Abstract
This review introduces a research strategy that may radically transform the pursuit of new anxiolytics, via the use of human models of anxiety in healthy individuals. Despite enormous investments in developing novel pharmacological treatments for anxiety disorders, pharmacotherapy for these conditions remains suboptimal. Most candidate anxiolytics from animal studies fail in clinical trials. We propose an additional screening step to help select candidate anxiolytics before launching clinical trials. This intermediate step moves the evidence for the potential anxiolytic property of candidate drugs from animals to humans, using experimental models of anxiety in healthy individuals. Anxiety-potentiated startle is a robust translational model of anxiety. The review of its face, construct, and predictive validity as well as its psychometric properties in humans establishes it as a promising tool for anxiolytic drug development. In conclusion, human models of anxiety may stir a faster, more efficient path for the development of clinically effective anxiolytics.
Keywords: anxiolytics, anxiety, drug development, human models, startle
1. Introduction
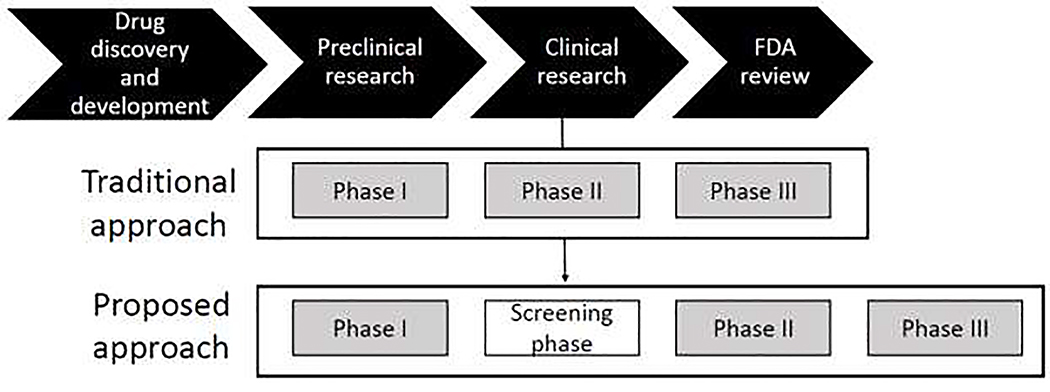
Anxiety disorders are the most common psychiatric disorders, with an estimated lifetime prevalence of over 28% of the population (Kessler et al., 2005). These conditions are debilitating, impair quality of life, and are costly (Bandelow and Michaelis, 2015). Unfortunately, the treatment of anxiety disorders is a significant challenge. Current treatments consist of a few key agents that are helpful, but with limitations (Griebel and Holmes, 2013; Insel and Scolnick, 2006; Papassotiropoulos and de Quervain, 2015). The challenge remains to develop more effective anxiolytic treatments. A considerable impediment to drug development is the long, arduous, and costly process through which a candidate drug moves from the discovery stage into an actual treatment. Currently, this process entails multiple steps, from 1) identifying biological targets, developing molecules that interact with these targets, and screening candidate agents in animal models, 2) establishing safety of potentially effective doses in healthy humans in phase I clinical trials, 3) moving to clinical trials in patients in phase II trials to detect pharmacodynamics activity and determine effective doses and large-scale multicenter phase III trials to determine drug efficacy, and 4) finally requesting approval for New Drug Application from the Food and Drug Administration (Fig. 1). Unfortunately, positive results in animals do not always translate to humans and most promising agents fail to be effective in patients (Griebel and Holmes, 2013). Drug sponsors are then faced with the dilemma of selecting among various candidate anxiolytics for costly and time-consuming clinical trials. There is a need to improve the drug development process to quickly identify agents that deserve clinical testing. The present paper argues for the benefit of adding a step before moving from Phase I to Phase II clinical trials, i.e., testing the anxiolytic property of candidate drugs in healthy volunteers (Fig. 1). This step avoids the instances of launching clinical trials with agents that seem to be effective in animals but end up showing no anxiolytic property in humans. This review focuses on the description and validation of such an intermediate step in drug development.
Figure 1.
Drug development process. See text for more details. The proposed approach adds a screening phase (between Phases I and II) in healthy subjects to assess the efficacy of new drugs.
A powerful experimental model of anxiety has already been well-validated for human research.(Davis et al., 2010). This model, which was directly translated from animal work, rests on the physiological manifestations of anxiety that can reliably be measured as changes in the startle reflex (Davis et al., 2010). The startle reflex is an involuntary whole-body response provoked by a sudden and intense stimulus. It is used in various experimental models to study habituation, sensitization, pre-pulse inhibition, and fear-potentiation (Davis, 1984). Anxiety-potentiated startle (APS) is the increase in startle response during anticipation of temporally unpredictable aversive events. It is a readout of a sustained anxiety state. Critically, this model has shown to help identify and screen candidate anxiolytics (Davis et al., 2010; Grillon et al., 2006b; Grillon et al., 2009a; Grillon et al., 2015; Kaye et al., 2019).
This review will demonstrate how APS in humans could enhance early phases of drug development by providing data on anxiolytic properties of new agents in humans, and in turn assist drug developers in making the crucial go/no go decision before moving to a clinical trial.
2. Experimental model of anxiety in healthy humans: rationale
Once a promising molecule is identified via basic research, its potential anxiolytic property is tested in animals. If the molecule is deemed to have the preliminary requisites for further testing, the feasibility of moving to humans is tested. Dosage, side effects and mode of administration are evaluated in healthy humans. If the drug passes these initial steps, it is recommended for clinical drug trials. A glaring gap, at this point, is the absence of data on anxiolytic effects in humans. Evidence of efficacy in animal models does not readily translate into efficacy in humans. We argue that this information can be easily obtained using an experimental of model of anxiety in healthy humans.
