Abstract
Objectives
The aim of this study was to describe the causes, clinicopathologic features and outcomes of feline protein-losing nephropathy (proteinuria secondary to glomerular disease [PLN]).
Methods
Kidney biopsy/necropsy samples from proteinuric cats submitted to the International Veterinary Renal Pathology Service were retrospectively reviewed. Diagnoses based on histopathology were categorized by primary disease compartment. Clinicopathologic variables at diagnosis, development of hypoalbuminemia, anemia, hypertension, azotemia and effusion/edema, and survival were compared between cats with immune-complex glomerulonephritis (ICGN) and other causes of PLN.
Results
Fifty-eight percent (n = 31/53) of proteinuric cats had ICGN and 74% (n = 31/42) of cats with PLN had ICGN. Cats with glomerular diseases other than ICGN had a higher median urine protein:creatinine ratio than ICGN cats (14.5 vs 6.5; P <0.001). Onset of PLN occurred at a young age; median age at diagnosis was 3.5 years in ICGN cats vs 1.3 years in cats with other glomerular diseases (P = 0.026). Development of complications such as hypoalbuminemia, anemia, hypertension, azotemia and effusion/edema were common, regardless of the cause of PLN, and were not different between ICGN and cats with other glomerular diseases. Male cats were over-represented in the ICGN group (P = 0.003). Median survival time (MST) for all cats with PLN was 94 days (range 3–1848 days). Survival was not different between cats with ICGN and cats with other glomerular diseases. MST in ICGN cats that developed effusion was shorter (94 days) than cats that did not (700 days; P = 0.035). MST in IGCN cats that received immunosuppressive medications was longer (244 days) than cats that did not (17 days, P = 0.039).
Conclusions and relevance
Taken together, these data suggest that clinical suspicion for glomerular proteinuria should increase in young, male cats with higher degrees of proteinuria, and immune-mediated disease is common. Further studies are needed to determine the effect of immunosuppression on morbidity and mortality in cats with ICGN.
Keywords: Proteinuria, glomerular disease, renal biopsy, protein-losing nephropathy, ICGN, immune-complex glomerulonephritis
Introduction
Renal proteinuria in dogs and cats can be caused by both tubular and glomerular diseases, and has been associated with the development of azotemia, progression of disease and decreased survival.1–4 Primary glomerular disease, clinically referred to as protein-losing nephropathy (PLN), is the result of abnormalities in or damage to the glomerular filtration barrier and is more common in canine patients. 5 In contrast, PLN is relatively uncommon in cats, and feline chronic kidney disease (CKD) is more classically characterized as tubulointerstitial (TI) inflammation and fibrosis.6,7 In the setting of CKD, proteinuria is typically secondary to tubular dysfunction and glomerulosclerosis, which increase in prevalence with disease severity. 6
In dogs, causes and outcomes of PLN have been previously described.1,8–10 PLN can be further subcategorized as immune-mediated vs non-immune, and approximately 50% of dogs biopsied for suspected glomerular disease have immune complex deposition within the glomerulus of the kidney (immune-complex glomerulonephritis; ICGN).8,9 In these cases, treatment options are not limited to symptomatic therapies aimed at decreasing proteinuria (ie, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers). Instead, canine ICGN can be treated with immunosuppressive agents to directly target the underlying immune-mediated process. Although no prospective studies exist evaluating immunosuppressive therapy in canine or feline ICGN, in human medicine immunosuppressive protocols are known to induce remission of ICGN and reduce the chance of progression to end-stage renal failure. 11
Currently, there are relatively few data available regarding the presentation and expected outcomes of feline PLN, as it is much less common in cats than in dogs. Therefore, the primary aim of this study was to describe the population, clinical features, clinicopathologic abnormalities and underlying pathologic diagnoses associated with renal proteinuria by retrospectively evaluating feline renal samples that had been submitted to the International Veterinary Renal Pathology Service (IVRPS). A secondary aim was to assess clinical and survival outcomes in cats with PLN. We hypothesized that the percentage of proteinuric cases caused by immune-complex diseases would be similar in cats and dogs, and that development of sequelae such as anemia, azotemia, hypertension, peripheral edema or ascites, hypercholesterolemia and hypoalbuminemia would be common in cats with PLN.
Materials and methods
Feline renal samples submitted to the IVRPS from 12 January 2006 to 1 December 2017 were retrospectively reviewed. Cats with measured urine protein:creatinine ratios (UPC) classified as proteinuric (UPC >0.4) or borderline proteinuric (UPC 0.2–0.4) according to International Renal Interest Society (IRIS) staging guidelines were considered eligible for inclusion in the study. 12
Diagnoses were based on comprehensive evaluation of tissue, including histopathology (hematoxylin and eosin) with special stains (Periodic acid–Schiff, Masson’s trichrome, Jones methenamine silver [with or without Congo red as indicated]). Transmission electron microscopy (TEM) and immunofluorescence for IgG, IgM, IgA and Lambda light chain were also performed, with the exception of 11 cats that did not have TEM performed as it was not considered necessary for the diagnosis (amyloidosis, n = 2; renal lymphoma, n = 1; collagenofibrotic glomerulopathy, n = 1; membranous glomerulonephritis [MGN] or membranoproliferative glomerulonephritis [MPGN] in which definitive evidence of immune deposits were readily identifiable on trichrome and silver stains on light microscopy, n = 7).
