Summary:
Monitoring of glucose levels is essential to effective diabetes management. Over the past 100 years, there have been numerous innovations in glucose monitoring methods. The most recent advances have centered on continuous glucose monitoring (CGM) technologies. Numerous studies have demonstrated use of continuous glucose monitoring confers significant glycemic benefits on individuals with type 1 diabetes (T1DM) and type 2 diabetes (T2DM). Ongoing improvements in accuracy and convenience of CGM devices have prompted increasing adoption of this technology. The development of standardized metrics for assessing CGM data has greatly improved and streamlined analysis and interpretation, enabling clinicians and patients to make more informed therapy modifications. However, many clinicians many be unfamiliar with current CGM and how use of these devices may help individuals with T1DM and T2DM achieve their glycemic targets. The purpose of this review is to present an overview of current CGM systems and provide guidance to clinicians for initiating and utilizing CGM in their practice settings.
Keywords: Continuous glucose monitoring, CGM, real-time CGM, rtCGM, intermittently scanned CGM, isCGM, type 1 diabetes, type 2 diabetes, HbA1c, Ambulatory Glucose Profile, AGP
1. Introduction
Innovations in glucose monitoring have resulted in the development of continuous glucose monitoring (CGM) systems, which have been shown to confer significant benefits in improving glycemic control. [1–11] Although adoption of CGM is steadily increasing, use of this technology has been centered mainly in endocrinology and diabetes specialty practices. As such, many primary care physicians may be less familiar with CGM and how it can benefit their patients with diabetes. The purpose of this review is to present an overview of current CGM systems and provide guidance to clinicians for initiating and utilizing CGM in their practice settings.
2. Evolution of Glucose Monitoring
The field of glucose monitoring has progressed significantly over the last 100 years, since Stanley Rossiter Benedict published his seminal work on analytical methods for measuring urinary glucose.[12] The Benedict’s Solution/Assay was the main test for diabetes monitoring for the next 50 years, until the glucose-oxidase based reactions were discovered in the late 1950’s, [13] and later adapted to be used in clinical laboratories to measure plasma glucose – initially manually, and currently by automated methods [14]
Since then, there were several advances in the development of glucose test strips, but it was not until the late 1970s to early 1980s that the concept of self-monitoring of blood glucose (SMBG) with glucose meters became more widely applied. [13] The next stage was the development of continuous glucose monitoring (CGM) around this time, but the first CGM was not commercially available until 1999.[15] However, these initial CGMs had limited use in clinical settings.
Several revolutionary advances in CGM technology have occurred with improved accuracy, use of smaller and less invasive devices, extended sensor life, approval for insulin dose decisions and with the elimination of finger-sticks need for capillary glucose measurements in factory-calibrated CGMs, thereby decreasing the patient’s burden of diabetes care. All these advances have led to better patient’s satisfaction and adherence with device use and medication therapy, increased awareness by clinicians and a significant increase in CGM use, mostly in patients with T1DM, but also in patients with T2DM treated with intensive insulin regimens.[16–19]
Improvements in CGM technology has also permitted remarkable advances in the integration with continuous subcutaneous insulin infusion (CSII) or insulin pumps, and further development of automated insulin delivery systems, or “closed-loop systems”, along with the creation and validation of newer CGM-based glycemic metrics, beyond SMBG and HbA1c.[20]
3. CGM
3.1. Rationale for CGM
Although SMBG remains an important tool that guides glycemic management strategies and decision-making for patients with DM and their clinicians, it can provide point-in-time measurements of current glucose levels, with no predictive information about impending glucose levels. Whereas, CGM devices present both the current glucose level and rate of change (ROC) trend arrows, indicating the direction and rapidity of changing glucose. These data enable patients to respond immediately to mitigate or prevent acute glycemic events and allow patients to make better informed decisions in their medication requirements and other areas of their daily diabetes self-management. Moreover, historical data can be viewed in the device reader/receiver or smartphone app and downloaded for retrospective analysis by patients with diabetes and their clinicians.
3.2. Personal CGM Technologies
There are currently two types of personal CGM system technologies available: real-time CGM (rtCGM) and intermittently scanned CGM (isCGM), which is often referred to as “flash” CGM. Current rtCGM systems include the Dexcom G6 (Dexcom, Inc., San Diego, USA), Medtronic Guardian Sensor 3 (Medtronic, Inc., Northridge, USA) and Senseonics Eversense (Senseonics, Inc., Germantown, USA). Relevant features of the current personal CGM devices are presented in Table 1.
Table 1.
Key features of current personal CGM systems
| Feature | FreeStyle Libre 2 | Dexcom G6 | Medtronic Guardian Connect | Eversense |
|---|---|---|---|---|
| Indication, years of age | ≥4 | ≥2 | ≥7 | ≥18 |
| Sensor wear, days | 14 | 10 | 7 | 90 (US), 180 (Europe) |
| Requires fingerstick test calibration | no | no | 2 x/day | 2x/day |
| Warm up period (hrs) | 1 | 2 | 2 | 24 (upon insertion of the sensor)* |
| Requires confirmatory fingerstick test for insulin dosing | no | no | yes | no |
| Active alarms/alerts | yes | yes | yes | yes |
| Real-time remote monitoring (data sharing) | yes | yes | yes | yes |
| Connects with insulin pump | no | yes | yes | no |
| Accuracy: Overall’ MARD, % | 9.3 [28] | 9.0[29] | 10.4[30] | 9.0[31] |
| Chemical substances Interferences | Ascorbic Acid; Salicylic Acid [32] | Hydroxyurea; [29] repeated doses of APAP [33] | Acetaminophen; Ethanol/Wine; Albuterol; Lisinopril; Atenolol; Atorvastatin; Ascorbic Acid [34] | Tetracycline; Mannitol [35] |
| Interferences from Radiological Studies | Limited evidence of in-vitro exposure to X-Ray and RT, CT or MRI did not impact the data recorded by Libre Pro in 10 sensors [36] | No impact from X-Ray, CT or angiography among hospitalized patients (n=49); [37] there is some migration from MRI [38] | Medtronic recommends that users remove the sensor in the presence of X-ray, CT, MRI PET, Airport scanners [39] | Close contact with direct electromagnetic interference (EMI) may interfere with the smart transmitter’s ability to send data to the mobile device. [40] |
The U.S. Food & Drug Administration cautions that readings from the first 12 hours of use should not form the basis of treatment decisions. MARD : Mean Absolute Relative Difference
The Guardian Sensor 3 and Dexcom G6 systems comprise three components: a disposable wired sensor inserted in the subcutaneous tissue; a transmitter that is attached to the sensor; and a receiver (handheld or smartphone) that displays the glucose data. The Dexcom G6 can be used with the receiver and smartphone app, whereas, the Guardian Connect is used only with a smartphone app. The Eversense rtCGM consists of a sensor implanted by a clinician into the subcutaneous tissue in the upper arm; an external transmitter that is secured above the sensor; and a smartphone app, which serves as the receiver. The most current isCGM system is the FreeStyle Libre 2 (Abbott Diabetes Care, Alameda, USA). The system comprises two components: a sensor/transmitter unit that is inserted in the upper arm; and a handheld touchscreen reader device or a smartphone app (LibreLink). Both the rtCGM and isCGM technologies are available as standalone devices. Furthermore, Guardian 3 and Dexcom G6 can be linked to sensor-augmented insulin infusion pumps or automatic insulin delivery (AID) systems.
