Abstract
Objectives
The aim of this study was to develop an evidence-based, clinically expedient checklist to identify cats likely to have degenerative joint disease (DJD)-associated pain.
Methods
Data were compiled from previously conducted studies that employed a standardized subjective outcome measure consisting of a series of questions. These studies included a prevalence study (with DJD non-informed owners) and therapeutic trials (with DJD-informed owners). For each cat, and each question, response scores were converted to ‘impaired’ and ‘unimpaired’. Cats were categorized as ‘DJD pain’ and ‘non-DJD’ based on orthopedic pain and radiographic DJD scores. These binary data were compared between cat phenotypes (non-DJD and DJD pain) for each question. Sensitivity and specificity of each question were calculated using the binary data; based on this, potential questions for the checklist were selected. Sensitivity and specificity across this group of questions were calculated, and questions sequentially removed to optimize length, sensitivity and specificity. Finally, the proposed checklist was applied to a novel data set to evaluate its ability to identify cats with DJD pain.
Results
In total, 249 DJD pain cats and 53 non-DJD cats from five studies were included. Nine questions with adequate sensitivity and specificity were initially identified. Following sequential removal of questions, a checklist with six binary questions was proposed. Based on the data from the cohorts of DJD-informed and DJD non-informed owners, the sensitivity and specificity of the proposed checklist were approximately 99% and 100%, and 55% and 97%, respectively.
Conclusions and relevance
The proposed checklist represents a data-driven approach to construct a screening checklist for DJD pain in cats. This checklist provides a clinically expedient tool likely to increase veterinarians’ ability to screen for DJD pain in cats. The identified behaviors comprising the checklist may further provide a foundation for increasing awareness of DJD pain among cat owners.
Keywords: Osteoarthritis, pain, checklist, musculoskeletal disease, degenerative joint disease, screening, behavioral change
Introduction
Degenerative joint disease (DJD) is associated with negative consequences, including pain, mobility impairment and decreased quality of life.1–3 In cats, the prevalence of DJD is high – an estimated 90% of cats have radiographic signs of DJD, with at least 40% of these cats showing clinical signs related to pain.1,4 However, in spite of this high prevalence, DJD in cats remains underdiagnosed and undertreated.5,6 Several reasons for this discrepancy have been suggested, including the decrease in cat visits to the veterinarian and the difficulty of diagnosis. Another reason may be the lower prevalence of single-limb lameness as a major clinical sign of DJD in cats. 7 In our experience, owners are familiar with lameness (usually referred to as ‘limping’ by owners) as a classical sign of joint or other limb pain; in the absence of this sign, they may attribute other behavioral signs of DJD to normal aging. In cats, this is particularly problematic as cats generally do not perform the same behaviors in the veterinary clinic as they do at home. This lowers the opportunity for veterinarian observation of important behavioral signs such as difficulty navigating stairs. Thus, along with veterinary orthopedic examination and radiographs, owners remain a critical part of the diagnosis and monitoring of DJD-associated pain.8–10
Given the importance of owner monitoring of behaviors associated with pain in cats, several metrology instruments have been described for clinical or research use in cats. The most commonly used are the Feline Musculoskeletal Pain Index (FMPI), 11 Client-Specific Outcome Measures,12,13 and the Montreal Instrument for Cat Arthritis (MI-CAT). 14 However, these tools may be best applied once the presence of DJD has been confirmed, rather than for clinically expedient screening. Indeed, these tools have been developed and used with the assumption that DJD-associated pain has been diagnosed.While screening tools have been developed for dogs (https://www.zoetisus.com/oa-pain/img/pdf/zoetis-canine-oa-checklist-printable-version.pdf; https://www.previcox.com/assets/documents/PVX15EXAMPOSTIT.PDF), currently there are no tools available for screening client-owned cats for the presence of DJD-associated pain. A screening checklist to identify cases of DJD-associated pain would fill a critical gap in the detection of DJD in cats. To be valuable, however, such a checklist must be developed using a data-driven approach, and with adequate accuracy. The principal parameters for measuring accuracy are sensitivity, specificity and predictive value. Sensitivity and specificity are the ability of a test to correctly identify patients with or without the disease, respectively. Positive predictive value (PPV) refers to the probability that a patient with a positive screening test truly has the disease, while negative predictive value (NPV) refers to the probability that a patient with a negative screening test truly does not have the disease. Therefore, the aim of this study was to develop a checklist with adequate accuracy that could be completed by cat owners and used to identify cats likely to have DJD-associated pain.
Materials and methods
This study used data collected previously at the Translational Research in Pain (TRiP) Program (formally called the Comparative Pain Research Laboratory) at North Carolina State University (NCSU). All data were collected in accordance with the Institutional Care and Use Committee guidelines (IACUC numbers 06-056; 08-124; 08-125; 11-102; 14-009; and 14-043), and procedures were carried out with informed, written owner consent. The work in the original studies involved the use of non-experimental (owned) animals only, and followed established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care. All prior studies from which the data were collected were performed under the NC State Institutional Animal Care and Use Committee approval.
