Abstract
In anti-neutrophilic cytoplasmic antibody (ANCA)-associated vasculitis (AAV) genetic predisposition, ANCA autoantibodies, neutrophil extracellular traps (NETs), complement activation, and toll-like receptor signaling are implicated in AAV pathogenesis. Heat shock proteins (HSPs), a highly conserved group of small-sized molecular chaperones, take part in protein folding during cellular stress. Although HSPs were initially observed intracellularly, it has been shown that they can be secreted in the extracellular space and modulate the immune response in various autoimmune diseases including AAV. The scope of the present study is to investigate the role of heat shock protein 60 (HSP60) and 70 (HSP70) in the long renal effects in an ANCA vasculitis cohort. In this cohort of ANCA-associated vasculitis, 29 patients were followed up over 20 years. At diagnosis, immunohistochemistry was performed for HSP60 and HSP70 within the various nephron compartments. Higher renal HSP60 expression was associated with increased interstitial inflammatory infiltrates at diagnosis, while HSP70 expression was associated with a greater extent of interstitial fibrosis at diagnosis. Notably, intense tissue expression of HSP70 at the time of biopsy was associated with a worsened kidney survival. Renal HSP70 expression was associated with poor renal outcomes during long-term follow-up. This finding may indicate a role of HSPs in renal disease progression in ANCA vasculitis. Further validating studies are needed to verify a causative association between HSP70 expression and renal outcomes in ANCA-associated vasculitis.
Keywords: ANCA-vasculitis, Heat shock protein, Pauci-immune glomerulonephritis, Interstitial inflammation, Interstitial fibrosis
Introduction
Heat shock proteins (HSPs), a highly conserved group of cellular chaperones, are involved in transmembrane protein transport and protein folding under cellular stress conditions (Jindal et al. 1989). They are classified into different families according to their molecular weight. While HSP60 is located in the cytosol and the mitochondria, HSP70 shows a wider distribution including localization within the nucleus, the mitochondria, and the cytoplasm (O’Neill et al. 2014). Although HSPs were initially detected within the cytoplasm and their function has been thought to be restricted within the cell itself, it has been shown that they can be additionally secreted into the extracellular space, where they have an active role in immune response modulation. For example, HSP72 release within exosomes leads to upregulate CD83 expression and stimulates IL-12 release by naive dendritic cells (Bausero et al. 2005). HSP60 interacts as a ligand with toll-like receptors TLR2 and TLR4 (Vabulas et al. 2002). Furthermore, HSP60 as well as HSP70 (and anti-HSP70 antibodies) circulate in plasma of normal subjects (Pockley et al. 1998). HSP60 as well as HSP70 seem to exert both inhibitory and stimulatory effects on an immune response (Venkataseshan and Marquet 1996). Patients with borderline hypertension, peripheral vascular disease, patients with end-stage renal disease, as well as patients with cardiovascular disease present increased levels of circulating HSP60, HSP70, and anti-HSP70 antibodies respectively and are associated with disease progression (Pockley et al. 2000; Prohaszka et al. 2001; Wright et al. 2000; O’Neill et al. 2014). Decrease of anti-HSP70 antibodies is associated with lower complications of diabetic microangiopathy (Gruden et al. 2009). HSPs have been implicated in the pathogenesis of several vasculitis syndromes. In Kawasaki disease, an increase in plasma HSP70/HSP60 has been described in the subacute phase of the disease (Lu et al. 1998). Extracellular HSP60 seems to play a regulatory role in the subacute phase of the disease (Yin Ji et al. 2007). Anti-HSP70 antibodies are involved in the pathogenesis of Cogan syndrome, a rare form of vasculitis (Bonaguri et al. 2014). In Behçet disease patients, HSP60 binds with HSP65 and seems to induce a Th1 cell response (Imamura et al. 2005).
ANCA-associated vasculitis (AAV) belongs to primary small vessel vasculitis syndromes including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA), which are associated with elevated serum anti-neutrophil cytoplasm antibodies (ANCA) (Jennette 2013). Although the understanding of the underlying mechanisms leading to kidney injury in these patients is limited, ANCA antibodies are directly involved in the pathophysiology of AAV. The activated ANCA-expressing neutrophils adhere to endothelial cells inducing the release of pro-inflammatory cytokines and the production of reactive oxygen species. ANCA antibodies induce crescent formation in animal models of ANCA-associated vasculitis and cause glomerulonephritis and lung hemorrhage in neonates through transplacental transfer (Rowaiye et al. 2015). In a subset of microscopic polyangiitis patients, Slot et al. (2006) could show an increase of anti-HSP60 antibodies. Furthermore, HSP60/70 antibodies are associated with increased immunological background in microscopic polyangiitis when compared with SLE or RA patients (Komiya et al. 2011). Nevertheless, it seems that circulating antibodies against HSP can be classified according to their specificity on causing specific disease patterns (Alard et al. 2008). Endothelial cells can express HSP60 on their plasma membrane. Multiple studies have verified that antibodies against HSP60 expressed on the surface of endothelial cells can be cytotoxic to endothelial cells (Alard et al. 2007; Jamin et al. 2005; Schett et al. 1995). Anti-HSP60 antibodies from patients with microscopic polyangiitis or with granulomatosis with polyangiitis can induce apoptosis in endothelial cells. Soluble HSP70 could bind with toll-like receptor 2(TLR2) and thus inducing apoptosis (Jamin et al. 2005). Furthermore, HSP60 can bind to surface ATPase of endothelial cells. Antibodies against HSP60/ATPase complex can induce apoptosis in endothelial cells in vasculitis patients (Alard et al. 2011). Tissue expression of HSP70 in various forms of glomerular disease has recently been associated with an intense inflammatory response. It is proposed that HSP70 can switch its anti-inflammatory effect to a pro-inflammatory effect (Chebotareva et al. 2018).
In the light of the aforementioned studies, we examine the relation of renal HSP60 and HSP70 expression in the various nephron compartments at diagnosis, with renal function and renal survival in a cohort of AAV patients during a period of 20 years.
Methods
Patients
This prospective study was conducted in the department of Nephrology, Heraklion University Hospital, a tertiary referral center in Crete, Greece. Twenty-nine patients with ANCA-associated vasculitis, diagnosed from 1995 to 1999, were prospectively followed up until 2015. Informed consent was obtained from all study participants. The study was conducted in compliance with EU Directive 2010/63 of the European Parliament. Patient data were anonymized. We could not obtain a control group because of ethical considerations (i.e., need for renal biopsy in healthy subjects) as well as matching difficulties (reduced renal mass in nephrectomized patients).
