Abstract
Background:
Although potential neurotoxicity of perfluoroalkyl and polyfluoroalkyl substances (PFAS) is suggested, previous epidemiologic studies have reported a ‘protective’ association between serum PFAS concentration and cognition function. Poor outcome assessment, residual confounding, non-monotonic dose-responses (NMDRs), and the role of reduced kidney function in PFAS excretion may be alternative explanations of these findings.
Objectives:
We examined the association of perfluoroalkyls with cognitive functions assessed using the Consortium to Establish a Registry for Alzheimer’s Disease word learning and recall; the Animal Fluency; and the Digit Symbol Substitution tests.
Methods:
We included 903 adults aged ≥60 years from the National Health and Nutrition Examination Survey (NHANES) 2011-2014. We computed a composite z-score as an average of four individual cognitive z-scores and used it as the outcome. Linear and generalized additive models were used to evaluate linear and non-linear associations.
Results:
With the linearity assumption, perfluorooctanoate (PFOA) and perfluorononanoate (PFNA) were significantly positively associated with composite z-score after adjustment for age, sex, race/ethnicity, education, smoking, poverty-income ratio, health insurance, food security, alcohol, and physical activity. Smoothing plots suggested NMDRs, especially for perfluorooctane sulfonate (PFOS) with a U-shape dose-response. When restricting to participants without chronic kidney disease (CKD) (n=613), the positive associations for PFOA and PFNA observed in the whole population diminished, whereas PFOS was inversely and significantly associated with composite z-score. Also, negative confounding effects of fish/seafood consumption seem to be substantial. Effect estimates of composite z-score were −0.055 (95% CI: −0.097, −0.012, P=0.01) for a doubling increase in PFOS.
Discussion:
These findings suggest that the previous epidemiologic findings of a ‘protective’ association between PFAS and cognition may be explained by CKD, NMDRs and confounding by fish consumption. PFOS at the current population exposure level in the U.S. may be a risk factor for cognitive decline in older adults with normal kidney function.
INTRODUCTION
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a family of man-made compounds extensively used in surface coating and protectant formulations (ATSDR 2018). Please notice that PFAS is used in this paper only if a set of chemicals analyzed includes both perfluoroalkyls and polyfluoroalkyls. These “forever chemicals”, especially long-chain PFAS, have been a significant public health concern given their non-biodegradable property in the environment (Post et al., 2017; Prevedouros et al., 2006) and potential human exposure through contaminated drinking water and food (Sunderland et al., 2019). Numerous epidemiologic studies have reported potential links between PFAS, mainly perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS), and a wide range of adverse health endpoints including increased lipid levels, liver damage, and thyroid dysfunction (ATSDR 2018). Activation of peroxisome proliferator-activated receptor-alpha (PPARα), a ligand-activated transcription factor that plays a role in lipid homeostasis, inflammation, and peroxisome proliferation, is an established mode of action for PFAS health effects (Ren et al., 2009; Takacs and Abbott, 2007; Wolf et al., 2008) although other putative mechanisms have been proposed, such as gap junctional inhibition to disrupt cell-cell communication, mitochondrial dysfunction, interference of protein binding, partitioning into lipid bilayers, oxidative stress, alterations in calcium homeostasis and related signaling pathways (Lau 2015). However, there is limited human data on the neurotoxicity of PFAS. To date, only three epidemiologic studies have evaluated the association between PFAS exposure and cognition in adults (Gallo et al., 2013; Power et al., 2013; Shrestha et al., 2017).
A cross-sectional study using data from participants from the 1999-2000 and 2003-2008 National Health and Nutrition Examination Surveys (NHANES) (n=1,766, age 60-85 years) reported non-significant inverse associations between perfluoroalkyls and self-reported cognitive limitation due to difficulty remembering or periods of confusion (Power et al., 2013). These associations were stronger and became statistically significant among non-medicated diabetics. Another cross-sectional study with 21,024 older adults (aged ≥50 years) who were exposed to high levels of PFOA from a chemical facility in the Mid-Ohio Valley, West Virginia and participated in the C8 Health Project reported statistically significant inverse associations of self-reported short-term memory loss with PFOS, PFOA, and perfluorohexane sulfonate (PFHxS) (Gallo et al., 2013). A study of 126 older adults aged 55–74 years and living in upper Hudson River communities which utilized various neuropsychological assessment tools (e.g., the California Verbal Learning Test, the Wechsler Memory Scale, the Wisconsin Card Sorting Test) also observed that higher PFOA and PFOS concentrations were associated with better performance in memory and learning, executive function and visuospatial function (Shrestha et al., 2017). These studies postulated that the observed neuroprotective associations could be due to the activation of PPARs (PPARα and PPARγ) and their anti-inflammatory effects (Gallo et al., 2013; Power et al., 2013; Shrestha et al., 2017) as PFAS have affinity for PPARs and thus serve as PPAR agonists (Jiang 2015). However, the epidemiologic evidence of increased serum lipid levels, particularly total cholesterol and low-density lipoprotein (LDL) cholesterol, contradicts the PPAR agonist hypothesis (Eriksen et al., 2013; Frisbee et al., 2010; Lin et al., 2019; Nelson et al., 2010; Starling et al., 2014; Steenland et al., 2009).
The present study explored if there are other possible explanations that can address the “protective” association between perfluoroalkyls and cognitive function: 1) use of more valid outcome measurements; 2) adjustment for residual confounding; 3) non-monotonic dose-responses (NMDRs); and 4) the role of reduced kidney function in PFAS excretion. First, except for Shrestha et al. (2017), the other two studies relied on self-reported, binary, cognitive limitation or short-term memory loss (Gallo et al., 2013; Power et al., 2013), raising potential outcome misclassification. Second, the previous findings could also be subject to potential negative confounding, such as fish consumption which may be a protective factor for cognitive decline (Kokubun et al., 2020; Zhang et al., 2015) but a major dietary source of PFAS exposure (Christensen et al., 2017; Fromme et al., 2009; Jain, 2014). Other residual confounding may include tobacco smoke and nicotine (Campos et al., 2017; Swan and Lessov-Schlaggar, 2007). Third, PFAS as endocrine disrupting chemicals may have NMDRs, i.e., the shapes of dose-response curves are non-linear, such as U- or J-shapes (Vandenberg et al., 2012). Conventional regression modeling with the linearity assumption may not capture NMDRs. Finally, reduced kidney function or chronic kidney disease (CKD) may play a role in evaluating the association between PFAS and cognition, given that reduced kidney function can lead to alterations in serum concentrations of PFAS. Reduced kidney function may decrease PFAS excretion, resulting in higher PFAS serum concentrations which are the consequences of reduced kidney function, not the causes (i.e., reverse causation) (Dhingra et al., 2017; Shankar et al., 2011). Another study reported an inverted U-shaped association between PFAS and declining estimated glomerular filtration rate (eGFR) of advancing stages of kidney disease (Jain and Ducatman, 2019). Individuals at different stages of kidney disease may have altered balance of glomerular secretion and reabsorption which influences the serum concentrations of PFAS (Han et al., 2012). Additionally, CKD has been associated with poor cognitive function (Berger et al., 2016; Brodski et al., 2019; Etgen et al., 2012). Therefore, reduced kidney function/CKD may be another potential confounder that was not considered in the previous studies. On the other hand, PFAS exposure has been associated with a lower eGFR and a higher prevalence of CKD (Conway et al., 2018; Watkins et al., 2013). Hence, evaluation of the PFAS health effects may be complicated if the population of interest includes affected individuals whose proxy exposure (serum PFAS concentrations) do not necessarily reflect true exposure. Restriction to individuals with normal kidney function may provide an ideal population that allows us to evaluate causal effects of PFAS in older populations.