The tissue evaluations were performed with the routine methods used by the IVRPS for diagnosis, which have been described in detail elsewhere. 9 Briefly, in addition to microscopic examination to diagnose the glomerular lesions, biopsies were evaluated for interstitial fibrosis and lipid. Biopsies were assessed for the presence of TI fibrosis, confirmed with Masson’s trichrome stain, and severity was scored based on a semiquantitative scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). Lastly, additional microscopic features included the presence of lipid, free within the interstitium and/or within tubular lumens (ie, casts). Glomerulosclerosis was defined effacement of glomerular capillary lumens by extracellular matrix with consolidation of the tuft. It was further characterized as segmental (<75% of the tuft is consolidated) or global (>75% of the tuft is affected). The percentage of globally sclerotic glomeruli in the sample was recorded.
The IVRPS database was searched for signalment, weight, packed cell volume (PCV) or hematocrit, blood urea nitrogen (BUN), creatinine, albumin, cholesterol, urine specific gravity (USG), UPC and systolic blood pressure (SBP); these values were recorded if available. When possible, referring veterinarians were contacted for follow-up information on clinicopathologic data, treatments attempted, disease progression and overall survival. Diagnostic test results were reported for the results obtained closest to times of initial diagnosis and biopsy/necropsy. Values were included if they occurred within 8 weeks of these events; otherwise, values were reported as unknown. For some cats, data at diagnosis of proteinuria and data at biopsy were the same, as diagnosis and biopsy occurred closely together in time.
Cats were staged according to IRIS CKD guidelines based on available clinicopathologic data. 12 If follow-up information was available, the medical record was examined to determine whether disease progression to IRIS CKD stage 2 or greater, hypertension, anemia, hypoalbuminemia, hypercholesterolemia and/or cavitary effusion/peripheral edema developed within the cat’s lifetime. Hypoalbuminemia, hypercholesterolemia and anemia were defined according to the normal ranges of the reference laboratories where laboratory work was performed. If reference intervals were not available (ie, spun PCV was performed rather than complete blood count), anemia was defined as a PCV <30%. Hypertension was defined as SBP >160 mmHg as measured via Doppler or oscillometric methods. If the patient was hypertensive at any point during their renal disease, they were considered hypertensive, even if their hypertension was later controlled with medical management. Cats that were not identified as hypertensive but which had medical records indicating that they were receiving amlodipine were also defined as hypertensive. Cavitary effusion or peripheral edema was considered present if noted in the medical record on physical examination or diagnostic imaging.
Date of diagnosis of proteinuria was defined as the first day proteinuria was documented on urinalysis, microalbuminuria testing or UPC in the absence of known pre- or post-renal contributions such as pyuria or a positive urine culture. Presence of bacteria on microscopic urine sediment was not considered significant unless accompanied by >2–5 white blood cells per high-powered field or a concurrent positive urine culture; when present, these cats were excluded from the study. If a patient was known to be deceased, but the exact date of death was not noted in the record, the date of death was defined as the date of the patient’s last recheck evaluation. Survival was defined as the number of days from the date of diagnosis to the date of death or last known follow-up. Patient age at diagnosis and death/last follow-up were also obtained from the medical record, if available.
Continuous variables were assessed for normality via visual assessment of histograms and Shapiro–Wilk tests. Normally distributed variables (weight at sampling) are reported as mean ± SD. Non-normally distributed variables are reported as median (range [ie, minimum–maximum]). Owing to the small number of cats with TI disease and the variability in etiology (eg, obstruction, acute tubular injury), statistical comparison was not performed between groups with PLN vs TI disease. Sex distribution of ICGN cats was evaluated for male or female predilection via a 1 × 2 contingency Fisher’s exact test (http://www.kisnet.or.jp/nappa/software/star-e/freq/1x2.htm). Continuous and ordinal variables were compared between cats with ICGN and cats with all other types of glomerular diseases with a Mann–Whitney U-test. Categorical variables were compared between cats with ICGN and cats with all other glomerular diseases via Fisher’s exact test. Kaplan–Meier analysis was used to calculate median survival time from proteinuria diagnosis, as well as median age at diagnosis and death or last known follow-up. Cats that were still alive at the time of manuscript preparation or lost to follow-up were right-censored at time of last known follow-up. A log-rank test was used to compare survival between cats with ICGN and cats with other glomerular diseases and survival of cats according to medications received, degree of TI scarring and severity of glomerulosclerosis. Two cats with ICGN had renal transplants and were excluded from survival analysis. Statistics were performed with commercial statistics software (SPSS [IBM]; Prism 7 [GraphPad Software]).
Results
Signalment, clinicopathologic data, microscopic diagnoses and sequelae
In total, 164 feline renal biopsies were submitted to the IVRPS for review during the study period. One hundred and six cats were excluded for the following reasons: no UPC reported (n = 95); no clinicopathologic data within 8 weeks of biopsy (n = 2); UPC <0.2 (n = 4); lack of TEM prevented definitive diagnosis (n = 2); proteinuria suspected to be pre-renal secondary to a hepatic plasma cell tumor (n = 1); microscopy performed only on paraffin-embedded samples as a second opinion (n = 1); and incomplete database information (n = 1). A total of 58 cats were included for retrospective analysis. Of these, five cats were borderline proteinuric and 53 cats were proteinuric. Four borderline proteinuric cats had primary diseases in the tubular compartment; the remaining cat had disease in both the TI and glomerular compartments.
Of the cats included (n = 58), 15 were females (two intact) and 43 were males (two intact). Represented breeds included domestic shorthair (n = 34), domestic longhair (n = 3), Maine Coon (n = 3), Siamese (n = 2), domestic mediumhair (n = 2), Burmese (n = 2), Abyssinian (n = 2) and one each of American Shorthair, Bengal, Birman, Devon Rex, Oriental, Persian, Ragdoll and mixed-breed cats. Information on breed was unavailable for two cats. Forty-four cats were located in the USA, and 14 cats were located outside of the USA (UK, n = 10 cats; Canada, n = 2 cats; Australia, n = 1 cat; Taiwan, n = 1).