A key differentiator between the two CGM technologies is how data are delivered to the user. RtCGM systems automatically transmit data to the patient’s receiver and/or smartphone. In contrast, isCGM systems require the patient to “swipe” the receiver close to the sensor to obtain current and historical glucose data. However, if more than 8 hours occur between scans, only the most recent 8 hours of data will be retained and available for review.
An important feature of both rtCGM and isCGM systems is the ability to alert users when glucose levels are rising above or falling below the target glucose range. Both technologies allow users to set high and low alarms/alerts. In addition, the Dexcom G6 system has a predictive “urgent low soon” (ULS) glucose alarm at 55 mg/dL [3.1 mmol/L].[21] This advanced warning has the ability to alert users when glucose is predicted to drop below 55 mg/dL [3.1 mmol/L] and been shown to be effective in reducing hypoglycemia among rtCGM-experienced users.[22] The Guardian Connect CGM System (Medtronic, Northridge, CA, USA), including the Enlite™ glucose sensor and the Guardian Connect Transmitter has similar predictive low glucose alerts plus a predictive high alert feature as well.[23]
An added safeguard of some current CGM systems is the ability to share data with clinicians, caregivers and family/friends in which glucose data and alarms/alerts are shared with family/friends and caregivers. A recent retrospective analysis of device usage and glycemic control in 15,000 children and adult found that real-time sharing CGM data was associated with improved device utilization and glycemic control.[24]
3.3. Professional CGM Technologies
Professional CGM consists of a real-time or masked CGM that is worn by patients for short periods (typically 6–14 days). The clinician uses the CGM data to assess patient glycemic status, make changes to treatment regimens to achieve glycemic targets and provide patient education. Three professional CGM systems are currently available: Dexcom G6 Pro CGM system; Medtronic iPro2 Professional CGM system; and FreeStyle Libre Pro system. The Dexcom G6 Pro CGM system can be programmed to provide real-time masked or unmasked data over 10 days. [25] Accordingly, it can also be used by patients as a trial for personal CGM or to get near immediate feedback about the impact of lifestyle or therapy decisions on glycemia. The iPro2 system provides masked retrospective data for up to 6 days. [26] The FreeStyle Libre Pro system provides masked retrospective data up to 14 days. [27]
4.0. Evidence of Efficacy and Safety
4.1. rtCGM
The clinical efficacy, safety and other benefits of rtCGM use in individuals with T1DM and T2D, regardless of the insulin delivery method used, have been demonstrated in numerous studies. [1, 2, 41–50] The large, randomized DIAMOND trials showed improved HbA1c, reduced time spent in the hypoglycemic and hyperglycemic ranges and reductions in moderate to severe hypoglycemia in individuals with multiple daily injections (MDI)-treated T1DM and T2DM using rtCGM compared with traditional SMBG.[1, 2] Investigators also reported significant reductions in diabetes-related distress and greater hypoglycemic confidence among the rtCGM users, [45] Importantly, findings from a recent randomized trial found that rtCGM use significantly increased time spent in normoglycemia and reduced severe hypoglycemia in among individuals with impaired hypoglycemia awareness.[48]
Similar results were shown in the recent HypoDE study, which investigated rtCGM use vs. SMBG in MDI-treated T1DM adults with problematic hypoglycemia (e.g., impaired hypoglycemia awareness, frequent severe and/or nocturnal hypoglycemia).[8] Compared with SMBG, rtCGM use was associated with fewer low glucose events and episodes of severe hypoglycemia.
Studies have also that rtCGM as a component of sensor-augmented insulin pumps (SAP) with predictive low glucose suspend (PLGS) functionality reduces the incidence and severity of hypoglycemia,[51–53] suggesting that automated suspension of insulin infusion in response to impending low glucose can assist individuals with T1DM avoid hypoglycemia without significantly increasing hyperglycemia.[51] Use of a hybrid closed-loop (HCL) insulin delivery system also showed notable reductions in HbA1c, glycemic variability and time spent in the low glycemic ranges, as well as improved time in target range.[52]
Advances in automated insulin delivery research have led to the development of hybrid and advanced hybrid closed-loop control (CLC) systems, which utilize integrated CGM-insulin pump systems with algorithm-driven controllers that automatically control delivery of basal insulin and correction boluses. Randomized controlled trials and meta-analyses have consistently shown that use of CLC insulin delivery systems can improve glycemic control, increase time in range and reduce hypoglycemia risk in pediatric and adult T1DM patients.[54–58] Most recently Brown et al. investigated use of a CLC system in 168 adolescent and adult patients with T1DM who were randomized 2:1 to CLC or sensor-augmented insulin pump therapy and followed for 6 months.[59] Investigators reported that use of the CLC system was associated with a higher percentage of time in target glycemic range compared with sensor-augmented pump (SAP) in experienced users. In a follow-up trial, 109 CLC-users from the previous study were randomly assigned to CLC or treatment with a hybrid closed-loop (HCL) system with low glucose suspend (LGS) and followed for an additional 3 months.[60] Switching to the HCL system resulted in reduced time in range and with increased HbA1c; however, similar reductions in hypoglycemia were observed in both groups. A recent study by Breton et al. showed that use of a CLC system resulted in a higher percentage of time in glucose range in T1D children compared with a sensor-augmented insulin pump.[61] The next step in CLC research is the development of systems that provide full closed-loop control that alleviates the need for meal announcements, alerts/alarms and ongoing maintenance.
4.2. isCGM
Use of isCGM has been associated with reductions in hypoglycemia, increased time in range, lower glycemic variability and improved patient satisfaction in individuals have been associated with isCGM use in randomized controlled trials with well-controlled T1DM [4] and T2DM [6] who were treated with intensive insulin therapy. In the IMPACT study, use of an earlier generation FreeStyle Libre system was associated with a 38% reduction in time spent in hypoglycaemia (<70 mg/dL), with increased time in range and reductions in glycemic variability.[4] Similar results were reported in the REPLACE study, which showed an association between FreeStyle Libre use and a 43% reduction in time spent in hypoglycemia in a large T2DM population treated with intensive insulin therapy.[6] Although reductions in HbA1c were not seen in either of these two randomized controlled trials, recent prospective, observational studies have demonstrated significant reductions in HbA1c [9–11, 62, 63] and hypoglycemia [9–11, 62] compared with SMBG within large T1DM and T2DM populations. Moreover, some of these studies also showed significant reductions in hospitalizations for hypoglycemia [9, 10] and absenteeism.[9, 10]
5. Beyond HbA1c: Rationale for Clinical Use of CGM Metrics
HbA1c has been long considered the gold standard in assessing the risk for long-term microvascular and macrovascular complications. However, the accuracy of HbA1c test results can be falsely high or low in numerous conditions such as iron deficiencies,[64] anemia,[65] hemoglobinopathies[66] and chronic kidney disease. [67] Pregnancy[68] and ethnic and racial differences in glycation rates[69–71] are also linked to inaccuracies in test results. It has also been shown that a single HbA1c value may encompass a wide glucose range.[72] For example, whereas the mean glucose range for an HbA1c value of 8.0% (64 mmol/mol) is 155 to 218 mg/dL, the mean glucose for an HbA1c value of 7.0% (53 mmol/mol) ranges from 128 to 190 mg/dL.[72] Moreover, HbA1c testing provides no information about the frequency and magnitude of acute intra-day glycemic excursions or overall glycemic variability. Despite these limitations, HbA1c testing is a valuable tool when used in conjunction with CGM data. Moreover, HbA1c remains an accepted quality metric for assessing effectiveness and quality of care by HEDIS (HealthCare Effectiveness Data and Information Set), with potential impact on reimbursement.