Disease onset was defined as the time point at which the patient was referred to the nephrology department. Renal involvement was initially diagnosed through elevated serum creatinine levels and reduced eGFR along with urine sediment examination followed by kidney biopsy. The presence of glomerular hematuria (≥ 3 acanthocytes/high-power field) and/or proteinuria served as an indicator of disease activity. The initiation of renal replacement therapy (renal death) was defined as primary endpoint of the study. At the time of diagnosis, all patients were treated with 1 g (i.v.) methylprednisolone day−1 over 3 days followed by oral methylprednisolone at a dosage of 1 mg kg body weight−1 (BW) over 30 days at a maximum dose of 80 mg. A tapering strategy was followed for 5 months until the establishment of 5 mg (p.o.) methylprednisolone for 2–5 years. Furthermore, all patients received induction therapy with i.v. cyclophosphamide at a dose of 15 mg kg−1 BW−1 (renal function adjusted) monthly for 6 months, followed by oral azathioprine at a dose of 2 mg kg−1 BW−1 day−1 as maintenance treatment.
Each patient was evaluated monthly for the first 6 months after treatment initiation and, thereafter, at annual visits. At each patient visit, the presence of disease-associated symptoms and signs (hypertension, fever, malaise, arthritis, hemorrhagic exanthema, hemoptysis, mucosal lesions) was evaluated. Evaluation included laboratory markers of disease activity (erythrocyte sedimentation rate (ESR), CRP, p-ANCA, c-ANCA antibodies, hemoglobin, glomerular hematuria, presence of casts, estimation of serum creatinine, and glomerular filtration rate (modification of diet in renal disease (MDRD)) (Levey et al. 1999). Chest X-ray was obtained at diagnosis and/or when clinical suspicion of lung involvement was present. The findings were then confirmed with computerized tomography (CT) of the lung.
Renal histopathology
Each biopsy specimen was evaluated by a renal pathologist (FS) blinded to clinical findings, laboratory profile, and renal function of the patient. Renal glomeruli were evaluated for intracapillary hyperplasia, ischemia, necrosis, and crescent formation. Interstitial inflammation was evaluated as the presence of inflammatory cells (neutrophils, macrophages) within the renal interstitium and graded according to its extent as mild (0–50%) or severe (≥ 50%). Interstitial fibrosis was assessed by the presence of collagen within renal interstitium utilizing Masson’s trichrome stain as mild (0–50%) or severe (≥ 50%). The biopsies were assigned according to the classification of Berden et al. (2010) into the following four categories:
Sclerotic (≥ 50% globally sclerotic glomeruli),
Focal (≤ 50% globally sclerotic glomeruli and ≥ 50% normal glomeruli),
Crescentic (≤ 50% globally sclerotic glomeruli ≤ 50% normal glomeruli and ≥ 50% cellular crescents), and
Mixed (≤ 50% globally sclerotic glomeruli, ≤ 50% normal glomeruli, and ≤ 50% cellular crescents) (Berden et al. 2010).
Immunohistochemical studies
Paraffin sections were incubated with monoclonal antibodies against CD3 (T lymphocytes marker), CD20 (B lymphocytes marker), CD68 (macrophages marker Santa Cruz Biotechnology, Dallas TX), HSP60 (StressGen Biotechnologies Corp., San Diego, CA), HSP70 (Novocastra Laboratories, Ltd. UK), epithelial membrane antigen (EMA) as a distal tubular marker, Fas serving as an apoptosis marker, vimentin serving as a cytoskeleton marker of glomerular epithelial cells, and MNF-116 serving as cytokeratin marker of epithelial cells within glomerular crescents (Abcam Plc., UK). Immunoperoxidase, alkaline phosphatase-anti-alkaline-phosphatase (APAAP), and avidin-biotin-complex staining methods (Dako, Denmark) were applied. Immunohistochemical staining was quantified according to staining intensity by a renal pathologist blinded to clinical findings (FS). Renal tissue from nephrectomized patients (ten patients) for reasons other than kidney disease or neoplasia was utilized as negative control. Due to ethical reasons, there was no control group over the observation time period. Results were evaluated by a renal histopathologist (FS) blinded to clinical findings, laboratory profile, and patient renal function.
Statistical analysis
All data were tested for normality. In the case of non-normality, logarithmic transformation was performed. Survival analyses were performed utilizing Kaplan-Meier algorithms. Fisher’s exact test or Chi-square test when applied was used for categorical variables. A two-tailed bivariate analysis was used for detecting clinicopathological correlations. p < 0.05 was considered statistically significant. The analysis was performed utilizing SPSS 20 (SPSS Inc. Chicago IL).
Results
Demographical data are presented in Table 1. The cohort comprises 29 patients. The mean age at baseline was 57.8 ± 19.2 years. All patients were of Caucasian ancestry.
Table 1.
Cohort characteristics
| Number of patients | Percent | Mean | Standard deviation | |
|---|---|---|---|---|
| Sex | ||||
| Male | 11 | 37.90 | ||
| Female | 18 | 62.10 | ||
| Age (years) | 58 | 19 | ||
| Arterial hypertension | 14 | 48.30 | ||
| Nephritic urinary sediment | 27 | 93.10 | ||
| Malaise | 22 | 75.90 | ||
| Pulmonary involvement | 15 | 51.70 | ||
| Purpura, necrotic skin lesions | 12 | 41.40 | ||
| Mucosal lesions | 1 | 3.40 | ||
| Fever | 12 | 41.40 | ||
| Ear osteochondritis | 1 | 3.40 | ||
| Central nervous system involvement | 0 | 0.00 | ||
| Hepatic involvement | 2 | 6.90 | ||
| pANCA | 13 | 44.80 | ||
| cANCA | 6 | 20.70 | ||
| ANCA negative | 10 | 34.50 | ||
| ESR (mmol h−1) | 107 | 29 | ||
| CRP (mg dl−1) | 6.36 | 3.16 | ||
| Hematocrit (%) | 31 | 4,76 | ||
| Serum creatinine at diagnosis (mg dl−1) | 3.93 | 2.93 | ||
| Glomerular filtration rate (ml min−1 1.73 m−2 body surface area) | 24.47 | 18.08 | ||
Clinical data (Table 1)
The main clinical characteristics of the patients at the time of inclusion were malaise (N = 22), arthralgia (N = 23), pulmonary involvement (N = 15), and arterial hypertension (N = 14). Fever and hemorrhagic exanthem or necrotic skin lesions were present in 12 patients (N = 12), respectively. Interestingly, none of the patients presented with or developed diabetes during the observation period. Mean serum creatinine at presentation was 3.9 mg dl−1, and the estimated mean glomerular filtration rate utilizing MDRD-equation was 24.47 ml min−1 1.73 m−2 BSA. Most patients presented with anemia (mean hematocrit, 31.1%) and elevated C-reactive protein (CRP) (mean CRP, 6.3 mg dl−1). Thirteen patients showed positivity for p-ANCA and six for c-ANCA; the rest of the patients (ten) were p-ANCA/c-ANCA negative. Twenty-seven patients had a nephritic urine sediment.
