/downloads/Hsp90facts.pdf). We also examined differences in cochaperone expression level and tissue distribution, identifying cellular processes or tissues where groups of Hsp90 cochaperones have been found to have shared functions.
P23-like cochaperones that contain CS domains
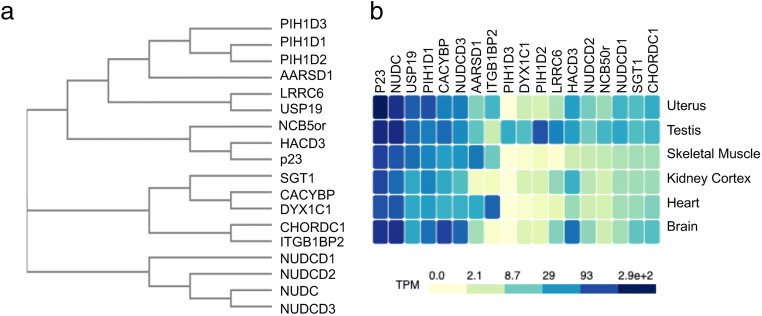
The CS domain of p23 domain consists of a 7 strand antiparallel β-sandwich that is similar to crystallin (Ali et al. 2006). A total of 18 proteins that contain p23-like sequences have been identified. The structures of many of these proteins, or specifically the isolated CS domains, have been determined (Table S1 and (Garcia-Ranea et al. 2002)). Figure 2a shows a phylogenetic tree of the CS domains of the cochaperones aligned with Clustal Omega (Madeira et al. 2019). Many of these proteins contain additional functional domains. Two families of proteins that are present in this group are the NudC (Nuclear distribution gene C) family and the PIH (protein interacting with Hsp90) family (Fu et al. 2016; Yamamoto et al. 2010). Two additional closely related proteins in this group are CHORDC1 and ITGB1BP2, also known as Morgana and Melusin (Ferretti et al. 2011).
Fig. 2.
a Phylogenetic tree showing homology of cochaperones with p23-like CS domains. The sequences corresponding to the CS domain of human cochaperones were aligned with Clustal Omega. The accession sequences for each cochaperone are listed in Table S1. b The Genotype-Tissue Expression (GTEx) database (gtexportal.org) was used to compare mRNA expression data for each of the cochaperones across a range of human tissues. Brain = frontal cortex, heart = atrial appendage. The hatched polygons show the mean transcripts per million (TPM) of the mRNA encoding the indicated cochaperone in the tissue shown. Darker colors represent higher expression levels
Although these proteins share a common domain and are known or predicted to bind to the amino-terminus of Hsp90, they have different properties with regard to both Hsp90 interaction and client interaction. p23 binds the amino-terminus of Hsp90 while it is in the closed-ATP bound conformation (Ali et al. 2006), and it is able to weakly inhibit ATPase activity (McLaughlin et al. 2006). However, Sgt1 and CHORDC1 appear to bind different conformations of Hsp90 and have differing effects on ATPase activity (reviewed in (Shirasu 2009)). p23, NudC, NUDCD3 and CACYBP are chaperones able to protect other proteins from aggregating (Freeman et al. 1996; Fu et al. 2016; Goral et al. 2016; Zheng et al. 2011). A comparative analysis suggests that each of these cochaperones may have unique client specificity. For example, Sgt1, NudC, NUDCD1, NUDCD2, and NUDCD3 exhibited preferential interaction with different types beta-propeller folds (Taipale et al. 2014).
Until recently, it has been difficult to compare the relative expression of the different cochaperones across a wide range of tissues. However, the Genotype-Tissue Expression (GTEx) project provides RNA sequence data for non-disease human tissue sites across multiple individuals (https://www.gtexportal.org/home/). We used that data to compare the overall transcript abundance of the cochaperones in a limited set of tissues. This approach allows us to examine how the composition of Hsp90 complexes may change in a tissue-specific manner. p23 was one of the first cochaperones identified, and it binds steroid hormone receptors, telomerase, certain chromatin remodelers, and other proteins (Echtenkamp et al. 2016). As shown in Fig. 2b, p23 is expressed abundantly and ubiquitously. Other cochaperones with CS domains are expressed at similar levels, suggesting they may function with a wide range of Hsp90 clients or interact with clients that are abundantly expressed. This includes NudC, USP19, PIH1D1, CACYBP, and NUDCD3. NudC has important roles in regulating dynein and actin organization during mitosis and cell migration, while the functions of NUDCD3 are largely unknown (Boitet et al. 2019; Zhu et al. 2010). PIH1D1 is part of a large R2TP complex involved in assembly of diverse protein complexes (Rivera-Calzada et al. 2017). USP19 is a deubiquitinase involved in a variety of functions (He et al. 2016). CACYBP is a multi-domain and multi-functional protein (reviewed in (Topolska-Wos et al. 2016)).
The remaining proteins in this group exhibit lower overall expression or strong tissue-specific expression patterns. Sgt1 is required for the function of nucleotide-binding domain and leucine-rich repeat-containing proteins (NLR) proteins that are required for innate immunity. Sgt1 also has roles in kinetochore complex formation, activation of the leucine-rich repeat adenylyl cyclase Cyr1, and formation of the SCF ubiquitin-ligase complex (reviewed in (Shirasu 2009)). CHORDC1 has important roles in regulating genomic stability by regulating the centrosome cycle (Ferretti et al. 2010). NUDCD2 was recently shown to play a role in modulating sister chromatin cohesion during mitosis (Yang et al. 2019), and NUDCD1 is involved in dynein signaling (He et al. 2018).
The expression of some of these cochaperones is tissue- specific. LRRC6, PIH1D2, PIH1D3, and DYX1C1 are highly expressed in the testis (Dong et al. 2014), while Aarsd1 and ITGB1BP2 are highly expressed in muscle tissues (Echeverria et al. 2016; Tarone and Brancaccio 2015). Two additional family members in this group have varied expression and poorly understood functions. HACD3 has a role in viral replication and was shown to share some functions with p23 (Taguwa et al. 2008). The reason it may be highly expressed in the brain is unknown, but it may be related to its interaction with Rac, a member of the Ras superfamily (Courilleau et al. 2000). NCB5or (NADH-cytochrome b5 oxidoreductase) is implicated in diabetes and neurological diseases, but the role of Hsp90 in these functions is unclear (Benson et al. 2019).