5.1. New CGM-Based Glycemic Metrics
In 2017, an expert panel met to develop consensus recommendations for interpreting CGM data and identified 14 key CGM metrics for assessing glycemic status.[73] In 2019, the panel reconvened to develop specific CGM targets relevant to these metrics in order to assist clinicians and patients with diabetes in interpreting and utilizing CGM data in routine clinical care.[74] (Table 2)
Table 2.
CGM Metrics for Use in Clinical Care
| 1. Number of Days CGM Worn (recommend 14 days) [75, 76] | |
| 2. Percentage of time CGM is active (recommend 70% of data from 14 days) | |
| 3. Mean Glucose | |
| 4. Glucose Management Indicator (GMI) [77] | |
| 5. Glycemic Variability (%CV) Target ≤36% [78] | |
| 6. Time Above Range (TAR) - % of readings and time >250 mg/dL (>13.9 mmol/L) | Level 2 |
| 7. Time Above Range (TAR) - % of readings and time 181–250 mg/dL (10.1–13.9 mmol/L) | Level 1 |
| 8. Time In Range (TIR) - % of readings and time 70–180 mg/dL (3.9–10.0 mmol/L) | In Range |
| 9. Time Below Range (TBR) - % of readings and time 54–69 mg/dL (3.0–3.8 mmol/L) | Level 1 |
| 10. Time Below Range (TBR) - % of readings and time <54 mg/dL (<3.0 mmol/L) | Level 2 |
Among the 10 core metrics selected for use in clinical care, the panel identified three metrics that could be used as an initial starting point for assessing glycemic status: time in range (TIR: 70–180 mg/dL [3.9–10 mmol/L]), time below range (TBR: <70 mg/dL [<3.9 mmol/L]; <54 mg/dL [<3 mmol/L]); and time above range (TAR: >180 mg/dL [>10 mmol/L]; >250 mg/dL [>13.9 mmol/L]).[74] As reported by Beck et al. [79] and Vigersky et al., [80] TIR has been shown to closely correlate with HbA1c values. (Table 3) TIR has also been to closely correlate with peripheral neuropathy in T2DM and chronic renal disease, [81] and it has been recommended as an outcome measure for future clinical trials. [82]
Table 3.
| TIR 70–180 mg/dL (3.9–10.0 mmol/L) |
Estimated A1C % (mmol/mol) |
TIR 70–180 mg/dL (3.9–10.0 mmol/L) |
Estimated A1C % (mmol/mol) |
|---|---|---|---|
| 20% | 9.4 (79) | 20% | 10.6 (92) |
| 30% | 8.9 (74) | 30% | 9.8 (84) |
| 40% | 8.4 (68) | 40% | 9.0 (75) |
| 50% | 7.9 (63) | 50% | 8.3 (67) |
| 60% | 7.4 (57) | 60% | 7.5 (59) |
| 70% | 7.0 (53) | 70% | 6.7 (50) |
| 80% | 6.5 (48) | 80% | 5.9 (42) |
| 90% | 6.0 (42) | 90% | 5.1 (32) |
| Every 10% increase in TIR = ~0.5% (5.5) A1C reduction | Every 10% increase in TIR = ~0.8% (8.7) A1C reduction | ||
To further simplify data interpretation, the panel recommended use of a composite metric, TIR/TBR, focusing primarily on reducing TBR while increasing TIR. This approach would reinforce the need to reduce time in hypoglycemia, and at the same time, reduce time spent in hyperglycemia. As shown in Table 4, the specific targets time spent in these ranges were adjusted according to hypoglycemia risk. For example, the tighter target for time spent <70 mg/dL (<3.9 mmol/L) among older and/or high-risk patients was offset by a more relaxed target for time spent >250 mg/dL (>13.9 mmol/L).
Table 4.
Targets for assessment of glycemic control: Type 1 / Type 2 and Older/High-Risk Individuals [74]
| Diabetes Group |
Time in Range (TIR) | Time Below Range (TBR) | Time Above Range (TAR) | |||
|---|---|---|---|---|---|---|
| Within Target Range | % of readings time/day | Below Target Level | % of readings time/day | Above Target Level | % of readings time/day | |
| Type 1* / Type 2 | 70–180 mg/dL 3.9–10 mmol/L |
>70% >16hr, 48 min |
<70 mg/dL <3.9 mmol/L |
<4% <1 hr |
>180 mg/dL >10 mmol/L |
<25% <6 hr |
| <54 mg/dL <3.0 mmol/L |
<1% <15 min |
>250 mg/dL >13.9 mmol/L |
<5% <1 hr, 12 min |
|||
| Older/High-Risk | 70–180 mg/dL | >50% | <70 mg/dL | <1% | >250 mg/dL | <10% |
For age <25 yr., if the A1C goal is 7.5% then set TIR target to approximately 60%.
5.2. Standardized CGM Data Presentation
In their consensus guidelines for use of CGM metrics, the expert panel recommended adoption of the ambulatory glucose profile (AGP) as a standardized template for displaying CGM metrics. [83, 84] (Figure 1) The AGP report facilitates rapid assessment of TIR, TBR and TAR, as well as other metrics, such as average glucose and the glucose management indicator (GMI) calculation. [77] Glucose variability is presented as a percentage coefficient of variation (%CV), with the recommended goal of <36%. [78] The composite glucose profile and daily profiles allow clinicians and patients to quickly identify problematic glucose patterns, which facilitate more informed clinical decision making and enhance patient-clinician collaboration. Many CGM manufacturers and third-party developers have adopted the AGP as a template for their own download software.
Figure 1.