Histopathological characteristics
The histopathological characteristics of the cohort are shown in Table 2. The mean number of glomeruli obtained per biopsy was 27. Ninety percent of glomeruli showed morphological alterations. Crescents were shown in 30% of examined glomeruli. Intracapillary hyperplasia was shown in 25% of examined glomeruli. Glomerular ischemia was shown in 12% of glomeruli. Global glomerular sclerosis as well as segmental glomerular sclerosis were relatively rare (10.8 and 6.2%, respectively). Absence of interstitial inflammatory infiltrates was a rare phenomenon (6.9% of patients). Fifty-one percent of patients showed inflammatory infiltrates in more than 25% of renal interstitium. On the contrary, severe interstitial fibrosis (≥ 50% of interstitial area) was present in 10.3% of patients. Acute tubular necrosis and vascular hyalinosis were also uncommon phenomena (3.4 and 10.4% of patients, respectively).
Table 2.
Histopathological characteristics
| Mean | Standard deviation | Sum | Number of patients | Patients (%) | |
|---|---|---|---|---|---|
| Number of glomeruli | 27 | 10 | 787 | ||
| Number of diseased glomeruli | 25 | 11 | 720 | ||
| % of diseased glomeruli | 90.14 | 16.85 | |||
| Number of glomeruli with global sclerosis | 3 | 6 | 85 | ||
| % of glomeruli with global sclerosis | 9.1 | 16.77 | |||
| Number of glomeruli with segmental sclerosis | 2 | 2 | 49 | ||
| % of glomeruli with segmental sclerosis | 9.76 | 19.28 | |||
| Number of glomeruli with intracapillary hyperplasia | 7 | 9 | 201 | ||
| % of glomeruli with intracapillary hyperplasia | 26.10 | 30.77 | |||
| Number of ischemic glomeruli | 3 | 2 | 95 | ||
| % of ischemic glomeruli | 13.45 | 9.55 | |||
| Number of crescentic glomeruli | 9 | 9 | 250 | ||
| % of crescentic glomeruli | 31.62 | 28.66 | |||
| ANCA Vasculitis Lesion Classification | |||||
| Focal | 1 | 3.4 | |||
| Crescentic | 6 | 20.7 | |||
| Mixed | 19 | 65.5 | |||
| Sclerotic | 3 | 10.3 | |||
| Without crescents | 0 | 0.0 | |||
| Interstitial fibrosis | |||||
| 0% | 18 | 62.1 | |||
| < 25% | 6 | 20.7 | |||
| > 25 to < 50% | 2 | 6.9 | |||
| > 50% | 3 | 10.3 | |||
| Interstitial inflammation | |||||
| 0% | 2 | 6.9 | |||
| < 25% | 12 | 41.4 | |||
| > 25 to < 50% | 4 | 13.8 | |||
| > 50% | 11 | 37.9 | |||
ANCA Vasculitis Lesion Classification according to Berden et al. (2010)
According to the classification proposed by Berden et al. (2010), 3% of patients had focal lesions. Sclerotic lesions were present in 10.3% of patients. Crescentic lesions were observed in 20.7% of patients. The vast majority of patients (65.5%) presented with mixed lesions.
HSP expression in control and AAV biopsies
All control biopsies showed negative or weak expression of HSP60 or HSP70 (Fig. 1). HSP60 and HSP70 expression within crescents (Fig. 2), in proximal tubule and distal tubule as well as in renal interstitium was statistically significantly higher in patients with AAV compared with the control group (Table 3).
Fig. 1.
Healthy control section for HSP60 (a); staining for HSP60 within proximal tubule epithelium and cells within a newly forming glomerular crescent (arrowhead, b), EMA-HSP60 colocalization. Staining of HSP60 within distal tubule (c); intense staining of proximal tubules for HSP60 (d). Bars, 1.5 μm; Hsp60: brown color; epithelial membrane antigen (EMA–distal tubular marker): red color
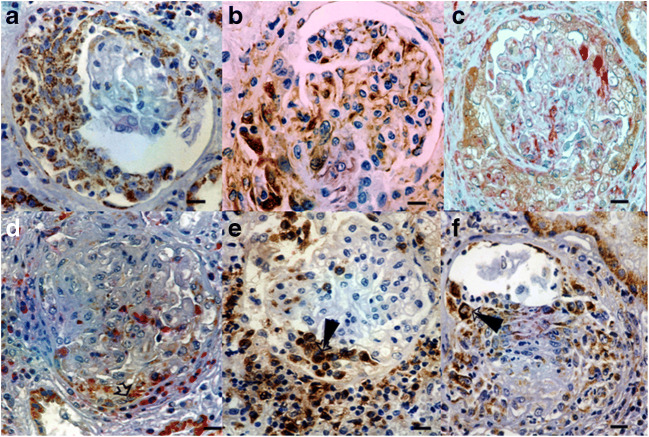
Fig. 2.
HSP60 (brown) staining within crescent (a), Vimentin (glomerular epithelial cells-brown) stained cells (crescent, glomerular tuft, b), HSP60-Vimentin (glomerular epithelial cells - red) colocalization indicating expression of HSP60 within glomerular epithelial cells (crescent, glomerular tuft, c), CD68 (Macrophage indicator-red)-HSP60 colocalization (crescent, glomerular tuft, arrowhead, d), Periglomerular Macrophages (CD68 positive cells, e), Macrophages presenting HSP60 staining (CD68 positive cells arrowhead, f) Bars: 8 um)
Table 3.
Expression of heat shock proteins between patients and control subjects
| Control patients | ANCA vasculitis patients | Fisher’s exact test sig. | |
|---|---|---|---|
| Interstitial Hsp70 expression | |||
| Yes | 0 | 16 | |
| No | 10 | 13 | 0.002 |
| Interstitial HSP60 expression | |||
| Yes | 0 | 10 | |
| No | 10 | 19 | 0.04 |
| Crescentic expression of HSP60a | |||
| Yes | 0 | 9 | |
| No | 10 | 20 | 0.047a |
| Crescentic expression of HSP70 | |||
| Yes | 0 | 17 | |
| No | 10 | 12 | 0.002 |
| Distal tubular expression of HSP60 | |||
| Yes | 0 | 12 | |
| No | 10 | 17 | 0.017 |
| Distal tubular expression of HSP70 | |||
| Yes | 0 | 18 | |
| No | 10 | 11 | 0.001 |
| Proximal tubular expression of HSP60 | |||
| Yes | 0 | 22 | |
| No | 10 | 7 | < 0.001 |
| Proximal tubular expression of HSP70 | |||
| Yes | 0 | 16 | |
| No | 10 | 13 | 0.002 |
aStrong expression of HSP60 within glomerular crescents was found to be of statistical significance. In that case, LR = 6.21; p = 0.045
Localization of HSP in the renal tissue of AAV biopsies (Fig. 3)
Fig. 3.