TPR-containing cochaperones
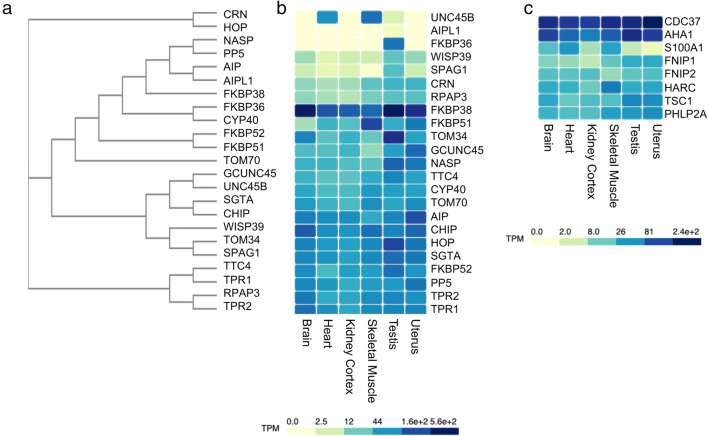
The tetratricopeptide repeat (TPR) is a degenerate 34 amino acid sequence (D'Andrea and Regan 2003; Scheufler et al. 2000). Figure 3a shows a phylogenetic tree of the TPR domains of the cochaperones aligned with Clustal Omega. TPR-containing cochaperones vary in the number of TPR repeats in a tandem array, and some cochaperones, such as Hop, contain more than one TPR domain. Thus, alternate alignments are possible. The structure of the TPR domains of many of these cochaperones has been determined (Table S2). Most of the 23 TPR cochaperones are known or predicted to function primarily by binding to the EEVD sequence at the carboxy-terminus of Hsp90, but some may also bind the conserved sequence in cytosolic Hsp70. A discussion of the determinants of the specificity of TPR cochaperones may be found elsewhere (Assimon et al. 2015). TPR cochaperones may bind different conformations of Hsp90: Hop is known to preferentially interact with the nucleotide-free form of Hsp90, while others, such as FKBP51, FKBP52, and Cyp40, preferentially interact with the ATP-bound form of Hsp90 (Johnson et al. 1994; Richter et al. 2003). TPR cochaperones also have varied effects on the Hsp90 ATPase activity (Blundell et al. 2017; Prodromou et al. 1999; Schopf et al. 2017). Each monomer of Hsp90 can bind one TPR protein at a time, and competition for TPR binding sites on Hsp90 appears to shape chaperone activity (Assimon et al. 2015).
Fig. 3.
a Phylogenetic tree showing homology of cochaperones containing TPR domains. The sequences corresponding to the TPR domains of human cochaperones were aligned with Clustal Omega. The accession sequences for each cochaperone are listed in Table S2. b, c The Genotype-Tissue Expression (GTEx) database (gtexportal.org) was used to compare mRNA expression data for each of the cochaperones across a range of human tissues. Brain = frontal cortex, heart = atrial appendage. The hatched polygons show the mean transcripts per million (TPM) of the mRNA encoding the indicated cochaperone in the tissue shown. Darker colors represent higher expression levels
The TPR cochaperones are diverse, and many of them have domains that confer additional functions, such as peptidyl-prolyl isomerase, protein phosphatase, or ubiquitin ligase activity (Golden et al. 2008; Guy et al. 2015; Zhang et al. 2005). In some cases, client activity is known to vary depending on the identity of the bound cochaperone. For instance, activity of the GR is positively affected by FKBP52 interaction, but not by FKBP51 interaction, and changes in the relative levels of cochaperones may be a method to regulate client activity or degradation (Connell et al. 2001; Erlejman et al. 2014; Reynolds et al. 1999). The range of functions of many of these cochaperones is not known, but it is likely that they have differing specificity for clients and/or distinct effects on client activity (Guy et al. 2015; Taipale et al. 2014).
Data obtained from the GTEx project (https://www.gtexportal.org/home/) shows variation in the expression levels and tissue distribution of human TPR cochaperones (Fig. 3b). Surprisingly, the one that is expressed at the highest level across a range of tissue is FKBP38, a membrane-anchored protein distributed predominantly in mitochondria (Blundell et al. 2017; Shirane and Nakayama 2003). Many of the other TPR cochaperones are expressed at similar levels across a range of tissues. This includes most of the proteins identified in complex with steroid hormone receptors: Hop, PP5, FKBP52, Cyp40, GCUNC45, SGTA, CHIP, and TPR2 (Chadli et al. 2006; Connell et al. 2001; Moffatt et al. 2008; Paul et al. 2014; Pratt and Toft 1997). Other cochaperones without a clear tissue specificity that are expressed at varying levels include TPR1, which is involved in Ras signaling (Kwan et al. 2012); aryl hydrocarbon receptor interacting protein (AIP) (Morgan et al. 2012); TOM70, part of the mitochondrial outer membrane complex essential for protein import into mitochondria (Young et al. 2003); TTC4, which interacts with the licensing factor Cdc6 (Crevel et al. 2008); NASP, a linker histone-binding protein (Alekseev et al. 2005); RPAP3, which, like PIH1D1, is part of the large R2TP complex (Rivera-Calzada et al. 2017); CRN (Hatakeyama et al. 2004); and WISP39, which plays a role in cell migration and regulation of the cell cycle (Howell et al. 2015; Jascur et al. 2005).
Similar to the CS-CHORD cochaperones, some TPR cochaperones exhibit tissue-specific expression. Levels of FKBP51, FKBP36, SPAG1, and TOM34 were highest in the testis (Jarczowski et al. 2009; Lin et al. 2001; Trcka et al. 2014). In contrast, levels of the Unc45B, a myosin-specific cochaperone (Srikakulam et al. 2008), were highest in muscle cells. Finally, although not highly expressed in any of the tissues shown, AIPL1 has critical roles in the retina (Hidalgo-de-Quintana et al. 2008).
Other cochaperones
The remaining cochaperones do not contain either CS or TPR domains, and most of them interact with the middle domain of Hsp90. Structural information of some of the cochaperones in this category is available (Table S3). A comparison of the mRNA levels in the GTEx project shows that one of the most highly expressed is Cdc37 (Fig. 3c). Cdc37 and Hsp90 are required for the folding and stabilization of approximately 60% of protein kinases (Taipale et al. 2012). The structure of Cdc37 in complex with both Hsp90 and a protein kinase shows extensive contacts between the three proteins (Verba et al. 2016). Harc, which is homologous to Cdc37 but appears to have different specificity, was expressed at the highest levels in skeletal muscle (Scholz et al. 2001; Taipale et al. 2014).