To address these gaps, we examined the association of perfluoroalkyls with cognitive functions assessed using the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) word learning and recall tests, the Animal Fluency test, and the Digit Symbol Substitution test which are widely used as sensitive tests for detecting mild cognitive impairment (Shankle et al., 2005), in older adults of the general United States (U.S.) population.
METHODS
Study population
The NHANES is a complex, multistage survey combining interviews and physical examinations together to assess health and nutritional status on the noninstitutionalized U.S. population. We used data from the 2011-2012 and 2013-2014 cycles where the CERAD cognition assessments were conducted. Participants aged 60 years and older were eligible as cognitive assessments were only administered in this age range. For this analysis, 988 older adults who participated in the sub-study of the perfluoroalkyls measurement were eligible. Among them, 85 participants who did not complete any of the four cognitive function test batteries described below were excluded, yielding the final sample size to 903 participants.
Cognitive Function Assessment
Three cognitive assessments were conducted in NHANES 2011-2014 including the CERAD Word Learning subtest, the Animal Fluency test, and the Digit Symbol Substitution test (DSST). The CERAD Word Learning subtest, consisting of three consecutive learning trials and a delayed recall, evaluates immediate and delayed learning ability for new verbal information (Morris, 1989). For the learning trials, participants read aloud 10 unrelated words, one at a time, as they were presented in large bolded letters on a computer monitor. Right after the tenth word, participants recalled as many words as possible. The order of the 10 words was changed in each trial. The delayed world recall trial was conducted after the other two cognitive tests (Animal Fluency and DSST) were completed. The maximum score on each trial is 10. The Animal fluency test examines categorical verbal fluency, a component of executive function in which participants were asked to name as many animals as possible in one minute (Strauss et al., 2006). The DSST, a component of the Wechsler Adult Intelligence Test (WAIS-III), is used to evaluate the brain function on processing speed, sustained attention, and working memory (Wechsler, 1997). The exercise was conducted using a paper form that has a key at the top containing nine numbers paired with symbols. Participants drew symbols corresponding to nine numbers in the 133 boxes for 2 minutes. The score was the number of correctly drawn symbols. The maximum score is 133. Generally higher scores indicate better cognitive function.
In this study, we computed “global cognition” as a summary score of the four cognitive tests (CERAD Word List Learning Test, CERAD Word List Recall Test, Animal Fluency Test and DSST) because individual cognitive assessments have several limitations such as inter- and intra-individual variations, and floor and ceiling effects (Li et al., 2018). Global cognition was created by the average of the standardized z-scores of each test.
Perfluoroalkyls assessment
Serum perfluoroalkyl concentrations were measured in a one-third randomly selected participants aged 12 years and older. Details of samples collection, storage, preparation, and detection are discussed in elsewhere (CDC/NCHS, 2013, 2012). Briefly, perfluoroalkyls were measured using online-solid phase extraction coupled to High Performance Liquid Chromatography-Turbo Ion Spray ionization-tandem Mass Spectrometry. The lower limit of detection (LLOD) was 0.1 ng/mL for all analytes. We selected four perfluoroalkyl compounds, PFOS, PFOA, PFHxS, and perfluorononanoate (PFNA), which were detected in almost all participants (the detection rates >98%). The concentrations below LLOD were replaced with a value of LLOD/√2 (0.07 ng/mL). In NHANES 2013-2014, linear PFOA (n-PFOA), sum of branched isomers of PFOA (Sb-PFOA, branched PFOA isomers), linear PFOS (n-PFOS), and sum of perfluoromethylheptane sulfonate isomers (Sm-PFOS, monomethyl branched PFOS isomers) were measured instead of total concentrations. We calculated the sum of isomers for PFOA and PFOS, respectively, as NHANES suggested that the sum of isomers from 2013-2014 cycle was comparable to the total concentrations reported in previous cycles. Comprehensive quality assurance (QA)/quality control (QC) procedures were conducted including analysis of QC pools. The coefficients of variation were 10.3-11.9% for the low QC pools; and 8.6-12.8% for the high QC pools (CDC/NCHS, 2013, 2012).
Covariates
Sociodemographic and behavioral factors were obtained by self-reported questionnaires. We considered a group of covariates as per Power et al. (2013): age (continuous, years), sex (male, female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and other), education (<high school, high school, >high school), poverty-income ratio (<1, 1-1.25, 1.25-<2, 2-<4, ≥4), health insurance (none or single service, government, private, multiple), food security (fully secure, not fully secure), smoking status (never, former, current), alcohol consumption (Never, ≤1 drink per day, >1 drink per day), total recreational physical activity (continuous, metabolic equivalent (MET) score), and NHANES survey cycles (2011-2012, 2013-2014). We additionally considered the following potential confounders as they were identified to be associated with serum PFAS concentrations and cognition: 1) measures of tobacco smoke and nicotine other than smoking status: smoking intensity (pack-years: 0, <20, ≥20) and serum cotinine concentrations (Campos et al., 2017; Swan and Lessov-Schlaggar, 2007); and 2) fish and shellfish consumption (frequency during the past 30 days: never, 1 to <4 times, ≥4 times) (Christensen et al., 2017; Fromme et al., 2009; Jain, 2014). CKD status was determined using either eGFR <60 mL/min/1.73 m2 or urinary albumin to creatinine ratio (ACR) ≥30 mg/g (Vassalotti et al., 2007). eGFR was estimated using the CKD-EPI Creatinine Equation (Levey and Stevens, 2010).
Statistical analysis
Analyses incorporated primary sampling units (clusters), strata and sampling weights as per guidelines to account for the complex sampling design of NHANES (NCHS, 2011). Participant characteristics and composite z-score of cognition were summarized using means or geometric means (standard errors, SEs) for continuous variables, and percentages for categorical variables. Detection rates, geometric means (95% confidence intervals, 95% CIs), and quantiles of PFAS concentrations were calculated. There were missing observations in the covariates listed above. To maximize the sample size and power and to achieve valid inference, we used multiple imputations by fully conditional specification (also known as multiple imputation by chained equation, MICE) with PROC MI in SAS (van Buuren, 2007). This procedure imputed plausible data values from a distribution specifically generated for each data point. Twenty datasets were created with imputations for missing covariates and computed pooled parameter estimates and SEs using PROC MIANALYZE in SAS.
To determine the relationship between serum concentrations of PFAS and cognition z-score, we utilized the PROC SURVEYREG procedure in SAS. Separate sequential models were constructed to check if the previously observed protective effects of perfluoroalkyls on cognition could be explained by different potential confounders. Initial models (Model 1) were adjusted for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational physical activity and NHANES cycles, in line with Power et al. (2013). We additionally included smoking pack-years and serum cotinine (log-transformed) (Model 2); fish and shellfish consumption (Model 3); and CKD status (Model 4). Serum concentrations of PFAS were log-transformed with base 2 and modeled as linear continuous variables. Thus, effect estimates were presented for a doubling increase in perfluoroalkyl concentrations. To reduce the possibility of residual confounding, all of the following analyses were adjusted for covariates as controlled for in Model 4. As a sensitivity analysis, we repeated analyses using observed data allowing missing values. We assessed potential effect modification by sex by including an interaction term between perfluoroalkyls and sex.