Differential expression of HSP70 and HSP60 in nephron segments
HSP70 in glomerular crescents was expressed in most patients. Bowman’s capsule as well as glomerular capillaries showed non-prevalent staining for HSP60 or HSP70. Proximal tubule was stained positive for HSP60 (Figs. 1c, d and 3) while distal tubule was stained positive for HSP70 in the majority of patients (Figs. 3 and 4). Renal interstitial macrophages were stained positive for HSP60 (Fig. 5). Interstitial T lymphocytes showed a slight predominance for HSP70 staining (Figs. 3 and 5). Severe interstitial injury was accompanied by HSP70-positive cell debris. HSP70 intense staining was observed in tubular epithelial cells demonstrating apoptosis (Fig. 4b). Interestingly, necrotic areas did not show any HSP staining. The surrounding inflammatory reaction involved mainly macrophages (Fig. 6).
Fig. 4.
HSP70 staining in proximal tubules along with severe interstitial insult (a),HSP70-FAS colocalization, HSP70 intense staining was observed in tubular epithelial cells demonstrating apoptosis (arrowhead, b). Bars: 1.5 um, HSP70: brown color, FAS (Apoptosis indicator): red color
Fig. 5.
HSP60 (brown) – macrophage (CD68 positive cells) colocalization (red, arrowhead, a), HSP60 (brown) – T lymphocytes (CD3 positive cells) colocalization (red, arrowhead, b) Bars: 8 um
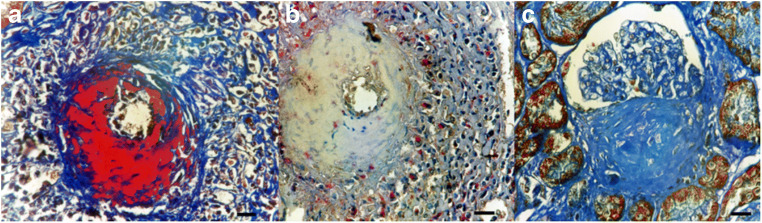
Fig. 6.
Necrotizing vasculitis interlobular artery (Masson Trichrome Stain, Anti HSP60 stain, a), Area of necrosis without HSP60 staining, macrophages (CD68-red) present HSP60 staining (brown, b), Fibrosis within crescent negative for HSP60 (c) Bars: 14 um
Association of HSP expression with renal function, interstitial fibrosis, and interstitial inflammation at diagnosis
Increased HSP70 expression within the renal interstitium was associated with worsened renal function at the time of diagnosis (Pearson’s R = 0.414; p = 0.026). On the contrary, HSP70 expression in proximal tubule (Pearson’s R = − 0.337; p = 0.044) as well as distal tubule (Pearson’s R = − 0.435; p = 0.019) was associated with reduced interstitial fibrosis. HSP60 expression within distal tubular epithelial cells is associated with increased interstitial inflammation (Pearson’s R = 0.672; p < 0.001). The presence of nephritic sediment did not correlate with the expression of HSP60 or HSP70 in nephron segments (Table 4).
Table 4.
Clinicopathological associations
| Serum creatinine (mg dl−1) | Nephritic sediment | Interstitial fibrosis | Interstitialinflammation | |
|---|---|---|---|---|
| HSP70 interstitial expression | ||||
| Pearson’s R | 0.414 | 0.109 | 0.521 | 0.014 |
| p | 0.026* | 0.574 | 0.004* | 0.943 |
| HSP60 interstitial expression | ||||
| Pearson’s R | − 0.025 | − 0.089 | 0.008 | 0.025 |
| p | 0.897 | 0.647 | 0.966 | 0.897 |
| HSP60 crescentic expression | ||||
| Pearson’s R | − 0.247 | 0.183 | − 0.017 | − 0.201 |
| p | 0.197 | 0.343 | 0.931 | 0.297 |
| HSP70 crescentic expression | ||||
| Pearson’s R | − 0.251 | 0.324 | − 0.055 | − 0.029 |
| p | 0.189 | 0.086 | 0.775 | 0.881 |
| HSP70 distal tubular expression | ||||
| Pearson’s R | − 0.044 | 0.068 | − 0.435 | − 0.096 |
| p | 0.82 | 0.727 | 0.019* | 0.613 |
| HSP60 distal tubular expression | ||||
| Pearson’s R | − 0.309 | − 0.048 | 0.055 | 0.672 |
| p | 0.103 | 0.806 | 0.775 | < 0.001* |
| HSP60 proximal tubular expression | ||||
| Pearson’s R | − 0.061 | − 0.154 | 0.073 | − 0.1 |
| p | 0.753 | 0.427 | 0.707 | 0.605 |
| HSP70 proximal tubular expression | ||||
| Pearson’s R | − 0.38 | 0.028 | − 0.337 | − 0.1 |
| p | 0.844 | 0.884 | 0.044 | 0.604 |
p is the statistical significance of Pearson’s R values
Survival analysis (Table 5; Figs. 7, 8, 9, and 10)
Table 5.
Association of variables used in survival analysis (interstitial fibrosis, percentage of glomerular crescents, expression of interstitial HSP70) with patient data
| Factor | HSP70 | Interstitial fibrosis | Crescent (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strong expression | Weak expression | Sig. | 0–25% | ≥ 25% | Sig. | ≤ 50 | > 50 | Sig | |
| Age (years) | |||||||||
| ≤ 66 | 4 | 14 | 0.129 | 15 | 3 | 0.646 | 14 | 4 | 1.000 |
| > 66 | 0 | 11 | 8 | 3 | 8 | 3 | |||
| Nephritic sediment | |||||||||
| Present | 4 | 23 | 0.739 | 21 | 6 | 1.000 | 20 | 7 | 1.000 |
| Absent | 0 | 2 | 2 | 0 | 2 | 0 | |||
| p-ANCA | |||||||||
| Present | 1 | 12 | 0.606 | 8 | 5 | 0.063 | 10 | 3 | 1.000 |
| Absent | 3 | 13 | 15 | 1 | 12 | 4 | |||
| c-ANCA | |||||||||
| Present | 1 | 5 | 0.627 | 6 | 0 | 0.295 | 4 | 2 | 0.612 |
| Absent | 3 | 20 | 14 | 5 | 18 | 5 | |||
| ANCA negative | |||||||||
| Present | 2 | 8 | 0.429 | 9 | 1 | 0.302 | 8 | 2 | 0.341 |
| Absent | 2 | 17 | 14 | 5 | 14 | 5 | |||
| Hypertension | |||||||||
| Present | 2 | 12 | 1.000 | 13 | 1 | 0.169 | 11 | 3 | 1.000 |
| Absent | 2 | 13 | 10 | 5 | 11 | 3 | |||
| Arthrits | |||||||||
| Present | 3 | 20 | 1.000 | 19 | 4 | 0.575 | 18 | 5 | 0.612 |
| Absent | 1 | 5 | 4 | 2 | 4 | 2 | |||
| Pulmonary involvement | |||||||||
| Present | 3 | 12 | 0.326 | 11 | 4 | 0.651 | 11 | 4 | 1.000 |
| Absent | 1 | 13 | 12 | 2 | 11 | 3 | |||
| Ischemic glomeruli | |||||||||
| < 8% | 1 | 9 | 0.364 | 8 | 2 | 0.667 | 7 | 3 | 0.604 |
| 9–19% | 3 | 10 | 11 | 2 | 11 | 2 | |||
| > 20% | 0 | 6 | 4 | 2 | 4 | 2 | |||
| Tubular necrosis | |||||||||
| Present | 0 | 1 | 0.862 | 1 | 0 | 1.000 | 1 | 0 | 1.000 |
| Absent | 4 | 24 | 22 | 6 | 21 | 7 | |||
| Intima media necrosis | |||||||||
| Present | 1 | 8 | 1.000 | 5 | 4 | 0.056 | 6 | 3 | 0.369 |
| Absent | 3 | 17 | 18 | 2 | 16 | 4 | |||
| Vascular hyalinosis | |||||||||
| Present | 0 | 3 | 2 | 1 | 0.515 | 2 | 1 | 1.000 | |
| Absent | 4 | 12 | 1.000 | 21 | 5 | 20 | 6 | ||
| Periglomerular inflammation | |||||||||
| 0% | 3 | 24 | 0.109 | 22 | 5 | 0.376 | 20 | 7 | 0.428 |
| 1–6% | 1 | 0 | 0 | 1 | 1 | 0 | |||
| > 7% | 0 | 1 | 1 | 0 | 1 | 0 | |||
Fig. 7.
