Abstract
Background
Biologically important curcuminoids viz curcumin, demethoxycurcumin, and bisdemethoxycurcumin in turmeric rhizome have several health benefits. Pharmaceutical industries synthesize curcuminoids manipulating gene expressions in vitro or in vivo because of their medicinal importance. In this experiment, we studied the gene expressions involved in the curcuminoid biosynthesis pathway in association with curcuminoid yield in turmeric rhizome to study the impact of individual gene expression on curcuminoid biosynthesis.
Results
Gene expressions at the different growth stages of turmeric rhizome were association tested with respective curcuminoid contents. Gene expression patterns of diketide-CoA synthase (DCS) and multiple curcumin synthases (CURSs) viz curcumin synthase 1 (CURS1), curcumin synthase 2 (CURS2), and curcumin synthase 3 (CURS3) were differentially associated with different curcuminoid contents. Genotype and growth stages showed a significant effect on the gene expressions resulting in a significant impact on curcuminoid balance in turmeric rhizome. DCS and CURS3 expression patterns were similar but distinct from CURS1 and CURS2 expression patterns in turmeric rhizome. DCS expression had a significant positive and CURS3 expression had a significant negative association with curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid in turmeric rhizome. CURS1 expression had a negative association with curcumin whereas CURS2 expression showed a positive association with demethoxycurcumin.
Conclusions
The effects of DCS and CURS expressions are not always positive with different curcuminoid contents in turmeric rhizome. DCS expression has a positive and CURS3 expression has a negative association with curcuminoids. CURS1 and CURS2 are also associated with curcumin and demethoxycurcumin synthesis. This mechanism of co-expression of diketide-CoA synthase and multiple curcumin synthases in turmeric rhizome has a significant effect on curcuminoid balance in different turmeric cultivars. Further experiment will explore more insights; however, association-based test results from this experiment can be useful in improving curcuminoid yield in turmeric rhizome or in vitro through the application of genetic engineering and biotechnology.
Graphical abstract
Associating gene expressions with curcuminoid biosynthesis in turmeric
Keywords: Association, Curcuminoids, Diketide-CoA synthase, Gene regulation, Multiple curcumin synthases
Background
Turmeric (Curcuma longa L. syn. Curcuma domestica Val.) is a rhizomatous, herbaceous, and perennial plant belonging to the Zingiberaceae family which includes almost 110 species [1]. Turmeric is also known by different names in different countries such as Haldi in India and Besar in Nepal. Turmeric is widely cultivated in the tropical and subtropical regions of the world including India, China, Bangladesh, Nepal, Sri Lanka, Pakistan, Asian subcontinents, and the Caribbean and Latin American regions [2]. The use of turmeric rhizome is mentioned in the Vedic culture in Indian subcontinents and still has extensive use as a culinary spice, coloring agent, food preservative, natural dye in the food industry, cosmetics, medicine, and religious rituals [3, 4]. The first evidence of biological effects of turmeric have been attributed to its constituent curcumin, discovered and isolated almost two centuries ago [5]; however, recent studies suggest that most of the pharmaceutical properties of turmeric depend on its biologically active constituents, curcuminoids viz curcumin, demethoxycurcumin, and bisdemethoxycurcumin [6]. The turmeric rhizome contains 3–5% curcuminoids and up to 5% essential oils and resins [7] where commercially available turmeric powder is generally composed of about 77% curcumin, 17% demethoxycurcumin, and 3% bisdemethoxycurcumin [6]. Curcumin, a major curcuminoid from turmeric rhizome, is proven for its significance as anti-inflammatory, antioxidant, antimutagenic, antidiabetic, antibacterial, antiviral, antifungal, hepatoprotective, kidney disease treatment, expectorant, anti-cancerous, treating conditions like arthritis, inflammation to Alzheimer’s disease, and influencing gene transcription and expression, cell signaling pathway, and epigenetic mechanisms [3, 8–12]. Turmeric is a minor crop in terms of its contribution towards food security; however, the curcuminoids from this spice crop are attractive targets for metabolic engineering and pharmaceutical applications [13].
Curcuminoids are biologically synthesized through a phenylpropanoid pathway in turmeric [14]; however, several studies were also carried out successfully for producing curcuminoids in vitro through an artificial curcuminoid biosynthesis pathway [13, 15, 16]. There are several other upstream section enzyme genes involved in the curcuminoid biosynthesis pathway which catalyze the synthesis of early substrates (feruloyl-CoA/p-coumaroyl-CoA) [14, 17, 18]; however, downstream section enzyme genes involved in the curcuminoid biosynthesis pathway play a major role in catalyzing the formation of curcuminoid scaffold in turmeric rhizome. Diketide-CoA synthase (DCS), a type III PKS, and multiple curcumin synthases (CURSs) viz curcumin synthase1 (CURS1), curcumin synthase2 (CURS2), and curcumin synthase3 (CURS3) involved in the downstream section of the curcuminoid biosynthesis pathway in turmeric were identified and characterized where DCS synthesizes feruloyldiketide-CoA/p-coumaroyldiketide CoA and CURS1, CURS2, and CURS3 then converts diketide-CoA esters into different curcuminoid scaffolds viz curcumin, demethoxycurcumin, and bisdemethoxycurcumin [19, 20]. The authors also reported that relative expression of DCS, CURS1, and CURS2 were higher in rhizome than leaf whereas CURS3 expression was almost similar in rhizome and leaf. The gene expression studies suggested that the content of curcuminoids in turmeric cultivars depends on the expression balance of these four major enzyme genes (DCS, CURS1, CURS2, and CURS3) in turmeric rhizome in addition to the availability of the substrates for curcuminoid scaffold biosynthesis. Transcriptomics studies in turmeric have shed light on the identification and validation of multiple curcumin synthases involved in the curcuminoid biosynthetic pathway in turmeric [17, 18, 21]. More novel type III PKS genes have been identified recently, such as CLPKS9 and CLPKS10 [22], CLPKS1and CLPKS2 [18], and CLPKS11 [23] which may have a potential role in curcuminoid scaffold biosynthesis; however, the difference in the contents of curcuminoids among the species can be explained by the changes in the expression of genes encoding diketide-CoA synthase and multiple curcumin synthases at the branching point of the curcuminoid biosynthesis pathway in turmeric [24]. The variation in curcuminoid content among the various lines and cultivars of C. longa was reported to be caused by hybridization and introgression [25], agro-climatic variation [26], genotype and environment interaction [27], and microenvironment (below soil surface near root zone) and macroenvironment (above soil surface) interaction [28]; however, the effects of tissue-specific gene expressions on curcuminoid biosynthesis are still unknown. Gene expressions of diketide-CoA synthases and multiple curcumin synthases in turmeric were also found to be influenced by tissue-specific (leaves and rhizome) and temporal (growth stages) variations within a cultivar [29]; this may have an influence on curcuminoid balance. CURS expression was found regardless of the variation in the curcumin content in different turmeric cultivars at different agroclimatic regions, and expressions were positively correlated with curcumin content in turmeric cultivars within an environment at different stages of growth [30]; however, the effect of individual DCS and CURS gene expression on curcuminoid yield is still unexplored. The first-stage study of this experiment revealed that differential DCS and CURS expressions can be predictively associated with curcuminoid content in different turmeric cultivars to study the relationship between target gene expressions and curcuminoid yield in turmeric rhizome [31]. Specific genes associated with target quantitative traits are commonly known as trait-associated genes (TAGs) which can be defined by the robust linear regression analysis through an association test between continuous traits and mRNA expression [32]. Huber’s M-estimator-based robust regression method [33] is useful for identifying TAGs. In this experiment, we studied DCS and CURS gene expressions in association with variable curcuminoid content in turmeric rhizome at different stages of growth to find out the impact of individual gene expression on curcuminoid yield in terms of magnitude and direction using a statistical model. Gene expressions or transcript abundance was regarded as genotypic values whereas associated changes in curcuminoids were regarded as phenotypic values at different growth stages while performing the association-based test. The association-based test results from this experiment can also be useful for improving curcuminoid yield in turmeric rhizome or in vitro through the application of genetic engineering and biotechnology.