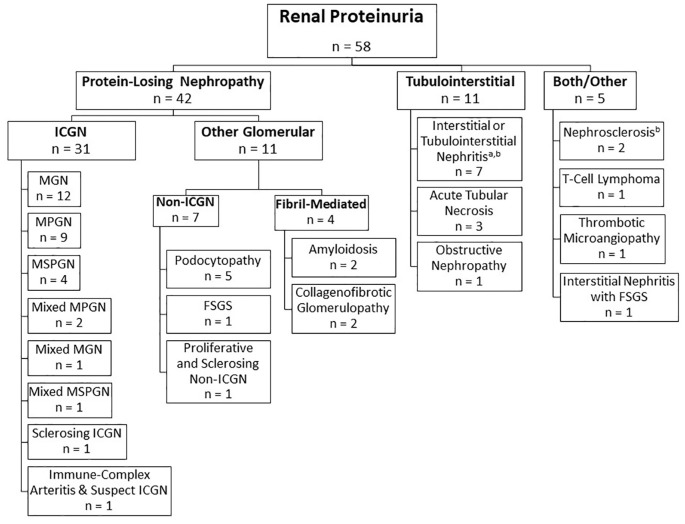
Disease categories are classified according to primary disease compartment in Figure 1. Overall, considering only cats with a UPC >0.4, 79% (n = 42/53) had primary glomerular diseases (meeting our definition of PLN) and 58% (31/53 cats) of proteinuric cats had ICGN. Thus, 74% (31/42 cats) of cats with PLN were diagnosed with ICGN.
Figure 1.
Histopathologic classifications of feline proteinuric disease. Cats are divided into subgroups according to the disease compartment containing the primary histopathologic lesion. Both/other category refers to cats in which there are apparent primary lesions in both compartments, or in which the primary lesion cannot be determined. aFive cats with tubulointerstitial nephritis had glomerular obsolescence (n = 1), glomerulosclerosis (n = 3) or nephrosclerosis (n = 1), which was secondary to disease in the tubulointerstitial compartment. bSome cats in these categories had only borderline proteinuria. MGN = membranous glomerulonephritis; MPGN = membranoproliferative glomerulonephritis; MSPGN = mesangioproliferative glomerulonephritis; ICGN = immune-complex glomerulonephritis; FSGS = focal segmental glomerulosclerosis
Signalment, weight, clinicopathologic characteristics and development of secondary pathologies (hypoalbuminemia, anemia, hypertension, azotemia and effusion/edema, etc) for cats with PLN vs cats with TI disease are presented in Table 1. These characteristics are compared between cats with ICGN and cats with other glomerular diseases in Table 2. There were significant differences in age at initial diagnosis of proteinuria, weight at time of biopsy or necropsy, and UPC at time of biopsy or necropsy between cats with ICGN and cats with other glomerular diseases (Table 2). A significant male predilection was identified for ICGN (n = 24/31 cats, P = 0.003), and specifically for MGN) (n = 10/12 cats; P = 0.02). Cats with glomerular diseases other than ICGN had a higher median UPC than ICGN cats (14.5 vs 6.5; P <0.001), as well as a younger age at diagnosis (1.3 vs 3.5 years; P = 0.015) and lower body weight (3.0 vs 4.0 kg; P = 0.003). No significant differences were identified in BUN, creatinine, cholesterol, USG, albumin, PCV or SBP at the time of sampling between cats with ICGN and cats with other glomerular diseases. No significant differences were identified in the lifetime development of hypertension, hypoalbuminemia, hypercholesterolemia, anemia, disease progression to IRIS CKD stage 2 or greater, or the development of ascites/effusion or peripheral edema between ICGN and cats with other types of glomerular diseases.
Table 1.
Signalment and clinicopathologic variables of cats with primary tubulointerstitial (TI) disease vs cats with protein-losing nephropathy (PLN)
| TI, borderline (n = 4) |
Range | TI, proteinuric (n = 7) |
Range | PLN (n = 43) |
Range | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female spayed | 1 | – | 2 | − | 10 | − |
| Female intact | − | – | − | − | 1 | − |
| Male castrated | 4 | – | 4 | − | 31 | − |
| Male intact | − | – | 1 | – | 1 | − |
| Age at proteinuria diagnosis (years) | 12.8 (4) | 2.5–16.9 | 2.0 (7) | 0.3–16.9 | 3.3 (39) | 0.2–9.9 |
| Weight at biopsy/necropsy (kg) | 3.8 (3) | 3.4–4.4 | 3.7 (3) | 1.7–4.2 | 3.7 (18) | 0.8–7.0 |
| Sample type | ||||||
| Biopsy | 3 | – | 3 | − | 34 | − |
| Necropsy | 1 | 4 | − | 8 | − | |
| Values at biopsy/necropsy | ||||||
| PCV or HCT (%) | 28 (4) | 21–33 | 32 (5) | 20–33 | 24 (31) | 12–40 |
| BUN (mg/dl) | 46 (4) | 33–153 | 114 (7) | 42–220 | 69 (30) | 20–358 |
| Creatinine (mg/dl) | 2.5 (4) | 1.3–7.4 | 7.5 (7) | 2.4–22.5 | 2.6 (42) | 0.7–16.3 |
| Albumin (g/dl) | 3.4 (4) | 2.8–3.6 | 3.1 (6) | 2.3–3.7 | 2.1 (41) | 1.1–3.6 |
| Cholesterol (mg/dl) | 149 (3) | 129–200 | 219 (3) | 183–223 | 250 (24) | 120–423 |
| SBP (mmHg) | 132 (4) | 105–202 | 138 (5) | 120–170 | 150 (37) | 74–240 |
| USG | 1.014 (5) | 1.012–1.052 | 1.017 (4) | 1.012–1.024 | 1.020 (26) | 1.010–1.050 |
| UPC | 0.26 (4) | 0.2–0.3 | 2.2 (7) | 1.0–5.8 | 8.38 (42) | 0.45–30 |
| Cats that developed | ||||||
| Anemia | 3/4 | − | 4/6 | − | 29/33 | − |
| ⩾IRIS stage 2 CKD | 4/4 | − | 7/7 | − | 34/42 | − |
| Hypoalbuminemia | 0/4 | − | 2/6 | − | 34/41 | − |
| Hypercholesterolemia | 0/3 | − | 1/3 | − | 14/25 | − |
| Hypertension | 1/4 | − | 4/6 | − | 22/37 | − |
| Effusion/edema | 0/3 | − | 1/6 | − | 22/37 | − |
Values are n or median (n). Cats where primary disease compartment could not be determined are not represented (n = 5)
PCV = packed cell volume; HCT = hematocrit; BUN = blood urea nitrogen; SBP = systolic blood pressure; USG = urine specific gravity; UPC = urine protein:creatinine ratio; IRIS = International Renal Interest Society; CKD = chronic kidney disease
Table 2.