Study overview
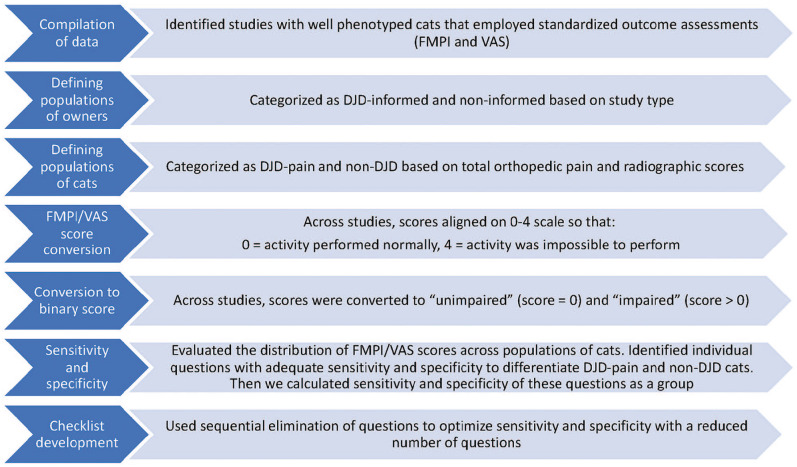
In order to generate a binary response (yes/no) checklist to identify cats that potentially have DJD-associated pain, we used previously generated owner questionnaire data. Included data were generated at the screening visits from previous studies of DJD in cats. These included a study on the prevalence of DJD, as well as studies focused on outcome measure development and studies evaluating the efficacy of potential therapeutics. Across these studies, all owners had been asked the same questions about their cats’ activity (Pain Behaviour Visual Analog Scale Scoring; FMPI; available in the supplementary material), with each question graded on a scale of normal to severely impaired. For each cat and each question, these scores were then converted to ‘unimpaired’ and ‘impaired’ responses, with ‘impaired’ reflecting that there was some degree of problem in performing that particular activity. These binary data were compared between cat phenotypes (healthy non-DJD and DJD pain) in order to develop a set of questions to comprise a checklist based on ‘yes/no’ answers. The study flow is displayed (Figure 1) and described in detail in the following sections.
Figure 1.
Study overview for the various stages of checklist development. FMPI = Feline Musculoskeletal Pain Index; VAS = visual analog scale; DJD = degenerative joint disease
Populations of owners and cats
In order to evaluate potential checklist questions in owners who both were and were not aware of DJD and associated pain, we included two populations of owner/cat dyads (DJD-informed and DJD non-informed). Across both population groups, all cats received a standardized physical, orthopedic, neurologic and radiographic examination; all owners completed a questionnaire about their cat’s activities. Populations, examination and questionnaire details are described below.
DJD-informed group
To generate this group, we used data from four clinical studies that included client-owned cats with DJD (DJD pain) and employed a standardized subjective outcome measure – the FMPI. These included a trial of a nutraceutical (unpublished data), a study to evaluate a clinical metrology instrument, 11 a study of a low-dose non-steroidal anti-inflammatory drug 15 and a study of an anti-nerve growth factor antibody. 16 In addition, data from an NCSU study utilizing client-owned cats without clinical signs of DJD (healthy, non-DJD cats) were included for comparison. 17 These owners were specifically recruited as having ‘healthy cats without musculoskeletal pain’ (ie, free from signs of DJD).Owners of cats in these data sets were considered DJD-informed owners because they had, in some way, been educated about the presence of, or lack of, DJD in cats (through their veterinarian, advertising, recruitment strategies, TV pieces, etc).
DJD non-informed
Data were used from a DJD prevalence study of 100 cats randomly selected from a single veterinary practice. 4 Owners of these cats competed a questionnaire (a visual analog scale [VAS]) about their cat’s activity and mobility with identical or similar questions to the FMPI. Owners in this group were unaware of why their cats were being evaluated, other than a general health screening, and were not informed about DJD or signs of DJD in cats. These owners made up our DJD non-informed owner group and represented an unbiased assessment of their cat’s activity/mobility.
Examinations
All cats contributing data to this checklist development study had been evaluated for systemic disease, orthopedic pain, and radiographic evidence of DJD. During screening, all cats received a physical examination, followed by an orthopedic examination, during which each joint or spinal segment was palpated and gently manipulated, and scored for the presence and severity of pain, as described previously. 18 Briefly, pain was scored on the following scale: 0 = no resentment; 1 = mild withdrawal, mild resistance to manipulation; 2 = moderate withdrawal, body tenses, may orient to site, may vocalize or increase vocalization; 3 = orients to site, forcible withdrawal from manipulation, may vocalize, hiss or bite; and 4 = tries to escape or prevent manipulation, bites or hisses, marked guarding of site. Total pain (TPain) scores were calculated as the sum of the scores for individual joints, with a possible range of 0–80.