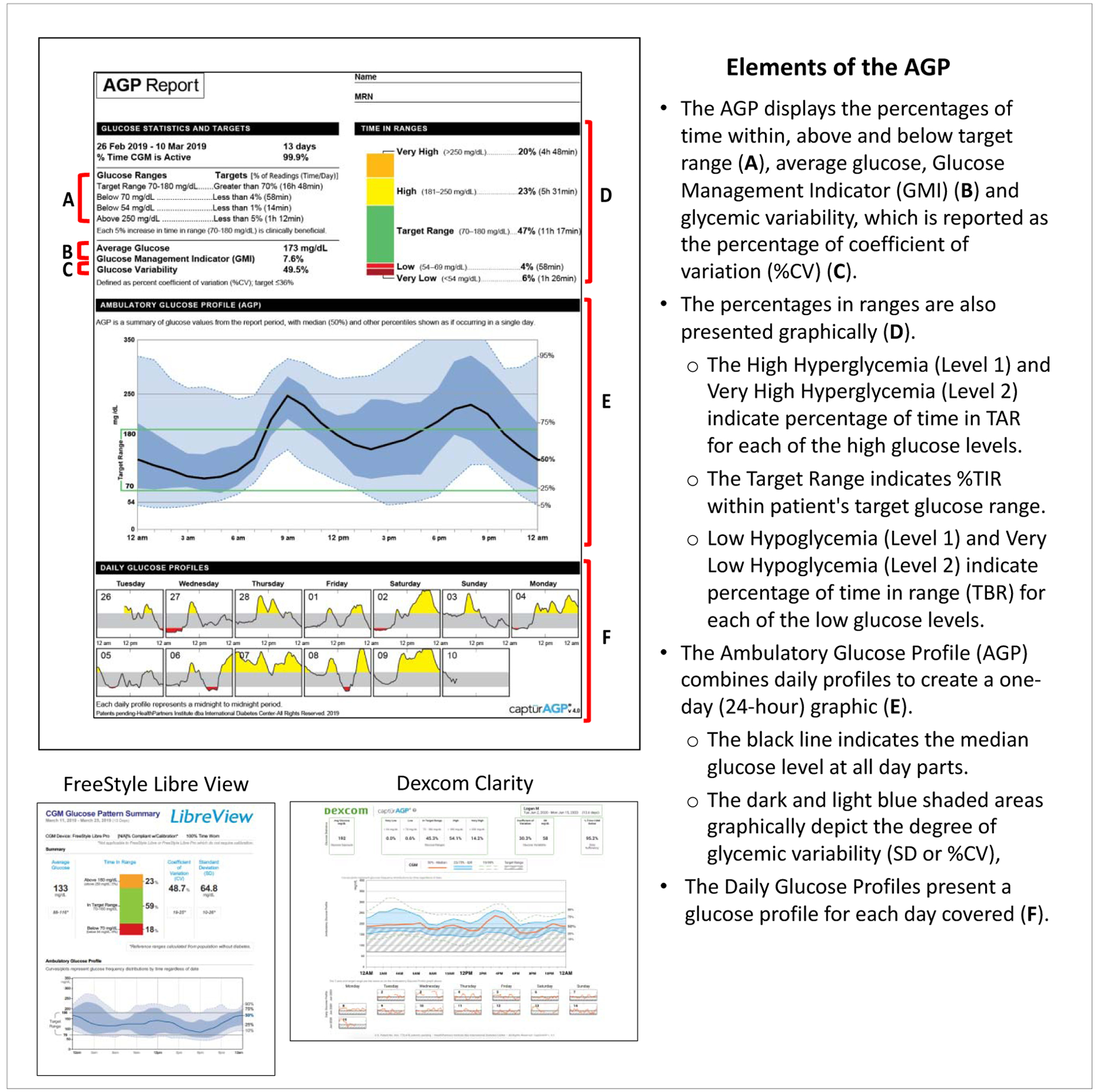
Ambulatory Glucose Profile (AGP)
5.3. CGM Data Interpretation
An important feature of current CGM systems is the ability to transmit glucose data to clinicians for retrospective analysis to identify problematic glycemic patterns that require attention. Clinicians can then provide feedback via in-clinic visits or telehealth technologies (e.g., phone, text, video conference) to counsel patients as needed. Although several approaches for interpreting data have been proposed,[85–89] clinicians who are less familiar with CGM may want to consider a 5-step process for interpreting AGP reports. (Table 5)
Table 5.
CGM Data Interpretation Process
| Step 1 | Confirm that sufficient CGM data are available for analysis. A minimum of 14 days of CGM use, covering at least 70% (10 days), is needed for accurate interpretation. |
| Step 2 | Evaluate the average glucose and Glucose Management Indicator (GMI) and then review the TIR, TBR and TAR statistics. |
| Step 3 | Pattern identification, focusing first on indicators of hypoglycemia. If the data show problematic time below range, it is important to formulate strategies to resolve this issue (e.g., adjust medication, modify health behaviors) before addressing any other concerns. The 24-hour glucose profile will show the time(s) when hypoglycemia is occurring. If a pattern exists, it may be necessary to review multiple days to identify any particular day(s) or time (s) when the patterns are most significant. |
| Step 4 | Pattern identification, addressing hyperglycemia. If the data indicate that time in range is not at the desired target level, clinicians are advised to review the 24- hour glucose profile to identify the time(s) when hyperglycemia is occurring. This is particularly important when the standard deviation is elevated. If a pattern exists, it may be necessary to review multiple days to identify any particular day(s) when the patterns are most significant. |
| Step 5 | Assess the basal insulin dose (if applicable). For patients treated with intensive insulin regimens, clinicians are advised to determine the appropriateness of the basal dose. It is also important that clinicians talk to patients to determine how they are dosing for meals and correcting for elevated glucose values. |
6. Implementing CGM in Clinical Practice
6.1. Workflow and Staff Training
The first step in CGM implementation is establishing a defined workflow that identifies the individual(s) who will be responsible for downloading/obtaining CGM data, displaying the data for analysis, entering data in patient records (scanned or electronically) and printing out reports for each patient. Areas of proficiency should include:
Device setup, troubleshooting and awareness of common questions, problems and concerns.
Ability to download and interpret device data (e.g., glucose, insulin administration), change device settings as needed, and adjust therapy.
All CGM manufacturers offer comprehensive, on-line training/education materials for their device. Guidance documents for interpreting/utilizing data, specifically, use of ROC trend arrows, are also available. These resources are presented in Supplementary Table 1.
6.2. Patient Selection
Although use of CGM was initially focused patients treated with intensive insulin therapy, a growing body of evidence is now demonstrating its value in patients on less-intensive treatment regimens.[90–93] Because the various CGM systems offer different features and functionalities, it is important to collaborate with patients to help them select the system that best meets their clinical needs, lifestyle, motivational level, cognitive capabilities and socioeconomic status. The following is a list of patient characteristics that may benefit from CGM use:
Treated with intensive insulin regimens (MDI or insulin pump).[1–3]
Increased risk for hypoglycaemia, impaired hypoglycaemia awareness, frequent nocturnal hypoglycaemia, frequent severe hypoglycaemia.[8, 48, 94]
Pregnant with pre-existing T1DM,[95, 96] T2DM [95] or gestational diabetes.[97]
Newly diagnosed T2DM (for episodic use as an educational tool). [93]
T2DM patients not on intensive insulin regimens who are under good control but may benefit from full-time or episodic CGM as an alternative to SMBG. [93]
6.3. Patient Education and Training
Training is essential to optimizing use of CGM,[98–100] and it should begin with “refresher” instruction on strategies for prevention and treatment of hypoglycemia and hyperglycemia. For patients treated with intensive insulin regimens, clinicians should also confirm each patient’s skills in calculating insulin doses, utilizing current glucose levels and individualized insulin parameters (e.g., insulin-to carbohydrate ratio(s) [I:CHO], insulin sensitivity factor(s) [ISF]) and anticipated carbohydrate intake.