To evaluate NMDRs between PFAS exposure and cognition, we applied generalized additive models (GAMs) with penalized splines to visualize the shape of the associations. Penalized spline was chosen because it determines smoothness (or wiggliness) of the dose-response curves without any a priori shapes based on the bias-variance tradeoff and the minimum prediction errors (Wood, 2006). The gam() function from the mgcv package in R (version 4.0; R Foundation for Statistical Computing) was used. Sampling weights were incorporated into the GAMs.
To evaluate the impact of CKD, the analysis was restricted to participants without CKD. Potential NMDRs were also evaluated in this subpopulation. We also included an interaction term between perfluoroalkyls and CKD status and evaluated effect modification by CKD.
RESULTS
A total of 903 adults aged 60 years and older were included for analysis. The mean age of the study participants was 69.4 (SE=0.3) years and 54.2% were females (Table 1). The composite z-score for global cognition had a normal distribution with the mean of 0.27 (SE=0.04) and ranged from −2.19 to 2.10. Geometric means of serum concentrations were 8.38 ng/mL (95% CI: 7.69-9.13) for PFOS; 2.62 ng/mL (95% CI: 2.45-2.80) for PFOA; 1.84 ng/mL (95% CI: 1.68-2.02) for PFHxS; and 0.95 ng/mL (95% CI: 0.87-1.03) for PFNA. More details of the distributions of perfluoroalkyls are presented in Table S1.
Table 1.
Survey-weighted characteristics of study participants aged 60 years 674 and older (n=903).
| Characteristic | Unweighted N | Weighted mean (95% CI) or % |
|---|---|---|
| Perfluorooctane sulfonate (PFOS), ng/mL | 903 | 8.38* (7.69-9.13) |
| Perfluorooctanoate (PFOA), ng/mL | 903 | 2.62* (2.45-2.80) |
| Perfluorohexane sulfonate (PFHxS), ng/mL | 903 | 1.84* (1.68-2.02) |
| Perfluorononanoate (PFNA), ng/mL | 903 | 0.95* (0.87-1.03) |
| Global cognition (composite z-score) | 903 | 0.27 (0.18, 0.36) |
| Age, years | 903 | 69.4 (68.8, 70.0) |
| Body mass index, kg/m2 | 891 | 28.8 (28.2, 29.3) |
| Physical activity, metabolic equivalent score | 902 | 10.1 (7.59, 12.7) |
| Serum cotinine, ng/mL | 903 | 0.077* (0.052, 0.11) |
| Sex | ||
| Male | 443 | 45.8% |
| Female | 460 | 54.2% |
| Race/ethnicity | ||
| Non-Hispanic white | 432 | 80.7% |
| Non-Hispanic black | 218 | 8.0% |
| Mexican American | 77 | 3.1% |
| Other Hispanics | 94 | 3.7% |
| Non-Hispanic Asian | 73 | 3.2% |
| Others | 9 | 1.3% |
| Education | 902 | |
| <High school | 247 | 18.1% |
| High school or equivalent | 212 | 22.6% |
| Some college | 246 | 32.1% |
| College graduate | 197 | 27.2% |
| Poverty-income ratio | 813 | |
| <1 | 134 | 8.7% |
| 1-1.25 | 86 | 7.3% |
| 1.25-<2 | 144 | 14.7% |
| 2-<4 | 224 | 31.7% |
| ≥4 | 225 | 37.6% |
| Health insurance | 902 | |
| None or single service plan | 77 | 5.0% |
| Government insurance | 345 | 29.2% |
| Private insurance | 208 | 26.9% |
| Multiple insurance | 272 | 38.9% |
| Food security | 898 | |
| Not fully secured | 174 | 11.3% |
| Fully secured | 724 | 88.7% |
| Smoking status | 902 | |
| Never smoker | 428 | 48.1% |
| Former smoker | 356 | 41.3% |
| Current smoker | 118 | 10.6% |
| Alcohol consumption | 716 | |
| Never | 274 | 31.3% |
| ≤1 drink per day | 359 | 55.9% |
| >1 drink per day | 83 | 12.8% |
| NHANES cycles | 903 | |
| 2011-2012 | 392 | 45.0% |
| 2013-2014 | 511 | 54.9% |
| Smoking intensity | 878 | |
| Never smoker | 428 | 49.1% |
| <20 pack-years | 268 | 27.5% |
| ≥20 pack-years | 182 | 23.4% |
| Fish consumption | 828 | |
| Never | 174 | 18.9% |
| 1-<4 times in the past 30 days | 332 | 38.8% |
| ≥4 times in the past 30 days | 322 | 42.3% |
| Shellfish consumption | 830 | |
| Never | 434 | 48.9% |
| 1-<4 times in the past 30 days | 276 | 35.5% |
| ≥4 times in the past 30 days | 120 | 15.6% |
| Chronic kidney disease | 903 | |
| No | 613 | 72.2% |
| Yes | 290 | 27.8% |
Geometric mean.
We first constructed a model for the association between perfluoroalkyls and the composite z-score for global cognition that is comparable to the model by Power et al. (2013). After adjustment for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational physical activity, and NHANES survey cycles (Model 1), PFOA and PFNA were, when these variables were fit as a logarithmic form, statistically significantly, positively associated with the composite z-score: 0.067 (95% CI: 0.009, 0.12, P=0.02) for a doubling increase in PFOA; 0.065 (95% CI: 0.023, 0.11, P=0.002) for PFNA (Table 2). PFOS and PFHxS were not significantly associated with the composite z-score. Additional adjustment for smoking pack-years and serum cotinine (Model 2) did not change the significances and magnitudes of the associations. Additional adjustment for fish and shellfish consumption (Model 3) shifted the directionality of the associations to the left: positive associations for PFOA and PFNA toward the null (0.059 (95% CI: 0.001, 0.12, P=0.05) for a doubling increase in PFOA; 0.057 (95% CI: 0.014, 0.10, P=0.01) for PFNA), whereas the null association for PFOS away from the null (−0.009 (95% CI: −0.049, 0.031, P=0.66) for PFOS). The results essentially remained the same with additional adjustment for CKD (Model 4). In sensitivity analyses, the results were similar with wider confidence intervals when observed data instead of imputed data were used (Table S2). There was no significant evidence of effect modification by sex although the magnitudes of the associations for PFOA and PFNA were larger in females (Table S3).
Table 2.