Following the physical and orthopedic examinations, cats were sedated using an individually tailored protocol and orthogonal digital radiographs were made of each joint and spinal segment. Radiographs were evaluated by a single investigator (BDXL) and scored for the presence and severity of DJD using previously published criteria. 4 Scores were ascribed according to a 10-point scale where 0 = no evidence of DJD and 10 = ankylosis of the joint. Total radiographic DJD (TDJD) scores were calculated as the sum of the scores for individual joints, with a possible range of 0–200.
Using summary scores for TPain and TDJD, cats were categorized according to previously published criteria (Table 1). 19 Cats with negligible/normal or mild scores for both TPain and TDJD were categorized as non-DJD; cats with at least one moderate or high score on TPain or TDJD were categorized as DJD pain. TPain and TDJD scores were only used to categorize cats as non-DJD or DJD pain.
Table 1.
Total pain (TPain) and total radiographic degenerative joint disease (TDJD) scores were used to categorize cats using previously published criteria as shown 19
| Non-DJD |
DJD pain |
|||
|---|---|---|---|---|
| Negligible/normal | Mild | Moderate | High | |
| TPain | 0–2 | 2–4 | 5–9 | >10 |
| TDJD | 0–3 | 4–12 | 13–24 | >25 |
Cats were considered non-DJD if they had negligible/normal and mild scores for both TPain and TDJD and DJD pain if they had moderate or high scores. A score of 2 was negligible/normal if no single joint received a score of 2 and was mild if a 2 was scored for a single joint
FMPI/VAS score conversion
In the included studies, scoring of the FMPI data had been performed in various ways (for statistical reasons). To align each study, the scoring for each study was converted to a 0–4 scale (0 = activity performed normally, 4 = activity was impossible to perform). For example, if the original study employed a 5 (normal) to 1 (impossible) scale for each question, the data were converted as shown in Table 2. The VAS has been shown to be highly correlated with a five-point verbal descriptive scale. 20 Thus, the responses on the VAS were converted from a 0 to 100 scale (100 = activity performed normally, 0 = activity was impossible to perform) to a 0–4 scale (0 = activity performed normally, 4 = activity was impossible to perform) and VAS scores were then categorized as shown in Table 2.
Table 2.
Summary of how different scoring for similar question responses was aligned to a 0–4 scale
| Converted FMPI/VAS score | Original FMPI score | Description in FMPI | Original VAS score |
|---|---|---|---|
| 0 | 5 | Normal | >80–100 |
| 1 | 4 | Not quite normal | >60–80 |
| 2 | 3 | Somewhat worse than normal | >40–60 |
| 3 | 2 | Barely, or with great effort | >20–40 |
| 4 | 1 | Not at all | 0–20 |
FMPI = Feline Musculoskeletal Pain Index; VAS = visual analog scale
Calculation of the percentage of cats in each study scored as impaired on each question
As the ideal checklist for clinical use would consist of dichotomously scored questions (ie, yes/no responses), across all studies and cats, the FMPI/VAS scores for each question (0–4) were then converted to 0 (unimpaired) or 1 (impaired) by designating a score on any individual question of 1 or more as impaired, which would correspond to a response of ‘no’ on a dichotomous checklist.
Checklist development
Using data from these populations, checklist development was then conducted in two phases.
Phase 1
Phase 1 consisted of (a) an evaluation of percentages of impaired scores for each question; (b) a calculation of sensitivity and specificity of questions using data generated from DJD-informed owners; and (c) a comparison with DJD non-informed owners.
Phase 2
Phase 2 consisted of selection of questions for the checklist and determination of sensitivity and specificity across varying numbers of questions, and application of the proposed checklist to a novel data set.
Phase 1
The data from questions scored at the screening visit were analyzed to determine which questions best identified impaired cats as impaired and healthy cats as unimpaired; this was carried out for both DJD-informed and DJD non-informed populations. Comparing these percentages for each question highlighted the gap between informed and uninformed owners, and allowed us to identify questions of high salience to owners of cats with DJD. Of note, the FMPI included two questions – ‘Jump up to kitchen-counter height in one try’ and ‘Lie and/or sit down’ – which were not present on the VAS. The VAS, however, had a question about ‘Height of jumping up’. For the purposes of comparison, we considered the VAS ‘Height of jumping up’ and the FMPI ‘Jump up to kitchen-counter height in one try’ as similar because ‘height’ was meant to refer to a high place in the house, such as a kitchen counter, and the kitchen counter was added to the FMPI to provide this context for owners.