When providing training on the selected device, clinicians should make sure that patients understand that the CGM measures glucose in the interstitial space and the values may be different than a blood glucose number. Typically, these are within 20% above 100 mg/dL (5.6 mmol/L) or < 20/mg/dl when glucose is less than 100 mg/dl (5.6 mmol/L). Training for using the CGM does not need to be complex. A simple 1-page instruction sheet was developed to support Engagement with CGM systems and to help optimize glycemic outcomes.[101] The initial topics to cover during the training session include:
Procedures for setting the target range(s) and alarms/alerts.
Sensor placement and insertion technique.
Situations that will require confirmatory fingerstick test.
Significance and functionality of the trend arrows.
Procedure for downloading CGM data for personal use and for transmission to the clinician.
How to use set up and use the data share function (if available)
How to check for skin problems, sensitivity and allergic reactions that may be caused by the sensor adhesion material.
Clinicians should schedule close follow-up training to focus on use of trend arrows for insulin dosage and adjustment and activity/nutrition modification, interpretation and use of retrospective CGM data and use of data sharing functions. It is important that clinicians manage patient expectations in terms of what CGM can and cannot do and the time and effort required to integrate use of CGM into their daily lives.
7. Looking to the Future
7.1. Pregnancy
Another area where the value of CGM has been demonstrated is pregnancy. In a recent 12-month, randomized controlled study of 325 T1DM women who were pregnant or planning pregnancy, use of CGM vs. SMBG was associated with significantly increased TIR compared (p=0.0034) and shorter hospital stays (p=0.0091), with few neonatal intensive care admissions with >24-hour duration (p=0.0157), less neonatal hypoglycemia (p=0.0250) and lower incidence of large for gestational age (p=0.0210). [96] Although CGM use in pregnancy was historically been considered to be off-label, the Dexcom G6, FreeStyle Libre 2 and Guardian Connect are approved for use in pregnancy in some European countries, but not in the U.S.
7.2. CGM Use in Renal Disease
It is well recognized that glucose and insulin metabolism in patients with diabetes are negatively impacted by end-stage renal disease (ESRD).[67] Although HbA1c testing in conjunction with SMBG is the recommended approach to assess glycemic control in diabetes patients with ESRD,[102] the accuracy and utility of these testing methods is severely limited due to susceptibility to numerous ESRD-related factors that can impact accuracy of results.[67] Use of CGM has the potential to overcome these limitation and facilitate identification of problematic glucose patterns and improve therapy management in these patients;[67, 103] however, CGM devices are not currently approved for use in dialysis patients.
In a 12-week pilot study by Joubert [104] et al., 15 dialysis patients with diabetes were monitored with SMBG three times daily for six weeks and then transitioned to CGM for an additional six weeks. Glucose profiles from both testing methods were used to guide therapy. [104] Mean CGM glucose dropped from 8.3±2.5 mmol/l to 8.2±1.6 mmol/l after SMBG but then significantly to 7.7±1.6 mmol/l (P<0.05) at the end of the CGM period without increased risk of hypoglycemia. Investigators also reported more frequent treatment changes using CGM vs. SMBG data.
7.3. Use of CGM during Radiologic Procedures
Concerns about component damage and patient safety have prompted most CGM manufacturers to warn users against wearing their devices when undergoing radiologic procedures, including magnetic resonance imaging (MRI), computed tomography (CT), and diathermy. [21, 32, 39] However, as stated in their safety labeling, their devices have never been tested under those conditions. The only exception is the Eversense CGM system, for which “non-clinical” testing has shown that the implanted sensor is not affected by MRI, as long as the strength of the static magnetic field remains within certain limits. [40]
The effects of radiologic procedures on CGM technologies have not been well studied. However, a recent in vitro study by Thomas et al. casts considerable doubt on the necessity for removing CGM devices when undergoing these procedures. [38] Investigators assessed the accuracy and functional integrity of wearable components of the Dexcom G6 system when exposed to therapeutic x-ray at a cumulative dose of 80 Gy and MRI, using radiofrequency fields (RF) oscillating at 64 or 128 MHz and magnetic fields of 1.5 or 3 T. Following these exposures, the reported glucose concentrations were similar to those displayed in the unexposed devices. The displacement force of 306g during the MRI exposure did not dislodge the sensor from the substrate. Moreover, the glucose data stored in the transmitter before exposure to MRI and x-ray remained intact. In addition, in a recent study of the Dexcom G6 sensor during elective abdominal surgery, investigators reported that glucose values were consistent and acceptable, suggesting that use of the device may be appropriate for peri-operative glucose management. [105] An in-vitro and small (n=10 sensors) study of the FreeStyle Libre Pro sensor showed no interferences from CT, MRI, x-ray or radiotherapy (RT).[36]
7.4. CGM Use in Hospital Settings
The COVID-19 pandemic has placed significant pressure on hospital staff to provide care to a growing number of individuals who present with severe SARS-CoV-2 infection. Diabetes and stress-induced hyperglycemia is a common scenario and is associated with poor outcomes in patients with COVID-19, [106–108]
Although early studies of CGM in hospitalized patients have been limited mostly to intravenous CGM technologies, [109–113] use of minimally-invasive and factory-calibrated CGM technologies have not been well studied. Nevertheless, in April 2020. the U.S. Food and Drug Administration (FDA) issued a new policy to expand the availability and capability of non-invasive remote monitoring devices [e.g., CGM] to facilitate patient monitoring while reducing patient and healthcare provider contact and exposure to COVID-19 for the duration of the COVID-19 public health emergency. [114] Albeit, this does not represent FDA approval for use of CGM in the hospital. In a recent study by Galindo et al, use of isCGM in hospitalized T2DM patients was associated with higher detection rate of hypoglycemic events (nocturnal and prolonged events) compared with standard point-of-care blood glucose testing. [115] In addition to this s, the advantages of using rtCGM devices with remote monitoring features (e.g., Dexcom G6 Follow and Abbott FreeStyle LibreLinkUp apps) reduces both staff exposure to infection and utilization of personal protective equipment.[116] A few studies using the Dexcom G6 system have shown reductions in hypoglycemia [117] and hyperglycemia among non-critically ill hospitalized patients. [118] Although CGM has the potential to become widely accepted for monitoring glycemic status in hospitalized patients, additional studies are needed to support continued use.
8. Summary
Over the last 100 years, advances in glucose monitoring have led to an array of innovative tools that have enabled patients with DM to improve their glycemic status and the quality of their lives. With ongoing improvements in CGM and insulin delivery technologies, patients and their healthcare providers now have the ability to fine tune therapy and, at the same time, reduce the burden of diabetes.
The recent adoption of standardized CGM metrics and integration of the AGP template into data download software has greatly simplified data interpretation, thereby increasing the feasibility of CGM use in primary care settings. As emerging evidence continues to demonstrate the benefits of CGM among patients with diabetes treated with less-intensive or non-insulin therapies, CGM may soon become a standard of care within the broader diabetes population. Moreover, primary care clinicians or their staffs will need to acquire requisite knowledge and skills to be able to effectively manage patients using CGM technologies.