Another highly expressed cochaperone in this group is Aha1, which stimulates ATP hydrolysis by promoting a conformational change that allows a catalytic loop of the middle domain to associate with the amino-terminus (reviewed in (LaPointe et al. 2020)). Aha1 plays a critical role in the quality control process that leads to degradation of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) (Wang et al. 2006), and other interactors have been identified (Sun et al. 2015). The remaining cochaperones are expressed at varied and lower levels. FNIP1 and FNIP2 are homologous cochaperones that compete with Aha1 for binding to Hsp90. TSC1 similarly regulates Hsp90 complexes by blocking Aha1 interaction (Woodford et al. 2016; Woodford et al. 2017). S100A1, which binds calcium (Okada et al. 2004), was expressed at highest levels in muscle tissues. PhLP2a, which interacts with an angiogenic VEGF receptor and functions in apoptosis (Krzemien-Ojak et al. 2017; Srinivasan et al. 2013), showed relatively high and ubiquitous expression levels.
Cochaperone functions in cancer, neurodegeneration, and dynein assembly
Hsp90 is overexpressed in cancer cells and the role of Hsp90 in cancer has been the subject of recent reviews (Calderwood and Gong 2016; Vartholomaiou et al. 2016). Many of the cochaperones are also overexpressed in cancer cells or have been shown to have other roles in tumorigenesis (Buchanan et al. 2007; Calderwood 2013; Chen et al. 2009; Crevel et al. 2008; De Leon et al. 2011; Dunn et al. 2015; Ferretti et al. 2010; Golden et al. 2008; Han et al. 2018; McDowell et al. 2009; Muller et al. 2019; Pearl 2005; Pei et al. 2009; Srinivasan et al. 2013; Woodford et al. 2016; Woodford et al. 2017). The role of Hsp90 cochaperones in neurodegenerative disease has also been the subject of recent reviews, including one that summarizes findings in which cochaperones that are upregulated in cancer are downregulated in neurodegenerative disease (Calderwood and Murshid 2017). Specific cochaperones implicated in neurodegeneration are p23, Sgt1, CACYBP, CHORDC1, USP19, FKBP51, FKBP52, CYP40, Hop, PP5, CHIP, Cdc37, PhLP2a, and Aha1. Changes in the expression of some of the cochaperones during aging have also been noted (Bohush et al. 2019; Brehme et al. 2014; Herold et al. 2016; Lackie et al. 2017; Shelton et al. 2017).
The composition of Hsp90 complexes varies between the testis and muscle
The expression levels of many of the cochaperones are different upon comparison of the muscle and testis (Table 1). Tissue-specific cochaperone expression may allow greater control of more widely expressed clients, such as GR (Connell et al. 2001; Reynolds et al. 1999; Riggs et al. 2004). For example, Aarsd1 is a muscle-specific cochaperone that interacts with Hsp90 during muscle differentiation (Echeverria et al. 2016). Aarsd1 displaces p23, resulting in reduced GR activity. Further analysis of how interacting cochaperones alter client activity may provide new insights in to how altered cochaperone levels during differentiation, aging, or disease impacts activity of a wide range of Hsp90 clients. Hsp90 is very abundant, and there are two isoforms of human Hsp90, Hsp90 alpha (encoded by HSP90AA1), and Hsp90 beta (encoded by HSP90AB1). The relative levels of the two isoforms also vary between the testis and muscle. Hsp90 beta is more predominant in muscle, while Hsp90 alpha is expressed at high levels in the testis. Some of the cochaperones, such as GCUNC45 and Aarsd1, preferentially interact with one isoform or the other, contributing to the specificity of their effects (Chadli et al. 2008; Echeverria et al. 2016).
Table 1.
Differential expression of Human Hsp90 isoforms and cochaperones in the muscle and testis
| Cochaperone | Skeletal muscle Mean TPM | Testis Mean TPM | Function |
|---|---|---|---|
| FKBP51 | 176.7 | 28.8 | Promotes myogenesis |
| S100A1 | 21.7 | 2.8 | Localizes to Z-discs and sarcoplasmic reticulum |
| ITGB1BP2 | 7.7 | 2.4 | Integrin binding protein |
| UNC45B | 83.7 | 2.2 | Myosin chaperone |
| AARSD1 | 38.4 | 9.1 | Muscle wasting and GR activity |
| HARC | 40.5 | 18.0 | Mutations linked to heart failure |
| NASP | 15.4 | 106.4 | testis- and sperm-specific histone-binding protein |
| TOM34 | 12.6 | 293.0 | |
| PIH1D2 | 0.6 | 66.0 | Dynein assembly |
| FKBP36 | 0 | 76.7 | |
| HACD3 | 2.2 | 21.1 | |
| NUDCD1 | 3.7 | 18.2 | Dynein assembly |
| NCB5or | 2.7 | 12.9 | |
| LRRC6 | 0.1 | 32.1 | Dynein assembly |
| DYX1C1 | 0.2 | 11.0 | Dynein assembly |
| SPAG1 | 0.3 | 16.5 | Dynein assembly |
| PIH1D3 | 0 | 16.2 | Dynein assembly |
| HSP90AA1 | 239.4 | 902.0 | Hsp90 alpha |
| HSP90AB1 | 929.3 | 541.8 | Hsp90 beta |
TPM, transcripts per million (gtexportal.org)
Hsp90-specific functions in the muscle
Some Hsp90 cochaperones are likely required to assist client proteins unique to those tissues. As shown in Table 1, the levels of the mRNAs encoding FKBP51, S100A1, ITGB1BP2, Unc45B, Aarsd1, and Harc are elevated in the skeletal muscle, and most are also elevated in the heart tissue. These cochaperones have known muscle-specific functions. ITGB1BP2 binds integrin as part of costameres that regulate the interaction between the cytoskeleton and the extracellular matrix and has been shown to have cardioprotective effects (reviewed in (Tarone and Brancaccio 2015)). Unc45B assists the folding myosin (Srikakulam et al. 2008), and S100A1 is a regulator of muscle contractility (Most et al. 2001). FKBP51 has roles in myoblast differentiation and regulating muscle mass (Ruiz-Estevez et al. 2018; Shimoide et al. 2016). A recent study suggests that mutations in the gene encoding Harc may be associated with heart failure (Huang et al. 2018). Cochaperones that are more widely expressed, such as Sgt1, USP19, FNIP1, and Cdc37, also have established roles in the heart or muscle function (Coyne et al. 2018; Kapustian et al. 2013; Ota et al. 2010; Reyes et al. 2015). Hsp90 cooperates with Aha1, Aarsd1, Hop, and Cdc37 during myoglobulin maturation (Ghosh et al. 2019). FKBP38, Aha1, Hop, and Hsp90 are involved in the folding of the CLC-1 chloride channel in the skeletal muscle (Peng et al. 2016). These results suggest that muscle tissues may be an excellent model system to study how variations in Hsp90 complexes affect client function.