Many reasons can explain why an anti-anxiety effect in animals may not be readily translated to the clinic. For example, brain complexity and cognitive function are most obvious. The brain is far more complex in humans than in animals. Disturbance of evolutionary recent circuits in humans are implicated in anxiety disorders (Nestler and Hyman, 2010). In addition, animals have limited higher-order cognitive functions, which critically interacts with emotion processes (LeDoux and Pine, 2016). This interaction is highly relevant to anxiety, whose etiology, expression and maintenance is supported by the joint contribution of cognitive and emotional mechanisms (Pessoa, 2014). It is important to recognize that the crucial role of cognition in anxiety may play a role in the lack of clinical efficacy of agents with anti-anxiety effects in animal models (LeDoux and Pine, 2016). Experimental models in humans allows a new approach that considers the interplay of cognition and emotion.
Furthermore, the rationale for human models is provided by current dimensional conceptualization of psychopathology: the same underlying cognitive and emotional processes that contribute to normal anxiety are also implicated in pathological anxiety (Cuthbert and Insel, 2013). This conceptualization assumes a continuum from normal to pathological anxiety, and therefore a similar continuity for the underlying mechanisms. The implication of this view is that excessive anxiety arises from dysregulation of psychological processes and neurocircuits involved in normative responses to threat. It is therefore within these processes and neurocircuits that one must look for clues as to dysfunctional mechanisms and treatment targets.
3. Anxiety-potentiated startle to unpredictable threat
The development of experimental models of psychiatric disorders requires a careful consideration of the targeted symptoms and their measurement. Two key symptoms are relevant to anxiety disorders, fear and anxiety. Fear and anxiety are distinct defensive responses to different types of threats. Fear is a short-duration response, a surge of autonomic arousal necessary for fight or flight, in response to an imminent danger (Association, 2013; Davis et al., 2010). Anxiety is a long-duration state of vigilance, tension, and caution in preparation for an uncertain future threat (Association, 2013; Davis et al., 2010).
There are excellent translational models of fear using Pavlovian (cued) fear conditioning. During fear conditioning, a short-duration cue (e.g., a light) is repeatedly paired with an aversive stimulus (e.g., a shock), making the cue a reliable predictor of danger. Subsequent presentation of the cue evokes a conditioned fear response, while the absence of the cue signals safety. Fear conditioning is useful to develop new treatment strategies targeting fear, but not anxiety (Norberg et al., 2008).
The sustained defensive response that characterizes anxiety can be evoked by unpredictable threat, i.e. a threat not signaled by a cue. This can be accomplished with either conditioning procedures (e.g. context conditioning) in humans and animals or verbal threat in humans (Grillon et al., 2006a; Luyten et al., 2011). During verbal threat, subjects are informed that an aversive stimulus (e.g., shock) will be delivered unpredictably.
Anxiety can be measured with the startle reflex. The startle reflex is a widely used translational measure of defensive response in laboratory animals and in humans (Davis et al., 2010). What makes the startle reflex an ideal readout of anxiety resides in its use as a probe to track changes in emotional states over sustained periods of time (Grillon et al., 1991; Grillon et al., 1993). The startle reflex can be used in animals and in humans to study both fear and anxiety. In these studies, fear-potentiated startle (FPS, i.e. fear) is operationally defined as the increased in startle reactivity in the presence of a cue (e.g. a light) that predicts an aversive stimulus (e.g. a shock) (Davis et al., 2010). Anxiety-potentiated startle (APS, i.e. anxiety) is operationally defined as the increase in startle reactivity during sustained aversive states evoked by unpredictable threat. In rodents, FPS is mediated by the central nucleus of the amygdala (CeA), while APS reflects activation of the bed nucleus of the stria terminalis (BNST) by corticotropin releasing factor (Davis et al., 2010).
Anxiety-potentiated startle paradigms in humans rely on verbal threat (Schmitz and Grillon 2012) or context conditioning procedures (Glotzbach-Schoon et al., 2013; Grillon et al., 2006a). In a typical experiment, anxiety is assessed using startle during relatively long periods (e.g. 2 min) of unpredictable aversive stimuli and compared to a control (safe) condition. A variation of this experiment adds to these two conditions (safe and unpredictable) a third condition, during which the aversive stimuli are predictable, i.e. signaled by a cue. This so-called NPU threat test consists of three different conditions, Neutral (i.e. safe), Predictable, and Unpredictable, each lasting about 120 s, and repeated 2 or 3 times each (Schmitz and Grillon 2012). The advantage of the NPU threat test is that the features of anxiety (unpredictable condition) and fear (predictable condition) can be compared.
4. Anxiety-potentiated startle: validity criteria
Anxiety-potentiated startle satisfies several validity criteria as a human model of anxiety. The traditional validity criteria for animal models are face validity, predictive validity, and construct validity (Willner, 1986). For human models, one can add 1) clinical validity, the degree to which the model discriminates patients from non-patients and can be used as an index of disease (i.e. clinical biomarker), and 2) practicality for clinical trial and tolerability for the subjects validation (Green et al., 2004). Finally, animal and human models should show good test-retest reliability and utility as a repeated measure test (Green et al., 2004).
4.1. Face validity
Face validity refers to the phenomenological similarity between the model and the “real-life” symptoms of anxiety. The threat of unpredictable shock evokes many of the sustained and persistent symptoms of anxiety and stress disorders, including subjective anxiety, physiological arousal (heightened APS), and hypervigilance (Cornwell et al., 2017; Herrmann et al., 2016a; MacNamara and Barley, 2018). These symptoms, especially increased startle (i.e. APS) and autonomic arousal, are found more prominently in individuals with panic disorder and PTSD (Association, 2013; Grillon et al., 2008; Grillon and Morgan, 1999; Grillon et al., 2009b). Beyond startle, the attentional, cognitive, and behavioral effects induced by unpredictable threat also bear resemblance with the symptoms of anxiety disorders. Unpredictable shock increases attentional bias for threat (Robinson et al., 2014), weakens attention control (Sarapas et al., 2017), increases distractibility (Vytal et al., 2012), and promotes caution and avoidance (Robinson et al., 2013).