Signalment, clinicopathologic variables of cats with immune-complex glomerulonephritis (ICGN) vs cats with other glomerular diseases
| ICGN (n = 31) |
Range | Other glomerular (n = 11) |
Range | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female spayed | 7 | − | 3 | − | − |
| Female intact | − | − | 1 | − | − |
| Male castrated | 24 | − | 6 | − | − |
| Male intact | − | − | 1 | − | − |
| Age at diagnosis (years) | 3.5 (30) | 1.7–9.9 | 1.3 (9) | 0.2–5.7 | 0.015 |
| Weight at biopsy/necropsy (kg) | 4.0 (12) | 3.1–4.9 | 3.0 (6) | 0.8–4.1 | 0.003 |
| Sample type | |||||
| Biopsy | 26 | − | 9 | − | − |
| Necropsy | 5 | − | 3 | − | − |
| Values at biopsy/necropsy | |||||
| PCV or HCT (%) | 24 (23) | 12–36 | 24 (8) | 15–40 | 0.557 |
| BUN (mg/dl) | 66 (22) | 20–358 | 77 (8) | 21–234 | 0.559 |
| Creatinine (mg/dl) | 2.4 (31) | 0.7–13.0 | 4.4 (11) | 0.8–16.3 | 0.616 |
| Albumin (g/dl) | 2.1 (30) | 1.1–3.6 | 2.1 (11) | 1.4–2.8 | 0.699 |
| Cholesterol (mg/dl) | 242 (16) | 120–418 | 258 (8) | 179–423 | 0.664 |
| SBP (mmHg) | 151 (26) | 110–240 | 140 (11) | 74–180 | 0.433 |
| USG | 1.020 (19) | 1.010–1.050 | 1.021 (7) | 1.011–1.049 | 0.854 |
| UPC | 6.5 (31) | 1.81–13.3 | 14.5 (11) | 10.1–30 | <0.001 |
| Cats that developed: | |||||
| Anemia | 21/25 | − | 8/8 | − | 0.550 |
| ⩾IRIS stage 2CKD | 25/31 | − | 9/11 | − | 1.000 |
| Hypoalbuminemia | 24/30 | − | 10/11 | − | 0.651 |
| Hypercholesterolemia | 10/17 | − | 4/8 | − | 1.000 |
| Hypertension | 17/26 | − | 5/11 | − | 0.295 |
| Effusion/edema | 17/27 | − | 5/10 | − | 0.708 |
Values are n or median (n). Continuous variables compared via Mann–Whitney U-test; categorical variables compared via Fisher’s exact test
PCV = packed cell volume; HCT = hematocrit; BUN = blood urea nitrogen; SBP = systolic blood pressure; USG = urine specific gravity; UPC = urine protein:creatinine ratio; IRIS = International Renal Interest Society; CKD = chronic kidney disease
Secondary microscopic features
Fifty-seven cats had data available regarding the degree of TI fibrosis and presence of lipid in the tubules and/or the interstitium (one cat had light microscopy performed at its presenting institution, and TEM was performed on the glomerular compartment only). All 58 cats had data available regarding the degree of global glomerulosclerosis. The degree of TI fibrosis and global glomerulosclerosis varied widely among the cats. There was no statistically significant relationship between the primary disease process (eg, ICGN) and the severity of TI fibrosis in the biopsy sample. Approximately one-quarter of renal biopsy samples from proteinuric/borderline proteinuric cats contained free lipid within the interstitium and approximately two-thirds contained lipid casts with tubular lumens.
Disease management and survival
Twenty cats did not have follow-up data available beyond the time of biopsy or did not have a known date of proteinuria diagnosis; thus, survival data were only available for 40 cats (14 cats with necropsy submissions and 26 cats with biopsy submissions). Additionally, two cats that received renal transplants at the time of biopsy were excluded from the survival analysis, leaving 38 cats that were ultimately included in the survival analysis. Seven of these 38 cats were censored (four owing to loss to follow-up and three still alive at time of writing).
Age at initial diagnosis of proteinuria was known for 52 of all cats with proteinuria/borderline proteinuria; median age at diagnosis was 3.3 years (range 0.2–16.9 years). Age at death or last follow-up was known for 40 cats; median age was 4.8 years (range 0.3–19.2 years). Median survival time of all included cats (n = 38) was 94 days (range 0–1888 days) from initial diagnosis. Survival information for cats with TI lesions was not reported owing to the small number of cases and wide variety of associated disease processes (CKD, AKI, obstruction).