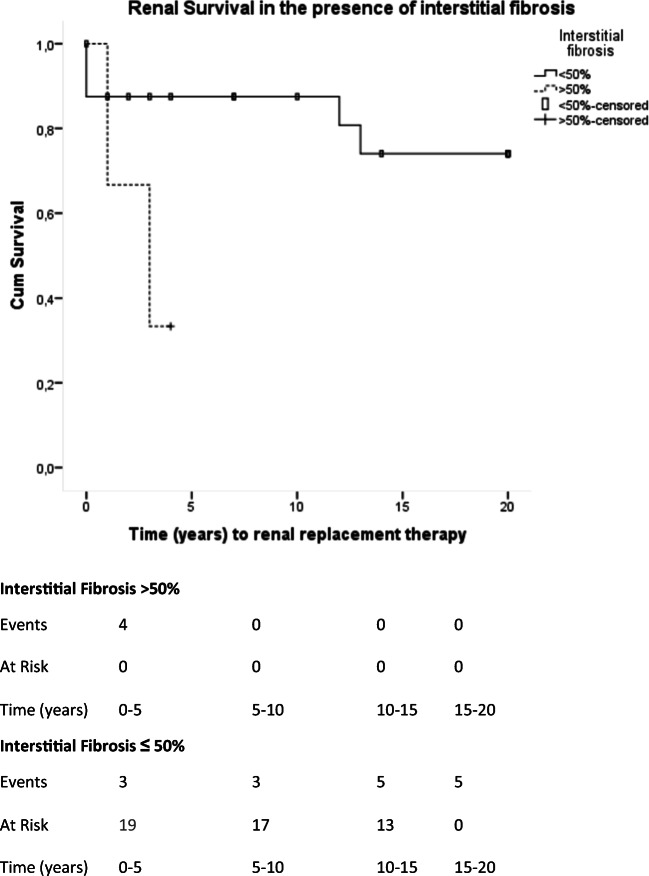
Interstitial fibrosis is related with renal function deterioration. Mean time to renal replacement therapy initiation was 2.6 years (95% CI, 1.2–4.1 years) when renal fibrosis was more than 50% of biopsy area. Mean time to renal replacement therapy initiation was 16.49 years (95% CI, 13.68–19.29 years). Chi-Square (Log-Rank) = 4.325; p = 0.038
Fig. 8.
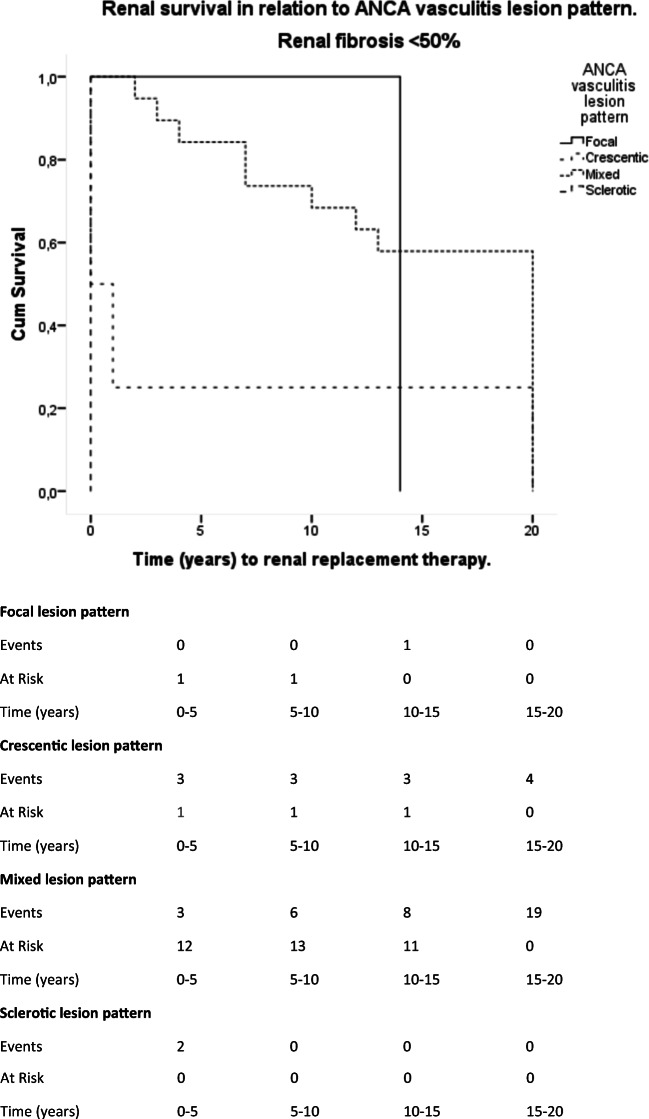
ANCA vasculitis lesion pattern is associated with renal survival in patients with renal fibrosis < 50% of biopsy area. Patients with a crescentic lesion pattern have a mean time to renal replacement therapy of 5.25 years (95% CI, 0–14.89 years). Patients with a mixed lesion pattern have a mean time to renal replacement therapy less of 14.63 years (95% CI, 11.5–17.7 years). Patients with a sclerotic lesion pattern have a mean time to renal replacement therapy of less than 1 year (95% CI, 0 year). There was a statistically significant difference in patients with a mixed lesion pattern and patients with a crescentic lesion pattern (Test Chi-Square [Log Rank]) = 5.22, p = 0.022) as well as with patients presenting with a sclerotic lesion pattern (Test Chi-Square [Log Rank] = 20; p < 0.001)
Fig. 9.