In conclusion, the many similarities between the effect of unpredictable threat and the symptoms of pathological anxiety support the face validity of APS.
4.2. Construct validity
The definition of construct validity is complex and depends on theories about the nature of the disorders and dysfunctional processes, as well as etiological mechanisms (Willner, 1986). Construct validity can also be examined from the perspective of underlying neurocircuits (Luyten et al., 2011).
It has long been proposed that exposure to unpredictable stress recapitulates many symptoms of anxiety and stress-related disorders (Mineka and Kihlstrom, 1978). Etiological theories postulate that the feelings of helplessness and apprehension about potential future danger are central to pathological anxiety (Barlow, 2000). Underlying such feelings is a perceived sense of unpredictability and a lack of control over aversive stimuli, which lead to a behavioral state of sensitization (Barlow, 2000; Grillon, 2002). This view is consistent with findings from animal research. In animals, unpredictable, but not predictable, aversive stimuli have debilitating behavioral and somatic effects, which resemble those of pathological anxiety (Maier et al., 2005; Seligman and Binik, 1977). When noxious stimuli are unpredictable, the organism remains in a constant state of hypervigilance to seek pertinent information, and a sustained state of hyperarousal to maintain readiness to respond. This protracted, and highly distressing process turns into a progressive behavioral sensitization characterized by enhanced responding to mild threat (Davis et al., 2010; Maier et al., 2005). In rodents, behavioral sensitization, following successive days of exposure to unpredictable shocks, leads to a progressive increase in startle reactivity, which is mediated by the BNST (Davis et al., 2010). Anxiety-potentiated startle is a readout of this behavioral sensitization and underlying neurocircuit activity (Davis et al., 2010; Grillon et al., 2019).
Construct validity is also supported by studies of anxiety-related neurocircuits. Animal models provide clues as to basic neurocircuits that can be extrapolated to humans, and human models inform on potential circuits implicated in patients (Grillon et al., 2019). Regions implicated in anxiety disorders include the amygdala, anterior cingulate, anterior insula, and medial and lateral prefrontal cortex (Bandelow et al., 2016; Duval et al., 2015). These regions are also activated by unpredictable threat in healthy subjects (Buff et al., 2016; Shankman et al., 2014). In addition, studies in rodents have long pointed to a key role of the BNST in APS (Davis et al., 2010). The BNST has subsequently been found to be activated by unpredictable threat in humans and implicated in anxiety and stress-related disorders (Brinkmann et al., 2017a; Brinkmann et al., 2017b; Buff et al., 2017; Herrmann et al., 2016b; Somerville et al., 2010). The overlap of the neurocircuits involved in response to unpredictable threat and in the pathophysiology of anxiety strongly attests to the validity of APS as a human model.
To summarize, the construct validity of APS is supported by data from experimental psychopathology and brain circuits. In animals, unpredictable stressors lead to symptoms that are akin to those experienced by individuals with anxiety and stress-related disorders. Brain studies show that the neural structures implicated in pathological anxiety are also activated by unpredictable threat.
4.3. Predictive validity
Predictive validity is critical for drug development (Geyer and Markou, 1995). It refers to pharmacologic effects, the ability of the model to correctly predict the clinical efficacy of a psychopharmacological treatment without false positive (errors of commission) or failure to identify clinically efficient treatment (errors of omission) (Willner, 1986).
Evidence from studies in healthy subjects using the NPU threat test shows that clinically effective anxiolytics selectively down-regulate anxiety (APS), without affecting fear (FPS) (see Table). Benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) are the most widely used anxiolytics (Craske et al., 2017). Acute administration of the benzodiazepine alprazolam (Grillon et al., 2006b; Grillon et al., 2015) and semi-chronic (two weeks) treatment with the SSRI citalopram selectively reduce APS without affecting FPS (Grillon et al., 2009a). In addition, alprazolam, but not citalopram, reduces overall startle reactivity (Grillon et al., 2006b; Grillon et al., 2015). This is consistent with the observation that alprazolam, but not citalopram, causes sedation. Furthermore, acute citalopram is anxiogenic in the NPU test (i.e. it increases APS) (Grillon et al., 2007), an effect in line with clinical evidence of anxiogenic reactions shortly following treatment initiation with SSRIs in anxiety patients (Gorman et al., 1987). Finally, alcohol, which is used to self-medicate anxiety, also reduces APS without affecting FPS (Moberg and Curtin, 2009). Taken together, these results indicate that the NPU threat test can detect the anxiolytic, anxiogenic, and sedative effects of drugs.
Table.
Effect of established and candidate anxiolytics on potentiated startle
| Drugs | Doses | Acute/chronic | Baseline startle | FPS | APS | References |
|---|---|---|---|---|---|---|
| Alprazolam | .5 mg, 1 mg | Acute | ↓5 | − | ↓7 | Grillon et al., 2006b |
| Alprazolam | 1 mg | Acute | ↓ | − | ↓ | Grillon et al., 2015 |
| Citalopram | 20 mg/day | 14 days | − | − | ↓ | Grillon et al., 2009a |
| GSK5616791 | 50 mg, 400 mg | Acute | − | ↑6 | − | Grillon et al., 2015 |
| LY3547402 | 20 mg, 200 mg | Acute | − | − | ↓ | Grillon et al., 2003 |
| Oxytocin | 24 IU$ | Acute | − | ↑ | − | Grillon et al., 2013 |
| Prazosin3 | 2 mg | Acute | − | − | − | Kaye et al., 2019 |
| SRX2464 | 300 mg | 4–7 days | − | − | ↓ | Lago et al., 2020 |
CRF1 antagonist (Verucerfont)
Group II mGlu2/3 agonist
norepinephrine alpha-1 (NE-α1) antagonist
V1a receptor antagonist
1 mg only
400 mg only
nasal spray
These studies suggest that anxiolytics act, at least partially, by targeting sensitivity to unpredictable threat. Consistent with this hypothesis, a recent study examined APS and FPS using the NPU threat test in anxiety patients treated for 12 weeks with SSRIs or cognitive behavioral therapy (Gorka et al., 2017a). SSRIs had no effect on APS and did not improve the clinical symptoms. Cognitive behavioral therapy reduced APS and the magnitude of this reduction correlated with symptom improvement. These results suggest that positive clinical outcome may depend on reducing the behavioral sensitization process indexed by APS. The implication for the drug discovery process is that candidate anxiolytics that target response to unpredictable threat may have clinical efficacy.