Methods
General experimental conditions
Rhizome samples of turmeric cultivars (GNT-2, Pratibha, and NDH-98) were selected randomly for gene expression study and curcuminoid analysis at three different growth stages in field conditions. The first stage was determined to be the active vegetative growth stage (SI, 4 months after planting), the second stage was at the active rhizome development stage (SII, 5 months after planting), and the third stage was at the maturity or senescence stage (SIII, 6 months after planting). Three biological samples (rhizomes) from each cultivar at three stages of growth under study were taken out of the soil for RNA isolation, isolated RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA), and gene expression was studied through RT-qPCR in the Department of Genetics and Plant Breeding and Biotechnology Laboratory at NAU, Gujarat, India, during 2017–2018. The cultivars used in this experiment were denoted as biological sets NDH-98 (N), GNT-2 (G), and Pratibha (P). From each biological sample, two technical replicates were utilized for further downstream analysis through qPCR. The gene expression results obtained from the RT-qPCR assay were further used for association tests with curcuminoid yield in turmeric rhizome. Plant sampling procedures for RNA isolation were carried out following the recommended guidelines [34]. Technical sampling procedures and RT-qPCR procedures were followed as per the MIQE guidelines for the gene expression study [35, 36].
Rhizome sampling and curcuminoid content analysis
At each stage of study viz SI, SII, and SIII, rhizome fingers were excised from the mother rhizome with a sterile knife and washed, packed into aluminum foil along with identification number, and immediately dipped into liquid nitrogen (− 196 °C) container for RNA isolation, cDNA synthesis, and gene expression study in the laboratory. The remaining portions of individually sampled rhizomes were cleaned, sliced into pieces, sun-dried for 5–7 days, and made into a fine powder using the grinder for curcuminoid content analysis (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) through HPLC using individual curcuminoid standards (Sigma Aldrich) at the Food Quality Testing Laboratory (FQTL), NAU, Navsari, Gujarat. For curcuminoid analysis, 1.0 g turmeric powder of each cultivar was dissolved in 50 ml of 100% methanol separately and then mixed and vortexed for 30 min. The solution is then filtered and injected in the Thermo HPLC Surveyor instrument (Thermo Scientific) for individual curcuminoid measurement at 425-nm wavelength. Laboratory analysis was performed at room temperature (25–30 °C).
RNA isolation and cDNA synthesis
Total RNA was isolated at three different growth stages (SI, SII, and SIII) of three turmeric cultivars under study using the RNeasy® Plant Mini Kit (Qiagen, Germany) involving on-column genomic DNA digestion step of DNase I (Qiagen) treatment following the manufacturer’s protocol. The integrity and size distribution of the total RNA purified were checked by 1.2% denaturing formaldehyde agarose (FA) gel electrophoresis and staining with ethidium bromide visualizing two sharp bands of 28S rRNA to 18S RNA in a 2:1 ratio. RNA purity check and quantification were done using the Nanodrop 2000 instrument (Thermo Scientific) which showed the acceptable purity for each biological sample (A260/A280 of 1.9 to 2.1). From the total RNA, the mRNA with the poly-A tail was reverse transcribed into cDNA using oligo dT primers during the reverse transcription step. cDNA was synthesized in 20 μl reaction using 2 μl of total isolated RNA (1 ng in final solution) with 4 μl of 5X cDNA synthesis buffer (1X final), 2 μl of 2X dNTP mix (500 μM each), 1 μl of anchored oligo dT primer (for mRNA reverse transcription), 1 μl RT enhancer, 1 μl verso enzyme mix, and water up to 20 μl. cDNA synthesis protocol involved a single 30-min cycle of polymerase enzyme activity at 42 °C followed by one additional cycle of 2 min at 95 °C for inactivation of enzyme (Verso cDNA Synthesis Kit, Thermo Scientific).
Quantification of transcript abundance through RT-qPCR assays
RT-qPCR assays at three different stages were carried out along with gene-specific and reference gene primers (Additional file 1: Table S2) which were validated in turmeric RNA samples. All the qPCR assays of four target genes as well as reference genes at each stage were included in a single 96-well plate (Thermo Scientific) for minimizing error while quantifying the abundance of cDNA in technical replicates of each biological sample. A standard qPCR protocol and plate setup were performed on CFX96 real-time PCR thermocycler using the CFX Manager software (Bio-Rad). For finding out transcript abundance in different turmeric samples, qPCR assay comprised 10 μl of 2X PowerUp™ SYBR™ Green Master Mix (Applied Biosystems), 0.5 μM of each gene-specific forward and reverse primers along with reference gene Actin, 2 μl of 10-fold diluted cDNA as a template, and remaining quantity of nuclease-free water (HiMedia) to make a final volume of 20 μl. qPCR conditions for gene expression study were as follows: heat-labile uracil-DNA glycosylase (UDG) activation at 50 °C for 2 min followed by Dual-Lock™ DNA polymerase activation at 95 °C for 2 min, and 40 cycles of denaturation at 95 °C for 15 s followed by combined anneal/extend temperature of 60 °C for 1 min. For studying the specificity of the products, the final melting curve analysis of 65 to 95 °C with a 0.5 °C increment was used according to the manufacturer’s protocol (Bio-Rad, CFX96 instrument), and the products were also confirmed on 1.2% agarose gel electrophoresis. Quantification cycle (Cq) and normalized gene expression values obtained in the CFX Manager software were used for further analysis. Negative controls (no template control (NTC)) were also included to detect any false positives during reverse transcription (RT) and qPCR assays. The data generated along with the Cq values were subjected to the CFX Manager (v.3.1, Bio-Rad) software package which includes mathematical models for estimating the relative quantification and normalization of qPCR data for gene expression analysis [37, 38]. Gene study feature in the CFX Manager software was set to a regulation threshold of 2-fold change and a p value threshold of 0.05 for finding out any significant variations in the gene expressions. DCS and CURSs melt peak curves were studied for the specificity of the products, and gel electrophoresis was also performed for the validation of the qPCR products using the reference gene Actin.
Association-based test between gene expressions and curcuminoids
In this experiment, diketide-CoA synthase (DCS) and multiple curcumin synthases (CURS1, CURS2, and CURS3) or CURSs gene expression patterns were studied at three different growth stages viz SI (active vegetative stage), SII (active rhizome development stage), and SIII (maturity or senescence stage) among three turmeric cultivars significantly differing in curcuminoid (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) contents in the rhizome. The robust regression analysis was used by taking normalized gene expression values from the RT-qPCR analysis as explanatory or independent variables and curcuminoid contents (curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid) obtained from HPLC analysis as a response or dependent variables for identifying genes that are more closely associated with curcuminoid biosynthesis in turmeric, also known as trait-associated genes (TAGs). A separate association-based test was also done for the total curcuminoid content in turmeric rhizome which was estimated by summing up the individual curcuminoid content in each turmeric rhizome at each growth stage. The association between curcuminoids and mRNA expressions was analyzed using Huber’s M-estimator-based ROBUSTREG procedure [39] in SAS® University Edition (SAS® Studio 3.71, SAS® Institute Inc.). The general linear regression model for the association-based test [32] using gene expression data was as follows:
Traiti = β0 + β1 Expression1i + … + βp Covariatepi + εi, εi~(N, σ2)
where i represents the individuals; Expressioni indicates the normalized gene expression value; Traiti represents each continuous trait such as curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid; Covariatepi represents the covariate effects of factors other than error factors (εi); beta (β) in a linear regression model is a standardized coefficient indicating the magnitude of the correlation between an independent and dependent variable; N and σ2 represent the analysis of variance assumption of normal distribution and common standard deviation, respectively. Additional tests like the rho (ρ) test based on the robust F-test and Rn^2 test based on the Wald-type tests were also carried out to measure the association of covariates on response variables. The SAS codes and dataset used during the data analysis are given in Additional file 1: Annex 1.