Median age at diagnosis for cats with PLN (n = 40) was 3.3 years (range 0.2–9.9 years), and median age at death or last follow-up (n = 26) was 4.6 years (range 0.4–9.9 years). For cats with PLN that were included in the survival analysis (n = 26), median survival from proteinuria diagnosis was 94 days (range 3–1848 days). There was no difference in survival for cats with PLN that developed azotemia, anemia, hypertension, hypercholesterolemia, hypoalbuminemia or peripheral edema/cavitary effusion vs cats that did not. However, the development of these sequelae was very common, so the small number of cats that did not experience these secondary effects may preclude meaningful comparison.
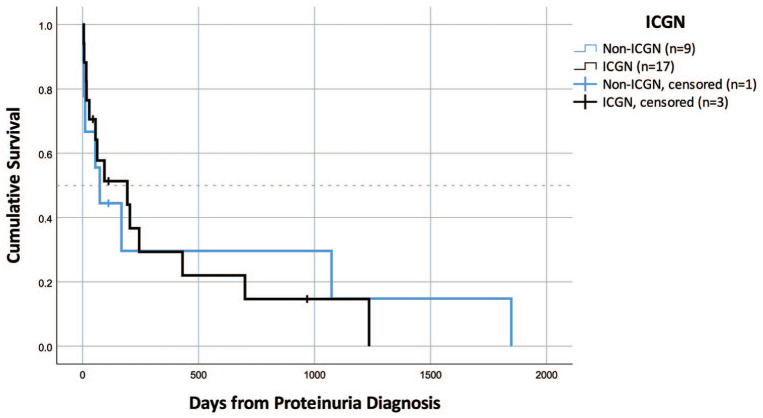
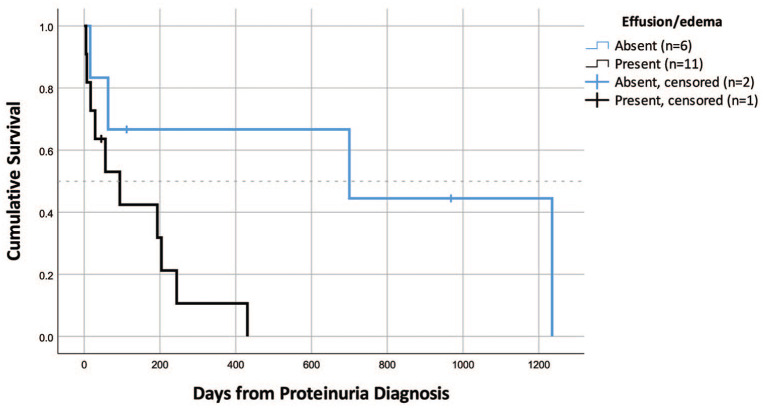
Survival data were available for 17 cats with ICGN and nine cats with other glomerular diseases. Median survival time for cats with ICGN was 193 days (range 5–1235 days) vs 74 days for cats with other glomerular diseases (range 3–1848 days); this difference was not statistically significant (Figure 2). Cats with ICGN that developed peripheral edema or cavitary effusion had shorter median survival times (MSTs; n = 11, MST = 94 days [range 5–431 days]) than cats that did not (n = 6, MST = 700 days [range 16–1235 days]; P = 0.035 [Figure 3]). There was no difference in survival for cats with ICGN that developed azotemia, anemia, hypertension, hypercholesterolemia or hypoalbuminemia, compared with cats that did not; however, again, the small number of cats that did not develop these sequelae may have under-powered this analysis.
Figure 2.
Survival in cats with immune-complex glomerulonephritis (ICGN) vs cats with other glomerular diseases (n = 26). There was no difference in median survival time between these groups
Figure 3.
Survival in cats with immune-complex glomerulonephritis (ICGN) based on presence or absence of peripheral edema or cavitary effusion (n = 17). Cats with effusions had a significantly shorter median survival time than cats without effusions (P = 0.035)
When the potential impact of TI and glomerular scarring on survival was assessed in cats with PLN and a known survival time, there was no significant difference in survival time between cats with ⩽25% sclerotic glomeruli (n = 22, MST = 94 days) and >25% sclerotic glomeruli (n = 4, MST = 63 days). Additionally, no survival difference was identified between cats with none-to-mild TI fibrosis (n = 13, MST = 193 days) vs moderate-to-severe TI fibrosis (n = 10, MST = 74 days). Two cats with primary glomerular disease (histopathologic diagnosis of MGN and MPGN) were still alive at the time of writing (survival 968 and 1184 days from diagnosis, respectively). The cat with MPGN that survived at least 1184 days had had a renal transplant, so this cat is not included in any survival analysis. An additional two cats with primary TI disease (acute tubular necrosis and one with interstitial nephritis) were still alive at last known follow-up (survival times 1511 and 492 days, respectively).