However, the ultimate test of predictive validity will be determined by the ability of APS to identify clinically effective novel candidate anxiolytics. This validation is still at an early stage and is facing many practical challenges. Among them, no new anxiolytic drug has been approved for the treatment of anxiety disorder in the last decade (Sartori and Singewald, 2019). In addition, candidate anxiolytics are not readily made available by drug sponsors for testing in experimental models in humans.
So far, five novel agents with an anti-anxiety profile in animal models have been tested using APS (and FPS) in healthy subjects (see Table). They include the corticotropin releasing factor (CRF1) antagonist GSK561679 (Grillon et al., 2015), the group II metabotropic glutamate receptor (mGluR2/3) agonist LY354740 (Grillon et al., 2003), oxytocin (Grillon et al., 2013), the norepinephrine alpha-1 (NE-α1) antagonist prazosin (Kaye et al., 2019), and the vasopressin receptor (V1a) antagonist SRX246 (Lago et al., 2020).
Corticotropin releasing factor (CRF1) antagonists were promising agents due to substantial evidence of their anti-anxiety effect in a wide array of animal models (Murrough and Charney, 2017). However, clinical trials have been negative, showing a lack of efficacy in generalized anxiety disorder (GAD), major depressive disorder, social anxiety disorder, and posttraumatic stress disorder (PTSD) (Murrough and Charney, 2017). Consistent with these findings, results in healthy subjects also do not support the anti-anxiety effect of CRF1 antagonists found in animals. The CRF1 antagonist GSK561679 (also known as Verucerfont) was tested acutely in the NPU threat test and was found to have no effect on APS (Dunlop et al., 2017; Grillon et al., 2015). These results suggest that the NPU threat test can detect false positive agents.
Since metabotropic glutamate (mGlu) receptors modulate neural excitation throughout the brain, down-regulation of glutamate signal is a potential target for anxiolytic treatments. Among these receptors, the group II mGlu2/3 receptor agonist LY354740 had robust anti-anxiety effect in several animal models (Schoepp et al., 2003). A similar anti-anxiety effect was confirmed in healthy humans: acute administration of LY354740 reduces APS in healthy controls (Grillon et al., 2003). However, results from clinical trials with LY544344 (which possesses greater bioavailability than LY354740) were mixed. LY544344 was effective in patients with GAD (Dunayevich et al., 2008) and in one (Levine et al., 2002) of two studies in patients with panic disorder (Bergink and Westenberg, 2005). Unfortunately, development of these compounds has been halted or slowed down following pre-clinical findings of convulsion (Dunayevich et al., 2008).
Oxytocin (OT) is best known for its pro-social effects and it also has anti-anxiety effects in animal models (Neumann and Slattery, 2016). However, only the pro-social effects have been confirmed in humans (Gottschalk and Domschke, 2018). Oxytocin may not be indicated to alleviate anxiety symptoms in humans as suggested by the fact that it is not anxiolytic in the NPU threat test (Grillon et al., 2013).
Prazosin, a norepinephrine alpha-1 (NE-α1) antagonist traditionally used as an antihypertensive drug, has anti-anxiety effects in animal models (Skelly and Weiner, 2014). It has been used in PTSD to alleviate the arousal symptoms of exaggerated startle and sleep disturbance (Zhang et al., 2020). Early studies showed positive clinical results in PTSD (Raskind et al., 2003; Roepke et al., 2017), but a recent large-scale multi-site study did not confirm these observations (Raskind et al., 2018). A recent study reported no effect of prazosin on APS in the NPU test (Kaye et al., 2019). Here again, like for CRF1 antagonists, animal models resulted in a false positive, but the APS test did not.
Arginine vasopressin (AVP) is a neuropeptide that modulates physiological and emotional responses to threat. The AVP system has become a target of anti-anxiety treatment because it is synthesized and released following threat and participates in the regulation of anxiety (Neumann and Landgraf, 2012). In addition, abnormal AVP levels are found in anxiety disorders (Peskind et al., 1998). Among its different receptors, the V1a receptors are heavily expressed in the amygdala and the BNST (Ross et al., 2019), and V1a receptor knock-out mice exhibit reduced anxiety in several models of anxiety (Neumann and Landgraf, 2012). Recently, Azevan Pharmaceuticals Inc. developed a V1a receptor antagonist, SRX246. This agent, SRX246, has yet to be tested in anxiety patients, but the results of a recent study using the NPU threat test in healthy humans found anxiolytic effects. A week of treatment with SRX246 reduced APS (Lago et al., 2020).
To summarize, among the agents with anti-anxiety effects in animal models, only the mGlu2/3 receptor agonist LY354740 (Grillon et al., 2003) and V1a antagonist SRX246 (Lago et al., 2020) had an anti-anxiety effect on APS. LY354740 showed some evidence of clinical effectiveness in patients with anxiety disorders (Dunayevich et al., 2008; Levine et al., 2002) and the effectiveness of SRX246 has yet to be evaluated. Collectively, these observations establish APS as a promising tool to help screen candidate anxiolytics. APS detects the anxiolytic activity of conventional anxiolytics. It is insensitive to agents that have anti-anxiety effects in animal models but have no clinical efficacy. The remaining challenge is to show that candidate anxiolytics with anti-anxiety effect on APS, such as the V1a receptor antagonist SRX246, are anxiolytic in patients.