In response to the COVID-19 pandemic, there is a rapidly growing interest in use of telemedicine technologies, which will further expand use of CGM and sensor-driven insulin delivery devices. The pandemic has also triggered, at least temporarily, the need for using CGM in hospital settings where its value in improving glycemic control is compounded by providing additional safeguards for hospital staff and reducing utilization of personal protective equipment. However, expanding use of CGM and other diabetes technologies into hospitals or into primary care practices will require healthcare professionals to restructure their treatment and workflow protocols to streamline data downloading, data interpretation and patient follow-up.
Supplementary Material
Acknowledgements
The authors wish to thank Christopher G. Parkin, MS, CGParkin Communications, Inc., Henderson, Nevada, USA, for his editorial assistance.
Funding
The authors received no financial support for developing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
RJG has received unrestricted research support to Emory for investigator-initiated studies from Novo Nordisk and Dexcom INC, and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk, Eli Lilly, and Valeritas. RJG is partially supported by research grants from NIH/NIDDK P30DK11102 and 1K23DK123384–01. GA has received research support from AstraZeneca, Dexcom, Eli Lilly, Insulet, Novo Nordisk, and is a consultant for Dexcom and Insulet.
References
- [1].Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017; 317:371–378. [DOI] [PubMed] [Google Scholar]
- [2].Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374. [DOI] [PubMed] [Google Scholar]
- [3].Šoupal J, Petruželková L, Grunberger G, et al. Glycemic Outcomes in Adults with T1D Are Impacted More by Continuous Glucose Monitoring Than by Insulin Delivery Method: 3 Years of Follow-Up from The COMISAIR Study. Diabetes Care 2020. January; 43(1): 37–43. [DOI] [PubMed] [Google Scholar]
- [4].Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263. [DOI] [PubMed] [Google Scholar]
- [5].Haak T, Hanaire H, Ajjan R, et al. : Use of flash glucose sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther 2017;8:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8(1):55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Krӧger J, Weitgasser R 6, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heinemann L, Freckmann G, Faber-Heinemann G, et al. Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: Results of the multicentre, randomised controlled HypoDE study. The Lancet 2018;391(10128):1367–1377. [DOI] [PubMed] [Google Scholar]
- [9].Charleer S, De Block C, Van Huffel L, et al. Quality of Life and Glucose Control After 1 Year of Nationwide Reimbursement of Intermittently Scanned Continuous Glucose Monitoring in Adults Living With Type 1 Diabetes (FUTURE): A Prospective Observational Real-World Cohort Study. Diabetes Care 2020;43(2):389–397. [DOI] [PubMed] [Google Scholar]
- [10].Fokkert M, van Dijk P, Edens M, et al. Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care 2019;7(1):e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tyndall V, Stimson RH 2,3, Zammitt NN, et al. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia 2019;62(8):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benedict SR. A Reagent for the Detection of Reducing Sugars. J Biol Chem 1909;5(6):485–487. [PubMed] [Google Scholar]
- [13].Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci 2012;69(2):83–93. [PubMed] [Google Scholar]
- [14].Hill JG, Kessler G. An Automated Determination of Glucose Utilizing a Glucose Oxidase-Peroxidase System. J Lab Clin Med 1961;57:970–80. [PubMed] [Google Scholar]
- [15].Didyuk O, Nicolas Econom N, Guardia A, Livingston K, Klueh U. Continuous Glucose Monitoring Devices: Past, Present, and Future Focus on the History and Evolution of Technological Innovation. J Diabetes Sci Technol 2020. January 13;1932296819899394. doi: 10.1177/1932296819899394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ajjan RA, Jackson N, Thomson SA. Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial. Diab Vasc Dis Res 2019;16:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].New JP, Ajjan R, Pfeiffer AF, Freckmann G. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med 2015;32:609–617. [DOI] [PubMed] [Google Scholar]
- [18].Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther 2007;9(3):203–210. [DOI] [PubMed] [Google Scholar]
- [19].Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. J Diabetes Investig. 2018;9(4):713–725. doi: 10.1111/jdi.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beyond A1C Writing Group. Need for Regulatory Change to Incorporate Beyond A1C Glycemic Metrics. Diabetes Care 2018;41(6):e92–e94. [DOI] [PubMed] [Google Scholar]
- [21].Dexcom, Inc. Dexcom G6 Continuous Glucose Monitoring System Safety Information. https://www.dexcom.com/safety-information. Accessed July 11, 2020.
- [22].Puhr S, Derdzinski M, Welsh JB, Parker AS, Walker T, Price DA. Real-World Hypoglycemia Avoidance with a Continuous Glucose Monitoring System’s Predictive Low Glucose Alert. Diabetes Technol Ther. 2019. April;21(4):155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abraham SB, Arunachalam S, Zhong A, Agrawal P, Cohen O, McMahon CM. Improved Real-World Glycemic Control With Continuous Glucose Monitoring System Predictive Alerts. J Diabetes Sci Technol 2019. July 4;1932296819859334. doi: 10.1177/1932296819859334. https://europepmc.org/article/med/31272204. Accessed September 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Welsh JB, Derdzinski M, Parker AS, et al. Real-Time Sharing and Following of Continuous Glucose Monitoring Data in Youth. Diabetes Ther 2019. April;10(2):751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dexcom, Inc. Dexcom G6 Pro. https://provider.dexcom.com/faqs-categories/dexcom-g6-pro. Accessed July 14, 2020.
- [26].Medtronic, Inc. Medtronic iPro2 Professional CGM system; https://www.medtronic.com/us-en/healthcare-professionals/products/diabetes/continuous-glucose-monitoring-systems/ipro2-professional.html. Accessed July 14, 2020.
- [27].Abbott Diabetes Care. FreeStyle Libre Pro system. https://provider.myfreestyle.com/freestyle-libre-pro-product.html. Accessed July 14, 2020.
- [28].Abbott Diabetes Care. FreeStyle Libre 2 User Guide. 2020
- [29].Dexcom G6 Continuous Glucose Monitoring Systems User Guide. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf.
- [30].Christiansen MP, Garg SK, Ronald Brazg R, et al. Accuracy of a Fourth-Generation Subcutaneous Continuous Glucose Sensor. Diabetes Technol Ther. 2017;19(8):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Christiansen MP, Klaff LJ, Brazg RR, et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20(3):197–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abbott Diabetes Care. FreeStyle Libre 2 Continuous Glucose Monitoring System Safety Information. https://www.freestylelibre.us/safety-information.html. Accessed July 11, 2020.