Results
Curcuminoid analysis in turmeric cultivars
Based on the high-performance liquid chromatography (HPLC) analysis, turmeric cultivars “GNT-2” and “Pratibha” were not significantly different for curcumin and total curcuminoid content whereas “NDH-98” was with relatively lower curcumin and lower total curcuminoid content than “GNT-2” and “Pratibha” (Table 1). There was no significant difference among the cultivars for demethoxycurcumin content; however, the highest bisdemethoxycurcumin was in “GNT-2” followed by “Pratibha” and below detection level in “NDH-98” turmeric rhizome. Irrespective of the curcuminoid contents, the rhizome yield of short-growth-duration cultivar “NDH-98” was the highest (33–34 t ha−1) followed by long-growth-duration cultivars “GNT-2” (28–29 t ha−1) and “Pratibha” (23–24 t ha−1) (Table 1). The results showed that the higher turmeric rhizome yield does not necessarily mean higher curcuminoids in the turmeric cultivars. The chromatograms showing individual curcuminoid detection in the HPLC system are given in Additional file 2.
Table 1.
Curcuminoid contents in turmeric cultivars at different stages under study
| Sample ID | Curcumin (%) | Demethoxy curcumin (%) | Bisdemethoxycurcumin (%) | Total curcuminoid (%) | Rhizome yield (t ha−1) | Growth duration (days) |
|---|---|---|---|---|---|---|
| NDH-98_SI | 0.27 | 0.76 | BDL | 1.03 | ||
| NDH-98_SII | 0.1 | 0.36 | BDL | 0.46 | ||
| NDH-98_SIII | 0.04 | 0.08 | BDL | 0.12 | ||
| Average NDH-98 | 0.14b (25%) | 0.40a (75%) | BDL | 0.54b (100%) | 33–34 | 240 |
| GNT-2_SI | 2.31 | 0.71 | 1.14 | 4.16 | ||
| GNT-2_SII | 0.76 | 0.07 | 0.15 | 0.98 | ||
| GNT-2_SIII | 0.81 | 0.18 | 0.24 | 1.23 | ||
| Average GNT-2 | 1.29a (61%) | 0.32a (15%) | 0.51a (24%) | 2.12a (100%) | 28–29 | 256 |
| Pratibha_SI | 0.56 | 0.11 | 0.14 | 0.81 | ||
| Pratibha_SII | 1.29 | 0.17 | 0.14 | 1.6 | ||
| Pratibha_SIII | 1.33 | 0.22 | 0.24 | 1.79 | ||
| Average Pratibha | 1.06a (76%) | 0.17a (12%) | 0.17b (12%) | 1.40ab (100%) | 23–24 | 256 |
“a” and “b” are the significance letters which indicate that values with the same letters in a column are statistically not significant from each other at p < 0.05. [NDH-98 has lower curcumin (25%), higher demethoxycurcumin (75%), and negligible bisdemethoxycurcumin; GNT-2 has higher curcumin (61%) and lower bisdemethoxycurcumin (24%) and demethoxycurcumin (15%); Pratibha has higher curcumin (76%) and lower demethoxycurcumin (12%) and bisdemethoxycurcumin (12%); GNT-2 and Pratibha have similar and higher total curcuminoid yield than NDH-98]
NDH-98, GNT-2, and Pratibha turmeric cultivars
BDL Below the detection limit during HPLC analysis, SI 4 months after planting, SII 5 months after planting, SIII 6 months after planting
Gene expressions in turmeric rhizome at different growth stages
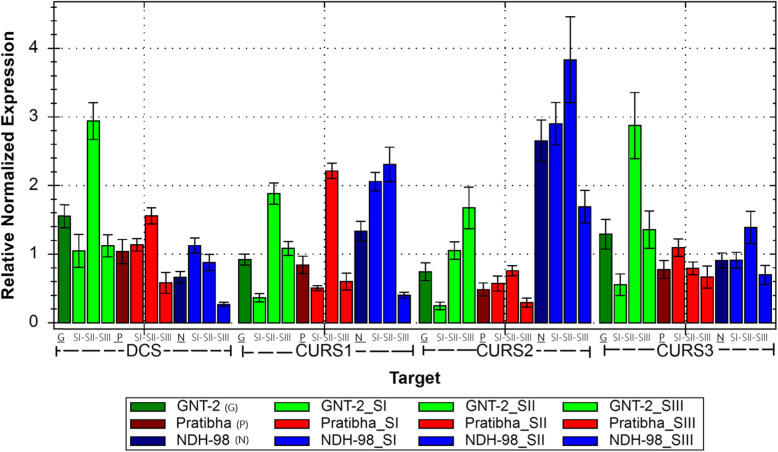
There were no off products in the qPCR analysis based on the melt peak curves which was further confirmed by gel electrophoresis showing a single band of PCR product, and the data was considered reliable for further analysis. The results of the qPCR analysis are given in Additional file 2. Comparing the gene expression profiles in different turmeric cultivars, DCS (2.94-fold) and CURS3 (2.87-fold) expressions were upregulated at SII as compared with SI and SIII; however, CURS1 (1.88-fold) and CURS2 (1.05 fold) were expressed at a lower level in GNT-2. In NDH-98, DCS (0.88-fold) and CURS3 (1.39-fold) expressions were lower; however, CURS1 (2.30-fold) and CURS2 (3.83-fold) expressions were higher at SII as compared with SI and SIII. In Pratibha, DCS (1.56-fold), CURS2 (0.76-fold), and CURS3 (0.79-fold) were expressed at a lower level; however, CURS1 (2.22 fold) expression was upregulated at SII as compared with SI and SIII (Fig. 1 and Additional file 1: Table S1). Generally, the gene expressions were increasing from active vegetative stage to rhizome development stage (SI to SII) and decreasing at maturity or senescence stage (SIII) under study except that DCS expression was decreasing throughout the growth stages in NDH-98, CURS2 expression was increasing throughout the growth stages in GNT-2, and CURS3 expression was decreasing throughout the growth stages in Pratibha (Fig. 1). These patterns in each cultivar might have some significant role to play for curcuminoid yield in different turmeric cultivars.
Fig. 1.
Comparative gene expression analysis in turmeric cultivars at three stages of growth. Gene expressions were normalized to the reference gene Actin. Green colored bars represent GNT-2, red-colored bars represent Pratibha, and dark blue colored bars represent NDH-98. The first bar in each gene family is the average of the three stages SI, SII, and SIII of each cultivar, i.e., GNT-2 (G), Pratibha (P), and NDH-98 (N). In NDH-98, DCS expression is decreasing at all stages of growth from SI to SIII, CURS1, and CURS2 expression is higher at SI and SII and decreased at SIII whereas higher expression of CURS3 at SII stages under study. In the case of GNT-2, DCS, CURS1, and CURS3 are highly expressed at SII than SI and SIII, the CURS2 expression level is increasing throughout the growth stages from SI to SIII where DCS and CURS3 have almost equal expression level at SII in GNT-2. For Pratibha, DCS, CURS1, and CURS2 gene expressions are higher at SII as compared with SI and SIII, and CURS3 gene expression is decreasing at all stages of growth from SI to SIII. A significant amount of gene expression variation was observed during SII (5 months after planting) among the cultivars
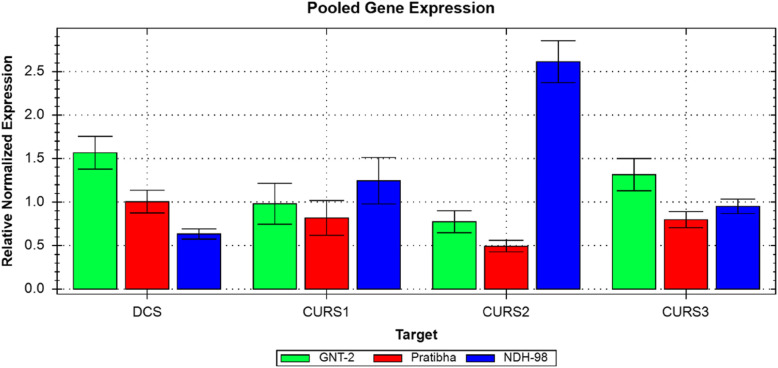
Pooled gene expressions in turmeric cultivars
The highest DCS expression was in higher curcumin yielding cultivar GNT-2 (1.55-fold) followed by Pratibha (1.04-fold) and the least in low curcumin yielding cultivar NDH-98 (0.66-fold) over the growth stages under study. CURS1 and CURS2 gene expressions were highest in NDH-98 (1.34-fold and 2.65-fold, respectively) followed by GNT-2 (0.92-fold and 0.74-fold, respectively) and Pratibha (0.84-fold and 0.49-fold, respectively). CURS3 expressions were lower than DCS expressions in all the cultivars over growth stages with the highest expression in GNT-2 (1.29-fold) followed by NDH-98 (0.91-fold) and Pratibha (0.77-fold). Among all the genes under study, CURS2 expressions were lower in Pratibha (0.48-fold) and GNT-2 (0.74-fold), however significantly higher in NDH-98 (2.65-fold). The results of pooled gene expressions over growth stages are in Fig. 2 and Additional file 1: Table S1. The results showed that GNT-2 and Pratibha, higher curcuminoid yielding cultivars, had a higher DCS expression with relatively lower CURS1, CURS2, and CURS3 expressions opposite to that of NDH-98, relatively lower curcuminoid yielding cultivar, which depicted lower DCS expression with relatively higher CURS1, CURS2, and CURS3 expressions (Fig. 2). There was no significant change in DCS, CURS1, CURS2, and CURS3 gene expressions between GNT-2 and Pratibha (higher curcumin and total curcuminoid yielding cultivars) at all stages of growth: however, their expressions differed in NDH-98 (lower curcumin and total curcuminoid yielding cultivar) (Table 2). DCS expression was found downregulated (− 2.35- and –1.57-fold) whereas CURS2 expression was found upregulated (3.57- and 5.46-fold) in lower curcumin and lower total curcuminoid yielding cultivar (NDH-98) as compared with higher curcumin and higher total curcuminoid yielding cultivars (GNT-2 and Pratibha) and vice versa (Table 2). There was no significant change in the CURS3 expression (< 2-fold change) among the cultivars.