4.4. Clinical validity
If exposure to unpredictable stressors plays a role in the development of behavioral sensitization in pathological anxiety (Construct validity section), one would expect that APS can serve as a useful phenotypic readout of this behavioral sensitization and help distinguish patients from non-patients. Consistent with this assumption, there is increased evidence of exaggerated APS in anxiety and stress-related disorders across a range of threat manipulations (context conditioning, verbal threat, shocks, unpleasant pictures) and laboratories. Exaggerated APS has been documented in PTSD during context conditioning (Grillon and Morgan, 1999) and verbal threat (Brinkmann et al., 2017b; Grillon et al., 2009b; Lieberman et al., 2020; Simmons et al., 2013). Similar results have been found in panic disorder (Brinkmann et al., 2017a; Gorka et al., 2017b; Grillon et al., 1994; Grillon et al., 2008; Shankman et al., 2013). Exaggerated APS is also associated with a family history of panic disorder, suggesting such response may be a familial risk factor for panic disorder (Nelson et al., 2013). Findings in individuals with GAD have been mixed. Anxiety-potentiated startle is not increased in GAD (Gorka et al., 2017b; Grillon et al., 2009b) but there is evidence of increased contextual anxiety (Grillon et al., 2009b) and enhanced reactivity to unpredictable threat in GAD at the neural level (Buff et al., 2017). One possibility for this discrepancy is that sensitivity to threat is expressed differently in GAD compared to other anxiety disorders. The enhanced APS found in PTSD and panic disorder may be suppressed by worry, the cardinal symptom of GAD (Borkovec et al., 2004). Of note, the increased responses to unpredictable threat in these disorders have been documented in the context of a normal response to predictable threat.
Taken together, these results attest to the clinical validity of APS. There is a pharmacological parallel between the experimental model of unpredictable threat and anxiety disorders: anxiolytics that are used to treat anxiety disorders reduce APS. In addition, APS is exaggerated in pathological anxiety, and is sensitive to conventional drug treatments. This raises the possibility that APS could be used to evaluate targeted treatments. Theoretically, exaggerated startle reflects a sensitized behavioral state characterized by hyperarousal and hypervigilance. A key question is whether agents that reduce APS preferentially reduce arousal and vigilance symptoms.
4.5. Test-retest reliability and reproducibility
The poor reproducibility of animal studies is a major concern for the pharmacological industry (Prinz et al., 2011), but scientific and clinical investigations in humans face the same problem (Collaboration, 2012; Ioannidis, 2005; Prinz et al., 2011). It is therefore imperative to ensure that tasks used for proof-of-concept studies have sound psychometric properties (Hajcak and Patrick, 2015; Kaye et al., 2016). The psychometric properties of APS have been examined recently with a large sample size (N=128) (Kaye et al., 2016). The measures investigated included the stability of the effect size over two testing sessions separated by approximately a week, internal consistency, and temporal stability within-subjects (see also(Lieberman et al., 2017; Shankman et al., 2013) for converging evidence). Results showed that the APS yielded robust, “exceptionally large potentiation of startle” (e.g., 7–10 standard t scores) with large (partial eta-squared) effect sizes (range .56–.77). Anxiety-potentiated startle displayed adequate-to-good internal consistencies. The authors concluded that the task was “appropriate for research questions that require multiple administrations across time”.
4.6. Practicality and tolerability
Practicality is the experience of administrating the test from the experimenter point of view. It includes consideration of difficulties in test setup, staff training, administration, and scoring. The psychophysiological equipment for APS is inexpensive (about $10,000-$15,000) and relatively easy to set up. The training to administer the test and score the data is rapid.
Tolerability refers to the experience of the test from the participant’s point of view. It considers the length of the test and features that make the test unpleasant. Of course, the shock is mildly painful, and the white noise uses to elicit startle is also unpleasant. However, this is not problematic when testing healthy controls or even patients. In most studies, subjects set the level of shock themselves. Shocks have been used in large number of studies that investigate drug effects in healthy subjects, including studies requiring repeated testing over times. The many studies conducted in patients (see Clinical validity) also attest of the possible application of APS for clinical trials.
5. The Way Forward: Beyond anxiety-potentiated startle
A great advantage of APS is its translational nature. It can be evaluated in laboratory animals, healthy humans, and patients. It is a readout of a defensive response to unpredictable threat. However, anxiety is multifaceted with behavioral, emotional, and cognitive symptoms that reflect the expression of distinct underlying neurocircuits. These different symptoms probably respond to different treatments. Targeting these symptoms requires a better understanding of their underlying mechanisms, interaction, and dysfunction. This can be accomplished by extending the approach presented in this review beyond the study of defensive responses such as APS to include emotional, behavioral, and cognitive functions that are altered by anxiety. We have previously described such an approach which consists in combining an anxiety-inducing procedure with cognitive (e.g. working memory) or behavioral (e.g. go/nogo) tasks that probe core cognitive (e.g. distractibility) or behavioral (e.g. poor decision making, response inhibition) deficits in anxiety patients (Grillon et al., 2019).