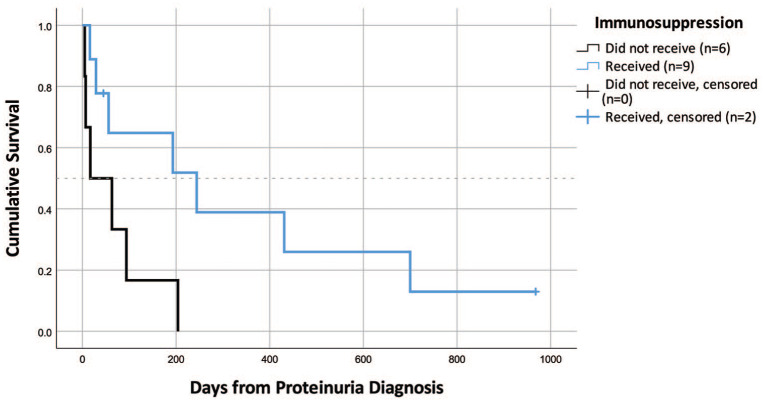
Data on medications administered before and/or after sampling were available for 52 cats (26 ICGN cats; nine other glomerular disease cats; 12 TI cats; 5 both/other cats). Cats received a median of four different categories of medications or therapies (range 1–28); the 20 most common medications or therapies administered are presented in Table 3. Fifteen cats with ICGN had sufficient data available to determine survival and whether immunosuppressive therapy was attempted. Of these, nine received immunosuppressive medications and six did not. Medications prescribed included prednisolone (n = 6), dexamethasone sodium phosphate (n = 1), ciclosporin (n = 5), chlorambucil (n = 4) and mycophenolate (n = 1). Four ICGN cats received steroids in combination with, or followed by, other immunosuppressive medications. Those that received immunosuppressive medications had a median survival time of 244 days vs 17 days for the cats that had ICGN but did not receive immunosuppressive medications (Figure 4). This difference was statistically significant (P = 0.039).
Table 3.
Twenty most commonly administered medications based on follow-up data (n = 52)
| Medication | Total cats (n = 52) |
ICGN (n = 26) |
Other glomerular (n = 8) |
TI (n = 11) |
Both/other (n = 5) |
|---|---|---|---|---|---|
| Antibiotics | 32 | 17 | 6 | 7 | 2 |
| Renal diet | 24 | 13 | 4 | 7 | – |
| Benazepril | 16 | 12 | 3 | 1 | – |
| Intravenous fluids | 14 | 8 | 2 | 4 | – |
| Aluminum hydroxide | 13 | 7 | 4 | 2 | – |
| Glucocorticoids | 12 | 7 | 2 | 2 | 1 |
| Subcutaneous fluids | 11 | 5 | 2 | 2 | 2 |
| Mirtazapine | 10 | 4 | 2 | 4 | – |
| Famotidine | 8 | 1 | 4 | 2 | 1 |
| Amlodipine | 8 | 3 | 2 | 2 | 1 |
| Enalapril | 8 | 4 | 2 | 2 | – |
| Maropitant | 8 | 4 | 2 | 2 | – |
| Blood transfusion | 8 | 6 | 2 | – | – |
| Buprenorphine | 7 | 6 | 1 | – | – |
| Ciclosporin | 7 | 5 | 1 | – | 1 |
| Potassium gluconate | 4 | 1 | 1 | 2 | – |
| Cobalamin | 4 | 2 | 1 | 1 | – |
| Omeprazole | 4 | 4 | – | – | – |
| Clopidogrel | 4 | 4 | – | – | – |
| Chlorambucil | 4 | 4 | – | – | – |
ICGN = immune-complex glomerulonephritis; TI = tubulointerstitial nephritis
Figure 4.
Survival in cats with immune-complex glomerulonephritis (ICGN) based on whether or not immunosuppressive therapies were administered. ICGN cats that did not receive immunosuppressive therapies had a significantly shorter median survival time than cats that did (P = 0.039)
Discussion
This study described the underlying pathologies, clinicopathologic characteristics and outcomes of feline PLN. In dogs, approximately 50% biopsied for a suspicion of glomerular disease had ICGN.8,9 The present study revealed similar percentages in cats, with 31/60 (52%) of proteinuric cats having a diagnosis of ICGN. However, when only cats with PLN (defined as proteinuria due to primary glomerular disease) were considered, 72% of cats were diagnosed with ICGN. It is important to note that PLN, as defined here, required a biopsy to determine whether or not the glomerulus was the primary compartment that was injured. The UPC values ranged widely in both the PLN cats and cats with primary TI disease and differentiating between the two groups was not possible based on clinicopathologic values alone.
MGN, a complement-driven inflammatory disease in which immune complexes are deposited on the abluminal (subepithelial) side of the glomerular basement membrane, was the most common cause of feline PLN. This finding is consistent with the available literature on ICGN in cats, in which MGN is described more often than other causes.13,14 An apparent male predilection exists for MGN; in the present study, 10/12 affected cats were males, similar to 8/11 and 11/18 affected cats described in previous studies.10,14 MPGN was the second-most common cause of feline PLN in this study, similar to a previous report from Europe. 10 MPGN differs from MGN in that immune complexes are deposited beneath the endothelium, and in other species may be a primary or post-infectious, paraneoplastic or drug-related autoimmune disease. 5 In contrast to studies performed in dogs, focal segmental glomerulosclerosis was a relatively rare finding in our population of cats. 9
The signalment of the cats described in this study differs greatly from the ‘typical’ population of cats with CKD. In a study evaluating histopathologic changes in the kidneys of cats with CKD due to TI nephritis, the mean age at death of cats with IRIS stage 1–4 CKD ranged from 11 to 15 years. 6 In contrast, the median age at death for cats with PLN described in the current study was 4.62 years. In a retrospective study evaluating European cats with ICGN, a much older population of cats was identified (median age 8.9 years). 10 This disagreement in signalment associated with ICGN underscores the need for further identification and study of this disease process in feline patients.