Second, the sensitivity and specificity of each question were calculated, separately for each population. As we were interested in the ability of the checklist to identify cats with DJD in a general population (ie, not a targeted population), potential questions for inclusion in the checklist were selected using the data from the DJD non-informed owners. Initial questions were selected if they had >25% sensitivity and >80% specificity. Sensitivity and specificity were calculated using following equations:
Phase 2
After selection of potential questions for inclusion in the checklist, we combined these questions and calculated their sensitivity and specificity as a combined set. Sensitivity, specificity, predictive values and accuracy of collective groups of questions were calculated. The number of questions was reduced gradually by sequentially eliminating questions with the lowest sensitivity. We aimed to select approximately six questions: this number of questions would be of reasonable length as a screening tool, yet have adequate sensitivity and specificity.We began by calculating the sensitivity and specificity of the proposed checklist for the data from DJD non-informed owners as these represent the general population of cat owners (ie, without specific education on DJD-associated pain and behavior). We then calculated the sensitivity and specificity of the proposed checklist for the data from DJD-informed owners, highlighting the impact that education about the behavioral signs of DJD can have on owners’ ability to detect changes in their cats. Finally, the proposed checklist was applied to a recent clinical study to determine whether the checklist would be able to identify impaired cats in this well-phenotyped group (unpublished data).
Results
Animals
Demographic data are reported in the relevant publications and available from the authors on request.4,11,15–17 In this report, we simply report on the number of cats in each category for the DJD-informed and DJD non-informed populations.
DJD-informed population
All cats included in the four clinical trials were categorized as DJD pain cats. After reviewing the orthopedic examination results and radiographic data of 22 potentially healthy, non-DJD cats (from the normal activity study), 16 cats met the stringent inclusion criteria of non-DJD cats for this study, while six cats were re-categorized as DJD pain cats (see Table 1 for score descriptions). In total, this gave us 186 DJD pain cats and 16 non-DJD cats in this population.
DJD non-informed population
After categorization into DJD pain and non-DJD as described above, 63/100 cats were categorized as DJD pain and 37 cats as non-DJD.
Part 1
Comparison of impaired and unimpaired for each question
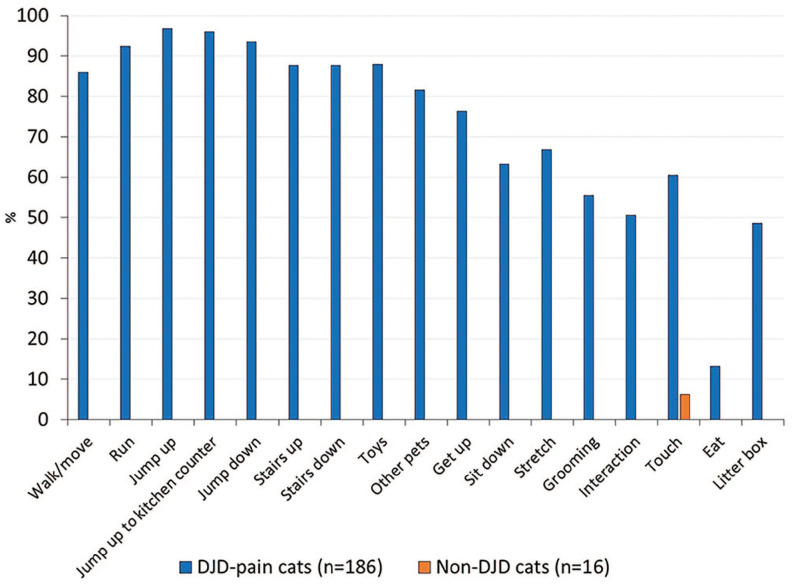
Using data from the DJD-informed population, we found that a high percentage of DJD pain cats were rated as impaired (ie, a score above 0) for many of the activities queried. This was in contrast to the percentage of non-DJD cats rated as impaired for those activities. Four questions were rated as impaired for >90% of the DJD pain cats (run, jump up, jump up to kitchen counter height and jump down); an additional five questions were rated as impaired for >80% of the DJD pain cats (walk/move, stairs up, stairs down, playing with toys and interacting with other pets) (Figure 2). For the non-DJD cats, only one question (touch) was rated as impaired by any cat owner. It is important to note, however, that these were ratings made by owners recruited specifically for studies based on the presence or absence of DJD pain. In this analysis, no single question was rated as impaired for all the DJD pain cats; this means that there was no universally altered owner-rated behavior across all these cats.
Figure 2.