Pooled effect estimates (βs)* and 95% Confidence Intervals (CIs) of composite z-score for global cognition† per doubling increase in serum perfluoroalkyl concentrations among 903 adults 60 years and older.
| β (95% CI) | P value | |
|---|---|---|
| PFOS | ||
| Model 1a | −0.001 (−0.040, 0.038) | 0.97 |
| Model 2b | −0.001 (−0.040, 0.038) | 0.96 |
| Model 3c | −0.009 (−0.049, 0.031) | 0.66 |
| Model 4d | −0.009 (−0.048, 0.030) | 0.64 |
| PFOA | ||
| Model 1a | 0.067 (0.009, 0.12) | 0.02 |
| Model 2b | 0.065 (0.008, 0.12) | 0.02 |
| Model 3c | 0.059 (0.001, 0.12) | 0.05 |
| Model 4d | 0.059 (0.003, 0.12) | 0.04 |
| PFHxS | ||
| Model 1a | 0.026 (−0.013, 0.064) | 0.20 |
| Model 2b | 0.026 (−0.012, 0.064) | 0.18 |
| Model 3c | 0.021 (−0.017, 0.059) | 0.28 |
| Model 4d | 0.021 (−0.016, 0.058) | 0.27 |
| PFNA | ||
| Model 1a | 0.065 (0.023, 0.11) | 0.002 |
| Model 2b | 0.066 (0.026, 0.11) | 0.001 |
| Model 3c | 0.057 (0.014, 0.10) | 0.01 |
| Model 4d | 0.057 (0.015, 0.10) | 0.008 |
PFOS, perfluorooctane sulfonate; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate.
Model 1 was adjusted for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational activity, and NHANES cycles. Complex survey designs were taken into account in the analyses
Model 2: Model 1 + smoking pack-years, serum cotinine (log-transformed)
Model 3: Model 2 + fish and shellfish consumption
Model 4: Model 3 + CKD status.
Estimates were pooled with 20 imputed data.
Global cognition was created by the average of the standardized z-scores of the four cognitive tests (CERAD Word List Learning Test, CERAD Word List Recall Test, Animal Fluency Test and Digit Symbol Substitution Test).
We evaluated potential non-linearity using smoothing plots (Figure 1). For PFOA, the dose-response shape was monotonic and positive until the median (2 ng/mL, log2-transformed value=1) and then leveled off. There was a clear linear positive association between PFNA and composite z-score. PFHxS had a weak, positive association. Whereas, a non-monotonic, inverted U-shape dose-response relationship was seen for PFOS: a positive association between 1 ng/mL (log2-transformed value=0) and 8 ng/mL (log2-transformed value=3, approximately the 40th percentile) and then a decline through the end. This inverted U-shape association seemed to result in an overall null association with the linearity assumption reported in Table 2.
Figure 1.
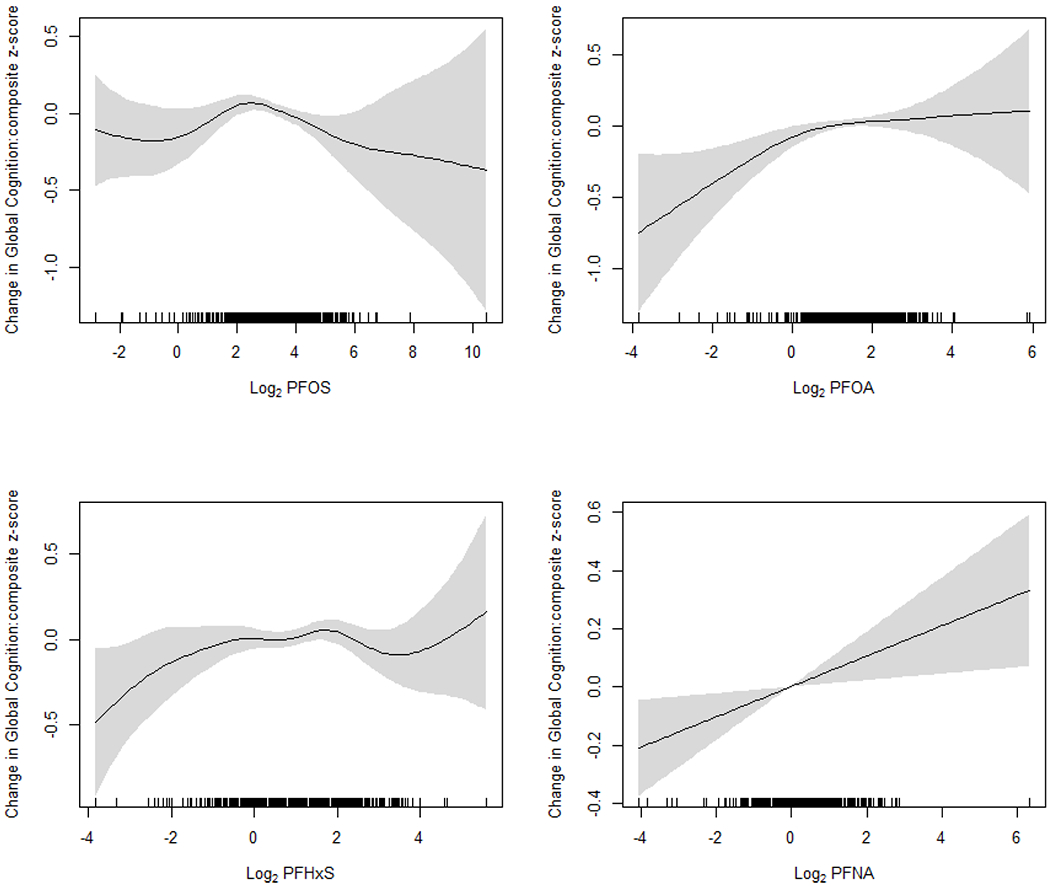
Smoothing plots of the association between serum perfluoroalkyl concentrations (transformed with log base 2) and global cognition (composite z-score). All models were adjusted for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational activity, NHANES cycles, smoking pack-years, serum cotinine (log-transformed), fish and shellfish consumption, and chronic kidney disease status. Perfluoroalkyl variables were fit using penalized splines in generalized additive models. The regression models were weighted with the sampling weights to account for the NHANES sampling design.
When analysis was restricted to participants without CKD (n=613) (Table 3), the positive associations for PFOA and PFNA observed in the whole population diminished and were no longer statistically significant, whereas PFOS was inversely and significantly associated with composite z-score. Also, confounding effects of fish and seafood consumption seem to be substantial. In fully adjusted models including fish and seafood consumption (Model 3), effect estimates of composite z-score were −0.055 (95% CI: −0.097, −0.012, P=0.01) for a doubling increase in PFOS; 0.023 (95% CI: −0.060, 0.11, P=0.59) for PFOA; 0.016 (95% CI: −0.025, 0.058, P=0.44) for PFNA; and −0.005 (95% CI: −0.055, 0.046, P=0.85) for PFHxS. Smoothing plots confirm the inverse association for PFOS and weak associations for other compounds (Figure 2). A test for effect modification by CKD status using the model including an interaction between perfluoroalkyls and CKD revealed that the difference in effects for PFOS between participants with and without CKD was statistically significant (P for interaction=0.02) (Table S4).
Table 3.
Pooled effect estimates (βs)* and 95% Confidence Intervals (CIs) of composite z-score for global cognition† per doubling increase in serum perfluoroalkyl concentrations among 613 adults without chronic kidney disease.
| β (95% CI) | P value | |
|---|---|---|
| PFOS | ||
| Model 1a | −0.036 (−0.079, 0.007) | 0.10 |
| Model 2b | −0.037 (−0.081, 0.006) | 0.09 |
| Model 3c | −0.055 (−0.097, −0.012) | 0.01 |
| PFOA | ||
| Model 1a | 0.043 (−0.039, 0.13) | 0.30 |
| Model 2b | 0.037 (−0.047, 0.12) | 0.39 |
| Model 3c | 0.023 (−0.060, 0.11) | 0.59 |
| PFHxS | ||
| Model 1a | 0.007 (−0.047, 0.060) | 0.81 |
| Model 2b | 0.004 (−0.048, 0.055) | 0.89 |
| Model 3c | −0.005 (−0.055, 0.046) | 0.85 |
| PFNA | ||
| Model 1a | 0.037 (−0.009, 0.084) | 0.12 |
| Model 2b | 0.036 (−0.010, 0.082) | 0.13 |
| Model 3c | 0.016 (−0.025, 0.058) | 0.44 |
PFOS, perfluorooctane sulfonale; PFOA, perfluorooctanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate.