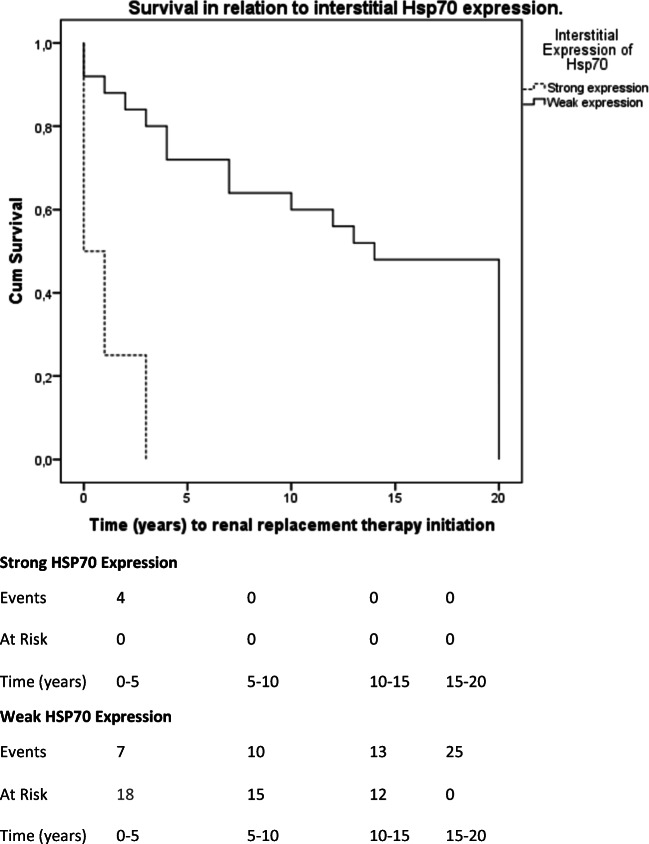
Strong interstitial HSP70 expression is associated with worsened renal survival. Mean renal survival in patients with strong interstitial expression of HSP70 was 1 year (95% CI, 0–2.38 years). Mean renal survival in patients with weak interstitial expression of Hsp70 was 12.68 years (95% CI, 9.54–15.81 years). The differences observed are of statistical significance (Log Rank Test Chi-Square = 14.41; p < 0.001)
Fig. 10.
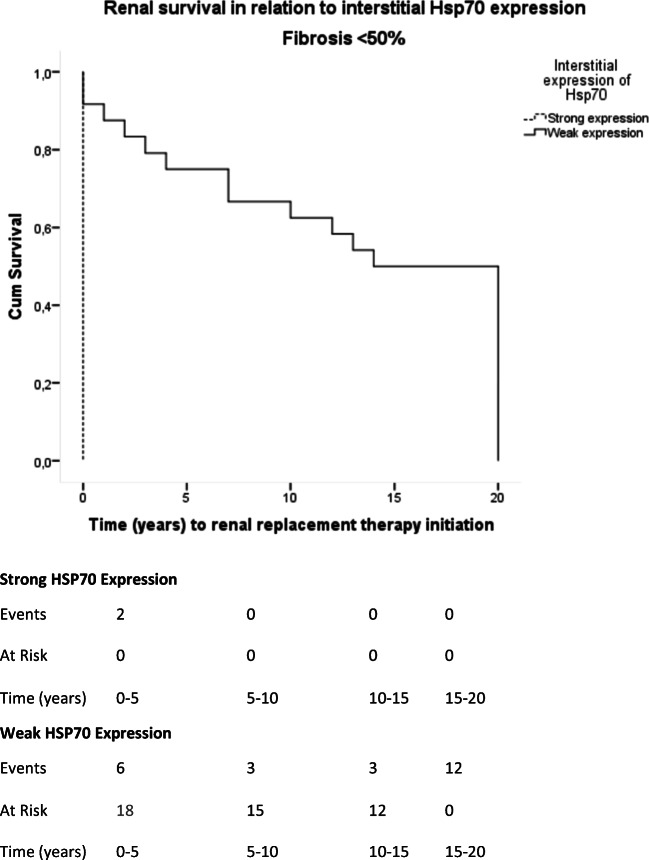
Strong interstitial HSP70 expression is associated with worsened renal survival when renal fibrosis is < 50% of biopsy area. Mean renal survival in patients with strong interstitial expression of HSP70 was < 0 years (95% CI, 0 years). Mean renal survival in patients with weak interstitial expression of HSP70 was 11.45 years (95% CI, 9.86–16.22 years). The differences observed are of statistical significance (Log-Rank Test Chi-Square = 11.45; p = 0.001)
Renal fibrosis at presentation was associated with adverse renal outcomes. Extensive renal fibrosis (> 50%) was associated with rapid renal function loss (mean time to renal replacement therapy was 2.6 years (95% CI, 1.2–4.1 years), log-rank Chi-square = 4.33; p = 0.038; Fig. 7).
Presence of ≥ 50% global sclerotic glomeruli at the time of diagnosis correlated with reduced renal survival (mean renal survival of < 1 year (95% CI, 0 years, log-rank Chi-square = 20; p < 0.001) in comparison with patients with mixed lesion pattern. Patients with crescentic lesion pattern at diagnosis showed also a rapid deterioration of renal function (mean renal survival of 5.25 years (95% CI, 0–14.89 years), log-rank Chi-square = 5.22; p = 0.022; Fig. 8) when compared with patients with mixed lesion pattern.
Importantly, strong interstitial expression of HSP70 at diagnosis was associated with worsened renal survival in patients presenting interstitial fibrosis < 50% of biopsy area (mean time to renal replacement therapy initiation, 0 years (95% CI, 0), log-rank Chi-square = 11.45; p = 0.001; Figs. 9 and 10) when compared with weak interstitial expression of HSP70.
In the multifactorial analysis, interstitial fibrosis, percentage of glomerular crescents, and the intensity of HSP70 expression did not show any significant association with the following parameters: age, nephritic sediment, ANCAs, arterial hypertension, arthritis, pulmonary involvement, ischemic glomeruli, tubular necrosis, intima media necrosis, vascular hyalinosis, and periglomerular inflammation (Table 5).
Discussion
In the present study, we could show an increased expression of HSP70 and HSP60 in renal tissue of patients with ANCA-vasculitis in comparison with healthy controls. The majority of patients showed positivity for p-ANCA, and the main clinical presentation was a rapidly progressive nephritic syndrome. Histologically, most patients showed a mixed lesion pattern in glomeruli as well as interstitial inflammation. Severe interstitial fibrosis was uncommon. Interstitial expression of HSP70 was associated with an increased extent of interstitial fibrosis. Extensive interstitial fibrosis as well as sclerotic and crescentic class at the time of diagnosis were associated with decreased renal survival. In the present cohort, we could not demonstrate a relation between HSP60 and renal survival. The most interesting finding in the present study is that increased HSP70 expression within the renal interstitium was associated with decreased renal survival independently of the extent of renal fibrosis.