Percentage of cats scored as impaired by their owners for each question on the Feline Musculoskeletal Pain Index (FMPI), plotted for cats with and without degenerative joint disease (DJD) pain in the DJD-informed cohort. No single question was impaired for all cats
Comparison of impaired and unimpaired for each question on the VAS
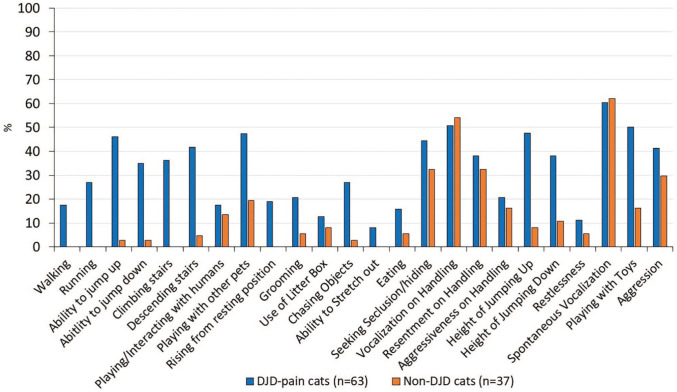
Using the responses to questions in the DJD non-informed population, a higher percentage of DJD pain than non-DJD cats were rated as impaired for several of the activities queried. In particular, this difference was most pronounced for activities that required mobility, such as running and jumping. Other behaviors that have been noted to occur in cats with DJD-associated pain, such as difficulty using the litter box and aggression, were not good differentiating questions for this population (Figure 3).
Figure 3.
Percentage of degenerative joint disease (DJD) pain and non-DJD cats scored as impaired by their owners for each question by the DJD non-informed cohort of owners.
VAS = visual analog scale
Comparison of the distribution of responses to questions for DJD-informed and DJD non-informed
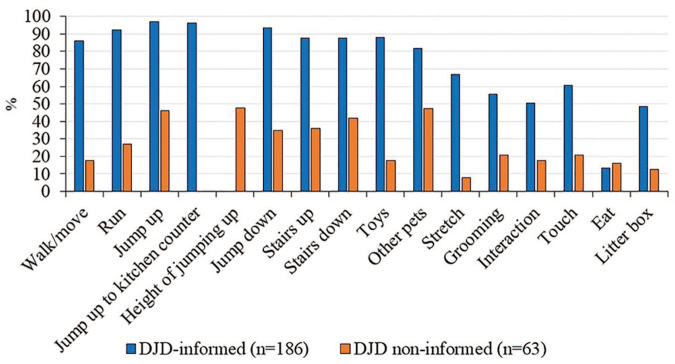
Finally, we compared the distribution of responses for all questions between the two DJD pain groups (DJD-informed and DJD non-informed). A higher percentage of DJD-informed owners scored their cats as impaired for every question compared with owners in the DJD non-informed population (Figure 4). The difference between the percentages represents an opportunity for targeted education about the behavioral signs of DJD in cats.
Figure 4.
Comparison of the percentage of degenerative joint disease (DJD) pain cats scored as impaired by their owners for each question for DJD-informed owners and DJD non-informed owners. As all cats had clinically identified DJD-associated pain (ie, both pain on examination and radiographic evidence of DJD), differences between the populations can be attributed, in part, to owner awareness of DJD in cats
Sensitivity and specificity
The results for the calculation of sensitivity and specificity for each question are shown in Table 3. Based on these results, 10 questions with >25% sensitivity and >80% specificity were initially selected for further evaluation as potential questions to include in the checklist. As the question ‘Height of jump down’ was included in the VAS but had no comparable question on the FMPI it was excluded, leaving nine questions for initial evaluation.
Table 3.
Sensitivity and specificity for each visual analog scale question based on 63 degenerative joint disease (DJD) pain cats and 37 non-DJD cats
| Question | Sensitivity | Specificity |
|---|---|---|
| Walking | 17.5 | 100 |
| Running* | 27.0 | 100 |
| Ability to jump up* | 46.0 | 97.3 |
| Ability to jump down* | 34.9 | 97.3 |
| Climbing stairs* | 36.2 | 100 |
| Descending stairs* | 41.7 | 95.5 |
| Playing/interacting with humans | 17.5 | 86.5 |
| Playing with other pets* | 47.3 | 80.6 |
| Rising from a resting position | 19.0 | 100 |
| Grooming | 20.6 | 94.6 |
| Use of litter box | 12.7 | 91.9 |
| Chasing objects (toys, prey, etc)* | 27.0 | 97.3 |
| Ability to stretch out | 7.9 | 100 |
| Eating | 15.9 | 94.6 |
| Seeking seclusion/hiding | 44.4 | 67.6 |
| Vocalization on handling | 50.8 | 45.9 |
| Resentment on handling | 38.1 | 67.6 |
| Aggressiveness on handling | 20.6 | 83.8 |
| Height of jumping up* | 47.6 | 91.9 |
| Height of jumping down* | 38.1 | 89.2 |
| Restlessness | 11.1 | 94.6 |
| Spontaneous vocalization | 60.3 | 37.8 |
| Playing with toys* | 50 | 83.8 |
| Aggression | 41.3 | 70.3 |
Questions with >25% sensitivity and >80% specificity; these represent potential questions for inclusion on the checklist
Phase 2
Evaluation of checklist performance
Starting with the nine questions identified in phase 1, the sensitivity, specificity, predictive values and accuracy of the questions as a group were evaluated. To achieve a clinically useful and expedient checklist, a goal of approximately six questions was set, balancing for optimal sensitivity and specificity. Results for sequential elimination of questions with the lowest sensitivity are shown in the supplementary materials (Tables S1–S3); sensitivity, specificity, predictive value and accuracy for the final six questions are shown in Table 4.