Model 1 was adjusted for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational activity, and NHANES cycles. Complex survey designs were taken into account in the analyses
Model 2: Model 1 + smoking pack-years, serum cotinine (log-transformed)
Model 3: Model 2 + fish and shellfish consumption.
Estimates were pooled with 20 imputed data.
Global cognition was created by the average of the standardized z-scores of the four cognitive tests (CERAD Word List Learning Test, CERAD Word List Recall Test, Animal Fluency Test and Digit Symbol Substitution Test).
Figure 2.
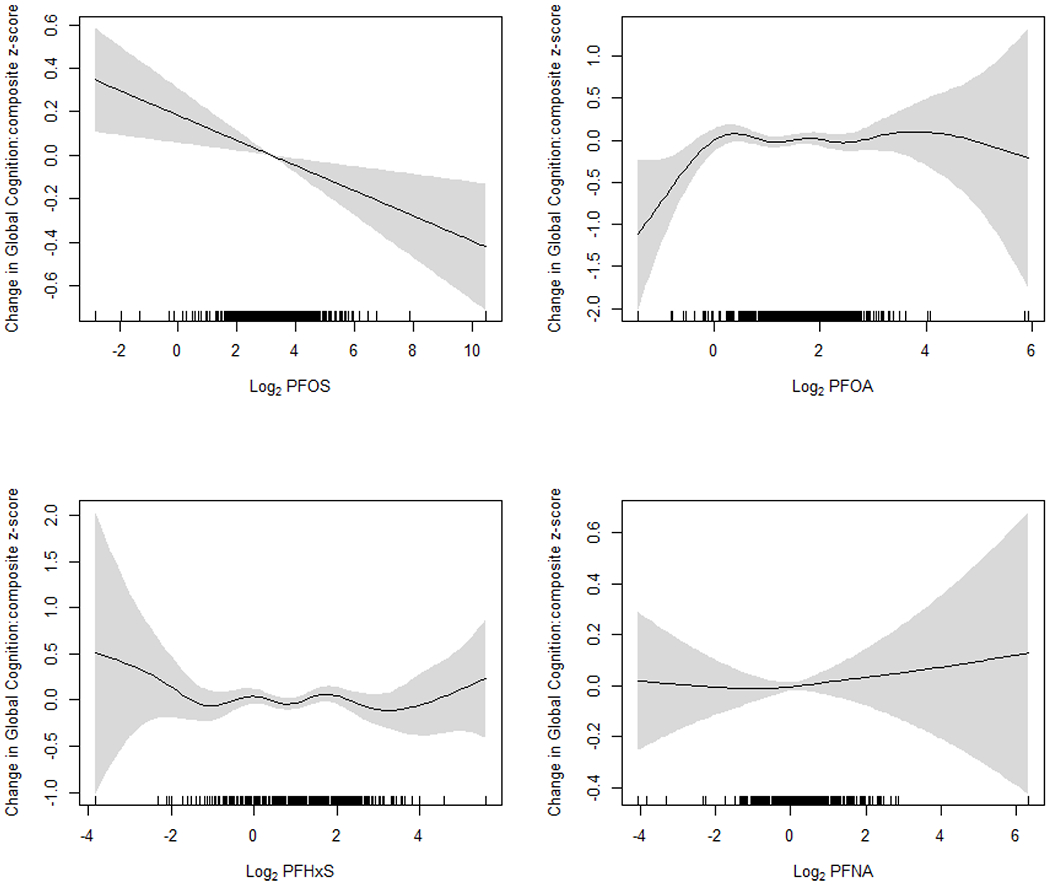
Smoothing plots of the association between serum perfluoroalkyl concentrations (transformed with log base 2) and global cognition (composite z-score) in participants without chronic kidney disease. All models were adjusted for age, sex, race/ethnicity, education, poverty-income ratio, health insurance, food security, smoking status, alcohol consumption, total recreational activity, NHANES cycles, smoking pack-years, serum cotinine (log-transformed), fish and shellfish consumption. Perfluoroalkyl variables were fit using penalized splines in generalized additive models. The regression models were weighted with the sampling weights to account for the NHANES sampling design.
DISCUSSION
Although biological plausibility of the association between PFAS and cognitive decline has been postulated, findings from previous epidemiologic studies were not supportive and even contradictory suggesting neuroprotective effects of PFAS. The present study explored if alternative approaches could explain such findings: 1) use of more valid outcome measurements; 2) accounting for residual confounding; 3) NMDRs; and 4) restriction to participants without CKD. We did not find much differences with the well-validated cognitive function test tools, the CERAD (Consortium to Establish a Registry for Alzheimer Disease) cognition test batteries. Confounding by fish and seafood consumption seems to be critical and should not be dismissed. The present study also revealed potential NMDR relationships, especially an inverted U-shape for PFOS, suggesting that neurotoxicity of PFAS may depend on dose or other susceptibility factors may play a role. Reduced kidney function/CKD could be one such factor.
The implication of kidney function and kidney failure for human studies of PFAS and disease states has been recently discussed by Jain and Ducatman (2019). They compared serum concentrations of perfluoroalkyls across the stages of glomerular function and found inverted U-shaped distributions, i.e., perfluoroalkyl concentrations increased through the stage-3A (eGFR ≥45 mL/min/1.73 m2) but decreased in the stage 3B and 4 combined (eGFR 15-44 mL/min/1.73 m2) (Jain and Ducatman, 2019). The inverted U-shaped association between serum perfluoroalkyl concentrations and eGFR stages could be attributed to altered balance of reabsorption and secretion in progressive kidney disease compared to healthy kidney. Therefore, kidney function should be taken into account in epidemiologic studies where PFAS are assessed in blood and the health outcomes of interest are associated with reduced kidney function and CKD (Jain and Ducatman, 2019). The present study supports this notion and suggests that epidemiologic results of the association between serum PFAS concentrations and cognitive decline in older adults may mislead true causal associations unless reduced kidney functions are not considered. In our case, CKD seems to be an effect modifier rather than a confounder. True causal associations between PFAS exposure and cognitive decline may be observed only if the analysis is restricted to individuals with normal kidney functions.