- [33].Denham D Effect of Repeated Doses of Acetaminophen on a Continuous Glucose Monitoring System with Permselective Membrane. J Diabetes Sci Technol 2020; July 29 https://journals.sagepub.com/doi/pdf/10.1177/1932296820948544. Accessed September 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Basu A, Slama MQ, Nicholson WT, et al. Continuous Glucose Monitor Interference With Commonly Prescribed Medications: A Pilot Study 2J Diabetes Sci Technol 2017;11(5):936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lorenz C, Sandoval W, Mortellaro M. Interference Assessment of Various Endogenous and Exogenous Substances on the Performance of the Eversense Long-Term Implantable Continuous Glucose Monitoring System. Diabetes Technol Ther 2018;20(5):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takatsu Y, Shiozaki T, Miyati T, Asahara M, Tani Y. Are the recorded data of flash glucose monitoring systems influenced by radiological examinations? Radiol Phys Technol 2019;12(2):224–229. [DOI] [PubMed] [Google Scholar]
- [37].Migdal AL, Spanakis EK, Galindo RJ, et al. Accuracy and Precision of Continuous Glucose Monitoring in Hospitalized Patients Undergoing Radiology Procedures. J Diabetes Sci Technol. 2020;1932296820930038. doi: 10.1177/1932296820930038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thomas C, Welsh JB, Lu S, Gray JM. Safety and Functional Integrity of Continuous Glucose Monitoring Components After Simulated Radiologic Procedures. J Diabetes Sci Technol 2020. April 22;1932296820920948. doi: 10.1177/1932296820920948. Accessed July 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Medtronic, Inc. Important safety information: indications, contraindications, warnings and precautions (630G). Available from https://www.medtronicdiabetes.com/important-safety-information#minimed-630g. Accessed January 21, 2018.
- [40].Senseonics, Inc. Eversense Continuous Glucose Monitoring System Safety Information https://www.eversensediabetes.com/safety-info. Accessed July 11, 2020.
- [41].Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensoraugmented pump therapy for A1C reduction (Star 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34:2403–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Aleppo G, Ruedy KJ, Riddlesworth TD, et al. REPLACE-BG: A randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40(4):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care 2003;26:163–167. [DOI] [PubMed] [Google Scholar]
- [44].Kuenen JC, Borg R, Kuik DJ, et al. ADAG Study Group. Does glucose variability influence the relationship between mean plasma glucose and HbA1c levels in type 1 and type 2 diabetic patients? Diabetes Care 2011;34:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Polonsky WH, Hessler D, Ruedy KJ, Beck RW, for the DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017; 40:736–741. [DOI] [PubMed] [Google Scholar]
- [46].Šoupal J, Petruželková L, Flekač M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. 2016;18(9):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abdelhafiz AH, Rodríguez-Mañas L, Morley JE, Sinclair AJ. Hypoglycemia in Older People - A Less Well Recognized Risk Factor for Frailty. Aging Dis 2015;6(20;156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. [DOI] [PubMed] [Google Scholar]
- [49].Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011; 343: d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lind M, Polonsky W, Hirsch IB. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317(4):379–387 [DOI] [PubMed] [Google Scholar]
- [51].Choudhary P, Olsen BS, Conget I, et al. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther 2016;18(5):288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- [53].Forlenza GP, Li Z, Buckingham BA, et al. Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children With Type 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Diabetes Care 2018. October;41(10):2155–2161. [DOI] [PubMed] [Google Scholar]
- [54].Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-Night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2016;39:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392(10155):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Karageorgiou V, Papaioannou TG, Bellos I, et al. Effectiveness of Artificial Pancreas in the Non-Adult Population: A Systematic Review and Network Meta-Analysis. Metabolism 2019. January;90:20–30. doi: 10.1016/j.metabol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [57].Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018; 361 doi: 10.1136/bmj.k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Weisman A, Bai J-W, Marina Cardinez M, Kramer CK, Perkins BA. Effect of Artificial Pancreas Systems on Glycaemic Control in Patients With Type 1 Diabetes: A Systematic Review and Meta-Analysis of Outpatient Randomised Controlled Trials. Lancet Diabetes Endocrinol 2017. July;5(7):501–512. [DOI] [PubMed] [Google Scholar]
- [59].Brown SA, Kovatchev BP, Raghinaru D, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med 2019. October 31;381(18):1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brown SA, Beck RW, Raghinaru D, et al. Glycemic Outcomes of Use of CLC Versus PLGS in Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care 2020. May 29;dc200124. doi: 10.2337/dc20-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Breton MD, Kanapka LG, Beck RW, et al. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N Engl J Med 2020;383:836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kröger J, Fasching P, Hanaire H. Three European Retrospective Real-World Chart Review Studies to Determine the Effectiveness of Flash Glucose Monitoring on HbA1c in Adults with Type 2 Diabetes. Diabetes Ther 2020;11(1):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of Flash Glucose Monitoring Technology on Glycemic Control and Treatment Satisfaction in Patients With Type 2 Diabetes. Diabetes Care. 2019;42(7):1178–1184. [DOI] [PubMed] [Google Scholar]
- [64].Ford ES, Cowie CC, Li C, Handelsman Y, Bloomgarden ZT. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J Diabetes 2011;3(1):67–73. [DOI] [PubMed] [Google Scholar]
- [65].National Diabetes Information Clearinghouse (NDIC). Sickle cell trait and other hemoglobinopathies and diabetes: important information for providers [Internet]. Available from https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/sickle-cell-trait-hemoglobinopathies-diabetes. Accessed September 12, 2020.
- [66].Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001;47(2):153–163. [PubMed] [Google Scholar]
- [67].Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic Monitoring and Management in Advanced Chronic Kidney Disease. Endocrine Reviews. 2020; bnaa017, 10.1210/endrev/bnaa017. Accessed July 10,2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2005;27(5):1200–1201. [DOI] [PubMed] [Google Scholar]
- [69].Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. [DOI] [PubMed] [Google Scholar]
- [70].Shipman KE, Jawad M, Sullivan KM, Ford C, Gama R. Ethnic/racial determinants of glycemic markers in a UK sample. Acta Diabetol 2015;52:687–692. [DOI] [PubMed] [Google Scholar]
- [71].Wolffenbuttel BHR, Herman WH, Gross JL, et al. Ethnic differences in glycemicmarkers in patientswith type 2 diabetes. Diabetes Care 2013;36:2931–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Danne T, Nimri R, Battelino T, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xing D, Kollman C, Beck RW, et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther 2011;13:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Riddlesworth TD, Beck RW, Gal RL, et al. Optimal Sampling Duration for Continuous Glucose Monitoring to Determine Long-Term Glycemic Control. Diabetes Technol Ther. 2018;20(4):314–316. [DOI] [PubMed] [Google Scholar]