Table 4.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy across six questions (running, jump up, jump down, stairs up, stairs down, chasing objects)
| Disease + | Disease – | |
|---|---|---|
| Checklist + | 35 | 1 |
| Checklist – | 28 | 36 |
| Value (%) | 95% CI (%) | |
| Sensitivity | 55.6 | 42.3–68.1 |
| Specificity | 97.3 | 85.8–99.9 |
| Positive predictive value | 97.2 | 83.3–99.6 |
| Negative predictive value | 56.3 | 49.3–63.0 |
| Accuracy | 71.0 | 61.1–79.6 |
CI = confidence interval
Based on the results of the sequential question elimination, the final six-question checklist was proposed (shown in Figure 5).
Figure 5.
Proposed Feline Musculoskeletal Pain Screening Checklist (Feline MiPSC). DJD = degenerative joint disease
Comparison of proposed checklist sensitivity in DJD-informed owners
In order to assess how many cats with DJD-informed owners would be identified using the six-question checklist, we calculated the same measures for this cohort (DJD-informed cohort) using the six proposed questions. We found that just these six questions would identify almost all (~99%) of the cats with clinically confirmed DJD (Table 5). Additionally, the proposed checklist identified all 109 cats enrolled into our recent clinical trial of a therapeutic for DJD pain that used the FMPI (sensitivity of 100%).
Table 5.
Sensitivity, specificity, positive predictive value, negative predictive value and accuracy across the six proposed checklist questions
| Value (%) | 95% CI (%) | |
|---|---|---|
| Sensitivity | 98.9 | 96.2–99.9 |
| Specificity | 100.0 | 79.4–100.0 |
| Positive predictive value | 100.0 | – |
| Negative predictive value | 88.9 | 66.8–97.0 |
| Accuracy | 99.0 | 96.5–99.9 |
CI = confidence interval
Discussion
In this study, we used a data-driven approach for the development of a clinically useful checklist to identify cats that are likely to have DJD-associated pain. The proposed checklist represents a starting point for discussion with owners and further veterinary investigation. We believe this checklist can be quickly completed by owners, and if ‘no’ is selected in answer to any question, this can prompt further evaluation with a more detailed screening (review of video in the home environment, in-clinic observation and orthopedic evaluation) and a severity assessment/monitoring tool, such as the full FMPI. In addition, the checklist could serve as an important educational tool, especially when coupled with pictures or videos of cats with and without DJD pain while they are performing the activities of daily living. Indeed, our results showed a wide gap between the responses of the DJD-informed and DJD non-informed owners of cats with clinically confirmed DJD for many checklist behaviors. While other factors may be involved, we believe this gap also represents an opportunity for engagement and education of owners with adult and senior cats. As cats are most likely to perform these behaviors at home, rather than at the clinic, owner engagement is critical to the detection and diagnosis of DJD and associated pain.
Based on the data from the cohort of DJD non-informed owners, the sensitivity and specificity of the proposed checklist were approximately 55% and 97%, respectively. While this sensitivity may not seem high, given the prevalence of DJD in cats, many cats with undiagnosed DJD would still be identified using the proposed checklist without any owner education about DJD in cats. A recently released statistic serves as a useful example: based on prevalence data,1,4,8,9,21 in a group of 10,000 cats, it is estimated that 9000 would have radiographic evidence of DJD and 4500 would have clinically relevant pain. Prior to any education about DJD in cats, the checklist would identify almost 2500 cats. For comparison, current data provided by the Banfield Pet Hospital database found that in 2017, only 90 cats per 10,000 (0.9%) were seen because of arthritis (https://www.banfield.com/state-of-pet-health). When the checklist is coupled with education and engagement in watching for behavioral signs of DJD-associated pain, the detection of DJD should improve even more.