PLN is traditionally thought to cause higher-magnitude proteinuria than tubular diseases, and previous consensus recommendations cite UPC values higher than 2.0 as consistent with a glomerular cause of proteinuria.15,16 However, more recent data indicate that this cut-off is not reliable. In the study evaluating histopathologic changes in the kidneys of cats with CKD due to TI nephritis, 24/33 cats with UPC data available had a UPC <0.4; however, three cats with IRIS stage 3 or 4 CKD had a UPC >2.0 (review of raw data). 6 Additionally, in a study that compared cats with ICGN with cats without ICGN (including both cats with non-ICGN glomerular disease, as well as cats with TI nephritis), a UPC cut-off of >3.8 was necessary to distinguish ICGN from other causes of proteinuria. 10 Both studies suggest that higher UPCs are more likely to be glomerular in origin but that this is not an absolute rule. Similarly, in the present study, the median UPC for cats with PLN was 7.9 vs 1.1 in cats with TI disease. However, one cat with PLN (specifically ICGN) had a UPC as low as 0.45 and a cat with TI disease had a UPC as high as 5.8. Specifically, 4/12 (33%) cats with TI primary disease in this study had a UPC >2.0, and 5/43 (12%) cats with primary glomerular lesions had UPCs <2.0. Two cats in the TI disease category with a UPC >2.0 were diagnosed with acute tubular necrosis; the other two were diagnosed with interstitial nephritis, one of which had progressed to the point of having secondary glomerulosclerosis. Four of the five cats in the glomerular category with a UPC <2.0 had ICGN; the other cat had focal segmental glomerulosclerosis. Based on our results, the authors advocate renal biopsy as the only currently available diagnostic test that can definitely distinguish glomerular from tubular proteinuria. Taken together, our data suggest that clinical suspicion for PLN should increase in young male cats with significantly elevated renal proteinuria but that a cut-off UPC value for distinguishing glomerular from tubular proteinuria would lack sensitivity and specificity.
Based on this study, the prognosis for cats with PLN is grave, with median survival times of <14 months from diagnosis, regardless of the underlying cause. The short survival period after diagnosis may indicate that these diseases progress rapidly once they develop. Alternatively, this may indicate that cats are not diagnosed until quite late in the disease process, when much of the damage to the kidney may no longer be reversible. Potentially, earlier identification of feline PLN, prompting diagnostics such as renal biopsy, supportive therapies and immunosuppression, where indicated, might greatly improve the outcomes for some of these cats. This underscores the great importance of performing screening urinalyses, even in seemingly healthy cats with normal clinicopathologic values.
The population of cats with PLN in this study had variable severity of glomerulosclerosis and TI scarring, both of which are irreversible lesions. 17 Although the relationship between renal parenchymal scarring and progression to end-stage renal disease is documented in humans, a similar association could not be verified in this cohort. 18 Specifically, cats with a greater severity of glomerulosclerosis and TI fibrosis did not have statistically different median survival times. It is unclear whether the study population was simply too small to correctly identify this association. Alternatively, outcome data in humans with end-stage renal disease leading to dialysis and/or transplantation might not be applicable to veterinary medicine where options for euthanasia exist.
Renal biopsy is recommended in canine patients where primary glomerular disease is suspected to identify cases that may respond to immunosuppression, 16 and given the incidence of ICGN in cats, this recommendation is appropriate for this species as well. In the present study, cats with ICGN that received immunosuppressive medications survived longer than cats that did not receive immunosuppression (244 vs 17 days; P = 0.039). Given the retrospective nature of this study, it is possible that confounding factors contributed to this difference. Prospective studies regarding the effect of immunosuppression on prognosis are needed to confirm the efficacy of immunosuppressive protocols in cats with ICGN.
Problematically, however, the patient may not be stable enough for renal biopsy to be performed, or owners may not consent to the procedure. Unfortunately, without a biopsy, there are currently no other methodologies available to detect ICGN. Urine gel electrophoresis is a technique that is currently being optimized for characterization of proteinuria in cats and dogs, and in the future may help to better identify cats that likely have glomerular proteinuria. 19 However, this diagnostic is not yet available for feline practitioners. Given that our study found that 57% of proteinuric cats and 74% of cats with PLN had an immune-mediated underlying disease, empirical immunosuppression could be a reasonable treatment option if renal biopsy cannot be pursued, and similar recommendations have been made for canine patients. 20 Prospective studies are still needed to determine ideal protocols for immunosuppressive therapies in the specific disease processes that cause ICGN in cats.
This study has a number of limitations. The overall number of included cats was small, owing to the rarity (or under-diagnosis) of feline PLN. The number of cats with survival data available was also quite small, precluding useful statistical analysis on survival differences by specific disease category, clinicopathologic variables at presentation or administered medications. Additionally, data on medications administered, including dose and duration, varied widely in the level of detail for individual cats, further precluding useful analysis on the effectiveness of individual therapeutic protocols. For these reasons, univariate and multivariate survival analysis were not performed.
The retrospective nature of this study did not allow standardization of treatments or timing of diagnostics performed relative to biopsy. This means that hypertension and proteinuria may not have been documented to be persistent for all cats, and standardized diagnostics to rule out extra-renal causes of proteinuria may not have been performed in all cats. Therefore, some cats could have had only transient proteinuria, been proteinuric owing to causes unrelated to the kidney or proteinuric owing to undiagnosed hypertension (although the presence of confirmed histopathologic renal lesions and the desire of the clinician to perform renal biopsy lessens this likelihood). Additionally, details of sample collection for UPC analysis were not available for most cats; it is unlikely that UPC analyses were performed on pooled samples, and the daily variation in feline UPC values has not been evaluated as it has in dogs.