Hsp90-specific functions in the testis
A subset of cochaperones has recently been linked to the preassembly of dynein arms or regulation of dynein functions (reviewed in (Fabczak and Osinka 2019)), and dynein plays an important role in spermatogenesis (Wen et al. 2018). This includes PIH1D1, PIH1D2, PIH1D3, RPAP3, SPAG1, DYX1C1, LRRC6, and NUDCD1. Although transcription of genes encoding NASP, TOM34, HACD3, and NCB5or is elevated in testis, details on their functions are scarce. However, loss of FKBP36 results in chromosome mispairing during meiosis and mutations are suspected to cause azoospermia (Crackower et al. 2003; Zhang et al. 2007). FKBP52 is required for androgen receptor signaling (Cheung-Flynn et al. 2005), and Hsp90 and cochaperones are required for the function of testis-specific serine/threonine kinases essential for male fertility (Jha et al. 2013). Altered function of a pathway that includes TSC1 has also been shown to cause disruption of spermatogenesis (Tanwar et al. 2012). In addition, the Hsp90 alpha isoform is highly expressed in the testis, and loss of Hsp90 alpha causes infertility in mice (Grad et al. 2010). Part of this effect of Hsp90 alpha may be due to its role in fetal PIWI-interacting RNAs (piRNA) in male germ cells (Ichiyanagi et al. 2014). These results demonstrate that the Hsp90 machinery plays a critical role in male fertility.
Concluding remarks
Hsp90 is a complicated molecular chaperone machine that directly or indirectly impacts the function of 10–15% of all proteins in humans (Wu et al. 2012). Cochaperones have a wide variety of functions, such as modulating Hsp90 ATPase activity or conformational changes and targeting clients to Hsp90. The benefit of such a wide variety of cochaperones is largely unknown. The most likely explanation is that the large cohort of cochaperones enables Hsp90 to interact with hundreds of diverse proteins that do not share any sequence or structural similarity. A few studies have undertaken the daunting task of matching up client-cochaperone pairs (Echeverria et al. 2011; Taipale et al. 2014). However, in most cases, the individual roles of cochaperones in client folding remain unknown. The number and types of Hsp90 cochaperones varies from species to species, suggesting a correlation between client diversity and the range of available cochaperones (Johnson and Brown 2009). Analysis of yeast cochaperones suggests that a few cochaperones are required for core Hsp90 functions, while others have more specialized functions (Biebl et al. 2020; Sahasrabudhe et al. 2017).
Despite the discovery of an unexpectedly large cohort of cochaperones, the current model of the Hsp90 folding cycle is very similar to the model that was first established over 20 years ago. Early in the cycle, clients are transferred to Hsp90 from Hsp70 in a process mediated by cochaperones. Nucleotide binding and hydrolysis results in additional changes in the types of bound cochaperones and/or Hsp90 conformation. The expanded list of cochaperones increases the likelihood that different clients bind different cochaperones throughout the cycle. Further analysis Hsp90 and cochaperone complexes with diverse clients will help identify the core features of the human Hsp90 folding machine. In contrast, a better understanding of cochaperones that have selective client interactions will provide new insights into how cochaperones contribute to client folding and broaden the scope of Hsp90 functions. This may lead to novel approaches to selectively inhibiting a subset of Hsp90 clients without disrupting essential functions. Another area of research is understanding how post-translational modifications of Hsp90 provide directionality to the folding process by promoting progression to the next cochaperone complex in the folding pathway (Mollapour and Neckers 2012; Sager et al. 2019). More studies are also needed to understand how changes in the composition of Hsp90-complexes due to aging, development, or disease affect client function.
Electronic supplementary material
(PDF 122 kb)
Acknowledgments
The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 04/13/20. Additional information about proteins that interact with Hsp90 is available at http://www.picard.ch/downloads/Hsp90facts.pdf.
Authors’ contributions
MED helped gather data and assisted with manuscript preparation. JLJ conceived the idea for the publication and wrote the manuscript.