Of note, the specificity of the proposed checklist was >97% for both the DJD-informed and DJD non-informed owners. This high specificity is important as the checklist is not useful for veterinarians if it generates many false positives; at this level of specificity, if a cat scores abnormally on any of the questions, that cat is likely to have DJD-associated pain.One potential limitation of the proposed checklist is that two of the questions ask about stairs, while not all owners have stairs in their home. However, when sensitivity and specificity were calculated using only the four other questions, both remained essentially unchanged. This means that the lack of stairs in the home environment does not significantly affect the ability of the checklist to detect cats with DJD pain. However, these questions were retained in the proposed checklist as they are highly salient for owners who do have stairs. Another weakness of our study is that we did not evaluate the checklist in cats with other diseases. It is possible that positive responses to questions on the checklist would be generated for cats with cardiovascular or neurological disease, for example. The number of healthy non-DJD cats included in the present study was relatively low; while taken as a representative sample, only a few of these cats had other organ dysfunction. The specificity of the checklist for identifying DJD in cats in the DJD non-informed owner cohort suggests that the checklist is appropriate for routine clinical use as a screening tool, not as a diagnostic instrument. Further study in a broader clinical population will allow us to evaluate whether the presence of other organ dysfunction affects the results of the checklist.
Unlike sensitivity and specificity, PPV and NPV (the probability that a patient with a positive or negative screening test truly has or does not have the disease) are largely dependent on disease prevalence in an examined population. A test’s PPV increases and NPV decreases as the prevalence of a disease rises. 22 The PPV of the proposed checklist was >97% in both DJD-informed owners and DJD non-informed owners, which means that there were few false-positive screening test results. This is important as false-positive results can lead to unnecessary time, costs and discomfort for the cats. The NPV of the proposed checklist was moderate (56.3%) in the DJD non-informed cohort and strong (88.9%) in the DJD-informed cohort. This suggests that, especially when owners are not aware of the behavioral effects of DJD in cats, cats with negative results on the checklist may still have DJD; this also highlights the importance of owner education and engagement. The numerical value of accuracy represents the proportion of both true positives and negatives. The accuracy is also affected by the prevalence of disease (a lower prevalence is associated with improved accuracy). Although there are no data to compare our results against, the accuracy of the proposed checklist was 71% in the DJD non-informed cohort and 99% in the DJD-informed cohort, suggesting it is appropriate for the use as a ‘screening checklist’.
Several behaviors and characteristics that have been noted to be altered in cats with DJD-associated pain were not good at discriminating between the DJD pain and non-DJD cats. These include behaviors associated with aggression, handling, vocalization, seeking seclusion and use of a litter box. This is a logical finding, as cats can show these behaviors in the absence of DJD; these behaviors are associated with other factors, such as temperament and social and environmental stressors. 23 However, given that these behaviors are detectable by owners, and have been shown to improve with analgesic treatment, 24 these remain an important part of the discussion with owners. Whether or not a cat can perform these behaviors normally (as indicated on the checklist) may be less informative than whether there has been a change in these behaviors for an individual cat. Therefore, we suggest that the checklist is coupled with brief questions about changes in behaviors related to social and emotional wellbeing. 25
Conclusions
In the effort to increase awareness of this highly prevalent disease, the absence of a clinically useful, easily completed checklist to screen for cats with DJD-associated pain was a critical missing tool. By using data from well-phenotyped cats with and without DJD, we were able to design and test a set of six questions to fit this need. When coupled with educational tools designed to engage owners in monitoring their cats for behaviors associated with painful DJD, this proposed checklist will serve two purposes: first, it will be able to increase veterinarians’ ability to screen for DJD in a clinically expedient manner; second, it will provide a foundation for increasing awareness of DJD among cat owners. This initial screening can then be followed with other tools, such as the FMPI 11 or MI-CAT, 14 which are better suited for monitoring progression and response to treatment.
Supplemental Material
Supplemental material, Enomoto_Development_of_FMPC-_R1_-_Supplemental_File_1 for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery
Supplemental material, Enomoto_Development_of_FMPC-_R1_-_Supplemental_File_2 for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery
Supplemental material, Enomoto_Development_of_FMPC_Supplementary_Tables for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery
Acknowledgments
The authors acknowledge all the cat owners and referring veterinarians that participated in these studies. They especially thank Andrea Thomson for her assistance in conducting these studies.
Footnotes
Accepted: 28 January 2020
Supplementary material: The following files are available online:
Supplementary file 1: Development of FMPC – Feline Musculoskeletal Screening Checklist.
Supplementary file 2: Development of FMPC–Pain Behavior Visual Analog Scale Scoring (feline).
Table S1: Sensitivity, specificity, positive predictive value, negative predictive value and accuracy across nine questions selected (running, jump up, jump down, stairs up, stairs down, playing with other pets, chasing objects, playing with toys, height of jumping).
Table S2: Sensitivity, specificity, positive predictive value, negative predictive value and accuracy across eight questions (running, jump up, jump down, stairs up, stairs down, chasing objects, playing with toys, height of jumping).
Table S3: Sensitivity, specificity, positive predictive value, negative predictive value and accuracy across seven questions (running, jump up, jump down, stairs up, stairs down, chasing objects, height of jumping).