Other important factors that may complicate evaluation of PFAS health effects in human populations are menstruation, parturition, breastfeeding, and other characteristics that influence the levels of PFAS elimination, especially among women of reproductive age (Ding et al., 2020; Park et al., 2019; Taylor et al., 2014; Wong et al., 2014). Menstrual bleeding, menopause, parity, and breastfeeding have been associated with lower blood PFAS concentrations (Ding et al., 2020; Park et al., 2019; Taylor et al., 2014; Wong et al., 2014). A study of the association between serum perfluoroalkyl concentrations and ovarian hormone concentrations in naturally cycling women reported a significant inverse association between PFOS and estradiol only among nulliparous women, but not parous women (Barrett et al., 2015). The authors proposed to examine nulliparous women for evaluating the effects of PFAS on ovarian function because nulliparous women can serve as a “cleaner” population for the possible ovarian toxicity of PFAS (Barrett et al., 2015). The present study did not further exclude female participants due to menopausal status, parity, or history of breastfeeding because eligible participants were aged 60 years and older and therefore their serum perfluoroalkyl concentrations may not be influenced by the aforementioned reproductive characteristics due to the reaccumulation of perfluoroalkyls. In fact, we did not see different perfluoroalkyl concentrations in relation to time since menopause or the number of live births in the included female participants (data not shown). Altogether, factors that are involved in the PFAS elimination pathways should be taken into account in PFAS epidemiologic studies in which PFAS exposure is determined in blood specimens; stratification and restriction can provide a clear picture of plausible causal associations instead of adjustment as a confounder; and which factors should be considered depend on the life stages of the population and clinical outcomes of interest.
Differential associations by CKD may not be the only reason for the observed potential NMDRs, especially for PFOS, in the present study. NMDR curves are defined as “a nonlinear relationship between dose and effect where the slope of the curve changes sign somewhere within the range of doses examined” (Vandenberg et al., 2012). This is a common feature of endocrine disrupting chemicals like PFAS. A few experimental and epidemiological studies have reported non-linear relationships with PFAS exposure (Bodin et al. 2016; Ding and Park 2020; Hu et al. 2014; Lauritzen et al. 2018; Melzer et al. 2010). Liao et al. (2009a) evaluated neurotoxicity of PFOS using cultured rat hippocampal neurons and observed that glutamate-activated currents were elevated in a low dose of PFOS (1 μM) but suppressed in high doses (10 and 100 μM), suggesting an inverted U-shape (Liao et al. 2009a). A similar NMDR shape was observed in the association between prenatal exposure to PFAS as maternal serum concentrations and BMI z-score and other adiposity measures in children (Braun et al. 2016; Lauritzen et al. 2018). On the other hand, other studies reported non-monotonic J-shaped curves, suggesting a threshold effect such that adverse effects start if exposure levels (doses) are greater than a certain threshold level. Hu et al. reported non-monotonic J-shaped curves in the associations of perfluoroalkyl acids individually and as mixtures with cellular viability of a human liver cell line (Hu et al., 2014). A recent study using NHANES data showed NMDR relationships with thresholds between PFNA and PFDA and hearing impairment, suggesting that ototoxicity of long chain perfluoroalkyl acids may be manifested when exposure exceeds certain levels (Ding and Park, 2020).
Several potential mechanisms have been proposed to demonstrate NMDRs including cytotoxicity, cell- and tissue-specific receptors and cofactors, receptor selectivity, receptor down-regulation and desensitization, receptor competition, and endocrine negative feedback loops (Vandenberg et al., 2012). The nervous system appears to be one of the most sensitive targets of PFAS toxicity. Previous reviews of mechanistic studies have raised concerns that PFAS are neurotoxic chemicals (Mariussen, 2012; Wang et al., 2019). Activation of PPARα or PPARγ that plays a role in lipid homeostasis, inflammation, and peroxisome proliferation, is a putative underlying mechanism demonstrating protective neurologic effects of PFAS (Ren et al., 2009; Takacs and Abbott, 2007; Wolf et al., 2008). PFAS may also disrupt the thyroid system, calcium homeostasis and its related signal pathway, synaptogenesis and synaptic plasticity, neurotransmitters as well as neural cell proliferation and differentiation (Mariussen, 2012; Wang et al., 2019). Some of these effects of PFAS are independent of PPAR activation. Thyroid hormones play key roles in the central nervous system that can manifest as changes in cognitive function (Bavarsad et al., 2019). Low thyroxine (T4) levels were associated with cognitive decline in euthyroid older women (Volpato et al., 2002). It is of interest that the thyroid system seems particularly vulnerable to disruption by PFAS in humans (Bloom et al. 2010; Ji et al. 2012; Lewis et al. 2015; Melzer et al. 2010; Knox et al. 2011; Shrestha et al. 2015; Wang et al. 2014). PFOA and PFOS have been associated with dysregulation of Ca2+ homeostasis (the most important universal second messenger of cells) in hippocampal neurons, which in turn results in extracellular influx and release of intracellular calcium store and hence excitotoxicity and neuronal loss (Mariussen, 2012; Wang et al., 2019). Whether these different mechanisms turn on and off depending on different doses of PFAS found in the human population and hence leading to non-monotonic responses in neuronal cells remain uncertain.
The present study shows an adverse cognitive effect only with PFOS, but not with PFOA, PFNA, and PFHxS. This raises a question, does neurotoxicity of PFAS depend on the functional group, carboxylate vs. sulfonate, and/or the carbon chain lengths? Liao et al. (2009b) tested differential neurotoxicity (e.g., synaptic transmission, calcium current, and neurite growth) of PFAS with varying chain lengths and the functional group on cultured rat hippocampal neurons and observed that larger adverse effects were exhibited in PFAS with longer chain lengths and the sulfonate functional group (PFOS over PFOA), which is in line with our findings (Liao et al. 2009b) Another study using cultured rat cerebellar granule neurons also confirmed that the cytotoxicity of these neurons increases with increasing carbon chain lengths and the sulfonate functional group leads to a greater toxicity than the carboxylate group if the chain length is the same (Berntsen et al., 2017). It should be noted that the doses used in these experimental studies are at the range of or higher than serum concentrations found in occupationally exposed workers. Also, serum concentrations of PFOS (geometric mean=8.38 ng/mL) in the present study were 3-8 times higher than those of PFOA (2.62 ng/mL), PFHxS (1.84 ng/mL) and PFNA (0.95 ng/mL) (Table 1). Therefore, the observed differences in the present study may not necessarily reflect differential responses to chain lengths or functional groups but dose-dependent non-monotonic responses.
The present study also suggests that potential confounding by fish and shellfish consumption could be more than negligible. This phenomenon was more substantial when the analysis was restricted to participants without CKD. For example, the effect estimates changed from −0.037 (95% CI, −0.081, 0.0006; P=0.09) to −0.055 (95% CI, −0.097, −0.012; P=0.01) of the composite z-score for PFOS among participants without CKD (Table 2), approximately a 50% change in the magnitude and a change in statistical significance at α=0.05. Negative confounding by fish consumption or omega-3 fatty acids is well understood in epidemiologic research on environmental toxicants (Choi et al., 2008). Neurotoxicity and cardiovascular toxicity of methylmercury could be masked if potential negative confounding by beneficial nutrients, omega-3 fatty acids, that share the common sources of exposure with methylmercury, fish consumption (Choi et al., 2009, 2008; Guallar et al., 2002). Fish consumption is a major dietary source of PFAS exposure (Christensen et al., 2017; Fromme et al., 2009; Jain, 2014), whereas it is an important source of beneficial nutrients including omega-3 fatty acids against cognitive decline (Kokubun et al., 2020; Zhang et al., 2015). Our findings add to the current literature that negative confounding by fish consumption in the evaluation of neurotoxicants should not be ignored to make unconfounded causal inference.