Funding
Research in the Johnson Lab is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM127675. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alekseev OM, Widgren EE, Richardson RT, O'Rand MG. Association of NASP with HSP90 in mouse spermatogenic cells: stimulation of ATPase activity and transport of linker histones into nuclei. J Biol Chem. 2005;280:2904–2911. doi: 10.1074/jbc.M410397200. [DOI] [PubMed] [Google Scholar]
- Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimon VA, Southworth DR, Gestwicki JE. Specific binding of tetratricopeptide repeat proteins to heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) is regulated by affinity and phosphorylation. Biochemistry. 2015;54:7120–7131. doi: 10.1021/acs.biochem.5b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DR, Lovell S, Mehzabeen N, Galeva N, Cooper A, Gao P, Battaile KP, Zhu H. Crystal structures of the naturally fused CS and cytochrome b5 reductase (b5R) domains of Ncb5or reveal an expanded CS fold, extensive CS-b5R interactions and productive binding of the NAD(P)(+) nicotinamide ring. Acta Crystallogr D Struct Biol. 2019;75:628–638. doi: 10.1107/S205979831900754X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl MM, Riedl M, Buchner J. Hsp90 Co-chaperones form plastic genetic networks adapted to client maturation. Cell Rep. 2020;32:108063. doi: 10.1016/j.celrep.2020.108063. [DOI] [PubMed] [Google Scholar]
- Blundell KL, Pal M, Roe SM, Pearl LH, Prodromou C. The structure of FKBP38 in complex with the MEEVD tetratricopeptide binding-motif of Hsp90. PLoS One. 2017;12:e0173543. doi: 10.1371/journal.pone.0173543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohush A, Bieganowski P, Filipek A (2019) Hsp90 and its co-chaperones in neurodegenerative diseases. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- Boitet ER, Reish NJ, Hubbard MG, Gross AK. NudC regulates photoreceptor disk morphogenesis and rhodopsin localization. FASEB J. 2019;33:8799–8808. doi: 10.1096/fj.201801740RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014;9:1135–1150. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G, Ricciardelli C, Harris JM, Prescott J, Yu ZC, Jia L, Butler LM, Marshall VR, Scher HI, Gerald WL, et al. Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein alpha. Cancer Res. 2007;67:10087–10096. doi: 10.1158/0008-5472.CAN-07-1646. [DOI] [PubMed] [Google Scholar]
- Calderwood SK. Molecular cochaperones: tumor growth and cancer treatment. Scientifica (Cairo) 2013;2013:217513. doi: 10.1155/2013/217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Gong J. Heat shock proteins promote cancer: it's a protection racket. Trends Biochem Sci. 2016;41:311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A. Molecular chaperone accumulation in cancer and decrease in Alzheimer's disease: the potential roles of HSF1. Frontiers in Neuroscience. 2017;11:192. doi: 10.3389/fnins.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A, Graham JD, Abel MG, Jackson TA, Gordon DF, Wood WM, Felts SJ, Horwitz KB, Toft D. GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol Cell Biol. 2006;26:1722–1730. doi: 10.1128/MCB.26.5.1722-1730.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A, Felts SJ, Toft DO. GCUNC45 is the first Hsp90 co-chaperone to show alpha/beta isoform specificity. J Biol Chem. 2008;283:9509–9512. doi: 10.1074/jbc.C800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao M, Wang S, Chen J, Wang Y, Cao Q, Zhou W, Liu J, Xu Z, Tong G, Li J. A novel role for DYX1C1, a chaperone protein for both Hsp70 and Hsp90, in breast cancer. J Cancer Res Clin Oncol. 2009;135:1265–1276. doi: 10.1007/s00432-009-0568-6. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Courilleau D, Chastre E, Sabbah M, Redeuilh G, Atfi A, Mester J. B-ind1, a novel mediator of Rac1 signaling cloned from sodium butyrate-treated fibroblasts. J Biol Chem. 2000;275:17344–17348. doi: 10.1074/jbc.M000887200. [DOI] [PubMed] [Google Scholar]
- Coyne ES, Bedard N, Wykes L, Stretch C, Jammoul S, Li S, Zhang K, Sladek RS, Bathe OF, Jagoe RT, Posner BI, Wing SS. Knockout of USP19 deubiquitinating enzyme prevents muscle wasting by modulating insulin and glucocorticoid signaling. Endocrinology. 2018;159:2966–2977. doi: 10.1210/en.2018-00290. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Kolas NK, Noguchi J, Sarao R, Kikuchi K, Kaneko H, Kobayashi E, Kawai Y, Kozieradzki I, Landers R, Mo R, Hui CC, Nieves E, Cohen PE, Osborne LR, Wada T, Kunieda T, Moens PB, Penninger JM. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science. 2003;300:1291–1295. doi: 10.1126/science.1083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel G, Bennett D, Cotterill S. The Human TPR protein TTC4 is a putative Hsp90 co-chaperone which interacts with CDC6 and shows alterations in transformed cells. PLoS ONE. 2008;3:e0001737. doi: 10.1371/journal.pone.0001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A. 2011;108:11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Shinohara K, Botilde Y, Nabeshima R, Asai Y, Fukumoto A, Hasegawa T, Matsuo M, Takeda H, Shiratori H, Nakamura T, Hamada H. Pih1d3 is required for cytoplasmic preassembly of axonemal dynein in mouse sperm. J Cell Biol. 2014;204:203–213. doi: 10.1083/jcb.201304076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DM, Woodford MR, Truman AW, Jensen SM, Schulman J, Caza T, Remillard TC, Loiselle D, Wolfgeher D, Blagg BS, et al. c-Abl mediated tyrosine phosphorylation of Aha1 activates its co-chaperone function in cancer cells. Cell Rep. 2015;12:1006–1018. doi: 10.1016/j.celrep.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Bernthaler A, Dupuis P, Mayer B, Picard D. An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PLoS One. 2011;6:e26044. doi: 10.1371/journal.pone.0026044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria PC, Briand PA, Picard D. A remodeled Hsp90 molecular chaperone ensemble with the novel cochaperone Aarsd1 is required for muscle differentiation. Mol Cell Biol. 2016;36:1310–1321. doi: 10.1128/MCB.01099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, Garabedian M, Freeman BC. Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol Cell. 2011;43:229–241. doi: 10.1016/j.molcel.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenkamp FJ, Gvozdenov Z, Adkins NL, Zhang Y, Lynch-Day M, Watanabe S, Peterson CL, Freeman BC. Hsp90 and p23 molecular chaperones control chromatin architecture by maintaining the functional pool of the RSC chromatin remodeler. Mol Cell. 2016;64:888–899. doi: 10.1016/j.molcel.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlejman AG, Lagadari M, Harris DC, Cox MB, Galigniana MD. Molecular chaperone activity and biological regulatory actions of the TPR-domain immunophilins FKBP51 and FKBP52. Curr Protein Pept Sci. 2014;15:205–215. doi: 10.2174/1389203715666140331113753. [DOI] [PubMed] [Google Scholar]