Fig. 2.
Pooled gene expression in three turmeric cultivars. GNT-2, Pratibha, and NDH-98 are turmeric cultivars. DCS, diketide-CoA synthase. CURS1, CURS2, and CURS3 are multiple curcumin synthases. Pooled gene expressions are the averages of three stage (SI, SII, and SIII) expressions in each cultivar. DCS and CURS3 expressions are higher in GNT-2, a higher curcuminoid yielding cultivar, whereas CURS1 and CURS2 expressions are higher in NDH-98, a lower curcuminoid yielding cultivar
Table 2.
DCS and CURSs gene regulation in turmeric rhizome over growth stages
| Target | Experimental sample | Regulation | p value | Exceeds p value threshold | Compared to regulation threshold | Control sample |
|---|---|---|---|---|---|---|
| Actin | GNT-2 | NA | 0.790985 | Yes | No change | Pratibha |
| CURS1 | GNT-2 | 1.09 | 0.179227 | Yes | No change | Pratibha |
| CURS2 | GNT-2 | 1.53 | 0.192329 | Yes | No change | Pratibha |
| CURS3 | GNT-2 | 1.66 | 0.003796 | No | No change | Pratibha |
| DCS | GNT-2 | 1.50 | 0.369600 | Yes | No change | Pratibha |
| Actin | NDH-98 | NA | 0.496892 | Yes | No change | Pratibha |
| CURS1 | NDH-98 | 1.59 | 0.730277 | Yes | No change | Pratibha |
| CURS2 | NDH-98 | 5.46 | 0.000001 | No | Upregulated | Pratibha |
| CURS3 | NDH-98 | 1.17 | 0.395052 | Yes | No change | Pratibha |
| DCS | NDH-98 | − 1.57 | 0.044860 | No | No change | Pratibha |
| Actin | NDH-98 | NA | 0.689920 | Yes | No change | GNT-2 |
| CURS1 | NDH-98 | 1.45 | 0.036328 | No | No change | GNT-2 |
| CURS2 | NDH-98 | 3.57 | 0.000027 | No | Upregulated | GNT-2 |
| CURS3 | NDH-98 | − 1.43 | 0.022002 | No | No change | GNT-2 |
| DCS | NDH-98 | − 2.35 | 0.000368 | No | Downregulated | GNT-2 |
“+” or no sign in the regulation column represents upregulation (higher expression) of a gene in the current sample as compared with the control sample
“−” sign indicates downregulation (lower expression) of a gene in the current sample as compared with the control sample
Upregulation of a “gene” in experimental sample as compared with control also indicates the downregulation of that same “gene” in the control sample as compared with experimental sample and vice versa. Gene expressions at different stages of growth viz SI, SII, and SIII for each cultivar were pooled together for comparative gene regulation study
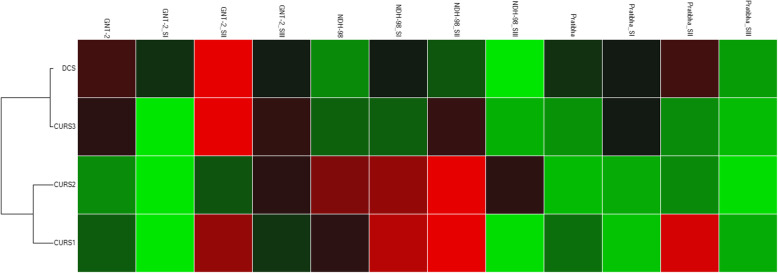
Cluster analysis of DCS and CURSs gene expressions
Cluster analysis of the gene expressions of three turmeric cultivars at three different growth stages revealed that DCS and CURS3 expression patterns were similar but distinct from CURS1 and CURS2 expression patterns in turmeric rhizome (Fig. 3). Similar results were obtained when gene expression was studied in two turmeric cultivars, GNT-2 and NDH-98, after 6 months of planting in field conditions [31].
Fig. 3.
Cluster gram showing the association between the gene expressions in turmeric at three growth stages. NDH-98, GNT-2, and Pratibha are three turmeric cultivars. SI, 4 months after planting; SII, 5 months after planting; SIII, 6 months after planting. Upregulation (higher expression) is shown by a red square, downregulation (lower expression) by a green square, and no regulation by a black square. The lighter the shade of color, the greater the relative expression difference. Gene expression patterns of DCS and CURS3 were more closely associated and distant from CURS1 and CURS2 gene expression patterns among the genotypes at all stages of growth. The upregulation of most of the genes was found at SII as compared with other states under study. GNT-2, NDH-98, and Pratibha expressions are the average expressions of all three stages
DCS and CURSs gene expressions associated with curcuminoid biosynthesis in turmeric
There was a significant effect of DCS, CURS1, and CURS3 gene expressions on curcumin content at p < 0.01. DCS had a positive linear association whereas CURS1 and CURS3 expressions had a negative linear association with curcumin. The covariate effect of CURS1 and CURS2 expressions on curcumin was also found significant at p < 0.01 although the correlation was significant at p < 0.08. Thus, DCS had a significantly positive whereas CURS1 and CURS3 expressions had a significantly negative association with curcumin yield in turmeric rhizome. Co-regulated expressions of CURS1 and CURS2 were also found significantly associated with curcumin synthesis in turmeric rhizome (Table 3).
Table 3.
Association-based test of DCS and multiple curcumin synthases with curcumin
| Parameter | Class label | Main effects | CURS1 and CURS2 as a covariate | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Chi-square | Pr > ChiSq | Estimate | Chi-square | Pr > ChiSq | ||
| Intercept | 1.1091 | 486.32 | < .0001 | 1.4185 | 327.39 | < .0001 | |
| Growth stage | SI | 0.1039 | 4.05 | 0.0442 | − 0.1792 | 4.53 | 0.0333 |
| Growth stage | SII | 0.4701 | 57.26 | < .0001 | 0.0073 | 0.01 | 0.936 |
| Growth stage | SIII | 0 | 0 | ||||
| Genotype | GNT-2 | 1.0375 | 342.2 | < .0001 | 1.1168 | 161.13 | < .0001 |
| Genotype | NDH-98 | − 0.2774 | 14.84 | 0.0001 | − 0.616 | 61.29 | < .0001 |
| Genotype | Pratibha | 0 | 0 | ||||
| DCS | 0.9126 | 82.3 | < .0001 | 0.6182 | 26.03 | < .0001 | |
| CURS1 | − 0.3392 | 12.48 | 0.0004 | 0 | |||
| CURS2 | − 0.0033 | 0.01 | 0.9371 | 0 | |||
| CURS3 | − 1.3922 | 335.16 | < .0001 | − 1.2216 | 153.92 | < .0001 | |
| Reduced parameter robust linear test for curcumin | |||||||
| Rho | 4.1143 | 5.16 | 0.0759 | ||||
| Rn^2 | 27.0998 | 27.1 | < .0001 | ||||
Pr < ChiSq approximately predicts the associated probability level after the selected variable has been entered or removed
Pr Probability, ChiSq Chi-square, Rho association or correlation, Rn^2 robust Wald-type association test
For demethoxycurcumin, DCS and CURS2 expressions had a significant positive association whereas CURS3 expressions had a significant negative association with demethoxycurcumin at p < 0.01. The covariate effect of CURS1 and CURS2 expressions on demethoxycurcumin was also found highly significant (p < 0.01). Thus, DCS and CURS2 expressions had significant positive associations whereas CURS3 expression had a significant negative association with demethoxycurcumin. Co-regulated expression of CURS1 and CURS2 also had a significant impact on the demethoxycurcumin content in the turmeric rhizome (Table 4).