The threat of unpredictable shock is an efficient way to induce a sustained state of anxiety, but there are other models of anxiety, including pharmacological challenges such as cholecystokinin (CCK) (Eser et al., 2009) and yohimbine (Charney et al., 1987), and physiological challenges such as carbon dioxide (CO2). While inhalation of high concentration of CO2 triggers an immediate feeling of fear and bodily symptoms that resembles naturally occurring panic attacks, inhalation of low-level of CO2 (5–7.5%) has been proposed as a model of generalized anxiety disorder (GAD) (Bailey et al., 2011). The objective of this review was not to discuss and compare the merit of these experimental models. Reviews concerning these approaches can be found elsewhere (Bailey et al., 2011). Given the diversity of anxiety disorders and of anxiety symptoms, research in humans will likely benefit from a multi-prompt approach using different experiment models.
6. Conclusion
It is striking that the screening of candidate anxiolytics is still conducted uniquely in animal models, while human models do not play any role beyond phase I of clinical trials. The limited efficacy of animal models in the drug development process and the need for alternatives have long been pointed out (Griebel and Holmes, 2013; Rodgers, 1997). We believe it is time to invest in a new approach, namely using human models of anxiety to test candidate anxiolytics. There is strong evidence suggesting that experimental models of anxiety in humans, and more specifically APS, has a great potential as an experimental tool that can provide a rapid readout of drug efficacy and help drug developers decide whether the drug warrants a clinical trial.
There are two main arguments in favor of APS as a human model to screen candidate anxiolytics. APS meets many of the validation and criteria required for an experimental model; 1) it has strong face, construct, and clinical validity, 2) it is a sensitive and reliable measure of anxiety states with good test-retest reliability and reproducibility, and 3) it is easy to implement and well tolerated. Second, it is well-established that animal models are necessary based on ethical and safety considerations, but the rationale for using only animal models and not human models to determine if candidate anxiolytics have anxiolytic effects is not clear. Why would reducing anxiety in an experimental model of anxiety in laboratory animals be a better predictor of clinical efficacy than reducing anxiety in humans? We believe that patients are more likely to benefit from a drug with anti-anxiety effects in humans than in animals.
Testing candidate anxiolytics in human models of anxiety prior to a clinical trial presents several advantages beside informing the decision to conduct a clinical trial. Human models can be used to test a range of issues relevant to drug testing. For example, human models may be able to reveal sex differences in the effects of a candidate anxiolytic; sex differences have historically received less consideration in animal models (An et al., 2011; Craske et al., 2017; Kokras and Dalla, 2014) and is now encouraged by the NIMH (https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html). The purpose of animal models is also to help determine the dose of drug administration. However, this is not an easy task (Nair and Jacob, 2016). Conceivably, human models could evaluate the dose-response relationship and inform optimum drug dosage for clinical trials.
However, several issues need to be tackled before APS can deliver on its potential. A crucial issue for drug sponsors is predictive validity. Will candidate anxiolytic drugs with anti-anxiety effects on APS reduce anxiety in patients? And if so, will the test help make predictions about individual differences in treatment response? Will patients with high APS levels most likely benefit from the treatment? To answer these questions, drug developers and sponsors may benefit from testing candidate anxiolytics in human models.
These issues can be addressed satisfactorily with combined efforts from interested parties. Drug developers are encouraged to make candidate anxiolytic available for testing in human models. Grant and funding mechanisms should promote integrated translational projects of candidate anxiolytics in laboratory animals, healthy volunteers, and patients (with U19 mechanisms, for example, which was used for a collaborative study to test the CRH antagonist GSK561679). It is our belief that human models will lead to a better understanding and treatment of anxiety and stress-related disorders.
Highlights.
There is a need to improve the drug screening process for anxiolytics
Experimental models of anxiety in humans should be used as an additional screening step to help select candidate anxiolytics before launching clinical trials.
Anxiety-potentiated startle fulfill several key validity criteria as an experimental model of anxiety in humans
Anxiety-potentiated startle is a promising tool for anxiolytic drug development
Acknowledgements
This work was supported by the Intramural Research Program, National Institute of Mental Health (grant number ZIAMH002798, NCT00026559).
Footnotes
Disclosure statement
Drs. Grillon and Ernst have nothing to disclose.
Disclaimer: The views expressed in this article do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An X-L, Zou J-X, Wu R-Y, Yang Y, Tai F-D, Zeng S-Y, Jia R, Zhang X, Liu E-Q, Broders H, 2011. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Experimental Animals 60, 111–123. [DOI] [PubMed] [Google Scholar]
- Association AP, 2013. Diagnostic and statistical manual of mental disorders: DSM-5, 5th ed. American Psychiatric Association, Washingron, DC. [Google Scholar]
- Bailey JE, Dawson GR, Dourish CT, Nutt DJ, 2011. Validating the inhalation of 7.5% CO2 in healthy volunteers as a human experimental medicine: a model of generalized anxiety disorder (GAD). Journal of Psychopharmacology 25, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Baldwin D, Abelli M, Altamura C, Dell’Osso B, Domschke K, Fineberg NA, Grünblatt E, Jarema M, Maron E, Nutt D, Pini S, Vaghi MM, Wichniak A, Zai G, Riederer P, 2016. Biological markers for anxiety disorders, OCD and PTSD – a consensus statement. Part I: Neuroimaging and genetics. The World Journal of Biological Psychiatry 17, 321–365. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, 2015. Epidemiology of anxiety disorders in the 21st century. Dialogues in clinical neuroscience 17, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, 2000. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist 55, 1247–1263. [DOI] [PubMed] [Google Scholar]