- Fabczak H, Osinka A (2019) Role of the novel Hsp90 co-chaperones in Dynein Arms' Preassembly. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- Ferretti R, Palumbo V, Di Savino A, Velasco S, Sbroggio M, Sportoletti P, Micale L, Turco E, Silengo L, Palumbo G, et al. Morgana/chp-1, a ROCK inhibitor involved in centrosome duplication and tumorigenesis. Dev Cell. 2010;18:486–495. doi: 10.1016/j.devcel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Ferretti R, Sbroggio M, Di Savino A, Fusella F, Bertero A, Michowski W, Tarone G, Brancaccio M. Morgana and melusin: two fairies chaperoning signal transduction. Cell Cycle. 2011;10:3678–3683. doi: 10.4161/cc.10.21.18202. [DOI] [PubMed] [Google Scholar]
- Forafonov F, Toogun OA, Grad I, Suslova E, Freeman BC, Picard D. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol. 2008;28:3446–3456. doi: 10.1128/MCB.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- Fu Q, Wang W, Zhou T, Yang Y. Emerging roles of NudC family: from molecular regulation to clinical implications. Sci China Life Sci. 2016;59:455–462. doi: 10.1007/s11427-016-5029-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Ranea JA, Mirey G, Camonis J, Valencia A. p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. 2002;529:162–167. doi: 10.1016/s0014-5793(02)03321-5. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Dai Y, Biswas P, Stuehr DJ. Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase. FASEB J. 2019;33:9885–9896. doi: 10.1096/fj.201802793RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden T, Swingle M, Honkanen RE. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008;27:169–178. doi: 10.1007/s10555-008-9125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral A, Bieganowski P, Prus W, Krzemien-Ojak L, Kadziolka B, Fabczak H, Filipek A. Calcyclin binding protein/Siah-1 interacting protein is a Hsp90 binding chaperone. PLoS One. 2016;11:e0156507. doi: 10.1371/journal.pone.0156507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, De Massy B, Nef S, Picard D. The molecular chaperone Hsp90alpha is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One. 2010;5:e15770. doi: 10.1371/journal.pone.0015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy NC, Garcia YA, Sivils JC, Galigniana MD, Cox MB. Functions of the Hsp90-binding FKBP immunophilins. Subcell Biochem. 2015;78:35–68. doi: 10.1007/978-3-319-11731-7_2. [DOI] [PubMed] [Google Scholar]
- Han B, Zhang YY, Xu K, Bai Y, Wan LH, Miao SK, Zhang KX, Zhang HW, Liu Y, Zhou LM. NUDCD1 promotes metastasis through inducing EMT and inhibiting apoptosis in colorectal cancer. Am J Cancer Res. 2018;8:810–823. [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S, Matsumoto M, Yada M, Nakayama KI. Interaction of U-box-type ubiquitin-protein ligases (E3s) with molecular chaperones. Genes Cells. 2004;9:533–548. doi: 10.1111/j.1356-9597.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- He WT, Zheng XM, Zhang YH, Gao YG, Song AX, van der Goot FG, Hu HY. Cytoplasmic ubiquitin-specific protease 19 (USP19) modulates aggregation of polyglutamine-expanded Ataxin-3 and Huntingtin through the HSP90 chaperone. PLoS One. 2016;11:e0147515. doi: 10.1371/journal.pone.0147515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Dai J, Wang X, Qian X, Zhao J, Wang H, Xu D. NudCD1 affects renal cell carcinoma through regulating LIS1/Dynein signaling pathway. Am J Transl Res. 2018;10:519–524. [PMC free article] [PubMed] [Google Scholar]
- Herold C, Hooli BV, Mullin K, Liu T, Roehr JT, Mattheisen M, Parrado AR, Bertram L, Lange C, Tanzi RE. Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer's disease with OSBPL6, PTPRG, and PDCL3. Mol Psychiatry. 2016;21:1608–1612. doi: 10.1038/mp.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, van der Spuy J. The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Invest Ophthalmol Vis Sci. 2008;49:2878–2887. doi: 10.1167/iovs.07-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M, Brickner H, Delorme-Walker VD, Choi J, Saffin JM, Miller D, Panopoulos A, DerMardirossian C, Fotedar A, Margolis RL, Fotedar R. WISp39 binds phosphorylated Coronin 1B to regulate Arp2/3 localization and Cofilin-dependent motility. J Cell Biol. 2015;208:961–974. doi: 10.1083/jcb.201410095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Luo B, Wang B, Wu Q, Liang Y, He Y. Identification of potential gene interactions in heart failure caused by idiopathic dilated cardiomyopathy. Med Sci Monit. 2018;24:7697–7709. doi: 10.12659/MSM.912984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyanagi T, Ichiyanagi K, Ogawa A, Kuramochi-Miyagawa S, Nakano T, Chuma S, Sasaki H, Udono H. HSP90alpha plays an important role in piRNA biogenesis and retrotransposon repression in mouse. Nucleic Acids Res. 2014;42:11903–11911. doi: 10.1093/nar/gku881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczowski F, Jahreis G, Erdmann F, Schierhorn A, Fischer G, Edlich F. FKBP36 is an inherent multifunctional glyceraldehyde-3-phosphate dehydrogenase inhibitor. J Biol Chem. 2009;284:766–773. doi: 10.1074/jbc.M709779200. [DOI] [PubMed] [Google Scholar]
- Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, Fotedar R, Fotedar A. Regulation of p21(WAF1/CIP1) stability by WISp39, a Hsp90 binding TPR protein. Mol Cell. 2005;17:237–249. doi: 10.1016/j.molcel.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Jha KN, Coleman AR, Wong L, Salicioni AM, Howcroft E, Johnson GR. Heat shock protein 90 functions to stabilize and activate the testis-specific serine/threonine kinases, a family of kinases essential for male fertility. J Biol Chem. 2013;288:16308–16320. doi: 10.1074/jbc.M112.400978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Brown C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress & Chaperones. 2009;14:83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Beito TG, Krco CJ, Toft DO. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol. 1994;14:1956–1963. doi: 10.1128/mcb.14.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustian LL, Vigontina OA, Rozhko OT, Ryabenko DV, Michowski W, Lesniak W, Filipek A, Kroupskaya IV, Sidorik LL. Hsp90 and its co-chaperone, Sgt1, as autoantigens in dilated cardiomyopathy. Heart Vessels. 2013;28:114–119. doi: 10.1007/s00380-011-0226-1. [DOI] [PubMed] [Google Scholar]
- Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien-Ojak L, Goral A, Joachimiak E, Filipek A, Fabczak H. Interaction of a novel chaperone PhLP2A with the heat shock protein Hsp90. J Cell Biochem. 2017;118:420–429. doi: 10.1002/jcb.25669. [DOI] [PubMed] [Google Scholar]
- Kwan DH, Yung LY, Ye RD, Wong YH. Activation of Ras-dependent signaling pathways by G(14) -coupled receptors requires the adaptor protein TPR1. J Cell Biochem. 2012;113:3486–3497. doi: 10.1002/jcb.24225. [DOI] [PubMed] [Google Scholar]
- Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML, Prado VF, Prado MAM. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Frontiers in Neuroscience. 2017;11:254. doi: 10.3389/fnins.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe P, Mercier R, Wolmarans A. Aha-type co-chaperones: the alpha or the omega of the Hsp90 ATPase cycle? Biol Chem. 2020;401:423–434. doi: 10.1515/hsz-2019-0341. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhou X, Zhang M, Li Y, Miao S, Wang L, Zong S, Koide SS. Expression and function of the HSD-3.8 gene encoding a testis-specific protein. Mol Hum Reprod. 2001;7:811–818. doi: 10.1093/molehr/7.9.811. [DOI] [PubMed] [Google Scholar]
- Lorenz OR, Freiburger L, Rutz DA, Krause M, Zierer BK, Alvira S, Cuellar J, Valpuesta JM, Madl T, Sattler M, et al. Modulation of the hsp90 chaperone cycle by a stringent client protein. Mol Cell. 2014;53:941–953. doi: 10.1016/j.molcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CL, Bryan Sutton R, Obermann WM. Expression of Hsp90 chaperome proteins in human tumor tissue. Int J Biol Macromol. 2009;45:310–314. doi: 10.1016/j.ijbiomac.2009.06.012. [DOI] [PubMed] [Google Scholar]
- McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, Jackson SE. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol. 2006;356:746–758. doi: 10.1016/j.jmb.2005.11.085. [DOI] [PubMed] [Google Scholar]
- Moffatt NS, Bruinsma E, Uhl C, Obermann WM, Toft D. Role of the cochaperone Tpr2 in Hsp90 chaperoning. Biochemistry. 2008;47:8203–8213. doi: 10.1021/bi800770g. [DOI] [PubMed] [Google Scholar]
- Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2012;1823:648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RM, Hernandez-Ramirez LC, Trivellin G, Zhou L, Roe SM, Korbonits M, Prodromou C. Structure of the TPR domain of AIP: lack of client protein interaction with the C-terminal alpha-7 helix of the TPR domain of AIP is sufficient for pituitary adenoma predisposition. PLoS One. 2012;7:e53339. doi: 10.1371/journal.pone.0053339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most P, Bernotat J, Ehlermann P, Pleger ST, Reppel M, Borries M, Niroomand F, Pieske B, Janssen PM, Eschenhagen T, et al. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci U S A. 2001;98:13889–13894. doi: 10.1073/pnas.241393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Coates PJ, Nenutil R, Trcka F, Hrstka R, Chovanec J, Brychtova V, Vojtesek B. Tomm34 is commonly expressed in epithelial ovarian cancer and associates with tumour type and high FIGO stage. J Ovarian Res. 2019;12:30. doi: 10.1186/s13048-019-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Hatakeyama T, Itoh H, Tokuta N, Tokumitsu H, Kobayashi R. S100A1 is a novel molecular chaperone and a member of the Hsp70/Hsp90 multichaperone complex. J Biol Chem. 2004;279:4221–4233. doi: 10.1074/jbc.M309014200. [DOI] [PubMed] [Google Scholar]
- Ota A, Zhang J, Ping P, Han J, Wang Y. Specific regulation of noncanonical p38alpha activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ Res. 2010;106:1404–1412. doi: 10.1161/CIRCRESAHA.109.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Garcia YA, Zierer B, Patwardhan C, Gutierrez O, Hildenbrand Z, Harris DC, Balsiger HA, Sivils JC, Johnson JL, Buchner J, Chadli A, Cox MB. The cochaperone SGTA (small glutamine-rich tetratricopeptide repeat-containing protein alpha) demonstrates regulatory specificity for the androgen, glucocorticoid, and progesterone receptors. J Biol Chem. 2014;289:15297–15308. doi: 10.1074/jbc.M113.535229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15:55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Huang JJ, Wu HH, Hsieh HY, Wu CY, Chen SC, Chen TY, Tang CY. Regulation of CLC-1 chloride channel biosynthesis by FKBP8 and Hsp90beta. Sci Rep. 2016;6:32444. doi: 10.1038/srep32444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Prodromou C. The 'active life' of Hsp90 complexes. Biochim Biophys Acta. 2012;1823:614–623. doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C, Siligardi G, O'Brien R, Woolfson DN, Regan L, Panaretou B, Ladbury JE, Piper PW, Pearl LH. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. Embo J. 1999;18:754–762. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes NL, Banks GB, Tsang M, Margineantu D, Gu H, Djukovic D, Chan J, Torres M, Liggitt HD, Hirenallur SD, et al. Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112:424–429. doi: 10.1073/pnas.1413021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J Biol Chem. 2003;278:10328–10333. doi: 10.1074/jbc.M213094200. [DOI] [PubMed] [Google Scholar]
- Riggs D, Cox M, Cheung-Flynn J, Prapapanich V, Carrigan P, Smith D. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
- Rivera-Calzada A, Pal M, Munoz-Hernandez H, Luque-Ortega JR, Gil-Carton D, Degliesposti G, Skehel JM, Prodromou C, Pearl LH, Llorca O. The structure of the R2TP complex defines a platform for recruiting diverse client proteins to the HSP90 molecular chaperone system. Structure. 2017;25(1145-1152):e1144. doi: 10.1016/j.str.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Estevez M, Staats J, Paatela E, Munson D, Katoku-Kikyo N, Yuan C, Asakura Y, Hostager R, Kobayashi H, Asakura A, et al. Promotion of myoblast differentiation by Fkbp5 via Cdk4 isomerization. Cell Rep. 2018;25(2537-2551):e2538. doi: 10.1016/j.celrep.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager RA, Woodford MR, Mollapour M. The mTOR independent function of Tsc1 and FNIPs. Trends Biochem Sci. 2018;43:935–937. doi: 10.1016/j.tibs.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager RA, Woodford MR, Backe SJ, Makedon AM, Baker-Williams AJ, DiGregorio BT, Loiselle DR, Haystead TA, Zachara NE, Prodromou C, et al. Post-translational regulation of FNIP1 creates a rheostat for the molecular chaperone Hsp90. Cell Rep. 2019;26(1344-1356):e1345. doi: 10.1016/j.celrep.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe P, Rohrberg J, Biebl MM, Rutz DA, Buchner J. The plasticity of the Hsp90 co-chaperone system. Mol Cell. 2017;67(947-961):e945. doi: 10.1016/j.molcel.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Scholz GM, Cartledge K, Hall NE. Identification and characterization of Harc, a novel Hsp90-associating relative of Cdc37. J Biol Chem. 2001;276:30971–30979. doi: 10.1074/jbc.M103889200. [DOI] [PubMed] [Google Scholar]
- Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nature reviews Molecular cell biology. 2017;18:345–360. doi: 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- Shelton LB, Koren J, 3rd, Blair LJ. Imbalances in the Hsp90 chaperone machinery: implications for tauopathies. Frontiers in Neuroscience. 2017;11:724. doi: 10.3389/fnins.2017.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoide T, Kawao N, Tamura Y, Morita H, Kaji H. Novel roles of FKBP5 in muscle alteration induced by gravity change in mice. Biochem Biophys Res Commun. 2016;479:602–606. doi: 10.1016/j.bbrc.2016.09.126. [DOI] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat Cell Biol. 2003;5:28–37. doi: 10.1038/ncb894. [DOI] [PubMed] [Google Scholar]
- Shirasu K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol. 2009;60:139–164. doi: 10.1146/annurev.arplant.59.032607.092906. [DOI] [PubMed] [Google Scholar]
- Smith JR, Clarke PA, de Billy E, Workman P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 2009;28:157–169. doi: 10.1038/onc.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulam R, Liu L, Winkelmann DA. Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS ONE. 2008;3:e2137. doi: 10.1371/journal.pone.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Meyer RD, Lugo R, Rahimi N. Identification of PDCL3 as a novel chaperone protein involved in the generation of functional VEGF receptor 2. J Biol Chem. 2013;288:23171–23181. doi: 10.1074/jbc.M113.473173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Hartson SD, Matts RL. Identification of proteins associated with Aha1 in HeLa cells by quantitative proteomics. Biochim Biophys Acta. 2015;1854:365–380. doi: 10.1016/j.bbapap.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Taguwa S, Okamoto T, Abe T, Mori Y, Suzuki T, Moriishi K, Matsuura Y. Human butyrate-induced transcript 1 interacts with hepatitis C virus NS5A and regulates viral replication. J Virol. 2008;82:2631–2641. doi: 10.1128/JVI.02153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Tucker G, Peng J, Krykbaeva I, Lin ZY, Larsen B, Choi H, Berger B, Gingras AC, Lindquist S. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell. 2014;158:434–448. doi: 10.1016/j.cell.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar PS, Kaneko-Tarui T, Zhang L, Teixeira JM. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum Mol Genet. 2012;21:4394–4405. doi: 10.1093/hmg/dds272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G, Brancaccio M. The muscle-specific chaperone protein melusin is a potent cardioprotective agent. Basic Res Cardiol. 2015;110:10. doi: 10.1007/s00395-015-0466-9. [DOI] [PubMed] [Google Scholar]
- Topolska-Wos AM, Chazin WJ, Filipek A. CacyBP/SIP—structure and variety of functions. Biochim Biophys Acta. 2016;1860:79–85. doi: 10.1016/j.bbagen.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Trcka F, Durech M, Man P, Hernychova L, Muller P, Vojtesek B. The assembly and intermolecular properties of the Hsp70-Tomm34-Hsp90 molecular chaperone complex. J Biol Chem. 2014;289:9887–9901. doi: 10.1074/jbc.M113.526046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartholomaiou E, Echeverria PC, Picard D. Unusual suspects in the twilight zone between the Hsp90 interactome and carcinogenesis. Adv Cancer Res. 2016;129:1–30. doi: 10.1016/bs.acr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Verba KA, Wang RY, Arakawa A, Liu Y, Shirouzu M, Yokoyama S, Agard DA. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science. 2016;352:1542–1547. doi: 10.1126/science.aaf5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, III, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Wen Q, Tang EI, Lui WY, Lee WM, Wong CKC, Silvestrini B, Cheng CY. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am J Physiol Endocrinol Metab. 2018;315:E924–E948. doi: 10.1152/ajpendo.00114.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford MR, Dunn DM, Blanden AR, Capriotti D, Loiselle D, Prodromou C, Panaretou B, Hughes PF, Smith A, Ackerman W, Haystead TA, Loh SN, Bourboulia D, Schmidt LS, Marston Linehan W, Bratslavsky G, Mollapour M. The FNIP co-chaperones decelerate the Hsp90 chaperone cycle and enhance drug binding. Nat Commun. 2016;7:12037. doi: 10.1038/ncomms12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford MR, Sager RA, Marris E, Dunn DM, Blanden AR, Murphy RL, Rensing N, Shapiro O, Panaretou B, Prodromou C, Loh SN, Gutmann DH, Bourboulia D, Bratslavsky G, Wong M, Mollapour M. Tumor suppressor Tsc1 is a new Hsp90 co-chaperone that facilitates folding of kinase and non-kinase clients. EMBO J. 2017;36:3650–3665. doi: 10.15252/embj.201796700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Moghaddas Gholami A, Kuster B. Systematic identification of the HSP90 candidate regulated proteome. Mol Cell Proteomics. 2012;11(M111):016675. doi: 10.1074/mcp.M111.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Hirono M, Kamiya R. Discrete PIH proteins function in the cytoplasmic preassembly of different subsets of axonemal dyneins. J Cell Biol. 2010;190:65–71. doi: 10.1083/jcb.201002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang W, Li M, Gao Y, Zhang W, Huang Y, Zhuo W, Yan X, Liu W, Wang F, Chen D, Zhou T. NudCL2 is an Hsp90 cochaperone to regulate sister chromatid cohesion by stabilizing cohesin subunits. Cell Mol Life Sci. 2019;76:381–395. doi: 10.1007/s00018-018-2957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH. Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang S, Xiao C, Yang Y, Zhoucun A. Mutation screening of the FKBP6 gene and its association study with spermatogenic impairment in idiopathic infertile men. Reproduction. 2007;133:511–516. doi: 10.1530/REP-06-0125. [DOI] [PubMed] [Google Scholar]
- Zheng M, Cierpicki T, Burdette AJ, Utepbergenov D, Janczyk PL, Derewenda U, Stukenberg PT, Caldwell KA, Derewenda ZS. Structural features and chaperone activity of the NudC protein family. J Mol Biol. 2011;409:722–741. doi: 10.1016/j.jmb.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Liu X, Jin Q, Cai Y, Yang Y, Zhou T. The L279P mutation of nuclear distribution gene C (NudC) influences its chaperone activity and lissencephaly protein 1 (LIS1) stability. J Biol Chem. 2010;285:29903–29910. doi: 10.1074/jbc.M110.105494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 122 kb)
Data Availability Statement
Not applicable.