- [77].Bergenstal RM, Beck RW, Close KL, et al. Glucose Management Indicator (GMI):a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838. [DOI] [PubMed] [Google Scholar]
- [79].Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, Rodbard D. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J Diabetes Sci Technol. 2019. January 13:1932296818822496. doi: 10.1177/1932296818822496. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vigersky RA, McMahon C. The Relationship of Hemoglobin A1C to Time-in-Range in Patients with Diabetes. Diabetes Technol Ther. 2019;21(2):81–85. [DOI] [PubMed] [Google Scholar]
- [81].Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diab Res Care. 2020;8(1):e000991 https://drc.bmj.com/content/8/1/e000991.abstract. Accessed July 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42(3): 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15(3):198–211. [DOI] [PubMed] [Google Scholar]
- [84].International Diabetes Center. AGP - Ambulatory Glucose Profile: AGP reports. http://www.agpreport.org/agp/agpreports. Accessed July 10, 2020
- [85].Johnson ML, Martens TW, Criego AB, Carlson AL, Bergenstal RM. Utilizing the ambulatory glucose profile (AGP) to standardize and implement continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2019. June;21(S2):S217–S225. [DOI] [PubMed] [Google Scholar]
- [86].Aleppo G, Webb K. Continuous Glucose Monitoring Integration in Clinical Practice: A Stepped Guide to Data Review and Interpretation. J Diabetes Sci Technol 2019;13(4):664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kruger DF, Edelman SV, Hinnen DA, Parkin CG. Reference guide for integrating continuous glucose monitoring into clinical practice. Diabetes Educ. 2019. February;45(suppl 1):3S–20S. [DOI] [PubMed] [Google Scholar]
- [88].Hammond P Interpreting the ambulatory glucose profile. Br J Diabetes 2016;16(Suppl1):S10–S15. [Google Scholar]
- [89].Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. J Lab Med 2020; 44(2):71–79. [Google Scholar]
- [90].Miller E, Brandner L, Wright E. HbA1c Reduction After Initiation of the FreeStyle Libre® System in Type 2 Diabetes Patients on Long-Acting Insulin or Non-Insulin Therapy. Diabetes 2020. June; (Supplement 1):84–LB. [Google Scholar]
- [91].Miller E, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Wright E. FreeStyle Libre® System Use Associated with Reduction in Acute Diabetes Events and All-Cause Hospitalizations in Patients with Type 2 Diabetes Without Bolus Insulin. Diabetes 2020. June; (Supplement 1):85–LB [Google Scholar]
- [92].Wright E, Kerr MSD, Reyes I, Nabutovsky Y, Miller E. HbA1c Reduction Associated with a FreeStyle Libre® System in People with Type 2 Diabetes Not on Bolus Insulin Therapy. Diabetes 2020. June; (Supplement 1):78–LB-P [Google Scholar]
- [93].Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A Randomized Clinical Trial of the Effect of Continuous Glucose Monitoring on Nocturnal Hypoglycemia, Daytime Hypoglycemia, Glycemic Variability, and Hypoglycemia Confidence in Persons with Type 1 Diabetes Treated with Multiple Daily Insulin Injections (GOLD-3) Diabetes Technol Ther. 2018. April 1; 20(4): 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yu F, Lv L, Liang Z, et al. Continuous Glucose Monitoring Effects on Maternal Glycemic Control and Pregnancy Outcomes in Patients With Gestational Diabetes Mellitus: A Prospective Cohort Study. J Clin Endocrinol Metab 2014. December;99(12):4674–4682. [DOI] [PubMed] [Google Scholar]
- [98].Bailey TS, Walsh J, Stone JY. Emerging technologies for diabetes care. Diabetes Technol Ther 2018; 20: S278–S284. [DOI] [PubMed] [Google Scholar]
- [99].Majeed W, Thabit H. Closed-loop insulin delivery: current status of diabetes technologies and future prospects. Expert Rev Med Devices 2018; 15: 579–590. [DOI] [PubMed] [Google Scholar]
- [100].Tanenbaum ML, Adams RN, Hanes SJ, et al. Optimal use of diabetes devices: clinician perspectives on barriers and adherence to device use. J Diabetes Sci Technol 2017; 11: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Barnard-Kelly K, Polonsky WH. Development of a Novel Tool to Support Engagement With Continuous Glucose Monitoring Systems and Optimize Outcome Journal of Diabetes Science and Technology 2020;14(1)151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 2012. November;60(5):850–886. [DOI] [PubMed] [Google Scholar]
- [103].Chantrel G, Sissoko H, L Képénékian L, et al. Influence of Dialysis on the Glucose Profile in Patients With Diabetes: Usefulness of Continuous Glucose Monitoring. Horm Metab Res 2014;46(11):810–813. [DOI] [PubMed] [Google Scholar]
- [104].Joubert M, Fourmy C, Henri P, et al. Effectiveness of Continuous Glucose Monitoring in Dialysis Patients With Diabetes: The DIALYDIAB Pilot Study. Diabetes Res Clin Pract 2015. March;107(3):348–354. [DOI] [PubMed] [Google Scholar]
- [105].Tripyla A, Herzig D, Joachim D, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes Metab 2020;22(9):1678–1682. [DOI] [PubMed] [Google Scholar]
- [106].Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the seattle region — case series. NEJM.org. 2020. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region – Case Series. N Engl J Med 2020;382(21):2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bochicchio GV, Nasraway S, Moore L, et al. Results of a multicenter prospective pivotal trial of the first inline continuous glucose monitor in critically ill patients. J Trauma Acute Care Surg. 2017;82:1049–1054. [DOI] [PubMed] [Google Scholar]
- [110].Righy Shinotsuka C, Brasseur A, Fagnoul D, So T, Vincent J-L, Preiser J-C. Manual versus automated monitoring accuracy of glucose II (MANAGE II). Crit Care 2016;20:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nohra E, Buckman S, Bochicchio K, et al. Results of a near continuous glucose monitoring technology in surgical intensive care and trauma. Contemp Clin Trials 2016;50:1–4. [DOI] [PubMed] [Google Scholar]
- [112].Barassi A, Umbrello M, Ghilardi F, et al. Evaluation of the performance of a new OptiScanner™ 5000 system for an intermittent glucose monitoring. Clin Chim Acta 2015;438:252–254. [DOI] [PubMed] [Google Scholar]
- [113].Umbrello M, Salice V, Spanu P, et al. Performance assessment of a glucose control protocol in septic patients with an automated intermittent plasma glucose monitoring device. Clin Nutr 2014;33:867–87. [DOI] [PubMed] [Google Scholar]
- [114].U.S. Food & Drug Administration. Enforcement Policy for Non-Invasive Remote Monitoring Devices Used to Support Patient Monitoring During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (Revised). https://www.fda.gov/media/136290/download. Accessed September 20, 2020.
- [115].Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro Flash Continuous Glucose Monitoring (CGM) System and Point-of-Care Capillary Glucose Testing (POC) in Hospitalized Patients With Type 2 Diabetes (T2D) Treated With Basal-Bolus Insulin Regimen. Diabetes Care 2020. July: dc192073 10.2337/dc19-2073. Accessed July 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Galindo RG, Aleppo G, Klonoff DC, et al. Implementation of Continuous Glucose Monitoring in the Hospital: Emergent Considerations for Remote Glucose Monitoring During the COVID-19 Pandemic J Diabetes Sci Technol 2020. June 14;1932296820932903. doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Singh LG, Satyarengga M, Isabel Marcano I, et al. Reducing Inpatient Hypoglycemia in the General Wards Using Real-Time Continuous Glucose Monitoring: The Glucose Telemetry System, a Randomized Clinical Trial. Diabetes Care 2020. August 5;dc200840. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fortmann AL, Spierling Bagsic SR, Talavera L, et al. Glucose as the Fifth Vital Sign: A Randomized Controlled Trial of Continuous Glucose Monitoring in a Non-ICU Hospital Setting. Diabetes Care 2020. August 27;dc201016. doi: 10.2337/dc20-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