Mean age of disease onset in our study was 60 years. Age did not correlate with the extent of histological alterations or with renal replacement therapy. ANCA-associated vasculitis shows a peak at the age of 65–74 years (Ntatsaki et al. 2010). Moving from northern to southern hemisphere attributes a shift towards p-ANCA vasculitis predominance. In the island of Crete, there is a p-ANCA predominance (Ioannidou et al. 2002; Panagiotakis et al. 2009). Previous studies have shown that c-ANCA-negative patients present with more severe clinical phenotype and experience a rapid progression of disease (Mahr et al. 2013).
Heat shock proteins are conserved throughout the animal kingdom (Li and Srivastava 2004). This finding states their importance in cell function. There is cumulating evidence showing that heat shock proteins can modulate not only the intracellular but also the extracellular stress response (Kim et al. 2016; Tsen et al. 2013; Zuo et al. 2016). HSP70 has a constitutional as well as an inducible expression. Strong tissue expression of HSP70 can induce cell necrosis (Daniels et al. 2004). Heat shock proteins can be excreted through active or passive mechanisms and thus have additional roles in modulating the immunologic response in the extracellular space (Calderwood et al. 2019). The effects of increased heat shock protein expression are addressed in various models of renal disease (Hernadez-Pando et al. 1995; O’Neill et al. 2014; Tsuji et al. 2009). Patients with autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, and dermatomyositis show increased levels of circulating HSP70 and HSP60 as well as increased levels of antibodies against HSP70 or HSP60 (Dhillon et al. 1991; Georgopoulos and McFarland 1993; Jorgensen et al. 1998; Lang et al. 2005).
Heat shock response may have a multifaceted role in the kidney of patients with AAV. Increased anti-HSP60 or anti-HSP70 antibodies have been described in subsets of AAV patients (Jamin et al. 2005; Komiya et al. 2011; Slot et al. 2006). Alard et al. (2009) showed that HSP60 could interact with HSP70 and induce cell damage through toll-like receptor 2 activation. Furthermore, in renal tissue of patients with glomerulonephritis-increased HSP70 expression exerts a proinflammatory effect (Chebotareva et al. 2018). On the other hand, HSP60 expression in immune-mediated glomerulonephritis has been associated with a worsened prognosis (Lang et al. 2005). We observed a differential expression of HSP60 and HSP70 within the various nephron segments. HSP70 was associated with apoptosis within the distal tubule. HSP60 expression could be shown mainly within interstitial macrophages, macrophages surrounding glomerular crescents, as well as in proximal tubular cells. HSP60 has been shown to provoke a proinflammatory reaction (Choi et al. 2015). Interestingly, necrotic areas lack HSP expression thus implicating heat shock protein response in the early stages of renal injury. Increased infiltration of renal interstitium with inflammatory cells, the small percentage of glomeruli with segmental glomerular sclerosis or glomerular ischemia and the small percentage of patients presenting with extensive interstitial fibrosis, acute tubular necrosis, or vascular hyalinosis when compared with other studies (Hauer et al. 2002) depicts the early diagnosis of the disease.
The observation that interstitial HSP70 overexpression is associated with worse renal outcomes, could imply that heat shock protein system has a role in the prognosis of ANCA-associated vasculitis. To our knowledge, this is the first study that shows an association of renal heat shock protein expression and renal outcomes in an ANCA vasculitis cohort.
The study limitations are the small patient number as well as the fact that it was a single-center study thus underlying the necessity of larger confirmatory studies. Furthermore, c-ANCA patients were underrepresented thus limiting the extrapolation of present observations in c-ANCA-positive patients.
Acknowledgments
The contribution of Dr. F. Sotsiou and Prof. D. Emmanouil to this work was invaluable.
Author’s contribution
I.P.: data analysis, statistical analysis, and manuscript review. A.A.: manuscript preparation, manuscript review, and data analysis. S.S.: data analysis and manuscript review). E.D. and K.S.: manuscript review and conceptual planning.
Funding
This study is supported by ELKE Research Committee, University of Crete (Research Program Nr. 2976).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Availability of data
Data available in SPSS format. Renal tissue is not available.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alard JE, Dueymes M, Mageed RA, Saraux A, Youinou P, Jamin C. Mitochondrial heat shock protein (HSP) 70 synergizes with HSP60 in transducing endothelial cell apoptosis induced by anti-HSP60 autoantibody. FASEB J. 2009;23:2772–2779. doi: 10.1096/fj.08-128785. [DOI] [PubMed] [Google Scholar]
- Alard JE, Dueymes M, Youinou P, Jamin C. Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun Rev. 2007;6:438–443. doi: 10.1016/j.autrev.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Alard JE, Dueymes M, Youinou P, Jamin C. HSP60 and anti-HSP60 antibodies in vasculitis: they are two of a kind. Clin Rev Allergy Immunol. 2008;35:66–71. doi: 10.1007/s12016-007-8062-x. [DOI] [PubMed] [Google Scholar]
- Alard JE, Hillion S, Guillevin L, Saraux A, Pers JO, Youinou P, Jamin C. Autoantibodies to endothelial cell surface ATP synthase, the endogenous receptor for hsp60, might play a pathogenic role in vasculatides. PLoS One. 2011;6:e14654. doi: 10.1371/journal.pone.0014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- Bonaguri C, et al. Cogan’s syndrome: anti-Hsp70 antibodies are a serological marker in the typical form. Isr Med Assoc J. 2014;16:285–288. [PubMed] [Google Scholar]
- Calderwood SK, Repasky EA, Neckers L, Hightower LE. The IXth CSSI international symposium on heat shock proteins in biology and medicine: stress responses in health and disease: Alexandria Old Town, Alexandria, Virginia, November 10-13, 2018. Cell Stress Chaperones. 2019;24:1–6. doi: 10.1007/s12192-018-00966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebotareva N, Bobkova I, Lysenko L, Neprinzeva N, Vinogradov A, Moiseev S. Heat shock protein 70 and anti-heat shock protein 70 antibodies in patients with chronic glomerulonephritis. Cell Stress Chaperones. 2018;23:1229–1235. doi: 10.1007/s12192-018-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Choi M, Park C, Lee EK, Kang DH, Lee DJ, Yeom JY, Jung Y, Kim J, Lee S, Kang SW. Cytosolic Hsp60 orchestrates the survival and inflammatory responses of vascular smooth muscle cells in injured aortic vessels. Cardiovasc Res. 2015;106:498–508. doi: 10.1093/cvr/cvv130. [DOI] [PubMed] [Google Scholar]