The calculated median survival time may be subject to bias, as the cats biopsied in this study may have been more severely affected than cats that did not undergo biopsy, and therefore more likely to die sooner. Additionally, 14 cats were included based on necropsy sampling, and may have been in later stages of disease than the cats that were biopsied. We were unable to control for time of biopsy or necropsy sampling relative to the development and diagnosis of proteinuria, meaning that end-stage changes (fibrosis, interstitial nephritis and nephrosclerosis) may have overshadowed a primary glomerular lesion in some cats. Lastly, the use of a population of cats that had renal biopsies submitted to a tertiary institution may have biased the distribution of diagnoses. For example, it is possible that the prevalence of amyloidosis was underestimated in our study, as clinicians may assume that Abyssinian cats with proteinuria have amyloidosis and elect not to perform renal biopsy.
Unfortunately, these limitations cannot be overcome with a retrospective study design, and prospective evaluations of treatment strategies and outcomes in feline PLN are needed to further characterize and address this diverse cohort of diseases.
Conclusions
This retrospective study revealed that approximately 57% of proteinuric cats with renal tissues submitted to the IVRPS were diagnosed with ICGN, and considering only PLN cats, in which proteinuria was secondary to glomerular disease, approximately 74% were diagnosed with ICGN. Cats with ICGN tended to be young in age, and males appeared to be over-represented. Prognosis for proteinuric cats was grave, with a median survival time of 94 days, regardless of the underlying cause. Further studies are needed to determine whether earlier identification of glomerular disease and immunosuppression in cases of ICGN may improve outcome.
Acknowledgments
This study was supported by Hill’s Pet Nutrition and Bayer Animal Health under the auspices of the World Small Animal Veterinary Association (WSAVA). The WSAVA contributed to the creation and maintenance of the veterinary renal pathology database used in this study.
Footnotes
Accepted: 1 April 2020
Author note: The results of this study were presented, in part, as a research report at the American College of Veterinary Internal Medicine Annual Forum, Phoenix, AZ, 2019.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was supported by the International Veterinary Renal Pathology Service and Buttons Fund for Feline CKD Research.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Jessica M Quimby  https://orcid.org/0000-0002-1388-0452
https://orcid.org/0000-0002-1388-0452
References
- 1. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc 2005; 226: 393–400. [DOI] [PubMed] [Google Scholar]
- 2. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009; 23: 806–813. [DOI] [PubMed] [Google Scholar]
- 3. King JN, Tasker S, Gunn-Moore DA, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–916. [PubMed] [Google Scholar]
- 4. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 5. Littman MP. Protein-losing nephropathy in small animals. Vet Clin North Am Small Anim Pract 2011; 41: 31–62. [DOI] [PubMed] [Google Scholar]
- 6. McLeland SM, Cianciolo RE, Duncan CG, et al. A comparison of biochemical and histopathologic staging in cats with chronic kidney disease. Vet Pathol 2015; 52: 524–534. [DOI] [PubMed] [Google Scholar]
- 7. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013; 50: 147–155. [DOI] [PubMed] [Google Scholar]
- 8. Cianciolo RE, Mohr FC, Aresu L, et al. World Small Animal Veterinary Association Renal Pathology Initiative: classification of glomerular diseases in dogs. Vet Pathol 2016; 53: 113–135. [DOI] [PubMed] [Google Scholar]
- 9. Schneider SM, Cianciolo RE, Nabity MB, et al. Prevalence of immune-complex glomerulonephritides in dogs biopsied for suspected glomerular disease: 501 cases (2007–2012). J Vet Intern Med 2013; 27 Suppl 1: S67–S75. [DOI] [PubMed] [Google Scholar]
- 10. Rossi F, Aresu L, Martini V, et al. Immune-complex glomerulonephritis in cats: a retrospective study based on clinico-pathological data, histopathology and ultrastructural features. BMC Vet Res 2019; 15: 303. DOI: 10.1186/s12917-019-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12: 983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Renal Interest Society. Algorithm for staging of chronic kidney disease in cats. http://www.iris-kidney.com/pdf/003-5559.001-iris-website-staging-of-ckd-pdf_220116-final.pdf#page=7 (accessed February 7, 2018).
- 13. Nash AS, Wright NG, Spencer AJ, et al. Membranous nephropathy in the cat: a clinical and pathological study. Vet Rec 1979; 105: 71–77. [DOI] [PubMed] [Google Scholar]
- 14. Wright NG, Nash AS, Thompson H, et al. Membranous nephropathy in the cat and dog: a renal biopsy and follow-up study of sixteen cases. Lab Invest 1981; 45: 269–277. [PubMed] [Google Scholar]
- 15. Lees GE, Brown SA, Elliott J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 16. Littman MP, Daminet S, Grauer GF, et al. Consensus recommendations for the diagnostic investigation of dogs with suspected glomerular disease. J Vet Intern Med 2013; 27 Suppl 1: S19–S26. [DOI] [PubMed] [Google Scholar]
- 17. Brown CA, Elliott J, Schmiedt CW, et al. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet Pathol 2016; 53: 309–326. [DOI] [PubMed] [Google Scholar]
- 18. Hewitson TD, Smith ER, Samuel CS. Qualitative and quantitative analysis of fibrosis in the kidney. Nephrology (Carlton) 2014; 19: 721–726. [DOI] [PubMed] [Google Scholar]
- 19. Hokamp JA, Leidy SA, Gaynanova I, et al. Correlation of electrophoretic urine protein banding patterns with severity of renal damage in dogs with proteinuric chronic kidney disease. Vet Clin Pathol 2018; 47: 425–434. [DOI] [PubMed] [Google Scholar]
- 20. Pressler B, Vaden S, Gerber B, et al. Consensus guidelines for immunosuppressive treatment of dogs with glomerular disease absent a pathologic diagnosis. J Vet Intern Med 2013; 27 Suppl 1: S55–S59. [DOI] [PubMed] [Google Scholar]