Our study still has numerous limitations. This study was conducted using NHANES data which is an important strength in terms of generalizability of the findings, but its cross-sectional nature raises an issue of validity of causal inference including reverse causation (Dhingra et al., 2017). Restriction to participants without CKD could be an analytic approach to addressing such a limitation. Epidemiologic studies of PFAS and longitudinal trajectories of cognitive function are urgently needed to address this issue and replicate our findings.
In conclusion, our study suggests that the previous epidemiologic findings of a ‘protective’ association between PFAS and cognition may be explained by CKD, NMDRs and confounding by fish consumption. Taken all these into account, exposure to PFOS at the current population exposure level in the US may be an unrecognized risk factor for cognitive decline in older adults with normal kidney function. The present study suggests important implications for designing and analyzing epidemiologic studies of PFAS and neurological outcomes. Epidemiologists should consider data collection/measurements on factors influencing serum concentrations through the PFAS elimination pathways. For age-related outcomes such as cognitive decline, kidney function measures and CKD are a critical factor. Dose-response studies beyond linearity should be part of data analysis. It is important to include a wide range of PFAS exposures during the design stage which can allow us to distinguish between biologically relevant NMDRs vs. sampling issues. Finally, negative confounding by fish consumption should be included to make a proper causal inference.
Supplementary Material
We examined the association of perfluoroalkyls with cognitive functions
Non-monotonic dose-responses were observed
Perfluorooctane sulfonate (PFOS) had a U-shape dose-response
Substantial negative confounding effects of fish/seafood consumption were observed
PFOS may be a risk factor for cognitive decline in older adults with normal kidney function
Acknowledgements
Dr. Sung Kyun Park was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no competing financial interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- ATSDR, 2018. Toxicological Profile for Perfluoroalkyls. (Draft for Public Comment). Atlanta, GA: Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Barrett ES, Chen C, Thurston SW, Haug LS, Sabaredzovic A, Fjeldheim FN, Frydenberg H, Lipson SF, Ellison PT, Thune I, 2015. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril 103, 1261–1270. e3 10.1016/J.FERTNSTERT.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavarsad K, Hosseini M, Hadjzadeh MAR, Sahebkar A, 2019. The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol 10.1002/jcp.28198 [DOI] [PubMed] [Google Scholar]
- Berger I, Wu S, Masson P, Kelly PJ, Duthie FA, Whiteley W, Parker D, Gillespie D, Webster AC, 2016. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 14, 206 10.1186/s12916-016-0745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen HF, Bjørklund CG, Audinot JN, Hofer T, Verhaegen S, Lentzen E, Gutleb AC, Ropstad E, 2017. Time-dependent effects of perfluorinated compounds on viability in cerebellar granule neurons: Dependence on carbon chain length and functional group attached. Neurotoxicology 63, 70–83. 10.1016/j.neuro.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Bloom MS, Kannan K, Spliethoff HM, Tao L, Aldous KM, Vena JE, 2010. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol. Behav 99, 240–245. 10.1016/j.physbeh.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Bodin J, Groeng EC, Andreassen M, Dirven H, Nygaard UC, 2016. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicol. Reports 3, 664–672. 10.1016/j.toxrep.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodski J, Rossell SL, Castle DJ, Tan EJ, 2019. A systematic review of cognitive impairments associated with kidney failure in adults before natural age-related changes. J. Int. Neuropsychol. Soc 10.1017/S1355617718000917 [DOI] [PubMed] [Google Scholar]
- Campos MW, Serebrisky D, Castaldelli-Maia JM, 2017. Smoking and Cognition. Curr. Drug Abuse Rev. 9, 76–79. 10.2174/1874473709666160803101633 [DOI] [PubMed] [Google Scholar]
- CDC/NCHS, 2013. Laboratory Procedure Manual (Polyfluoroalkyl chemicals) Available: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/pfc_g_met.pdf [accessed 15 June 2020].
- CDC/NCHS, 2012. NHANES 2011-2012: Polyfluoroalkyl Chemicals Data Documentation, Codebook, and Frequencies Available: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PFC_G.htm [accessed 15 June 2020].
- Choi AL, Cordier S, Weihe P, Grandjean P, 2008. Negative confounding in the evaluation of toxicity: The case of methylmercury in fish and seafood. Crit. Rev. Toxicol 10.1080/10408440802273164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen TP, Murata K, Nielsen HP, Petersen MS, Askham J, Grandjean P, 2009. Methylmercury exposure and adverse cardiovascular effects in Faroese Whaling men. Environ. Health Perspect 117, 367–372. 10.1289/ehp.11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Blackowicz M, Liu Y, Thompson BA, Anderson HA, Turyk M, 2017. Perfluoroalkyl substances and fish consumption. Environ. Res 154, 145–151. 10.1016/J.ENVRES.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Conway BN, Badders AN, Costacou T, Arthur JM, Innes KE, 2018. Perfluoroalkyl substances and kidney function in chronic kidney disease, anemia, and diabetes. Diabetes, Metab. Syndr. Obes. Targets Ther 11, 707–716. 10.2147/DMSO.S173809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K, 2017. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Env. Heal. Perspect 125, 416–421. 10.1289/EHP273EHP273 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK, 2020. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women’s Health Across the Nation. Environ. Int 135, 105381 10.1016/j.envint.2019.105381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Park S, 2020. Perfluoroalkyl Substances Exposure and Hearing Impairment in US Adults. Environ. Res 187, 109686 10.1016/j.envres.2020.109686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjønneland A, Overvad K, Sørensen M, 2013. Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population. PLoS One 8, e56969 10.1371/journal.pone.0056969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen T, Chonchol M, Frstl H, Sander D, 2012. Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am. J. Nephrol 10.1159/000338135 [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM, 2010. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 health project. Arch. Pediatr. Adolesc. Med 164, 860–869. 10.1001/archpediatrics.2010.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D, 2009. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Env. Heal 212, 239–270. 10.1016/j.ijheh.2008.04.007S1438-4639(08)00030-8 [pii] [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Brayne C, Armstrong B, Fletcher T, 2013. Serum perfluoroalkyl acids concentrations and memory impairment in a large cross-sectional study. BMJ Open 3 10.1136/bmjopen-2012-002414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, Van’T Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ, 2002. Mercury, fish oils, and the risk of myocardial infarction. N. Engl. J. Med 347, 1747–1754. 10.1056/NEJMoa020157 [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW, 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chem. Res. Toxicol 10.1021/tx200363w [DOI] [PubMed] [Google Scholar]
- Hu J, Li J, Wang J, Zhang A, Dai J, 2014. Synergistic effects of perfluoroalkyl acids mixtures with J-shaped concentration-responses on viability of a human liver cell line. Chemosphere 96, 81–88. 10.1016/j.chemosphere.2013.07.033 [DOI] [PubMed] [Google Scholar]
- Jain RB, 2014. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003-2008. Int J Hyg Env. Heal 217, 52–61. 10.1016/j.ijheh.2013.03.008S1438-4639(13)00046-1 [pii] [DOI] [PubMed] [Google Scholar]
- Jain RB, Ducatman A, 2019. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ. Res 169, 476–482. 10.1016/j.envres.2018.11.033 [DOI] [PubMed] [Google Scholar]
- Ji K, Kim Sunmi, Kho Y, Paek D, Sakong J, Ha J, Kim Sungkyoon, Choi K, 2012. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Environ. Int 45, 78–85. 10.1016/j.envint.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Jiang Q, Gao H, Zhang L, 2015. Chapter 7. Metabolic Effects of PFAS In: DeWitt JC (Ed.), Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Springer International Publishing, Switzerland, pp. 177–202. [Google Scholar]