- Daniels GA, Sanchez-Perez L, Diaz RM, Kottke T, Thompson J, Lai M, Gough M, Karim M, Bushell A, Chong H, Melcher A, Harrington K, Vile RG. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- Dhillon V, Latchman D, Isenberg D. Heat shock proteins and systemic lupus erythematosus. Lupus. 1991;1:3–8. doi: 10.1177/096120339100100102. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, McFarland H. Heat shock proteins in multiple sclerosis and other autoimmune diseases. Immunol Today. 1993;14:373–375. doi: 10.1016/0167-5699(93)90135-8. [DOI] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Pinach S, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Cavallo-Perin P, EURODIAB Prospective Complications Study Group ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/ macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med. 2009;266:527–536. doi: 10.1111/j.1365-2796.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Hauer HA, Bajema IM, van Houwelingen H, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002;61:80–89. doi: 10.1046/j.1523-1755.2002.00089.x. [DOI] [PubMed] [Google Scholar]
- Hernadez-Pando R, et al. Histological and subcellular distribution of 65 and 70 kD heat shock proteins in experimental nephrotoxic injury. Exp Toxicol Pathol. 1995;47:501–508. doi: 10.1016/s0940-2993(11)80337-4. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Kurokawa MS, Yoshikawa H, Nara K, Takada E, Masuda C, Tsukikawa S, Ozaki S, Matsuda T, Suzuki N. Involvement of Th1 cells and heat shock protein 60 in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2005;139:371–378. doi: 10.1111/j.1365-2249.2005.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidou DJ, Krasagakis K, Daphnis EK, Perakis KE, Sotsiou F, Tosca AD. Cutaneous small vessel vasculitis: an entity with frequent renal involvement. Arch Dermatol. 2002;138:412–414. doi: 10.1001/archderm.138.3.412. [DOI] [PubMed] [Google Scholar]
- Jamin C, Dugué C, Alard JÉ, Jousse S, Saraux A, Guillevin L, Piette JC, Youinou P. Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum. 2005;52:4028–4038. doi: 10.1002/art.21401. [DOI] [PubMed] [Google Scholar]
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603–606. doi: 10.1007/s10157-013-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989;9:2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen C, Gedon E, Jaquet C, Sany J. Gastric administration of recombinant 65 kDa heat shock protein delays the severity of type II collagen induced arthritis in mice. J Rheumatol. 1998;25:763–767. [PubMed] [Google Scholar]
- Kim EY, Durai M, Mia Y, Kim HR, Moudgil KD. Modulation of adjuvant arthritis by cellular and humoral immunity to Hsp65. Front Immunol. 2016;7:203. doi: 10.3389/fimmu.2016.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya I, Arimura Y, Nakabayashi K, Yamada A, Osaki T, Yamaguchi H, Kamiya S. Increased concentrations of antibody against heat shock protein in patients with myeloperoxidase anti-neutrophil cytoplasmic autoantibody positive microscopic polyangiitis. Microbiol Immunol. 2011;55:531–538. doi: 10.1111/j.1348-0421.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- Lang A, Benke D, Eitner F, Engel D, Ehrlich S, Breloer M, Hamilton-Williams E, Specht S, Hoerauf A, Floege J, von Bonin A, Kurts C. Heat shock protein 60 is released in immune-mediated glomerulonephritis and aggravates disease: in vivo evidence for an immunologic danger signal. J Am Soc Nephrol. 2005;16:383–391. doi: 10.1681/ASN.2004040276. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Li Z, Srivastava P (2004) Heat-shock proteins. Curr Protoc Immunol Appendix 1:Appendix 1T doi:10.1002/0471142735.ima01ts58 [DOI] [PubMed]
- Lu W, Cheng P, Chen S. HSP60, HSP70 in the pathogenesis of Kawasaki disease: implication and action. J Tongji Med Univ. 1998;18:145–148. doi: 10.1007/BF02888523. [DOI] [PubMed] [Google Scholar]
- Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Höglund P, Merkel PA, Pagnoux C, Rasmussen N, Westman K, Jayne DRW, for the French Vasculitis Study Group (FVSG) and the European Vasculitis Society (EUVAS) Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–1010. doi: 10.1136/annrheumdis-2012-201750. [DOI] [PubMed] [Google Scholar]
- Ntatsaki E, Watts RA, Scott DG. Epidemiology of ANCA-associated vasculitis. Rheum Dis Clin North Am. 2010;36:447–461. doi: 10.1016/j.rdc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- O'Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J. Heat-shock proteins and acute ischaemic kidney injury. Nephron Exp Nephrol. 2014;126:167–174. doi: 10.1159/000363323. [DOI] [PubMed] [Google Scholar]
- Panagiotakis SH, Perysinakis GS, Kritikos H, Vassilopoulos D, Vrentzos G, Linardakis M, Bertsias G, Glaser K, Daphnis E, Boumpas DT. The epidemiology of primary systemic vasculitides involving small vessels in Crete (southern Greece): a comparison of older versus younger adult patients. Clin Exp Rheumatol. 2009;27:409–415. [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Duba J, Horvath L, Csaszar A, Karadi I, Szebeni A, Singh M, Fekete B, Romics L, Fust G. Comparative study on antibodies to human and bacterial 60 kDa heat shock proteins in a large cohort of patients with coronary heart disease and healthy subjects. Eur J Clin Invest. 2001;31:285–292. doi: 10.1046/j.1365-2362.2001.00819.x. [DOI] [PubMed] [Google Scholar]
- Rowaiye OO, Kusztal M, Klinger M. The kidneys and ANCA-associated vasculitis: from pathogenesis to diagnosis. Clin Kidney J. 2015;8:343–350. doi: 10.1093/ckj/sfv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot MC, Theunissen R, van Paassen P, Damoiseaux JG, Tervaert JW. Evaluation of antibodies against human HSP60 in patients with MPO-ANCA associated glomerulonephritis: a cohort study. J Autoimmune Dis. 2006;3:4. doi: 10.1186/1740-2557-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsen F, Bhatia A, O'Brien K, Cheng CF, Chen M, Hay N, Stiles B, Woodley DT, Li W. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol Cell Biol. 2013;33:4947–4959. doi: 10.1128/MCB.00559-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, Ito H, Fujigaki Y, Hishida A. The dimethylthiourea-induced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol. 2009;234:202–208. doi: 10.1016/j.taap.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- Venkataseshan VS, Marquet E. Heat shock protein 72/73 in normal and diseased kidneys. Nephron. 1996;73:442–449. doi: 10.1159/000189108. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]
- Yin Ji X, Kang MR, Choi JS, Jeon HS, Han HS, Kim JY, Son BR, Lee YM, Hahn YS. Levels of intra- and extracellular heat shock protein 60 in Kawasaki disease patients treated with intravenous immunoglobulin. Clin Immunol. 2007;124:304–310. doi: 10.1016/j.clim.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Zuo D, Subjeck J, Wang XY. Unfolding the role of large heat shock proteins: new insights and therapeutic implications. Front Immunol. 2016;7:75. doi: 10.3389/fimmu.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]