- Bergink V, Westenberg HGM, 2005. Metabotropic glutamate II receptor agonists in panic disorder: a double blind clinical trial with LY354740. International Clinical Psychopharmacology 20, 291–293. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Alcaine OM, Behar E, 2004. Avoidance theory of worry and generalized anxiety disorder, in: Heimberg RG, Turk CL, Mennin DS (Eds.), Generalized anxiety disorder: advances in research and practice. Guilford, New York, pp. 77–108. [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak SV, Becker MPI, Herrmann MJ, Straube T, 2017a. Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychological Medicine, 1–14. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Neumeister P, Tupak SV, Becker MPI, Herrmann MJ, Straube T, 2017b. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Human Brain Mapping 38, 2190–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Bruchmann M, Becker MPI, Tupak S, Herrmann MJ, Straube T, 2017. Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Social Cognitive and Affective Neuroscience 12, 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Neumeister P, Feldker K, Heitmann C, Gathmann B, Andor T, Straube T, 2016. Specifically altered brain responses to threat in generalized anxiety disorder relative to social anxiety disorder and panic disorder. NeuroImage: Clinical 12, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR, 1987. Neurobiological mechanism of panic anxiety: biochemical and behavioral correleates of yohimbine-induced panic attacks. American Journal of Psychiatry 144, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Collaboration OS, 2012. An open, large-scale, collaborative effort to estimate the reproducibility of psychological science. Perspectives on Psychological Science 7, 657–660. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Garrido MI, Overstreet C, Pine DS, Grillon C, 2017. The un-predictive brain under threat: a neuro-computational account of anxious hypervigilance. Biol Psychiatry 82, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen H-U, 2017. Anxiety disorders. Nature Reviews 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1984. The mammalian startle response, in: Eaton RC (Ed.), Neural Mechanisms of Startle Behavior. Plenum Press, New York, pp. 287–351. [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacol 35, 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD, 2008. Efficacy and tolerability of an mglu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology 33, 1603–1610. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Iosifescu D, Mathew SJ, Neylan TC, Pape JC, Carrillo-Roa T, Green C, Kinkead B, Grigoriadis D, Rothbaum BO, Nemeroff CB, Mayberg HS, 2017. Corticotropin-Releasing Factor Receptor 1 Antagonism Is Ineffective for Women With Posttraumatic Stress Disorder. Biological Psychiatry 82, 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, Liberzon I, 2015. Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management 11, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Leicht G, Lutz J, Wenninger S, Kirsch V, Schule C, Karch S, Baghai T, Pogarell O, Born C, Rupprecht R, Mulert C, 2009. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum Brain Mapp, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A, 1995. Animal models of psychiatric disorders, in: Bloom FE, Kupfer DJ (Eds.), Psychopharmacology: the fourth generation in progress. Raven Press, LTD, New York, NY, pp. 787–798. [Google Scholar]
- Glotzbach-Schoon E, Andreatta M, Mühlberger A, Pauli P, 2013. Context conditioning in virtual reality as a model for pathological anxiety, e-Neuroforum, p. 63. [Google Scholar]
- Gorka SM, Lieberman L, Klumpp H, Kinney KL, Kennedy AE, Ajilore O, Francis J, Duffecy J, Craske MG, Nathan J, Langenecker S, Shankman SA, Phan KL, 2017a. Reactivity to unpredictable threat as a treatment target for fear-based anxiety disorders. Psychological Medicine 47, 1–11. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA, 2017b. Startle Potentiation to Uncertain Threat as a Psychophysiological Indicator of Fear-Based Psychopathology: An Examination Across Multiple Internalizing Disorders. J Abn Psychol 126, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, Davies SO, Klein DF, 1987. An open trial of fluoxetine in the treatment of panic attacks. Journal of clinical psychopharmacology 7, 329–332. [PubMed] [Google Scholar]
- Gottschalk MG, Domschke K, 2018. Oxytocin and Anxiety Disorders, in: Hurlemann R, Grinevich V (Eds.), Behavioral Pharmacology of Neuropeptides: Oxytocin. Springer International Publishing, Cham, pp. 467–498. [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RSE, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR, 2004. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry 56, 301–307. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A, 2013. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12, 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, 2002. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry 51, 851–858. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods S, Davis M, 1994. Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry 35, 431–439. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M, 1991. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M, 1993. Measuring the time-course of anxiety using the fear-potentiated startle reflex. Psychophysiology 30, 340–346. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Cornwell BR, Johnson L, 2006a. Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predictability. Biol Psychiatry 60, 752–759. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J, 2006b. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry 60, 760–766. [DOI] [PubMed] [Google Scholar]
- Grillon C, Chavis C, Covington MS, Pine DS, 2009a. Two-week treatment with citalopram reduces contextual anxiety but not cued fear. Neuropsychopharmacol 34, 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine L, Morgan CA III., 2003. Anxiolytic effects of the group II metabotropic glutamate receptors LY354740 in the fear-potentiated sartle paradigm in humans. Psyhopharmacology 168, 446–454. [DOI] [PubMed] [Google Scholar]
- Grillon C, Hale E, Lieberman L, Davis A, Pine DS, Ernst M, 2015. The CRH1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacol 40, 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B, 2013. Oxytocin increases anxiety to unpredictable threat Mol Psychiatry 18, 958–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS, 2007. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacol 32, 225–231. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS, 2008. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry 165, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, 1999. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf war veterans with posttraumatic stress disorder. J Abn Psychol 108, 134–142. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M, 2009b. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry 66, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Robinson OJ, Cornwell B, Ernst M, 2019. Modeling anxiety in healthy humans: A key intermediate bridge between basic and clinical sciences. Neuropsychopharmacol 44, 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Patrick CJ, 2015. Situating psychophysiological science within the Research Domain Criteria (RDoC) framework. International Journal of Psychophysiology 98, 223–226. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Beier JS, Simons B, Polak T, 2016a. Transcranial Direct Current Stimulation (tDCS) of the Right Inferior Frontal Gyrus Attenuates Skin Conductance Responses to Unpredictable Threat Conditions. Frontiers in Human Neuroscience 10, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MPI, Tupak SV, Guhn A, Schmidt B, Brinkmann L, Straube T, 2016b. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping 37, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM, 2006. Cure therapeutics and strategic prevention: raising the bar for mental health research. Molecular psychiatry 11, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA, 2005. Why Most Published Research Findings Are False. PLOS Medicine 2, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ, 2016. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology 53, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Fronk GE, Zgierska AE, Cruz MR, Rabago D, Curtin JJ, 2019. Acute prazosin administration does not reduce stressor reactivity in healthy adults. Psychopharmacology 236, 3371–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu W, Demler O, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month dsm-iv disorders in the national comorbidity survey replication. Archives of General Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, Dalla C, 2014. Sex differences in animal models of psychiatric disorders. British journal of pharmacology 171, 4595–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago T, Brownstein M, Beydler E, Manbeck A, Beale A, Simon N, Ernst M, Grillon C, 2020. Effects of SRX246, a vasopressin 1a receptor (V1a) antagonist, on an experimental model of fear and anxiety in humans. Biol Psychiatry 87, S167–S168. [Google Scholar]