Table 4.
Association-based test of DCS and multiple curcumin synthases with demethoxycurcumin
| Parameter | Class label | Main effects | CURS1 and CURS2 as a covariate | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Chi-square | Pr > ChiSq | Estimate | Chi-square | Pr > ChiSq | ||
| Intercept | − 0.1443 | 32.09 | < .0001 | − 0.1497 | 12.45 | 0.0004 | |
| Growth stage | SI | 0.3705 | 200.58 | < .0001 | 0.3895 | 73.06 | < .0001 |
| Growth stage | SII | 0.2073 | 43.38 | < .0001 | 0.2591 | 27.78 | < .0001 |
| Growth stage | SIII | 0 | 0 | ||||
| Genotype | GNT-2 | 0.4197 | 218.18 | < .0001 | 0.4175 | 76.88 | < .0001 |
| Genotype | NDH-98 | 0.3327 | 83.19 | < .0001 | 0.4616 | 117.48 | < .0001 |
| Genotype | Pratibha | 0 | 0 | ||||
| DCS | 0.2596 | 25.95 | < .0001 | 0.2913 | 19.72 | < .0001 | |
| CURS1 | 0.0296 | 0.37 | 0.5431 | 0 | |||
| CURS2 | 0.0535 | 6.44 | 0.0111 | 0 | |||
| CURS3 | − 0.4059 | 110.98 | < .0001 | − 0.4188 | 61.76 | < .0001 | |
| Reduced parameter robust linear test for demethoxycurcumin | |||||||
| Rho | 10.1158 | 12.68 | 0.0018 | ||||
| Rn^2 | 19.0271 | 19.03 | < .0001 | ||||
Pr < ChiSq approximately predicts the associated probability level after the selected variable has been entered or removed
Pr Probability, ChiSq Chi-square, Rho association or correlation, Rn^2 robust Wald-type association test
DCS (p < 0.09) depicted a positive effect whereas CURS3 (p < 0.01) showed a significant negative effect on bisdemethoxycurcumin content. The covariate effects of CURS1 and CURS2 expression on bisdemethoxycurcumin were also found non-significant at p < 0.05. Thus, DCS expression had a positive effect and CURS3 expression had a significant negative effect on bisdemethoxycurcumin content in turmeric rhizome (Table 5).
Table 5.
Association-based test of DCS and multiple curcumin synthases with bisdemethoxycurcumin
| Parameter | Class label | Main effects | CURS1 and CURS2 as a covariate | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Chi-square | Pr > ChiSq | Estimate | Chi-square | Pr > ChiSq | ||
| Intercept | 0.3381 | 9.58 | 0.002 | 0.3586 | 12.19 | 0.0005 | |
| Growth stage | SI | 0.1514 | 1.82 | 0.1771 | 0.1847 | 2.8 | 0.094 |
| Growth stage | SII | 0.1877 | 1.93 | 0.1644 | 0.0847 | 0.51 | 0.4768 |
| Growth stage | SIII | 0 | 0 | ||||
| Genotype | GNT-2 | 0.5534 | 20.63 | < .0001 | 0.6544 | 32.23 | < .0001 |
| Genotype | NDH-98 | − 0.0154 | 0.01 | 0.9216 | − 0.1401 | 1.85 | 0.1741 |
| Genotype | Pratibha | 0 | 0 | ||||
| DCS | 0.3727 | 2.91 | 0.0881 | 0.0342 | 0.05 | 0.8294 | |
| CURS1 | − 0.2976 | 2.03 | 0.1537 | 0 | |||
| CURS2 | 0.0424 | 0.22 | 0.6389 | 0 | |||
| CURS3 | − 0.618 | 13.99 | 0.0002 | − 0.4367 | 11.46 | 0.0007 | |
| Reduced parameter robust linear test for bisdemethoxycurcumin | |||||||
| Rho | 1.5189 | 1.9 | 0.3859 | ||||
| Rn^2 | 2.7031 | 2.7 | 0.2588 | ||||
Pr < ChiSq approximately predicts the associated probability level after the selected variable has been entered or removed
Pr Probability, ChiSq Chi-square, Rho association or correlation, Rn^2 robust Wald-type association test
Only DCS and CURS3 expressions had a significant effect (p < 0.01) on the total curcuminoid content in turmeric rhizome where DCS had a significant positive linear association and CURS3 had a significant negative linear association with total curcuminoid. The covariate effect of CURS1 and CURS2 expression was found non-significant at p < 0.05. Thus, DCS had a significant positive and CURS3 had a significant negative association with total curcuminoid yield in turmeric rhizome (Table 6).
Table 6.
Association-based test of DCS and multiple curcumin synthases with total curcuminoid
| Parameter | Class label | Main effects | CURS1 and CURS2 as a covariate | ||||
|---|---|---|---|---|---|---|---|
| Estimate | Chi-square | Pr > ChiSq | Estimate | Chi-square | Pr > ChiSq | ||
| Intercept | 1.0927 | 45.46 | < .0001 | 1.2257 | 128.64 | < .0001 | |
| Growth stage | SI | 0.7981 | 23 | < .0001 | 0.9247 | 63.49 | < .0001 |
| Growth stage | SII | 1.0051 | 25.2 | < .0001 | 0.8874 | 50.23 | < .0001 |
| Growth stage | SIII | 0 | 0 | ||||
| Genotype | GNT-2 | 2.1593 | 142.72 | < .0001 | 2.4428 | 405.65 | < .0001 |
| Genotype | NDH-98 | 0.1852 | 0.64 | 0.4247 | − 0.2492 | 5.28 | 0.0216 |
| Genotype | Pratibha | 0 | 0 | ||||
| DCS | 1.545 | 22.71 | < .0001 | 0.5921 | 12.57 | 0.0004 | |
| CURS1 | − 0.5897 | 3.63 | 0.0567 | 0 | |||
| CURS2 | 0.0863 | 0.41 | 0.5201 | 0 | |||
| CURS3 | − 2.4214 | 97.63 | < .0001 | − 1.838 | 183.38 | < .0001 | |
| Reduced parameter robust linear test for total curcuminoid | |||||||
| Rho | 3.1409 | 3.94 | 0.1396 | ||||
| Rn^2 | 4.772 | 4.77 | 0.092 | ||||
Pr < ChiSq approximately predicts the associated probability level after the selected variable has been entered or removed
Pr Probability, ChiSq Chi-square, Rho association or correlation, Rn^2 robust Wald-type association test
Discussion
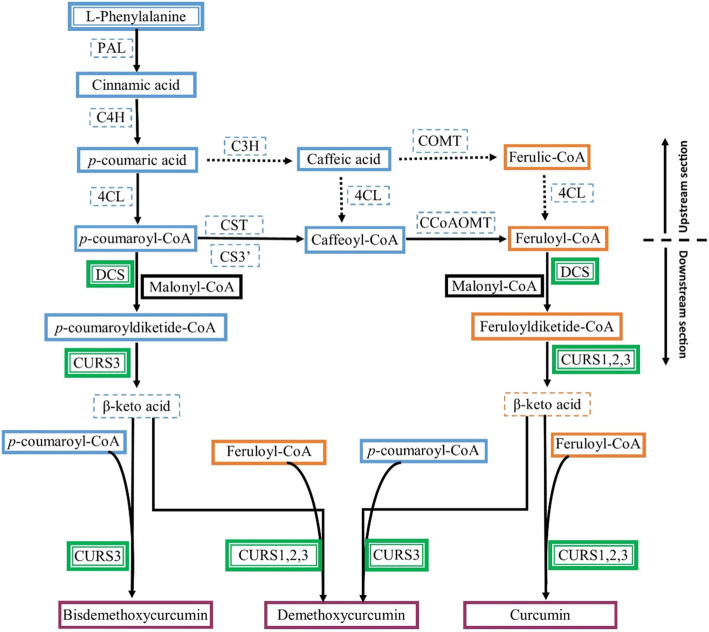
Gene expression studies can quantify the amount of functional mRNA (messenger ribonucleic acid) transcript in the experimental sample measured in terms of reverse-transcribed cDNA (complementary deoxyribonucleic acid) concentration which codes for amino acids and functional proteins. After the identification and characterization of multiple type III PKS enzyme genes involved in the curcuminoid biosynthesis pathway [19, 20], it is now possible to study the differential gene expression patterns using reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) assays in addition to protein-enzyme and product assays in different tissues of turmeric. The role of DCS and CURSs for curcuminoid biosynthesis in turmeric has been described by different studies (Fig. 4); however, the correlation or directional association between the gene expression level and curcuminoids level in turmeric rhizome is still unfolded. In this experiment, three cultivars differing in curcuminoid contents were evaluated for DCS and CURSs gene expressions, and normalized expression values are association tested with curcuminoid contents (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) measured at three different growth stages in turmeric cultivars.