MEG and BDXL are both paid consultants for Zoetis. MEG has current funding from Zoetis for other (unrelated) research. BDXL has a $0 (zero dollar) collaborative research agreement with Zoetis for other (unrelated) work. Zoetis did not participate in the development of the concept nor design of the study, nor in the analysis of the data or writing of the manuscript.
Funding: This study was funded by Zoetis in the form of salary support for ME for analysis of previously collected data.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not necessarily required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. All prior studies from which the data were collected were performed with full owner consent. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Masataka Enomoto  https://orcid.org/0000-0003-2516-635X
https://orcid.org/0000-0003-2516-635X
B Duncan X Lascelles  https://orcid.org/0000-0002-2950-9009
https://orcid.org/0000-0002-2950-9009
Margaret E Gruen  https://orcid.org/0000-0002-6036-8849
https://orcid.org/0000-0002-6036-8849
References
- 1. Slingerland LI, Hazewinkel HA, Meij BP, et al. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011; 187: 304–309. [DOI] [PubMed] [Google Scholar]
- 2. Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–445. [DOI] [PubMed] [Google Scholar]
- 3. Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Feline Med Surg 2009; 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lascelles BD, Henry JB, 3rd, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 5. Taylor PM, Robertson SA. Pain management in cats – past, present and future. Part 1. The cat is unique. J Feline Med Surg 2004; 6: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett D, Zainal Ariffin SM, Johnston P. Osteoarthritis in the cat: 1. how common is it and how easy to recognise? J Feline Med Surg 2012; 14: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lascelles BD. Feline degenerative joint disease. Vet Surg 2010; 39: 2–13. [DOI] [PubMed] [Google Scholar]
- 8. Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec 2005; 157: 793–799. [DOI] [PubMed] [Google Scholar]
- 9. Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J Am Vet Med Assoc 2002; 220: 628–632. [DOI] [PubMed] [Google Scholar]
- 10. Suter E, Herzog W, Leonard TR, et al. One-year changes in hind limb kinematics, ground reaction forces and knee stability in an experimental model of osteoarthritis. J Biomech 1998; 31: 511–517. [DOI] [PubMed] [Google Scholar]
- 11. Benito J, Hansen B, Depuy V, et al. Feline Musculoskeletal Pain Index: responsiveness and testing of criterion validity. J Vet Intern Med 2013; 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 12. Gingerich DA, Strobel JD. Use of client-specific outcome measures to assess treatment effects in geriatric, arthritic dogs: controlled clinical evaluation of a nutraceutical. Vet Ther 2003; 4: 376–386. [PubMed] [Google Scholar]
- 13. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of Client-Specific Outcome Measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 14. Klinck MP, Monteiro BP, Lussier B, et al. Refinement of the Montreal Instrument for Cat Arthritis Testing, for use by veterinarians: detection of naturally occurring osteoarthritis in laboratory cats. J Feline Med Surg 2018; 20: 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS One 2015; 10: e0131839. DOI: 10.1371/journal.pone.0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruen ME, Thomson AE, Griffith EH, et al. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Intern Med 2016; 30: 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gruen ME, Alfaro-Cordoba M, Thomson AE, et al. The use of functional data analysis to evaluate activity in a spontaneous model of degenerative joint disease associated pain in cats. PLoS One 2017; 12: e0169576. DOI: 10.1371/journal.pone.0169576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease-associated pain in cats. Am J Vet Res 2010; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 19. Gruen ME, Messenger KM, Thomson AE, et al. Evaluation of serum cytokines in cats with and without degenerative joint disease and associated pain. Vet Immunol Immunopathol 2017; 183: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis 1978; 37: 378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godfrey DR. Osteoarthritis in cats: a retrospective radiological study. J Small Anim Pract 2005; 46: 425–429. [DOI] [PubMed] [Google Scholar]
- 22. Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Health 2017; 5: 307. DOI: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellis SL, Rodan I, Carney HC, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg 2013; 15: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klinck MP, Rialland P, Guillot M, et al. Preliminary validation and reliability testing of the Montreal Instrument for Cat Arthritis Testing, for use by veterinarians, in a colony of laboratory cats. Animals (Basel) 2015; 5: 1252–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noble CE, Wiseman-Orr LM, Scott ME, et al. Development, initial validation and reliability testing of a web-based, generic feline health-related quality-of-life instrument. J Feline Med Surg 2019; 21: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Enomoto_Development_of_FMPC-_R1_-_Supplemental_File_1 for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery
Supplemental material, Enomoto_Development_of_FMPC-_R1_-_Supplemental_File_2 for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery
Supplemental material, Enomoto_Development_of_FMPC_Supplementary_Tables for Development of a checklist for the detection of degenerative joint disease-associated pain in cats by Masataka Enomoto, B Duncan X Lascelles and Margaret E Gruen in Journal of Feline Medicine and Surgery