- LeDoux JE, Pine DS, 2016. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 173, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Levine L, Gaydos B, Sheehan D, Goddard A, Feighner J, Potter W, D., S., 2002. The mGlu2/3 receptor agonist, LY354740, reduces panic anxiety induced by a CO2 challenge in patients diagnosed with panic disorder. Neuropharmacology 43. [Google Scholar]
- Lieberman L, Funkhouser CJ, Gorka SM, Liu H, Correa KA, Berenz EC, Phan KL, Shankman SA, 2020. The Relation Between Posttraumatic Stress Symptom Severity and Startle Potentiation to Predictable and Unpredictable Threat. The Journal of Nervous and Mental Disease 208, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ, Weinberg A, Sarapas C, Huggins AA, Shankman SA, 2017. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. International Journal of Psychophysiology 114, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Vansteenwegen D, van Kuyck K, Gabriëls L, Nuttin B, 2011. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cogn Affect Behav Neurosci 11, 228–244. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Barley B, 2018. Event-related potentials to threat of predictable and unpredictable shock. Psychophysiology 55, e13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Steven F, Watkins LR, Linda R, 2005. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29, 829–841. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF, 1978. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. J Abnorm Psychol 87, 256–271. [DOI] [PubMed] [Google Scholar]
- Moberg C, Curtin J, 2009. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology 118, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Charney DS, 2017. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call It quits? Biological Psychiatry 82, 858–860. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Gorka SM, Katz AC, Shankman SA, 2013. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J Abn Psychol 122, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, 2010. Animal models of neuropsychiatric disorders. Nat Neurosci 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R, 2012. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in Neurosciences 35, 649–659. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA, 2016. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biological Psychiatry 79, 213–221. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF, 2008. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry 63, 1118–1126. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, de Quervain DJF, 2015. Failed drug discovery in psychiatry: time for human genome-guided solutions. Trends in Cognitive Sciences 19, 183–187. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, Wilkinson CW, Raskind MA, 1998. Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biological Psychiatry 44, 1007–1016. [DOI] [PubMed] [Google Scholar]
- Pessoa L, 2014. Précis on The Cognitive-Emotional Brain. Behavioral and Brain Sciences 38, e71. [DOI] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K, 2011. Believe it or not: how much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery 10, 712–712. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, Hart KL, McFall M, Mellman TA, Reist C, Romesser J, Rosenheck R, Shih M-C, Stein MB, Swift R, Gleason T, Lu Y, Huang GD, 2018. Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. New England Journal of Medicine 378, 507–517. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Tröster K, Thomas RG, McFall MM, 2003. Reduction of Nightmares and Other PTSD Symptoms in Combat Veterans by Prazosin: A Placebo-Controlled Study. American Journal of Psychiatry 160, 371–373. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Grillon C, 2013. The impact of induced anxiety on response inhibition. Front Hum Neurosci 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C, 2014. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. The lancet. Psychiatry 1, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, 1997. Animal models of ‘anxiety’: where next? Behavioural Pharmacology 8, 477–496. [DOI] [PubMed] [Google Scholar]
- Roepke S, Danker-Hopfe H, Repantis D, Behnia B, Bernard F, Hansen ML, Otte C, 2017. Doxazosin, an α−1-adrenergic-receptor antagonist, for nightmares in patients with posttraumatic stress disorder and/or borderline personality disorder: A chart review. Pharmacopsychiatry 50, 26–31. [DOI] [PubMed] [Google Scholar]
- Ross AP, McCann KE, Larkin TE, Song Z, Grieb ZA, Huhman KL, Albers HE, 2019. Sex-dependent effects of social isolation on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT) and serotonin (5HT) 1a receptor binding and aggression. Hormones and Behavior 116, 104578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Weinberg A, Langenecker SA, Shankman SA, 2017. Relationships among attention networks and physiological responding to threat. Brain and Cognition 111, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori SB, Singewald N, 2019. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacology & Therapeutics 204, 107402. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C, 2012. Assessing fear and anxiety in humans using threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protoc 7, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ, 2003. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress 6, 189–197. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Binik YM, 1977. The safety signal hypothesis, in: Davis H, Hurwitz HMB (Eds.), Operant-Pavlovian interactions. Hillsdale, New York, pp. 165–187. [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O, 2014. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport 25, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, McGowan SK, Katz AC, Gorka SM, 2013. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J Abn Psychology 122, 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Flagan TM, Wittmann M, Strigo IA, Matthews SC, Donovan H, Lohr JB, Paulus MP, 2013. The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. pdf2 puberty 146, 426–432. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL, 2014. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain and Behavior 4, 468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L, Whalen P, Kelley W, 2010. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry 68, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C, 2012. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology 49, 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, 1986. Validation criteria for animal models of human mental disorders: Learned helplessness as a paradigm case. Progress in Neuro-Psychopharmacology and Biological Psychiatry 10, 677–690. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren R, Sanford LD, Yang L, Ni Y, Zhou J, Zhang J, Wing Y-K, Shi J, Lu L, Tang X, 2020. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Medicine 67, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]