Fig. 4.
Curcuminoid biosynthesis pathway in turmeric [adapted and modified from Ramirez-Ahumada et al. [14] and Katsuyama et al. [19, 20]]. Cinnamic acid is synthesized from phenylalanine by phenylalanine ammonia lyase (PAL) and converted into coumaric acid by cinnamate-4-hydroxylase (C4H). Then, 4-coumarate-CoA ligase (4CL) converts coumaric acid into coumaroyl-CoA, and p-coumaroyl shikimate transferase (CST), p-coumaroyl 5-O-shikimate 3-hydroxylase (CS3=H), and caffeoyl-CoA-O-methyltransferase (CCoAOMT) convert it into feruloyl-CoA. Coumaroyl-CoA and feruloyl-CoA are then converted by diketide-CoA synthase (DCS) into diketide-CoAs by condensation with malonyl-CoA. In the end, multiple curcumin synthases (CURS1, CURS2, and CURS3) catalyze the formation of curcuminoids by condensing the diketide-CoAs with coumaroyl-CoA and feruloyl-CoA. Depending on the combination, different curcuminoids are produced, namely curcumin, demethoxycurcumin, and bisdemethoxycurcumin. The route indicated by solid arrows corresponds to the central phenylpropanoid pathway that occur in vivo, in C. longa
The curcuminoid analysis results showed that a higher turmeric rhizome yield does not necessarily mean higher curcuminoid contents in the turmeric cultivars. The curcuminoid contents were higher in long-growth-duration cultivar than short-growth-duration cultivar irrespective of the rhizome yield (Table 1). Differential gene expression patterns were also observed in three turmeric cultivars yielding different amounts of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin). In general, higher gene expressions were during active vegetative and rhizome development stage (SI–SII) of turmeric cultivars than reduced expression at maturity or senescence stage (SIII) under study except that DCS expression was decreasing in NDH-98 and CURS3 expression was decreasing in Pratibha throughout the growth stages (Fig. 1). Differential expression of CURSs genes was also reported to be positively correlated with the curcumin content in Curcuma spp. during the 3rd and 6th months (active vegetative and rhizome developmental stages); however, during the 9th month (maturity stage), the curcumin content increased and the putative CURSs gene expressions were downregulated [40]. These patterns in each cultivar might have some significant role to play for curcuminoid biosynthesis in different turmeric cultivars suggesting that the genotype and growth stages affect the DCS and CURSs expressions at different developmental stages in turmeric. It was also reported that the highest expression of multiple curcumin synthases in Curcuma caesia Roxb. was found at the peak of its vegetative stage and expression levels of CURSs decreased at the time of maturity [29]. Among the three cultivars under study, there was no significant difference in DCS, CURS1, CURS2, and CURS3 gene expressions between GNT-2 and Pratibha (higher curcumin and total curcuminoid yielding cultivars); however, their expressions differed in NDH-98 (lower curcumin and total curcuminoid yielding cultivar) (Table 2). DCS expression was downregulated whereas CURS2 expression was upregulated in lower curcumin and lower total curcuminoid yielding cultivar (NDH-98) as compared with higher curcumin and higher total curcuminoid yielding cultivars (GNT-2 and Pratibha) and vice versa (Table 2). There was no significant change in the CURS3 expression (< 2-fold change) among the cultivars. CURS3 expressions were also found lower in all the turmeric species differing in curcuminoid yield [24]. These results showed that there is a significant variation in DCS and CURSs gene expressions in turmeric rhizome also affected by genotype and growth stages.
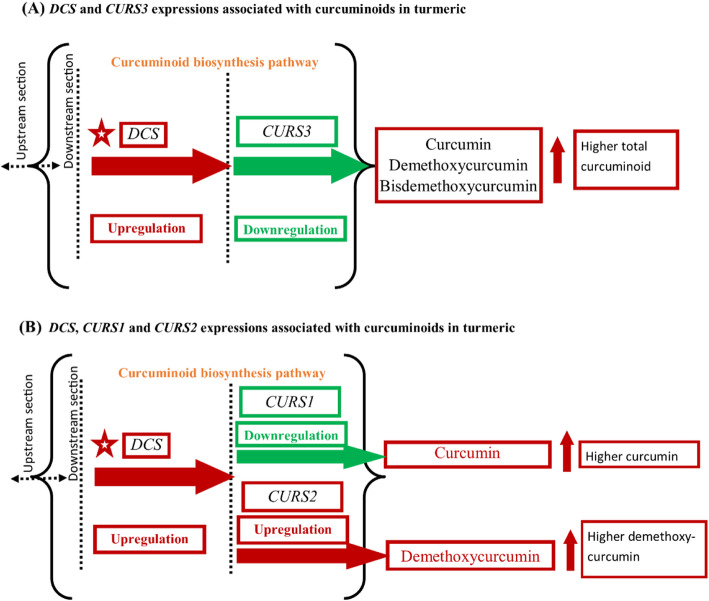
The expressions of CURSs genes were also found to be affected by soil, climate, and harvesting time; however, CURSs expressions were found positively correlated with the curcumin content at a particular agroclimatic zone [30]. The expression of curcumin synthase genes (CURSs) and subsequent curcumin production varied at the different developmental stages of the plant in Curcuma spp. [29, 40]; however, we did not find any publications concerning DCS and CURSs expressions together in association with curcuminoid yield in turmeric to date. CURS1 and CURS2 expressions were also clustered as a distant group from DCS and CURS3 in this experiment (Fig. 3). Additionally, the gene expression studies alone cannot explain the magnitude and direction of the association between genes (DCS and CURSs) and gene products (curcuminoids). Thus, a more robust association-based test (robust regression) was applied for finding out the magnitude and direction of the associations. DCS and CURSs expressions in turmeric rhizome samples at three different growth stages were studied in association with biologically synthesized curcuminoids viz curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid contents in turmeric rhizome samples for identifying any significant association between them. A robust regression analysis using the M-estimator method was used for the association-based test which detects the outliers in the observed dataset and provides more precise information [32]. Association-based tests between the gene expression and curcuminoids revealed that differential expressions of DCS, CURS1, CURS2, and CURS3 are responsible for the differential curcuminoid balance in turmeric rhizome, and their expressions are not always positively associated or correlated with curcuminoid yield. DCS expression had a significant positive but CURS3 expression had a significant negative association with curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid yield in turmeric rhizome (Table 7).
Table 7.