- Kokubun K, Nemoto K, Yamakawa Y, 2020. Fish Intake May Affect Brain Structure and Improve Cognitive Ability in Healthy People. Front. Aging Neurosci 12, 76 10.3389/fnagi.2020.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, 2015. Chapter 1. Perfluorinated Compounds: An Overview In: DeWitt JC (Ed.), Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Springer International Publishing, Switzerland, pp. 1–21. [Google Scholar]
- Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland JO, Van De Bor M, Jacobsen GW, 2018. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environ. Heal. A Glob. Access Sci. Source 17, 9 10.1186/s12940-017-0338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, 2010. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am. J. Kidney Dis 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Johns LE, Meeker JD, 2015. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011-2012. Int. J. Environ. Res. Public Health 12, 6098–6114. 10.3390/ijerph120606098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang Z, Fu Z, Yan M, Wu N, Wu H, Yin P, 2018. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open 8 10.1136/bmjopen-2017-020533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Cui L, Zhou Q, Duan S, Jiang G, 2009a. Effects of perfluorooctane sulfonate on ion channels and glutamate-activated current in cultured rat hippocampal neurons. Environ. Toxicol. Pharmacol 27, 338–344. 10.1016/j.etap.2008.11.013 [DOI] [PubMed] [Google Scholar]
- Liao C, Wang T, Cui L, Zhou Q, Duan S, Jiang G 2009b. Changes in synaptic transmission, calcium current, and neurite growth by perfluorinated compounds are dependent on the chain length and functional group. Environ. Sci. Technol 43, 2099–2104. doi: 10.1021/es802985e [DOI] [PubMed] [Google Scholar]
- Lin PID, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, Calafat AM, Webster TF, Horton ES, Oken E, 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults—longitudinal analysis of the diabetes prevention program outcomes study. Environ. Int 129, 343–353. 10.1016/j.envint.2019.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, 2012. Neurotoxic effects of perfluoroalkylated compounds: Mechanisms of action and environmental relevance. Arch. Toxicol 10.1007/s00204-012-0822-6 [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS, 2010. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ. Health Perspect 118, 686–692. 10.1289/ehp.0901584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N, 1989. Spatial monitoring in visual working memory. Br. J. Psychol 80, 333–349. 10.1111/j.2044-8295.1989.tb02324.x [DOI] [PubMed] [Google Scholar]
- NCHS (National Center for Health Statistics), 2011. NHANES: Aalytic Guidelines, 2011-2014 and 2015-2016. [Google Scholar]
- Nelson JW, Hatch EE, Webster TF, 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect 118, 197–202. 10.1289/ehp.0901165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD, 2019. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res 175, 186–199. 10.1016/j.envres.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, Gleason JA, Cooper KR, 2017. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS Biol. 15, e2002855 10.1371/journal.pbio.2002855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Webster TF, Baccarelli AA, Weisskopf MG, 2013. Cross-sectional association between polyfluoroalkyl chemicals and cognitive limitation in the national health and nutrition examination survey. Neuroepidemiology. 10.1159/000342310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH, 2006. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol 10.1021/es0512475 [DOI] [PubMed] [Google Scholar]
- Ren H, Vallanat B, Nelson DM, Yeung LWY, Guruge KS, Lam PKS, Lehman-McKeeman LD, Corton JC, 2009. Evidence for the involvement of xenobiotic-responsive nuclear receptors in transcriptional effects upon perfluoroalkyl acid exposure in diverse species. Reprod. Toxicol 27, 266–277. 10.1016/j.reprotox.2008.12.011 [DOI] [PubMed] [Google Scholar]
- S. Knox S, Jackson T, J. Frisbee S, Javins B, M. Ducatman A, 2011. Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J. Toxicol. Sci 36, 403–410. 10.2131/jts.36.403 [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A, 2011. Perfluoroalkyl Chemicals and Chronic Kidney Disease in US Adults. Am. J. Epidemiol 174, 893–900. 10.1093/aje/kwr171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankle WR, Romney AK, Rara J, Fortier D, Dick MB, Chen JM, Chan T, Sun X, 2005. Methods to improve the detection of mild cognitive impairment. Proc. Natl. Acad. Sci. U. S. A 102, 4919–4924. 10.1073/pnas.0501157102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Bloom MS, Yucel R, Seegal RF, Rej R, McCaffrey RJ, Wu Q, Kannan K, Fitzgerald EF, 2017. Perfluoroalkyl substances, thyroid hormones, and neuropsychological status in older adults. Int. J. Hyg. Environ. Health 220, 679–685. 10.1016/j.ijheh.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Shrestha S, Bloom MS, Yucel R, Seegal RF, Wu Q, Kannan K, Rej R, Fitzgerald EF, 2015. Perfluoroalkyl substances and thyroid function in older adults. Environ. Int 75, 206–214. 10.1016/j.envint.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbø M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, Longnecker MP, 2014. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ. Int 62, 104–112. 10.1016/j.envint.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V, 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol 170, 1268–1278. 10.1093/aje/kwp279 [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O, 2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Appl. Neuropsychol 14, 62–63. 10.1080/09084280701280502 [DOI] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol 10.1038/s41370-018-0094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, 2007. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev 10.1007/s11065-007-9035-9 [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD, 2007. Activation of mouse and human peroxisome proliferator-activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci 95, 108–117. 10.1093/toxsci/kfl135 [DOI] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL, 2014. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ. Health Perspect 122, 145–50. 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, 2007. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16, 219–242. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 33, 378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalotti JA, Stevens LA, Levey AS, 2007. Testing for Chronic Kidney Disease: A Position Statement From the National Kidney Foundation. Am. J. Kidney Dis 50, 169–180. 10.1053/j.ajkd.2007.06.013 [DOI] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ, 2002. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology 58, 1055–1061. 10.1212/WNL.58.7.1055 [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP, Wang SL, 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect 122, 529–534. 10.1289/ehp.1306925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang L, Chang W, Zhang Yinfeng, Zhang Yuan, Liu W, 2019. Neurotoxic effects of perfluoroalkyl acids: Neurobehavioral deficit and its molecular mechanism. Toxicol. Lett 10.1016/j.toxlet.2019.01.012 [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA, 2013. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ. Health Perspect 121, 625–630. 10.1289/ehp.1205838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. Wechsler Adult Intelligence Scale. 3rd Edition Psychol. Corp; San Antonio [Google Scholar]
- Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD, 2008. Activation of Mouse and Human Peroxisome ProliferatorÀActivated Receptor Alpha by Perfluoroalkyl Acids of Different Functional Groups and Chain Lengths. Toxicol. Sci 106, 162–171. 10.1093/toxsci/kfn166 [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT, 2014. Enhanced Elimination of Perfluorooctane Sulfonic Acid by Menstruating Women: Evidence from Population-Based Pharmacokinetic Modeling. Environ. Sci. Technol 48, 8807–8814. 10.1021/es500796y [DOI] [PubMed] [Google Scholar]
- Wood SN, 2006. Generalized additive models: an introduction with R. Chapman & Hall/CRC [Google Scholar]
- Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J, 2015. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies 1–3. Am. J. Clin. Nutr 103, 330–340. 10.3945/ajcn.115.124081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