Summary of the association-based tests between gene expression and curcuminoids
| Gene ID | Association test results | Remarks | |||
|---|---|---|---|---|---|
| Curcumin | Demethoxy-curcumin | Bisdemethoxy-curcumin | Total curcuminoid | ||
| DCS | +, *** | +, *** | +, ¥ | +, *** | DCS has a strong positive association with all the curcuminoids. |
| CURS1 | −, *** | NS | NS | −, ¥ | CURS1 expression is negatively associated with curcumin. |
| CURS2 | NS | +, * | NS | NS | CURS2 expression has a positive association with demethoxycurcumin. |
| CURS3 | −, *** | −, *** | −, *** | −, *** | CURS3 expression has a strong negative association with all the curcuminoids. |
| CURS1 and CURS2 | ¥ | *** | NS | ¥ | Covariate effect of CURS1 and CURS2 have significant impact on demethoxycurcumin. |
On the other hand, the covariate effects of CURS1 and CURS2 were also found to be associated with curcuminoid balance in turmeric rhizome. CURS1 expression had a significant negative association with curcumin yield; however, CURS2 expression showed a significant positive association with demethoxycurcumin. The co-regulated expression of CURS1 and CURS2 were also found significantly associated with demethoxycurcumin content in turmeric rhizome (Table 7). It was also reported that DCS and CURSs were capable of synthesizing curcuminoids through the major curcuminoid biosynthesis pathway in turmeric [19, 20] where CURS3 is capable of synthesizing all three curcuminoids; however, CURS1 and CURS2 are mainly responsible for the curcumin and demethoxycurcumin biosynthesis [20]. The results in this study suggested that the level of expression of DCS and CURSs genes have different individual effects on curcuminoid biosynthesis.
The results showed that higher curcuminoid yield in turmeric rhizome is associated with higher DCS and relatively lower CURS3 expressions, and vice versa. Similarly, higher CURS1 expression is associated with lower curcumin yield; however, higher CURS2 expression is associated with a higher demethoxycurcumin in turmeric rhizome, and vice versa (Table 7). This mechanism of co-expression of diketide-CoA synthase and multiple curcumin synthase genes involved in the curcuminoid biosynthesis pathway in turmeric rhizome revealed a significant effect on curcuminoid balance in different turmeric cultivars. Thus, diketide-CoA synthase and multiple curcumin synthase gene expressions are differentially associated with curcuminoid yield in turmeric rhizome, not always positively associated with individual curcuminoid. Further, enzymatic study regarding gene regulation of DCS and multiple curcumin synthases through genetic engineering and biotechnology approach will help to enhance curcuminoid content in turmeric rhizome or in vitro.
Conclusions
Gene expressions of diketide-CoA synthase and multiple curcumin synthases involved in the curcuminoid biosynthesis pathway in turmeric rhizome are influenced by genotype and growth stages. DCS and CURS3 expression patterns were closely associated and distinct from CURS1 and CURS2 expression patterns in turmeric rhizome. The association-based tests showed that DCS and CURSs gene expressions are not always positively associated with curcuminoid yield in turmeric rhizome. DCS expression had a significant positive but CURS3 expression had a significant negative association with curcumin, demethoxycurcumin, bisdemethoxycurcumin, and total curcuminoid yield. Curcumin, a major curcuminoid, is also found negatively associated with CURS1 expression. The co-regulated expressions of CURS1 and CURS2 were significantly associated with demethoxycurcumin content in turmeric rhizome. The gene regulation study of DCS co-expressed with multiple curcumin synthases (CURSs) and the overexpression of DCS through genetic engineering approach will help to enhance curcuminoid content in turmeric rhizome or in vitro for medicinal and industrial use.
Supplementary Information
Additional file 1: Table S1. DCS and multiple curcumin synthases gene expressions at different stages in turmeric rhizome. Table S2. Gene specific and reference primers used in qPCR assay. Table S3. Duncan’s mean comparison of DCS, CURS1, CURS2 and CURS3 expressions among turmeric cultivars. Annex 1. SAS codes for association-based test using ROBUSTREG procedure.
Additional file 2: Fig S1. Chromatograms and calibration curves for curcumin, demothoxycurcumin and bisdemethoxycurcumin standards using HPLC. Fig S2. Chromatograms showing individual curcuminoid contents measured using HPLC at three stages of growth of three turmeric cultivars. Fig S3. Melt peak curves and gel electrophoresis results for DCS, CURS1, CURS2, CURS3 and reference gene Actin. In melt peak curves, single peak of each gene specific product was observed in each sample. In gel image, N represents ‘NDH-98’; G represents, ‘GNT-2’; and P represents ‘Pratibha’ cultivars of turmeric under study.
Acknowledgements
The research work and laboratory analysis were supported by the Department of Genetics and Plant Breeding, N. M. College of Agriculture, Navsari Agricultural University (NAU), Gujarat, India. The research opportunity provided by the Nepal Aid Fund Scholarship Scheme 2016-17 from the Government of India is highly acknowledged. We are privileged by the excellent coordination and laboratory facility available at Forest Biotechnology Laboratory in ASPEE College of Horticulture and Forestry, Sugarcane Research Station, and Food and Quality Testing Laboratory (FQTL), NAU. Suggestions and guidance during research work from Dr. A. V. Narwade, Dr. C. V. Kapadiya, Dr. Madhu Bala, Shreya Sen, Haidar Abbas, and the Qiagen Support team are highly acknowledged. Assistance during laboratory work from colleagues Ajay Gamit, Gaurav Patel, Vrunda Rawal, and Harshal Modi is acknowledged with appreciation.
Abbreviations
- CoA
Coenzyme A
- cDNA
Complementary deoxyribonucleic acid
- CURS1
Curcumin synthase 1
- CURS2
Curcumin synthase 2
- CURS3
Curcumin synthase 3
- DCS
Diketide-CoA synthase
- HPLC
High-performance liquid chromatography
- mRNA
Messenger ribonucleic acid
- PCR
Polymerase chain reaction
- PKS
Polyketide synthase
- RT-qPCR
Reverse transcription quantitative real-time PCR
- SAS
Statistical Analysis System
- UDG
Uracil DNA glycosylase
Authors’ contributions
DKA did the laboratory work, data analysis, and manuscript writing. KM guided the laboratory works, interpreted the results, and proofread the manuscript. VP guided the RT-qPCR, curcuminoid analysis, data analysis, and interpretation. RP ensured the funding for the laboratory work and guided the research work. VR and AB assisted during laboratory analysis and manuscript writing. Further, all the authors have read and approved the final manuscript and agreed for submitting the manuscript for publication in the Journal of Genetic Engineering and Biotechnology.
Funding
This research work was supported by the Department of Genetics and Plant Breeding, Navsari Agricultural University, Gujarat, India, for studying the gene expression in association with curcuminoid biosynthesis in turmeric cultivars. The research fund was utilized for purchasing reagents and doing laboratory works.
Availability of data and materials
The dataset supporting the conclusions of this article are included within the article and its additional file: Additional file 1: Table S1, Table S2, and Annex 1.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s43141-020-00101-2.
References
- 1.Sun W, Wang S, Zhao W, Wu C, Guo S, Gao H, Tao H, Lu J, Wang Y, Chen X. Chemical constituents and biological research on plants in the genus curcuma. Crit Rev Food Sci Nutr. 2017;57(7):1451–1523. doi: 10.1080/10408398.2016.1176554. [DOI] [PubMed] [Google Scholar]
- 2.Plotto A (2004) Turmeric: post-production management. Food and Agriculture Organization http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Turmeric.pdf
- 3.Krup V, Prakash L, Harini A. Pharmacological activities of turmeric (Curcuma longa Linn): a review. J Homeop Ayurv Med. 2013;2(4):133. doi: 10.4172/2167-1206.1000133. [DOI] [Google Scholar]
- 4.Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet Resour. 2005;3(2):230–251. doi: 10.1079/PGR200574. [DOI] [Google Scholar]
- 5.Vogel A, Pelletier J. Examen chimique de la Racine de Curcuma. J Pharm. 1815;1:289–300. [Google Scholar]
- 6.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010;30(5):818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin-from molecule to biological function. Angew Chem Int Ed. 2012;51(22):5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Wu D, Zhang Y, Zhou H, Lai T, Ding W. RNA-Seq analysis reveals candidate targets for curcumin against Tetranychus cinnabarinus. Biomed Res Int. 2016;2016:2796260. doi: 10.1155/2016/2796260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathaur P, Raja W, Ramteke P, John SA. Turmeric: the golden spice of life. Int J Pharm Sci Res. 2012;3(8):1987. [Google Scholar]
- 13.Katsuyama Y, Matsuzawa M, Funa N, Horinouchi S. Production of curcuminoids by Escherichia coli carrying an artificial biosynthesis pathway. Microbiol. 2008;154(9):2620–2628. doi: 10.1099/mic.0.2008/018721-0. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Ahumada MC, Timmermann BN, Gang DR. Biosynthesis of curcuminoids and gingerols in turmeric (Curcuma longa) and ginger (Zingiber officinale): identification of curcuminoid synthase and hydroxycinnamoyl-CoA thioesterases. Phytochemistry. 2006;67(18):2017–2029. doi: 10.1016/j.phytochem.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Katsuyama Y, Matsuzawa M, Funa N, Horinouchi S. In vitro synthesis of curcuminoids by type III polyketide synthase from Oryza sativa. J Biol Chem. 2007;282(52):37702–37709. doi: 10.1074/jbc.M707569200. [DOI] [PubMed] [Google Scholar]
- 16.Kita T, Imai S, Sawada H, Kumagai H, Seto H. The biosynthetic pathway of curcuminoid in turmeric (Curcuma longa) as revealed by 13C-labeled precursors. Biosci Biotechnol Biochem. 2008;72(7):1789–1798. doi: 10.1271/bbb.80075. [DOI] [PubMed] [Google Scholar]
- 17.Koo HJ, McDowell ET, Ma X, Greer KA, Kapteyn J, Xie Z, Descour A, Kim H, Yu Y, Kudrna D, Wing RA, Soderlund CA, Gang DR. Ginger and turmeric expressed sequence tags identify signature genes for rhizome identity and development and the biosynthesis of curcuminoids, gingerols and terpenoids. BMC Plant Biol. 2013;13(1):27. doi: 10.1186/1471-2229-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheeja TE, Deepa K, Santhi R, Sasikumar B. Comparative transcriptome analysis of two species of Curcuma contrasting in a high-value compound curcumin: insights into genetic basis and regulation of biosynthesis. Plant Mol Biol Report. 2015;33(6):1825–1836. doi: 10.1007/s11105-015-0878-6. [DOI] [Google Scholar]
- 19.Katsuyama Y, Kita T, Funa N, Horinouchi S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J Biol Chem. 2009;284(17):11160–11170. doi: 10.1074/jbc.M900070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuyama Y, Kita T, Horinouchi S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 2009;583(17):2799–2803. doi: 10.1016/j.febslet.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Annadurai RS, Neethiraj R, Jayakumar V, Damodaran AC, Rao SN, Katta MA, Gopinathan S, Sarma SP, Senthilkumar V, Niranjan V, Gopinath A, Mugasimangalam RC. De novo transcriptome assembly (NGS) of Curcuma longa L. rhizome reveals novel transcripts related to anticancer and antimalarial terpenoids. PLoS One. 2013;8(2):e56217. doi: 10.1371/journal.pone.0056217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resmi MS, Soniya EV. Molecular cloning and differential expressions of two cDNA encoding type III polyketide synthase in different tissues of Curcuma longa L. Gene. 2012;491(2):278–283. doi: 10.1016/j.gene.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Deepa K, Sheeja T, Rosana O, Srinivasan V, Krishnamurthy K, Sasikumar B. Highly conserved sequence of ClPKS11 encodes a novel polyketide synthase involved in curcumin biosynthesis in turmeric (Curcuma longa L.) Ind Crop Prod. 2017;97:229–241. doi: 10.1016/j.indcrop.2016.12.003. [DOI] [Google Scholar]
- 24.Li D, Ono N, Sato T, Sugiura T, Altaf-Ul-Amin M, Ohta D, Suzuki H, Arita M, Tanaka K, Ma Z, Kanaya S. Targeted integration of RNA-Seq and metabolite data to elucidate curcuminoid biosynthesis in four Curcuma species. Plant Cell Physiol. 2015;56(5):843–851. doi: 10.1093/pcp/pcv008. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa H, Minaniya Y, Ito K, Yamamoto Y, Fukuda T. Difference of curcumin content in Curcuma longa L.(Zingiberaceae) caused by hybridization with other Curcuma species. Am J Plant Sci. 2011;2(02):111–119. doi: 10.4236/ajps.2011.22013. [DOI] [Google Scholar]
- 26.Sandeep IS, Kuanar A, Akbar A, Kar B, Das S, Mishra A, Sial P, Naik PK, Nayak S, Mohanty S. Agroclimatic zone based metabolic profiling of turmeric (Curcuma Longa L.) for phytochemical yield optimization. Ind Crop Prod. 2016;85:229–240. doi: 10.1016/j.indcrop.2016.03.007. [DOI] [Google Scholar]
- 27.Anandaraj M, Prasath D, Kandiannan K, Zachariah TJ, Srinivasan V, Jha A, Singh B, Singh A, Pandey V, Singh S. Genotype by environment interaction effects on yield and curcumin in turmeric (Curcuma longa L.) Ind Crop Prod. 2014;53:358–364. doi: 10.1016/j.indcrop.2014.01.005. [DOI] [Google Scholar]
- 28.Sandeep IS, Sanghamitra N, Sujata M. Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma) Indian J Exp Biol. 2015;53(6):406–411. [PubMed] [Google Scholar]
- 29.Behar N, Tiwari K, Jadhav S. Semi-quantitative expression studies of genes involved in biosynthesis of curcuminoid in Curcuma caesia Roxb. Indian J Biotechnol. 2016;15(4):491–494. [Google Scholar]
- 30.Sandeep IS, Das S, Nasim N, Mishra A, Acharya L, Joshi RK, Nayak S, Mohanty S. Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): towards site specific cultivation for high curcumin yield. Plant Physiol Biochem. 2017;118:348–355. doi: 10.1016/j.plaphy.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Ayer D, Modha K, Parekh V, Patel R, Ramtekey V, Bhuriya A. Comparative gene expression study between two turmeric (Curcuma longa L.) cultivars. JOSAC. 2018;27(2):131–137. [Google Scholar]
- 32.Seo M, Kim K, Yoon J, Jeong JY, Lee H-J, Cho S, Kim H. RNA-seq analysis for detecting quantitative trait-associated genes. Sci Rep. 2016;6:24375. doi: 10.1038/srep24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousseeuw PJ, Leroy AM (2005) Robust regression and outlier detection. Wiley
- 34.MacRae E. Extraction of plant RNA. Methods Mol Biol. 2007;353:15–24. doi: 10.1385/1-59745-229-7:15. [DOI] [PubMed] [Google Scholar]
- 35.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 36.Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT-qPCR—publishing data that conform to the MIQE guidelines. Methods. 2010;50(4):S1–S5. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SAS . The ROBUSTREG procedure. Cary: Statistical Analysis System; 2015. [Google Scholar]
- 40.Santhoshkumar R, Yusuf A (2020) Comparative differential expression of CURS genes and determination of curcumin content at different growth stages of Curcuma longa L. and its wild relative C. zanthorrhiza Roxb. Genet Resour Crop Evol. 10.1007/s10722-020-00970-z
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. DCS and multiple curcumin synthases gene expressions at different stages in turmeric rhizome. Table S2. Gene specific and reference primers used in qPCR assay. Table S3. Duncan’s mean comparison of DCS, CURS1, CURS2 and CURS3 expressions among turmeric cultivars. Annex 1. SAS codes for association-based test using ROBUSTREG procedure.
Additional file 2: Fig S1. Chromatograms and calibration curves for curcumin, demothoxycurcumin and bisdemethoxycurcumin standards using HPLC. Fig S2. Chromatograms showing individual curcuminoid contents measured using HPLC at three stages of growth of three turmeric cultivars. Fig S3. Melt peak curves and gel electrophoresis results for DCS, CURS1, CURS2, CURS3 and reference gene Actin. In melt peak curves, single peak of each gene specific product was observed in each sample. In gel image, N represents ‘NDH-98’; G represents, ‘GNT-2’; and P represents ‘Pratibha’ cultivars of turmeric under study.
Data Availability Statement
The dataset supporting the conclusions of this article are included within the article and its additional file: Additional file 1: Table S1, Table S2, and Annex 1.